Abstract
Most forms of suprathreshold sensory stimulation perturb sleep. In contrast, presentation of pure olfactory or mild trigeminal odorants does not lead to behavioral or physiological arousal. In fact, some odors promote objective and subjective measures of sleep quality in humans and rodents. The brain mechanisms underlying these sleep-protective properties of olfaction remain unclear. Slow oscillations in the electroencephalogram (EEG) are a marker of deep sleep, and K complexes (KCs) are an EEG marker of cortical response to sensory interference. We therefore hypothesized that odorants presented during sleep will increase power in slow EEG oscillations. Moreover, given that odorants do not drive sleep interruption, we hypothesized that unlike other sensory stimuli odorants would not drive KCs. To test these hypotheses we used polysomnography to measure sleep in 34 healthy subjects (19 women, 15 men; mean age 26.5 ± 2.5 yr) who were repeatedly presented with odor stimuli via a computer-controlled air-dilution olfactometer over the course of a single night. Each participant was exposed to one of four odorants, lavender oil (n = 13), vetiver oil (n = 5), vanillin (n = 12), or ammonium sulfide (n = 4), for durations of 5, 10, and 20 s every 9–15 min. Consistent with our hypotheses, we found that odor presentation during sleep enhanced the power of delta (0.5–4 Hz) and slow spindle (9–12 Hz) frequencies during non-rapid eye movement sleep. The increase was proportionate to odor duration. In addition, odor presentation did not modulate the occurrence of KCs. These findings imply a sleep-promoting olfactory mechanism that may deepen sleep through driving increased slow-frequency oscillations.
Keywords: odor, olfaction, sleep, smell
sleep is characterized by decreased responsiveness to external stimuli. Despite this diminished responsiveness, external stimuli are processed during sleep and affect brain activity (Bastuji et al. 2002; Portas et al. 2000), often resulting in arousals. Numerous studies confirm that auditory, somatosensory, and visual stimuli perturb sleep by inducing arousals, disrupting sleep length and architecture (Terzano et al. 1990; Vallet and Mouret 1984; Velluti 1997). In turn, a growing body of evidence indicates that unlike sensory stimuli in other modalities, purely olfactory or mildly trigeminal odorants do not lead to arousal or wake from sleep (Arzi et al. 2010; Badia et al. 1990; Carskadon and Herz 2004; Hummel et al. 1991; Keverne et al. 1986; Stuck et al. 2007). In fact, there is some evidence that several odors promote sleep: In humans, lavender oil presented during sleep improved sleep efficiency, increased total sleep time, elevated vigor the following morning (Fismer and Pilkington 2012; Goel et al. 2005), and promoted sleep in patients with insomnia (Hardy et al. 1995). Jasmine odor diffused in the room during nocturnal sleep led to greater sleep efficiency (Raudenbush et al. 2003), and cedar extract administered during daytime naps shortened sleep latency (Sano et al. 1998). In animals, inhalation of valerian oil shortened sleep latency and prolonged total sleep time (Komori et al. 2006); cedar extract increased the amount of non-rapid eye movement (NREM) sleep (Sano et al. 1998); and, finally, α-pinene (a woody resinous odor) increased the duration of paradoxical sleep in rats (Yamaoka et al. 2005). These studies support the notion that odors can improve both objective and subjective sleep quality. However, the brain mechanisms underlying this effect remain unclear.
There are various measures of instantaneous changes in sleep depth. For example, a shift from low to high frequencies in the electroencephalogram (EEG) indicates a brief arousal, and a shift from higher to low frequencies indicates a transition into deeper stages of sleep. A second relevant EEG measure is the K complex (KC). KCs are large singular waveforms that occur spontaneously (Davis et al. 1939) during stage 2 sleep, heralding the onset of slow-wave sleep (Nicholas et al. 2002). KCs can also be evoked by external stimuli (Loomis et al. 1938) such as auditory stimulation, respiratory obstruction (Colrain 2005), or limb movements, thus reflecting a cortical response to interference (Halasz 2005). It has been proposed that the mechanism reflected by KCs allows the maintenance of a constant state of low or no arousal when internal or external stimuli occur, thereby reflecting a “sleep-protective” mechanism (Bastien and Campbell 1992; Colrain 2005; Hairston et al. 2010; Halasz 2005; Nicholas et al. 2002). Indeed, healthy sleepers show an increase in KCs during presentation of auditory stimuli, while individuals with primary insomnia do not invoke this protective mechanism (Hairston et al. 2010).
Given the behavioral evidence for improved sleep in the presence of odors, we hypothesized that odors would increase slow-wave oscillations resulting in increased low-frequency power. Moreover, given that odors do not interfere with sleep, we hypothesized that, unlike other sensory modalities, they would not drive increased KCs. To investigate these hypotheses, we applied EEG during a full night sleep in humans exposed to odorants. Because some odorants stimulate the olfactory nerve endings alone (pure olfactants) and others stimulate both the olfactory and trigeminal nerves, we tested our hypotheses with both types of odorants.
METHODS
The data used in this study were collected as part of a study that also examined nasal airflow parameters in sleep and was published independently (Arzi et al. 2010).
Participants.
Thirty-five healthy subjects (19 women, 16 men), ranging in age from 22 to 36 yr (mean = 26.5 ± 2.5 yr) participated in the study after providing written informed consent to the procedures approved by the Committee for the Protection of Human Subjects at Assuta Hospital. Participants reported normal sleep-wake cycles and general good physical and mental health. On the day of the experiment, participants were instructed to not ingest alcohol- or caffeine-containing beverages 6 h before arriving at the lab. Exclusion criteria were irregular breathing patterns, insufficient sleeping time, use of medication, or evidence of sleep apnea. One subject failed to meet the study criteria and was therefore excluded from analysis.
Odorants.
An odor was presented to the subjects while they were sleeping in a specified protocol (see below). Each participant was exposed to only one of four odorants: undiluted lavender oil (Sensale, Ramat-Gan, Israel; n = 13), considered pleasant and mildly trigeminal; undiluted vetiver oil (Givaudan; n = 5), considered unpleasant and mildly trigeminal; vanillin, 3% (vol/vol) diluted in water (CAS 121-33-5, Sigma-Aldrich; n = 12), considered pleasant and purely olfactory; and ammonium sulfide, 1% (vol/vol) diluted in water (CAS 12135-76-1, Sigma-Aldrich; n = 4), considered unpleasant and purely olfactory.
Odor delivery.
We used a computer-controlled air-dilution olfactometer (Johnson and Sobel 2007; Sobel et al. 1997) that delivers pulses of odorant embedded in a constant flow of clean air into a small nasal mask. Stimulation is without visual, auditory, tactile, humidity, or thermal cues to otherwise indicate the onset and offset of odorant presentation. The subject bed was in a room specially designed for olfaction experiments, where stainless steel-coated walls prevent odor contamination over time. The olfactometer was located in an adjacent room, with tubes entering the experimental room through waveguides to further prevent potential nonolfactory signals from the olfactometer body.
Polysomnography recordings.
Physiological measurements were acquired with the PowerLab 16SP Monitoring System (ADInstruments, Bella Vista, NSW, Australia) at a sampling rate of 1 kHz with a 50-Hz notch filter. EEG was obtained with two circular electrodes located at positions C3 and C4 according to the 10-20 system, referenced to electrodes on the opposite mastoids (A2 and A1, respectively). Signals were amplified with a preamplifier (Octal Bio Amp ML138, ADInstruments). Electroocculogram was obtained with two circular Ag/AgCl conductive adhesive electrodes, placed 1 cm above or below and laterally of each eye and referenced to electrodes on the opposite mastoids. Electromyogram was obtained with two circular Ag/AgCl conductive adhesive electrodes, located bilaterally adjacent to the submentalis muscles. Electrocardiogram was obtained with two circular Ag/AgCl conductive adhesive electrodes, placed on left and right sides of the abdomen and referenced to a ground electrode placed on the left foot. In addition, blood oxygenation was measured with a finger oximeter probe (MLT321 SpO2 Finger Clip Sensor, ADInstruments), and nasal respiration was measured with separate pneumotachometers (high-sensitivity flowmeter model 4719, Hans Rudolph) attached in line with the vent ports of the nasal mask. Differential pressure was converted to voltage signal with a spirometer (ML141, ADInstruments) that delivered the voltage to the instrumentation amplifier. In five participants oral respiration was measured in addition to nasal respiration. Participants were monitored from an adjacent room via IR video camera and a one-way observation window.
Procedure.
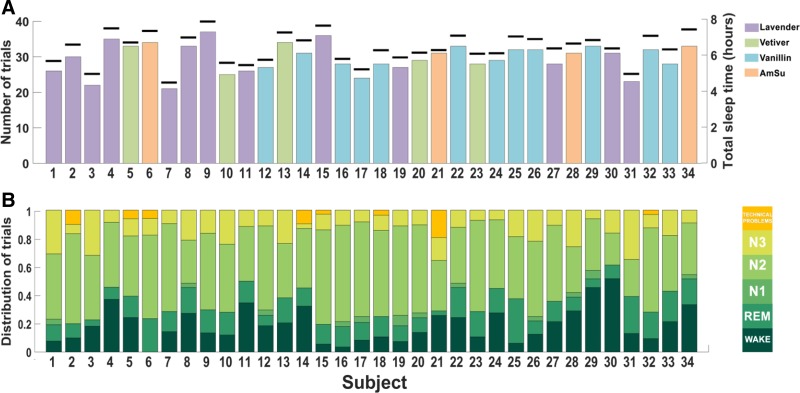
All participants slept one night in the laboratory. They arrived at the laboratory at a self-selected time, ∼1 h prior to their typical sleep time, signed the informed consent form, and were led to the experimental room. After polysomnography (PSG) sensors were applied, and a comfortable positioning in bed ensured, participants were left alone in the room to be observed from the neighboring control room. Experimenters observed the real-time PSG reading and initiated the computer-controlled odor delivery ∼20 min after the onset of stage 2 sleep (18.54 ± 8.12 min). Odor stimulus durations were 5, 10, or 20 s with an interstimulus interval of 9, 12, or 15 min pseudorandomized in an automated manner, regardless of sleep stage. This resulted in an average of 29.7 ± 4.0 repeats per subject (range: 21–37; Fig. 1A, Table 1).
Fig. 1.
Sleep and stimulation patterns. A: number of trials (primary axis) and total sleep time (black markers, secondary axis). Bar color denotes the type of odor used per subject. B: distribution of sleep stages for all subjects who participated in the study.
Table 1.
Distribution of trials across sleep stages
| No. of Trials | N2 Sleep | N3 Sleep | REM Sleep | N1 Sleep | Arousal and Wakes | Technical Problems | Odor | Trigeminality | |
|---|---|---|---|---|---|---|---|---|---|
| Subject 001 | 26 | 12 | 8 | 3 | 1 | 2 | 0 | Lavender | Trigeminal |
| Subject 002 | 30 | 19 | 2 | 3 | 0 | 3 | 3 | Lavender | Trigeminal |
| Subject 003 | 22 | 10 | 7 | 1 | 0 | 4 | 0 | Lavender | Trigeminal |
| Subject 004 | 35 | 16 | 3 | 3 | 0 | 13 | 0 | Lavender | Trigeminal |
| Subject 005 | 33 | 14 | 4 | 5 | 0 | 8 | 2 | Vetiver | Trigeminal |
| Subject 006 | 34 | 20 | 4 | 8 | 0 | 0 | 2 | AmSu | Olfactory |
| Subject 007 | 21 | 13 | 2 | 3 | 0 | 3 | 0 | Lavender | Trigeminal |
| Subject 008 | 33 | 10 | 7 | 6 | 1 | 9 | 0 | Lavender | Trigeminal |
| Subject 009 | 37 | 20 | 6 | 6 | 0 | 5 | 0 | Lavender | Trigeminal |
| Subject 010 | 25 | 12 | 6 | 4 | 0 | 3 | 0 | Vetiver | Trigeminal |
| Subject 011 | 26 | 10 | 3 | 4 | 0 | 9 | 0 | Lavender | Trigeminal |
| Subject 012 | 27 | 16 | 3 | 2 | 1 | 5 | 0 | Vanillin | Olfactory |
| Subject 013 | 34 | 13 | 8 | 6 | 0 | 7 | 0 | Vetiver | Trigeminal |
| Subject 014 | 31 | 13 | 1 | 4 | 0 | 10 | 3 | Vanillin | Olfactory |
| Subject 015 | 36 | 24 | 4 | 5 | 0 | 2 | 1 | Lavender | Trigeminal |
| Subject 016 | 28 | 19 | 3 | 4 | 1 | 1 | 0 | Vanillin | Olfactory |
| Subject 017 | 24 | 16 | 2 | 3 | 1 | 2 | 0 | Vanillin | Olfactory |
| Subject 018 | 28 | 17 | 3 | 4 | 0 | 3 | 1 | Vanillin | Olfactory |
| Subject 019 | 27 | 17 | 3 | 3 | 2 | 2 | 0 | Lavender | Trigeminal |
| Subject 020 | 29 | 18 | 3 | 3 | 1 | 4 | 0 | Vetiver | Trigeminal |
| Subject 021 | 31 | 11 | 5 | 1 | 0 | 8 | 6 | AmSu | Olfactory |
| Subject 022 | 33 | 13 | 4 | 7 | 1 | 8 | 0 | Vanillin | Olfactory |
| Subject 023 | 28 | 18 | 2 | 5 | 0 | 3 | 0 | Vetiver | Trigeminal |
| Subject 024 | 29 | 14 | 2 | 5 | 0 | 8 | 0 | Vanillin | Olfactory |
| Subject 025 | 32 | 14 | 6 | 10 | 0 | 2 | 0 | Vanillin | Olfactory |
| Subject 026 | 32 | 17 | 7 | 3 | 1 | 4 | 0 | Vanillin | Olfactory |
| Subject 027 | 28 | 15 | 3 | 4 | 0 | 6 | 0 | Lavender | Trigeminal |
| Subject 028 | 31 | 10 | 8 | 3 | 1 | 9 | 0 | AmSu | Olfactory |
| Subject 029 | 33 | 12 | 2 | 2 | 2 | 15 | 0 | Vanillin | Olfactory |
| Subject 030 | 31 | 7 | 5 | 3 | 0 | 16 | 0 | Lavender | Trigeminal |
| Subject 031 | 23 | 6 | 8 | 6 | 0 | 3 | 0 | Lavender | Trigeminal |
| Subject 032 | 32 | 19 | 3 | 6 | 0 | 3 | 1 | Vanillin | Olfactory |
| Subject 033 | 28 | 11 | 5 | 6 | 0 | 6 | 0 | Vanillin | Olfactory |
| Subject 034 | 33 | 12 | 3 | 6 | 1 | 11 | 0 | AmSu | Olfactory |
| Average | 29.71 | 14.35 | 4.27 | 4.32 | 0.41 | 5.79 | 0.56 | ||
| Total | 1010 | 488 | 145 | 147 | 14 | 197 | 19 |
Values are the number of trials in each sleep stage for each subject. AmSu, ammonium sulfide.
Data analysis.
Sleep stages were visually scored off-line according to Rechtschaffen and Kales criteria (Rechtschaffen and Kales 1968) (Fig. 1B, Table 2). A change in EEG frequency and/or a brief increase in electromyogram amplitude lasting >3 s or >15 s was classified as an arousal or a wake, respectively, as defined by the Atlas Task Force of the American Sleep Disorder Association (Iber 2007). Next, PSG data were scanned for automatically marked odor onset triggers. Thirty seconds of EEG recordings was extracted before odor onset (“before” epoch) and 30 s immediately after the odor onset (“after” epoch), for C3 and C4 channels and respiration. If an arousal or wake occurred within a 60-s time period surrounding the odor pulse (i.e., 30 s before or after odor onset), the trial was excluded from analysis.
Table 2.
Sleep architecture
| Wake, % | REM, % | N1, % | N2, % | N3, % | Total Night Time, h | |
|---|---|---|---|---|---|---|
| Subject 001 | 6.17 | 10.57 | 2.42 | 50.96 | 29.88 | 05:42:29.3 |
| Subject 002 | 3.62 | 12.47 | 1.49 | 67.53 | 14.89 | 06:36:50.5 |
| Subject 003 | 9.15 | 6.08 | 1.68 | 55.16 | 27.93 | 04:59:24.2 |
| Subject 004 | 19.26 | 7.68 | 2.49 | 60.40 | 10.17 | 07:30:42.0 |
| Subject 005 | 16.75 | 17.15 | 3.05 | 48.62 | 14.43 | 06:43:44.0 |
| Subject 006 | 11.66 | 18.46 | 0.51 | 51.54 | 17.83 | 07:22:07.1 |
| Subject 007 | 9.84 | 12.73 | 2.39 | 51.39 | 23.64 | 04:30:46.6 |
| Subject 008 | 9.54 | 15.28 | 1.76 | 51.19 | 22.23 | 07:00:42.1 |
| Subject 009 | 4.35 | 18.75 | 2.86 | 52.51 | 21.53 | 07:53:20.7 |
| Subject 010 | 6.64 | 16.38 | 1.13 | 51.49 | 24.36 | 05:36:12.5 |
| Subject 011 | 30.68 | 10.59 | 1.99 | 45.64 | 11.10 | 05:28:54.6 |
| Subject 012 | 15.79 | 11.89 | 2.56 | 56.33 | 13.43 | 05:46:33.4 |
| Subject 013 | 11.47 | 21.68 | 0.85 | 44.57 | 21.43 | 07:17:03.4 |
| Subject 014 | 15.89 | 22.05 | 5.74 | 51.45 | 4.87 | 06:50:47.7 |
| Subject 015 | 2.90 | 14.34 | 3.25 | 64.04 | 15.46 | 07:39:43.0 |
| Subject 016 | 4.75 | 15.75 | 6.81 | 57.10 | 15.61 | 05:49:38.6 |
| Subject 017 | 5.70 | 15.21 | 2.05 | 66.00 | 11.03 | 05:14:45.9 |
| Subject 018 | 2.94 | 15.26 | 0.53 | 64.27 | 16.99 | 06:18:01.4 |
| Subject 019 | 7.04 | 12.10 | 1.36 | 65.39 | 14.11 | 05:54:19.0 |
| Subject 020 | 6.67 | 11.89 | 2.84 | 66.27 | 12.34 | 06:10:07.4 |
| Subject 021 | 13.35 | 18.41 | 0.42 | 49.40 | 18.42 | 06:18:40.5 |
| Subject 022 | 10.22 | 18.80 | 11.09 | 46.66 | 13.23 | 07:06:23.6 |
| Subject 023 | 15.42 | 15.03 | 1.19 | 63.43 | 4.93 | 06:06:26.0 |
| Subject 024 | 10.39 | 12.08 | 1.87 | 66.77 | 8.88 | 06:08:11.8 |
| Subject 025 | 3.99 | 29.00 | 2.89 | 43.28 | 20.83 | 07:03:50.4 |
| Subject 026 | 9.30 | 14.26 | 4.45 | 54.73 | 17.26 | 06:55:13.5 |
| Subject 027 | 5.45 | 12.39 | 2.27 | 62.50 | 17.39 | 06:24:04.5 |
| Subject 028 | 14.37 | 11.87 | 6.49 | 45.14 | 22.13 | 06:40:25.6 |
| Subject 029 | 45.13 | 6.00 | 4.86 | 37.65 | 6.36 | 06:51:47.4 |
| Subject 030 | 18.08 | 13.51 | 1.30 | 48.22 | 18.88 | 06:24:22.0 |
| Subject 031 | 4.10 | 24.04 | 0.55 | 48.44 | 22.87 | 04:59:39.5 |
| Subject 032 | 10.25 | 15.49 | 1.98 | 61.34 | 10.94 | 07:06:09.3 |
| Subject 033 | 6.69 | 18.00 | 0.23 | 56.19 | 18.89 | 06:20:59.2 |
| Subject 034 | 13.86 | 15.29 | 5.56 | 56.40 | 8.89 | 07:27:24.3 |
Values are the total sleep time and % of time each subject spent in each sleep stage.
Spectral analysis.
Thirty-second epochs before and after odor onset were extracted, and the mean frequency power in the delta (0.5–4 Hz), theta (4–8 Hz), slow spindle (9–12 Hz), fast spindle (12–15 Hz), and beta (16–24 Hz) frequency bands was calculated for each epoch, with a custom MATLAB R2011a (MathWorks, Natick, MA) script. The segregation into slow or fast spindles was based on criteria from a previous study (Molle et al. 2011). For each odor presentation, the power percent change was calculated by dividing the power following odor onset by the power preceding odor onset (power after odor onset/power before odor onset) for each electrode separately. Then, the percent change in power was averaged across the two electrodes, ultimately reduced into single values denoting bilateral spectral power ratio for each frequency band in each trial. Next, for each subject the percent change values were geometrically averaged across trials. Finally, a grand average of percent change values was calculated with geometric averaging across subjects for display purposes. Note that geometric averaging (unlike arithmetic averaging) weighs increases and decreases equivalently when averaging percent change values, regardless of sign. Spectrograms were calculated on the power percent change values (power after odor/power before odor onset) with a 5-s Hamming window with 4.5-s overlap. To assess the influence of odor duration on EEG activity we conducted the same analysis for 5-, 10-, 15-, 45-, and 60-s epochs before and after odor onset.
Slow-wave activity (SWA) increases over time during NREM sleep. For example, power in the low frequency range rises exponentially within the first 20 min of a typical sleep episode (Dijk et al. 1990). Thus, to control for spectral shifts and other phenomena that may have taken place during sleep regardless of odor presentation, we conducted a control analysis. In this control analysis we selected a random “no odor” point between every two presentations of odor. For these “no odor” points we applied the same analysis described above and compared the power percent change for the “odor” and “no odor” epochs.
K complex analysis.
KC scoring was conducted with both C3 and C4. Epochs classified as N2 were manually inspected for KCs by two experienced sleep technicians, who independently marked KC locations on right and left EEG recordings on 30 epochs before and after odor onset. A KC was defined as a negative high-voltage peak, with an amplitude equal or greater than −75 μV, with the characteristic morphology. KC occurrences reported by the two investigators underwent a separate statistical analysis yielding nearly identical results, and therefore the results presented here are an average of the two.
RESULTS
Odor distribution across sleep stages.
We presented odors to sleeping humans during a complete night sleep. Odor distribution across sleep stages and sleep parameters of the participants are presented in Fig. 1 and in Tables 1 and 2. Each presentation of odor to a subject is considered a trial, and a total of 1,010 such trials were obtained from the 34 participants who took part in this study. Two hundred sixteen trials were excluded because of arousals/wakes (n = 197), EEG artifacts (n = 15), or technical problems with odor delivery or respiration recording (n = 4), retaining 794 trials. Of these 794 trials, 14 occurred in N1 (1.8%), 488 in N2 (61.5%), 145 in N3 (18.3%), and 147 in rapid eye movement (REM) sleep (18.5%). Sleep architecture varied between subjects (Fig. 1B, Table 2), and therefore the number of NREM (N2 + N3) sleep trials per subject varied, ranging between 12 and 28 (mean = 18.6 ± 3.7 NREM trials; Fig. 1B, Table 1). To prevent skew driven by individuals with a small pool of NREM trials, we included only subjects with >15 NREM trials, retaining 27 of 34 subjects and 536 of 633 trials.
Odors enhanced slow oscillations during sleep.
To test whether odors modulated brain rhythms during sleep we first calculated the percentage of power change in 60-s epochs centered on odor onset in the delta (0.5–4 Hz), theta (4–8 Hz), slow spindle (9–12 Hz), fast spindle (12–15 Hz), and beta (16–24 Hz) frequency bands during NREM sleep. The power change was calculated by averaging (geometric average) across trials the power in each frequency band during a 30-s epoch following odor presentation divided by the average power in each frequency band at 30 s preceding odor presentation. We conducted a repeated-measures ANOVA on the power percent change with condition of frequency band [delta (0.5–4 Hz)/theta (4–8 Hz)/slow spindle (9–12 Hz)/fast spindle (12–15 Hz)/beta (16–24 Hz)] and found a main effect of frequency band [F(4,26) = 6.74, P < 0.00005; Fig. 2A]. Follow-up one-sample t-tests revealed a significant increase of 9.79 ± 18.0% in the delta band power [t(26) = 3.22, P < 0.005] and 5.16 ± 7.81% in the slow spindle power [t(26) = 3.91, P < 0.001]. No significant differences were found in any other frequency bands (all P > 0.07). In addition, the same analysis during REM sleep revealed no change in power in any of the five frequency bands (all P > 0.16; Fig. 2B). These findings imply that odor administration during sleep increased low-frequency power.
Fig. 2.
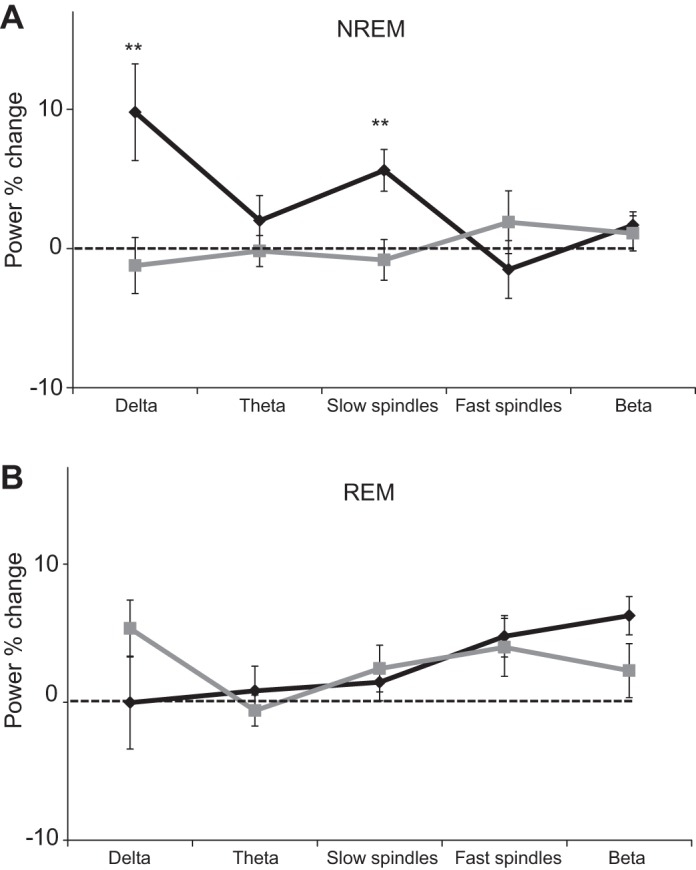
Odor-induced brain modulation in sleep: power percent change in a 30-s time window following odor administration during nocturnal human sleep (black line) and “no odor” condition (gray line) during non-rapid eye movement (NREM, N2+N3; A) and rapid eye movement (REM; B) sleep. Error bars are SE. **P < 0.005.
Because SWA rises within each NREM episode (Dijk et al. 1990), it is possible that greater power in the low frequencies in epochs following odor onset compared with epochs preceding odor onset was merely due to their temporal relationship. In other words, the observed increase in low-frequency power could be alternatively explained by the normal structure of sleep, regardless of odor presentation. To test whether the increase in power reflects the natural structure of sleep, we conducted a control analysis. In this analysis we selected 30-s epochs before and after a random time point between every two presentations of odor in which no odor was presented. For these “no odor” epochs we applied the same analysis described above and compared the power percent change for the “odor” and “no odor” epochs. An ANOVA on the power percent change with conditions odor (“odor”/“no odor”) and frequency band [delta (0.5–4 Hz)/theta (4–8 Hz)/slow spindle (9–12 Hz)/fast spindle (12–15 Hz)/beta (16–24 Hz)] revealed a main effect of odor [F(1,26) = 6.82, P < 0.05], a main effect of frequency band [F(4,104) = 2.94, P < 0.05], and a significant interaction between odor and frequency band [F(4,104) = 5.54, P < 0.0005; Fig. 2A]. Post hoc follow-up t-tests revealed a significant enhancement in the delta [t(26) = 3.21, P < 0.005] and slow spindles [t(26) = 2.20, P < 0.05] after odor presentation compared with “no odor” control epochs. The results of this analysis suggest that the enhancement in slow oscillations is odor induced and does not merely reflect naturally occurring deepening of sleep, and further support the hypothesis that odor presentations during sleep increase power in slow frequencies.
Odor-induced slow oscillations during sleep were independent of odor trigeminality.
Four odors were used in the experiment, yet each participant was exposed to only one of the following odors: vanillin (considered pure olfactory), ammonium sulfide (considered pure olfactory), lavender (considered mildly trigeminal), and vetiver (considered mildly trigeminal). To test whether the odor-induced slow oscillations during sleep were related to trigeminality we divided the participants into two groups according to odor trigeminality: a trigeminal group (n = 14) and an olfactory group (n = 13). An ANOVA on the power percent change with conditions of trigeminality (trigeminal/olfactory) and frequency band [delta (0.5–4 Hz)/theta (4–8 Hz)/slow spindle (9–12 Hz)/fast spindle (12–15 Hz)/beta (16–24 Hz)] revealed no main effect of trigeminality [F(1,25) = 0.74, P > 0.39; Fig. 3], a main effect of frequency band [F(4,100) = 6.54, P < 0.0001], and no significant interaction between trigeminality and frequency band [F(4,100) = 0.74, P > 0.39]. Moreover, planned comparisons revealed no difference in power percent change in the delta [t(25) = 0.63, P > 0.53] and slow spindles [t(25) = 0.51, P > 0.73] between the olfactory and mild trigeminal groups, while one-sample one-tailed t-test showed a significant enhancement in both groups in the delta and slow spindles (all P < 0.05). These findings suggest that the mild odor trigeminality in this study did not influence the odor-induced slow oscillations during sleep.
Fig. 3.
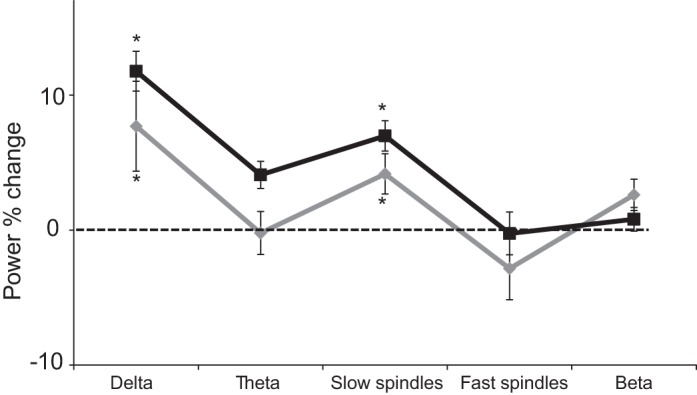
Odor-induced brain modulation in sleep was independent of mild trigeminality: power percent change in a 30-s time window after trigeminal odor administration (black line) and olfactory odor administration (gray line) during NREM sleep. Error bars are SE. *P < 0.05.
One may suggest that although odor trigeminality did not influence odor-induced changes in EEG power, it may have influenced sleep architecture by changing the time spent in each sleep stage. Thus, to further test the influence of odor trigeminality on sleep architecture, we compared the sleep parameters between the two levels of trigeminality and found no difference between the groups in the time they spent in either wake or the different sleep stages or in the total sleep time (all P > 0.11). These findings further suggest that the mild odor trigeminality in this study did not modulate sleep architecture.
Another aspect of odor quality that was different across odorants, besides trigeminality, was odor pleasantness. A comparison of the delta and slow spindle power change in relation to odor pleasantness revealed no differences [delta pleasant: 13.1 ± 16.2%, delta unpleasant: 6.7 ± 19.6%, t(25) = 0.83, P > 0.41; slow spindles pleasant: 13.1 ± 16.2%, slow spindles unpleasant: 6.7 ± 19.6%, t(25) = 0.98, P > 0.33]. In addition, no differences were observed between individual odors (vanillin, ammonium sulfide, lavender, vetiver) in either delta or slow spindles (all P > FDR corrected). However, unlike in the case of trigeminality, where the participants were distributed evenly between groups (olfactory n = 13, trigeminal n = 14), the distributions of participants according to pleasantness [pleasant (vanillin and lavender): n = 19, unpleasant (vetiver and ammonium sulfide): n = 8] and odor type [vanillin: n = 10, lavender: n = 9, ammonium sulfide: n = 3, vetiver: n = 5] were uneven. Therefore, a comparison of the EEG power percent change according to these odor properties should be interpreted with caution as it might be influenced by group size and unreliably imply a lack of differences between groups.
Odor-induced slow oscillations during sleep were influenced by odor duration.
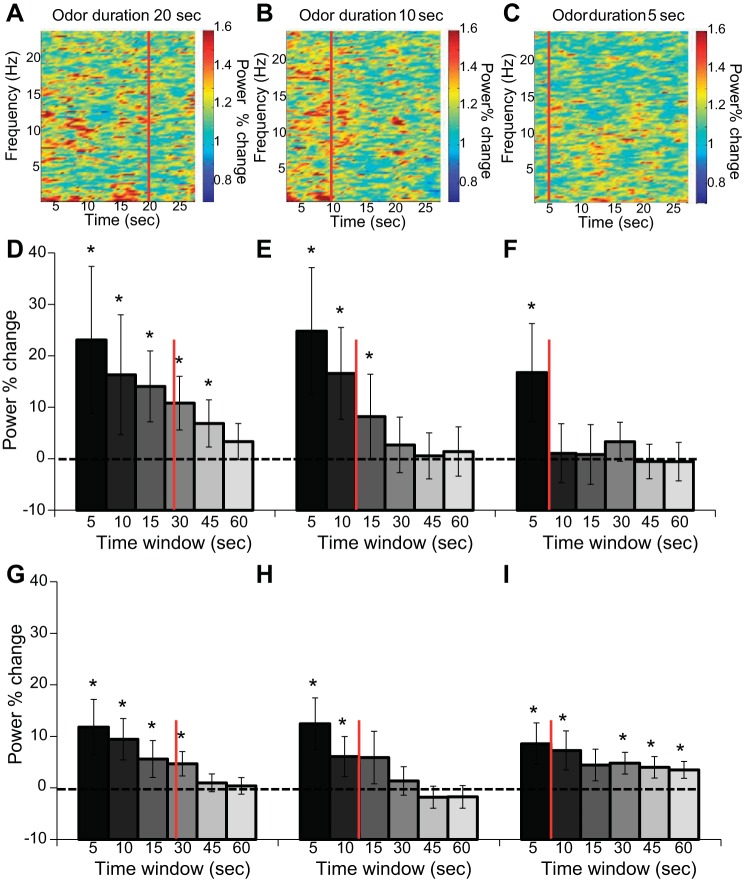
Odors were delivered randomly for one of three possible durations: 5, 10, and 20 s. In the above analyses we averaged across all odor durations and included only participants with >15 NREM sleep trials. Here, as we divided the trials into three categories of odor duration we included all 633 trials. To test the influence of odor duration on neural oscillations during sleep, and to test how the odor-induced oscillations evolved over time, we divided all trials according to odor duration and analyzed the power percent change in six time windows of 5, 10, 15, 30, 45, and 60 s centered around odor onset. An ANOVA on the delta power percent change with conditions of odor duration (5/10/20 s) and time window (5/10/15/30/45/60 s) revealed no main effect of odor duration [F(2,66) = 1.55, P > 0.21], a main effect of time window [F(5,165) = 9.69, P < 0.00001], and no interaction between odor duration and time window [F(10,330) = 0.61, P > 0.79; Fig. 4] The same ANOVA conducted on the slow spindle power percent change with conditions of odor duration (5/10/20 s) and time window (5/10/15/30/45/60 s) revealed no main effect of odor duration [F(2,66) = 0.11, P > 0.89], a main effect of time window [F(5,165) = 8.56, P < 0.00001], and no interaction between odor duration and time window [F(10,330) = 0.93, P > 0.50; Fig. 4]. These results suggest that the duration of odor presentation did not significantly modulate the increase in slow oscillation but did influence the period in which the enhancement was significant. Specifically, the duration of enhancement of delta was proportional to the odor presentation duration and lasted no more than twice the duration of presentation. In slow spindles, the same phenomenon was observed in the 20- and 10-s odor durations. The 5-s odor duration enhancement of slow spindles was found in almost all six time windows (Fig. 4I).
Fig. 4.
Odor-induced brain modulation in sleep was dependent on odor duration. A–C: power percent change spectrograms of 30-s time window after odor administration for 20 s (A), 10 s (B), and 5 s (C) during NREM sleep. Red line indicates offset of the odor administration. D–F: power percent change in the delta (0.5–4 Hz) frequency band in 6 time windows of 5, 10, 15, 30, 45, and 60 s after odor administration for 20 s (D), 10 s (E), and 5 s (F) during NREM sleep. G–I: power percent change in the slow spindle (9–12 Hz) frequency band in 6 time windows of 5, 10, 15, 30, 45, and 60 s after odor administration for 20 s (G), 10 s (H), and 5 s (I) during NREM sleep. Error bars are SE. *P < 0.05.
To further test whether beyond transient impact odors had a persistent or a cumulative effect on brain rhythms, we conducted two additional analyses. First, we compared the power percent change in 30-s epochs between the first and second halves of the night across all odor durations. We divided the trials of each subject into two groups and conducted a paired t-test across all subjects comparing the power percent change between the halves. We found no significant difference in the power between the first and second halves in either delta [t(33) = 0.19, P > 0.84] or slow spindles [t(33) = 0.36, P > 0.71]. Furthermore, to ask whether there was a cumulative or long-lasting effect of repeated odor presentation that was carried over from one trial to the next, we correlated the number of odor presentations and the change in power in 30-s epochs and found no significant correlation in either delta or slow spindles (all P > 0.15). These findings point against a continuous or long-lasting effect of odor throughout the night.
Odor administration during sleep did not modulate the occurrence of K complexes.
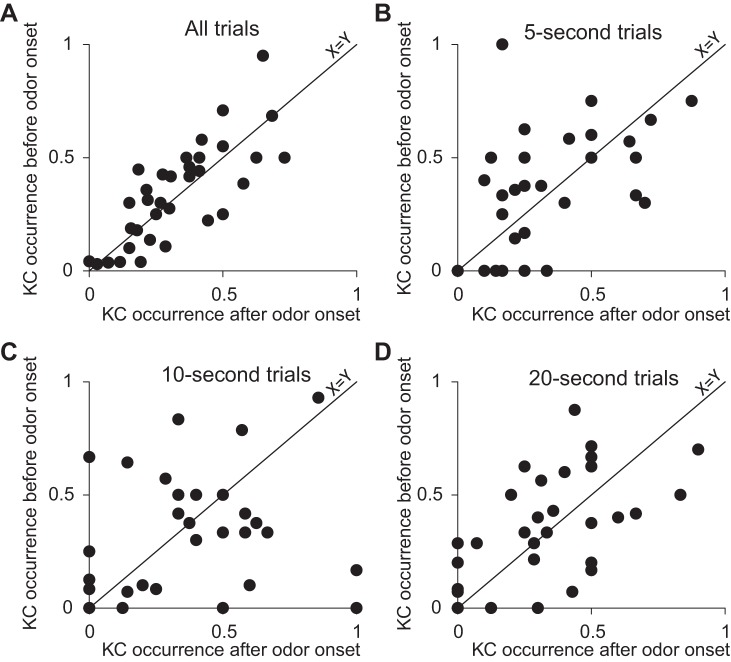
We tested whether the presentation of odors during sleep increased the prevalence of KCs. For this we summated the number of KCs during 30 s before and 30 s after odor onset in a total of 488 N2 epochs in 34 subjects. As the number of trials ranged between subjects, we divided the number of KCs by the number of trials for each subject. We found no significant difference in the occurrence of KCs before and after odor onset [before: 0.33 ± 0.19 KCs per trial, after: 0.34 ± 0.22 KCs per trial, t(33) = 0.14, P > 0.55; Fig. 5]. These results suggest that odors did not influence the prevalence of KCs during N2 sleep. To further substantiate that the null hypothesis was not rejected, we used a Bayesian paired t-test [JASP (https://jasp-stats.org/)], comparing the probability that odor presentation had no effect on the number of KCs (H0) to the alternate hypothesis that the number of KCs after odor presentation will be greater than the number of KCs before (H1). The results suggested that H0 moderately explained the data better than H1 (Jarosz and Wiley 2014) [prior = 0.7; Bayes factor for H0 (BF01) = 6.61, 1/BF01 = 0.15; 95% CI: −0.104, −0.004].
Fig. 5.
Odor administration in sleep did not modulate K complex (KC) occurrence: scatterplots of the occurrence of KCs in 30-s time window before odor onset (y-axis) and after odor onset (x-axis) in all trials (A), 5-s odor administration trials (B), 10-s odor administration trials (C), and 20-s odor administration for trials (D). Diagonal line is a unit slope line, and each data point represents a subject. If a dot is located above the line it means that the average number of KCs before odor onset was larger than after odor onset in that subject.
Next, we tested whether odor duration influenced the prevalence of KCs. We calculated the occurrence of KCs for each of the three odor durations (5, 10, and 20 s) during 30 s preceding and following odor onset in N2. No significant difference was found in the occurrence of KCs before and after odor onset in all three odor durations (all P > 0.43; Fig. 5). These findings may reflect that because odors do not induce arousals the sleep protection property of KCs is not necessary and KC occurrence remained unchanged.
Finally, to test whether odor trigeminality modulated KC occurrence, we conducted an ANOVA on KC occurrence with conditions of trigeminality (trigeminal/olfactory) and odor onset (before/after). We found a main effect of trigeminality [F(1,32) = 4.16, P = 0.05] that may reflect a baseline difference between groups in KC occurrence, no main effect of odor onset [F(1,32) = 0.36, P = 0.55], and, importantly, no significant interaction between odor onset and trigeminality [F(1,32) = 0.09, P = 0.77], reflecting that odor trigeminality did not influence KC occurrence.
To conclude, consistent with our hypotheses we found that odor administration during nocturnal human sleep enhanced delta and slow spindle power and did not modulate the occurrence of KCs. These findings support the existence of an odor-induced sleep-protective brain mechanism.
DISCUSSION
Whereas salient sensory information presented during sleep typically wakes, this is not the case with odors. Here we investigated brain electrical activity markers that may reflect this phenomenon. We found that odor presentation during sleep enhanced low-frequency power without modifying the power in high frequency bands. Specifically, we observed a significant power increase in delta (0.5–4 Hz) during NREM sleep. We previously observed a similar odor-induced increase in delta recorded from central electrodes and averaged across NREM and REM sleep (Arzi et al. 2012). Additional studies observed increased delta only if the odor was previously introduced as part of a wake memory task (Rihm et al. 2014) or increased high delta (2.5–4 Hz) after odor presentation in N3 (Hauner et al. 2013). Despite some differences across studies, they combine to imply that olfactory stimulation during sleep induces slow-wave oscillations. Shifts in power toward low frequencies imply a transition into deeper stages of sleep (Blake and Gerard 1937; Landolt et al. 1996). Therefore, an enhancement in the delta power following odor administration implies that odors may deepen, and thus improve, sleep.
Our results indicate a positive relationship between stimulus duration and SWA enhancement. The longer an odorant was presented, the longer the increase in SWA power lasted. We did not observe such straightforward interaction between odor duration and power of slow sleep spindles: When the odor was presented for 5 s we observed an increase in power of slow spindles, which remained significant within most of the time windows inspected (10, 30, 45, and 60 s, but not 15 s). Conversely, we did not observe such a long-lasting effect following odor presentation of longer duration. A possible, though speculative, explanation is that there may exist some interaction between delta and spindle activity; SWA is perhaps able to moderate the emergence of spindle activity. However, when the odor is presented for only 5 s, the increased SWA it elicits is not sufficient to attenuate spindle activity. Corroboration of such a mechanism will obviously require additional experimentation specifically aimed at this question.
Apart from the enhancement in SWA, we also found a significant increase in the slow spindle (9–12 Hz) power following odor administration during sleep but not in fast spindles (12–15 Hz). Sleep spindles are bursts of oscillatory brain activity of thalamic origin. Therefore it was previously suggested that they play a role in sleep maintenance by gating synaptic transmission of sensory information arriving at the thalamus (Dang-Vu et al. 2011). Thus odor-induced slow spindles can be regarded as an additional sleep protection mechanism elicited by olfactory stimulation. The relation between odor delivery during human sleep and sleep spindles was previously examined only in the context of memory reactivation, where odors were used as memory cues; a recent study reported that odor cuing during sleep significantly modulated fast spindles (13–16 Hz) and questionably slow spindles (10–13 Hz) (Cox et al. 2014). In another memory reactivation paradigm, odor reexposure during sleep significantly increased fast spindles (13–15 Hz), but not slow spindles (11–13 Hz), while exposure to novel odors did not (Rihm et al. 2014). Notably, the aforementioned studies focused on the role of sleep in learning and memory and hence often harnessed odor as a cue for memory reactivation or alternatively as an unconditioned stimulus. Therefore, the reported increases in EEG power could have been part of an ongoing consolidation process. Given that our study did not include a learning component, we find it less likely that the observed enhancement in power following odor presentation was a manifestation of such consolidation processes. Moreover, an integration of our results with those of the aforementioned studies may suggest that slow spindles reflect sensory processing during sleep whereas fast spindles are linked with cognitive processing.
The occurrence of KCs, distinguished waveforms often induced by sensory information, was not influenced by odor stimulation. Particularly, odor presentation did not modulate the overall number of KCs within the observed time window, regardless of odor duration. This finding is in contrast with most forms of salient sensory stimuli presented sparsely during sleep (Colrain et al. 1999; Riedner et al. 2011). A limitation of this study, however, is that we did not use different sensory modalities in these subjects and therefore cannot determine with certainty that they would have evoked KCs under other circumstances.
When addressing this finding, one must bear in mind the unique structure and circuitry of the primary olfactory cortex compared with other sensory modalities. Specifically, sensory information from the nose is relayed directly to the primary olfactory cortex and not mediated by the thalamus (Courtiol and Wilson 2014; Price 1990; Sela and Sobel 2010). Therefore, a possible explanation for the lack of odor-induced KCs here is that odors do not evoke the neural cascade typical of other sensory cortices. This alternate processing route may provide an explanation as to why odor stimulation during sleep does not evoke the dual “Janus-faced” KC, i.e., the combination of a stimulus-dependent cortical excitation with an induced cortical slow wave (Halász 2015). There is debate in the sleep literature as to the function of KCs. The view that KCs reflect an arousal is supported by the fact that often they are accompanied by other physiological measures of arousal (i.e., increase of heart rate or muscle tone). Conversely, the link between KC incidence and the dynamics of SWA in NREM sleep (De Gennaro and Ferrara 2003; Nicholas et al. 2002) has been seen as evidence that KCs protect or promote sleep (Halász 2015). A recent fMRI study suggests that both views may be true, i.e., that stimulus-evoked KCs were associated with subcortical arousal, reflected in low-level activation of the default-mode network, combined with sleep-preserving counteractivity in cerebral midline structures (Jahnke et al. 2012). This observation supports the interpretation that KCs manifest the brain's “decision” not to wake up (Halász 2015). In light of these findings, we think it will be highly informative to compare the processing of odorants and other sensory modalities during sleep within the fMRI environment. Moreover, given that the probability of eliciting KCs is dependent on the intensity of the stimulus (Bastien and Campbell 1992; Halasz et al. 1985), it would be of interest to test whether increasing odor concentration will increase the probability of KC occurrence in future experimentation.
A limitation of this study was the imbalance of different odorants tested across subjects. Some odorants may interfere with normal sleep (Komori et al. 2006; Norrish and Dwyer 2005). Here we used only four different odorants, and a different number of subjects with each. Thus we cannot make strong claims on any odorant-specific effects and acknowledge the possibility that, in contrast to the sleep-promoting effects of the present odorants, other odors may act differently. We can conclude, however, that olfactory stimulation during nocturnal human sleep increased delta oscillation and slow spindles and did not evoke KCs, suggesting a unique processing pathway that may improve sleep quality.
GRANTS
This work was funded by the James S. McDonnell Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: O.P., A.A., N.S., and I.S.H. conception and design of research; O.P., A.A., and L. Sela performed experiments; O.P., A.A., L. Secundo, Y.H., P.S., and I.S.H. analyzed data; O.P., A.A., L. Secundo, N.S., and I.S.H. interpreted results of experiments; O.P. and A.A. prepared figures; O.P. and A.A. drafted manuscript; O.P., A.A., L. Secundo, A.O., N.S., and I.S.H. edited and revised manuscript; O.P., A.A., and N.S. approved final version of manuscript.
REFERENCES
- Arzi A, Sela L, Green A, Givaty G, Dagan Y, Sobel N. The influence of odorants on respiratory patterns in sleep. Chem Senses 35: 31–40, 2010. [DOI] [PubMed] [Google Scholar]
- Arzi A, Shedlesky L, Ben-Shaul M, Nasser K, Oksenberg A, Hairston IS, Sobel N. Humans can learn new information during sleep. Nat Neurosci 15: 1460–1465, 2012. [DOI] [PubMed] [Google Scholar]
- Badia P, Wesensten N, Lammers W, Culpepper J, Harsh J. Responsiveness to olfactory stimuli presented in sleep. Physiol Behav 48: 87–90, 1990. [DOI] [PubMed] [Google Scholar]
- Bastien C, Campbell K. The evoked K-complex: all-or-none phenomenon? Sleep 15: 236–245, 1992. [DOI] [PubMed] [Google Scholar]
- Bastuji H, Perrin F, Garcia-Larrea L. Semantic analysis of auditory input during sleep: studies with event related potentials. Int J Psychophysiol 46: 243–255, 2002. [DOI] [PubMed] [Google Scholar]
- Blake H, Gerard RW. Brain potentials during sleep. Am J Physiol 119: 692–703, 1937. [Google Scholar]
- Carskadon MA, Herz RS. Minimal olfactory perception during sleep: why odor alarms will not work for humans. Sleep 27: 402–405, 2004. [PubMed] [Google Scholar]
- Colrain IM. The K-complex: a 7-decade history. Sleep 28: 255–273, 2005. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response? J Sleep Res 8: 273–280, 1999. [DOI] [PubMed] [Google Scholar]
- Courtiol E, Wilson DA. Thalamic olfaction: characterizing odor processing in the mediodorsal thalamus of the rat. J Neurophysiol 111: 1274–1285, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R, Hofman WF, de Boer M, Talamini LM. Local sleep spindle modulations in relation to specific memory cues. Neuroimage 99: 103–110, 2014. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Bonjean M, Schabus M, Boly M, Darsaud A, Desseilles M, Degueldre C, Balteau E, Phillips C, Luxen A, Sejnowski TJ, Maquet P. Interplay between spontaneous and induced brain activity during human non-rapid eye movement sleep. Proc Natl Acad Sci USA 108: 15438–15443, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H, Davis PA, Loomis AL, Hervey E, Hobart G. Electrical reactions of the human brain to auditory stimulation during sleep. J Neurophysiol 2: 500–514, 1939. [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev 7: 423–440, 2003. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Brunner DP, Borbely AA. Time course of EEG power density during long sleep in humans. Am J Physiol Regul Integr Comp Physiol 258: R650–R661, 1990. [DOI] [PubMed] [Google Scholar]
- Fismer KL, Pilkington K. Lavender and sleep: a systematic review of the evidence. Eur J Integr Med 4: e436–e447, 2012. [Google Scholar]
- Goel N, Kim H, Lao RP. An olfactory stimulus modifies nighttime sleep in young men and women. Chronobiol Int 22: 889–904, 2005. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Talbot LS, Eidelman P, Gruber J, Harvey AG. Sensory gating in primary insomnia. Eur J Neurosci 31: 2112–2121, 2010. [DOI] [PubMed] [Google Scholar]
- Halasz P. K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment. Sleep Med Rev 9: 391–412, 2005. [DOI] [PubMed] [Google Scholar]
- Halász P. The K-complex as a special reactive sleep slow wave—a theoretical update. Sleep Med Rev 29: 34–40, 2015. [DOI] [PubMed] [Google Scholar]
- Halasz P, Pal I, Rajna P. K-complex formation of the EEG in sleep. A survey and new examinations. Acta Physiol Hung 65: 3–35, 1985. [PubMed] [Google Scholar]
- Hardy M, Kirk-Smith MD, Stretch DD. Replacement of drug treatment for insomnia by ambient odour. Lancet 346: 701, 1995. [DOI] [PubMed] [Google Scholar]
- Hauner KK, Howard JD, Zelano C, Gottfried JA. Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nat Neurosci 16: 1553–1555, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Pietsch H, Kobal G. Kallmann's syndrome and chemosensory evoked potentials. Eur Arch Otorhinolaryngol 248: 311–312, 1991. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: Am. Acad. Sleep Med, 2007. [Google Scholar]
- Jahnke K, von Wegner F, Morzelewski A, Borisov S, Maischein M, Steinmetz H, Laufs H. To wake or not to wake? The two-sided nature of the human K-complex. Neuroimage 59: 1631–1638, 2012. [DOI] [PubMed] [Google Scholar]
- Jarosz AF, Wiley J. What are the odds? A practical guide to computing and reporting Bayes factors. J Problem Solving 7: 2, 2014. [Google Scholar]
- Johnson BN, Sobel N. Methods for building an olfactometer with known concentration outcomes. J Neurosci Methods 160: 231–245, 2007. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Murphy CL, Silver WL, Wysocki CJ, Meredith M. Non-olfactory chemoreceptors of the nose: recent advances in understanding the vomeronasal and trigeminal systems. Chem Senses 11: 119–133, 1986. [Google Scholar]
- Komori T, Matsumoto T, Motomura E, Shiroyama T. The sleep-enhancing effect of valerian inhalation and sleep-shortening effect of lemon inhalation. Chem Senses 31: 731–737, 2006. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res 738: 205–212, 1996. [DOI] [PubMed] [Google Scholar]
- Loomis AL, Harvey EN, Hobart GA. Distribution of disturbance-patterns in the human electroencephalogram with special reference to sleep. J Neurophysiol 1: 413–430, 1938. [Google Scholar]
- Molle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep 34: 1411–1421, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CL, Trinder J, Colrain IM. Increased production of evoked and spontaneous K-complexes following a night of fragmented sleep. Sleep 25: 882–887, 2002. [PubMed] [Google Scholar]
- Norrish MI, Dwyer KL. Preliminary investigation of the effect of peppermint oil on an objective measure of daytime sleepiness. Int J Psychophysiol 55: 291–298, 2005. [DOI] [PubMed] [Google Scholar]
- Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron 28: 991–999, 2000. [DOI] [PubMed] [Google Scholar]
- Price J. Olfactory system. In: The Human Nervous System (2nd ed). San Diego, CA: Academic, 1990, p. 979–998. [Google Scholar]
- Raudenbush B, Koon J, Smith J, Zoladz P. Effects of odorant administration on objective and subjective measures of sleep quality, post-sleep mood and alertness, and cognitive performance. N Am J Psychol 5: 181–192, 2003. [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington DC: US Government Printing Office (National Institutes of Health Publication), 1968. [Google Scholar]
- Riedner BA, Hulse BK, Murphy MJ, Ferrarelli F, Tononi G. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. Prog Brain Res 193: 201–218, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihm JS, Diekelmann S, Born J, Rasch B. Reactivating memories during sleep by odors: odor specificity and associated changes in sleep oscillations. J Cogn Neurosci 26: 1806–1818, 2014. [DOI] [PubMed] [Google Scholar]
- Sano A, Sei H, Seno H, Morita Y, Moritoki H. Influence of cedar essence on spontaneous activity and sleep of rats and human daytime nap. Psychiatry Clin Neurosci 52: 133–135, 1998. [DOI] [PubMed] [Google Scholar]
- Sela L, Sobel N. Human olfaction: a constant state of change-blindness. Exp Brain Res 205: 13–29, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. A method for functional magnetic resonance imaging of olfaction. J Neurosci Methods 78: 115–123, 1997. [DOI] [PubMed] [Google Scholar]
- Stuck BA, Stieber K, Frey S, Freiburg C, Hormann K, Maurer JT, Hummel T. Arousal responses to olfactory or trigeminal stimulation during sleep. Sleep 30: 506–510, 2007. [DOI] [PubMed] [Google Scholar]
- Terzano MG, Parrino L, Fioriti G, Orofiamma B, Depoortere H. Modifications of sleep structure induced by increasing levels of acoustic perturbation in normal subjects. Electroencephalogr Clin Neurophysiol 76: 29–38, 1990. [DOI] [PubMed] [Google Scholar]
- Vallet M, Mouret J. Sleep disturbance due to transportation noise: ear plugs vs. oral drugs. Experientia 40: 429–437, 1984. [DOI] [PubMed] [Google Scholar]
- Velluti RA. Interactions between sleep and sensory physiology. J Sleep Res 6: 61–77, 1997. [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Tomita T, Imaizumi Y, Watanabe K, Hatanaka A. Effects of plant-derived odors on sleep-wakefulness and circadian rhythmicity in rats. Chem Senses 30, Suppl 1: i264–i265, 2005. [DOI] [PubMed] [Google Scholar]