ABSTRACT
The genetic metabolic disease mucopolysaccharidosis III type C (MPS IIIC, Sanfilippo disease type C) causes progressive neurodegeneration in infants and children, leading to dementia and death before adulthood. MPS IIIC stands out among lysosomal diseases because it is the only one caused by a deficiency not of a hydrolase but of HGSNAT (heparan--glucosaminide N-acetyltransferase), which catalyzes acetylation of glycosaminoglycan heparan sulfate (HS) prior to its hydrolysis.
KEYWORDS: acetyl-CoA, α-glucosaminide N-acetyltransferase, glycosaminoglycans, heparan sulfate, knockout mouse model, lysosome, mucopolysaccharidosis
We generated the first mouse model for MPS IIIC, a functional knockout of the Hgsnat locus in C57Bl/6N mice (Hgsnat-Geo mice). Hgsnat mRNA and activity levels in the tissues of Hgsnat-Geo mice are reduced to 0.6–1.5% as compared to wild type (WT), i.e. below or close to the detection level. Hgsnat-Geo mice are viable, have normal growth and do not have symptoms of the disease until the age of 11–12 mo when they start showing weight loss, loss of fur, and abnormal gait. At ∼65 wk mice have to be euthanized due to urinary retention. Hyperactivity and reduced anxiety are detected by open field test starting from 6 mo, but by the age of 10 mo the signs of hyperactive behavior diminish whereas defects in memory and spatial learning capability are detected by the Morris Water Maze test, resembling the disease progression in human patients.
In contrast to human MPS III patients who show rapid reduction of cortical volume and surface area, mouse brain cortex has normal stratification even at the age of 12 mo, but quantification of neurons in somatosensory cortex shows that neuronal loss in homozygous Hgsnat-Geo mice starts at 4 mo resulting in the >30% reduction of neuronal density by 12 mo. Besides which, gradually increasing levels of activated microglia cells and astrocytes detected throughout the brain and the increased expression of inflammation markers, CCL3/MIP1α and TNF/TNFα, observed starting from 10 d after birth are indicative of progressive neuroinflammation.
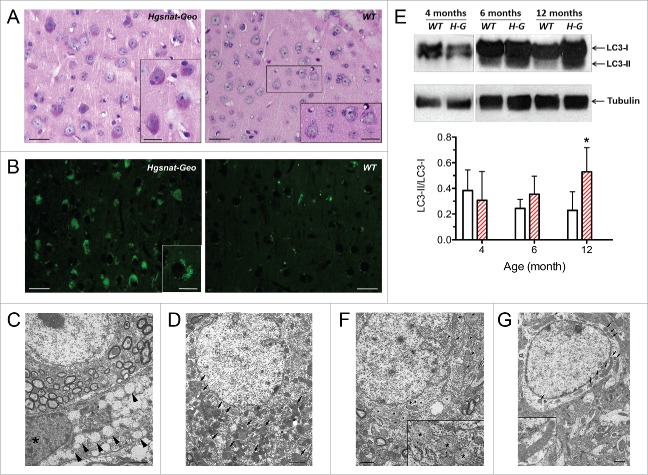
Multiple foam microglia cells with storage vacuoles (Fig. 1A) are present in both gray and white matter as early as at 2 mo of age preceding pathological changes in neurons, showing progressive cytoplasmic accumulation of autofluorescent granular material (Fig. 1B). At 5 mo storage is restricted to individual neurons, but at 12 mo it affects the whole neuronal population especially the cells in deep cortical layers, hippocampus, and cerebellum. Electron microscopy analysis shows that neurons contain densely packed fibrillary deposits of a rectilinear and/or fingerprint type resembling those detected in neuronal ceroid lipofuscinoses, and are different from storage vacuoles in microglia, which have a uniform electron-lucent appearance (Fig. 1C, D). Similar heterogeneous storage and the presence of “fingerprint-like” structures identified as the aggregates of ATP5G/subunit C of the mitochondrial ATP synthase were previously reported for other MPS mouse models.
Figure 1.
Pathological changes in the brains of Hgsnat-Geo mice. (A) Accumulation of PAS-positive granular material in neurons in the brain of a Hgsnat-Geo mouse. Microglia show the presence of storage, and have a foam-like appearance. Somatosensory cortex (layer V) of 12-mo-old Hgsnat-Geo and WT mice. Bars: 50 µm; bars in the inserts: 30 µm. (B) Accumulation of granular autofluorescent materials in neurons of somatosensory cortex (layer V) of 12-mo-old Hgsnat-Geo and WT mice. Bars: 100 µm; bar in the insert: 30 µm. (C) Storage pattern in microglia (asterisk): massive accumulation of vacuoles with single limiting membranes and a sparse fine content in the cytoplasm. Bar: 2 µm. (D) Lysosomal system in a cortical neuron is expanded and massively overloaded by electron dense material (marked by arrows). Bar: 2 µm. (E) Increased LC3-II levels in the brain tissues of Hgsnat-Geo (H-G) mice at 6 and 10 mo. Inset graph shows ratios (means and SD) of signal intensities for LC3-II and LC3-I. * P < 0.05 in unpaired 2-tailed t test. (F) At 12 mo mitochondria in neurons of Hgsnat-Geo mice are displaying swelling and disorganization of their inner membranes (marked by arrowheads and asterisks). Bar: 1 µm. (G) Mitochondrial population in neurons of WT mice at 12 mo is relatively uniform and is composed of normally shaped mitochondria. Bar: 1 µm. Reproduced from Martins et al. Brain. 2015; 138:336–55, with permission of the publisher.
Immunohistochemistry confirmed that most of HS storage is detected in activated foam microglia cells, whereas neurons accumulate mostly secondary storage products including GM2 and GM3 gangliosides. Stored gangliosides only partially colocalize with the lysosomal marker LAMP1 suggesting that they are also accumulated in the compartments having a nonlysosomal origin. Similar to neurons of other MPS III mouse models, neurons of Hgsnat-Geo mice also contain ATP5G aggregates, increased levels of ubiquitin and protein markers of Alzheimer disease and other tauopathies such as lysozyme, hyperphosphorylated MAPT/tau, GSK3B/Gsk3β, and β amyloid suggestive of altered autophagy. Indeed, starting from 6 mo of age elevated levels of LC3-phosphatidylethanolamine conjugate (LC3-II) are detected in brain tissues indicating increased autophagosomal genesis or decreased macroautophagic flux (Fig. 1E).
One of the most striking pathological changes in neurons observed in all parts of the brain is progressive accumulation of pleomorphic, swollen mitochondria containing disorganized or reduced cristae. Neurons containing swollen mitochondria are present as early as at 5 mo of age, and by the age of 12 mo the mitochondrial damage is observed in the majority of neurons (Fig. 1F, G).
Consistent with progressive mitochondrial defects observed by electron microscopy, activities of mitochondrial complex IV (COX) and complex II (SDH) enzymes and the total content of COQ10/coenzyme Q10 in the brain tissues are significantly lower in Hgsnat-Geo mice than in the corresponding WT controls at the ages of 8 and 12 mo, respectively. Besides, the activities of complex II (SDH), complex II+III and CS (citrate synthase) in Hgsnat-Geo mice decrease significantly with age, whereas no such dependence is detected for the WT animals.
Further studies are necessary to determine the mechanism leading to neuronal death in MPS IIIC, but based on the above results we hypothesize that it can be at least partially mediated by pathological changes in the mitochondrial system. Accumulation of HS and HS-derived oligosaccharides in microglia presumably released by exocytosis of lysosomes induces general inflammation reactions in the brain by activating TLR receptors of microglia cells, resulting in release of multifunctional cytokines. We speculate that these cytokines known to cause mitochondrial damage through formation of ROS and oxidative stress eventually lead to neuronal death. Progressive accumulation of ganglioside and ceroid-type densely packed protein aggregates detected in neurons at the advanced stages of the disease can result from increased mitophagy and impaired catabolism of autophagosomal content. While our data suggest autophagic alterations in the brain cells of Hgsnat-Geo mouse, it remains to be determined whether autophagy is indeed impaired since the detected LC3-II accumulation can reflect both increased autophagy and defective proteolysis following formation of the autophagosome. Further studies are also necessary to determine if progressive neuroinflammation/mitochondrial dysfunction, which we defined here for MPS IIIC, may represent a common phenomenon for metabolic neurodegenerative diseases.
Abbreviations
- GAG
glycosaminoglycans
- HGSNAT
heparan-α-glucosaminide N-acetyltransferase
- HS
heparan sulfate
- MPS
mucopolysaccharidosis
- ROS
reactive oxygen species
- WT
wild type
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.