Abstract
The effect of interaural time difference (ITD) and interaural level difference (ILD) on wave 4 of the binaural and summed monaural auditory brainstem responses (ABRs) as well as on the DN1 component of the binaural interaction component (BIC) of the ABR in young and old Mongolian gerbils (Meriones unguiculatus) was investigated. Measurements were made at a fixed sound pressure level (SPL) and a fixed level above visually detected ABR threshold to compensate for individual hearing threshold differences. In both stimulation modes (fixed SPL and fixed level above visually detected ABR threshold) an effect of ITD on the latency and the amplitude of wave 4 as well as of the BIC was observed. With increasing absolute ITD values BIC latencies were increased and amplitudes were decreased. ILD had a much smaller effect on these measures. Old animals showed a reduced amplitude of the DN1 component. This difference was due to a smaller wave 4 in the summed monaural ABRs of old animals compared to young animals whereas wave 4 in the binaural-evoked ABR showed no age-related difference. In old animals the small amplitude of the DN1 component was correlated with small binaural-evoked wave 1 and wave 3 amplitudes. This suggests that the reduced peripheral input affects central binaural processing which is reflected in the BIC.
Keywords: Binaural hearing, superior olivary complex, BIC, mammal, ITD, ILD
1. Introduction
Binaural hearing has relevance for localizing sound sources (Middlebrooks and Green, 1991) and perceiving signals in a noisy environment (e.g., speech signals; Hawley et al., 2004). With advanced age humans may show difficulties in binaural hearing without revealing any abnormalities in the audiogram (Pichora-Fuller and Souza, 2003; Abel et al., 2000). One possible explanation for the deficiencies is a peripheral pathology known as a “hidden hearing loss” being attributed to a loss of inner hair cell (IHC) synaptic ribbons and spiral ganglion neurons (e.g., Plack et al., 2014; Moore, 2014) resulting in a reduced input to the central auditory pathway. In addition, degeneration processes in the auditory brainstem (Gleich and Strutz, 2002; Gleich et al., 2004) and a reduced availability of inhibitory neurotransmitters with increasing age (Caspary et al., 2008) may affect neuronal structures involved in processing binaural cues. Appropriate animal models are essential to provide the link between the physiological changes with age and the perceptual deficits in binaural hearing (Plack et al., 2014).
One technique to test hearing abilities in an objective and non-invasive way is the auditory brainstem response (ABR). The different ABR waves can be assigned to the activity of different stages within the auditory pathway. In all mammals, wave 1 likely represents the auditory nerve activity (Boettcher, 2002). The occurrence of wave 3 correlates with the integrity of the MNTB or its output (Wada and Starr, 1989, 1983; Jalabi et al., 2013) although Boettcher (2002) suggested that it is based on the activity of the cochlear nucleus. Wave 4 in the gerbil and cat and wave 5 in humans are attributed to more central stages of the auditory pathway already representing binaural cues like neurons in the medial superior olive (MSO) and the lateral superior olive (LSO) and fibers from the superior olivary complex (SOC) to the lateral lemniscus (LL; Boettcher, 2002; Riedel and Kollmeier, 2002; Ungan and Yagcioglu 2002; Grothe et al., 2010). In humans (Riedel and Kollmeier, 2002), cats (Ungan et al., 1997; Ungan and Yagcioglu 2002) and rodents (Goksoy et al., 2005), a specific binaural interaction component (BIC) of the ABR has been observed. Latency and amplitude measures of the ABR waves and BIC allow drawing conclusions about the functioning of certain stages within the auditory pathway.
The BIC depicts the difference between the ABR derived by binaural stimulation and the sum of both the left and right monaural ABR and it reflects binaural interaction. The binaurally evoked wave 4 response was shown to be smaller than the sum of the monaural responses in several animal models such as guinea pigs (Dobie and Berlin, 1979; Goksoy et al., 2005) and cats (Ungan et al., 1997) and similar effects were observed in wave 5 of humans (Riedel and Kollmeier, 2002) resulting in a prominent negative peak of the BIC termed DN1 reflecting the processing of binaural cues. The two main cues relevant for binaural hearing are interaural time (ITD) and interaural level differences (ILD) being processed in MSO and LSO, respectively (Grothe et al., 2010). In humans and in animal models, these cues have been shown to affect the DN1 component of the BIC.
In clinical populations, BIC measurements have been employed to evaluate the integrity of binaural processing (e.g., Furst et al., 1985; Gordon et al., 2012). The effect of age on the BIC representing the binaural hearing has so far received only little attention (Fowler, 2004), and a systematic investigation how the effect of the binaural cues on the BIC is modified with age is lacking. Here we present data on the BIC in the Mongolian gerbil (Meriones unguiculatus), a rodent animal model that has a high sensitivity in the low frequency range (1 to 10 kHz) being similar to that of humans (Ryan, 1976) and is a prime species for studying the effects of aging (Cheal, 1986). Old gerbils (> 36 months) show a similar pattern of hearing loss as 60–65 year old male humans (e.g. Gleich et al., 2003; Mills et al., 1990).Here we investigated how ITD and ILD affected the BIC in young and old gerbils and draw conclusion whether changes in the periphery or more central parts of the auditory pathway are more likely to be related to the perceptual deficits typically found in binaural hearing of elderly human subjects. To this end, the results can serve to establish the gerbil as a suitable model species for a causal analysis of binaural perceptual deficits.
2. Material and Methods
2.1 Animals
Data were obtained in 11 young (2–10 months; 3 females) and 11 old (36–39 months; 2 females) Mongolian Gerbils (Meriones unguiculatus). ABRs were recorded from anesthetized animals using a mixture of ketamine (70mg/kg) and xylazine (3mg/kg). An anesthesia maintenance dose of around 1/3 of the initial dose was given if necessary. The core body temperature was maintained by an electronically controlled heating blanket.
The care and treatment of the gerbils were in accordance with the procedures of animal experimentation approved by the Government of Lower Saxony, Germany. All procedures were performed in compliance with the NIH Guide on Methods and Welfare Consideration in Behavioral Research with Animals (National Institute of Mental Health, 2002).
2.2 Stimuli
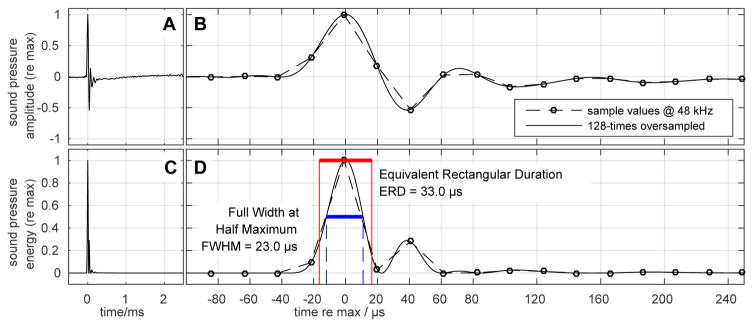
Monaural and binaural click evoked ABRs were recorded. The click stimulus (Fig. 1) had a duration of about 33 μs (equivalent rectangular duration, measured acoustically, 20.8 μs electrically) and a flat frequency spectrum between 1 and 24 kHz. The most important component of a click stimulus is the steep onset to elicit a strong and clear ABR signal (Beutelmann et al., 2015). Therefore, the signals were filtered by a 128th order minimum phase FIR filter before presentation, which equalizes the amplitude spectrum and compresses the impulse response duration at the ear canal entrance. The setup and generation of the stimuli were described in detail in (Beutelmann et al., 2015). Briefly, sampling rate for playback and recording of responses through an RME Hammerfall Multiface II sound card was 48 kHz. Playback and recording were controlled by custom software using MATLAB (The Mathworks, Inc., Natick, MA). The click stimuli were amplified by a Harman-Kardon HK6350 amplifier and presented by Vifa XT300/K4 speakers. The speakers were coupled to the ear canal of the gerbil by a custom built combination of exponential (100 mm length) and conical (20 mm length) horns (for detail of system calibration see Beutelmann et al., 2015). Acoustical crosstalk was measured between both speakers and the corresponding opposite Etymotic ER-7C probe microphone positioned at a place 2 mm into the ear canal. The average interaural attenuation across the relevant frequency range was between 30 and 50 dB, depending on animal and setup preparation. It was never below 30 dB.
Figure 1.
Waveform (A, B) of the click (90 dB pSPL) measured with an Etymotic ER-7C probe microphone at a place 2 mm into the gerbil ear canal and the corresponding sound energy trace (C, D).
Responses were recorded at 90 dB peak SPL (pSPL) and 20 dB above visual ABR detection threshold (abbreviated as 20 dB AVT; see table 1 in supplementary material). The stimuli were pre-generated, filtered and scaled for spectral and level calibration, and played with randomized interstimulus time intervals. The average inter-stimulus interval was 30 ms, with a standard deviation of 10 ms. The stimuli were played with alternating signs (condensation and rarefaction clicks) in order to cancel out the stimulus artifact in the recording.
A recording session started with determining the visual detection threshold of the ABR for monaural presentation by playing the click at different intensities. ABR recordings for monaural and binaural presentation (ITD = 0, ILD = 0) were then obtained for a range of sound intensities up to 100 dB pSPL in steps of 10 dB. Then monaural and binaural-evoked ABRs were also measured in separate series varying the click ITD and ILD, respectively. To obtain symmetrical effects from stimulation of the left and right ear on the ABR (see Ungan et al., 1997), the ITDs were shifted by half the total ITD in opposite directions in each ear. For the different ILDs the sound pressure level was increased by ILD/2 at the one ear and decreased by ILD/2 at the other ear. The ITD series included ITDs from - 1000 μs (left side leading) to + 1000 μs (right side leading) in steps of 250 μs and ITDS of ± 2000 μs (except one young animal that was only stimulated with ITDs of 0, ± 500, ± 1000 and ± 2000 μs). The ILD series included ILDs of 0, ± 10, ± 20 and ± 30 dB SPL, with negative and positive ILDs indicating the higher level at the left or right ear, respectively. The presentation of binaural and corresponding monaural stimuli was randomly mixed. Each stimulus was repeated until at least 500 of the corresponding recorded ABR intervals were artifact-free.
2.3 Recording and processing of responses
The ABR was recorded between needle electrodes placed at the vertex (active) and at the neck. A butterfly syringe for maintenance of anesthesia served as the ground electrode (electrodes moistened with physiological saline solution). The electrode signal was amplified by a factor of 10000 (World Precision Instruments ISO-80 Bio-amplifier) filtered between 300 and 3000 Hz and recorded on hard disk through the RME sound card. A recording interval started −4 ms before the click and had a total duration of 15 ms. All intervals were stored on disk regardless of the maximum response amplitude. Intervals of the recording with a maximum response amplitude less than about 25 μV were considered free of artifact due to contribution of heart or muscle potentials. For further analysis, the raw recordings were averaged using the procedure proposed by Riedel et al. (2001) and Granzow et al. (2001). In this two-step averaging procedure, the recordings are averaged with weights reciprocal of their signal-to-noise ratio. In the first step, the variance of each interval is used as a coarse estimate. In the second step, the weights are calculated after subtracting the first step average from each interval. Details on the signal processing are presented in Beutelmann et al., 2015.
The binaural interaction component was calculated by subtracting the sum of the two monaural ABRs from the binaural-evoked response: BIC = Binaural ABR − (left ABR + right ABR). For the calculation of the sum of the monaural ABRs for different ITDs the monaural ABRs were shifted with half of the ITD identical to the binaural stimulation. For the ILD conditions the monaural ABRs with the sound pressure level corresponding to the binaural stimulation were used. ABR waves in the binaural and summed potential were identified as local maxima and BDP waves as local minima by a custom MATLAB procedure. With this software it was possible to analyze all local maxima and minima by shifting the cursor on the recorded traces to the appropriate peak. For this study wave 1, wave 3, wave 4 and DN1 were analyzed. By visual inspection of the recorded potentials for all ILD conditions (ITD = 0 μs) wave 4 in the binaural as well as in the summed monaural ABR recording was identified correlating in latency with the prominent DN1 component (Fig. 2). For the different non-zero ITD conditions wave 4 was tracked comparing the waveform with that obtained for an ITD of 0 μs. Usually, the latency of the wave 4 response is found in a ± 0.5 ms interval centered at a latency shifted by an amount corresponding to the imposed ITD. If no local maxima could be detected in one of the potentials wave 4 latency was set manually to the latency of wave 4 in the summed potential or the binaural potential, respectively. The DN1 was identified as a local minimum in an interval of ± 1 ms starting at the latency of the corresponding binaural-evoked wave 4. In separate measurement, the amplitude and latency of wave 1 and 4 of the monaural, summed monaural and binaural potentials were determined for different intensities starting from the visually detected threshold for an ITD of 0 μs and an ILD of 0 dB. Wave 1 was always clearly identifiable for the highest sound pressure level measured as the first local maximum. In general, a latency shift of 0.2 ms per 10 dB step was found and wave 1 was identified as the maximum peak within a range of ± 0.45 ms of this general shift. For wave 4 the range in which the peak could be observed was ± 0.75 ms. The latency for all waves was referenced to the onset of the leading stimulus and the amplitude was referenced to 0 μV.
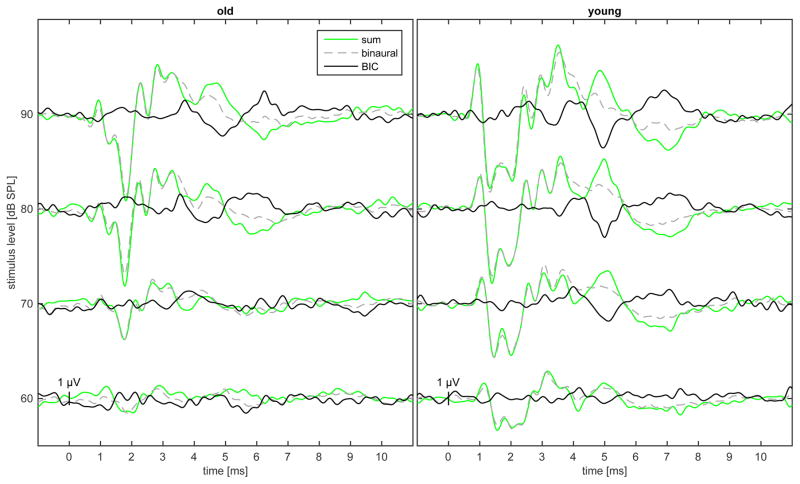
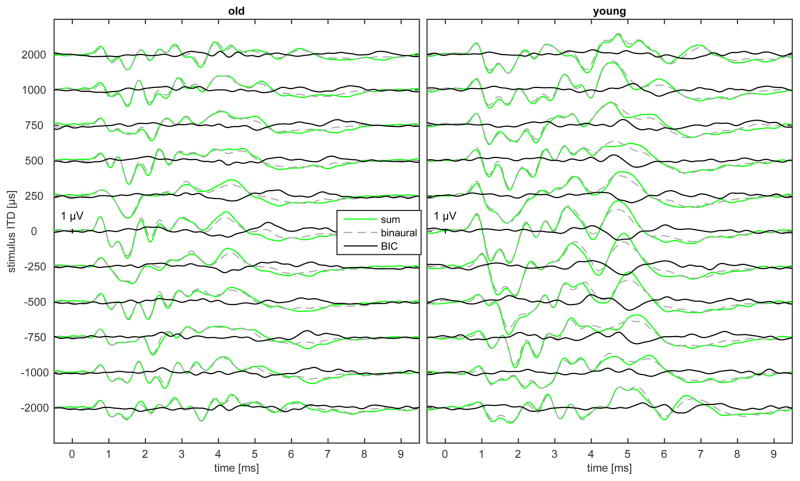
Figure 2.
Exemplary ABR in one old and one young gerbil for different ITDs ranging from-2000 μs to + 2000 μs at 90dB pSPL measured with the click stimulus. The green line represents the sum of the two monaural responses whereas the dashed grey line indicates the binaural ABR obtained by stimulation of both ears. The black line displays the binaural interaction component (BIC) which is the subtraction from both ABRs. The most prominent BDP component DN1 is marked with a x.
3. Results
We measured wave 1, 3 and wave 4 of the ABR and the BIC in the Mongolian gerbil and showed that also in this species there was a discernable DN1 component corresponding in latency to wave 4 of the ABR (Boettcher et al., 1993; Boettcher, 2002). The most prominent component of the BIC was the negative DN1 potential resulting from a smaller wave 4 in the binaural-evoked ABR in comparison to the summation of the monaural ABRs (Fig. 3).
Figure 3.
Exemplary ABR in one old and one young gerbil for different intensities ranging from 60 dB pSPL to 90 dB pSPL measured with the click stimulus. The green line represents the sum of the two monaural responses whereas the dashed grey line indicates the binaural ABR obtained by stimulation of both ears. The black line displays the binaural interaction component (BIC) which is the subtraction from both ABRs. Wave 1, 3 and 4 are marked with the corresponding number. The most prominent BDP component DN1 is labeled.
3.1 Effects of binaural cues and age on ABR wave 4 and the BIC at a fixed sound pressure level
We analyzed the effect of ITD and ILD on the latency and amplitude of the DN1 component as well as on the corresponding ABR wave 4 in the binaural and summed monaural potentials for a fixed stimulus level of 90 dB pSPL in young and old gerbils. For each of these response components (i.e, DN1, binaural and summed monaural ABR wave 4) the effects of ITD and age (fixed factors) as well as of ILD and age (fixed factors) were analyzed separately by a Generalized Linear Mixed Model (GLMM) ANOVA with latency or amplitude (response measures) as the dependent variable and animal subject being an additional random factor (for excluding effects of animals subject in the repeated measurements).
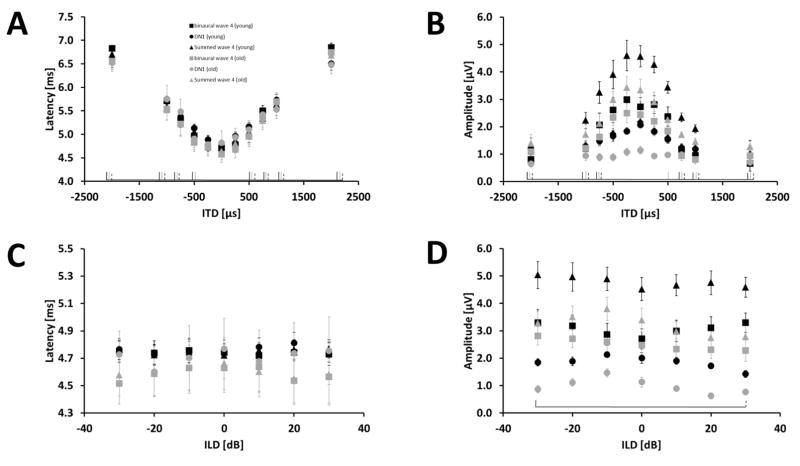
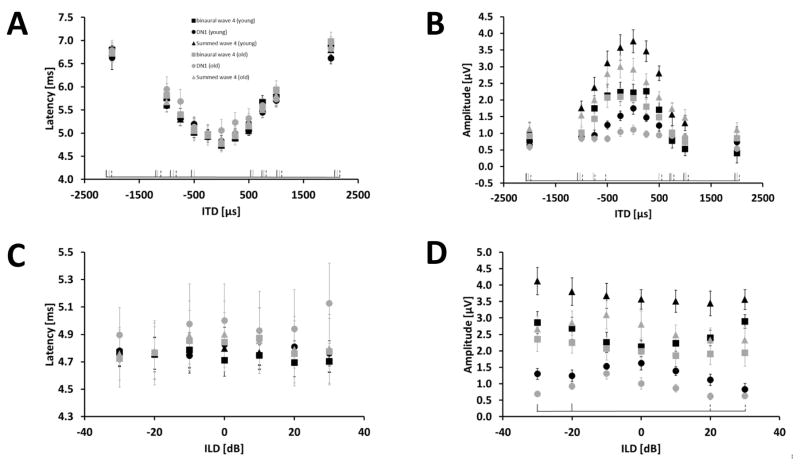
In the GLMM ANOVA focusing on ITD and age, ITD had a significant effect on the latency of the binaural-evoked wave 4, the summed wave 4 and the DN1 component (binaural-evoked wave 4 (BIN): p < 0.001, F(10,156) = 311.508; summed monaural wave 4 (SUM): p < 0.001, F(10,156) = 459.159; DN1: p < 0.001, F(10,156) = 94.190). Age had no significant effect on any of the analyzed ABR wave 4 and the DN1 component latencies (p > 0.05). The latency in all analyzed response components increased with increasing absolute ITD value from about 4.7 ms for a “centrally located” stimulus with an ITD of 0 μs to about 6.7 ms for an ITD of ± 2000 μs (Fig. 2 and 4 A). There were no significant interactions between the main effects. The GLMM ANOVA revealed a significant effect of ITD on the amplitude of wave 4 in the binaural as well as in the summed monaural ABR and the DN1 component (BIN: p < 0.001, F(10,156) = 33.892; SUM: p < 0.001, F(10,156) = 51.212; DN1: p < 0.001, F(10,156) = 10.195). A decrease in amplitude for all analyzed response components with increasing deviation of the ITD from 0 μs was observed (Fig. 4 B). In addition, age had a significant effect on the amplitude of DN1 (p < 0.001, F(1,16) =19.891) and the summed wave 4 (p = 0.05, F(1,16) = 4.496) but not on the amplitude of the binaural-evoked ABR wave 4. The DN1 component and summed wave 4 in old animals was smaller than in the younger animals. This difference was about 45 % for the DN1 component and 27 % for summed wave 4 at the central ITD of 0 μs. At the highest ITD of ± 2000 μs the difference was about 34 % for the DN1 component and close to 0 % for the summed monaural ABR wave 4, respectively. For these two response components also the interaction between ITD and age was significant (SUM: p = 0.002, F(10,156) = 2.939; DN1: p = 0.005, F(10,156) = 2.687). In young animals ITD affected the amplitude of both response components more than in old animals.
Figure 4.
Effects of ITD and ILD and age on the latency and amplitude on wave 4 in the binaural ABR and summed monaural ABRs as well as the DN1 component at a stimulus intensity of 90 dB pSPL. Results for wave 4 in the binaural ABR are indicated by squares and in the summed monaural ABR by triangles. Results for the DN1 component are indicated by circles. The different age groups are represented by black and grey for young and old gerbils, respectively. At the bottom of each subplot, significant differences to ITD of 0 μs or ILD of 0 dB are indicated by black solid lines for binaural-evoked wave 4, with grey solid lines for wave 4 in the summed monaural ABRs and with black dashed lines for the DN1 component. (A) Latency in ms (± S.E.M) for different ITDs in μs. (B) Amplitude in μV (± S.E.M) for different ITDs in μs. (C) Latency in ms (± S.E.M) for different ILDs in dB SPL. (D) Amplitude in μV (± S.E.M) for different ILDs in dB SPL. Sample size: ITD: Young: 9 animals for 0,± 500, ± 1000, ± 2000; 8 animals for ± 250, ± 750. Old: 9 animals. ILD: Young and Old: 9 animals.
In the GLMM ANOVA focusing on ILD and age, ILD as well as age had no effect on the latency of all tested response components (DN1 component, wave 4 in the binaural and summed monaural ABRs; Fig. 4 C). There was a significant main effect of the ILD on the amplitude of all analyzed response components (BIN: p = 0.001, F (6,96) = 4.317; SUM: p = 0.001, F(6,96) = 4.190; DN1: p < 0.001, F(6,96) = 5.881). For both age groups the amplitude of the DN1 component decreased with increasing ILD with a maximum amplitude at −10 dB SPL ILD (Fig. 4 D). Age had a significant effect on the amplitude of the summed wave 4 and the DN1 component (SUM: p = 0.015, F(1,16) = 7.486; DN1: p < 0.001, F(1,16) =50.571). Old animals displayed a reduced amplitude for wave 4 in the summed potential and the DN1 component of 25 % and 44 %, respectively, in comparison to the young animals at an ILD of 0 dB. This reduction increased to 44 % and 50 % for the summed wave 4 and the DN1 component, respectively, at the highest ILD values. In addition, the GLMM ANOVA revealed a significant interaction of ILD and age for the binaural-evoked wave 4 amplitude (p = 0.003; F(6,96) = 3.559). In the young animals the amplitude increased with increasing deviation of ILD from 0 dB whereas no such trend can be seen in the data from old animals.
3.2 Effects of binaural cues and age on ABR wave 4 and the BIC at a fixed level above visual detected ABR threshold
To account for possible differences in the hearing thresholds between individuals and especially age groups, we also analyzed the effects of ITDs and ILDs on the latency and amplitude of the different response components (DN1 component, ABR wave 4 in different types of stimulation (binaural and summed monaural)) at an intensity of 20 dB AVT. The effects of ITD and age as well as the effects of ILD and age on the different analyzed ABR response component amplitudes and latencies were analyzed by separate GLMM ANOVAs with latency or amplitude being the dependent variable, age and one of the binaural cues as fixed factors and animal subject as a random factor.
In the GLMM ANOVA focusing on ITD and age, ITD had a significant effect on the latency for all three examined ABR response components (BIN: p < 0.001, F(10,150) = 201.7; SUM: p < 0.001, F(10,150) = 343.658; DN1: p < 0.001, F(10,150) = 84.237). Age had no significant effect on any of the analyzed ABR response component latencies (p > 0.05). The latency increase was similar to that observed for stimulating with 90 dB pSPL clicks (i.e., about 4.8 ms for an ITD of 0 μs and about 6.7 ms for an ITD of ± 2000 μs, Fig. 5 A). ITD also had a significant effect on the amplitude of all three ABR response components (BIN: p < 0.001, F(10,150) = 28.827; SUM: p < 0.001, F(10,150) = 57.653; DN1: p < 0.001, F(10,150) = 16.783). As for the fixed stimulation level, the amplitude for all examined response components decreased with increasing ITD in both age groups (Fig. 5 B). In addition, the GLMM ANOVA revealed a significant interaction between ITD and age for wave 4 in the summed monaural ABRs and the DN1 component. (SUM: p < 0.001, F(10,150) = 3.440; DN1: p < 0.001, F(10,150) =3.536). The reduction in amplitude with increased ITD of the summed wave 4 (young: 74 %; old: 62 %) as well as for the DN1 component (young: 61 %; old: 49 %) was larger in the young animals in comparison to the old animals. The old animals showed a reduced amplitude for the DN1 component of about 37 % in comparison of young animals at an ITD of 0 μs which decreased to about 16% for the highest ITD values. The effect of age on the DN1 amplitude failed to reach statistical significance (p = 0.073, F(1,15) = 3.711).
Figure 5.
Effects of ITD and ILD and age on the latency and amplitude on wave 4 in the binaural ABR and summed monaural ABRs as well as the DN1 component at a stimulus intensity of 20 dB AVT above visual detected ABR threshold. Results for wave 4 in the binaural ABR are indicated by squares and in the summed monaural ABRs by triangles. Results for the DN1 component are indicated by circles. The different age groups are represented by black and grey for young and old gerbils, respectively. At the bottom of each subplot, significant differences to ITD of 0 μs or ILD of 0 dB are indicated by black solid lines for binaural-evoked wave 4, with grey solid lines for wave 4 in the summed monaural ABRs and with black dashed lines for the DN1 component. (A) Latency in ms (± S.E.M) for different ITDs in μs. (B) Amplitude in μV (± S.E.M) for different ITDs in μs. (C) Latency in ms (± S.E.M) for different ILDs in dB SPL. (D) Amplitude in μV (± S.E.M) for different ILDs in dB SPL. Sample size: ITD: Young and Old: 8 animals. ILD: Young: 7 animals. Old: 8 animals.
In the GLMM ANOVA focusing on ILD and age, ILD as well as age had no effect on the latency of wave 4 in the binaural and summed monaural ABRs as well as the DN1 component (Fig. 5 C). The GLMM ANOVA revealed a significant effect of ILD on the amplitude of all three ABR response components (BIN: p > 0.001, F(6,78) = 5.747; SUM: p = 0.001, F(6,78) = 4.050; DN1: p < 0.001, F(6,78) = 8.941). The amplitude of the DN1 component decreased with increasing ILD for both age groups (Fig. 5 D). Age had an significant influence on the amplitude of the DN1 component (p = 0.006, F(1,13) = 10.529). The effect of age on the amplitude of wave 4 in the summed monaural potential failed to reach statistical significance (p = 0.062, F(1,13) = 4.209). The old animals showed a reduced amplitude for the DN1 component of about 38 % in comparison to the young animals at an ILD of 0 dB which decreased to about 36 % for the highest ILD values.
3.3 Effects of age and sound pressure level on wave 1, wave 3 and wave 4
To investigate if the age effect on the DN1 component seen in the ITD and ILD measurements is due to changes in the monaural peripheral (e.g., auditory nerve, cochlear nucleus, etc.) or the binaural (LSO, MSO, etc.) central part of the auditory pathway we examined the latency and amplitude of wave 1, wave 3 and wave 4 in relation to the click sound pressure level for the different types of stimulation: left and right monaural, binaural and summed monaural measurement in the young and old animals. Wave 1 is due to the activity in the auditory nerve and therefore represents the peripheral part of the auditory pathway whereas wave 4 is likely to arise in fibers from the SOC (which include the binaural nuclei the LSO and MSO) to LL and represents the central part of the auditory pathway (Boettcher, 2002). Previous studies have related wave 3 to the integrity of the MNTB or its output (Wada and Starr, 1989, 1983, Jalabi et al 2013).
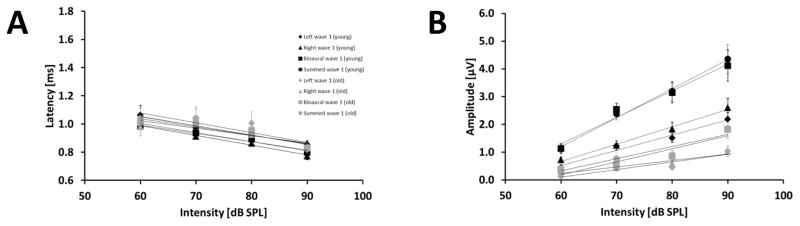
GLMM ANOVAs with the latency or amplitude as the dependent variable, age and sound pressure level as fixed factors and animal as a random factor were conducted for all types of stimulation (left and right monaural, binaural and summed monaural ABR) separately. The GLMM ANOVA revealed a significant effect of sound pressure level on wave 1 latency for all types of stimulation (Left monaural ABR (LMON): p < 0.001, F(3,43) = 8.325; Right monaural ABR (RMON): p < 0.001, F(3,42) = 52.363; BIN: p < 0.001, F(3,42) = 172.734; SUM: p < 0.001, F(3,42) = 25.632). The latency of wave 1 in the binaural, summed monaural and, left and right monaural stimulation type decreased with increasing sound pressure level in both age groups (Fig. 6 A). Older animals revealed longer wave 1 latencies for all types of stimulation in comparison to young animals at intensities of 60, 70 and 80 dB pSPL (Fig. 6 A). For all types of stimulation, the wave 1 latency-intensity function are very similar between young and old animals (Young: −0.0067 (LMON), −0.0069 (RMON), −0.0065 (BIN), −0.0059 (SUM); Old: −0.007 (LMON), −0.0053 (RMON), −0.0059 (BIN), −0.0065 (SUM)). The GLMM ANOVA also revealed a significant effect of the sound pressure level on wave 1 amplitude for all types of stimulation (LMON: p < 0.001, F(3,43) = 32.376; RMON: p < 0.001, F(3,45) = 33.557; BIN: p < 0.001, F(3,45) = 40.334; SUM: p < 0.001, F(3,46) = 36.983). Figure 6 B shows that the amplitude of wave 1 in the binaural, summed and monaural ABRs increased with increasing sound pressure level. Age had a significant effect on wave 1 amplitude for all stimulation types (LMON: p < 0.001, F(1,22) = 22.141; RMON: p < 0.001, F(1,21) = 21.176; BIN: p < 0.001, F(1,21) = 26.864; SUM: p < 0.001, F(1,21) = 28.082). The younger animals showed a larger wave 1 amplitude for all types of stimulation in comparison to the old animals at intensities of 60, 70, 80 and 90 dB pSPL. Furthermore also the interaction of age and sound pressure level was significant for the amplitude of wave 1 in the right monaural, binaural and summed monaural ABR (RMON: p = 0.003, F(3,45) = 5.497; BIN: p = 0.013, F(3,45) = 4.013; SUM: p = 0.005, F(3,46) = 4.911). The wave 1 amplitude-intensity function of young animals showed a steeper slope [μV/dB] (Young: 0.0548 (LMON), 0.0624 (RMON), 0.0957 (BIN), 0.1407 (SUM); Old: 0.0275 (LMON), 0.0232 (RMON), 0.0469 (BIN), 0.0441 (SUM)) for all types of stimulation in comparison to the old ones. There was no significant wave 1 difference between the left and right monaural potentials in the young and old animals, with the exception of the latency at 90 dB pSPL for young animals (p = 0.048, t = 2.144, t-test).
Figure 6.
Effect of sound pressure level and age on wave 1 in the binaural, summed monaural, left and right monaural ABR. Results for wave 1 in the binaural ABR are indicated by squares and in the summed monaural ABR by circles. Results for wave 1 in left and right monaural ABR are indicated by a diamond and triangle, respectively. The different age groups are represented by black and grey for young and old gerbils, respectively. Linear regression lines for all stimulation modes are shown in addition to the data points. See text for information about the slope of the functions. (A): Latency in ms (± S.E.M) of wave 1 for different intensities in dB pSPL. Left, right and binaural-evoked wave 1 are shown. Young animals are displayed in black and old animals are displayed in grey. (B) Amplitude in μV (± S.E.M) of wave 1 for different intensities in dB pSPL. Left, right and binaural-evoked wave 1 are shown. Young animals are displayed in black and old animals are displayed in grey. Sample size: Young: 60 dB: 11 animals, except left: 10 animals; 70 dB: 9 animals; 80 dB: 11 animals; 90 dB: 9 animals. Old: 60 dB: 4 animals, except left: 3 animals; 70 dB: 8 animals, except left: 7 animals; 80 dB: 8 animals; 90 dB: 10 animals.
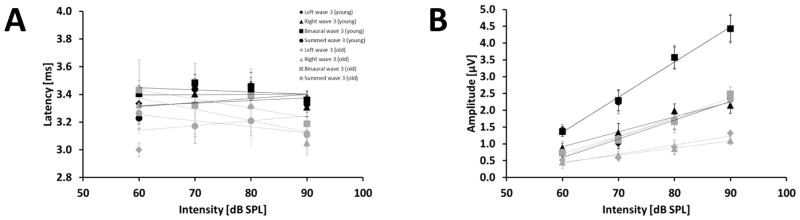
Figure 7 A showed that there was no effect of the intensity on latency of wave 3 for all types of stimulation in the young as well as in the old group (Fig. 7 A; age had no effect in the GLMM ANOVA). The wave 3 latency-intensity function of old animals revealed negative slopes [ms/dB] for the monaural right, binaural and summation of the monaural stimulations (Old: −0.0082 (RMON), −0.0067 (BIN), −0.0044 (SUM)), whereas the young animals only revealed a slightly shallower negative slope for the binaural stimulation type (Young: −0.0015 (BIN)). The wave 3 latency-intensity function of the other stimulation types showed a positive slope for the young animals (Young: 0.0002 (LMON), 0.0019 (RMON), 0.0029 (SUM)), whereby the slope of the function was steeper for the left monaural stimulation type in the old animals (Old: 0.0035 (LMON)). The latency intensity function for wave 3 in the binaural ABR showed a decrease in the old animals and an increase in the young animals supported by a significant interaction between age and intensity (p = 0.011, F(3,42) = 4.185). We also examined the effects of age and sound pressure level on the amplitude of wave 3 for all types of stimulation, and found a significant effect of sound pressure level on the amplitude (LMON: p < 0.001, F(3,43) = 42.168; RMON: p < 0.001, F(3,46) = 20.751; BIN: p < 0.001, F(3,44) = 76.756; SUM: p < 0.001, F(3,45) = 51.581). Figure 7 B shows that in both age groups the amplitude of wave 3 for all types of stimulation increased with increasing sound pressure level. The GLMM ANOVA also showed a significant main effect of age on the wave 3 amplitude for all types of stimulation (LMON: p = 0.002, F(1,23) = 12.355; RMON: p < 0.001, F(1,22) = 17.789; BIN: p <0.001, F(1,20) = 23.054; SUM: p < 0.001, F(1,21) = 22.708). The younger animals showed larger wave 3 amplitudes for all types of stimulation in comparison to the older animals for every sound pressure level. Differences in the monaural potentials could not be observed. The slopes [μV/dB] of the amplitude-intensity function of the different wave 3 response components showed only slightly steeper slopes [μV/dB] in the young group for the left and right monaural ABR (Young: 0.0562 (LMON), 0.0444 (RMON); Old: 0.027 (LMON), 0.0212 (RMON). For wave 3 in the binaural and summed monaural ABR the young animals revealed steeper slopes [μV/dB] in comparison to the old animals (Young: 0.1047 (BIN), 0.1402 (SUM); Old: 0.0581 (BIN), 0.0519 (SUM)). This is supported by a significant interaction between age and intensity for the amplitude wave 3 in the summed monaural and binaural ABR (BIN: p = 0.042, F(3,44) = 2.974; SUM: p = 0.017, F(3,45) = 3.747).
Figure 7.
Effect of sound pressure level and age on wave 3 in the binaural, summed monaural, left and right monaural ABR. Results for wave 3 in the binaural ABR are indicated by squares and in the summed monaural ABR by circles. Results for wave 3 in left and right monaural ABR are indicated by a diamond and triangle, respectively. The different age groups are represented by black and grey for young and old gerbils, respectively. Linear regression lines for all stimulation modes are shown in addition to the data points. See text for information about the slope of the functions (A): Latency in ms (± S.E.M) of wave 3 for different intensities in dB pSPL. Left, right and binaural-evoked wave 3 are shown. Young animals are displayed in black and old animals are displayed in grey. (B) Amplitude in μV (± S.E.M) of wave 3 for different intensities in dB pSPL. Left, right and binaural-evoked wave 3 are shown. Young animals are displayed in black and old animals are displayed in grey. Sample size: Young: 60 dB: 11 animals, except left: 10 animals; 70 dB: 9 animals; 80 dB: 11 animals; 90 dB: 9 animals. Old: 60 dB: 4 animals, except left: 3 animals; 70 dB: 8 animals, except left: 7 animals; 80 dB: 8 animals; 90 dB: 10 animals.
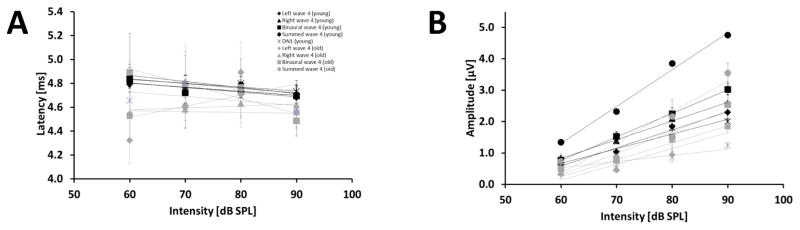
The GLMM ANOVA showed a significant effect of sound pressure level on the latency of the binaural and summed monaural ABR wave 4 as well as for the right monaural ABR (RMON: p = 0.004, F(3,42) = 5.179; BIN: p < 0.001, F(3,42) = 8.906; SUM: p = 0.008, F(3,42) = 4.478). The latency of wave 4 for all types of stimulation decreased with increasing intensity in the young group whereas the latency for all types of stimulation in the old group showed no consistent behavior, except for the binaural ABR wave 4 which showed a slight decrease with increasing intensity (Fig. 8 A). However, there was a significant interaction between the effects of age and sound pressure level on the latency for wave 4 for the binaural type of stimulation (BIN: p = 0.018, F(3,42) = 3.758). The latency-intensity function of old animals revealed a steeper negative slope [ms/dB] wave 4 in the binaural ABR in comparison to the young ones (Young: −0.0039; Old: −0.0124). The slope [ms/dB] of the latency intensity function for the DN1 component is comparable between age groups (Young: −0.0032; Old: −0.0038). For wave 4 in the summed monaural ABRs the young animals showed a slight decrease whereas in the old group no change was observed (Young: −0.0039; Old: 0.0017). For wave 4 of the monaural ABRs in the young group only a slight decrease was observed (Young: −0.0034 (LMON), −0.005 (RMON)), whereas for the old animals no consistent behavior was observed (Old: 0.0092 (LMON), −0.0007 (RMON)). We also examined the effects of age and sound pressure level on the amplitude of wave 4 for all types of stimulation and DN1, and found a significant effect of sound pressure level on wave 4 amplitude for all types of stimulation (LMON: p < 0.001, F(3,43) = 55.138; RMON: p < 0.001, F(3,44) = 48.544; BIN: p < 0.001, F(3,45) = 37.004; SUM: p < 0.001, F(3,43) = 89.049; DN1: p < = 0.001, F(3,51) = 27.710). Figure 8 B shows that in both age groups the wave 4 amplitude for all types of stimulation and DN1 component increased with increasing sound pressure level. The GLMM ANOVA also showed a significant main effect of age on the amplitude of wave 4 for all types of stimulation and the DN1 component (LMON: p = 0.001, F(1,22) = 13.446; RMON: p = 0.003, F(1,19) = 11.626; BIN: p = 0.038, F(1,20) = 4.973; SUM: p = 0.001, F(1,19) = 16.118; DN1: p < 0.001, F(1,23) = 37.141). The younger animals showed larger wave 4 amplitudes for all types of stimulation and the DN1 component in comparison to the older animals for every sound pressure level. Furthermore, for the DN1 component the interaction between age and sound pressure level was significant (p = 0.004; F(3,51) = 4.946). The amplitude-intensity function of young animals revealed a slight steeper slope [μV/dB] for the DN1 component in comparison to the old ones (0.0468 and 0.0195, respectively). For the other types of stimulation the slopes [μV/dB] of the wave 4 amplitude intensity functions are comparable between the age groups (Young: 0.0578 (LMON), 0.0596 (RMON), 0.0749 (BIN), 0.1175 (SUM); Old: 0.0502 (LMON), 0.0536 (RMON), 0.0683 (BIN), 0.0975 (SUM)). Differences in the monaural potentials could not be observed except for the amplitude of wave 4 at 70 dB SPL in the young group (p = 0.012, t = −2.887, t-test).
Figure 8.
Effect of sound pressure level and age on wave 4 in the binaural, summed monaural, left and right monaural ABR and DN1 component. Results for wave 4 in the binaural ABR are indicated by squares and in the summed monaural ABR by circles. Results for wave 4 in left and right monaural ABR are indicated by a diamond and triangle, respectively. Results for the DN1 component are indicated by a star. The different age groups are represented by black and grey for young and old gerbils, respectively. Linear regression lines for all stimulation modes and DN1 component are shown in addition to the data points. See text for information about the slope of the functions (A) Latency in ms (± S.E.M) for different intensities in dB pSPL for wave 4 in binaural ABR, in the summed monaural ABR, left and right monaural ABRs and DN1 component. (B) Amplitude in μV (± S.E.M) of for different intensities in dB pSPL for wave 4 in binaural ABR, in the summed monaural ABR, left and right monaural ABRs and DN1 component. Sample size: Young: 60 dB: 11 animals, except left: 10 animals; 70 dB: 9 animals; 80 dB: 11 animals; 90 dB: 9 animals. Old: 60 dB: 4 animals, except left: 3 animals; 70 dB: 8 animals, except left: 7 animals; 80 dB: 8 animals; 90 dB: 10 animals.
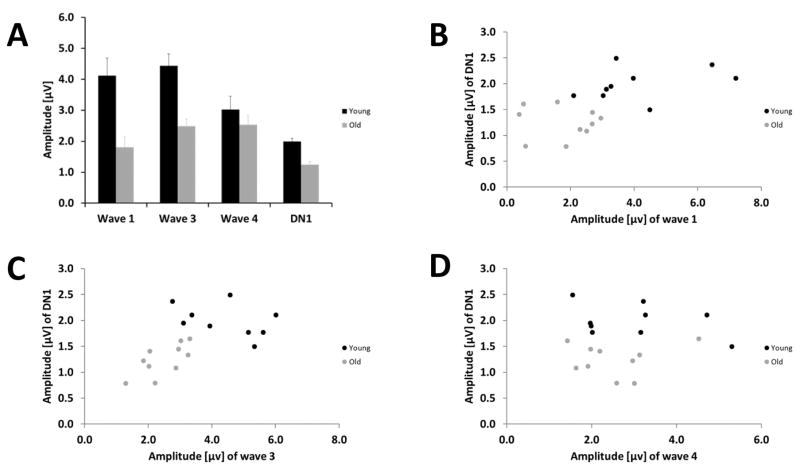
Since the binaural-evoked DN1 component reflects the central processing being driven by the input from the auditory periphery, we investigated for both age groups how the amplitude of binaural-evoked wave 1, wave 3 and wave 4 relate to the DN1 amplitude at a fixed sound pressure level. At the 90 dB pSPL stimulation level, wave 1, wave 3 as well as wave 4 were clearly discernable in both age groups. The amplitude of binaural-evoked wave 1 and 3 at a stimulation level of 90 dB pSPL was decreased in the old animals in comparison to the young group (wave 1: p = 0.003, t = 3.608; wave 3: p 0.001, t = 4.324, t-test), whereas the amplitude of binaural-evoked wave 4 was similar in both age groups (Fig. 9 A). Furthermore, with that level of stimulation the DN1 component was lower in amplitude in the old animals in comparison to the young ones (Fig. 8 A; p < 0.001, t = −5.307, t-test). Figures 9 B, C and D display the amplitude of the DN1 in relation to the amplitude of the binaural-evoked wave 1, wave 3 and wave 4, respectively, in young and old individuals. The lower DN1 amplitude was strongly correlated with the decrease in amplitude of binaural-evoked wave 1 and wave 3 with increased age (Fig. 9 B and C; wave 1: Pearson: CC: −0.614; p = 0.005; wave 3: Pearson: CC: −0.583, p = 0.009), but it was not correlated with the binaural-evoked wave 4 amplitude (Fig. 9 D; Pearson: CC: −0.076; p = 0.756).
Figure 9.
(A) Mean amplitude (± S.E.M.) of binaural-evoked wave 1, wave 3 and 4 and the DN1 component for young and old gerbils. (B) Correlation of binaural-evoked wave 1 amplitude and DN1 amplitude in dependence of age. (C) Correlation of binaural-evoked wave 3 amplitude and DN1 amplitude in dependence of age. (D) Correlation of binaural-evoked wave 4 amplitude and DN1 amplitude in dependence of age. For all figures the young gerbils are displayed in black and the old gerbils are displayed in grey. The measurements were taken at 90 dB pSPL.
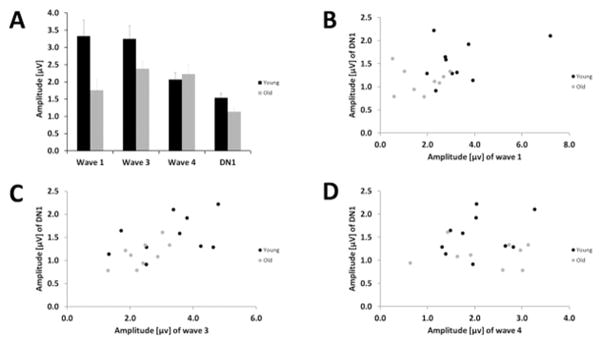
To control for the potential confounding effects of threshold differences between young and old animals, this analysis was repeated for binaural-evoked waves 1, 3 and 4 at a fixed 20 dB level above ABR threshold of each individual. The amplitude of binaural-evoked wave 1 at a level of 20 dB above ABR threshold was decreased in the old animals in comparison to the young group (p = 0.014, t = 2.784, t-test), whereas the amplitude of binaural-evoked wave 3 failed to reach a significant difference between the two age groups (p = 0.064, t = 2.017). Binaural-evoked wave 4 was similar in both age groups (Fig. 10 A). Furthermore, with that level of stimulation the DN1 component was lower in amplitude in the old animals in comparison to the young ones (Fig. 10 A; p = 0.025, t = −2.487, t-test). Figures 10 B, C and D display the amplitude of the DN1 in relation to the amplitude of the binaural-evoked wave 1, wave 3 and wave 4, respectively, in young and old individuals. Also for a fixed level above ABR threshold the lower DN1 amplitude was strongly correlated with the decrease in amplitude of binaural-evoked wave 1 and wave 3 with increased age (Fig. 10 B and C; wave 1: Pearson: CC: −0.492; p = 0.032; wave 3: Pearson: CC: −0.593, p = 0.007), but it was not correlated with the binaural-evoked wave 4 amplitude (Fig. 10 D; Pearson: CC: −0.068; p = 0.781).
Figure 10.
(A) Mean amplitude (± S.E.M.) of binaural-evoked wave 1, wave 3 and 4 and the DN1 component for young and old gerbils. (B) Correlation of binaural-evoked wave 1 amplitude and DN1 amplitude in dependence of age. (C) Correlation of binaural-evoked wave 3 amplitude and DN1 amplitude in dependence of age. (D) Correlation of binaural-evoked wave 4 amplitude and DN1 amplitude in dependence of age. For all figures the young gerbils are displayed in black and the old gerbils are displayed in grey. The measurements were taken at 20 dB AVT.
4. Discussion
This study investigates the binaural ABR in the gerbil to evaluate whether this species is a suitable animal model providing for an understanding of the processes underlying binaural hearing that in its ABR shows parallels to the measurement of the binaural ABR in human subjects. Here we focus on the BIC which is seen as an indicator for processing in the auditory brainstem.
4.1 ABR and BIC waveform
As in other species like guinea pig, cat and human (Goksoy et al., 2005; Ungan et al., 1997; Riedel and Kollmeier, 2002) the gerbil’s BIC with its most prominent negative component DN1 results from the amplitude of the relevant binaural-evoked ABR wave 4 (in animals) and wave 5 (in humans) being smaller than the corresponding sum of the left and right monaural ABRs. In comparison to other ABR studies conducted in the gerbil (e.g. Boettcher et al., 1993) up to two more peaks were observed in the monaural and binaural ABR measurements in the present study (Fig. 3). Such differences could be due to differences in the electrode placement (Beattie and Taggert, 1989; Dum et al., 1981), stimulus types (the previous study used tone pips whereas a click was used in the present study) or calibration (Beutelmann et al., 2015). The present study used a click since in human subjects it elicited a larger absolute BIC amplitude in comparison to the BIC observed for tone pips (Ito et al., 1988). Therefore we used an optimized click with a calibrated phase spectrum producing a sharp onset transient and a flat frequency spectrum to achieve high synchrony of the responding auditory nerve fibers (Beutelmann et al., 2015).
The DN1 latency measured in young gerbils with a click of 20 dB AVT was 4.8 ms being quite similar to that of guinea pigs (4.8 ms; Goksoy et al., 2005) and cats (4.2 ms; Ungan et al., 1997). Latencies of the DN1 component in humans range from 5.6 to 7.3 ms (e.g. Jones and Van der Poel, 1990; McPherson and Starr, 1995). The amplitude of the DN1 component of young gerbils of about 1.7 μV was smaller than in other animal models (5 μV in guinea pig, Goksoy et al., 2005; 2.3 μV in cat, Ungan et al., 1997). In humans the DN1 had a smaller amplitude ranging from 0.2 to 0.9 μV (e.g. Riedel and Kollmeier, 2002; McPherson and Starr, 1995).
As was observed in guinea pig and cat, the BIC in the gerbil was due to a reduced binaural-evoked wave 4 response in the ABR signal (Goksoy et al., 2005; Ungan et al., 1997) that possibly represents the activity of fibers in the LL originating from MSO and LSO (Boettcher, 2002; McPherson and Starr, 1993; Riedel and Kollmeier, 2002). The important role of the LL in generating the BIC is demonstrated by lesion studies which showed a complete loss of the BIC by bilateral lesions of the LL (e.g. Wada and Starr, 1989). The MSO consists mainly of so-called ‘EE’ neurons and receiving excitatory (‘E’) input from the spherical bushy cells (SBCs) of the anteroventral cochlea nuclei (AVCN) of both sides and some inhibitory input by the lateral and medial nucleus of the trapezoid body (LNTB and MNTB) which receive input by globular bushy cells (GBCs) from the ipsilateral and contralateral side, respectively. The LSO consists mainly of ‘EI’ neurons and receiving excitatory (‘E’) input from the ipsilateral side by SBCs of the AVCN and inhibitory (‘I’) input from the contralateral side via the MNTB that receives input by the GBCs of the AVCN (see Grothe, 2003; Tollin, 2003). Some studies suggest that the BIC, being due to a reduction in the binaural response in comparison to the sum of the monaural responses, results from an inhibitory mechanism as found in the LSO (e.g. Ungan et al., 1997). However, also the MSO receives some inhibitory input which has a role that is still under debate (see Grothe et al., 2010; Roberts et al., 2013; Van der Heijden et al., 2013). Furthermore, also EE based mechanisms could result in a negative value of the BIC if the underlying interaction shows a compressive nonlinearity (Gaumond and Psaltikidou, 1991). Gaumond and Psaltikidou (1991) suggested that the EI and EE mechanisms can be distinguished by the relation between the BIC and wave 4 and between BIC and an earlier ABR wave (e.g., wave 1 in our study). If a linear relation between the BIC and wave 4 is observed, this could be explained both by EI and EE mechanisms. A linear relation between wave 1 and BIC amplitude, however, can only be explained by EI mechanisms (Gaumond and Psaltikidou, 1991). In the gerbil, we observed a correlation based on a linear model between the wave 1 and the BIC amplitude (Fig. 8 B) that suggests an EI mechanism as the basis for the BIC typically found in the LSO. A compressive EE cell model describing MSO responses cannot explain the constant DN1/ABR wave 4 ratio for different intensities making MSO a less likely candidate for being the source of the BIC.
4.2 Effects of ITD and ILD on the BIC
The duplex theory of sound implies that the two cues for sound localization in the horizontal plane (ITDs and ILDs) are used in different frequency ranges (Rayleigh, 1907). ITDs are mainly encoded in the low frequency range (< 2 kHz) and ILDs are mainly encoded in the high frequency range (> 2 kHz). ITDs are predominantly processed in the MSO and ILDs in the LSO (see, Tollin, 2003; Grothe, 2003; Grothe et al., 2010). However, there is evidence for ITD processing low - and high –frequency neurons of the LSO in the cat which code for the binaural fine structure and the envelope cues provided by the signal, respectively (Joris and Yin, 1995; Tollin and Yin, 2005). Responses to low frequency ILDs have also been observed in LSO neurons of the gerbil (Sanes and Rubel, 1988) suggesting that this nucleus may also be involved in low-frequency ILD processing related to the computation of interaural time differences from the signal envelope. ILDs of the signal envelope could also result in ITDs due to the time-intensity trading in auditory nerve fibers that provide some timing information at high frequencies (Joris et al., 2008). The time-intensity trading also applies to broadband clicks in that it may well be that ITDs in the carrier and ITDs resulting from level differences (e.g., originating from level-dependent latencies in the auditory nerve, (Özmen and Ungan, 2009) are both processed by the LSO (Irvine et al., 2001; ) and no conclusion about the origin of the BIC can be derived from the relation between the BIC and ITD and ILD, respectively.
With increasing ITD in gerbils of both age groups a decrease in amplitude and an increase in latency were observed in the BIC and the SUM and BIN wave 4 ABR responses. The observation of a reduced DN1 component of the BIC is in accordance with studies in other species such as guinea pig, cat and human (Goksoy et al., 2005; Ungan et al., 1997; Riedel and Kollmeier, 2002; Riedel and Kollmeier, 2006). The reduced DN1 amplitude could be explained by fewer neurons responding to higher ITDs. Since the MSO as well as the LSO rely on exact timing information from both sides to process sound localization cues (Tollin, 2003), also a reduced coincidence in the action potentials arriving from left and right CN due to the ITD could account for a lower DN1 amplitude.
As could be expected from results in previous studies in other animal models (Ungan et al., 1997; Goksoy et al., 2005) we found little effects for ITDs of ± 250μs which is about double the natural acoustic range provided to the gerbil (~± 130 μs; Maki and Furukawa, 2005). Significant effects of ITD on DN1 latency or amplitude were found for values of ± 500μs and ± 750μs, respectively at a sound pressure level of 90 dB pSPL. Significant ITD effects extended up to ITDs of ± 2000μs. This is much larger than the gerbil physiological range of ITD provided by the acoustics, but is similar to the effects observed in other species (Goksoy et al., 2005; Ungan et al., 1997; Riedel and Kollmeier, 2006). Ungan and colleagues (1997) interpreted this as evidence against a coincidence detector mechanism with ITD represented by a neuronal map that would only include ITDs within the physiological range. In the gerbil, however, the MSO neurons have been found to respond to ITDs that are considerably larger than the physiological range (Brand et al., 2002, Pecka et al., 2008). An explanation for a similar ITD dependence of the BIC in a broad range of species including the human (guinea pig, cat and human) can be based on the similarity of time constants of the synaptic connections being involved; the previous studies simulated the ITD dependence of the BIC by applying a simple LSO model which predicted the DN1 latency and amplitude data very well (Goksoy et al., 2005; Ungan et al., 1997; Riedel and Kollmeier, 2006).
The effect of ILDs was not as clear as for ITDs. The gerbil DN1 component showed a significantly smaller amplitude for the highest absolute ILD values (+20 dB and +30 dB for the amplitude of the DN1 component at a level of 20 dB AVT and 90 dB pSPL, respectively) compared to an ILD of 0 dB, but no latency effect could be observed. Sanes and Rubel (1988) reported that LSO neurons in the gerbil respond best to a range of ILDs from −20 dB to + 20 dB indicating that a strongly responding population of neurons can drive the BIC in this range. In contrast, in cats the decrease of the DN1 amplitude was about 55 % for an ILD of 30 dB and in addition a trading of ITD and ILD of the DN1 component was observed (Ungan et al., 1997). Also in human subjects trading of ILD and ITD was observed in producing the BIC (Furst et al., 1985). A larger effect of ITD than of ILD could be explained by the trading ratio. In the auditory nerve a trading ratio of about 10 μs/dB was observed in single fiber recordings (Joris et al., 2008) which is close to the value of 20 μs/dB observed in human ABR (Özmen and Ungan, 2009). The electrophysiological trading ratio affecting the BIC being observed for an ILD range of up to 20 dB results in a similar ratio (20 μs/dB) as the cat ABR data (Ungan et al., 1997). An alternative explanation of small ILD effects could be that sound localization in the gerbil is mainly based on temporal analysis of the signal.
4.3 BIC in relation to the subject age
The older animals generally displayed a lower DN1 component amplitude in comparison to the young animals. For humans the observed effects of age on the BIC were quite diverse. Some studies showed an increase in BIC latency or a decrease in BIC amplitude with increased age (see Fowler, 2004) similarly to what we found for gerbils. As was found in humans (Abel et al., 2000) aged gerbils exhibited deteriorated sound localization in comparison to young gerbils (Maier et al., 2008). It is also reported that with increased age the sensitivity to the temporal fine structure as well to the envelope ITDs was decreased and that the effect of age seemed to be greater for ITD than for ILD (Moore, 2014) which would support the findings of the present study. Therefore the gerbil seems to be a suitable model to investigate age effects on binaural hearing and to examine the possible origins of pathologies which would allow drawing conclusions about the underlying mechanism in humans as well.
The reduced DN1 amplitude in old gerbils compared to young gerbils could have different origins. It is unlikely that a decreased amplitude could be explained by the loss of neurons in the SOC. Spongiform lesions reported in the MNTB of old gerbils (> 3 years) were limited to only of about 3 % of the total area, whereas young animals of about 1 year showed hardly any lesions. No loss of cells was reported in the MNTB although their soma size was reduced in the old animals. (Gleich and Strutz, 2002). Lesions in the LSO were observed only in some old gerbils but not to an extent as in the MNTB (Gleich et al., 2004). The observed changes with increasing age appear to be too small to have a substantial effect on the BIC.
In general, inhibition appears to be reduced in old rodents (Caspary et al., 2008). Since the inhibitory output of MNTB and LNTB plays an important role in creating differential responses in MSO and LSO that allow localizing a sound source accurately, one would expect that the reduced inhibition arising from MNTB could impede sound localization and likewise the amplitude of the DN1 component. In old gerbils Gleich and Strutz (2002) found only a non-significant decrease of glycine immune-reactive cells in the MNTB by 4 %. Furthermore, in the LSO mainly the size of the soma of high-frequency inhibitory neurons was reduced in old animals in comparison to young ones. However, in the high-frequency region of the LSO the number of GABA and glycine immuno-reactive cells was generally lower in comparison to the low- and mid-frequency region suggesting that inhibition plays a lesser role in processing of high frequency cues for sound localization (Gleich et al., 2004). In summary, the observed changes in inhibitory neurons of the MNTB and LSO were rather small making it rather unlikely to be affecting gross potentials such as the BIC.
Finally, the input to the SOC could be changed affecting the BIC. The observed reduction in the DN1 amplitude was correlated to a decreased amplitude in wave 1, also for compensated thresholds (Figure 9, 10 B) which suggests that a deteriorated peripheral input rather than deteriorated central mechanisms account for the reduced BIC in old gerbils. This view is supported by the observation that the binaural-evoked wave 4, which is based on activity in the SOC, is not reduced with increased age (Figure 9, 10 A). However, for 0 μs ITD the amplitude of the summed monaural ABR wave 4 was decreased in the old animals which indicate that there is an age effect on the monaural input into the auditory system. The effect of peripheral hearing loss on the wave 4 amplitude was similarly observed by Boettcher and colleagues (1993). The observed restoration of the binaural wave 4 amplitude could be explained by the reduced inhibition in the SOC that is due to the diminished input from the periphery (see also the observation in the Fmr1 mouse reported by Rothschafer et al., 2015). Such a compensation as seen for the binaural ABR wave 4 is not present at the level of wave 3 which showed a reduction in amplitude with increased age at 90 dB pSPL (Figure 9 A/C) that vanished after correction for hearing threshold differences (Figure 10 A/C). Wave 1 represents the activity of the auditory nerve. Already in young domesticated gerbils (~ 1 year) there was evidence for neuronal degeneration in the cochlear nucleus and the auditory nerve (McGinn and Faddis, 1998). It is possible that with increased age such effects may increase and lead to the decrease in wave 1 amplitude. Boettcher and colleagues (1993) and Schmiedt and colleagues (1996) reported that ABR thresholds of the old gerbils were slightly more elevated at higher frequencies than at lower frequencies. The increased thresholds and more shallow ABR growth functions in the gerbils were attributed to a loss of loss of hair cells, hair cell synapses or spiral ganglion cells (Schmiedt et al., 1996, Boettcher et al., 1993) or to a reduction of the endocochlear potential and degeneration of the stria vascularis (Boettcher et al., 1993). Increased CAP threshold for high frequencies have been observed in old gerbils showing no hair cell loss at all (Tarnowski et al., 1991). This corresponds well to findings of Sergeyenko and colleagues (2013) who showed a progression of cochlear synaptic loss in mice from 4 to 144 weeks of age even before changes in threshold or a hair cell loss were observed. Cochlear synaptic loss was followed by a loss of auditory nerve fibers. It has also been reported for old gerbils that a smaller number of low-spontaneous rate auditory nerve fibers in comparison to young gerbils was found which may be due to the reduced endocochlear potential and could account for deficits in binaural hearing (Schmiedt et al., 1996). Thus, auditory synaptopathy for which anatomical evidence has been found in rodents (Kujawa & Liberman 2009; Sergeyenko et al., 2013) and which has a physiological signature in the form of a reduced amplitude of the ABR wave 1 response, could be the relevant effect resulting in a reduced DN1 amplitude that is indicative of compromised binaural hearing.
Table 1.
Age in months and ABR threshold in dB pSPL for left and right ear for all tested gerbils. Threshold was measured in 10 dB steps therefore the higher intensity indicated the level at which a response was observed. This level was also used for calculating the level 20 dB above visual detected ABR threshold (20 dB AVT). Old animals are highlighted by grey
| Animal | Age | Left | Right |
|---|---|---|---|
| Dina | 2 | 50–60 | 40–50 |
| Giora | 2 | 50–60 | 40–50 |
| Barney | 4 | 40–50 | 40–50 |
| Gerbil | 4 | 60–70 | < 60 |
| Atilla | 6 | 50–60 | 50–60 |
| Toto | 6 | 40–50 | 40–50 |
| Jasper | 6 | 50–60 | 40–50 |
| Ramira | 5 | 50–60 | 40–50 |
| Ted | 5 | 50–60 | 50–60 |
| Fred | 7 | 40–50 | 40–50 |
| Gandalf | 10 | 40–50 | 40–50 |
| Alfred | 36 | 60–70 | 60–70 |
| Anton | 36 | 60–70 | 60–70 |
| Sally | 36 | 70–80 | 60–70 |
| Balthasar | 36 | 60–70 | 60–70 |
| Boris | 36 | 60–70 | 60–70 |
| Remus | 36 | 50–60 | 50–60 |
| Radik | 36 | 60–70 | < 60 |
| Marshal | 36 | 40–50 | 40–50 |
| Nick | 36 | 60–70 | 50–60 |
| Lara | 36 | 70–80 | 70–80 |
| Rubino | 39 | 60–70 | 50–60 |
Highlights.
Mongolian gerbils showed a binaural interaction component (BIC) in the ABR
Effects of ITD and ILD on the gerbil binaural ABR were similar to those in humans
Results suggest that synaptic mechanisms in the LSO cause the ITD effect
BIC and wave 4 in the summed monaural ABR were reduced in old gerbils
This reduction may be due to a compromised wave 1 response in old gerbils
Acknowledgments
This work was supported by NIH Grants R01-DC011555 (DJT) and by grants (EXC 1077, TRR31) from the Deutsche Forschungsgemeinschaft (GL, GMK, RB). Support was also provided by a fellowship from the Hanse Institute for Advanced Studies in Delmenhorst, Germany (DJT). Helmut Riedel provided software for stimulation and data analysis that we modified for the current experiments. We thank the anonymous reviewers for comments on the previous version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Geneviève Laumen, Email: genevieve.laumen@uni-oldenburg.de.
Daniel J. Tollin, Email: Daniel.Tollin@ucdenver.edu.
Rainer Beutelmann, Email: rainer.beutelmann@uni-oldenburg.de.
Georg M. Klump, Email: georg.klump@uni-oldenburg.de.
References
- Abel SM, Giguère C, Consoli A, Papsin BC. The effect of aging on horizontal plane sound localization. J Acoust Soc Am. 2000;108:743–752. doi: 10.1121/1.429607. [DOI] [PubMed] [Google Scholar]
- Beutelmann R, Laumen G, Tollin D, Klump GM. Amplitude and phase equalization of stimuli for click evoked auditory brainstem responses. J Acoust Soc Am. 2015;137:EL71–EL77. doi: 10.1121/1.4903921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher FA. Presbyacusis and the auditory brainstem response. J Speech Lang Hear Res. 2002;45:1249–1261. doi: 10.1044/1092-4388(2002/100). [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Norton BL, Schmiedt R. Age-related changes in auditory evoked potentials of gerbils. I Response amplitudes Hear Res. 1993;71:146–156. doi: 10.1016/0378-5955(93)90030-5. [DOI] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Burkard R, Boettcher F, Voigt H, Mills J. Comments on “Stimulus dependencies of the gerbil brain-stem auditory-evoked response (BAER). I: Effects of click level, rate and polarity”. J Acoust Soc Am. 1993;94:2441–2442. doi: 10.1121/1.407465. [J. Acoust. Soc. Am. 85, 2514–2525 (1989)] [DOI] [PubMed] [Google Scholar]
- Burkard R, Voigt HF. Stimulus dependencies of the gerbil brain-stem auditory-evoked response (BAER). I: Effects of click level, rate, and polarity. J Acoust Soc Am. 1989 doi: 10.1121/1.407465. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheal ML. The gerbil: a unique model for research on aging. Exp Aging Res. 1986;12:3–21. doi: 10.1080/03610738608259430. [DOI] [PubMed] [Google Scholar]
- Dobie RA, Berlin CI. Binaural interaction in brainstem-evoked responses. Arch Otolaryngol. 1979;105:391–398. doi: 10.1001/archotol.1979.00790190017004. [DOI] [PubMed] [Google Scholar]
- Dum N, Schmidt U, von Wedel H. Scalp distribution of the auditory evoked brainstem potentials in the guinea pig during monaural and binaural stimulation. Hear Res. 1981;5:271–284. doi: 10.1016/0378-5955(81)90051-4. [DOI] [PubMed] [Google Scholar]
- Fowler CG. Electrophysiological evidence for binaural processing in auditory evoked potentials: The binaural interaction component. Semin Hear. 2004;25:39–49. doi: 10.1055/s-2004-823046. [DOI] [Google Scholar]
- Furst M, Levine RA, McGaffigan PM. Click lateralization is related to the beta component of the dichotic brainstem auditory evoked potentials of human subjects. J Acoust Soc Am. 1985;78:1644–1651. doi: 10.1121/1.392802. [DOI] [PubMed] [Google Scholar]
- Gaumond RP, Psaltikidou M. Models for the generation of the binaural difference response. J Acoust Soc Am. 1991;89:454–456. doi: 10.1121/1.400482. [DOI] [PubMed] [Google Scholar]
- Gleich O, Hamann I, Klump GM, Kittel M, Strutz J. Boosting GABA improves impaired auditory temporal resolution in the gerbil. Neuroreport. 2003;14:1877–1880. doi: 10.1097/00001756-200310060-00024. [DOI] [PubMed] [Google Scholar]
- Gleich O, Strutz J. Age dependent changes in the medial nucleus of the trapezoid body in gerbils. Hear Res. 2002;164:166–178. doi: 10.1016/S0378-5955(01)00430-0. [DOI] [PubMed] [Google Scholar]
- Gleich O, Weiss M, Strutz J. Age-dependent changes in the lateral superior olive of the gerbil (Meriones unguiculatus) Hear Res. 2004;194:47–59. doi: 10.1016/j.heares.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Goksoy C, Demirtas S, Yagcioglu S, Ungan P. Interaural delay-dependent changes in the binaural interaction component of the guinea pig brainstem responses. Brain Res. 2005;1054:183–191. doi: 10.1016/j.brainres.2005.06.083. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Salloum C, Toor GS, van Hoesel R, Papsin BC. Binaural Interactions Develop in the Auditory Brainstem of Children Who Are Deaf: Effects of Place and Level of Bilateral Electrical Stimulation. J Neurosci. 2012;32:4212–4223. doi: 10.1523/JNEUROSCI.5741-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow M, Riedel H, Kollmeier B. Single-sweep-bades methods to improve the quality of auditory brain stem responses Part I: Optimized linear filtering. Z Audiol. 2001;40:32–44. [Google Scholar]
- Grothe B. New roles for synaptic inhibition in sound localization. Nat Rev Neurosci. 2003;4:540–550. doi: 10.1038/nrn1136. [DOI] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev. 2010;90:983–1012. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Hawley ML, Litovsky RY, Culling JF. The benefit of binaural hearing in a cocktail party: effect of location and type of interferer. J Acoust Soc Am. 2004;115:833–843. doi: 10.1121/1.1639908. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Park VN, McCormick L. Mechanisms underlying the sensitivity of neurons in the lateral superior olive to interaural intensity differences. J Neurophysiol. 2001;86:2647–2666. doi: 10.1152/jn.2001.86.6.2647. [DOI] [PubMed] [Google Scholar]
- Ito S, Hoke M, Pantev C, Lütkenhöner B. Binaural interaction in brainstem auditory evoked potentials elicited by frequency-specific stimuli. Hear Res. 1988;35:9–19. doi: 10.1016/0378-5955(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Jalabi W, Kopp-Scheinpflug C, Allen PD, Schiavon E, DiGiacomo RR, Forsythe ID, Maricich SM. Sound localization ability and glycinergic innervation of the superior olivary complex persist after genetic deletion of the medial nucleus of the trapezoid body. J Neurosci. 2013;33:15044–9. doi: 10.1523/JNEUROSCI.2604-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SJ, Van der Poel JC. Binaural interaction in the brain-stem auditory evoked potential: evidence for a delay line coincidence detection mechanism. Electroencephalogr Clin Neurophysiol. 1990;77:214–24. doi: 10.1016/0168-5597(90)90040-k. [DOI] [PubMed] [Google Scholar]
- Joris PX, Michelet P, Franken TP, McLaughlin M. Variations on a Dexterous theme: peripheral time-intensity trading. Hear Res. 2008;238:49–57. doi: 10.1016/j.heares.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences. J Neurophysiol. 1995;73:1043–1062. doi: 10.1152/jn.1995.73.3.1043. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki K, Furukawa S. Acoustical cues for sound localization by the Mongolian gerbil, Meriones unguiculatus. J Acoust Soc Am. 2005;118:872–886. doi: 10.1121/1.1944647. [DOI] [PubMed] [Google Scholar]
- McGinn MD, Faddis BT. Neuronal degeneration in the gerbil brainstem is associated with spongiform lesions. Microsc Res Tech. 1998;41:187–204. doi: 10.1002/(SICI)1097-0029(19980501)41:3<187::AID-JEMT3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- McPherson DL, Starr A. Auditory time-intensity cues in the binaural interaction component of the auditory evoked potentials. Hear Res. 1995;89:162–171. doi: 10.1016/0378-5955(95)00134-1. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Sound localization by human listeners. Annu Rev Psychol. 1991;42:135–159. doi: 10.1146/annurev.ps.42.020191.001031. [DOI] [PubMed] [Google Scholar]
- Mills JH, Schmiedt RA, Kulish LF. Age-related changes in auditory potentials of Mongolian gerbil. Hear Res. 1990;46:201–210. doi: 10.1016/0378-5955(90)90002-7. [DOI] [PubMed] [Google Scholar]
- Moore BC. Auditory processing of temporal fine structure Effects of age and hearing loss. World Scientific; 2014. [DOI] [PubMed] [Google Scholar]
- Morrison A, Evans H, Ator N, Nakamura R. Methods and welfare considerations in behavioral research with animals. Rockville, MD: Natl. Inst. Ment. Heal; 2002. [Google Scholar]
- Özmen B, Ungan P. Assessment of the role of the cochlear latency effect in lateralization of click sounds in humans. Psychophysiology. 2009;46:797–806. doi: 10.1111/j.1469-8986.2009.00828.x. [DOI] [PubMed] [Google Scholar]
- Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J Neurosci. 2008;28:6914–6925. doi: 10.1523/JNEUROSCI.1660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Souza PE. Effects of aging on auditory processing of speech. Interantional J Audiol. 2003;42:11–16. [PubMed] [Google Scholar]
- Riedel H, Granzow M, Kollmeier B. Single-sweep-based methods to improve the quality of auditory brain stem responses Part II: Averaging methods. Zeitschrift für Audiol. 2001;40:62–85. [Google Scholar]
- Riedel H, Kollmeier B. Interaural delay-dependent changes in the binaural difference potential of the human auditory brain stem response. Hear Res. 2006;218:5–19. doi: 10.1016/j.heares.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Riedel H, Kollmeier B. Auditory brain stem responses evoked by lateralized clicks: Is lateralization extracted in the human brain stem? Hear Res. 2002;163:12–26. doi: 10.1016/S0378-5955(01)00362-8. [DOI] [PubMed] [Google Scholar]
- Roberts M, Seeman S, Golding N. A mechanistic understanding of the role of feedforward inhibition in the mammalian sound localization circuitry. Neuron. 2013;78:923–935. doi: 10.1016/j.neuron.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer SE, Marshak S, Cramer KS. Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A. Hearing sensitivity of the mongolian gerbil, Meriones unguiculatis. J Acoust Soc Am. 1976;59:1222–1226. doi: 10.1121/1.380961. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Rubel EW. The ontogeny of inhibition and excitation in the gerbil lateral superior olive. J Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol. 1996;76:2799–2803. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–94. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnowski BI, Schmiedt RA, Hellstrom LI, Lee FS, Adams JC. Age-related changes in cochleas of mongolian gerbils. Hear Res. 1991;54:123–134. doi: 10.1016/0378-5955(91)90142-V. [DOI] [PubMed] [Google Scholar]
- Tollin DJ. The lateral superior olive: a functional role in sound source localization. Neuroscientist. 2003;9:127–143. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Yin TCT. Interaural Phase and Level Difference Sensitivity in Low-Frequency Neurons in the Lateral Superior Olive. J Neurosci. 2005;25:10648–10657. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungan P, Yagcioglu S, Özmen B. Interaural delay-dependent changes in the binaural difference potential in the cat auditory brainstem response: implications about the origin of the binaural interaction component. Hear Res. 1997;106:66–82. doi: 10.1016/s0378-5955(97)00003-8. [DOI] [PubMed] [Google Scholar]
- Ungan P, Yagcioglu S. Origin of the binaural interaction component in wave P4 of the short-latency auditory evoked potentials in the cat: Evaluation of serial depth recordings from the brainstem. Hear Res. 2002;167:81–101. doi: 10.1016/S0378-5955(02)00351-9. [DOI] [PubMed] [Google Scholar]
- Van der Heijden M, Lorteije JAM, Plauška A, Roberts MT, Golding NL, Borst JGG. Directional hearing by linear summation of binaural inputs at the medial superior olive. Neuron. 2013;78:936–948. doi: 10.1016/j.neuron.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S, Starr A. Anatomical bases of binaural interaction in auditory brainstem responses from guinea pig. Electroencephalogr Clin Neurophysiol. 1989;72:535–544. doi: 10.1016/0013-4694(89)90231-9. [DOI] [PubMed] [Google Scholar]
- Wada SI, Starr A. Generation of auditory brain stem responses (ABRs). III. Effects of lesions of the superior olive, lateral lemniscus and inferior colliculus on the ABR in guinea pig. Electroencephalogr Clin Neurophysiol. 1983;56:352–366. doi: 10.1016/0013-4694(83)90261-4. [DOI] [PubMed] [Google Scholar]