Abstract
Homeostatic intrinsic plasticity is a cellular mechanism for maintaining a stable neuronal activity level in response to developmental or activity-dependent changes. Type 1 metabotropic glutamate receptor (mGlu1 receptor) has been widely known to monitor neuronal activity, which plays a role as a modulator of intrinsic and synaptic plasticity of neurons. Whether mGlu1 receptor contributes to the compensatory adjustment of Purkinje cells (PCs), the sole output of the cerebellar cortex, in response to chronic changes in excitability remains unclear. Here, we demonstrate that the mGlu1 receptor is involved in homeostatic intrinsic plasticity through the upregulation of the hyperpolarization-activated current (Ih) in cerebellar PCs. This plasticity was prevented by inhibiting the mGlu1 receptor with Bay 36–7620, an mGlu1 receptor inverse agonist, but not with CPCCOEt, a neutral antagonist. Chronic inactivation with tetrodotoxin (TTX) increased the components of Ih in the PCs, and ZD 7288, a hyperpolarization-activated cyclic nucleotide-gated channel selective inhibitor, fully restored reduction of firing rates in the deprived neurons. The homeostatic elevation of Ih was also prevented by BAY 36–7620, but not CPCCOEt. Furthermore, KT 5720, a blocker of protein kinase A (PKA), prevented the effect of TTX reducing the evoked firing rates, indicating the reduction in excitability of PCs due to PKA activation. Our study shows that both the mGlu1 receptor and the PKA pathway are involved in the homeostatic intrinsic plasticity of PCs after chronic blockade of the network activity, which provides a novel understanding on how cerebellar PCs can preserve the homeostatic state under activity-deprived conditions.
Keywords: homeostatic plasticity, intrinsic excitability, mGlu1 receptor, Ih
homeostatic intrinsic plasticity maintains the stability of neuronal network activity against environmental or pathological destabilization, which includes the modulation of postsynaptic neurotransmitter receptors and the differential expression of ion channel genes (Desai et al. 1999; Lee et al. 2015; Naude et al. 2013). It therefore serves as a basis for the neural network to achieve an optimal activity range. Intrinsic cellular excitability, in particular, determines the total output of a neuron by integrating synaptic inputs and consecutively translating them into the firing of an action potential (AP). Thus, homeostatic intrinsic excitability plays a pivotal role in maintaining the network balance and maximizing information storage by tuning the average firing rate through the modulation of multiple neurotransmitter receptors and voltage-dependent channels (Stemmler and Koch 1999). Homeostatic intrinsic plasticity is a fascinating model on the plastic changes in neural circuits in both physiological and pathological conditions (Beraneck and Idoux 2012; Lambo and Turrigiano 2013; O'Leary et al. 2014). However, much of the detailed cellular and molecular basis for these regulatory mechanisms is largely unknown.
Metabotropic glutamate (mGlu) receptors monitor neuronal activity and can trigger either Hebbian or homeostatic synaptic plasticity using similar intracellular signaling cascades including calcium influx as well as induction of the immediate early gene Homer1a (Hu et al. 2010), Arc (Shepherd et al. 2006), and eukaryotic elongation factor 2 (Sutton et al. 2007). In addition, altered synaptic activity can also result in mGlu1 receptor-dependent changes in intrinsic excitability (Brager and Johnston 2007). A recent study (Lee et al. 2015) identified mGlu receptors among 873 novel chronic activity-regulated transcripts that had not previously been implicated in homeostatic intrinsic plasticity. Given the similarity in signaling between Hebbian and homeostatic synaptic plasticity, homeostatic regulation of intrinsic excitability could share a signal cascade with intrinsic plasticity following the Hebbian rule. Thus, we investigated whether mGlu1 receptors contribute to homeostatic intrinsic plasticity. We hypothesized that the type 1 metabotropic glutamate receptor (mGlu1 receptor) activity contributes to homeostatic intrinsic plasticity. To test this, we prepared organotypic slice cultures of rat cerebellum obtained by the membrane interface method and measured neuronal activities of Purkinje cells (PCs) by electrophysiological recordings. We found that the intrinsic excitability of cerebellar PCs was reduced by chronic activity deprivation. An mGlu1 receptor inverse agonist but not a neutral antagonist prevented these homeostatic changes, suggesting that the constitutive activation of the mGlu1 receptor results in decreases in intrinsic excitability. In addition, prolonged activity deprivation robustly increased the hyperpolarization-activated current (Ih) components, and the reduced excitability was rescued by an Ih antagonist, suggesting that the homeostatic intrinsic plasticity was dependent on Ih. The antagonism of the mGlu1 receptor also blocked the homeostatic upregulation of Ih and consequently attenuated the intrinsic excitability. Our observations indicate that homeostatic intrinsic excitability in cerebellar PCs is mediated by the agonist-independent activity of the mGlu1 receptor through regulation of Ih.
MATERIALS AND METHODS
Slice preparation and organotypic slice culture.
Experiments were performed according to methods approved by the Institutional Animal Care and Use Committee of Seoul National University College of Medicine and were in accordance with the ethical standards of the institutional research committee. After brain dissection, 250 μm cerebellar sagittal slices were made with a vibrating tissue slicer (Microm HM 650V) in ice-cold standard artificial cerebrospinal fluid (aCSF) solution: (in mM) 124 NaCl, 2.5 KCl, 1 NaH2PO4, 1.3 MgCl2, 2.5 CaCl2, 26.2 NaHCO3, and 20 d-glucose, bubbled with 95% O2/5% CO2, pH 7.4. Sagittal planes of cerebellar slices were transferred onto membrane of culture insert (pore size 0.4 μm) in six-well plastic plates. Culture medium (1 ml), composed of 50% basal medium with Earle's salts, 25% HBSS, 25% heat-inactivated horse serum, 1% l-glutaMaxTM-1 and 5 mg/ml glucose, was added into each well below the culture inserts. Cultured slices were incubated at 35°C in an atmosphere of humidified 5% CO2, and half of medium was replaced every 2–3 days.
Western blot analysis.
For Western blot, cultured slices were homogenized with homogenizing buffer (1% Triton X100, 0.1% SDS, 50 mM Tris·HCl, 0.3 M sucrose, 5 mM EDTA with protease inhibitor cocktail and pH 7.5) on ice. Lysates were boiled for 2 min at 60°C and loaded by 4–12% gradient SDS-PAGE gel. Separated proteins were transferred to PVDF membrane. The membrane blocked with 5% skim milk in TBS-T (24.7 mM Tris, 137 mM NaCl, 2.7 mM KCl, and 1% Tween 20, pH 7.4) for 1 h and incubated with anti-mGlu1 receptor-α (anti-mouse, 1:2,000, BD Bioscience), anti-β-actin (anti-mouse, 1:3,000, Sigma) for additional 1 h. After being washed with TBS-T, the membrane was incubated overnight at 4°C with horseradish peroxidase-conjugated appropriate goat IgG (1:2,000, Stressgen). The immunoblots were developed with enhanced chemiluminescence (ECL) solution (Invitrogen). For quantifying the band, Quantity One (Bio-Rad) was used.
Electrophysiology.
Whole cell patch-clamp configurations were made from 10–12 days in vitro slices. Slices were put onto a submerged recording chamber on the stage of Olympus microscope (BX50WI) and perfused with aCSF at 32°C and kept in place with a nylon-strung platinum anchor. All recordings were performed using multiclamp 700B patch-clamp amplifier (Axon Instruments) with a sampling frequency of 20 kHz, and signals were filtered at 2 kHz. For current clamp experiments, standard aCSF was used as extracellular solution described above; for voltage clamp experiments to isolate Ih, slices were incubated with extracellular solution composed of (in mM) 115 NaCl, 1.2 NaH2PO4, 5 KCl, 2 CaCl2, 1 MgCl2, 25 NaHCO3, 20 glucose, 1 BaCl2, 5 tetraethyl ammonium (TEA), 1 4-aminopyrimidine (4-AP), 1 NiCl2, 0.1 CdCl2, 0.01 μM 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), 0.1 picrotoxin, and 0.0005 tetrodotoxin (TTX), bubbled with 90% O2, 5% CO2, pH 7.4 (Nolan et al. 2003). Both excitatory and inhibitory synaptic inputs were all blocked by 10 μM NBQX and 100 μM picrotoxin, respectively. Patch pipettes (3–4 MΩ) were borosilicate glass and filled with internal solution containing (in mM), 9 KCl, 10 KOH, 120 K-gluconate, 3.48 MgCl2, 10 HEPES, 4 NaCl, 4 Na2ATP, 0.4 Na3GTP, and 17.5 sucrose, pH adjusted to 7.25. Electrophysiological recordings were started 5 min after obtaining the whole cell configuration to let the internal solution diffuse enough into the cytosol.
Data acquisition and analysis.
All data were acquired by Clampex software (Molecular Devices) and analyzed by IgorPro 8.1 (Wavemetrics). Otherwise we note, a cell was clamped at −70 mV with current injection and neurons with the injection current below −500 pA were discarded from this analysis. To evaluate the PC excitability, a series of current steps of 1 s duration ranging from +100 to +500 pA in 100 pA increments with a step interval of 4.5 s was applied to the cell from the membrane potential of −70 mV. Input resistance (Rin) was measured by injecting brief current (−200 pA or +100 pA; 100 ms) and was determined from the negative peak voltage deflection during current injection. Voltage threshold (Vthreshold) of AP was defined as the voltage where the dV/dt first exceeds 30–60 mV/ms. Membrane capacitance (Cm) was calculated by Cm = τ/R, at which the time constant (τ) and series resistance (RS) were calculated fitting a single exponentials to the voltage responses of the test pulse (−5 mV). Resting membrane potential (Vm) was measured when injected current was absent in current clamp mode with 1 μM TTX to prevent spontaneous AP firing. The AP waveform, including AP amplitude, half-width, 10–90% rise time, and first spike latency, was analyzed from the first evoked AP of the firing train when +400 pA of the depolarizing current was injected. AP amplitude was determined as difference between peak amplitude and the voltage threshold of the AP. Half-width and 10–90% rise time were the time duration at the half-maximal voltage, elevation time from 10 to 90% of the maximal AP voltage, respectively. The first spike latency was defined as the delay from beginning point of depolarizing current injection to the voltage threshold where the upstroke phase of the first spike was initiated. Fast afterhyperpolarization (fAHP) and medium afterhyperpolarization (mAHP) were measured by calculating the difference between voltage threshold and hyperpolarized negative peak voltage after the first AP or depolarizing square current injection, respectively.
The amount of voltage sag determined as difference between the maximum and steady-state voltage during the hyperpolarizing current injection from −100 pA to −600 pA with increments of −100 pA for 1 s with a step interval of 5 s. This sag amplitude was converted to sag percent, representing percentage change between two states [(VMax − VSteady state)/VMax] × 100. For Ih current isolation in voltage clamp, membrane potential was held at −45 mV and step voltage was applied from −50 mV to −120 mV with increments of −5 mV of 2.5 s.
Data are presented as means ± SE, and statistical evaluations were performed by two-sample t-test, two-way repeated-measures ANOVA with post hoc Tukey's test and Mann-Whitney U-test by Origin 8.5 and SigmaPlot 12.0 software, and the normal distribution was verified.
RESULTS
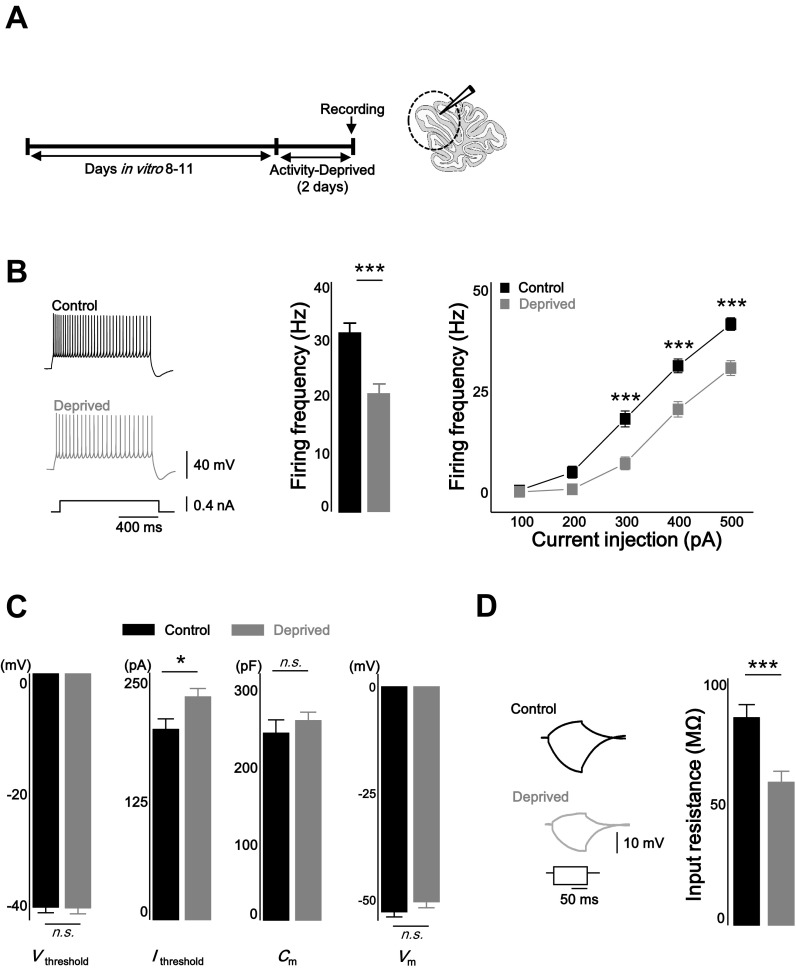
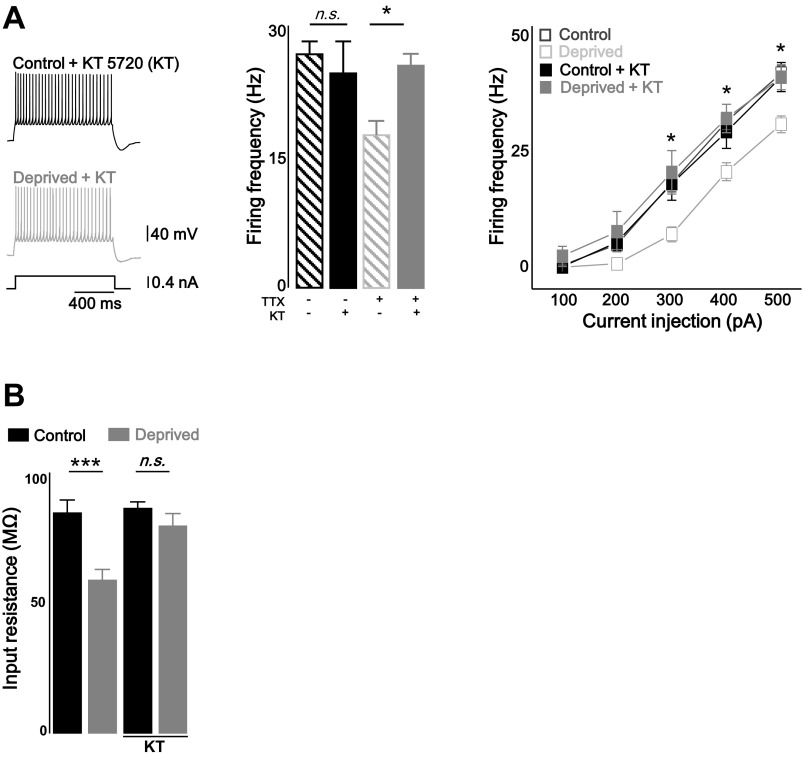
Rat organotypic cerebellar cultures were used rather than primary culture neurons to better preserve in vivo circuits. To investigate homeostatic intrinsic plasticity in cerebellar PCs, network activity of the cerebellar cortex was chronically deprived by applying TTX (1 μM) for 2 days (Fig. 1A). Evoked AP firing rates of control and TTX-treated (deprived) PCs in the presence of excitatory and inhibitory synaptic blockers were compared by injecting brief current steps from the membrane potential of about −70 mV (1 s, from +100 pA to +500 pA with an increment of 100 pA, step interval 4.5 s, see materials and methods). Activity deprivation reduced the intrinsic excitability of the PCs over most ranges of the current injection [Fig. 1B; firing frequency (Hz): control = 31.2 ± 1.7 at 400 pA injection, n = 24; deprived = 20.4 ± 1.9, n = 20; control vs. deprived: P < 0.001, two-way repeated-measured ANOVA]. The active properties of the neurons were analyzed from the first spike of the evoked spike train when +400 pA current was injected (Fig. 1C, Table 1). Activity deprivation increased the current threshold (Ithreshold) for evoking spikes, whereas Vthreshold did not change [Ithreshold (pA): control = 200.6 ± 10.7; deprived = 235 ± 8.2; control vs. deprived: P < 0.05; Vthreshold (mV): control = −40.2 ± 1.0; deprived = −40.3 ± 1.0; control vs. deprived: P = 0.95]. The passive membrane properties Cm and Vm were not altered by activity deprivation [Cm (pF): control = 242.6 ± 17.2; deprived = 259.1 ± 10.4; control vs. deprived: P = 0.4, Vm (mV): control = −52.9 ± 1.2; deprived = −50.6 ± 1.4; control vs. deprived: P = 0.2]. To measure Rin, voltage response was monitored when brief hyperpolarizing and subthreshold depolarizing current (−200 pA and +100 pA) were injected in the current clamp mode (Fig. 1D). Rin was significantly reduced in deprived neurons, and subsequently, voltage deflection was less in response to a brief input current (control = 85.8 ± 5.4 MΩ; deprived = 59.2 ± 4.5 MΩ; control vs. deprived: P < 0.001). The AP waveform, which included the AP amplitude (control = 55.3 ± 1.0 mV; deprived = 57.8 ± 1.0 mV), the half-width (control = 0.39 ± 0.01 ms; deprived = 0.39 ± 0.01 ms) and AP rise time (control = 0.25 ± 0.01 ms; deprived = 0.22 ± 0.01), first spike latency (control = 24.6 ± 1.7 ms; deprived = 28.8 ± 2.1 ms), and fAHP (control = 10.3 ± 0.8; deprived = 9.3 ± 1.0), was not altered in the activity-deprived condition (Table 1). mAHP was increased in the deprived neuron (Table 1; control = 9.8 ± 0.7; deprived = 12.7 ± 0.9; control vs. deprived: P < 0.05, statistical evaluation of all active and passive properties were done by two-sample t-test).
Fig. 1.
Chronic activity deprivation decreased intrinsic excitability of cerebellar Purkinje cells (PCs). A: experimental scheme. Network activity totally deprived by treatment of 1 μM tetrodotoxin (TTX) for 2 days in organotypic cerebellar slice culture. Electrophysiological recording was performed at anterior lobule (lobule III–V). B: representative traces (left), bar graphs at +400 pA injection (middle), and plots (right) showing that chronic activity-deprivation decreased intrinsic excitability of PCs. C: bar graph showing that chronic activity deprivation increased current threshold (Ithreshold), but voltage threshold (Vthreshold), membrane capacitance (Cm), and membrane potential (Vm) were not changed. D: representative traces (left) and summarizing graph (right) showing the input resistance (Rin) was decreased after chronic activity deprivation. Black, control; gray, deprived. Asterisks in B marked by post hoc Tukey's test, pairwise comparison followed by 2-way repeated-measures ANOVA; *P < 0.05, ***P < 0.001; n.s., no significance.
Table 1.
Parameters of AP properties and waveform
| Vthreshold, mV | Ithreshold, pA | AP Amplitude, mV | Half-width, ms | Rise Time, ms | First Spike Latency, ms | fAHP, mV | mAHP, mV | Rin, MΩ | Vm, mV | Cm, pF | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | −40.2 ± 1.0 | 200.6 ± 10.7 | 55.3 ± 1.0 | 0.4 ± 0.01 | 0.25 ± 0.05 | 24.6 ± 1.6 | 10.3 ± 0.8 | 9.8 ± 0.7 | 85.8 ± 5.4 | −52.9 ± 1.2 | 241.6 ± 17.2 |
| Deprived | −40.3 ± 1.0 | 235.0 ± 8.2* | 57.8 ± 1.0 | 0.4 ± 0.01 | 0.22 ± 0.01 | 28.8 ± 2.1 | 9.3 ± 1.0 | 12.7 ± 0.9* | 59.2 ± 4.5*** | −50.6 ± 1.4 | 259.1 ± 10.4 |
Among active membrane properties, current threshold (Ithreshold) was increased under deprived condition, whereas voltage threshold (Vthreshold) was not changed (see also Fig. 1). Action potential (AP) waveform, including AP amplitude, half-width, 10–90% rise time, and first spike latency, was monitored. The parameters were not affected by activity deprivation. Medium afterhyperpolarization (mAHP) was increased in deprived neurons, whereas fast afterhyperpolarization (fAHP) was not altered. Among the passive membrane properties [membrane capacitance (Cm), membrane potential (Vm), and input resistance (Rin)], only Rin was changed by activity deprivation. For comparison of the active and passive membrane properties, changes described here are from those shown in Fig. 1, C and D.
P < 0.05,
P < 0.001.
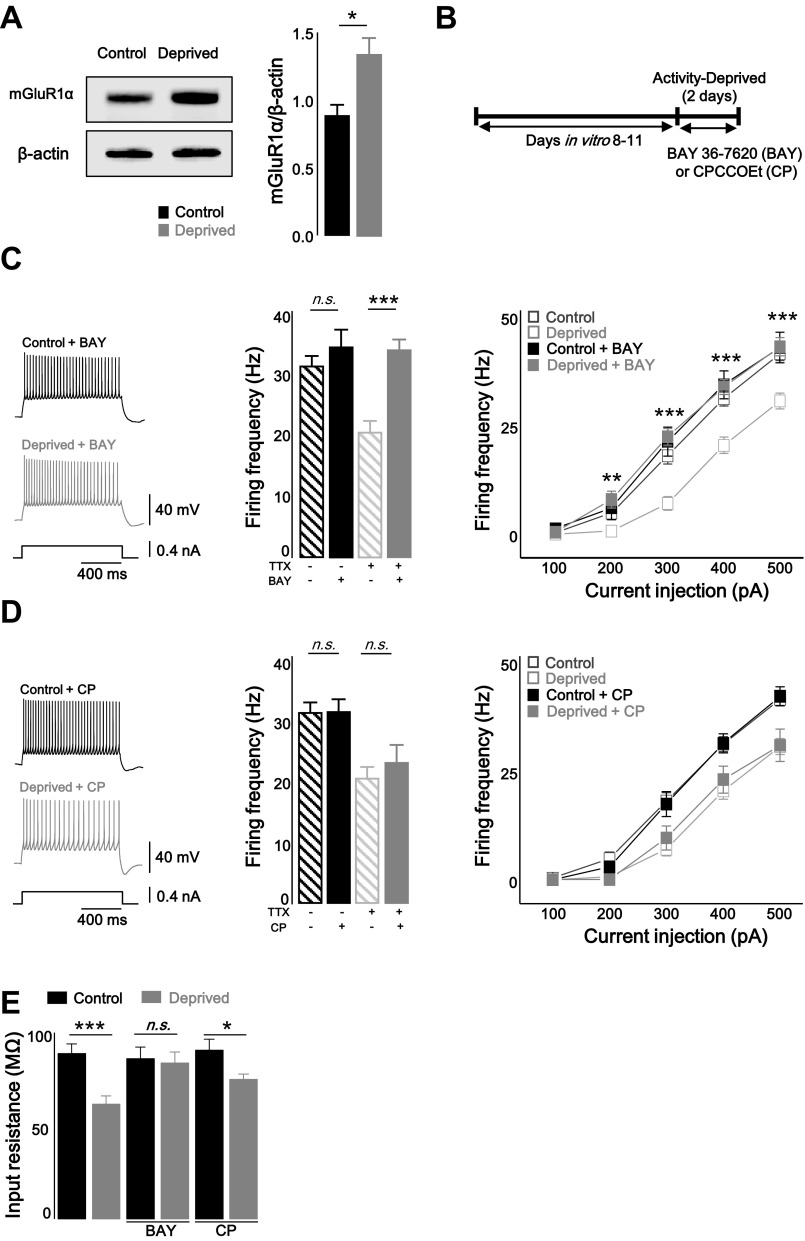
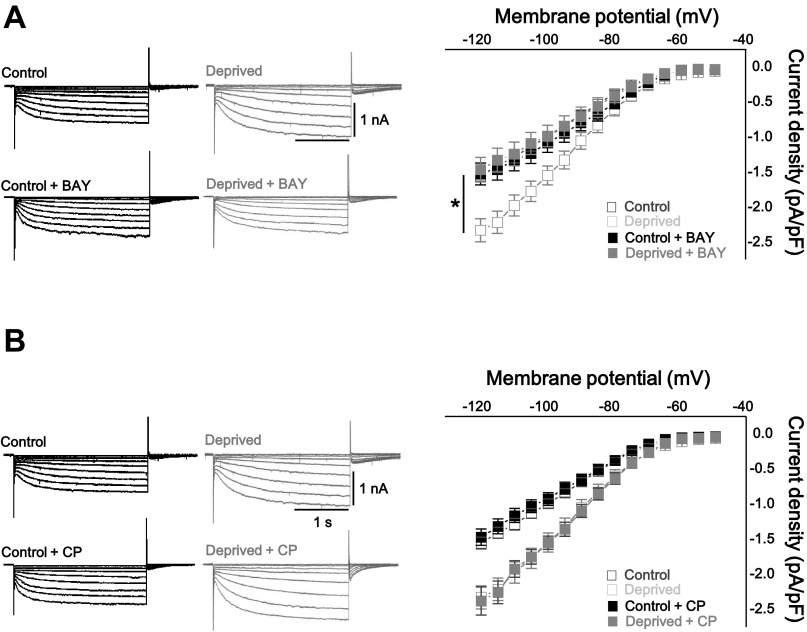
The mGlu receptor can induce plasticity of the intrinsic excitability by initiating a signal cascade resulting in the regulation of ion channels such as Ih, Ca2+ channel, and K+ channels (Brager and Johnston 2007; Kammermeier et al. 2000). Interestingly, agonist-independent (constitutive) activation of mGlu receptor is required for homeostatic synaptic plasticity, suggesting interplay between Hebbian and homeostatic synaptic plasticity (Hu et al. 2010). It has been unclear whether homeostatic intrinsic plasticity also requires agonist-independent activation of the mGlu1 receptor from a homeostatic perspective. To test the hypothesis that the constitutive activity of the mGlu1 receptor acts as the mechanism for the TTX-induced attenuated firing rates, the protein level of the mGlu1 receptor was assessed by Western blot. The expression level of mGlu receptors may change if these receptors contribute to homeostatic intrinsic plasticity because the facilitated agonist-independent activity of G protein-coupled receptors (GPCRs) corresponds with increased protein level (Smit et al. 1996). In addition, it has been observed that mGlu1 receptor expression is regulated in response to changes in network activity (Ehlers 2003). Consistent with this hypothesis, mGlu1 receptor expression was increased by activity deprivation (Fig. 2A; control vs. deprived: P < 0.05, Mann-Whitey U-test). To further examine our hypothesis, mGlu1 receptor inverse agonist, (3aS,6aS)-Hexahydro-5-methylene-6a-(2-naphthalenylmethyl)-1H-cyclopenta[c]furan-1-one (BAY 36–7620) (10 μM), or neutral antagonist, 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) (100 μM) was applied for 2 days to control and TTX-treated slices (Fig. 2B). Inverse agonist BAY 36–7620 (BAY) binds within the transmembrane domain of mGlu1 receptor and stabilizes the receptor in the inactive form (Nakashima et al. 2013). On the other hand, the other agent, CPCCOEt (CP), is classified as a neutral, noncompetitive antagonist that inhibits agonist binding in the NH2 terminus and suppression of agonist-induced signaling (Ango et al. 2001; Litschig et al. 1999). Blockade of the mGlu1 receptor with the inverse agonist prevented homeostatic intrinsic plasticity induced by chronic TTX treatment [Fig. 2C; control + BAY: firing frequency (Hz) = 34.3 ± 2.9 at 400 pA injection, n = 11; deprived + BAY: firing frequency (Hz) = 33.9 ± 1.8, n = 13; control vs. control + BAY: P = 0.31; deprived vs. deprived + BAY: P < 0.05, two-way repeated-measured ANOVA], whereas the homeostatic downregulation of firing rates was not prevented by the neutral antagonist (Fig. 2D; control + CP: firing frequency = 31.4 ± 2.2 at 400 pA injection, n = 11; deprived + CP: firing frequency = 23.0 ± 3.1, n = 10; control vs. control + CP: P = 0.69; deprived vs. deprived + CP: P = 0.58, two-way repeated-measured ANOVA). The Rin of the control and deprived slices was measured after treatment with BAY or CP for 2 days. Consistent with the results of the firing rates in Fig. 2, C and D, the antagonisms of the mGlu1 receptor by the inverse agonist prevented homeostatic downregulation of Rin (BAY: control + BAY = 86.1 ± 6.1 MΩ; deprived + BAY = 83.8 ± 5.7 MΩ; P > 0.05; control + CP = 90.6 ± 5.8 MΩ; deprived + CP = 74.9 ± 2.8 MΩ; P < 0.05, two-sample t-test; Fig. 2E). Taken together, our observation suggests that the homeostatic intrinsic plasticity of PCs requires the agonist-independent action of the mGlu1 receptor.
Fig. 2.
Homeostatic intrinsic plasticity of cerebellar PCs was required to agonist-independent activity of type 1 metabotropic glutamate receptor (mGlu1R). A: immunoblotting of mGlu1 receptor-α (mGluR1α) from control and deprived neuron (left) and summarizing bar graphs (right) showing that chronic activity deprivation increased the protein level of mGlu1Rα. B: experimental scheme. mGlu1 receptor inhibitors, BAY 36–7620 (BAY) or CPCCOEt (CP), were treated for 2 days in presence or absence of TTX. C: representative traces (left), summarizing bar graphs (middle; at +400 pA injection), and plots (right) showing that inverse agonist of mGlu1 receptor inhibited induction of homeostatic intrinsic plasticity. Closed black square, BAY only; closed gray square, deprived + BAY; open black square, control; open gray square, deprived. Control and deprived values are described in Fig. 1. D: representative traces (left) and summarizing bar graphs (middle; at +400 pA injection), and plots (right) showing that there were no effects of antagonizing mGlu1 receptor by CP on homeostatic intrinsic plasticity. Closed black square, CP only; closed gray square, deprived + CP; open black square, control; open gray square, deprived. Control and deprived values are as described in Fig. 1. E: bar graph showing that reduced Rin was recovered by inverse agonist, BAY, and neutral antagonist, CP, was not prevented downregulation of Rin. Asterisks in C and D marked by post hoc Tukey's test, pairwise comparison followed by 2-way repeated-measures ANOVA, compared with deprived and deprived + BAY or deprived + CP. Asterisks in E marked by 2-sample t-test. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., no significance.
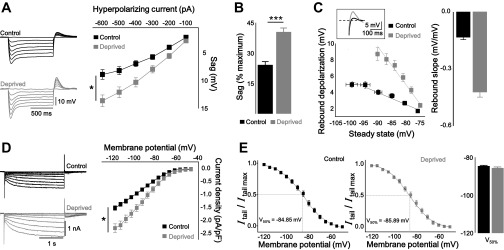
From the parameters of the AP waveform (Table 1), we found that the prolonged inhibition of the network activity robustly decreased the Rin, and consequently, excitability was downregulated (Figs. 1, C and D, and 2E). Among the various ion channels, we examined the hyperpolarization-activated cyclic nucleotide gated (HCN) channel because it has been widely postulated that Ih modulates the cellular excitability via contributing to Rin (Brager and Johnston 2007; Campanac et al. 2008; Rosenkranz and Johnston 2006). We speculated that activity deprivation caused the upregulation of Ih, resulting in reduced intrinsic excitability after chronic TTX treatment. Voltage sag and rebound depolarization were measured by hyperpolarizing step current injection (from −100 pA to −600 pA with an increment of −100 pA for 1 s with a step interval of 5 s) in the current clamp mode (Fig. 3, A and C). The voltage sag was normalized by the maximal negative voltage then recalculated as a percentage (Fig. 3B). In deprived neurons, all Ih components were elevated [voltage sag (mV): control = 6.7 ± 0.6 at −400 pA injection, n = 12; deprived = 10.2 ± 0.8 at −400 pA injection, n = 14; control vs. deprived: P < 0.05, two-way repeated-measured ANOVA; sag % (%): control = 24.0 ± 1.8; deprived = 40.3 ± 2.2 at −400 pA injection; P < 0.001, two-sample t-test; rebound depolarization: control = 4.0 ± 0.3; deprived = 7.3 ± 0.6 at −400 pA injection; P < 0.001, two-sample t-test]. We also observed the elevation of Ih in deprived neurons in the voltage clamp configuration (Fig. 3D; control Ih density = −1.0 ± 0.1 pA/pF at Vm = −100 mV, n = 15; deprived Ih density = −1.5 ± 0.1 pA/pF, n = 14; control vs. deprived: P < 0.05, two-way repeated-measures ANOVA). The tail current was normalized to the maximal amplitude, and then, the resulting data were fitted with a Boltzmann function (Fig. 3E). The half-maximal voltage (V50%) was not shifted (control = 84.9 ± 0.5 mV; deprived = −85.9 ± 0.8 mV), suggesting that voltage dependency was not affected by activity deprivation.
Fig. 3.
Activity deprivation resulted in enhanced hyperpolarization-activated current (Ih) activity. A: representative traces (left) and plots (right) showing that voltage sag was significantly increased in deprived neurons (gray) vs. control (black). B: bar graph of normalized sag voltage by maximal potential showing the increased voltage sag in deprived neurons. C: representative traces (inset), plot (bottom), and bar graph (right) showing relationship between rebound depolarization and steady-state voltage and calculated rebound slope from control and deprived. D: representative traces (left) and plots (right) showing that Ih density was increased in deprived neurons vs. control. E: representative plots (left) showing activation curve of Ih from control and deprived neurons. Tail current was normalized by maximal tail current amplitude and fitted by Boltzmann function. Bar graph (right) showing the half-maximal activation voltage of Ih in control and deprived neurons. Asterisks in A and D marked by 2-way repeated-measures ANOVA; *P < 0.05, ***P < 0.001.
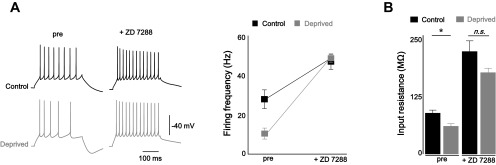
To test whether the downregulation of intrinsic excitability resulted from the elevation of Ih, the firing rates of the control and deprived neurons were compared before and after applying 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD 7288), an HCN channel selective inhibitor (Fig. 4A). Indeed, the decrease in the excitability of the deprived neurons was fully restored by Ih inhibition [control: pre firing frequency (Hz) = 26.7 ± 4.3, ZD 7288 = 45.4 ± 3.5; deprived: before firing frequency (Hz) = 11.7 ± 2.5, after = 46.7 ± 2.1]. Rin was robustly increased in neurons treated with ZD 7288; the difference between the control and deprived neurons was indisputably abolished by ZD 7288 [Fig. 4B; control: pre-ZD 7288 Rin (MΩ) = 89.3 ± 6.7, post-ZD 7288 = 224.2 ± 23.5, n = 6; deprived: pre-ZD 7288 Rin = 60.6 ± 6.0, post-ZD 7288 = 178.2 ± 9.7, n = 5; pre-ZD 7288 P < 0.05, post-ZD 7288 P = 0.12, two-sample t-test]. Thus, we conclude that homeostatic intrinsic plasticity in cerebellar PCs requires Ih regulation.
Fig. 4.
Blockade of Ih abolished the homeostatic downregulation of firing frequency. A: representative traces (left) and summarizing plot (right) showing that decrease in the intrinsic excitability of the deprived (gray) neurons was restored to control (black) in ZD 7288. B: bar graph showing the decrease in Rin in deprived neurons was abolished by ZD 7288. Asterisks in B marked by 2-sample t-test; *P < 0.05; n.s., no significance.
We asked whether homeostatic upregulation of Ih was dependent on the agonist-independent activity of the mGlu1 receptor. The Ih density in the control and deprived neurons was measured after treatment with BAY or CP as described in Fig. 2 (Fig. 5). Homeostatic regulation of Ih was restrained by treatment with the mGlu1 receptor inverse agonist, BAY (Fig. 5A; control + BAY Ih density = −1.1 ± 0.1 pF, n = 13; deprived + BAY Ih density = −1.0 ± 0.2 pF, n = 13, at Vm = −100 mV; control + BAY vs. deprived + BAY: P = 0.6; deprived vs. deprived + BAY: P < 0.001, two-way repeated-measured ANOVA). On the other hand, the mGlu1 receptor neutral antagonist did not prevent the homeostatic changes of Ih (Fig. 5B; control + CP Ih density = −0.9 ± 0.1 pF at Vm = −100 mV, n = 13; deprived + CP Ih density = −1.5 ± 0.1 pF, n = 13; control + CP vs. deprived + CP: P < 0.001; deprived vs. deprived + CP: P = 0.5, two-way repeated-measures ANOVA). These findings suggest that the agonist-independent activity of the mGlu1 receptor plays a pivotal role in homeostatic intrinsic plasticity through Ih.
Fig. 5.
Homeostatic modulation of Ih required agonist-independent activity of mGlu1 receptor. A: representative traces (left) and summarizing graph (right) showing that inverse agonist of mGlu1 receptor, BAY, prevented homeostatic downregulation of Ih. B: representative traces (left) and summarizing graph (right) showing that there were no effects of antagonizing mGlu1 receptor by CP on homeostatic Ih modulation. Closed black square, BAY only (A) or CP only (B); closed gray square, deprived + BAY (A) or deprived + CP (B); open black square, control; open gray square, deprived. Control and deprived values are as described in Fig. 3. Asterisks in A marked by 2-way repeated-measures ANOVA, compared with deprived and deprived + BAY; *P < 0.05.
Upstream regulators of Ih have been identified, including PKA (Narayanan et al. 2010), Ca2+ calmodulin-dependent protein kinase (CaMKII) (Fan et al. 2005) and auxiliary subunit TRIP8B (Santoro et al. 2004). Although the Gq-type of G protein is primarily involved in the excitatory responses of cerebellar PCs, coupling of the mGlu1 receptor to GS protein activates adenylyl cyclase, and thus, cAMP accumulates resulting in the activation of PKA (Aramori and Nakanishi 1992; Sugiyama et al. 2008; Tateyama and Kubo 2006). Therefore, we asked whether PKA activation is required for the homeostatic control of firing rates in cerebellar PCs. Organotypic slice cultures were chronically treated with a 500 nM PKA inhibitor, (5R,6S,8S)-hexyl 6-hydroxy-5-methyl-13-oxo-6,7,8,13,14,15-hexahydro-5H-16-oxa-4b,8a,14-triaza-5,8-methanodibenzo[b,h]cycloocta[jkl]cyclopenta[e]-as-indacene-6-carboxylate (KT 5720), in the presence or absence of TTX. The blockade of the PKA activity with KT 5720 (KT) prevented the homeostatic intrinsic plasticity induced by chronic TTX treatment [Fig. 6A; firing frequency (Hz): control + KT = 29 ± 3.6, n = 7; deprived + KT = 31.9 ± 3.6, n = 7 at + 400 pA injection; control + KT vs. deprived + KT, P = 0.9; deprived vs. deprived + KT, P < 0.05, two-way repeated-measures ANOVA]. In addition, KT also prevented the reduction of Rin in TTX-treated neurons (Fig. 6B; control + KT = 90.6 ± 2.5 MΩ, n = 7; deprived + KT = 83.6 ± 4.8 MΩ, n = 7; control + KT vs. deprived + KT, P = 0.2, deprived vs. deprived + KT, P < 0.005, two-sample t-test). This observation indicates that PKA activation mediates homeostatic intrinsic plasticity in cerebellar PCs.
Fig. 6.
Homeostatic intrinsic plasticity was dependent on PKA pathway. A: representative traces (left) and bar graph (middle; at +400 pA injection) and plots (right) showing that treatment of PKA inhibitor KT 5720 (KT) prevented homeostatic intrinsic plasticity in cerebellar PCs. B: bar graph showing the reduced Rin was recovered by KT (500 nM). Closed black square, KT only; closed gray square, deprived + KT; open black square, control; open gray square, deprived. Control and deprived values are as described in Fig. 1. Asterisks in A marked by post hoc Tukey's test, pairwise comparison followed by 2-way repeated-measures ANOVA, compared with deprived and deprived + KT. Asterisks in B marked by 2-sample t-test; *P < 0.05, ***P < 0.001; n.s., no significance.
DISCUSSION
The present study describes a novel mechanism by which homeostatic regulation of intrinsic excitability in PCs is dependent on the mGlu1 receptor under chronic activity deprivation. Interestingly, homeostatic changes in neuronal excitability were prevented by the mGlu1 receptor inverse agonist but not the neutral antagonist indicating that the homeostatic control of intrinsic excitability might require agonist-independent mGlu1 receptor signaling. Agonist-independent activation of group I mGlu receptors is inhibited by the selective noncompetitive antagonists (also called inverse agonists) Bay and 2-methyl-6-(phenylethynyl)-pyridine (MPEP) (Ango et al. 2001; Hu et al. 2010). Furthermore, we pharmacologically showed that the PKA activity, which is downstream of the mGlu1 receptor (Aramori and Nakanishi 1992; Sugiyama et al. 2008; Tateyama and Kubo 2006) in cerebellar PCs, is involved in homeostatic intrinsic plasticity in cerebellar PCs. This study shows how cerebellar PCs regulate their output signals in a homeostatic manner.
The intrinsic excitability of cerebellar PCs is downregulated by chronic activity deprivation. At first glance, this result is contradictory to conventional homeostatic regulation in which prolonged inactivation of the network activity induces boosting the intrinsic excitability or excitatory synaptic drive (Desai et al. 1999; Galante et al. 2000; Jang et al. 2015). However, in GABAergic neurons, it has been observed that synaptic strength (Chang et al. 2010) and firing rates (Sun 2009) decrease in response to days-long inhibition of network activity. Our results are in agreement with previous studies in that cerebellar PCs are GABAergic neurons. This reduced excitability observed in cerebellar PCs after chronic activity blockade with TTX can induce disinhibition of the silenced cerebellar cortex which could efficiently increase the network excitability against chronic activity deprivation. It is not simple to maintain the stability of the neuronal network activity against activity perturbation because neural activity is rather dynamically modulated (Hengen et al. 2013; Keck et al. 2013). For this reason, 2-day inhibition of network activity by TTX has been widely used to induce homeostatic plasticity, however, neural activity can change day to day. Furthermore, the time course to induce plasticity in vivo is greatly different from in vitro. This is the first observation of homeostatic control in cerebellar PCs. However, in vivo models have not yet been postulated and tested. Starting with this study, an in vivo model of homeostatic plasticity in the cerebellar cortex could be developed. Consequently, it could be possible to explore dynamically regulated neural activity in an activity-dependent manner.
In the present study, we suggest that the agonist-independent activity of the mGlu1 receptor is required for the homeostatic intrinsic plasticity of cerebellar PCs. Even in the absence of an agonist, the mGlu1 receptor can be spontaneously activated, similar to other GPCRs (Ango et al. 2001; Roosterman 2014; Scheer et al. 1996). A previous study has shown that the intracellular protein Homer regulates the agonist-independent constitutive activity of the mGlu1 receptor by interacting with partner proteins, such as the SHANK and MAGUK proteins (Tu et al. 1999). Neuronal excitation during synaptic plasticity or manifestation of convulsive seizures results in expression of the immediate early gene Homer1a (Brakeman et al. 1997; Kato et al. 1997), and the interaction between the mGlu1 receptor and long-form Homer proteins, subsequently, is disrupted leading to the constitutive activity of the receptor (Ango et al. 2001). In our experimental conditions in which the network activity is totally suppressed by TTX, there is a minute possibility that Homer 1a is involved in the activity deprivation-driven reduction of excitability because neuronal depolarization commonly induces Homer1a (Minami et al. 2003) but is more rather due to the downregulation of the long-form Homers, which induces the agonist-independent activation of mGlu1 receptors in cerebellar PCs. Indeed, knock-down of Homer3 facilitates the mGlu1 receptor activity by shifting equilibrium between its inactive and active conformation (Ango et al. 2001). The complex receptor-intracellular protein interactions in response to chronic changes in network activity due to neural plasticity and/or pathological conditions need to be further investigated.
The level of GPCR activity, a functional readout of the group I mGlu receptor activity, has been determined by the balance between the inactive (R) and active (R*) form of the receptor (Chidiac et al. 1994). Conformational changes of the receptor from the R to R* are regarded as one of the underlying mechanisms of constitutive activity (Scheer et al. 1996). Alternatively, an increased density of GPCRs enhances the agonist-independent activity of the receptor through an increase in the absolute amount of R* (Smit et al. 1996). We observed that activity-deprivation elevates mGlu1 receptor α, and this may reflect strengthened GPCR signaling through an increased amount of the R* form of the receptor. In addition, when the PKA pathway, downstream of the mGlu1 receptor (Aramori and Nakanishi 1992; Sugiyama et al. 2008; Tateyama and Kubo 2006), is inhibited under activity-deprived conditions, homeostatic intrinsic plasticity is prevented, suggesting that activity deprivation activates PKA. Given that the mGlu1 receptor activates adenylyl cyclase by the coupling of the receptor to the GS protein and cAMP subsequently accumulates (Tateyama and Kubo 2006), we can conclude that the chronic blockade of network activity activates mGlu1 receptor signaling resulting in the downregulation of firing rates through the PKA pathway. When mGlu1 receptor is activated, various cell responses are induced through coupling to several types of G proteins. Coupling of mGlu1 receptor to Gq11 can lead to the accumulation of inositol 1,4,5-trisphosphate (InsP3) resulting in the activation of the protein kinase C (PKC) pathway (Francesconi and Duvoisin 2000; Tateyama and Kubo 2006). A previous study reported that PKC activation inhibits Ih (Brager and Johnston 2007; Reetz and Strauss 2013). This leads to an increase in intrinsic excitability which contradicts our observation. Although GS and Gq11 coupling is simultaneously and independently triggered by mGlu1 receptor activation, PKA is a more plausible upstream regulator of the elevated Ih. For this reason, we exclude the PKC pathway in homeostatic intrinsic plasticity in cerebellar PCs.
Various ion channels determine the active and passive electrical properties of neuronal membranes, including membrane potential and AP threshold, and contribute to the synaptic integration and firing fidelity. Synaptic stimulus and/or somatic depolarization modifies neuronal excitability by changing the composition and conductance of ion channels (Belmeguenai et al. 2010; Hyun et al. 2013). From a homeostatic viewpoint, ion channels are dynamically regulated (Desai et al. 1999) to acquire network stability, and accordingly, AP firing rates are tuned within suitable ranges. Given the decreased Rin in deprived neurons, we focused on Ih among ion channels that contribute to neuronal excitability. However, the activity of many other ion channels need to be measured. A previous study showed that visual deprivation changes Ithreshold not Vthreshold, and Rin which is in agreement with our results (Fig. 1, Table 1) (Nataraj et al. 2010). Hence TEA-sensitive delayed-rectifier type K+ channel (KV 2.1) are a possible candidate for determining the excitability. Although Ca2+-activated K+ channels can be involved in homeostatic intrinsic plasticity, these are excluded because they have less of an effect on Rin and Ithreshold (Belmeguenai et al. 2010).
HCN channels are widely expressed in several brain regions (Notomi and Shigemoto 2004), and they contribute to the regulation of neural activity. Because Ih generates a tonic inward current at resting state, it is known as a pacemaker to initiate neuronal oscillation and rhythmic burst activity (Jahnsen and Llinas 1984; Llinas and Jahnsen 1982; Mccormick and Pape 1990). A previous study showed that the pharmacological blockade of Ih modifies membrane bistability, thereby inducing the spontaneous quiescence period (Williams et al. 2002). This indicates that Ih maintains the membrane potential, which enables tonic AP activity even when the activity of PCs is disrupted by hyperpolarizing inputs. Given that Ih stabilizes the cellular membrane potential within an appropriate range by its unusual gating properties (Nolan et al. 2007), activity-dependent regulation of Ih will be essential for homeostatic plasticity in an activity-disturbance condition. Homeostatically elevated Ih reduces Rin leading to a dampened membrane deflection to given current stimulation, and this will act as a cellular stabilizer to preserve the membrane potential. Furthermore, a potentiated rebound potential keeps the membrane potential close to the AP threshold and consequently leads to ‘history-independent integration’ (Nolan et al. 2003). Therefore, we suggest that the activity of cerebellar PCs is fine-tuned by the consequences of Ih modulation when the network activity is deprived.
Homeostatic plasticity has been regarded as a key mechanism of disease initiation (Friedman et al. 2014). Chronic activity-deprivation reduces the firing rates of cerebellar PCs, and it could be related to cerebellar disorders including Friedreich ataxia and spinocerebellar ataxias (SCAs) because lowered excitability is linked to the cellular phenomenon of disease (Hourez et al. 2011). Given that the constitutive activity of GPCRs is correlated to various human diseases, this work provides insight into possible therapeutic targets though modulation of GPCR-mediated homeostatic intrinsic plasticity. In addition, we also provide insight into the cellular basis of homeostatic control of firing rates of cerebellar PCs, and this finding broadens the understanding of homeostatic intrinsic plasticity of the cerebellar cortex.
GRANTS
This study was supported by National Research Foundation of Korea (NRF) Grant funded by the Korean government (MSIP) 2012R1A5A2A44671346; Korean Health Technology R&D Project, Ministry of Health & Welfare (A120476); and the Basic Science Research Program through the NRF funded by Ministry of Education Grant 2013R1A1A2013184.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.G.S., S.-S.J., J.M.P., and S.J.K. conception and design of research; H.G.S. and D.C.J. performed experiments; H.G.S. and D.C.J. analyzed data; H.G.S., D.C.J., J.M.P., and S.J.K. interpreted results of experiments; H.G.S., J.M.P., and S.J.K. prepared figures; H.G.S., D.C.J., J.M.P., and S.J.K. drafted manuscript; H.G.S., S.-S.J., D.C.J., Y.J., W.C., J.M.P., and S.J.K. edited and revised manuscript; H.G.S., S.-S.J., D.C.J., Y.J., W.C., J.M.P., and S.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Misun Mun, Geehoon Chung, and Michael Chang for proofreading the manuscript and providing scientific advice.
REFERENCES
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411: 962–965, 2001. [DOI] [PubMed] [Google Scholar]
- Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron 8: 757–765, 1992. [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, Hosy E, Bengtsson F, Pedroarena CM, Piochon C, Teuling E, He Q, Ohtsuki G, De Jeu MT, Elgersma Y, De Zeeuw CI, Jorntell H, Hansel C. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci 30: 13630–13643, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraneck M, Idoux E. Reconsidering the role of neuronal intrinsic properties and neuromodulation in vestibular homeostasis. Front Neurol 3: 25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci 27: 13926–13937, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386: 284–288, 1997. [DOI] [PubMed] [Google Scholar]
- Campanac E, Daoudal G, Ankri N, Debanne D. Downregulation of dendritic I(h) in CA1 pyramidal neurons after LTP. J Neurosci 28: 8635–8643, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci 13: 1090–1097, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidiac P, Hebert TE, Valiquette M, Dennis M, Bouvier M. Inverse agonist activity of beta-adrenergic antagonists. Mol Pharmacol 45: 490–499, 1994. [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515–520, 1999. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci 6: 231–242, 2003. [DOI] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h). Nat Neurosci 8: 1542–1551, 2005. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc Natl Acad Sci USA 97: 6185–6190, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han MH. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344: 313–319, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante M, Nistri A, Ballerini L. Opposite changes in synaptic activity of organotypic rat spinal cord cultures after chronic block of AMPA/kainate or glycine and GABAA receptors. J Physiol 523: 639–651, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron 80: 335–342, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourez R, Servais L, Orduz D, Gall D, Millard I, de Kerchove d'Exaerde A, Cheron G, Orr HT, Pandolfo M, Schiffmann SN. Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci: 11795–11807, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JH, Park JM, Park S, Xiao B, Dehoff MH, Kim S, Hayashi T, Schwarz MK, Huganir RL, Seeburg PH, Linden DJ, Worley PF. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron 68: 1128–1142, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun JH, Eom K, Lee KH, Ho WK, Lee SH. Activity-dependent downregulation of d-type K+ channel subunit Kv1.2 in rat hippocampal CA3 pyramidal neurons. J Physiol 591: 5525–5540, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electroresponsiveness and oscillatory properties of guinea-pig thalamic neurons invitro. J Physiol 349: 227–247, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SS, Royston SE, Xu J, Cavaretta JP, Vest MO, Lee KY, Lee S, Jeong HG, Lombroso PJ, Chung HJ. Regulation of STEP61 and tyrosine-phosphorylation of NMDA and AMPA receptors during homeostatic synaptic plasticity. Mol Brain 8: 55, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci 20: 7238–7245, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Hirai K, Inokuchi K. vesl, a gene encoding VASP/Ena family related protein, is upregulated during seizure, long-term potentiation and synaptogenesis. FEBS Lett 412: 183–189, 1997. [DOI] [PubMed] [Google Scholar]
- Keck T, Keller GB, Jacobsen RI, Eysel UT, Bonhoeffer T, Hubener M. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron 80: 327–334, 2013. [DOI] [PubMed] [Google Scholar]
- Lambo ME, Turrigiano GG. Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity. J Neurosci 33: 8810–8819, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Royston SE, Vest MO, Ley DJ, Lee S, Bolton EC, Chung HJ. N-methyl-d-aspartate receptors mediate activity-dependent down-regulation of potassium channel genes during the expression of homeostatic intrinsic plasticity. Mol Brain 8: 4, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litschig S, Gasparini F, Rueegg D, Stoehr N, Flor PJ, Vranesic I, Prezeau L, Pin JP, Thomsen C, Kuhn R. CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol Pharmacol 55: 453–461, 1999. [PubMed] [Google Scholar]
- Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurons in vitro. Nature 297: 406–408, 1982. [DOI] [PubMed] [Google Scholar]
- Mccormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurons. J Physiol 431: 319–342, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami I, Kengaku M, Smitt PS, Shigemoto R, Hirano T. Long-term potentiation of mGluR1 activity by depolarization-induced Homer1a in mouse cerebellar Purkinje neurons. Eur J Neurosci 17: 1023–1032, 2003. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Takeuchi H, Imai T, Saito H, Kiyonari H, Abe T, Chen M, Weinstein LS, Yu CR, Storm DR, Nishizumi H, Sakano H. Agonist-independent GPCR activity regulates anterior-posterior targeting of olfactory sensory neurons. Cell 154: 1314–1325, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Dougherty KJ, Johnston D. Calcium store depletion induces persistent perisomatic increases in the functional density of h channels in hippocampal pyramidal neurons. Neuron 68: 921–935, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj K, Le Roux N, Nahmani M, Lefort S, Turrigiano G. Visual deprivation suppresses L5 pyramidal neuron excitability by preventing the induction of intrinsic plasticity. Neuron 68: 750–762, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naude J, Cessac B, Berry H, Delord B. Effects of cellular homeostatic intrinsic plasticity on dynamical and computational properties of biological recurrent neural networks. J Neurosci 33: 15032–15043, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Dudman JT, Dodson PD, Santoro B. HCN1 channels control resting and active integrative properties of stellate cells from layer II of the entorhinal cortex. J Neurosci 27: 12440–12451, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin DQ, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 115: 551–564, 2003. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol 471: 241–276, 2004. [DOI] [PubMed] [Google Scholar]
- O'Leary T, Williams AH, Franci A, Marder E. Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron 82: 809–821, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reetz O, Strauss U. Protein kinase C activation inhibits rat and human hyperpolarization activated cyclic nucleotide gated channel (HCN)1-mediated current in mammalian cells. Cell Physiol Biochem 31: 532–541, 2013. [DOI] [PubMed] [Google Scholar]
- Roosterman D. Agonist-dependent and -independent dopamine-1-like receptor signalling differentially regulates downstream effectors. FEBS J 281: 4792–4804, 2014. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. J Neurosci 26: 3229–3244, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci 24: 10750–10762, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer A, Fanelli F, Costa T, De Benedetti PG, Cotecchia S. Constitutively active mutants of the alpha 1B-adrenergic receptor: role of highly conserved polar amino acids in receptor activation. EMBO J 15: 3566–3578, 1996. [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52: 475–484, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit MJ, Leurs R, Alewijnse AE, Blauw J, Van Nieuw Amerongen GP, Van De Vrede Y, Roovers E, Timmerman H. Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc Natl Acad Sci USA 93: 6802–6807, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmler M, Koch C. How voltage-dependent conductances can adapt to maximize the information encoded by neuronal firing rate. Nat Neurosci 2: 521–527, 1999. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Kawaguchi SY, Hirano T. mGluR1-mediated facilitation of long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. Eur J Neurosci 27: 884–896, 2008. [DOI] [PubMed] [Google Scholar]
- Sun QQ. Experience-dependent intrinsic plasticity in interneurons of barrel cortex layer IV. J Neurophysiol 102: 2955–2973, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron 55: 648–661, 2007. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Kubo Y. Dual signaling is differentially activated by different active states of the metabotropic glutamate receptor 1alpha. Proc Natl Acad Sci USA 103: 1124–1128, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23: 583–592, 1999. [DOI] [PubMed] [Google Scholar]
- Williams SR, Christensen SR, Stuart GJ, Hausser M. Membrane potential bistability is controlled by the hyperpolarization-activated current I(H) in rat cerebellar Purkinje neurons in vitro. J Physiol 539: 469–483, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]