Abstract
Rodents use their whiskers to explore the environment, and the superior colliculus is part of the neural circuits that process this sensorimotor information. Cells in the intermediate layers of the superior colliculus integrate trigeminotectal afferents from trigeminal complex and corticotectal afferents from barrel cortex. Using histological methods in mice, we found that trigeminotectal and corticotectal synapses overlap somewhat as they innervate the lower and upper portions of the intermediate granular layer, respectively. Using electrophysiological recordings and optogenetics in anesthetized mice in vivo, we showed that, similar to rats, whisker deflections produce two successive responses that are driven by trigeminotectal and corticotectal afferents. We then employed in vivo and slice experiments to characterize the response properties of these afferents. In vivo, corticotectal responses triggered by electrical stimulation of the barrel cortex evoke activity in the superior colliculus that increases with stimulus intensity and depresses with increasing frequency. In slices from adult mice, optogenetic activation of channelrhodopsin-expressing trigeminotectal and corticotectal fibers revealed that cells in the intermediate layers receive more efficacious trigeminotectal, than corticotectal, synaptic inputs. Moreover, the efficacy of trigeminotectal inputs depresses more strongly with increasing frequency than that of corticotectal inputs. The intermediate layers of superior colliculus appear to be tuned to process strong but infrequent trigeminal inputs and weak but more persistent cortical inputs, which explains features of sensory responsiveness, such as the robust rapid sensory adaptation of whisker responses in the superior colliculus.
Keywords: superior colliculus, trigeminal complex, neocortex, synaptic transmission, optogenetics, sensory pathways
the superior colliculus controls orienting eye movements (saccades) during visual exploration (Gandhi and Katnani 2011; Krauzlis et al. 2013; Sommer and Wurtz 2008; Sparks and Mays 1990). Rodents explore the environment using whisker movements (Carvell and Simons 1990; Gao et al. 2001; Kleinfeld et al. 2006; Kleinfeld and Deschenes 2011), and the superior colliculus may have a role during exploratory whisking similar to its role during visual exploration (Mitchinson and Prescott 2013). To decipher this role, it is important to characterize whisker-related pathways in the superior colliculus.
The rodent superior colliculus is divided into superficial (zonal, superficial gray, and optic layers), intermediate (intermediate gray and white layers), and deeper layers (deep gray and white layers). The superficial layers receive visual inputs from retina and primary visual cortex, whereas the intermediate and deeper layers receive somatosensory and auditory inputs, so that sensory maps lie in spatial register across layers (Drager and Hubel 1975a; Isa and Hall 2009; Stein et al. 1975; Triplett et al. 2012). Cells in the intermediate and deeper layers are responsive to passive touch of stationary whiskers (Cohen and Castro-Alamancos 2007, 2010b; Cohen et al. 2008; Grunwerg and Krauthamer 1990; Hemelt and Keller 2007; McHaffie et al. 1989) and to whisking (artificial) movement in air and on objects (Bezdudnaya and Castro-Alamancos 2014, 2011). Whisker responses in superior colliculus are driven first by inputs from the trigeminal complex (trigeminotectal) and later by inputs from the barrel cortex (corticotectal) in a temporally defined manner (Bezdudnaya and Castro-Alamancos 2014; Castro-Alamancos and Bezdudnaya 2015; Cohen et al. 2008). Although these characteristics have been defined in the rat, they have not yet been established in mice, which is becoming a species of choice for studies of neural circuitry and behavior. Thus one of our goals was to determine if whisker-evoked responses in the superior colliculus of mice resemble those observed in rats and have a similar dependence on corticotectal inputs.
The trigeminotectal pathway is formed by cells in the spinal subnuclei interpolaris (Sp5i) and oralis (Sp5o) (Erzurumlu et al. 2010), which have multiwhisker receptive fields and also project to the posterior nucleus of the thalamus (PO) and zona incerta, and by a portion of cells in the principal nucleus (Pr5), which have large multipolar somata with expansive dendritic trees and multiwhisker receptive fields (Bruce et al. 1987; Huerta et al. 1983; Killackey and Erzurumlu 1981; Rhoades et al. 1989; Veinante and Deschenes 1999). In contrast, the other spinal subnucleus, caudalis (Sp5c), has few projections to the superior colliculus (Killackey and Erzurumlu 1981). Thus the trigeminotectal pathway is formed by cells with multiwhisker receptive fields located in Pr5, Sp5i, and Sp5o. The whisker-related corticotectal pathway is formed by layer 5 cells in the barrel cortex (Killackey and Erzurumlu 1981; Triplett et al. 2012; Wise and Jones 1977) and whisker motor cortex (Miyashita et al. 1994) that project to the superior colliculus (McHaffie et al. 1993). Whereas it is well known that trigeminotectal and corticotectal afferents innervate the intermediate layers, it is not known if the projections of these afferents overlap and what laminar organization may exist between them. Thus we used a dual anterograde tracing method to address this issue.
It has not been possible to investigate the electrophysiological properties of trigeminotectal and corticotectal synaptic afferents that carry whisker-related information into the superior colliculus because these fibers do not form discrete bundles that can be selectively (electrically) stimulated. This limitation can now be overcome by using optogenetics (Zhang et al. 2006). In this study we used optogenetics and electrophysiological procedures in vivo and in vitro (slices) to characterize the properties of whisker-related trigeminotectal and corticotectal synaptic afferents in the mouse superior colliculus.
METHODS
AAV injections.
All procedures were reviewed and approved by the Animal Care Committee of Drexel University and conducted in adult (>8 wk) CD-1 mice. All CD-1 mice (n = 26) were anesthetized with ketamine-xylazine (100-5 mg/kg) and injected (0.2–0.4 μl) unilaterally or bilaterally with AAV9- or AAV5-hSyn-hChR2(H134R)-eYFP (UPenn Vector core) into Pr5 (n = 7), Sp5i (n = 9), or the barrel cortex (n = 10). The coordinates for Pr5 injections were (bregma-lambda plane) 5.3 mm posterior from bregma, 1.9 mm lateral from the midline, and 3.9 mm ventral from bregma (∼3.2 mm from the surface). The coordinates for Sp5i injections were 6.5 mm posterior from bregma, 1.8 mm lateral from the midline, and 5.0 mm ventral from bregma (∼4.0 mm from the surface). The coordinates for barrel cortex injections were 1.0 mm posterior from bregma, 2.6–3.0 mm lateral from the midline, and 0.6–0.7 mm ventral from the surface. A group of PV-Cre mice (n = 3) were injected (0.3 μl) with AAV9-EF1a-DIO-hChR2(H134R)-eYFP (UPenn Vector core) into the barrel cortex at the above coordinates. Animals were used for slice recordings or in vivo recordings starting 2 wk after the injections (range 2–3 wk).
In vitro (slice) recordings.
For slice preparation, mice were deeply anesthetized with an overdose of ketamine. Upon losing all responsiveness to a strong tail pinch, the animal was decapitated and the brain rapidly extracted. The brain stem was immediately placed in fixative for subsequent identification of the injection sites (see below). Slices (400 μm thick) were cut in the sagittal or coronal plane using a vibratome. Slices are collected in order and labeled starting with the first slice that shows a defining feature. For instance, sagittal slices were cut from lateral toward the midline, and slice 1 was the first 400-μm slice starting from the most lateral edge of the cortex. Recordings were derived from sagittal slices 7–9. All slices that employed AAV injections were sagittal. In a few cases, coronal slices were cut from posterior to anterior, and slice 1 was the first slice in which the inferior colliculus appeared after the cerebellum. Recordings were derived from coronal slices 3–6. Coronal slices provided additional cells for the characterization of intrinsic firing properties and for neuromodulation experiments. Slices were transferred to an interface chamber where they were bathed constantly (1–1.5 ml/min) with artificial cerebrospinal fluid (ACSF) at 32.5°C. The ACSF contained (in mM) 126 NaCl, 3.5 KCl, 1.25 NaH2Po4, 26 NaHCO3, 10 dextrose, 2 MgSO4·7H2O, and 2 CaCl2·2H2O. Field potential (FP) recordings were made using low-impedance (∼0.5 MΩ) glass pipettes filled with ACSF. Blind whole cell recordings were obtained from the superior colliculus intermediate layers using patch electrodes of 4- to 12-MΩ impedance. The electrodes were filled with internal solution containing (in mM) 135 K-gluconate, 4 KCl, 2 NaCl, 0.2 EGTA, 10 Tris-phosphocreatine, 0.3 TrisGTP, 10 HEPES, and 4 MgATP (290 mosM). Each slice was imaged using a compound fluorescent microscope to reveal the parts of the superior colliculus that contained enhanced yellow fluorescent protein (eYFP)-labeled fibers. This allowed direct targeting of the area containing trigeminotectal or corticotectal fibers that express channelrhodopsin 2 (ChR2). An FP electrode was then placed in that area to measure blue light-evoked responses. Whole cell recordings were initiated adjacent to the best FP evoked response location. At the end of each recording, we imaged the slice again (light and fluorescence) to record the location of the recording electrodes. In addition, in most cases the internal solution contained neurobiotin (0.2%) to label the recorded cells. In some cases, 6-cyano-7-nitroquinoxaline-2,3-dione (10–20 μM) and d-2-amino-5-phosphonopentanoic acid (50 μM) were dissolved in the ACSF to block glutamatergic synaptic responses evoked by blue light.
A 200-μm core diameter multimode optic fiber was used to apply pulses of blue light in the superior colliculus (around the recording site). The light source was a light-emitting diode (LED; ∼473 nm) driven by pulses of 1-ms duration. The blue light intensity was computer controlled by adjusting the output range of the light source. In addition, the intensity (irradiance) of the light beam exiting the optic fiber was measured by flashing a photodiode power sensor placed in the location of the slice. The light intensity range was 0–40.4 mW/mm2 (200-µm diameter), which corresponds to relative values of 0–5 V used to drive the LED (5 V is the maximum output of the LED). Single pulses or trains of blue light stimuli were applied at a minimum of 4 s apart.
Histology: slice experiments.
After each slice experiment, the slices were fixated in 4% paraformaldehyde with 1% glutaraldehyde, and later cryoprotected with sucrose (30%) and resectioned on a cryostat (80 μm). To further determine the extent of ChR2 expression, sections were mounted, coverslipped with 4,6-diamidino-2-phenylindole (DAPI) mounting medium, and photographed using a fluorescent microscope. Fluorescent sections that contained neurobiotin-filled cells were incubated in 0.2% Triton X-100 and 2% goat serum followed by DyLight 594-labeled streptavidin. Sections were then mounted, coverslipped with DAPI mounting medium, and photographed using a fluorescent microscope. The eYFP from the ChR2 expression appears greenish, the neurobiotin-filled cell appears reddish, and DAPI appears bluish (Favero and Castro-Alamancos 2013). Cells were reconstructed using Neurolucida (Microbrightfield, Williston, VT).
The brain stem of animals injected with adeno-associated viruses (AAVs) in Sp5i and Pr5 were collected during slice preparation, placed in fixative, and later sectioned in the coronal plane at the level of the trigeminal complex to identify the injection sites in Pr5 and Sp5i. The tissue was embedded in agar and sectioned with a vibratome. Coronal sections (100 μm) were mounted, coverslipped with DAPI mounting medium, and photographed using a fluorescent microscope. The eYFP from the ChR2 expression appears greenish, and DAPI appears blueish.
In vivo recordings.
We conducted two sets of in vivo experiments (see below). In both cases, anesthetized mice were placed in a stereotaxic frame. Skin incisions and frame contacts with the skin were injected with lidocaine (2%). Craniotomies and incisions of the dura were made over the target structures as necessary. Body temperature was automatically maintained constant with a heating pad at 37°C. The level of anesthesia was monitored with FP recordings (from the barrel cortex) and limb withdrawal reflexes. It was kept constant at about stage 3 (i.e., slow large-amplitude FP cortical oscillations, absence of pinch withdrawal reflex, absence of whisker movements) using supplemental doses. Whisker stimulation was delivered contralateral to the recording site. Mechanical whisker deflections were performed by placing multiple whiskers (6–12) in several glass pipettes (1/0.5-mm outer diameter/inner diameter) that were glued to the membrane of miniature speakers. Application of a 1-ms square current pulse to the speakers deflected the micropipette and the whiskers inside. The resulting whisker deflection is a very-low-amplitude (∼2°) and very-high-velocity (∼1,000°/s) stimulus. Each of the whisker stimulators was driven by counter/timer boards controlled with LabVIEW software (National Instruments, Austin, TX).
In the first set of in vivo experiments, we used CD-1 mice (n = 20) anesthetized with urethane (1.5 g/kg ip) to measure intracellular responses in superior colliculus cells evoked by multiwhisker stimulation. In these experiments, we also measured intracellular and extracellular responses evoked in superior colliculus cells by electrically stimulating corticotectal cells within the barrel cortex. Extracellular multiunit activity (MUA) and FP recordings were obtained with tungsten electrodes (1–5 MΩ). Intracellular recordings were obtained using high-impedance (80–120 MΩ) sharp electrodes filled with K-acetate (2 M). All intracellular recordings had overshooting (0 mV) action potentials and were stable for >15 min. Recording electrodes were located within the intermediate layers of the superior colliculus at ∼3.8–4.1 mm posterior from bregma, 1.5 mm lateral from the midline, and 1.5–2.2 mm ventral from the surface. To evoke corticotectal responses, a concentric bipolar stimulating electrode (125 μm in diameter) was placed in the barrel cortex at 1.0 mm posterior from bregma, 3.0 mm lateral from the midline, and ∼0.6 mm ventral from the surface.
In the second set of in vivo experiments, we used PV-Cre mice (n = 3) anesthetized with ketamine-xylazine (100-5 mg/kg ip; supplemented every ∼30 min) to determine the effect of inactivating a small portion of the barrel cortex on superior colliculus responses driven by multiwhisker stimulation. A 200-μm core diameter multimode optic fiber coupled to an LED (∼473 nm) was used to apply pulses of blue light in the barrel cortex at ∼1.0 mm posterior from bregma, 3.0 mm lateral from the midline, and 0.5 mm ventral from the surface. MUA and FP were recorded through tungsten electrodes located in the barrel cortex, adjacent to the optic fiber, and in the superior colliculus intermediate layers. The same LED as for the slice experiments was used at maximum power (1.27 mW).
Histology: dextran injections in vivo.
In a group of ketamine-xylazine-anesthetized CD-1 mice (n = 12), we did iontophoretic injections of biotinylated dextran amine (BDA; 3–10 kDa; Molecular Probes) in Sp5 or barrel cortex [10% in K-acetate (0.5 M); pH 7.4]. In two mice, the same animal received a BDA injection in Sp5 and a dextran fluorescein-labeled dextran (fluoro-emerald; Molecular Probes) injection in the opposite barrel cortex [5% in K-acetate (0.5 M); pH 7.4]. The iontophoretic injections (+1.5 μA; 5 s on/off; 5 min) were performed with a low-impedance glass electrode (∼1.5 MΩ) at several sites and depths within each location. In the barrel cortex, the four sites had the following anterior-posterior (−0.7 and −1.5) and lateral (1.5 and 3.0) coordinates (from bregma in mm). At each barrel cortex site, we iontophoretically injected at three depths (0.6, 0.8, and 1 mm from the surface). In Sp5, the two sites had the following anterior-posterior (−1.8, −2.6) and lateral (1.8) coordinates (from bregma in mm). At each Sp5 site, we iontophoretically injected at six depths (4.0, 4.2, 4.4, 4.6, 4.8, and 5.0 mm from the surface). Before injections, unit recordings were performed through the iontophoretic electrode at each site to assure that the sites responded to whisker stimulation.
After 3–6 days, mice were perfused through the heart using 4% paraformaldehyde with 1% glutaraldehyde. Sections (100 μm) were cut using a vibratome in the coronal or sagittal plane. Sections were incubated in 0.3% hydrogen peroxide, followed by 0.2% Triton X-100 and 2% goat serum. Incubation with ABC reagent (Vector Laboratories) occurred overnight. The following day, diaminobenzidine (DAB) was applied to the sections. After color development, sections were mounted and cleared in xylene. When fluoro-emerald was also injected in the same animal, sections were incubated in 0.3% hydrogen peroxide, followed by 0.2% Triton X-100 and 2% goat serum. We then used peroxidase anti-fluorescein (goat; Vector Laboratories) followed by DAB to reveal the fluoro-emerald (which appears brown). This was followed by 0.2% Triton X-100 and 2% goat serum, horseradish peroxidase-conjugated streptavidin (Vector Laboratories), and ImmpactSG (Vector Laboratories) to reveal the BDA (which appears bluish gray). Labeling from both dextrans was easily distinguished under the microscope.
Statistical analysis.
If the data were considered normally distributed, according to the Shapiro-Wilk normality test, we used parametric statistics. For two groups, we used the t-test (paired). For more than two groups (one factor), we tested for a significant main effect using the repeated-measures or independent ANOVA followed by comparisons with Tukey's test. A similar approach was used when there was more than one factor. In this case, reported P values correspond to significant Tukey's tests after a significant main effect or interaction. If the data were considered not normally distributed, we used nonparametric statistics consisting of the Wilcoxon signed ranks. When multiple comparisons were performed, P values were adjusted using a Bonferroni correction by multiplying the P value by the number of comparisons made. The alpha level used for significance was P < 0.05 or P < 0.01, as indicated.
RESULTS
Whisker-related afferents in mouse superior colliculus.
In a group of mice, we performed dextran iontophoretic injections in either barrel cortex (n = 5 mice), Sp5 (n = 5), or both (n = 2). Figure 1, A–C, shows parasagittal sections from two mice that were injected with BDA in barrel cortex. Figure 1A shows the injection sites in barrel cortex (2 of the 4 injections are visible at 2.4 mm from the midline), and Fig. 1B shows the resulting labeled fibers in PO, anterior pretectum (APT), and superior colliculus for the same animal. Corticotectal fibers arriving in superior colliculus from barrel cortex produced a dense patch of labeling in the upper part of the intermediate granular layer (InG) and sparse labeling in the deep granular layer (DpG). This is more evident in Fig. 1C, which shows a close-up of corticotectal fibers in superior colliculus from a different mouse with similar labeling. In Fig. 1C, the brightness was equalized to clearly reveal fibers arriving in both intermediate and deep layers. In all animals, corticotectal fibers appeared in two regions of superior colliculus, the central and posterior part of superior colliculus (red arrow in Fig. 1B) and the most anterior portion of superior colliculus at the border with APT (blue arrow in Fig. 1B). Labeling appeared in the central and posterior locations because central and medial portions of the barrel cortex (which we targeted with our injections) project to the central and posterior portions of superior colliculus (Triplett et al. 2012). The most anterior labeling (blue arrow) appears in coronal sections as a strip along the base of the superior colliculus at the border with anterior structures and seems to represent a concentration of corticotectal fibers entering the superior colliculus.
Fig. 1.
Photomicrographs of dextran-labeled trigeminotectal and corticotectal fibers in the superior colliculus. A: parasagittal section at showing 2 iontophoretic injections of BDA in the barrel cortex. B: more medial section showing corticofugal fibers in PO thalamus, APT, and superior colliculus (SC). Corticotectal fibers appeared in 2 regions of SC: the central and posterior part (red arrow) and the most anterior portion at the border with APT (blue arrow). IC, inferior colliculus. C: a similar section from a different mouse showing a close-up of the labeling in SC. In this photograph, the brightness was equalized to reveal the labeled corticotectal fibers in both intermediate (InG) and deep (DpG) layers. The different layers are identified. SuG, superficial gray layer; InWh, intermediate white layer. D: parasagittal section showing iontophoretic injections of BDA in the Sp5 at 2 different levels from the midline in an animal also subjected to a fluoro-emerald injection in the contralateral barrel cortex (not shown). E: 4 consecutive sections centered in the SC of the same animal as in D; sections are contralateral to the Sp5 injection and ipsilateral to the barrel cortex injection. Corticotectal fibers appear brown and trigeminotectal fibers appear bluish gray. The red asterisk in the third section denotes an area that is blown up in the photomicrograph shown below. F: simplified schematic of whisker-related trigeminotectal and corticotectal afferents in the superior colliculus. PrV, trigeminal nucleus principalis; SpVi, spinal trigeminal nucleus interpolaris; SpVo, spinal trigeminal nucleus oralis; Vg, trigeminal ganglion; VPM, ventral posteromedial nucleus.
Figure 1, D and E, shows parasagittal sections from a mouse that was injected with BDA in the contralateral Sp5 and with fluoro-emerald in the ipsilateral barrel cortex. Thus trigeminotectal fibers appear bluish gray (almost black), and corticotectal fibers appear brown. Figure 1D shows the injection sites in two brain stem sections that cover Sp5i. The cortical injections are similar to those in Fig. 1A (not shown). Figure 1E shows four consecutive sections centered in the superior colliculus of the same animal. Corticotectal fibers were located in the same locations as described above: upper portion of InG and sparsely in DpG. Trigeminotectal fibers overlapped these same locations. The Sp5i injection sites we used tended to produce labeling in the central and anterior portions of superior colliculus. When both corticotectal and trigeminotectal fibers were present in the same location, they overlapped extensively, but trigeminotectal fibers were located slightly deeper in InG (2/2 cases). For instance, a close-up of the arrangement in the InG layer revealed that the trigeminotectal fibers overlapped corticotectal fibers but were located slightly deeper than corticotectal fibers in this layer (see panel in Fig. 1E with red asterisk). In addition, both corticotectal and trigeminotectal fibers appeared in the most anterior location of superior colliculus at the border with APT, where trigeminotectal fibers were also slightly deeper. Figure 1F shows a schematic of the whisker-related projections to the superior colliculus. Thus our results show that trigeminotectal and corticotectal projections overlap in the intermediate layers of superior colliculus, with trigeminotectal slightly deeper. Below we describe the responses evoked by these synaptic connections both in vivo and in vitro.
Whisker-evoked responses in superior colliculus in vivo: cortical dependency.
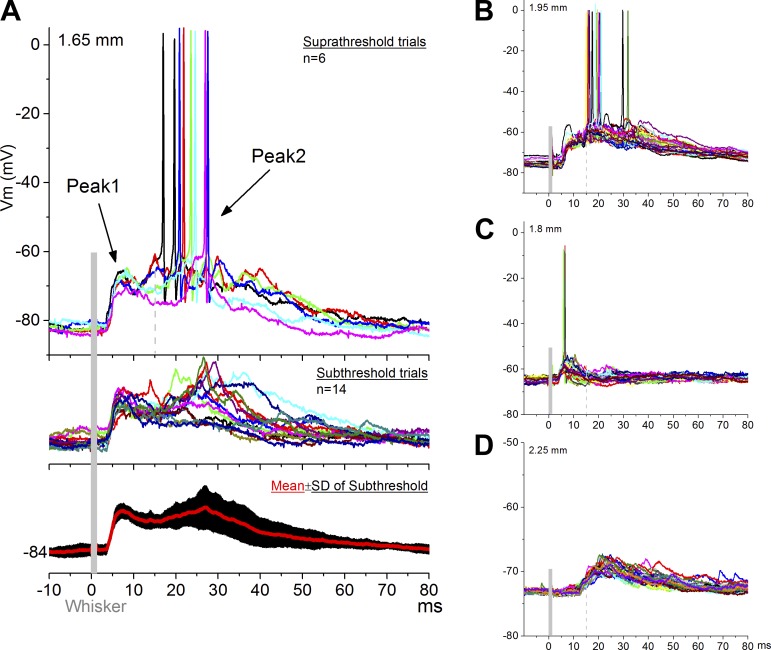
In rats, superior colliculus cells respond much more effectively to multiwhisker stimulation (i.e., simultaneous deflection of multiple whiskers; e.g., 6 whiskers) than to single-whisker stimulation. Multiwhisker stimulation produces two successive responses in superior colliculus, termed peak1 and peak2, that are mainly driven by trigeminotectal and corticotectal synapses, respectively (Cohen et al. 2008). In this experiment, we conducted intracellular and extracellular recordings from the superior colliculus of anesthetized mice and measured responses evoked by multiwhisker stimulation. Figure 2 shows intracellular recordings from four cells located in the superior colliculus (1.65–2.25 mm in depth; 1–1.5 mm lateral), which responded with peak1 (3–15 ms) and peak2 (15–40 ms) components. As in the rat, peak2 was more variable than peak1 (Fig. 2A; comparison of the SDs revealed that SD was significantly larger during peak2 than peak1; P < 0.001). We recorded from a total of 15 cells with similar responses. The majority of cells (13/15) responded with both peak1 and peak2 components (Fig. 2, A and B). One cell presented a peak1 response and appeared to be devoid of a peak2 response (Fig. 2C). Another cell was devoid of peak1, presenting only a peak2 response (Fig. 2D).
Fig. 2.
Intracellular recordings of SC cells driven by whisker deflections in vivo. A: typical cell responding with a peak1 and peak2 component to multiwhisker stimulation. Top, suprathreshold trials; middle, subthreshold trials; bottom, the variability (±SD) of peak2 is larger. Each colored trace is a single stimulus trial. B: another cell with a peak1 and peak 2 response. C: a cell with a peak1 response but devoid of peak2. D: a cell with a peak2 response but devoid of peak1.
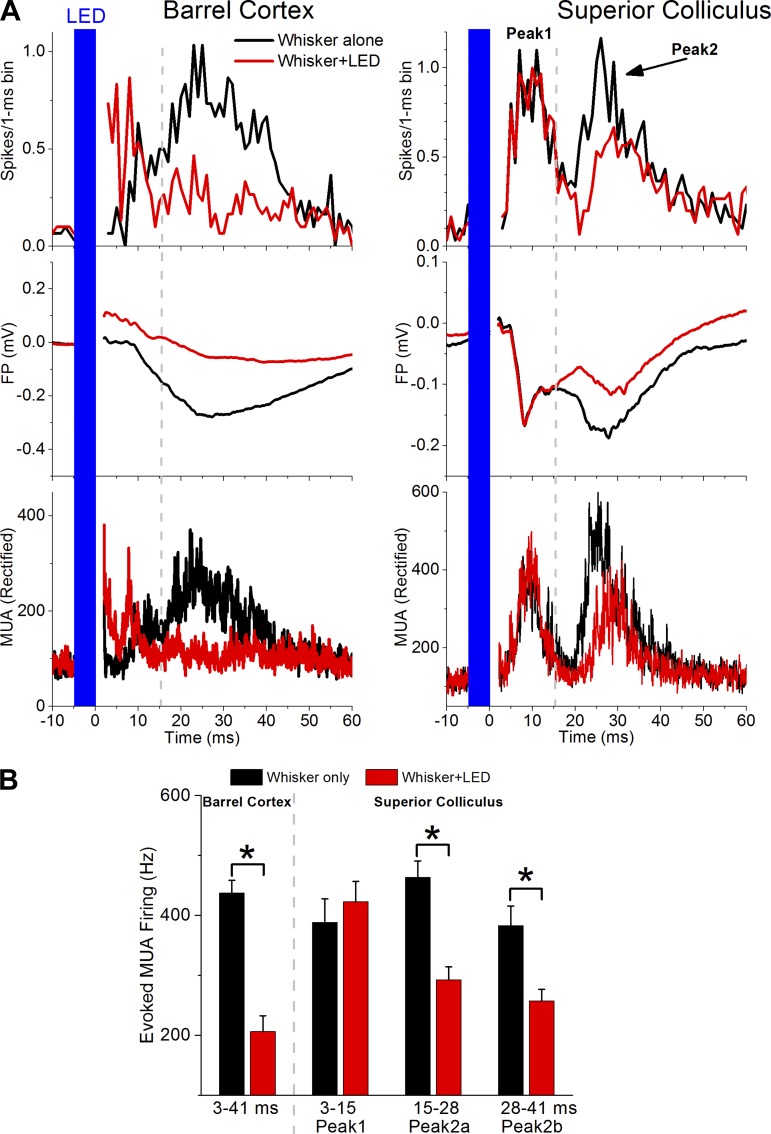
Peak1 and peak2 responses were also evident with extracellular FP and unit recordings (Fig. 3). In rats, peak2 is reversibly suppressed by pharmacological inactivation of barrel cortex, because of its dependency on corticotectal synapses (Cohen et al. 2008). To test if peak2 responses driven by whisker stimulation depend on barrel cortex, we used PV-Cre mice that express ChR2 in parvalbumin-expressing (PV+) cells of barrel cortex as a consequence of a prior AAV infusion (see methods). Animals were anesthetized, and both an optic fiber (200 μm in diameter) and a recording electrode were inserted into the barrel cortex. The optic fiber was lowered slightly into the cortex above layer 5. The recording electrode was inserted adjacent to the optic fiber within layer 5 to monitor MUA and FP responses driven by whisker stimulation. The location of the optic fiber was further adjusted until we found a location in which a 5-ms pulse of blue light, starting 5 ms before the whisker deflection, robustly (∼50%) inhibited the barrel cortex response evoked by whisker stimulation. At this point, the location of a recording electrode in the superior colliculus was adjusted until a whisker-evoked response was observed. We then tested the effect of inhibiting the cortical response driven by whisker stimulation on peak1 and peak2 whisker responses in superior colliculus. It is worth noting that application of pulses of blue light alone (5 ms) inhibited spontaneous firing in barrel cortex but also produced a transient excitatory response, which is evident in the red trace (whisker+LED) at the offset of the blue light (Fig. 3A, top left). This was not reflected in the superior colliculus and is likely caused by the firing of PV+ cortical cells driven by the blue light.
Fig. 3.
Transient inactivation of the barrel cortex with optogenetics selectively suppresses peak2 in vivo. A: MUA and FP recordings from the barrel cortex and SC of a PV-Cre mouse. Multiwhisker responses are evoked in the absence (whisker alone; black trace) or in the presence (whisker+LED; red trace) of blue light (LED) in the barrel cortex. The blue light drives PV+ cells that express ChR2, which robustly inhibits whisker-evoked responses in barrel cortex (left). At the same time, the barrel cortex inhibition leads to suppression of peak2 responses in SC (right). Top, poststimulus time histograms (PSTHs); middle, mean FP responses; bottom, rectified MUA (30 trials). The dashed vertical lines represent the border of the measurement window between peak1 and peak2. B: population data showing the effect of blue light (LED; red) on whisker-evoked responses measured in barrel cortex and SC at the indicated times poststimulus. *P < 0.01.
Figure 3A shows a typical experiment consisting of whisker stimulation alone (black traces) and whisker stimulation plus blue light in barrel cortex (red traces). Note that the blue light suppressed the whisker response in barrel cortex and at the same time selectively suppressed the peak2 response in superior colliculus; peak1 remained unaffected (Fig. 3A). This was repeated in different superior colliculus sites (n = 6) in three animals; the population results are shown in Fig. 3B. Although in the example shown it appears that only the earlier part of peak2 (termed peak2a; 15–28 ms) is being suppressed, this was not the case at other sites; both peak2a and peak2b (28–41 ms) were significantly suppressed when all sites were combined (Wilcoxon; P < 0.001). It is worth noting that our multiwhisker stimulation activates a large portion of the barrel cortex (many barrels). In contrast, we used only a 200-μm optic fiber coupled to an LED to inactivate the barrel cortex. Thus we would not expect under these conditions a complete abolishment of peak2. It is also worth noting that long-latency trigeminotectal inputs may contribute to the portion of peak2 remaining after cortical inactivation. In conclusion, whisker-evoked responses in mouse superior colliculus are characterized by peak1 and peak2 components driven by trigeminotectal and corticotectal synapses, respectively.
Corticotectal responses in vivo.
The previous results indicate that a significant portion of the whisker responses recorded in the superior colliculus, termed peak2, derive from the barrel cortex via corticotectal synapses. To characterize corticotectal responses in more detail, we conducted extracellular (MUA and FP) and intracellular recordings in the superior colliculus of anesthetized mice. To drive corticotectal synapses, a concentric bipolar stimulating electrode was placed in layer 5 of the barrel cortex.
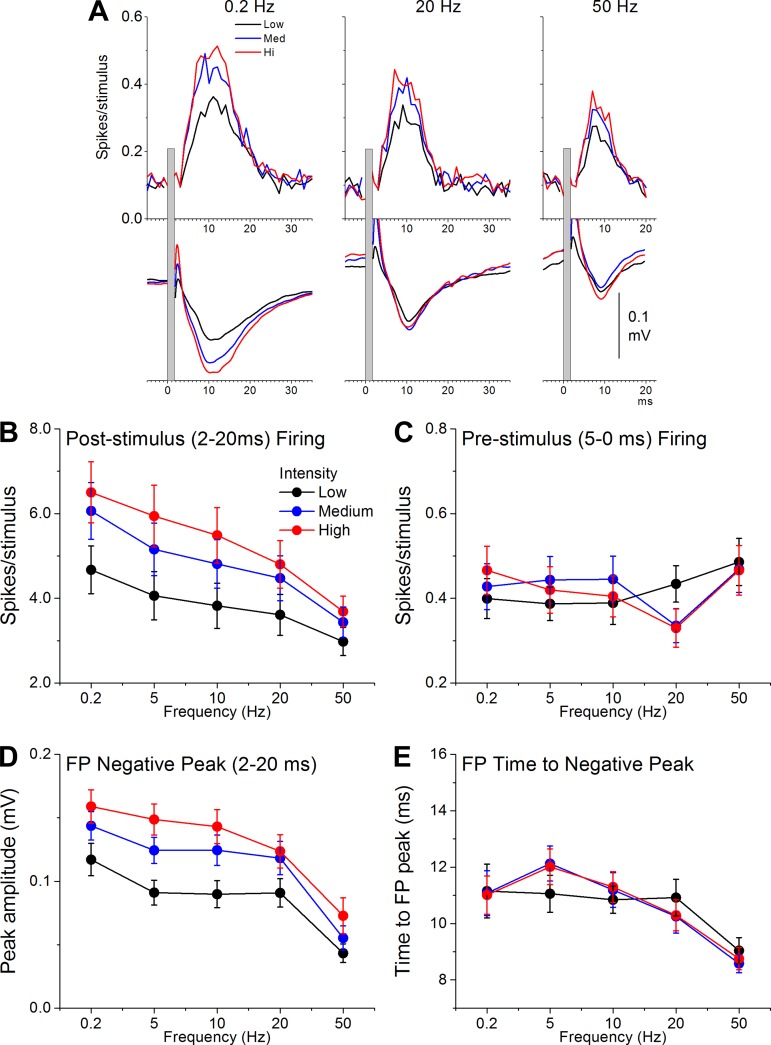
Figure 4 shows population data of MUA and FP responses evoked by electrical stimulation in layer 5 of barrel cortex (n = 26 sites from 20 mice). Stimuli consisted of five different frequencies (0.2, 5, 10, 20, and 50 Hz) delivered at three different intensities (mean ± SD low, medium, and high: 110 ± 50, 230 ± 70, and 350 ± 100 μA, respectively). Repeated-measures ANOVA revealed that there was a significant effect of frequency (P < 0.001) and intensity (P < 0.001) on MUA responses (spikes per stimulus; Fig. 4B) and FP responses (negative FP amplitude; Fig. 4D) measured between 2 and 20 ms poststimulus. Multiple comparisons (Tukey) revealed that increases in intensity significantly enhanced corticotectal responses between all of the intensities, whereas increases in frequency significantly depressed corticotectal responses between most of the frequencies (except 5 vs. 10 Hz and 10 vs. 20 Hz). We also measured the effect of intensity and frequency on firing before each stimulus (5-0 ms prestimulus firing; Fig. 4C). There was no effect of intensity on prestimulus firing (P = 0.9), but there was a significant effect of frequency (P = 0.001) and a slight interaction between intensity and frequency (P = 0.02). Multiple comparisons revealed that this effect was a suppression of prestimulus firing specifically for stimuli delivered at 20 Hz at the medium and high intensities. Finally, we measured the effect of intensity and frequency on the time to FP negative peak (Fig. 4E). There was no effect of intensity on time to FP peak (P = 0.97), but there was a significant effect of frequency (P < 0.001). Multiple comparisons revealed that the effect was a reduction of time to peak at the highest frequencies (20–50 Hz). These results indicate that corticotectal responses depress with frequency and are enhanced by recruitment of more corticotectal fibers with intensity (cooperativity). Thus cooperativity leads to larger corticotectal responses and higher presynaptic firing rates depress corticotectal responses.
Fig. 4.
Properties of extracellular corticotectal responses evoked in the SC by electrical stimulation in the barrel cortex in vivo. A: PSTHs showing MUA (top) and FP responses (bottom) in SC driven by electrical stimulation of barrel cortex (layer 5) at low, medium, and high intensities and at 3 different frequencies (0.2, 20, and 50 Hz). B–E: population data measuring the effect of frequency and intensity on MUA responses measured 2–20 ms poststimulus (B), on MUA responses measured 5-0 ms prestimulus (C), on FP responses measured 2–20 ms poststimulus (D), and on the time to FP negative peak measured poststimulus (E).
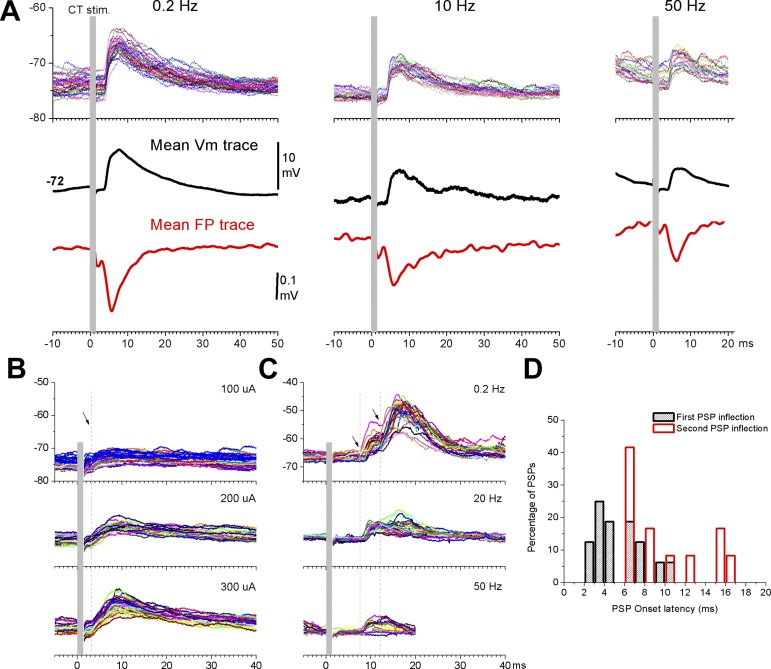
The previous results measured extracellular corticotectal responses in vivo. We next examined intracellular responses from single cells. Figure 5 shows intracellular responses from three different cells in superior colliculus evoked by electrical stimulation in barrel cortex. Figure 5A shows the effect of frequency on corticotectal postsynaptic potentials (PSPs; the cell was located at 1.66 mm in depth from the surface and 1.5 mm lateral from the midline) and simultaneously recorded FP responses. As described above for population responses, PSPs depressed with increases in frequency. Figure 5B shows the effect of intensity on another cell (1.75 mm in depth, 1.5 mm lateral). As expected, the slope and amplitude of the corticotectal PSPs increased with intensity indicating the recruitment of additional synaptic inputs (cooperativity). These characteristics were observed in all the cells examined (n = 16 cells). The main difference between the cells was the onset latency and the number of components (or peaks) present in the PSPs. Figure 5C shows a third cell (1.5 mm in depth; 1 mm lateral) that had a significantly longer onset latency (i.e., 7.5 ms). Moreover, a second response component is evident at an even longer latency (∼12 ms; note arrows in Fig. 5, B and C). As for cells with shorter onset latencies (<5 ms), intensity also caused long onset responses to increase and frequency caused them to depress (Fig. 5C). In Fig. 5D we plot the percentage of cells based on their PSPs onset latencies. When we measured only the onset latency of the earliest PSPs (black bars in Fig. 5D), we noticed that the cells were distributed within 2 peaks (between 2–5 and 6–8 ms) separated by about 4 ms. A third peak (between 9 and 11 ms) also may be present, but it includes few cells. This reflects cells responding with different onset latencies. Interestingly, for cells that had two response components, when we measured the onset of the second component, many of them roughly coincided with the second peak noted for the earliest onsets. Other cells had longer onset latencies. This suggests that corticotectal stimulation evokes a propagating wave of activity in superior colliculus that produces peaks separated by ∼4 ms, which may reflect the synaptic recurrence and laminar organization of the superior colliculus. To further examine the properties of whisker-related synaptic inputs to the superior colliculus, we next turned to studies using slices.
Fig. 5.
Intracellular corticotectal responses in SC cells driven by electrical stimulation of the barrel cortex in vivo. A: example intracellular and FP corticotectal responses evoked in the SC by electrical stimulation at 3 different frequencies. Single (top) and mean (middle) intracellular traces and mean FP traces (bottom) are shown. B: effect of intensity on corticotectal responses in a different cell. C: effect of frequency on another cell that had long-latency responses. D: population data showing the percentage of cells based on their PSP onset latencies. Black bars reflect onset latencies of the first PSP in the response. Red columns show onset latencies for the second PSP inflection in the response for cells that had 2 response components.
Neurons in the intermediate layers of superior colliculus slices.
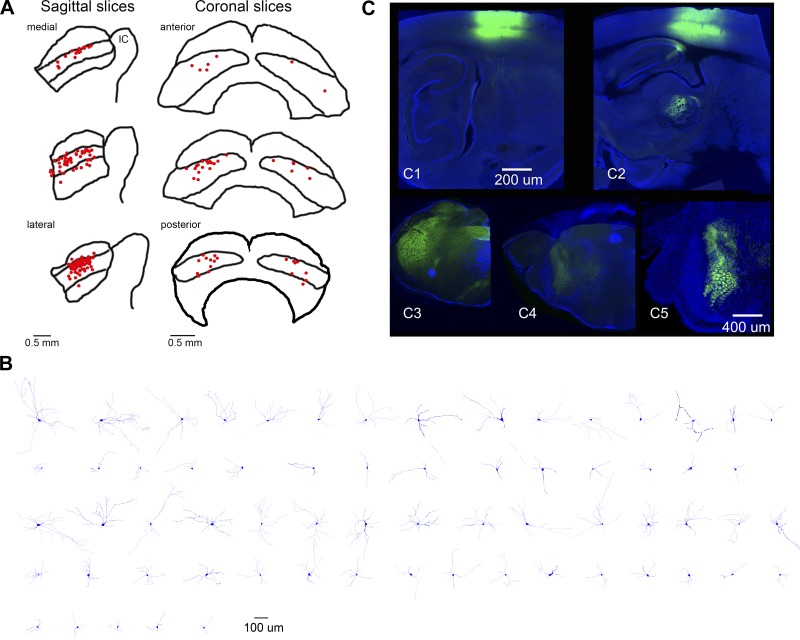
We performed whole cell recordings from cells in adult superior colliculus slices. Figure 6A shows the location of the patch electrode tip for all the cells included in the study (n = 134), at three different levels for slices cut in the sagittal and coronal planes. Some of these cells were filled with neurobiotin, labeled, and reconstructed. The electrophysiological and morphological properties of the cells we recorded were similar to those previously characterized in rodent slices, which have been classified according to the orientation of their dendrites (Isa and Hall 2009; Kaneda et al. 2008; Saito and Isa 1999). In adult animals, the intermediate layers contain wide-field vertical cells (cells that extend diverging dendritic trees dorsally), multipolar cells (cells that extend dendrites in all directions), and to a lesser extent, pyramidal, fusiform, and horizontal cells. Figure 6B shows a sample of 65 reconstructed cells classified according to their dendritic orientation and length. The first two rows show cells that were considered polarized because they had more than 75% of their dendrites in 2 of 4 quadrants on the basis of a polar analysis (Neurolucida). The last three rows represent cells that had a more even distribution among the four quadrants. In both groups, the cells are plotted in order according to the total dendritic length (from largest to smallest). Although some of the recorded cells could be unambiguously matched with the reconstructions, this was not the case for most cells because we opted for a high-density sampling (many cells in one slice) to reduce the number of animals used and to maximize the number of cells per slice. In this section, we describe the intrinsic electrophysiological properties of the recorded cells and the effect of a cholinergic agonist (carbachol) on some of them.
Fig. 6.
Histology for slice experiments. A: approximate location of the patch electrode tip (red dots) for all the cells included in the study. Sagittal (left) and coronal slices (right) are shown at 3 different medial-lateral and anterior-posterior levels, respectively. B: sample of 65 reconstructed cells in the intermediate layers. C: typical AAV injections in barrel cortex (C1, C2) and Sp5 (C3–C5). Top fluorescent images (green is eYFP and blue is DAPI) show 2 parasagittal sections (100 μm) from 1 animal injected in barrel cortex. The sections are ∼2.7 mm (C1) and 2.1 mm (C2) lateral from the midline. Bottom fluorescent images [green is eYFP and blue is the blue channel from a dark (C3, C4)- or brightfield image (C5)] show 3 coronal sections (100 μm) from another animal at the level of the brain stem. The sections are ∼3.3 mm posterior from bregma in Sp5i (C3), 1.3 mm posterior from bregma in Pr5 (C4), and a close-up (×2) of the adjacent section at 1.4 mm posterior from bregma in Pr5 (C5). Note the barrelettes in Pr5, denoting projections from Sp5. C1–C4 share the same scale bar. See Franklin and Paxinos (2008).
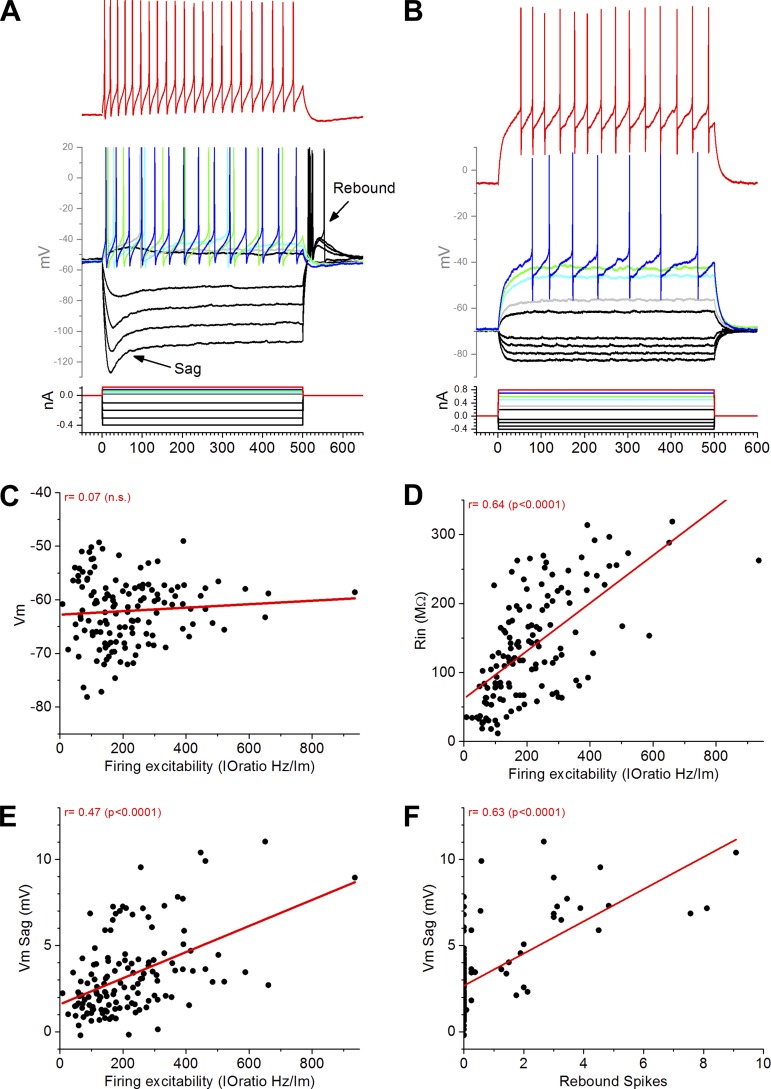
Figure 7, A and B, shows recordings from two cells with distinct responses to depolarizing and hyperpolarizing current pulses (500 ms). The first cell (Fig. 7A) shows two features that are prominent in some cells, namely, a depolarizing sag caused by hyperpolarizing pulses (the sag is measured by subtracting the steady voltage change), typically associated with a hyperpolarization-activated cation current, and a rebound depolarization after the hyperpolarization (Isa et al. 1998; Saito and Isa 1999). The second cell (Fig. 7B) lacks these features. Figure 7C shows the resting membrane potential (Vm) and the firing excitability for all the cells (n = 134). Firing excitability was calculated as the ratio between the firing rate (Hz) and the current injection (nA) applied to the cell (500-ms pulses). There was no significant correlation between these two variables, indicating that the firing excitability of a cell was not related to its resting Vm. However, firing excitability correlated strongly with the input resistance of the cell (r = 0.64; P < 0.0001; Fig. 7D) and with the depolarizing Vm sag caused by hyperpolarizing pulses (r = 0.47; P < 0.0001; Fig. 7E). Thus cells with higher input resistance are more excitable, and cells with a larger Vm sag are also more excitable. Finally, we found that there was a strong correlation between the Vm sag and the strength of the excitatory rebound after a hyperpolarizing pulse, measured as the number of rebound spikes (r = 0.63; P < 0.0001; Fig. 7F).
Fig. 7.
Intrinsic properties of cells recorded from intermediate layers of SC in slices. A and B: responses to depolarizing and hyperpolarizing intracellular current pulses (500 ms). One cell (A) shows a depolarizing sag caused by hyperpolarizing pulses and a rebound depolarization after the hyperpolarization. Another cell (B) lacks these properties. C–F: linear fit relationship of firing excitability with resting Vm (C), input resistance (Rin; D), and Vm sag (E) for all the cells and relationship between Vm sag and rebound spikes (F). The correlation between these variables is indicated in red; n.s., not significant.
We next tested the effect of the cholinergic agonist carbachol (CCh; 5 μM) on a group of these cells (n = 18). We tested carbachol because we recently found significant variability between cells in vivo regarding the effects of CCh on whisker-evoked firing (Bezdudnaya and Castro-Alamancos 2014). When considered together, cholinergic activation did not significantly affect the resting Vm (paired t-test; P = 0.11), the input resistance (P = 0.57), or the firing excitability to a positive current pulse (P = 0.29) of the tested cells. However, there was a strong correlation between the change in Vm caused by cholinergic activation and the change in input resistance (r = 0.79; P < 0.0001) or the change in firing rate (r = 0.8; P < 0.001) so that cells that were depolarized by CCh also tended to increase their input resistance and their firing rate in response to a current pulse. This implies the existence of two groups of cells that are differentially sensitive to cholinergic activation. Thus we created two groups of cells based on the effect of CCh on Vm. One group was depolarized by at least 0.8 mV (n = 8 cells; 6.8 ± 2 mV, mean ± SE), and the other group was hyperpolarized by at least the same amount (n = 6 cells; −2.2 ± 0.7 mV). When statistically compared (Mann-Whitney), these two groups of cells revealed significant differences in the effect of CCh on Vm (P < 0.001), but also on input resistance (P = 0.01) and firing excitability (P = 0.01). Thus, in one group of cells, cholinergic activation significantly depolarizes the Vm and increases input resistance and firing excitability. In a second group of cells, cholinergic activation hyperpolarizes the Vm and decreases firing excitability and input resistance. In conclusion, the effects of cholinergic activation on intermediate layer cells are heterogeneous. Two groups of cells are differentially sensitive to cholinergic activation.
Whisker-related synaptic responses in superior colliculus slices.
To characterize PSPs evoked in superior colliculus cells by trigeminotectal and corticotectal synapses, we expressed ChR2 in trigeminotectal or corticotectal fibers. To express ChR2 in corticotectal fibers we infused the AAV (see methods) in barrel cortex. To express ChR2 in trigeminotectal fibers, we infused the AAV into Sp5i or Pr5. Figure 6C shows examples of AAV injections in barrel cortex and Sp5i. Barrel cortex injections were typically well centered in layer 5. Inevitably cells in other layers were infected, but those cells do not project to the superior colliculus. Rarely, we observed some small expression in the underlying hippocampus, as shown in Fig. 6C2. The pattern of ChR2 expression in thalamus and superior colliculus after AAV injection in barrel cortex was very similar to that observed after BDA injections in barrel cortex. Thus, within the superior colliculus, AAV injections in the barrel cortex specifically express ChR2 in corticotectal fibers.
AAV injections in Sp5i lead to ChR2 expression that filled the Sp5i but could also partially encompass immediately adjacent structures such as the ventral cochlear nucleus, which is closer to Sp5o. This explains why in some slices from animals injected in Sp5i we also observe labeled fibers in the inferior colliculus (see below). Thus the AAV spread to Sp5o in some cases. The AAV injection did not appear to spread from Sp5i to Pr5. However, internuclear projections from Sp5i to Pr5 were robustly labeled in Pr5, because we typically observed barrelette patterns in Pr5 (Fig. 6, C4 and C5) (Erzurumlu et al. 2010; Furuta et al. 2008; Jacquin et al. 1990). AAV injections in Pr5 did not extend back to Sp5i (not shown) but usually infected the adjacent ventral cochlear nucleus consistently, and consequently we observed labeled projections in the inferior colliculus in almost every Pr5 injected slice. Within these targeted nuclei, the injections were not selective for whisker barrelettes, which are innervated by the infraorbital branch of the maxillary nerve. The fields innervated by mandibular, maxillary, and ophthalmic branches of the trigeminal ganglion (Erzurumlu et al. 2010) could also be infected. Thus some of the trigeminotectal synapses we studied may have originated from cells located in these fields of Pr5 and Sp5.
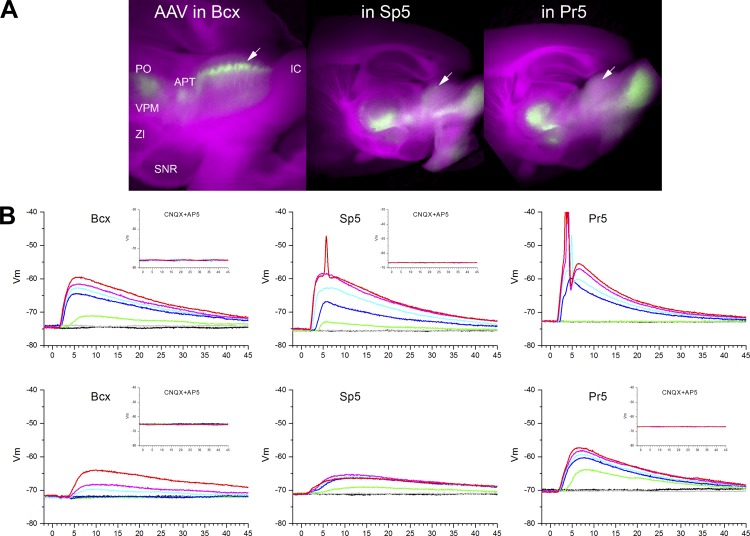
Two to three weeks after the infusion, we prepared coronal or sagittal slices and performed whole cell recordings from the intermediate layers of the superior colliculus in the area where there was ChR2 expression. We imaged the living slice to identify the area of the superior colliculus that shows eYFP fluorescence. Figure 8A shows images obtained from living sagittal slices during recordings. The images blend visible light of the slice (red and blue channels) with eYFP fluorescence (green channel) to reveal the location of ChR2 expression in the slice. Thus the slice appears purple, and the ChR2 expression appears green. In the superior colliculus, the strongest expression of ChR2 was observed in the intermediate layers in a fashion similar to BDA staining. As expected, other well-known targets of corticofugal and trigeminal fibers (e.g., thalamus) showed robust expression. In some cases, the inferior colliculus showed significant fluorescence due to spread of the AAV to ventral cochlear nuclei in the brain stem.
Fig. 8.
Trigeminotectal and corticotectal intracellular responses evoked in SC cells by optogenetics in slices. A: images obtained from living sagittal slices during recordings that blend visible light of the slice (red and blue channels) with eYFP fluorescence (green channel) to reveal the location of ChR2 expression in the slice. Examples are from slices with AAV injections in barrel cortex (left), Sp5 (middle), and Pr5 (right). The arrows point to the location where cells in the intermediate layers of SC were patched. SNR, substantia nigra pars reticulata; ZI, zona incerta. B: example intracellular responses obtained from 2 different cells per group evoked by pulses (1 ms) of blue light at 7 different intensities. Insets show the effect of glutamate receptor antagonists on the evoked responses. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; AP5, d-2-amino-5-phosphonopentanoic acid.
Our population data include three groups of cells; cells that responded to corticotectal fibers originating in barrel cortex (Bcx; n = 38), cells that responded to trigeminotectal fibers originating in Sp5i (Sp5; n = 44), and cells that responded to trigeminotectal fibers originating in Pr5 (Pr5; n = 35). An additional group of cells (n = 31 total: Bcx, n = 11; Sp5, n = 11; Pr5, n = 9) did not respond to the light and where not used in this analysis. For every cell, we applied blue light stimulation (1-ms duration) at seven different intensities (0.3–5; see methods). Figure 8B shows example responses obtained from two different cells per group. In all cases tested, block of glutamate receptors completely abolished the evoked responses (Fig. 8B, insets). We measured several variables from these PSP responses: onset latency, rising slope, peak amplitude (excluding action potentials), and decaying slope. We conducted two population analyses with these variables. The first analysis compared the mean responses evoked by the three groups as a function of the blue light intensity (2-way ANOVA in which intensity is a repeated measure). We determined if there was a significant effect of the group factor (3 levels) or an interaction with the intensity factor (7 levels). If that was the case, we conducted multiple comparisons between the groups at each of the seven intensities (Tukey). The second analysis looked at correlations between parameters within each group.
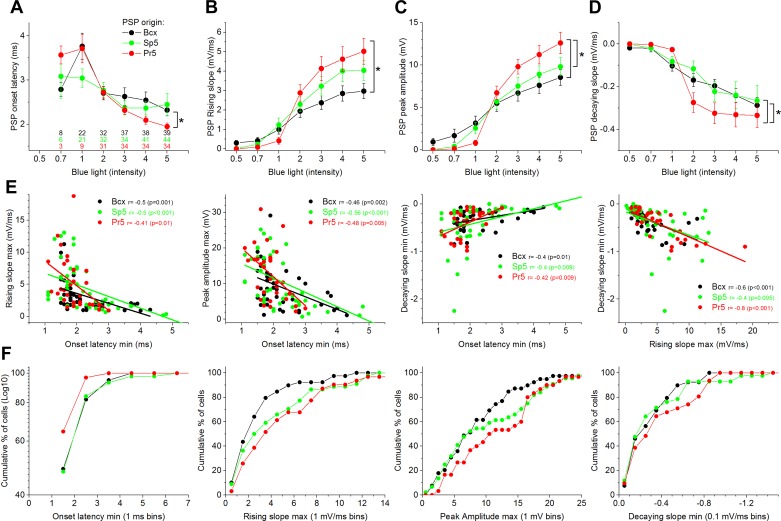
Figure 9A shows onset latency of the PSPs evoked by the different stimuli. The color-coded numbers represent the number of cells that produced measurable PSPs at that intensity. We found that a significant number of the responding cells produced measurable PSPs at an intensity of 2 (within the relative range of 0–5; see Methods), and as the intensity increased, more cells were recruited and the onset latency became shorter. However, although the three groups had similar onset latencies at an intensity of 2, cells that responded to Pr5 showed a sharper reduction in onset latency than cells that responded to either Sp5 or Bcx stimulation (P < 0.01). Thus, at the higher intensities, Pr5 evoked responses are more efficacious than either Sp5 or Bcx evoked responses. Figure 9, B–D, compares the rising slope, peak amplitude, and decaying slope of the PSPs between the three groups of responding cells. We found that PSPs driven by Pr5 synapses had significantly steeper slopes compared with those originating in Bcx at the three highest stimulation intensities (i.e., 3–5; P < 0.01), but not compared with those originating in Sp5. Pr5 synapses also produced significantly larger PSPs (peak amplitude) compared with Bcx synapses at the two highest intensities (P < 0.05) and compared with Sp5 synapses at the highest intensity (P = 0.02). Pr5 synapses also evoked PSPs that decayed significantly faster compared with Bcx and Sp5 synapses (P < 0.05), but this effect was only significant at intermediate stimulation intensities (2), not at the higher or lower intensities. None of the previous differences could be attributed to differences in resting Vm or input resistance between the cells since these variables were not significantly different between the groups of cells.
Fig. 9.
Population data of PSP measurements for the 3 groups of cells depending on the origin of the synaptic inputs. A–D: plots show the effect of blue light intensity on PSP onset latency (A), rising slope (B), peak amplitude (C), and decaying slope (D). E: correlations between the measured variables for each group. F: the percentage of cells as a function of the measured variable per group.
Figure 9E shows significant correlations between the different variables for each of the three groups of cells. Onset latency was negatively correlated with PSP rising slope and peak amplitude, and positively correlated with the PSP decaying slope. PSP rising slope was negatively correlated with PSP decaying slope. Thus cells with faster rising PSP slopes or larger PSP amplitudes had shorter onset latencies. Cells with a faster decaying slope had shorter onset latencies and faster rising slopes. Figure 9F plots the cumulative percentage of cells per group for ranges of values of the minimum onset latency, maximum rising slope, maximum peak amplitude and minimum decaying slope.
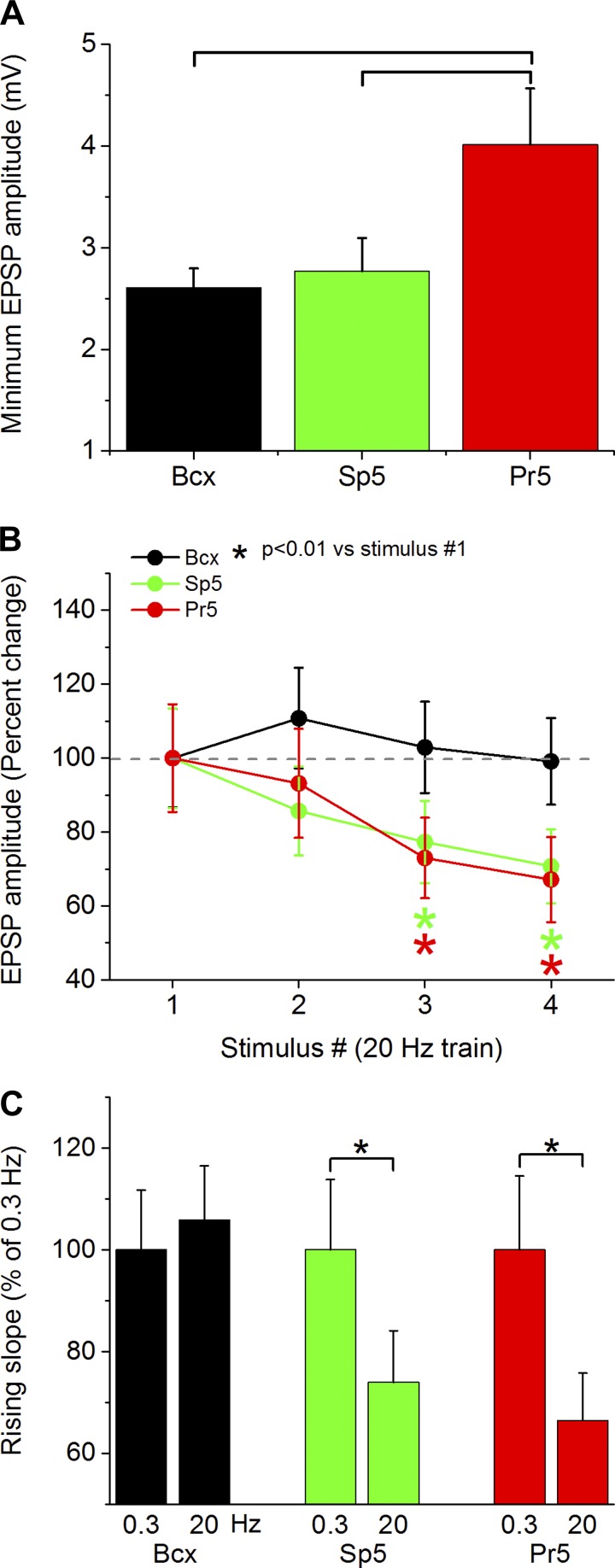
The previous results indicate that synaptic responses evoked by trigeminotectal fibers originating in Pr5 produce stronger responses in superior colliculus cells than trigeminotectal fibers originating in Sp5 or corticotectal fibers originating in Bcx. This result may be explained by two non-mutually exclusive scenarios. First, the synapses originating from single Pr5 fibers that converge onto one superior colliculus cell produce stronger synaptic responses than those converging from single Bcx or Sp5 fibers (i.e., synapses arriving from a single cell in Pr5 are stronger or more numerous). Second, superior colliculus cells receive larger numbers of converging synaptic contacts from different trigeminotectal fibers originating in Pr5 than from fibers originating in Sp5 or Bcx (i.e., the numbers and the strength of the synapses arriving from single cells does not vary, but superior colliculus cells are innervated by more cells from Pr5 than from the other sources). Finally, a combination of both scenarios would entail superior colliculus cells receiving stronger single-fiber synaptic contacts and larger numbers of synaptic contacts from Pr5 cells than from Bcx or Sp5 cells. The present study is not designed to differentiate between these options. However, to begin addressing these options, in the present study, we measured the amplitude of the minimum PSP response produced by our intensity curve for the three pathways. If the minimum PSP amplitude is different between the three pathways, and this denotes a single fiber being stimulated, it might suggest that the first scenario mentioned above is taking place. Indeed, we found (Fig. 10A) that the minimum synaptic response evoked by trigeminotectal fibers originating in Pr5 was significantly larger in amplitude than the minimum response evoked by stimulating trigeminotectal fibers originating in Sp5 (P = 0.04) or corticotectal fibers originating in Bcx (P < 0.01). This suggests that trigeminotectal synaptic afferents originating from single cells in Pr5 are more efficacious than those originating from single cells in Sp5 or Bcx. At a technical level, one may speculate that differences in the properties of the afferent fibers, in the ability of the AAV to infect certain cells, and the ability of the promoter to drive expression in specific cells types can make the depolarization elicited by the light more likely to trigger synaptic release in Pr5 fibers compared with corticotectal or Sp5 fibers. There may also be characteristics of ChR2-induced depolarization of afferent fibers that contribute to our findings. It is worth noting that ChR2 simply needs to depolarize the presynaptic membrane to the threshold of sodium channels, which is what drives the depolarization and synaptic release. Recordings from cortical cells that express ChR2 in these animals produce very large depolarizations (tens of millivolts in amplitude) in the presence of TTX that are much larger than the depolarization needed to reach firing threshold. Thus the cells producing the smallest synaptic responses in superior colliculus cells have very strong ChR2 expression. Another possibility is that the AAV may be infecting a greater percentage of fibers from one source than the others. However, if fluorophore expression in the superior colliculus is any indication of the number of fibers infected, it is clear that many more corticotectal fibers were infected compared with trigeminotectal fibers, because corticotectal fluorescence was always much stronger (Fig. 8A). Despite putatively having the largest number of ChR2 expressing fibers, the corticotectal pathway produced the smallest responses.
Fig. 10.
Distinct trigeminotectal and corticotectal synaptic responses. A: minimum synaptic response evoked by stimulation of trigeminotectal (Pr5 or Sp5) and corticotectal synapses. B: PSP response amplitude evoked by trigeminotectal and corticotectal synapses during a train of 4 pulses at 20 Hz. *P < 0.01 vs. stimulus 1. C: rising slope of the PSP evoked by the fourth pulse in a 20-Hz train compared with the first pulse at 0.3 Hz. *P < 0.01.
An actual difference in synaptic efficacy should be accompanied by differences in short-term synaptic plasticity. Thus we determined the effect of a train of four pulses at 20 Hz on each of the three pathways. We selected this frequency because ChR2 may have difficulty following pulses at higher frequencies (Zhang et al. 2006). Moreover, we only tested high-intensity pulses (>2) to ensure suprathreshold depolarization of presynaptic fibers during the first pulse. We found that both the amplitude and rising slope of trigeminotectal PSPs originating in Pr5 and Sp5 depressed significantly more than those originating in corticotectal synapses (Fig. 10, B and C).
DISCUSSION
The present study combined in vivo and slice experiments to characterize whisker-related inputs in the superior colliculus of adult mice transmitted by trigeminotectal and corticotectal fibers. Corticotectal fibers innervate the upper portion of the intermediate granular layer and, sparsely, the deeper granular layer. Trigeminotectal fibers overlap these same layers but innervate deeper portions of the intermediate granular layer. The intermediate layers contain primarily wide-field and multipolar cells, which orient many of their dendrites vertically toward the upper portion of the intermediate layers. Because the upper portion is innervated by corticotectal synapses and receives distal dendrites, it is possible that these cells will receive corticotectal synapses on distal dendrites and trigeminotectal synapses closer to the soma. Interestingly, trigeminotectal synapses produce stronger, faster rising, and more depressing PSPs than corticotectal synapses.
A portion of the cells in the intermediate layers are characterized by a prominent depolarizing sag and rebound excitation caused by hyperpolarization, whereas other cells respond to current pulses more linearly. Cells with higher input resistance are more excitable and cells with a larger depolarizing sag are also more excitable. The effects of cholinergic activation on intermediate layer cells are heterogeneous; one group of cells is excited whereas a second group is inhibited by cholinergic activation. This heterogeneity agrees with previous studies that have investigated the effect of cholinergic neuromodulation on superior colliculus cells both in slices (Li et al. 2004; Sooksawate and Isa 2006) and in vivo (Bezdudnaya and Castro-Alamancos 2014; Stubblefield et al. 2015).
Whisker-sensitive cells in superior colliculus.
Several studies have described superior colliculus responses evoked by deflecting stationary whiskers in the rat (Cohen and Castro-Alamancos 2007; Cohen et al. 2008; Fujikado et al. 1981; Grunwerg and Krauthamer 1990; Hemelt and Keller 2007; McHaffie et al. 1989; Stein and Dixon 1979), mouse (Drager and Hubel 1975b), and hamster (Chalupa and Rhoades 1977; Finlay et al. 1978; Larson et al. 1987; Rhoades et al. 1983, 1987; Stein and Dixon 1979). Intracellular and single-unit recordings in rats show that although superior colliculus cells respond relatively effectively to single whiskers, including a principal whisker and several adjacent whiskers, cells respond much more robustly to simultaneous, or nearly simultaneous, wide-field (multiwhisker) stimuli (Cohen et al. 2008). The enhanced multiwhisker response is temporally stereotyped, consisting of two short-latency excitatory peaks (peak1 and peak2) separated by ∼10 ms that have different characteristics and origins (Cohen et al. 2008). The spikes evoked during peak1 show little jitter and are driven by direct trigeminotectal PSPs. The spikes evoked during peak2 are much more dispersed and are driven by PSPs returning to the superior colliculus from the barrel cortex (corticotectal) (Cohen et al. 2008). In this study we found that multiwhisker responses evoked in the superior colliculus of mice are similar to those observed in rats. Optogenetic inhibition of corticotectal cells in barrel cortex during multiwhisker stimulation specifically reversibly suppresses peak2, indicating its cortical origin. Electrical stimulation of barrel cortex in vivo evokes PSPs in superior colliculus that increase with intensity and depress relatively modestly at frequencies up to 20 Hz, but more strongly at 40 Hz.
Optogenetic stimulation of trigeminotectal and corticotectal fibers in slices revealed that trigeminotectal synapses produce stronger, faster rising, and more depressing PSPs than corticotectal afferents, which could explain the sharpness and stability of peak1 (trigeminotectal) compared with peak2 (corticotectal) multiwhisker responses in vivo. Further work will need to address the cause of this synaptic difference, but our results suggest that single fibers innervating superior colliculus cells from Pr5 produce synaptic responses that are stronger than those driven by single corticotectal fibers. These strong trigeminotectal synapses resemble the fast-rising large-amplitude trigeminothalamic synapses that contact ventral posteromedial nucleus cells (Castro-Alamancos 2002a, 2002b), which consist of synaptic glomeruli containing many release sites close to the soma (Spacek and Lieberman 1974). Interestingly, corticotectal synapses are located in the upper most distal portion of the intermediate layers, whereas trigeminotectal synapses were located toward the middle of the layer. This implies that corticotectal synapses occur more commonly in distal portions of radiating superior colliculus cells, whereas trigeminotectal synapses occur closer to the soma. This difference may contribute to the weaker efficacy of corticotectal synapses, since synaptic inputs at distal dendrites can attenuate substantially at the soma. The experiments also show that trigeminotectal synapses depress much more strongly than corticotectal synapses. This may explain the strong frequency-dependent adaptation observed in superior colliculus during high-frequency deflection of passive whiskers (Cohen et al. 2008) or by artificial whisking (Bezdudnaya and Castro-Alamancos 2011, 2014). The response characteristics of corticotectal and trigeminotectal synapses imply that the intermediate layers of superior colliculus are set to process strong but infrequent whisker inputs from the periphery and weak but more persistent inputs from the barrel cortex. A main function of the superior colliculus is to produce orienting responses to salient sensory stimuli (Dean et al. 1989; Gandhi and Katnani 2011; Krauzlis et al. 2013; Mitchinson and Prescott 2013; Sparks and Mays 1990). This synaptic organization may allow the superior colliculus to be strongly driven by infrequent (e.g., novel) whisker stimuli, which produce orienting responses, and modulated by persistent neocortex activity depending on behavioral state (Cohen and Castro-Alamancos 2010a).
GRANTS
This work was supported by National Institutes of Health Grants MH096817 and NS059036 (to M. A. Castro-Alamancos).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.C.-A. conception and design of research; M.A.C.-A. and M.F. performed experiments; M.A.C.-A. and M.F. analyzed data; M.A.C.-A. interpreted results of experiments; M.A.C.-A. prepared figures; M.A.C.-A. drafted manuscript; M.A.C.-A. edited and revised manuscript; M.A.C.-A. and M.F. approved final version of manuscript.
REFERENCES
- Bezdudnaya T, Castro-Alamancos MA. Neuromodulation of whisking related neural activity in superior colliculus. J Neurosci 34: 7683–7695, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Castro-Alamancos MA. Superior colliculus cells sensitive to active touch and texture during whisking. J Neurophysiol 106: 332–346, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce LL, McHaffie JG, Stein BE. The organization of trigeminotectal and trigeminothalamic neurons in rodents: a double-labeling study with fluorescent dyes. J Comp Neurol 262: 315–330, 1987. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci 10: 2638–2648, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. J Physiol 539: 567–578, 2002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. J Neurophysiol 87: 946–953, 2002b. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Bezdudnaya T. Modulation of artificial whisking related signals in barrel cortex. J Neurophysiol 113: 1287–1301, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM, Rhoades RW. Responses of visual, somatosensory, and auditory neurones in the golden hamster's superior colliculus. J Physiol 270: 595–626, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Behavioral state dependency of neural activity and sensory (whisker) responses in superior colliculus. J Neurophysiol 104: 1661–1672, 2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Detection of low salience whisker stimuli requires synergy of tectal and thalamic sensory relays. J Neurosci 30: 2245–2256, 2010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. Early sensory pathways for detection of fearful conditioned stimuli: tectal and thalamic relays. J Neurosci 27: 7762–7776, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Hirata A, Castro-Alamancos MA. Vibrissa sensation in superior colliculus: wide-field sensitivity and state-dependent cortical feedback. J Neurosci 28: 11205–11220, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GW. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci 12: 137–147, 1989. [DOI] [PubMed] [Google Scholar]
- Drager UC, Hubel DH. Physiology of visual cells in mouse superior colliculus and correlation with somatosensory and auditory input. Nature 253: 203–204, 1975a. [DOI] [PubMed] [Google Scholar]
- Drager UC, Hubel DH. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol 38: 690–713, 1975b. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Murakami Y, Rijli FM. Mapping the face in the somatosensory brainstem. Nat Rev Neurosci 11: 252–263, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero M, Castro-Alamancos MA. Synaptic cooperativity regulates persistent network activity in neocortex. J Neurosci 33: 3151–3163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay BL, Schneps SE, Wilson KG, Schneider GE. Topography of visual and somatosensory projections to the superior colliculus of the golden hamster. Brain Res 142: 223–235, 1978. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Amsterdam: Elsevier/Academic, 2008. [Google Scholar]
- Fujikado T, Fukuda Y, Iwama K. Two pathways from the facial skin to the superior colliculus in the rat. Brain Res 212: 131–135, 1981. [DOI] [PubMed] [Google Scholar]
- Furuta T, Timofeeva E, Nakamura K, Okamoto-Furuta K, Togo M, Kaneko T, Deschenes M. Inhibitory gating of vibrissal inputs in the brainstem. J Neurosci 28: 1789–1797, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci 34: 205–231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Bermejo R, Zeigler HP. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J Neurosci 21: 5374–5380, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwerg BS, Krauthamer GM. Vibrissa-responsive neurons of the superior colliculus that project to the intralaminar thalamus of the rat. Neurosci Lett 111: 23–27, 1990. [DOI] [PubMed] [Google Scholar]
- Hemelt ME, Keller A. Superior sensation: superior colliculus participation in rat vibrissa system. BMC Neurosci 8: 12, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta MF, Frankfurter A, Harting JK. Studies of the principal sensory and spinal trigeminal nuclei of the rat: projections to the superior colliculus, inferior olive, and cerebellum. J Comp Neurol 220: 147–167, 1983. [DOI] [PubMed] [Google Scholar]
- Isa T, Endo T, Saito Y. The visuo-motor pathway in the local circuit of the rat superior colliculus. J Neurosci 18: 8496–8504, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol 102: 2581–2593, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin MF, Chiaia NL, Haring JH, Rhoades RW. Intersubnuclear connections within the rat trigeminal brainstem complex. Somatosens Mot Res 7: 399–420, 1990. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci 28: 11071–11078, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey HP, Erzurumlu RS. Trigeminal projections to the superior colliculus of the rat. J Comp Neurol 201: 221–242, 1981. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 435–444, 2006. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Deschenes M. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron 72: 455–468, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci 36: 165–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MA, McHaffie JG, Stein BE. Response properties of nociceptive and low-threshold mechanoreceptive neurons in the hamster superior colliculus. J Neurosci 7: 547–564, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Endo T, Isa T. Presynaptic muscarinic acetylcholine receptors suppress GABAergic synaptic transmission in the intermediate grey layer of mouse superior colliculus. Eur J Neurosci 20: 2079–2088, 2004. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Kao CQ, Stein BE. Nociceptive neurons in rat superior colliculus: response properties, topography, and functional implications. J Neurophysiol 62: 510–525, 1989. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Norita M, Dunning DD, Stein BE. Corticotectal relationships: direct and “indirect” corticotectal pathways. Prog Brain Res 95: 139–150, 1993. [PubMed] [Google Scholar]
- Mitchinson B, Prescott TJ. Whisker movements reveal spatial attention: a unified computational model of active sensing control in the rat. PLoS Comput Biol 9: e1003236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita E, Keller A, Asanuma H. Input-output organization of the rat vibrissal motor cortex. Exp Brain Res 99: 223–232, 1994. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Fish SE, Chiaia NL, Bennett-Clarke C, Mooney RD. Organization of the projections from the trigeminal brainstem complex to the superior colliculus in the rat and hamster: anterograde tracing with Phaseolus vulgaris leucoagglutinin and intra-axonal injection. J Comp Neurol 289: 641–656, 1989. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Jacquin MF. Complex somatosensory receptive fields of cells in the deep laminae of the hamster's superior colliculus. J Neurosci 3: 1342–1354, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Klein BG, Jacquin MF, Szczepanik AM, Chiaia NL. The structural and functional characteristics of tectospinal neurons in the golden hamster. J Comp Neurol 255: 451–465, 1987. [DOI] [PubMed] [Google Scholar]
- Saito Y, Isa T. Electrophysiological and morphological properties of neurons in the rat superior colliculus. I. Neurons in the intermediate layer. J Neurophysiol 82: 754–767, 1999. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci 31: 317–338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooksawate T, Isa T. Properties of cholinergic responses in neurons in the intermediate grey layer of rat superior colliculus. Eur J Neurosci 24: 3096–3108, 2006. [DOI] [PubMed] [Google Scholar]
- Spacek J, Lieberman AR. Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. J Anat 117: 487–516, 1974. [PMC free article] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Signal transformations required for the generation of saccadic eye movements. Annu Rev Neurosci 13: 309–336, 1990. [DOI] [PubMed] [Google Scholar]
- Stein BE, Dixon JP. Properties of superior colliculus neurons in the golden hamster. J Comp Neurol 183: 269–284, 1979. [DOI] [PubMed] [Google Scholar]
- Stein BE, Magalhaes-Castro B, Kruger L. Superior colliculus: visuotopic-somatotopic overlap. Science 189: 224–226, 1975. [DOI] [PubMed] [Google Scholar]
- Stubblefield EA, Thompson JA, Felsen G. Optogenetic cholinergic modulation of the mouse superior colliculus in vivo. J Neurophysiol 114: 978–988, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett JW, Phan A, Yamada J, Feldheim DA. Alignment of multimodal sensory input in the superior colliculus through a gradient-matching mechanism. J Neurosci 32: 5264–5271, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Deschenes M. Single- and multi-whisker channels in the ascending projections from the principal trigeminal nucleus in the rat. J Neurosci 19: 5085–5095, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Jones EG. Somatotopic and columnar organization in the corticotectal projection of the rat somatic sensory cortex. Brain Res 133: 223–235, 1977. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 3: 785–792, 2006. [DOI] [PubMed] [Google Scholar]