ABSTRACT
The endoplasmic reticulum (ER) is a major source for the generation of autophagosomes during macroautophagy. Our recent work in yeast shows that particular ER-derived vesicles are generated for the biogenesis of autophagosomes. These vesicles not only incorporate a SNARE protein that is largely ER-resident under nonstarving conditions, but also display COPII requirements for ER-exit that differ from conventional cargo-transporting vesicles. Our results suggest that specific intracellular traffic is launched at the ER for the transport of membranes to sites of autophagosome formation.
KEYWORDS: autophagy, COPII, membrane fusion, membrane traffic, SNARE proteins
The elaborate cellular ER network is a hallmark of all eukaryotic cells and is the hub for COPII vesicles that transport lipids as well as membrane proteins and soluble cargo from the ER to the Golgi apparatus. It is also known that the ER plays an important role for the biogenesis of autophagosomes, which have a characteristic double-layered membrane and form increasingly during periods of starvation. Our recent work with the yeast S. cerevisiae revealed a particular cellular trafficking pathway that emanates from the ER and constitutes one source of membranes for the formation of autophagosomes.
By investigating whether distinct ER membrane fusion machineries play a role in autophagy we found that the SNARE protein Ufe1, but not the atlastin-like Sey1, is required for this process. Interestingly, Ufe1, which was considered an ER-resident SNARE protein with known functions in ER-ER membrane fusion as well as in fusion of retrograde vesicles with the ER, is increasingly exported from the ER upon conditions that induce autophagy and ends up inside the vacuole. In mutants that block the vacuolar uptake of multivesicular bodies (MVBs), Ufe1 accumulates in extravacuolar structures. In ypt7Δ cells, where vacuolar uptake of both autophagosomes and of MVBs is blocked, Ufe1 colocalizes with Atg8 and Atg9 in punctate structures and shows physical interaction with these autophagy markers. These results combined lead to the hypothesis that Ufe1-containing ER vesicles are targeted to sites of autophagsome formation where they contribute membranes to emerging autophagosomes. Membrane fusion during these processes would be catalyzed in part by Ufe1, which would subsequently be forwarded to the vacuole via MVBs.
We performed a number of experiments to test our hypothesis. Previously published work from the laboratories of Fulvio Reggiori, Yoshinori Ohsumi and Daniel Klionsky demonstrated that the availability of membranes for autophagosome formation, in these cases Atg9-containing membranes, correlates with the number and size of autophagosomes. With this in mind we used thin-section electron microscopy and confocal laser scanning microscopy in combination with 3-dimensional reconstruction and measured the number and size of autophagosomes dependent on functional Ufe1. In cells with a temperature-sensitive allele of Ufe1 both the number and size of autophagosomes are reduced at the restrictive temperature when compared to control cells, showing a function of Ufe1 in membrane supply relevant for autophagosome biogenesis. Additional evidence came from the observed physical interactions of Ufe1 with SNARE proteins previously implicated in this process. We found that Ufe1, a Qa-SNARE, binds to Vti1 (Qb) and Ykt6 (R), both non-ER SNAREs, under conditions that induce autophagy.
In another series of experiments we addressed the requirements for ER exit of Ufe1 during starvation. Not too surprisingly, formation of vesicles that carry Ufe1 depend on the COPII complex. A connection of COPII vesicles to autophagosome formation in yeast has been demonstrated previously by the laboratories of Jodi Nunnari and Susan Ferro-Novick. We made the additional observation that the ER exit of Ufe1 is hypersensitive to the sec23-1 allele, which affects the inner layer of the COPII coat. At the permissive temperature the ER exit of conventional cargo is unaffected in sec23-1 cells upon starvation, whereas the ER exit of Ufe1 is strongly reduced. This provided us with a tool to test the correlation between the ER export of Ufe1 and the biogenesis of autophagosomes under conditions where the early secretory pathway is intact. Strikingly, we observed that sec23-1 cells are deficient in autophagy at the permissive temperature. On a subcellular level, using thin-section electron microscopy, we detected a decreased number of autophagosomes in sec23-1 cells when compared to wild-type cells or additional control cells, revealing a deficiency in membrane supply to autophagosome biogenesis.
The observed correlation between the specific COPII-dependent ER exit of Ufe1 during starvation and the number of generated autophagosomes in sec23-1 cells supports the model that one way of transporting membranes to sites of autophagosome formation relies on vesicles that contain Ufe1. Our data also indicate that these vesicles are specific COPII vesicles and that they differ from those that contain cargo destined for various places along the secretory pathway. One possibility for the observed sensitivity of formation of autophagy-specific COPII vesicles to the sec23-1 allele is that it might reflect subtle differences in coat arrangement. A recent report from work with mammalian cells could indicate that a similar system is also present in higher eukaryotes: the Schekman lab isolated specific COPII vesicles for the biogenesis of autophagosomes that bud from the ERGIC, an intermediate structure in mammalian cells that is functionally comparable to yeast ER exit sites. A striking feature of these autophagy-specific COPII vesicles is their markedly reduced diameter compared to COPII transport vesicles destined for the Golgi.
On a speculative note, we think it is possible that one class of ER vesicles routed to sites of autophagosome formation is generally free of lumenal cargo (Fig. 1, step 1). This could account for the observed differences in biophysical and structural features compared to cargo transporting vesicles destined for the Golgi. The main purpose of these vesicles would be to carry membranes along with the appropriate fusion machinery rather than lumenal cargo. Conversely, results from the group of Susan Ferro-Novick suggest that conventional transport vesicles en route to the Golgi can be diverted and used for autophagosome biogenesis as well (Fig. 1, step 2). An interesting and testable consequence of this scenario would be that ER lumenal cargo ends up permanently in between the autophagosomal membranes, or temporarily in case it is recycled from intermediate structures (Fig. 1). It remains to be seen whether the observed differences in COPII vesicles that are used for autophagosome biogenesis in distinct cell types or under distinct conditions indeed reflect different cellular pathways. Other interesting questions concern the mechanism of Ufe1 incorporation into ER vesicles and the particular fusion reactions that are catalyzed in connection with this SNARE protein, all of which will lead to crucial insight into the contribution of the ER to autophagosome formation by vesicular transport and its regulation.
Figure 1.
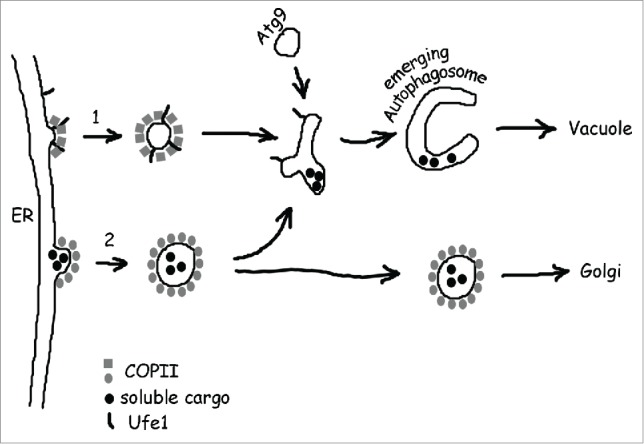
Current models for the contribution of ER-derived COPII vesicles to autophagosome biogenesis. (1) Upon cellular starvation, vesicles are produced that differ from conventional transport vesicles in several aspects. Our work suggests that in yeast, such vesicles incorporate the Qa-SNARE Ufe1 and have specific COPII requirements for budding, maybe due to subtle differences in coat arrangement. Likewise in mammals, starvation-specific and unusually small COPII vesicles are produced at the ERGIC. Based on these findings and the assumed functional role of such vesicles it is possible that they constitute a class of ER vesicles that is free of lumenal cargo and carries mainly membranes and fusion machinery instead, but evidence for this assumption is lacking. (2) Previous work with yeast suggests an alternative mechanism for the contribution of COPII vesicles to autophagosome biogenesis. In this scenario, transport vesicles are generated normally, but are diverted from their route to the Golgi and are targeted to sites of autophagosome formation by virtue of a starvation-specific membrane tethering factor. As a consequence, lumenal cargo might end up in between the 2 membranes that form an autophagosome.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work has been supported by an EMBO short-term fellowship (EMBO ASTF 293-2015) to L.L. and by grants of the Spanish Ministry of Science (BFU2009-07290) and (BFU2014-59309-P).


