Abstract
Kinematics of cat level walking recover after elimination of length-dependent sensory feedback from the major ankle extensor muscles induced by self-reinnervation. Little is known, however, about changes in locomotor myoelectric activity of self-reinnervated muscles. We examined the myoelectric activity of self-reinnervated muscles and intact synergists to determine the extent to which patterns of muscle activity change as almost normal walking is restored following muscle self-reinnervation. Nerves to soleus (SO) and lateral gastrocnemius (LG) of six adult cats were surgically transected and repaired. Intramuscular myoelectric signals of SO, LG, medial gastrocnemius (MG), and plantaris (PL), muscle fascicle length of SO and MG, and hindlimb mechanics were recorded during level and slope (±27°) walking before and after (10–12 wk postsurgery) self-reinnervation of LG and SO. Mean myoelectric signal intensity and frequency were determined using wavelet analysis. Following SO and LG self-reinnervation, mean myoelectric signal intensity increased and frequency decreased in most conditions for SO and LG as well as for intact synergist MG (P < 0.05). Greater elongation of SO muscle-tendon unit during downslope and unchanged magnitudes of ankle extensor moment during the stance phase in all walking conditions suggested a functional deficiency of ankle extensors after self-reinnervation. Possible effects of morphological reorganization of motor units of ankle extensors and altered sensory and central inputs on the changes in myoelectric activity of self-reinnervated SO and LG are discussed.
Keywords: nerve injury, muscle self-reinnervation, muscle length-dependent feedback, electromyography, locomotion
studies using an animal model have investigated the mechanisms of functional impairment and recovery following nerve transection and repair (Bodine-Fowler et al. 1993; Chan et al. 1982; Chen et al. 2010; Chang et al. 2009; Foehring et al. 1986b; Gordon et al. 1980; Maas et al. 2007; O'Donovan et al. 1985; Prilutsky et al. 2011; Sabatier et al. 2012). In the cat, transection of nerves to ankle extensors followed by immediate repair (muscle self-reinnervation) has demonstrated a return of the muscle's ability to be activated and generate force in response to electrical stimulation of the proximal nerve stump or ventral roots in 4–8 wk (Chan et al. 1982; Dum et al. 1985; Gordon and Stein 1982).
Functional recovery of ankle joint kinematics during level and upslope walking occurs ∼8–13 wk after nerve transection and repair of selected ankle extensors (Maas et al. 2007). However, during downslope walking increased ankle joint yield (flexion) is present in the first half of stance as long as 7–12 mo after self-reinnervation (Abelew et al. 2000; Maas et al. 2007). This has been attributed to the lack of stretch reflex in self-reinnervated muscles (Alvarez et al. 2010; Bullinger et al. 2011; Cope et al. 1994; Cope and Clark 1993), as ankle extensors are lengthening more in the stance phase of downslope walking compared with the stance phase of level or upslope walking (Gregor et al. 2006; Maas et al. 2009). However, this explanation alone is insufficient to account for the lack of altered locomotor kinematics during level walking.
Experimental data (Stein et al. 2000) and neuromechanical simulations (Yakovenko et al. 2004) indicate that, during the ankle yield phase in stance of level walking, stretch of ankle extensors accounts for about one-third of their activity due to the stretch reflex. The pattern of fascicle strains in soleus (SO) muscle in this phase of level walking (Maas et al. 2009) suggests the occurrence of substantial strain of muscle spindles, with associated increases in length-dependent afferent activity (Prochazka and Gorassini 1998). Given that SO is the main contributor to ankle extensor force production during level walking at low to medium speeds (Fowler et al. 1993; Hodgson 1983; Prilutsky et al. 1996; Walmsley et al. 1978), it is intriguing that removal of length-dependent feedback from SO, through self-reinnervation, does not result in increased ankle yield during level walking (Abelew et al. 2000; Maas et al. 2007).
It is possible that compensatory activity of intact ankle extensor synergists underpins recovery of level walking joint kinematics. Such a response is seen immediately after partial denervation of ankle extensors, with increased activation observed in their intact synergists (Maas et al. 2010; Pearson and Misiaszek 2000; Prilutsky et al. 2011). Although the mean magnitude of muscle activity of self-reinnervated muscles during locomotion does appear to recover within ∼11–14 wk of nerve transection and repair (Gregor et al. 2003, 2014; O'Donovan et al. 1985), little is known about the details of myoelectric activity of either self-reinnervated muscles or their intact synergists, i.e., time-dependent myoelectric signal intensity and frequency. This information may help explain the mechanisms of functional compensation of level walking kinematics after partial self-reinnervation of ankle extensors and clarify the role of stretch reflex in muscle activity.
The goal of this study was to determine the effect of self-reinnervation of the SO and lateral gastrocnemius (LG) on the intensity and frequency of myoelectric signals from these muscles and their intact synergists medial gastrocnemius (MG) and plantaris (PL) during level and slope locomotion as walking kinematics are restored. We expected larger changes in the myoelectric intensity and frequency characteristics in self-reinnervated SO and LG than in intact MG and PL due to the changed afferent input to reinnervated SO and LG motoneurons. Preliminary results of this work have been published in abstract form (Pantall et al. 2012b, 2013).
METHODS
Six adult female cats (Felis catus; mass: 2.7 kg-4.7 kg) were investigated in this study. All surgical and experimental procedures were in agreement with the Principles of Laboratory Animal Care (National Institutes of Health Publication No. 86-23, revised 1985) and approved by the Institutional Animal Care and Use Committee of the Georgia Institute of Technology. Surgical and experimental procedures were similar to those used in previous studies (Gregor et al. 2006; Hodson-Tole et al. 2012; Maas et al. 2007, 2009, 2010; Prilutsky et al. 2011), and therefore, only brief accounts of the procedures are reported below. Before surgery for implanting electromyographic electrodes (see below), each cat was trained to walk at self-selected speeds along a walkway for food reward for ∼3–5 wk. The walkway was enclosed with plexiglass walls and was inclined at level (0°), upslope (+50% or +27°), or downslope (−50% or −27°).
Surgical and Experimental Procedures
Each animal underwent two surgeries, which were performed under aseptic conditions using isoflurane anesthesia. Teflon-insulated multistranded stainless-steel wires (CW5402; Cooner Wire, Chatsworth, CA) for myoelectric recordings and, in three cats, piezoelectric crystals (2-mm diameter; Sonometrics, London, ON, Canada) for muscle fascicle length recordings were implanted in selected ankle extensors of the right hindlimb. Pairs of electromyographic electrodes (interelectrode distance: 5–10 mm) were implanted in the mid-bellies of SO, MG, and LG of all cats and in the distal portion of PL in two cats (Table 1). Piezoelectric sonomicrometry crystals were implanted near the origin and insertion of a fascicle of selected muscles, providing a direct measure of muscle fascicle length (Biewener 1998; Biewener et al. 1998) and indirect measure of muscle spindle strain (Maas et al. 2010). All transducers led to a connector, mounted on the head to enable collection of respective signals. SO was implanted with crystals in two cats (cats CO and NA) and MG in two cats as well (cats BL and CO). The cats recuperated for 10–14 days during which period postoperative analgesia and antibiotics were administered before recording recommenced.
Table 1.
Number of cycles analyzed for myoelectric signal analysis
| Pre-Nerve Cut and Repair |
Post-Self-Reinnervation |
||||||
|---|---|---|---|---|---|---|---|
| Cat | Down | Level | Up | Down | Level | Up | Total |
| Soleus | |||||||
| BL | 12 | 30 | 29 | 13 | 24 | 14 | 122 |
| CO | 10 | 19 | 11 | 16 | 13 | 12 | 81 |
| GE | 19 | 10 | 28 | 13 | 19 | 77 | 166 |
| IN | 37 | 43 | 13 | 9 | 21 | 23 | 146 |
| KO | 10 | 19 | 33 | 26 | 38 | 52 | 178 |
| NA | 12 | 17 | 42 | 17 | 42 | 39 | 169 |
| Lateral gastrocnemius | |||||||
| CO | 6 | 11 | 8 | 26 | 21 | 22 | 94 |
| GE | 20 | 8 | 48 | 19 | 17 | 79 | 191 |
| IN | 34 | 34 | 12 | 18 | 17 | 21 | 136 |
| KO | 7 | 5 | 7 | 25 | 39 | 30 | 113 |
| NA | 4 | 11 | 9 | 5 | 30 | 1 | 60 |
| Medial gastrocnemius | |||||||
| BL | 0 | N | 26 | 0 | 10 | 9 | 45 |
| CO | 13 | 27 | 23 | 20 | 15 | 21 | 119 |
| GE | 16 | 11 | 47 | 26 | 13 | 67 | 180 |
| IN | 35 | 47 | 13 | 16 | 15 | 16 | 142 |
| KO | 8 | N | 24 | 18 | 15 | 24 | 89 |
| NA | 4 | N | 19 | 0 | 12 | 35 | 70 |
| Plantaris | |||||||
| BL | 30 | 37 | 36 | 8 | 42 | 24 | 177 |
| CO | 11 | 21 | 19 | 15 | 19 | 9 | 94 |
| Total | 288 | 350 | 447 | 290 | 422 | 580 | 2,372 |
N, noisy signal.
After baseline data collection, transection and repair of the LG-SO nerve within the right hindlimb was performed using surgical procedures described previously (Huyghues-Despointes et al. 2003a,b; Prilutsky et al. 2011). The cats recovered for 3–5 days following which locomotor data collection continued.
After the electrode implantation surgery, we recorded myoelectric signals and walking mechanics (all cats), SO fascicle length (in cats CO and NA), and MG fascicle length (in cats BL and CO) before the second nerve transection-repair surgery and for 10–12 wk postnerve surgery because by the latter time point the magnitude of mean myoelectric activity of self-reinnervated muscles appears to recover its magnitude (Gregor et al. 2003, 2014).
Before each recording session small reflective markers were placed on the six anatomical landmarks of the right hindlimb (Gregor et al. 2006; Prilutsky et al. 2011). Marker positions were recorded by a six-camera motion capture system (Vicon Motion Systems, Oxford, UK) at a sampling rate of 120 Hz. The positional data were synchronized with the recorded ground reaction forces (GRF; Bertec, Columbus, OH; sampling rate 360 Hz), the myoelectric signals (sampling rate: 3,000 Hz) and sonomicrometry signals (sampling rate: 1,059 Hz). After completion of the experiments, the cats were euthanized by an overdose of pentobarbital sodium anesthetic (120–180 mg/kg iv).
Data Analysis
Walking trials were selected for analysis when the cat had walked at a steady, consistent speed from one end of the walkway to the other.
Wavelet analysis of myoelectric signals.
To obtain detailed information about intensity and frequency changes in locomotor myoelectric activity of ankle extensors after their partial self-reinnervation, we applied wavelet analysis to the myoelectric signals as described previously (Hodson-Tole et al. 2012). Wavelet analysis is commonly used for quantifying stochastic nonstationary signals, such as myoelectric signals, estimating their time-frequency parameters, and removing noise (von Tscharner 2000; Wakeling and Rozitis 2004). The main advantage of the wavelet analysis we used over traditional analyses of the signal magnitude and frequency content is that it resolves simultaneously the intensity and frequency of myoelectric signal in time space using optimally nonlinear scaled wavelets that accurately represent the shortest muscle physiological response time and signal total power (von Tscharner 2000).
Out of 19 (k = 0–18) nonlinear scaled wavelets approximating the recorded myoelectric signal, we removed the first 4 wavelets (k = 0–3) to reduce confounding effects of low-frequency noise due to movement artifacts, motor unit synchronization (Kleine et al. 2001; Lago and Jones 1981; Yao et al. 2000) or firing rate modulation of motor units (Lago and Jones 1981). In addition, as there was high-frequency noise present in some signals, we eliminated wavelets k = 15–18, leaving 11 wavelets for analysis (total bandwidth of 69.94–782.29 Hz). We selected stride cycles that displayed a physiological power spectral density according to the following criteria:
1) Instances when the intensity resolved by the fifth wavelet, k = 4, was less than the intensity resolved by the subsequent wavelet, k = 5.
2) When the mean intensity across all wavelets for a stride was more than 1.5 SD above the mean intensity during the swing phase for a specific slope/muscle/pre-nerve transection-repair or post-self-reinnervation condition for each cat.
3) When the maximum intensity of wavelets 4–11 was greater than 3 SD of the mean intensity of wavelets 13–14.
Removing the strides that did not satisfy these three criteria reduced the total number of strides for analysis by 5%, from 2,500 to 2,372.
Wavelet-transformed signals from each stride were then partitioned into 10% cycle durations and the mean value was taken for each time window. The total intensity of the signal for a given cycle was calculated as the sum of the total intensities of the selected k wavelets over the 10 cycle time bins. The instantaneous mean frequency of the myoelectric signal in the cycle (fm) was calculated from the sum of the products of the cycle intensity (I) and central frequency (cfk) of each k-th wavelet divided by the sum of intensities from all wavelets:
| (1) |
The mean myoelectric signal intensity from pre-nerve transection-repair upslope walking trials in each cat-muscle combination was used to normalize myoelectric intensity for all other conditions. Temporally, myoelectric data were normalized to the stride duration. Determination of paw-contact and paw-off times were derived from the marker position on the metatarsophalangeal joint with respect to the marker position on the greater trochanter as described previously (Pantall et al. 2012a).
Muscle-tendon unit and muscle fascicle length.
Muscle-tendon unit (MTU) and muscle fascicle length are measures of the kinematics of ankle extensors. The latter measure with its time derivative is also related to the muscle spindle strain and the type of muscle action (eccentric, concentric, isometric) and can influence myoelectric intensity and frequency (Doud and Walsh 1995; Pasquet et al. 2006; Trontelj 1993); see discussion. In addition, elongation of ankle extensors during the joint yield in the stance phase of walking has been used as an indicator of functional recovery after muscle nerve injury (Abelew et al. 2000; Maas et al. 2007, 2010). MTU length was obtained from recorded joint positions and a geometric model of the cat hindlimb (Goslow et al. 1973; Gregor et al. 2006; Whiting et al. 1984). MTU velocities were computed using the method of finite differences.
Muscle fascicle length was determined as the product of the measured time of ultrasound transmission between the two crystals implanted in the muscle and the speed of sound in vertebrate skeletal muscle (i.e., 1,540 m/s; see Biewener et al. 1998). Fascicle velocities were determined using the method of finite differences.
MTU and fascicle length and velocity data were time normalized with respect to the stance and swing duration determined from paw-contact and paw-off. MTU and fascicle length were amplitude normalized to the reference length (mean of the maximum and minimum lengths during swing phase of level walking for the pre-nerve transection condition) (Maas et al. 2009).
Detailed analysis of MTU length and fascicle length changes focused on the stance phase, when MG and SO are predominantly active during slope walking (Carlson-Kuhta et al. 1998; Gregor et al. 2006; Smith et al. 1998a). The magnitude of stretch in early stance (i.e., from paw-contact to time at peak muscle length) and shortening in later stance (i.e., from time at peak muscle length to paw-off) was assessed for both MTU and muscle fascicle. In addition, the mean length and peak of lengthening and shortening velocity in stance were determined for muscle fascicle and MTU.
Ankle joint moment.
The resultant muscle moment about the ankle joint in the sagittal plane, a measure of motor output of ankle extensors, was determined using the method of inverse dynamics (Fowler et al. 1993; Prilutsky et al. 2005). Moment data were time normalized using the same method as for MTU and muscle fascicle length. We determined the peak extension ankle moment and the mean extension moment during stance.
Statistical Analyses
A linear mixed-effects (MIXED) procedure based on restricted maximum likelihood method was used to test effects of independent factors (slope, muscle, presurgery-postreinnervation condition) on the dependent variables of the myoelectric frequency and intensity, joint moments, muscle fascicle, and MTU length and velocity (SPSS 19.0, IBM SPSS, Chicago, IL). Slope, muscle, and presurgery-postreinnervation condition were defined as fixed factors with cat and walking cycle as random factors. The covariate for the analyses was stride duration. When a statistical difference was identified (P < 0.05), a post hoc paired comparison with Bonferroni adjustment was performed.
RESULTS
Number of Trials, Stride Duration, and Walking Speed
Myoelectric signal analysis was performed on 2,372 strides (Table 1), analysis of ankle moments on 258 strides (Table 2), and fascicle and MTU length analysis on 181 strides (Table 2). The mean stride duration increased (P < 0.05) following muscle self-reinnervation in downslope walking from 698.7 ± 107.0 to 722.6 ± 99.3 ms (mean ± SD, 3% increase), in level walking from 738.1 ± 111.1 to 793.3 ± 95.1 ms (7% increase), and in upslope walking from 741.7 ± 99.0 to 764.1 ± 105.2 ms (3% increase). The changes in the cycle duration were relatively small, albeit significant. There was a corresponding trend of decreasing walking speed, determined as the mean horizontal velocity of the iliac crest marker in the cycle, although the speed changes were not significant (P > 0.05). Following self-reinnervation walking speed changed from 0.671 ± 0.095 to 0.651 ± 0.049 ms−1 for downslope (3% decrease), from 0.645 ± 0.079 to 0.619 ± 0.080 ms−1 for level (4% decrease), and from 0.586 ± 0.079 to 0.576 ± 0.076 ms−1 for upslope (2% decrease).
Table 2.
Number of cycles analyzed for ankle joint moments/muscle fascicle and MTU length
| Pre-Nerve Cut and Repair |
Post-Nerve Cut and Repair |
||||||
|---|---|---|---|---|---|---|---|
| Cat | Down | Level | Up | Down | Level | Up | Total |
| BL | 10/8 | 9/13 | 9/7 | 9/7 | 7/9 | 14/7 | 58/51 |
| CO | 7/12 | 8/15 | 4/6 | 5/11 | 10/15 | 9/11 | 43/70 |
| BL | 3 | 3 | 11 | 3 | 3 | 12 | 35 |
| CO | 8 | 12 | 7 | 8 | 7 | 10 | 52 |
| BL | 8 | 4 | 8 | 3 | 10 | 10 | 43 |
| CO | 2/8 | 3/11 | 2 | 6/8 | 1/11 | 13/8 | 27/60 |
| TOTAL | 38/28 | 39/39 | 41/27 | 34/26 | 38/35 | 68/26 | 258/181 |
MTU, muscle-tendon unit.
Myoelectric Signals
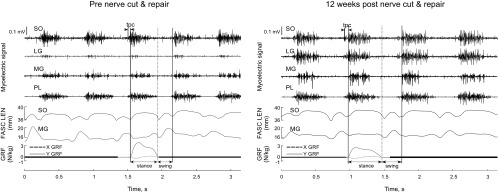
The raw myoelectric signals before wavelet analysis are illustrated for a 3-s period of level walking before nerve transection and post-self-reinnervation for cat CO (Fig. 1). Results for myoelectric signal parameters are described below and a summary of results is provided in Table 3.
Fig. 1.
Exemplar plots of raw myoelectric signal for soleus (SO), lateral gastrocnemius (LG), medial gastrocnemius (MG) and plantaris (PL); fascicle length (FASC LEN) for SO and MG; and ground reaction force (GRF) along the y (vertical)- and x (anterior-posterior)-axes during level walking before (left) and after (right) self-reinnervation of SO and LG muscles. Vertical solid and dashed lines indicate paw-contact and paw-off, respectively; tpc, duration of precontact myoelectric activity.
Table 3.
Summary of significant changes in selected myoelectric, muscle tendon unit and fascicle length parameters for soleus, lateral gastrocnemius, medial gastrocnemius, and plantaris muscles after self-reinnervation
| Soleus |
Lateral Gastrocnemius |
Medial Gastrocnemius |
Plantaris |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Downslope | Level | Upslope | Downslope | Level | Upslope | Downslope | Level | Upslope | Downslope | Level | Upslope | |
| MYO intensity | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||
| MYO precontact intensity | ||||||||||||
| MYO frequency | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||
| MYO PCI | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||
| MYO PCII | ↓ | |||||||||||
| MYO θ | ↑ | ↑ | ↑ | ↑ | ||||||||
| MTU lengthening | ↑ | - | - | - | - | - | - | |||||
| FAS lengthening | - | - | - | - | - | - | ||||||
| MTU shortening | - | - | - | - | - | - | ||||||
| FAS shortening | - | - | - | - | - | - | ||||||
| MTU lengthening velocity | ↑ | - | - | - | ↑ | - | - | - | ||||
| FAS lengthening velocity | ↑ | - | - | - | ↑ | - | - | - | ||||
| MTU shortening velocity | ↑ | - | - | - | ↑ | - | - | - | ||||
| FAS shortening velocity | ↓ | ↓ | - | - | - | ↑ | - | - | - | |||
MYO, myoelectric; FAS, fascicle; PCI and PCII, 1st and 2nd principal components.
Arrows indicate a significant (P < 0.05) increase or decrease in the variable following soleus-lateral gastrocnemius self-reinnervation.
Myoelectric signal intensity.
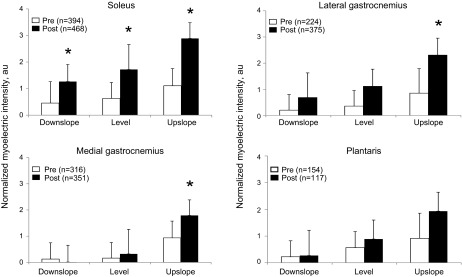
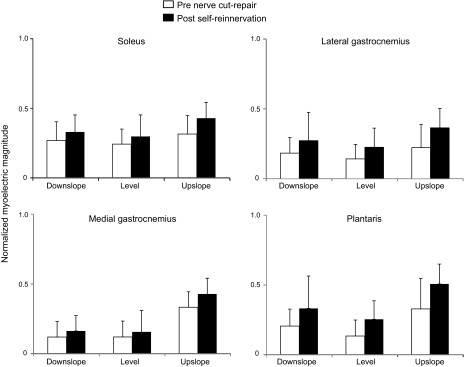
The myoelectric intensity was significantly affected by factors of self-reinnervation, walking slope, and muscle (F1,50 = 15.0, F2,45 = 12.5, and F3,45 = 3.8, respectively, P < 0.05). Specifically, following self-reinnervation of SO and LG, the mean normalized intensity of the myoelectric signal during a stride increased significantly (P < 0.05) in all slope walking conditions for SO (F1,48 = 17.7) and for the upslope condition for LG (F1,50 = 7.6). The signal intensity of intact muscles increased only in upslope condition for MG (P < 0.05, Fig. 2). The signal intensity across all muscles was significantly greater (P < 0.05) in upslope walking than in level and downslope for both pre- (F2,17 = 163.6) and post-self-reinnervation (F2,20 = 10.6) conditions.
Fig. 2.
Mean (±SD) normalized myoelectric intensity pre-nerve transection and post-self-reinnervation for 3 slope conditions; n = 6 cats for soleus, lateral gastrocnemius, medial gastrocnemius; n = 2 cats for plantaris; au, arbitrary units. *P < 0.05.
Myoelectric signal frequency.
The factors of self-reinnervation and muscle significantly affected the mean frequency (F1,45 = 17.2 and F3,42 = 7.2 respectively, P < 0.05). Following self-reinnervation of SO and LG, the mean frequency decreased significantly (P < 0.05) in all slope walking conditions for LG (on average from 258 ± 12 to 232 ± 13 Hz in downslope walking, from 263 ± 12 to 235 ± 12 Hz in level, and from 272 ± 12 to 248 ± 12 Hz in upslope) and for MG (from 257 ± 12 to 234 ± 13 Hz in downslope walking, from 264 ± 13 to 238 ± 11 Hz in level, and from 273 ± 11 to 251 ± 11 Hz in upslope; Table 3). The mean frequency of SO decreased after self-reinnervation for the downslope (from 227 ± 11 to 207 ± 11 Hz) and level (from 226 ± 11 to 204 ± 11 Hz) conditions (P < 0.05, Table 3). Post hoc tests showed significant differences (P < 0.05) in mean frequency between muscles, with SO displaying the lowest frequency averaged across all walking conditions, both before (228 ± 8 Hz) and after (208 ± 6 Hz) self-reinnervation. The corresponding frequencies for LG were 265 ± 8 and 238 ± 7 Hz; for MG, 264 ± 9 and 238 ± 7; and for PL, 245 ± 13 and 236 ± 11 Hz.
Mean frequencies computed for 10% time bins during periods of muscle activity showed no significant changes with time.
Kinetics and Kinematics of Ankle Extensors
To examine potentially confounding effects of muscle length and loading on myoelectric intensity and frequency, we recorded muscle fascicle length, hindlimb kinematics, and ground reaction forces (Fig. 1) and determined the resultant muscle moment at the ankle and MTU length of ankle extensors during level and slope walking.
Ankle joint moment.
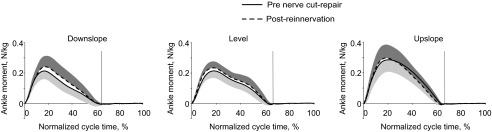
The peak (F1,5 = 2.0, P = 0.22) and mean (F1,5 = 1.1, P = 0.36) ankle extensor moment were unaffected by self-reinnervation of SO and LG (Fig. 3). There was however, a significant self-reinnervation × slope condition interaction for timing of the peak moment (F2,249 = 5.7, P < 0.05). Bonferroni post hoc tests indicated significantly earlier occurrence of peak ankle moment for upslope (35.6 ± 1.2% of stance before nerve transection-repair compared with 31.4 ± 1.0% after self-reinnervation of SO and LG; F1,249=15.8, P < 0.05).
Fig. 3.
Mean (±SD) normalized ankle moment pre-nerve transection and repair (continuous lines) and post-self-reinnervation of SO and LG (dashed lines) for 3 slope conditions; n = 6 cats. −SD shown for pre-nerve transection-repair; +SD shown for post-self-reinnervation for clarity. Vertical dashed lines indicate swing onset.
Fascicle length and MTU length.
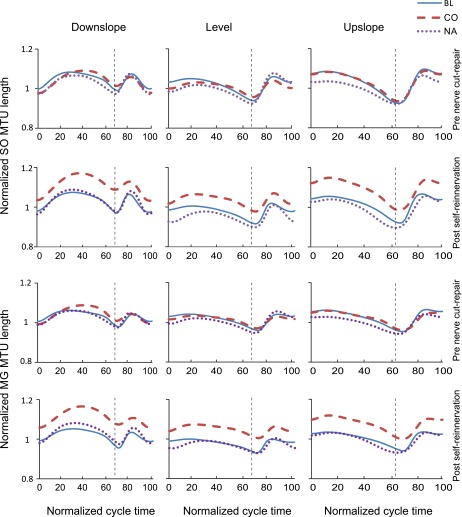
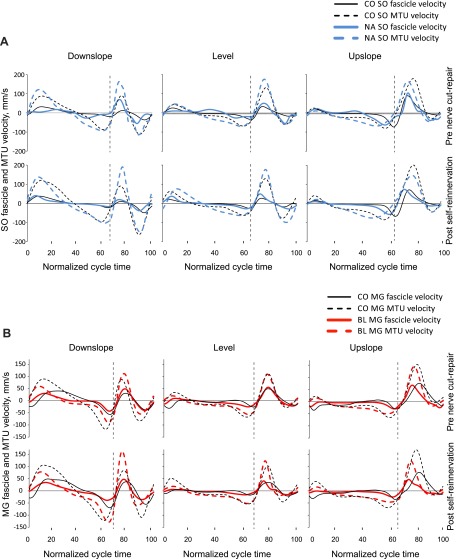
The MTU length patterns and fascicle velocities of SO and MG before nerve transection-repair (Figs. 4 and 5) were consistent with patterns reported previously for normal level and slope walking (Griffiths 1991; Hoffer et al. 1989; Maas et al. 2009). We assessed the similarity in waveforms of these variables before nerve transection-repair and after self-reinnervation using the coefficient of multiple correlation (CMC) (Kadaba et al. 1989). The similarity was generally high for most variables (CMC > 0.8) with the exception of SO MTU length for the downslope condition for two of the cats (CMC < 0.5).
Fig. 4.
Mean normalized muscle-tendon unit (MTU) length of SO and MG muscles as a function of the normalized cycle time for 3 slope walking conditions before LG-SO nerve transection-repair and post-self-reinnervation. Data of cats cats BL, CO, and NA. Vertical dashed lines separate stance and swing phases.
Fig. 5.
Mean fascicle and MTU velocity of SO (A) and MG (B) muscles as a function of the normalized cycle time for 3 slope walking conditions before LG-SO nerve transection-repair and post-self-reinnervation. Data of cats BL, CO, and NA. Vertical dashed lines separate stance and swing phases.
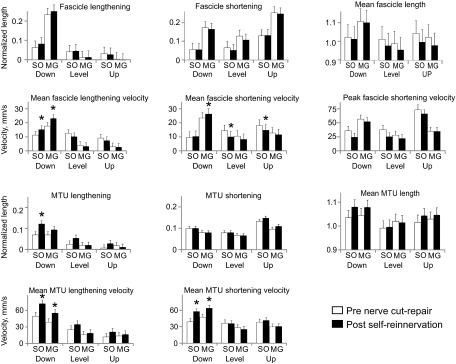
For 8 out of the 11 muscle length-dependent variables across muscles and surgery conditions, there was a significant slope effect. Figure 6 illustrates results found for 11 muscle length and velocity characteristics for SO and MG muscles and 3 slope conditions. The stretch of SO MTU in stance in downslope increased following self-reinnervation (F1,8 = 9.50, P < 0.05). The mean shortening fascicle velocity decreased for level and upslope conditions for SO (F1,4 = 16.0 and F1,7 = 6.1). However, for the downslope condition, SO and MG MTU shortening velocity and MG fascicle shortening velocity increased (F1,10 = 18.2, F1,7 = 17.9, and F1,6 = 6.8, respectively). Mean lengthening velocities of SO and MG also increased for both fascicle and MTU for the downslope condition after self-reinnervation. A summary of results for main fascicle and MTU parameters is provided in Table 3.
Fig. 6.
Mean (±SD) values of fascicle and MTU length parameters for SO and MG muscles for 3 slope conditions, pre-nerve transection and postreinnervation. *P < 0.05.
DISCUSSION
The goal of this study was to determine the effect of self-reinnervation of the SO and LG on the intensity and frequency of myoelectric signals from these muscles and their intact synergists MG and PL during level and slope locomotion. We found a significant change in the self-reinnervated muscles 12 wk after nerve transection and repair with SO exhibiting an increased intensity but decreased frequency for most conditions. LG and the intact synergist MG showed similar changes with an increase in intensity for the upslope condition and a frequency decrease for all conditions. We found no change in either intensity or frequency for the intact synergist PL, possibly due to fewer cats and strides being available for analysis (Table 1) resulting in lower statistical power. Despite the change in myoelectric activity, there was no change in ankle extensor moments for all conditions and most kinematics for level and upslope.
The results for myoelectric signal intensity and frequency obtained for pre-nerve injury conditions were similar to those reported in a previous study, despite only one cat (CO) out of six (Table 1) being common to both studies (Hodson-Tole et al. 2012). Myoelectric intensity was greater for the upslope walking for all studied muscles, which was in agreement with earlier findings (Carlson-Kuhta et al. 1998; Gregor et al. 2006; Hodson-Tole et al. 2012; Smith et al. 1998b). The mean frequency of SO (100% type I fibers; Ariano et al. 1973) for all walking conditions was significantly lower compared with other ankle extensors, whereas LG and MG with the smallest percentage of type I fibers (18 and 25%, respectively; Ariano et al. 1973) had the highest mean frequency.
Effects of SO and LG Self-Reinnervation on Myoelectric Intensity of Ankle Extensors
The increased intensity of myoelectric signals in self-reinnervated SO and LG during walking likely resulted from changes in self-reinnervated muscles and their neural control. Following self-reinnervation, loss of stretch reflex due to a dramatic reduction in synapses from Ia afferents on motoneurons has consistently been reported (Alvarez et al. 2011; Bullinger et al. 2011; Cope et al. 1994; Cope and Clark 1993; Huyghues-Despointes et al. 2003a,b; Maas et al. 2007). If all other synaptic inputs (e.g., from central drive, peripheral sources, Renshaw cells, etc.) remained the same, the lack of Ia excitatory signals during locomotion would be expected to reduce muscle activity by ∼30% (Stein et al. 2000; Yakovenko et al. 2004) due to the reduced number of recruited motor units in self-reinnervated SO and LG muscles and their intact synergists MG and PL that share SO-LG Ia monosynaptic excitatory inputs (Eccles et al. 1957; Nichols et al. 1999). However, the results showed an increase in signal intensity in self-reinnervated SO in all walking conditions and, for upslope, in self-reinnervated LG and intact synergist MG (Fig. 2 and Table 3), which contradicts the expectation.
Central drive.
It is conceivable that the loss of Ia afferent input may be compensated by increased central drive from the central pattern generator(s) to the ankle extensor motoneurons. This would increase recruitment of additional larger motor units at or above the preinjury level in addition to increasing firing frequency in the already recruited smaller motor units. To test this possibility, we compared the pre-paw-contact mean myoelectric intensity of each ankle extensor (from myoelectric signal onset in late swing to the instant of paw-contact determined using measured ground reaction forces; Fig. 1) (Gregor et al. 2006; Gritsenko et al. 2001) before nerve transection and repair and after self-reinnervation of SO and LG in a subset of analyzed myoelectric signal trials. Intensity of the pre-paw-contact muscle activity is considered a measure of central drive to extensor motoneurons without stance-related contributions from extensor muscle group I and II and cutaneous load sensitive afferents from the paw (Gritsenko et al. 2001; Maas et al. 2010). Five (n = 5) out of six cats had multiple strides with myoelectric signals and ground reaction forces available for all presurgery-postreinnervation/walking slope conditions. Results indicated that, following self-reinnervation, the pre-paw-contact intensity and thus the central drive to motoneurons of SO, LG, MG, and PL did not increase significantly (Fig. 7).
Fig. 7.
Mean (±SD) pre-paw-contact myoelectric intensity pre-nerve transection-repair and post-self-reinnervation for 3 slope conditions; n = 5 cats for soleus, lateral gastrocnemius, medial gastrocnemius; n = 2 cats for plantaris.
Feedback gain.
Another source of compensatory synaptic input after LG-SO self-reinnervation could be increased gain of afferent proprioceptive feedback (synaptic efficacy or increased fusimotor input to spindles) from intact ankle extensors (as for example, in MG several days after denervation of its synergists; Pearson et al. 1999; Pearson and Misiaszek 2000; Seburn and Cope 1998). This, however, also seems unlikely as stretch evoked reflex responses of intact ankle extensors become smaller or disappear not only in self-reinnervated muscles but also in their intact synergists (Cope et al. 1994; Horstman et al. 2011; Maas et al. 2007).
Motor unit size, synchronization, and clustering.
It has been well established that 4 mo after peripheral nerve injury and repair, size of motor units in reinnervated muscles is increased by up to 10 times (for review, see Brushart 2011) due to extensive branching (sprouting) of the regenerating axons into collateral fibers with subsequent innervation of additional muscle fibers (Gordon et al. 2011; Rafuse and Gordon 1996). This is also true for motor units formed by intact motoneurons in partially denervated muscles (ventral root cut) (Rafuse and Gordon 1996). Activation of a motoneuron therefore leads to the activation of a larger motor unit, i.e., the regenerated motoneuron activates a greater number of muscle fibers than before injury. However, there is a smaller total number of motor units in the reinnervated muscle as some motoneurons fail to reinnervate muscle fibers (Foehring et al. 1986a). The effect on myoelectric intensity of enlarged motor units may be, as synchronous action potentials are generated in the units' muscle fibers, a larger compound muscle potential detected by the implanted electrodes (Brown et al. 1981; Fu and Gordon 1995a,b; Rafuse et al. 1992). Over time, i.e., 12–24 mo, the size of motor units decreases (Krarup et al. 2002) with exercise promoting speedier reduction (Tam et al. 2002). In this study we only analyzed data at 10–12 wk postnerve repair, so the increased myoelectric intensity may revert at a later date to preinjury levels.
Although synchronization of action potentials in increased number of muscle fibers (and possibly in motor units innervating self-reinnervated muscles) leads to increased myoelectric intensity, it does not appear to change the mean force produced collectively by the synchronously activated muscle fibers or motor units (Clamann and Schelhorn 1988; Semmler et al. 2000; Yao et al. 2000). We did not find any change in the muscle moment at the ankle despite the increase in EMG intensity post-self-reinnervation, which appears to fit well with an increased number of muscle fibers synchronously activated by enlarged motor units.
A similar consideration is that clustering of muscle fibers within a motor unit area in the muscle develops as a result of the collateral sprouting of regenerating motor axons, leading to a potentially elevated myoelectric signal, depending on electrode location (Brown et al. 1981; Fu and Gordon 1995a,b; Rafuse et al. 1992).
Renshaw cells.
Fewer axon collaterals and collateral branches have been observed from regenerated MG motoneurons to themselves and motoneurons of synergists via Renshaw cells (Havton and Kellerth 1984, 1990; Obeidat et al. 2012). However, this reduction in collaterals and inhibitory synapses from Renshaw cells has not been found to reduce the recurrent inhibitory postsynaptic potential in axotomized MG motoneurons (Havton and Kellerth 1984, 1990). The effect of these changes in motoneuron collaterals on disinhibition of motoneurons innervating self-reinnervated muscles and their intact synergists remains open to debate and disinhibition may therefore possibly account for a part of increased myoelectric intensity following self-reinnervation.
Effects of SO and LG Self-Reinnervation on Myoelectric Frequency of Ankle Extensors
We observed a decrease in the mean myoelectric frequency of SO, LG, and MG in most walking conditions 10–12 wk after LG-SO nerve transection and repair. Myoelectric signal frequency is determined by a complex set of interacting factors. Such factors as length of muscle fibers (Doud and Walsh 1995; Trontelj 1993), type of muscle contraction, and velocity of muscle fascicle shortening (Pasquet et al. 2006), fatigue (Dimitrova and Dimitrov 2003) and distance between signal source and recording electrodes (Lateva 1988) could not account for the frequency decrease after SO-LG self-reinnervation found here. The mean length of fascicles (Table 3) and the types of muscle contraction, as demonstrated by fascicle velocity plots (Fig. 5), were similar pre- and post-self-reinnervation. Fatigue could also be excluded as a factor affecting myoelectric signal frequency in our experiments because, in addition to trials being collected across multiple days, after each crossing of the 3-m long walkway during locomotor data collection, the cat had a short break to consume food and then initiated the next trial itself.
Conduction velocity and shape of the action potential in slow muscle fibers.
It appears therefore that additional factors such as muscle fiber conduction velocity (Lindstrom et al. 1977; Solomonow et al. 1990) and the shape of the action potential (Kupa et al. 1995) that are closely related to the type of muscle fibers recruited for the motor task, could be responsible for the observed changes in the mean myoelectric frequency. For example, conduction velocity is 1.5 times lower in slow than in fast muscle fibers (Sadoyama et al. 1988), and the difference in shape of action potential between slow and fast muscle fibers (Luff and Atwood 1972) contributes to the higher myoelectric frequency content of muscles with a greater percentage of fast-twitch motor units (Kupa et al. 1995).
Thus the decrease in myoelectric signal frequency after self-reinnervation could be related to a greater contribution of slower motor units to the recorded signals. To evaluate this possibility in more detail, we applied a principal component (PC) analysis to the wavelet-transformed myoelectric signal to resolve major features of the intensity spectra and distinguish details of spectral shape (Wakeling and Rozitis 2004). The wavelet and PC analysis when applied to myoelectric signals recorded from populations of motor units of a known type [in fish with anatomically separated slow-twitch and fast-twitch muscle fibers (Wakeling et al. 2002), in goats with blocked recruitment of fast motor units (Lee et al. 2011) and in cat and rat ankle extensors with distinct muscle fiber composition (Hodson-Tole et al. 2012; Hodson-Tole and Wakeling 2008)] has consistently provided evidence of distinct frequency components within the myoelectric signal, which reflect the fiber type populations present in the muscles studied.
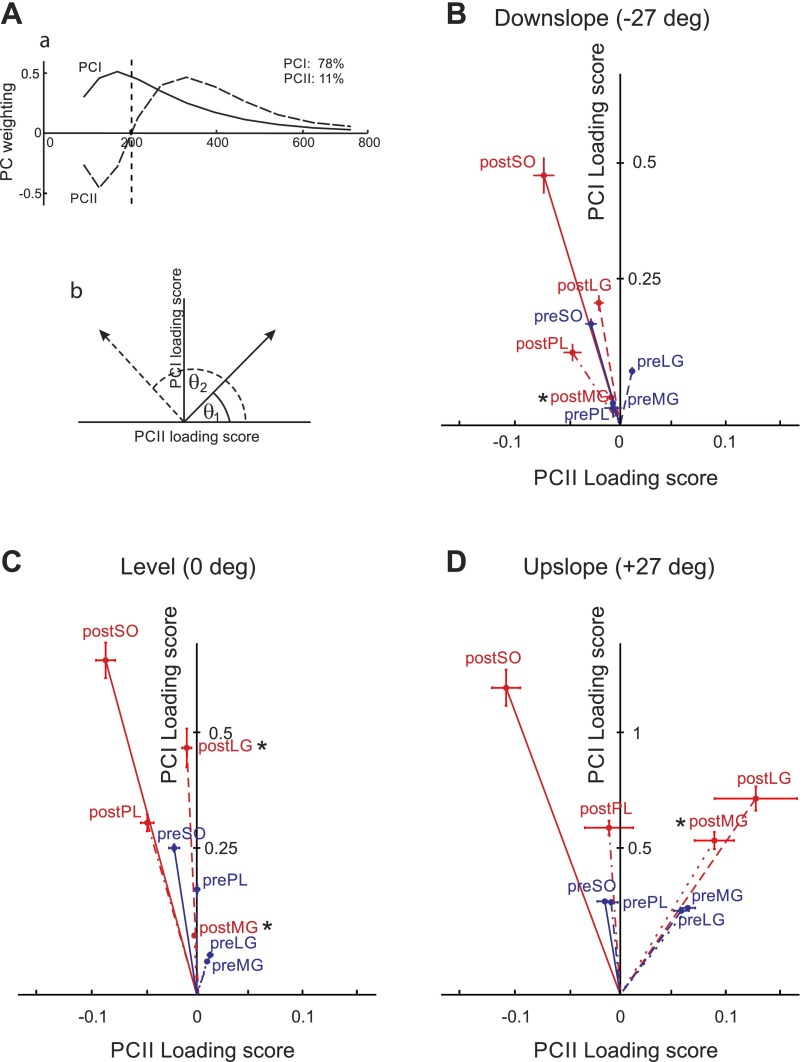
To apply a PC analysis, the wavelet-transformed, normalized, and time-window partitioned intensity spectra from all subjects, muscles, and experimental conditions were compiled into an 11 × 23,720 matrix A (11 wavelets × 2372 strides × 10 time windows). PC analysis was applied to the covariance matrix A with no prior subtraction of the data mean as described in detail previously (Hodson-Tole et al. 2012; Wakeling and Rozitis 2004). The first (PCI) and second (PCII) principal components described 89% of the signal (78 and 11%, respectively; Fig. 8Aa). The PCI loading scores were highly correlated with the total cycle myoelectric signal intensities computed for all 24 muscle/slope/pre-nerve transection-post-reinnervation combinations (the Pearson correlation coefficient r > 0.9, P < 0.05), whereas the PCII loading scores described the change of the signal relative to PCI and represented a measure of the relative frequency content within the signal. The angle formed between the PCI and PCII loading score vector and the PCII axis (θ, see Eq. 2) represented a quantitative measure of the contribution of low and high frequency content in the myoelectric signal (Fig. 8Ab) (Hodson-Tole et al. 2012; Hodson-Tole and Wakeling 2007; Wakeling and Rozitis 2004):
| (2) |
Angle θ significantly increased after self-reinnervation (F1,47 = 13.7, P < 0.05) and with changing walking slope from upslope to downslope (F2,43= 3.6, P < 0.05); see Fig. 8. Specifically, after self-reinnervation of SO and LG, angle θ for LG increased in the level walking condition; angle θ in intact ankle extensor MG increased significantly in all conditions (Fig. 8). Given the discussion above, the changes in angle θ suggest that the decrease in the mean myoelectric signal frequency after LG-SO self-reinnervation is partially caused by an increased contribution of slower motor unit populations in LG and MG muscles.
Fig. 8.
Results of principal component analysis on wavelet-transformed myoelectric signals. Aa: 1st 2 principal component weightings (PCI, solid line; PCII dashed line). The amount of the original myoelectric signal explained by each component is shown at top right. Ab: definition of angle theta (θ) that represents the angle between the PCI-PCII loading score vector and the PCII loading score axis. Larger θ values (θ2, dashed line) indicate relatively more low frequency signal content. B, C, and D: PCI-PCII loading score vector plots from whole stride data collected from intact soleus (preSO), lateral gastrocnemius (preLG), medial gastrocnemius (preMG), plantaris (prePL), and from the same muscles after self-reinnervation of soleus and lateral gastrocnemius: soleus (postSO), lateral gastrocnemius (postLG), medial gastrocnemius (postMG), and plantaris (postPL). Each point represents the mean ±SE loading score values for each condition: downslope walking (B: −50% or −27°), level walking (C: 0% or 0°), and upslope walking (D: +50% or +27°). Blue lines indicate vectors from the origin to pre-nerve transection points and red lines from the origin to points obtained post-self-reinnervation of SO and LG. Note that the y-axis scale for downslope and level walking conditions is different from Upslope condition. *Significant difference (P < 0.05) in angle θ compared with pre-nerve transection repair.
There was no change in angle θ for SO, although the SO mean frequency decreased for downslope and level walking conditions following self-reinnervation. It should be pointed out that while cat SO is a homogeneously slow-twitch muscle (Ariano et al. 1973), its motor unit properties (axon conduction velocity, contraction time, and maximum isometric force) may vary severalfold (McPhedran et al. 1965) and thus SO can exhibit a range of frequencies in its myoelectric signal, as observed during walking and paw-shake responses (Hodson-Tole et al. 2012). The difference between changes in angle θ and mean frequency of SO is likely caused by the fact that angle θ reflects major features in the frequency spectra (PCI and PCII components) without being affected by additional factors including artifacts, which is not the case in measures of the mean frequency (Wakeling 2009). Thus no changes in angle θ for SO strongly suggest that there were no additional lower frequency components in myoelectric signal following self-reinnervation.
Muscle fiber type composition.
Incomplete muscle self-reinnervation could potentially affect motor unit properties and muscle fiber type composition (Gordon et al. 2004) and thus the myoelectric signal frequency content. During the first 4–5 wk after muscle nerve transection and repair, the muscle is paralyzed (Gordon and Stein 1982; Gregor et al. 2003, 2014), which leads to changes in its fiber type composition towards a faster type as observed in long-term denervation experiments (Patterson et al. 2006; Roy et al. 1996). These changes would be expected to increase the relative contribution of the high-frequency content of the myoelectric signals. However, we consistently observed a decrease in signal frequency suggesting that substantial changes in SO and LG fiber composition are unlikely.
Inappropriate reinnervation.
Another possible explanation for the observed decrease in the myoelectric signal frequency could be related to inappropriate reinnervation of muscle fibers by regenerating motoneuron axons (English 2005; Thomas et al. 1987). Since this explanation could only explain the frequency decrease in LG due to its possible inappropriate reinnervation by small SO motoneurons but not the frequency decrease in self-reinnervated SO inappropriately innervated by large, on average, LG motoneurons, this explanation does not appear probable.
Scar tissue.
Forming scar tissue around implanted electrodes could potentially reduce myoelectric frequency. However, scar tissue would also decrease the signal intensity, contrary to what was observed (Fig. 2). Furthermore, our unpublished data on recorded myoelectric signals during walking of an intact cat over a 6-mo period revealed no significant change in the signal intensity or spectrum, suggesting scar tissue was unlikely to have a significant effect on myoelectric properties in our experiments.
Membrane properties and fiber diameter.
The decrease in myoelectric frequency after self-reinnervation might also be caused by the incompletely recovered membrane properties of reinnervated muscle fibers (Mackinnon et al. 1991; Rafuse and Gordon 1996) that could lead to changes in muscle conduction velocity (Fugleholm et al. 2000; Krarup et al. 2002) and myoelectric signal frequency. Decreased fast muscle fiber diameter as well as reduced conduction velocity in the regenerated nerve could also partially contribute to the reduction of the mean myoelectric frequency and to the increase in angle θ (Fugleholm et al. 2000; Krarup et al. 2002; Kupa et al. 1995; Lindstrom et al. 1977; Rafuse and Gordon 1996).
Functional and Clinical Significance
The greater SO MTU lengthening after self-reinnervation in downslope walking (Fig. 6) is consistent with the previous findings of exaggerated ankle joint yield in stance 2–12 mo after self-reinnervation of LG-MG or the whole triceps surae muscles (Abelew et al. 2000; Maas et al. 2007). This motor deficiency has previously been explained by the loss of length-dependent afferent input from self-reinnervated muscles that seemed to affect exclusively downslope walking due to much greater lengthening of muscle fascicles and therefore the spindles of ankle extensors in stance of intact walking (Maas et al. 2009).
The resultant muscle moment of ankle extensors did not change significantly after self-reinnervation (Fig. 3) even though the total myoelectric intensity of ankle extensors in stance of three walking conditions increased and no antagonistic coactivation of tibialis anterior was present (unpublished observations). This result may indicate a reduced “efficacy” of muscle force production per unit of muscle activity. If the relative contribution of slow-twitch motor unit populations in myoelectric activity increases after self-reinnervation, as the decrease in the myoelectric frequency and angle θ in LG and MG (Fig. 8) may suggest, one would anticipate a lower force output per unit of muscle activity.
In summary, following muscle self-reinnervation of SO and LG muscles, we observed an increase in intensity and a decrease in frequency of the myoelectric signal in the affected muscles during level and slope walking with similar changes in the neurologically intact synergist MG. Thus transection and repair of a nerve supplying a single muscle will not only impact that muscle but can have widespread ramifications modifying functions of other synergists. The increased myoelectric intensity of self-reinnervated ankle extensors during locomotion does not, however, change the extensor moment in all slope conditions or normalize an excessive yield at the ankle joint during downslope walking. The increased myoelectric intensity suggests that the absence of the stretch reflex does not reduce the activity of self-reinnervated muscles during walking. The mechanisms responsible for changes in the myoelectric signals may include the presence of larger motor units with synchronized activation of greater number of muscle fibers, incompletely recovered membrane properties of reinnervated muscle fibers, and an additional recruitment of slower motor units. These findings contribute to the understanding of why following repair of a transected peripheral nerve functional recovery is frequently incomplete with diminished motor output and difficulties generating and maintaining high levels of muscle force.
GRANTS
This research was supported by National Institute of Child Health and Human Development Grant HD-032571 (Supplement 5-41305-G1) and the Center for Human Movement Studies at Georgia Institute of Technology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.P., E.F.H.-T., and B.I.P. performed experiments; A.P. analyzed data; A.P., E.F.H.-T., R.J.G., and B.I.P. interpreted results of experiments; A.P. and B.I.P. prepared figures; A.P. and B.I.P. drafted manuscript; A.P., E.F.H.-T., R.J.G., and B.I.P. edited and revised manuscript; A.P., E.F.H.-T., R.J.G., and B.I.P. approved final version of manuscript; R.J.G. and B.I.P. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Timothy C. Cope for valuable comments on an earlier version of the manuscript. We thank Dr. Huub Maas, Dr. Margarita Bulgakova, Dr. Brad Farrell, and Ricky Mehta for assistance with data collection. We also thank Dr. Guay-haur Shue for technical assistance.
Present address of A. Pantall: College of Osteopathic Medicine, Michigan State University, East Lansing, MI 48038.
REFERENCES
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol 84: 2709–2714, 2000. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Bullinger KL, Titus HE, Nardelli P, Cope TC. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injuries. Ann NY Acad Sci 1198: 231–241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106: 2450–2470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano MA, Armstrong RB, Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21: 51–55, 1973. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp Biochem Physiol B Biochem Mol Biol 120: 73–87, 1998. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Konieczynski DD, Baudinette RV. In vivo muscle force-length behavior during steady-speed hopping in tammar wallabies. J Exp Biol 201: 1681–1694, 1998. [DOI] [PubMed] [Google Scholar]
- Bodine-Fowler SC, Unguez GA, Roy RR, Armstrong AN, Edgerton VR. Innervation patterns in the cat tibialis anterior six months after self-reinnervation. Muscle Nerve 16: 379–391, 1993. [DOI] [PubMed] [Google Scholar]
- Brown MC, Holland RL, Hopkins WG. Motor nerve sprouting. Ann Rev Neurosci 4: 17–42, 1981. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Nerve Repair. New York, NY: Oxford Univ. Press, 2011. [Google Scholar]
- Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J Neurophysiol 106: 2471–2485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79: 1687–1701, 1998. [DOI] [PubMed] [Google Scholar]
- Chan AK, Edgerton VR, Goslow GE Jr, Kurata H, Rasmussen SA, Spector SA. Histochemical and physiological properties of cat motor units after self-and cross-reinnervation. J Physiol 332: 343–361, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YH, Auyang AG, Scholz JP, Nichols TR. Whole limb kinematics are preferentially conserved over individual joint kinematics after peripheral nerve injury. J Exp Biol 212: 3511–3521, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang Y, Chen L, Sun C, English AW, Wolpaw JR, Chen XY. H-reflex up-conditioning encourages recovery of EMG activity and H-reflexes after sciatic nerve transection and repair in rats. J Neurosci 30: 16128–16136, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamann HP, Schelhorn TB. Nonlinear force addition of newly recruited motor units in the cat hindlimb. Muscle Nerve 11: 1079–1089, 1988. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol 71: 817–820, 1994. [DOI] [PubMed] [Google Scholar]
- Cope TC, Clark BD. Motor-unit recruitment in self-reinnervated muscle. J Neurophysiol 70: 1787–1796, 1993. [DOI] [PubMed] [Google Scholar]
- Dimitrova NA, Dimitrov GV. Interpretation of EMG changes with fatigue: facts, pitfalls, and fallacies. J Electromyogr Kinesiol 13: 13–36, 2003. [DOI] [PubMed] [Google Scholar]
- Doud JR, Walsh JM. Muscle fatigue and muscle length interaction: effect on the EMG frequency components. Electromyogr Clin Neurophysiol 35: 331–339, 1995. [PubMed] [Google Scholar]
- Dum RP, O'Donovan MJ, Toop J, Tsairis P, Pinter MJ, Burke RE. Cross-reinnervated motor units in cat muscle. II. Soleus muscle reinnervated by flexor digitorum longus motoneurons. J Neurophysiol 54: 837–851, 1985. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol 137: 22–50, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol 490: 427–441, 2005. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. I. Long-term reinnervation. J Neurophysiol 55: 931–946, 1986a. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. J Neurophysiol 55: 947–965, 1986b. [DOI] [PubMed] [Google Scholar]
- Fowler EG, Gregor RJ, Hodgson JA, Roy RR. Relationship between ankle muscle and joint kinetics during the stance phase of locomotion in the cat. J Biomech 26: 465–483, 1993. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci 15: 3876–3885, 1995a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci 15: 3886–3895, 1995b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugleholm K, Schmalbruch H, Krarup C. Post reinnervation maturation of myelinated nerve fibers in the cat tibial nerve: chronic electrophysiological and morphometric studies. J Peripher Nerv Syst 5: 82–95, 2000. [DOI] [PubMed] [Google Scholar]
- Gordon T, Hoffer JA, Jhamandas J, Stein RB. Long-term effects of axotomy on neural activity during cat locomotion. J Physiol 303: 243–263, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Stein RB. Time course and extent of recovery in reinnervated motor units of cat triceps surae muscles. J Physiol 323: 307–323, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Thomas CK, Munson JB, Stein RB. The resilience of the size principle in the organization of motor unit properties in normal and reinnervated adult skeletal muscles. Can J Physiol Pharmacol 82: 645–661, 2004. [DOI] [PubMed] [Google Scholar]
- Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci 31: 5325–5334, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow GE Jr, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol 141: 1–41, 1973. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Bulgakova MA, Maas H, Oliver A, Prilutsky BI. Locomotor activity of feline ankle extensors and kinematics during level and slope walking after removal of stretch reflex from soleus and lateral gastrocnemius by self-reinnervation. In: 2014 Neuroscience Meeting Planner, Program No. 827-01 (Online) Washington, DC: Soc. Neurosci, 2014. [Google Scholar]
- Gregor RJ, Prilutsky BI, Nichols TR, Smith W. EMG output in reinnervated medial gastrocnemius muscle during locomotion in the cat. In: 2003 Neuroscience Meeting Planner Program No 493.8 (Online) New Orleans, LA: Soc. Neurosci, 2003. [Google Scholar]
- Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. J Neurophysiol 95: 1397–1409, 2006. [DOI] [PubMed] [Google Scholar]
- Gritsenko V, Mushahwar V, Prochazka A. Adaptive changes in locomotor control after partial denervation of triceps surae muscles in the cat. J Physiol 533: 299–311, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havton L, Kellerth JO. Plasticity of recurrent inhibitory reflexes in cat spinal motoneurons following peripheral nerve injury. Exp Brain Res 79: 75–82, 1990. [DOI] [PubMed] [Google Scholar]
- Havton L, Kellerth JO. Retrograde effects of axotomy on the intramedullary axon collateral systems and recurrent inhibitory reflexes of cat spinal motoneurones. Neurosci Lett 52: 13–17, 1984. [DOI] [PubMed] [Google Scholar]
- Hodgson JA. The relationship between soleus and gastrocnemius muscle activity in conscious cats–a model for motor unit recruitment? J Physiol 337: 553–562, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson-Tole EF, Pantall AL, Maas H, Farrell BJ, Gregor RJ, Prilutsky BI. Task dependent activity of motor unit populations in feline ankle extensor muscles. J Exp Biol 215: 3711–3722, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson-Tole EF, Wakeling JM. Motor unit recruitment patterns 1: responses to changes in locomotor velocity and incline. J Exp Biol 211: 1882–1892, 2008. [DOI] [PubMed] [Google Scholar]
- Hodson-Tole EF, Wakeling JM. Variations in motor unit recruitment patterns occur within and between muscles in the running rat (Rattus norvegicus). J Exp Biol 210: 2333–2345, 2007. [DOI] [PubMed] [Google Scholar]
- Horstman GM, Nardelli P, Cope TC. Effectiveness of regenerated heterogenic stretch feedback. In: 2011 Neuroscience Meeting Planner Program No. 18102 (Online). Washington, DC: Soc. Neurosci, 2011. [Google Scholar]
- Huyghues-Despointes CM, Cope TC, Nichols TR. Intrinsic properties and reflex compensation in reinnervated triceps surae muscles of the cat: effect of activation level. J Neurophysiol 90: 1537–1546, 2003a. [DOI] [PubMed] [Google Scholar]
- Huyghues-Despointes CM, Cope TC, Nichols TR. Intrinsic properties and reflex compensation in reinnervated triceps surae muscles of the cat: effect of movement history. J Neurophysiol 90: 1547–1555, 2003b. [DOI] [PubMed] [Google Scholar]
- Kadaba MP, Ramakrishnan HK, Wootten ME, Gainey J, Gorton G, Cochran GV. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J Orthop Res 7: 849–860, 1989. [DOI] [PubMed] [Google Scholar]
- Kleine BU, Stegeman DF, Mund D, Anders C. Influence of motoneuron firing synchronization on SEMG characteristics in dependence of electrode position. J Appl Physiol 91: 1588–1599, 2001. [DOI] [PubMed] [Google Scholar]
- Krarup C, Archibald SJ, Madison RD. Factors that influence peripheral nerve regeneration: an electrophysiological study of the monkey median nerve. Ann Neurol 51: 69–81, 2002. [DOI] [PubMed] [Google Scholar]
- Kupa EJ, Roy SH, Kandarian SC, De Luca CJ. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J Appl Physiol 79: 23–32, 1995. [DOI] [PubMed] [Google Scholar]
- Lago PJ, Jones NB. Low-frequency spectral analysis of the EMG. Med Biol Eng Comput 19: 779–782, 1981. [DOI] [PubMed] [Google Scholar]
- Lateva ZC. Dependence of quantitative parameters of the extracellular potential power spectrum on propagation velocity, duration and asymmetry of action potentials. Electromyogr Clin Neurophysiol 28: 191–203, 1988. [PubMed] [Google Scholar]
- Lee SS, Miara Mde B, Arnold AS, Biewener AA, Wakeling JM. EMG analysis tuned for determining the timing and level of activation in different motor units. J Electromyogr Kinesiol 21: 557–565, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom L, Kadefors R, Petersen I. An electromyographic index for localized muscle fatigue. J Appl Physiol 43: 750–754, 1977. [DOI] [PubMed] [Google Scholar]
- Luff AR, Atwood HL. Membrane properties and contraction of single muscle fibers in the mouse. Am J Physiol 222: 1435–1440, 1972. [DOI] [PubMed] [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, English AW, Prilutsky BI. Locomotor changes in length and EMG activity of feline medial gastrocnemius muscle following paralysis of two synergists. Exp Brain Res 203: 681–692, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, Prilutsky BI. Distinct muscle fascicle length changes in feline medial gastrocnemius and soleus muscles during slope walking. J Appl Physiol 106: 1169–1180, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Prilutsky BI, Nichols TR, Gregor RJ. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res 181: 377–393, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL, O'Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve 14: 1116–1122, 1991. [DOI] [PubMed] [Google Scholar]
- McPhedran AM, Wuerker RB, Henneman E. Properties of motor units in a homogeneous red muscle (soleus) of the cat. J Neurophysiol 28: 71–84, 1965. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Cope TC, Abelew TA. Rapid spinal mechanisms of motor coordination. Exerc Sport Sci Rev 27: 255–284, 1999. [PubMed] [Google Scholar]
- O'Donovan MJ, Pinter MJ, Dum RP, Burke RE. Kinesiological studies of self- and cross-reinnervated FDL and soleus muscles in freely moving cats. J Neurophysiol 54: 852–866, 1985. [DOI] [PubMed] [Google Scholar]
- Obeidat AZ, Rotterman TM, Alvarez FJ, Cope TC. Global changes in recurrent inhibition following peripheral nerve repair. In: 2012 Neuroscience Meeting Planner Program No 78912 (Online). New Orleans, LA: Soc. Neurosci, 2012. [Google Scholar]
- Pantall A, Gregor RJ, Prilutsky BI. Feline soleus and lateral gastrocnemius self-reinnervation results in increased ankle extensor activity but no change in ankle extensor moment during upslope locomotion. In: Meeting of the American Society of Biomechanics. Omaha, NE: Am. Soc. Biomech, 2013. [Google Scholar]
- Pantall A, Gregor RJ, Prilutsky BI. Stance and swing phase detection during level and slope walking in the cat: Effects of slope, injury, subject and kinematic detection method. J Biomech 45: 1529–1533, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantall A, Hodson-Tole EF, Gregor RJ, Prilutsky BI. Changes in relative activity of faster and slower motor unit populations in feline ankle extensors during locomotion following self-reinnervation. In: 2012 Neuroscience Meeting Planner Program No. 478.20 (Online). New Orleans, LA: Soc. Neurosci, 2012b. [Google Scholar]
- Pasquet B, Carpentier A, Duchateau J. Specific modulation of motor unit discharge for a similar change in fascicle length during shortening and lengthening contractions in humans. J Physiol 577: 753–765, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MF, Stephenson GM, Stephenson DG. Denervation produces different single fiber phenotypes in fast- and slow-twitch hindlimb muscles of the rat. Am J Physiol Cell Physiol 291: C518–C528, 2006. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Fouad K, Misiaszek JE. Adaptive changes in motor activity associated with functional recovery following muscle denervation in walking cats. J Neurophysiol 82: 370–381, 1999. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE. Use-dependent gain change in the reflex contribution to extensor activity in walking cats. Brain Res 883: 131–134, 2000. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Herzog W, Allinger TL. Mechanical power and work of cat soleus, gastrocnemius and plantaris muscles during locomotion: possible functional significance of muscle design and force patterns. J Exp Biol 199: 801–814, 1996. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Maas H, Bulgakova M, Hodson-Tole EF, Gregor RJ. Short-term motor compensations to denervation of feline soleus and lateral gastrocnemius result in preservation of ankle mechanical output during locomotion. Cells Tissues Organs 193: 310–324, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol 94: 2959–2969, 2005. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol 507: 293–304, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. I. comparisons of the capacity for regenerating nerves to form enlarged motor units after extensive peripheral nerve injuries. J Neurophysiol 75: 268–281, 1996. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T, Orozco R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. J Neurophysiol 68: 1261–1276, 1992. [DOI] [PubMed] [Google Scholar]
- Roy RR, Eldridge L, Baldwin KM, Edgerton VR. Neural influence on slow muscle properties: inactivity with and without cross-reinnervation. Muscle Nerve 19: 707–714, 1996. [DOI] [PubMed] [Google Scholar]
- Sabatier MJ, To BN, Rose S, Nicolini J, English AW. Chondroitinase ABC reduces time to muscle reinnervation and improves functional recovery after sciatic nerve transection in rats. J Neurophysiol 107: 747–757, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoyama T, Masuda T, Miyata H, Katsuta S. Fibre conduction velocity and fibre composition in human vastus lateralis. Eur J Appl Physiol Occup Physiol 57: 767–771, 1988. [DOI] [PubMed] [Google Scholar]
- Seburn KL, Cope TC. Short-term afferent axotomy increases both strength and depression at Ia-motoneuron synapses in rat. J Neurosci 18: 1142–1147, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler JG, Steege JW, Kornatz KW, Enoka RM. Motor-unit synchronization is not responsible for larger motor-unit forces in old adults. J Neurophysiol 84: 358–366, 2000. [DOI] [PubMed] [Google Scholar]
- Smith JL, Carlson-Kuhta P, Trank TV. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol 79: 1702–1716, 1998a. [DOI] [PubMed] [Google Scholar]
- Smith JL, Carlson-Kuhta P, Trank TV. Motor patterns for different forms of walking: cues for the locomotor central pattern generator. Ann NY Acad Sci 860: 452–455, 1998b. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Baten C, Smit J, Baratta R, Hermens H, D'Ambrosia R, Shoji H. Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J Appl Physiol (1985) 68: 1177–1185, 1990. [DOI] [PubMed] [Google Scholar]
- Stein RB, Misiaszek JE, Pearson KG. Functional role of muscle reflexes for force generation in the decerebrate walking cat. J Physiol 525: 781–791, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SL, Archibald V, Tyreman N, Gordon T. Effect of exercise on stability of chronically enlarged motor units. Muscle Nerve 25: 359–369, 2002. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Stein RB, Gordon T, Lee RG, Elleker MG. Patterns of reinnervation and motor unit recruitment in human hand muscles after complete ulnar and median nerve section and resuture. J Neurol Neurosurg Psychiatry 50: 259–268, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trontelj JV. Muscle fiber conduction velocity changes with length. Muscle Nerve 16: 506–512, 1993. [DOI] [PubMed] [Google Scholar]
- von Tscharner V. Intensity analysis in time-frequency space of surface myoelectric signals by wavelets of specified resolution. J Electromyogr Kinesiol 10: 433–445, 2000. [DOI] [PubMed] [Google Scholar]
- Wakeling JM. Patterns of motor recruitment can be determined using surface EMG. J Electromyogr Kinesiol 19: 199–207, 2009. [DOI] [PubMed] [Google Scholar]
- Wakeling JM, Kaya M, Temple GK, Johnston IA, Herzog W. Determining patterns of motor recruitment during locomotion. J Exp Biol 205: 359–369, 2002. [DOI] [PubMed] [Google Scholar]
- Wakeling JM, Rozitis AI. Spectral properties of myoelectric signals from different motor units in the leg extensor muscles. J Exp Biol 207: 2519–2528, 2004. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol 41: 1203–1216, 1978. [DOI] [PubMed] [Google Scholar]
- Whiting WC, Gregor RJ, Roy RR, Edgerton VR. A technique for estimating mechanical work of individual muscles in the cat during treadmill locomotion. J Biomech 17: 685–694, 1984. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Gritsenko V, Prochazka A. Contribution of stretch reflexes to locomotor control: a modeling study. Biol Cybern 90: 146–155, 2004. [DOI] [PubMed] [Google Scholar]
- Yao W, Fuglevand RJ, Enoka RM. Motor-unit synchronization increases EMG amplitude and decreases force steadiness of simulated contractions. J Neurophysiol 83: 441–452, 2000. [DOI] [PubMed] [Google Scholar]