Abstract
The cerebellum was historically considered a brain region dedicated to motor control, but it has become clear that it also contributes to sensory processing, particularly when sensory discrimination is required. Prior work, for example, has demonstrated a cerebellar contribution to sensory discrimination in the visual and auditory systems. The cerebellum also receives extensive inputs from the motion and gravity sensors in the vestibular labyrinth, but its role in the perception of head motion and orientation has received little attention. Drawing on the lesion-deficit approach to understanding brain function, we evaluated the contributions of the cerebellum to head motion perception by measuring perceptual thresholds in two subjects with congenital agenesis of the cerebellum. We used a set of passive motion paradigms that activated the semicircular canals or otolith organs in isolation or combination, and compared results of the agenesis patients with healthy control subjects. Perceptual thresholds for head motion were elevated in the agenesis subjects for all motion protocols, most prominently for paradigms that only activated otolith inputs. These results demonstrate that the cerebellum increases the sensitivity of the brain to the motion and orientation signals provided by the labyrinth during passive head movements.
Keywords: cerebellum, vestibular, perception, motion, agenesis
the function of the cerebellum has historically been considered to be confined to motor control (e.g., Roostaei et al. 2014), but it also can be activated by sensory tasks, particularly those requiring sensory discrimination (Baumann et al. 2015; Gao et al. 1996). Recent work, for example, has demonstrated cerebellar contributions to visual (Handel et al. 2009), auditory (Parsons et al. 2009), and tactile (Tinazzi et al. 2013) sensory perception. Complex percepts of self-motion and orientation also rely in part on the cerebellum (Rondi-Reig et al. 2014), and these depend on multiple sensory inputs, most prominently those from the visual and vestibular systems. The visual system senses the position and motion of the visual scene relative to the retina, whereas the vestibular system senses angular and linear head motion and head orientation relative to gravity. Visual motion perception is clearly influenced by the cerebellum, because patients with cerebellar dysfunction have impaired ability to perceive the direction of visual motion patterns embedded in a noisy background (Cattaneo et al. 2014; Handel et al. 2009). In contrast, little is known about the role of the cerebellum in the perception of head motion and orientation.
Numerous studies have examined cerebellar processing of the vestibular inputs that underlie the vestibulo-ocular reflex (VOR; reviewed in Voogd et al. 2012), and more recently, thalamic neurons that carry vestibular signals generated in the cerebellum have been described (Meng et al. 2007). It is therefore logical to presume that the cerebellum affects the perception of head motion and orientation. Indeed, several recent studies have suggested that patients with cerebellar degeneration perceive head motion abnormally, since their percepts of rotation decayed faster than those of control subjects following constant-velocity, passive rotation in the dark (Bertolini et al. 2012; Bronstein et al. 2008). These studies, however, share a number of significant limitations. The regions of cerebellar dysfunction were not well defined and were described only qualitatively, making it difficult to correlate the perceptual and cerebellar deficits. Furthermore, these patients had normal cerebellar function for much of their lives, so central vestibular mechanisms that are controlled by the cerebellum but located elsewhere in the brain (e.g., the brain stem, where the floccular target neurons and velocity storage integrator are located; Katz et al. 1991; Ramachandran and Lisberger 2008) could be accurately calibrated before the onset of cerebellar degeneration. In addition, the motion stimuli in these studies were limited to yaw rotation, which only activates the lateral canals, so nothing is known about self-motion perception when the vestibular cues are provided by the otolith organs (e.g., during translation) or when the canals and otolith organs are activated concurrently (e.g., during head rotation about an off-vertical axis).
In this study, we measured perceptual thresholds for passive head motion in two patients with a very rare anomaly, congenital agenesis of the cerebellum (Chheda et al. 2002; Schmahmann et al. 2007). Because the threshold (by definition) is the smallest motion the brain can segregate from the noise inherent in peripheral and central vestibular processing, its measurement allows an assessment of the cerebellum's contribution to the discrimination of vestibular cues within a noisy neural environment (Grabherr et al. 2008; Valko et al. 2012). Furthermore, all cerebellar function was absent throughout the lives of the cerebellar agenesis subjects, allowing us to more definitively assess the cerebellum's role in the perception of head motion. We also extended prior studies that only used yaw-axis velocity steps by employing four motion paradigms (rotation, translations, and tilt) to activate different vestibular end organs in isolation or combination at three separate frequencies. Our results demonstrate that passive, self-motion perceptual thresholds were higher than normal in the cerebellar agenesis subjects for all motion profiles but were particularly elevated for translational motions that activated the otolith organs in isolation. These findings can be interpreted in the framework of inverse internal models of the labyrinthine end organs in the cerebellum that compensate for the mechanical deficiencies of these peripheral sensors and allow the brain to establish a more accurate estimate of head motion than is encoded in primary vestibular afferents.
MATERIALS AND METHODS
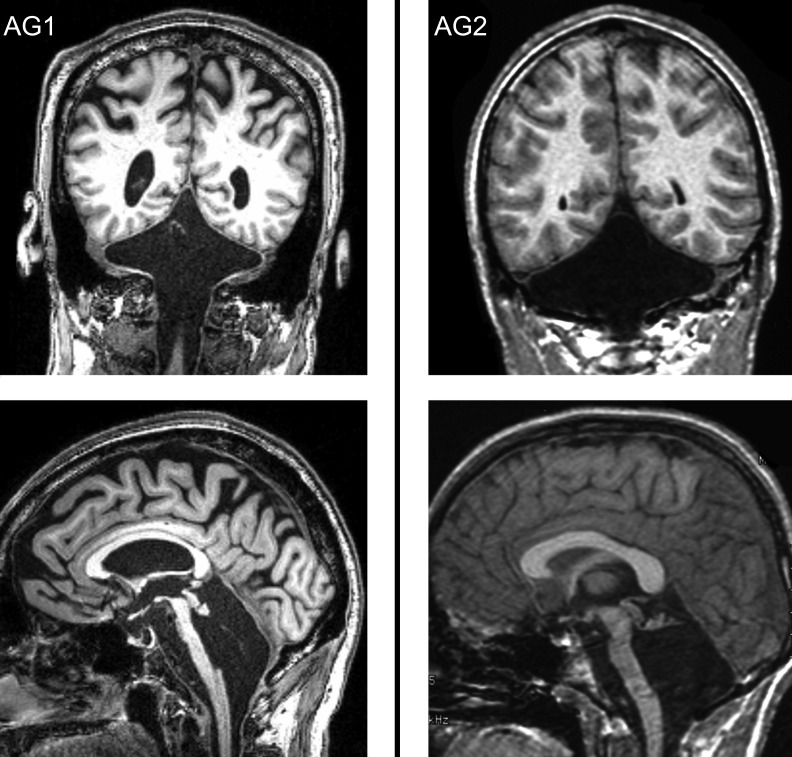
All methods were approved by the Institutional Review Board, and informed consent was obtained from each subject. Agenesis patient 1 (AG1) was 61 yr old, and agenesis patient 2 (AG2) was 30 yr old. MRI images (Fig. 1) show complete absence of the cerebellum in both subjects, except for a tiny residual nubbin superiorly, a finding documented also in previous reports of cerebellar agenesis (Rubinstein and Freeman 1940; Sener and Jinkins 1993; Velioglu et al. 1998). The anomaly includes hypoplasia of the basis pontis, reflecting absence of the pontine nuclei and of the middle cerebellar peduncles. The older subject (AG1) had age-appropriate mild decrease in cerebral volume. All brain structures were otherwise normal. Their medical histories were benign, and they had no other neurological or otological problems. Their physical examinations showed essentially normal gait, minimal appendicular ataxia with fine discriminative tests of motor function, and dysarthria that was mild in AG1 and moderate in AG2. Both had prominent cerebellar eye movement findings of impaired pursuit, fixation suppression of the vestibulo-ocular reflex (VOR), gaze holding, and saccadic accuracy (Lewis and Zee 1993). The VOR assessed with head thrusts was compensatory in amplitude and direction. Audiograms were unavailable, but both subjects reported that hearing was normal, auditory symptoms were absent, and detailed office neurological examination revealed no evidence of hearing impairment.
Fig. 1.
Magnetic resonance images of the 2 agenesis subjects (AG1 and AG2) demonstrating essentially complete absence of the cerebellum, with only a tiny nubbin of tissue remaining in the location of the superior medullary vellum. This remnant is entirely distinct from the vestibulo-cerebellum, which is located in the missing lobules IX and X (uvula, nodulus, and flocculus).
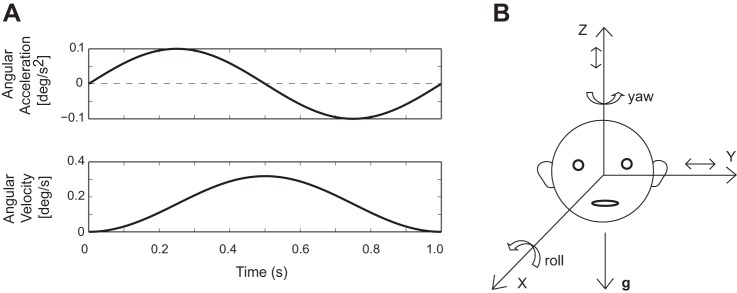
Head motion perceptual thresholds were measured using the methods previously employed in our laboratory (Grabherr et al. 2008; Valko et al. 2012). We used passive head motions (e.g., generated by en bloc movement of the head and body on a motor-driven motion platform) so that our analysis could focus specifically on cerebellar processing of sensory signals rather than the more complex interaction between sensory and motor signals that occurs during active, self-generated head movements (reviewed in Cullen et al. 2011). Subjects were seated upright in a chair mounted on a Moog motion platform, their bodies were restrained with a harness and stabilized in the chair with foam, the head was immobilized with a helmet, visual cues were eliminated since the room was dark and light-proof, and auditory cues were minimized with noise-cancelling headphones that provided a white noise background. Movements consisted of single-cycle sinusoidal accelerations performed at one of three frequencies, 0.5, 1.0, and 2.0 Hz, which generated bell-shaped velocity profiles (Fig. 2A). Motions (Fig. 2B) included yaw rotation or Z-axis (superior-inferior) translation using earth-vertical axes, and Y-axis (interaural) translation or roll tilt using earth-horizontal axes. Each motion/frequency was presented in a block of trials before the switch to a different movement, and the testing protocol followed the order used to collect our normative control data (Valko et al. 2012). Perceptual thresholds were determined using an adaptive forced-choice, “three-down one-up” paradigm (Leek 2001). An auditory tone was presented before and after each movement, and the subject pushed a button in the right hand if they perceived the motion as rightward (or upward) or in the left hand if it was perceived as leftward (or downward). They were required to guess if they were unsure of the correct response. The direction of motion was chosen randomly for each trial, and no information was provided or available about the accuracy of the subject's response. Results from the two agenesis subjects were compared with normative data previously obtained using identical methods from a population whose mean age was 36 yr (Valko et al. 2012). Because perceptual thresholds in healthy control subjects have a log-normal distribution, all threshold data were analyzed and plotted in logarithmic units. The VOR amplitude (gain), timing (phase), and symmetry (bias) were measured during sinusoidal yaw rotation about an earth-vertical axis at 0.5 and 1.0 Hz using standard video-based eye movement recordings and analysis (Wall 1990).
Fig. 2.
A: sample motion trajectory showing a single sinusoidal cycle of acceleration at 1.0 Hz with the associated bell-shaped velocity trajectory. B: schematic drawing of the head in the upright position. Rotations are about the earth-vertical Z-axis (yaw) or the earth-horizontal X-axis (roll); translations are along the earth-horizontal Y-axis or the earth-vertical Z-axis. g, Gravity.
RESULTS
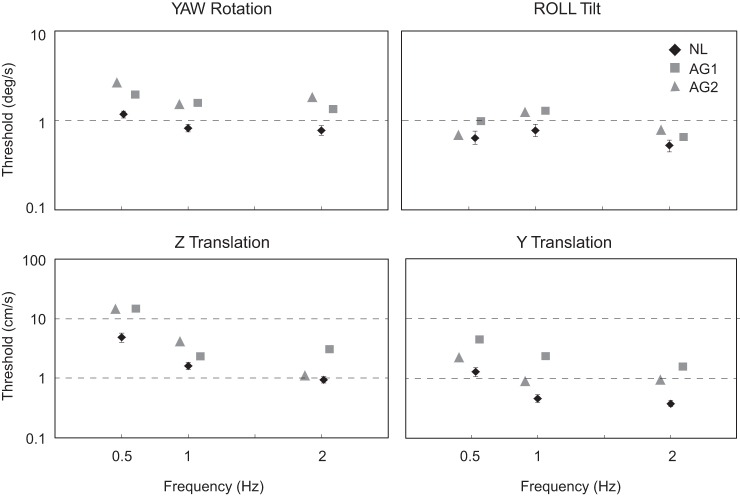
Figure 3 illustrates the perceptual thresholds measured in the two agenesis patients (gray icons) for the four motion paradigms and compares them with normative data (black icons). Thresholds in agenesis patients were uniformly higher than normal, although both agenesis and healthy control subjects displayed a similar dependence of threshold on the motion's axis and frequency. Comparing the two agenesis subjects, thresholds were higher in AG1 for 8 of 12 motions and in AG2 for 4 of 12 motions. The only consistent difference between the agenesis subjects was for Y translation, since AG1 had higher thresholds for each of the three frequencies.
Fig. 3.
Perceptual thresholds for the 2 agenesis subjects (AG1 and AG2, gray symbols) and the means and SE for thresholds measured in a healthy control group (NL; n = 11; black symbols). Four motion profiles were utilized, each at 3 frequencies, and thresholds are reported in °/s for yaw rotation (about a superior-inferior, earth-vertical axis) and roll tilt (about a naso-occipital, earth-horizontal axis) and in cm/s for Z (superior-inferior, earth-vertical axis) and Y translation (interaural, earth-horizontal axis). Symbols are separated horizontally for clarity, and some error bars for normal data are smaller than the icons and therefore not visible.
Three principal findings are evident in these results (Fig. 3): 1) thresholds were increased in the agenesis patients compared with healthy controls (ANOVA, P < 0.001); 2) thresholds in the agenesis subjects were higher (relative to healthy control subjects) for the two motions that activated otolith organs in isolation (Z and Y translation) compared with motions that activated the canals (yaw rotation, roll tilt; ANOVA, P < 0.001); and 3) among the six translation conditions, Z (up-down) translation at the lowest frequency (0.5 Hz) had the highest average threshold in both the healthy control (t-test, P < 0.001) and agenesis subjects (t-test, P < 0.01). Figure 4A summarizes the overall pattern of the results, showing the mean threshold for the agenesis patients for each motion axis, averaged across subjects and frequencies, and normalized by dividing the agenesis subject thresholds by the mean normal threshold for the appropriate motion (axis and frequency). In addition to recapitulating the observations that thresholds in the agenesis subjects were higher than normal for all motions and were higher for the two linear motions (Z and Y translation) compared with the two rotational motions (yaw and roll), Fig. 4A also shows that, when averaged across frequencies and subjects, Y-translation thresholds were increased the most in the agenesis patients compared with the normal control subjects (ANOVA, P < 0.001). Thresholds did differ between AG1 and AG2 for this motion profile, however, with AG1's thresholds higher at all three of the tested frequencies. VOR responses for yaw rotation are summarized in Fig. 4B and were characterized by reduced gains, elevated phase leads, and enlarged biases compared with normative data (dashed lines).
Fig. 4.
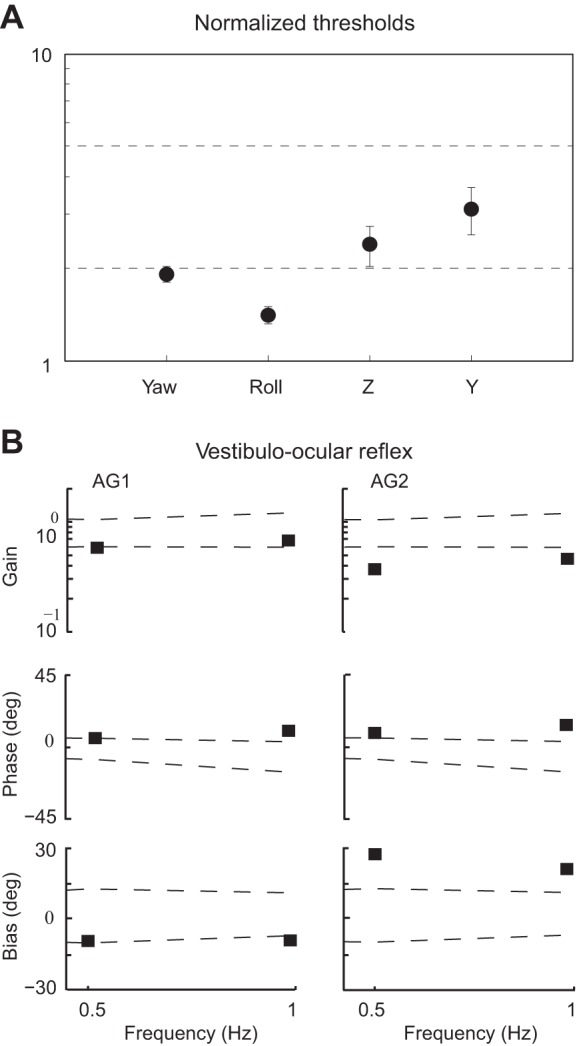
A: means and SE of the normalized perceptual thresholds measured in the agenesis subjects, compared with the mean thresholds in the healthy control population for each motion axis and frequency. Each symbol represents the average of 6 normalized threshold measurements, namely, each of the 2 agenesis thresholds divided by the normal mean for each of the 3 frequencies. B: gain, phase, and bias of the vestibulo-ocular reflex for the 2 agenesis subjects at 0.5 and 1.0 Hz, compared with the normal range for these values (dashed lines indicate the range between ±2 SD about the normal mean; Wall 1990).
DISCUSSION
Our principal finding is that perceptual thresholds for passive head movements are increased in cerebellar agenesis patients relative to healthy control subjects. Before concluding that the cerebellum contributes to the perception of self-motion, however, we must consider other possible explanations.
Potential explanations for elevated thresholds in cerebellar agenesis.
Abnormalities in the (noncerebellar) vestibular system could potentially be responsible for increased thresholds in the agenesis subjects, but this is an unlikely explanation for our findings. Pathological studies of congenital cerebellar agenesis have not shown vestibular abnormalities (Velioglu et al. 1998); the peripheral and central vestibular systems appeared normal on MRI for our subjects, so structural, developmental defects in the labyrinth or vestibular nerve were absent; and VOR responses were consistent with absent cerebellar function (Lewis and Zee 1993) but inconsistent with the severe vestibular hypofunction that would be required to produce the large threshold increases we observed (Hess et al. 1985; Valko et al. 2012). Indeed, perceptual thresholds were higher in agenesis subjects for several motions than were previously observed in patients with completely absent vestibular function (Valko et al. 2012). Overall, although we cannot exclude the possibility that impaired peripheral vestibular function contributed to our findings, it is very improbable that this was the sole cause of the increase in thresholds observed in the agenesis patients.
Hair cells die in the labyrinth during aging, and one agenesis subject (AG1) was substantially older than our normal control population. The effects of age on vestibular perceptual thresholds are unclear, since several studies found no age effects for yaw rotation (e.g., Chang et al. 2014) but possible effects for translational movements (Kingma 2005; Roditi and Crane 2012). Although age-related changes could contribute to the threshold elevation in the older AG1 relative to the younger AG2 for Y-axis translation (see below), it is notable that no similar pattern was evident for the other nine motions (3 axes × 3 frequencies), with higher threshold values almost equally distributed between the two agenesis subjects. Furthermore, AG2 was similar in age to the control population, but his thresholds were higher than normal for all 12 motions. Together, these observations indicate that the increased thresholds in the agenesis subjects cannot be explained by age effects.
Thresholds could also be increased because the cerebellum contributes to the decision-making process employed in the threshold paradigm. The cerebellum plays a role in decision-making (Rosenbloom et al. 2012; Schmahmann and Sherman 1998), but since threshold differences between agenesis and healthy control subjects were highly dependent on motion characteristics, interactions between the motion signals and the cerebellum appeared to have a prominent influence on perceptual thresholds beyond any effects that may reflect impaired decision-making.
Cerebellar mechanisms that could improve self-motion perception.
It is reasonable to conclude from our data set that the cerebellum contributes to the perception of head motion when the head and body are moved passively, particularly when the motion is translational rather than rotational. Because prior studies indicated that sensory signals provided by the vestibular labyrinth dominate head motion perception when visual signals are unavailable (Valko et al. 2012), we focus the interpretation of the agenesis results on labyrinthine rather than extralabyrinthine (e.g., somatosensory) motion cues, although certainly both vestibular and nonvestibular signals would be expected to contribute to the integrative processes used to determine the direction of head motion.
The increase in thresholds for all motion profiles in the agenesis subjects indicates that the cerebellum helps the brain extract the motion signal from the noise in the neural environment (since by definition the threshold is the smallest signal that can be segregated from the noise). These findings are consistent with prior studies of self-motion perception in patients with cerebellar degeneration (Bertolini et al. 2012; Bronstein et al. 2008), since a reduction in the relative signal-to-noise ratio that underlies the threshold elevation is predicted to shorten the time constant of the velocity storage integrator (Karmali and Merfeld 2012; MacNeilage et al. 2008). This would cause percepts of yaw rotation to decay more rapidly in cerebellar subjects relative to normal controls when they rotated in yaw at a constant velocity, as was previously observed.
Our results do not indicate what mechanism underlies the contribution of the cerebellum to the perception of self-motion. One simplifying feature is that by examining passive head motion, we can focus exclusively on central processing of sensory cues, primarily those provided by the vestibular labyrinth. In this sense, our results are similar to studies that showed cerebellar contributions to visual (e.g., Cattaneo et al. 2014) and auditory perception (Parsons et al. 2009). In contrast, active head movements are more complex, because they are associated with a motor command (efference copy) that could be used to predict the head motion and the associated labyrinthine afferent signal (via forward internal models of head/neck mechanical properties and labyrinthine dynamics). A variety of cerebellum-dependent mechanisms have been proposed that utilize the predicted/anticipated motion associated with active movements or predictions based on the pattern of sensory stimuli (see Shadmehr et al. 2010 for a review), such as error detection (comparison of predicted and actual movement; e.g., Xu-Wilson et al. 2009), state estimation (e.g., Molinari et al. 2009), and filtering sensory signals that are caused by volitional movements (e.g., Cullen et al. 2011; Requarth and Sawtell 2011). Because our paradigm used passive motions with the direction randomized, there was no relevant motor command and prediction of motion direction should be impossible. Furthermore, no feedback was available about the accuracy of the response, so no error information could be accessed. Assuming that the agenesis subjects can correctly determine that passive head movement (e.g., motion that occurs without an associated motor command) is external in origin and not self-generated, then cerebellar mechanisms that rely on forward models to predict/anticipate movement are not relevant.
Possible role of inverse models of the labyrinthine end organs.
Because the cerebellum appears to be a locus of internal models that process oculomotor and vestibular information (Angelaki et al. 2004; Lisberger 2009), it is reasonable to postulate that there may be a cerebellum-dependent inverse model of the labyrinthine sensors (e.g., a model that maps from sensor output to stimulus input) that uses the afferent signal to reconstruct an accurate internal estimate of head movement. During yaw-axis rotation, for example, the velocity storage integrator in the brain stem (which is controlled by the cerebellum; Waespe et al. 1985) generates an internal estimate of head angular velocity that more closely approximates the actual head motion than the signal encoding the movement in the afferent nerve. The cerebellum-controlled velocity storage network can be conceptualized as an inverse model of the semicircular canal dynamics (e.g., Laurens and Angelaki 2011; Zupan et al. 2002) that uses the afferent input to generate an estimate of head rotation, and this processing is dysfunctional if the cerebellum is damaged or absent (Wearne et al. 1998). Inverse models of the other vestibular end organs, which may be located in or controlled by the cerebellum, could allow the brain to reconstruct more accurate estimates of head motion than are carried by the vestibular afferents by compensating for the mechanical limitations of the peripheral transducers.
Otolith-mediated thresholds exceed canal-mediated thresholds.
Thresholds were substantially higher for motions that modulated otolith activity in isolation (Z and Y translation) compared with motions that modulate canal activity alone (yaw rotation) or canal and otolith activity (roll tilt, although the canal cues dominate perceptual thresholds for the frequencies we tested; Valko et al. 2012). The reasons for this threshold difference are uncertain, but the results are consistent with the hypothesis that cerebellar processing of sensory signals is more important when the complexity of the afferent signal or the difficulty of the discrimination task increases (Baumann et al. 2014; Petacchi et al. 2011). More specifically, 1) the canal afferent signal is relatively simple (all afferents that innervate a given canal modulate their firing rate in the same direction during head rotation) compared with the complexity of the otolith input (afferents modulate their activity during head tilt or translation in a complex pattern that reflects the radial orientation of the hair cells in the otolithic maculae, including the reversal at the striola; Uchino and Kushir 2011). 2) We are constantly exposed to gravity, a stimulus that activates the otolith organs, and these signals usually modulate at very low frequencies (e.g., when the head tilts). Because we spend much of the waking day with our heads upright, the saccules (which are aligned nearly parallel to gravity when the head is upright), as well as nonvestibular somatic sensors on the feet (standing) or the buttock and torso (sitting), chronically sense a nearly static 1-G force, so low-frequency accelerations aligned with gravity (e.g., along the Z-axis) may be particularly difficult to perceive correctly, since they generate small variations about a large (980 cm·s−1·s−1) baseline. Interestingly, thresholds for healthy control subjects were substantially higher on the 0.5-Hz Z-translation paradigm than the other translational motions, and of the 12 motion patterns we utilized (4 axes × 3 frequencies), agenesis patients had the highest thresholds relative to normal subjects for the 0.5-Hz Z-axis translation paradigm. Low-frequency up-down translation is therefore particularly difficult to discern in healthy controls, and thresholds on this motion paradigm are also highly dependent on cerebellar processing. 3) The cerebellar nodulus and uvula are crucial for distinguishing head tilt from translation, the two motion patterns that modulate otolith activity (Laurens et al. 2013a, 2013b). Because modulation in otolith activity during Z-axis translation is unambiguous (since it can only signal upward or downward acceleration) but is ambiguous during Y-axis translation (since it can reflect either roll tilt or interaural translation), the absence of normal cerebellar processing could affect Y more than Z translation, as was observed at all frequencies except for 0.5 Hz. Interestingly, Y translation was the only motion pattern that produced a consistent threshold difference between the two agenesis subjects (AG1 thresholds were substantially larger). The basis for increased thresholds in AG1 relative to AG2 is unknown, but since AG1 was substantially older than AG2, one possibility is that perception during this most ambiguous motion protocol is sensitive to the integrity of the relevant sensory inputs or noncerebellar brain structures. Age-dependent cerebral atrophy or reductions in utricular (Rosenhall 1973) or nonvestibular (e.g., somatosensory) signals may therefore have a more pronounced effect on Y-translation thresholds than on the other motion paradigms. Consistent with this hypothesis is the finding that perceptual thresholds for the similarly ambiguous X-axis (naso-occipital) translation, which can also reflect translation or (pitch) tilt, has been shown consistently to increase as a function of age (Kingma 2005; Roditi and Crane 2012).
Summary and conclusions.
Our results demonstrate that the cerebellum plays an important role in the identification of self-motion cues. These findings may help explain why patients with cerebellar dysfunction have difficulty navigating through space (Rondi-Reig et al. 2014) and why cerebellar ataxia patients often report that they have difficulty judging distances, since neither visual nor vestibular motion cues are extracted from neural afferents with normal efficiency when cerebellar function is impaired. More generally, our results support the contention that the cerebellum contributes to sensory processing in many realms when the behavioral goal is a discriminative rather than a motor task.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grant 1R01DC013069 and the Birmingham and MINDlink Foundations.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.D., Y.V., and R.F.L. analyzed data; K.D., J.D.S., and R.F.L. drafted manuscript; K.D., Y.V., J.D.S., and R.F.L. edited and revised manuscript; K.D., Y.V., J.D.S., and R.F.L. approved final version of manuscript; Y.V. performed experiments; Y.V., J.D.S., and R.F.L. interpreted results of experiments; R.F.L. conception and design of research; R.F.L. prepared figures.
ACKNOWLEDGMENTS
We thank Dan Merfeld and David Zee, and we acknowledge the assistance of Jason MacMore.
REFERENCES
- Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of nature. Nature 430: 560–564, 2004. [DOI] [PubMed] [Google Scholar]
- Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, Leggio M, Mattingley JB, Molinari M, Moulton EA, Paulin MG, Pavlova MA, Schmahmann JD, Sokolov AA. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum 14: 197–220, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini G, Ramat S, Bockisch C, Marti S, Straumann D, Palla A. Is vestibular self-motion perception controlled by the velocity storage? Insights from patients with chronic degeneration of the vestibulo-cerebellum. PLoS One 6: e36763, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AM, Grunfeld EA, Faldon M, Okada T. Reduced self-motion perception in patients with midline cerebellar lesions. Neuroreport 19: 691–693, 2008. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Renzi C, Casali S, Silvanto J, Vecchi T, Papagno C, D'Angelo E. Cerebellar vermis plays a causal role in visual motion discrimination. Cortex 58: 272–280, 2014. [DOI] [PubMed] [Google Scholar]
- Chang NY, Hiss MM, Sanders MC, Olomu OU, MacNeilage PR, Uchanski RM, Huller TE. Vestibular perception and the vestibule-ocular reflex in young and older adults. Ear Hear 35: 565–570, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chheda M, Sherman J, Schmahmann JD. Neurologic, psychiatric and cognitive manifestations in cerebellar agenesis. Neurology 58, Suppl 3: 356, 2002. [Google Scholar]
- Cullen KE, Brooks JX, Jamali M, Carriot J, Massot C. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res 210: 377–388, 2011. [DOI] [PubMed] [Google Scholar]
- Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science 272: 545–547, 1996. [DOI] [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res 186: 677–681, 2008. [DOI] [PubMed] [Google Scholar]
- Handel B, Their P, Haarmeier T. Visual motion perception deficits due to cerebellar lesions are paralleled by specific changes in cerebro-cortical activity. J Neurosci 29: 15126–15133, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess K, Baloh RW, Honrubia V, Yee RD. Rotational testing in patients with bilateral peripheral vestibular disease. Laryngoscope 95: 85–88, 1985. [DOI] [PubMed] [Google Scholar]
- Karmali F, Merfeld DM. A distributed, dynamic, parallel computational model: the role of noise in velocity storage. J Neurophysiol 108: 390–405, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, DeJong MBV, Buttner-Ennever JA, Cohen B. Effects of midline medullary lesions on velocity storage and the vestibulo-ocular reflex. Exp Brain Res 87: 505–520, 1991. [DOI] [PubMed] [Google Scholar]
- Kingma H. Thresholds for perception of direction of linear acceleration as a possible evaluation of otolith function. BMC Ear Nose Throat Disord 5: 5, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res 210: 407–422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens J, Meng H, Angelaki DE. Computation of linear acceleration through an internal model in the macaque cerebellum. Nat Neurosci 16: 1701–1708, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens J, Meng H, Angelaki DE. Neural representation of orientation relative to gravity in the macaque cerebellum. Neuron 80: 1508–1518, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys 63: 1279–1292, 2001. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Zee DS. Ocular motor disorders associated with cerebellar lesions: pathophysiology and localization. Rev Neurol 149: 665–677, 1993. [PubMed] [Google Scholar]
- Lisberger SG. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience 162: 763–776, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeilage PR, Ganesan N, Angelaki DE. Computation approaches to spatial orientation: from transfer functions to dynamic Bayesian inference. J Neurophysiol 100: 2981–2996, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, May PJ, Dickman JD, Angelaki DE. Vestibular signals in primate thalamus: properties and origins. J Neurosci 27: 13590–13602, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Restuccia D, Leggio MG. State estimation, response prediction, and cerebellar sensory processing for behavioral control. Cerebellum 8: 399–402, 2009. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Petacchi A, Schmahmann JD, Bower JM. Pitch discrimination in cerebellar patients: evidence for a sensory deficit. Brain Res 1303: 84–96, 2009. [DOI] [PubMed] [Google Scholar]
- Petacchi A, Kaernbach C, Ratnam R, Bower JM. Increased activation of the human cerebellum during pitch discrimination: a positron emission tomography (PET) study. Hear Res 282: 35–48, 2011. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Lisberger SG. Neural substrate of modified and unmodified pathways for learning in monkey vestibuloocular reflex. J Neurophysiol 100: 1868–1878, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requarth T, Sawtell NB. Neural mechanisms for filtering self-generated sensory signals in cerebellum-like circuits. Curr Opin Neurobiol 21: 602–608, 2011. [DOI] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Directional asymmetries and age effects in human self-motion perception. J Assoc Res Otolaryngol 13: 381–401, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondi-Reig L, Paradis AL, Lefort JM, Babayan BM, Tobin C. How the cerebellum may monitor sensory information for spatial representation. Front Syst Neurosci 8: 205, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roostaei T, Nazeri A, Sahraian MA, Minagar A. The human cerebellum: a review of physiologic neuroanatomy. Neurol Clin 32: 859–869, 2014. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MH, Schmahmann JD, Price BH. The functional neuroanatomy of decision-making. J Neuropsychiatry Clin Neurosci 24: 266–277, 2012. [DOI] [PubMed] [Google Scholar]
- Rosenhall U. Degenerative patterns in the aging human vestibular neuro-epithelia. Acta Otolaryngol 76: 208–220, 1973. [DOI] [PubMed] [Google Scholar]
- Rubinstein HS, Freeman W. Cerebellar agenesis. J Nerv Ment Dis 92: 489–502, 1940. [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain 121: 561–579, 1998. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum–insights from the clinic. Cerebellum 6: 254–267, 2007. [DOI] [PubMed] [Google Scholar]
- Sener RN, Jinkins JR. Subtotal agenesis of the cerebellum in an adult. MRI demonstration. Neuroradiology 35: 286–287, 1993. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Morgante F, Peretti A, Mariotti C, Panzeri M, Fiorio M, Fasano A. Impaired temporal processing of tactile and proprioceptive stimuli in cerebellar degeneration. PLoS One 8: e78628, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino Y, Kushir K. Differences between otolith- and semicircular canal-activated neural circuitry in the vestibular system. Neurosci Res 71: 315–327, 2011. [DOI] [PubMed] [Google Scholar]
- Valko Y, Lewis RF, Priesol AJ, Merfeld DM. Vestibular labyrinth contributions to human whole-body motion discrimination. J Neurosci 26: 13537–13542, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velioglu SK, Kuzevli K, Zmenoglu M. Cerebellar agenesis: a case report with clinical and MR imaging findings and a review of the literature. Eur J Neurol 15: 503–506, 1998. [DOI] [PubMed] [Google Scholar]
- Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw C. Visuomotor cerebellum in human and non-human primates. Cerebellum 11: 392–410, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waespe S, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science 228: 199–202, 1985. [DOI] [PubMed] [Google Scholar]
- Wall C., 3rd The sinusoidal harmonic acceleration rotary chair test. Theoretical and clinical basis. Neurol Clin 8: 269–285, 1990. [PubMed] [Google Scholar]
- Wearne S, Raphan T, Cohen B. Control of the spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol 79: 2690–2715, 1998. [DOI] [PubMed] [Google Scholar]