Abstract
Active sensing behaviors reveal what an animal is attending to and how it changes with learning. Gymnotus sp., a gymnotiform weakly electric fish, generates an electric organ discharge (EOD) as discrete pulses to actively sense its surroundings. We monitored freely behaving gymnotid fish in a large dark “maze” and extracted their trajectories and EOD pulse pattern and rate while they learned to find food with electrically detectable landmarks as cues. After training, they more rapidly found food using shorter, more stereotyped trajectories and spent more time near the food location. We observed three forms of active sensing: sustained high EOD rates per unit distance (sampling density), transient large increases in EOD rate (E-scans) and stereotyped scanning movements (B-scans) were initially strong at landmarks and food, but, after learning, intensified only at the food location. During probe (no food) trials, after learning, the fish's search area and intense active sampling was still centered on the missing food location, but now also increased near landmarks. We hypothesize that active sensing is a behavioral manifestation of attention and essential for spatial learning; the fish use spatial memory of landmarks and path integration to reach the expected food location and confine their attention to this region.
Keywords: spatial learning, active sensing, attention, weakly electric fish, landmark-based learning
many animals actively explore novel surroundings. Exploration involves both orienting movements toward salient objects (e.g., food) and stable spatial features (landmarks) and active sensing associated with these movements (Fonio et al. 2009; Gordon et al. 2014; Poucet and Herrmann 2001; Sofroniew and Svoboda 2015). For many vertebrates, exploration and active sensing are associated with spatial learning and result in a neural representation of their environment, often referred to as a spatial map (Alvernhe et al. 2012; Itti and Koch 2001; Knudsen 2007). Primates at least can select specific locations for detailed visual exploration, and this selection process is said to constitute “spatial attention” (Itti and Koch 2001; Knudsen 2007). Human psychophysical experiments have revealed a direct connection between active sensing, i.e., saccadic eye movements, and attention (Itti and Koch 2001; Knudsen 2007; Krauzlis et al. 2013; Schroeder et al. 2010).
Active sensing has previously been demonstrated in “wave type” electric fish, where specific movement patterns are associated with optimal prey capture (MacIver et al. 2001) and tracking behavior (Clarke et al. 2015; Stamper et al. 2012). Here we study spatial learning in relation to active electrosensing in a pulse type weakly electric fish (WEF). Pulse-type WEF, such as Gymnotus sp., actively and discretely electrosense their near environment by generating electric organ discharge (EOD) pulses (∼1 ms) at variable rates (∼20–150 Hz) and sensing spatially localized object-induced distortions (electric images) of the EOD-generated electric field with a cutaneous array of electroreceptors (Caputi et al. 2008). The electrosense is short range (see below), and it is, therefore, possible to more directly link the animal's exploratory (Early learning) and directed (Late learning) trajectories to the active sensing that occurs at specific locations during learning. We use the learning-related changes in active sensing to hypothesize that these fish have a cognitive process analogous to primate attention, and that, by observing active electrosensing, we can infer changes in the animal's foci of attention during learning.
We investigate how EOD sampling in these fish adapts over the course of learning by monitoring their movement trajectories and EOD rates (EODRs) during a food-searching task. To address how these animals use landmarks to navigate at different stages of learning, we trained them to find food placed at a fixed location in a large circular tank (1.5-m diameter) containing four distinct stable landmarks (e.g., plastic cylinders). After task performance reached a stable plateau, we removed food in randomly selected Probe trials and monitored the animals' navigation and EOD to observe how sampling, and putatively spatial attention, might be affected by the memory of landmark and food location.
In the results and discussion, we refer to the fish learning a “spatial map.” By this, we simply mean that they learn to estimate the trajectories (direction and distance) that effectively take them from their home location or a landmark to the food. We do not mean to imply that they have a “cognitive map” and can, e.g., devise novel short cuts from any point in the test arena to the food or their home location. Getting a deeper appreciation of the extent of these fish's spatial knowledge will require additional experiments beyond the scope of this study.
MATERIALS AND METHODS
Animals
All procedures, including housing and behavioral experiments, were approved by the University of Ottawa Animal Care Committee. We obtained South American pulse-type WEF (genus Gymnotus) of unknown species and sex from a local supplier (Big Al Aquarium Services, Ottawa, ON, Canada). Animals were initially housed in community tanks under 12-h light cycle and fed live blackworms. Prior to the behavioral experiments, four animals were transferred to the experimental chamber (Fig. 1) and individually housed in the corner compartments for at least 1 wk prior to starting the first trial. The length of fish ranged between 13.9 and 17.9 cm (Landmark trials: 15.5, 14.6, 13.9, 14.7 cm; Control trials: 15.9, 17.2, 15.9, 17.9 cm). Animals were fed live mealworms in their home compartments during the habituation period for familiarization; mealworms were used during the spatial experiments instead of blackworms, since mealworms were easier to restrain on suction cups using elastics. The tank water was maintained at 25 ± 1°C, pH 6.5–7.5 and 100 ± 10 μS/cm to closely match the natural habitat for these animals (Knudsen et al. 1975). Air ventilation was installed to expel excess humidity build-up caused by heating. The experimental chamber was under 12-h reverse light cycle to observe nocturnal behaviors. Experiments were performed during the dark cycle under infrared illumination (860–880 nm) invisible to these species (Douglas and Hawryshyn 1990; Fernald 1988) to permit visual observation. Water filtration was performed in between daily experiments by eight vertical filters (Whisper Internal Power Filter 40i, Tetra), and water from all compartments was mixed together during filtration to homogenize odorants. The filters were taken out of the central testing arena during experiments to prevent animals from using them as spatial landmarks.
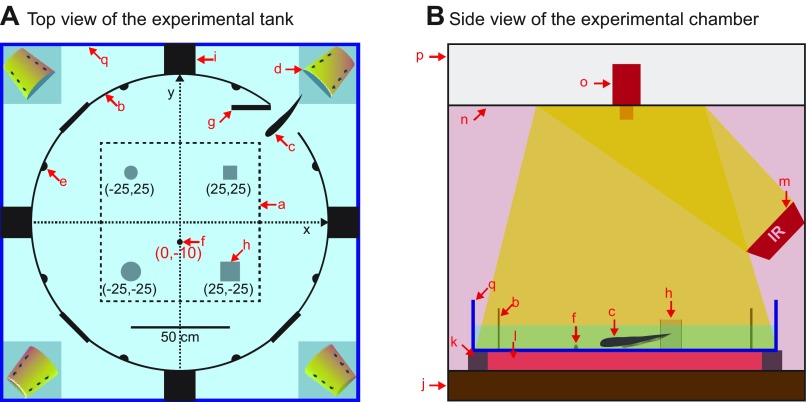
Fig. 1.
Overview of the experimental setup. A: top view of the experimental tank. The tank can house an individual in each of the four corner compartments, and the central arena was used as a testing area. Each compartment is watertight from the neighboring compartments to prevent the EOD of fish in their compartment from acting as a spatial cue for the fish learning the mealworm location. All analyses were performed in the active zone (a) positioned at least 15 cm away from the wall (b). Remotely controlled gate (g) lets an animal enter the central arena from the corner compartment. Red arrows indicate following objects. q, Glass tank (1.8 m × 1.8 m × 0.3 m); b, circular fence (heat-formed acrylic sheet); c, test fish; d, home base; e, one of eight graphite electrodes to measure the EOD; f, live mealworm restrained on a suction cup; g, remotely controlled gate; h, one of four landmarks; i, compartment separator. B: side view of the experimental chamber shows the aquarium tank (a) surrounded by the experimental chamber (p). The chamber wall (p) blocked external sources of light, sound and RF noise. The walls (p) were filled with fiberglass batts for soundproofing and surrounded by a Faraday cage. The chamber rested on multiple layers of vibration-absorbing materials (j). The aquarium was supported by aluminum frames on the edges (k) and was uniformly heated from below by a floor heater (l). Eight infrared illuminators (m) uniformly lit the central arena by reflecting off the ceiling (n). The ceiling panel hid an infrared-sensitive camera (o) to prevent its reflection on the water surface.
Experimental Setup
Video introduction of our experimental setup and further technical details are available from our laboratory's previous papers (Jun et al. 2014a; 2012; 2013). We constructed a glass tank (1.8 m × 1.8 m × 0.3 m) sufficiently large for animals to require spatial learning to efficiently find food. The tank rested on multiple layers of vibration-absorbing materials to minimize external vibratory stimuli. Fish swam in shallow water (10 ± 1 cm depth) to facilitate video tracking by restricting the animal movements mainly to two dimensions. The water temperature was closely regulated by a thermostat to prevent temperature-dependent EODR fluctuations (Ardanaz et al. 2001). The power-line noise from the floor heater underneath the tank was blocked by the Faraday cage. The tank was surrounded by a sensory-isolation chamber to block external sources of light, sound and radio-frequency noise. The tank was partitioned into a central test area and four corner compartments (Fig. 1A). The tank housed up to four animals at a time, and each compartment was made watertight to prevent electrocommunication between neighbors during experiments.
The tank was equipped with remotely operated gates to allow the animals to enter the test area (Jun et al. 2014a). While the animals were not being tested, the gates were shut and locked by nylon screws that pressed against the rubber gaskets. No metallic parts were used under the water, since our animals reacted sensitively to metal, which have higher conductivities than objects found in nature. The landmarks were built from a thin (1.6 mm) transparent acrylic sheet, and suction cups were attached below to fixate the landmarks on the glass floor. Four types of landmarks were built: two squares (5.6 and 9.0 cm/side) and two circles (7.6 cm and 10.2 cm diameter). White grid paper (5 cm spacing) was attached underneath the glass bottom to guide landmark and food placements, and to enhance the contrast.
EOD and Video Recordings
EOD signals were reliably and accurately captured by eight graphite electrodes (Mars Carbon 2-mm type HB; Staedtler, Germany) attached on the circular wall (Jun et al. 2012). The raw signals were amplified (30–100 × gain) and high-pass filtered (cut-off frequency = 300 Hz, second-order Butterworth). Lower gain was used for larger fish, which produced stronger EOD amplitudes, to prevent amplifier saturation. Four differentially amplified channels were digitized (CED mkII; Cambridge Electronic Design). The digitized signals were rectified, summed and root-mean-squared filtered to produce a unimodal pulse shape for accurate pulse timing detection (Jun et al. 2012). Video recordings were made by a USB camera after removing an infrared blocking filter (C910; Logitech). The camera provided adequate resolutions (1,600 × 1,200 or 1.4 mm/pixel) and frame-rate (15 frames/s) for accurate body tracking. The video recordings were synchronized to the concurrent EOD recordings by capturing a periodically blinking infrared LED (10-s intervals and 100-ms duration).
Automated movement tracking was performed by a custom-written Matlab script (Jun et al. 2014a), which reliably tracked the animals' head containing the highest density of electroreceptors on their body, thus serving as an electrosensory fovea (Bacelo et al. 2008; Carr et al. 1982; Castello et al. 2000). As animals swam forward, the tip of the head approached objects first. Our automated video-tracking software reliably discriminated the head from the tail (Jun et al. 2014a), and each automated tracking results were visually confirmed and manually corrected in the case of rare errors. Video tracking was performed from the moment the animal completely entered the test area until it found food, i.e., came within 1 cm of the food. The video-tracking data and the EODRs were aligned in time by resampling (spline interpolation) at 100 samples/s (Supplemental Video S1; Supplemental material for this article is available online at the journal website).
Experimental Protocol
Animals were trained to find a live mealworm restrained at a fixed location, and four unique landmarks were placed at stable locations to aid navigation. Before each trial, a single live mealworm was fastened on a suction cup using an elastic harness and attached to the glass bottom; the suction cup prevented contact of the mealworm and tank bottom. This was done to eliminate the chemical cues that might be created by such contact. In all experiments, and especially in the Probe trial (no food) experiments described below, we took extensive additional measures to eliminate possible chemical cues; we thoroughly vacuumed the tank bottom over a large region surrounding the area where the suction cup had been located so as to be sure that no remnants of the worm remained.
Trials were repeated up to four times per animal each day, and trials exceeding 15 min were aborted when animals failed to find the food. After successful feeding or at the end of the trials, we urged animals to get back to their home compartments by blinking visible light using eight bright LED lights attached on the circular wall. Concurrently, the gate was rapidly opened and closed to generate waves to encourage animals to get back. After each trial, remaining food particles were thoroughly removed, if found, to eliminate potential chemical cues as to the food location. Four animals were tested daily per experimental cycle, yielding up to 16 trials per day (4 trials/animal). Landmarks were positioned in a rotationally symmetric manner for all animals. Probe trials were conducted after the learning performance reached a stable plateau by removing the food and suction cup. We ran one Probe trial per day; for each fish, this trial was randomly selected from one of the four trials ran on that day (for the other three trials, food was present). A total of four Probe trials were tested per animal, and each trial lasted 5 min. After completing the training using four stable landmarks, we performed two additional Control trials with four new animals each. The control experiments were identical except that the None group was trained to find food located at a fixed location without any landmarks, using the same 12-session protocol.
Data Analysis and Statistical Tests
The spatial analysis was conducted in the 80-cm-square area in the center of the arena. This “active zone” excluded locations near the circular wall containing multiple features such as recording electrodes or gates which could act as landmarks. The separation distance between the wall and the perimeter of active zone was at least 18 cm, which is greater than typical body length of fish. Map-based analysis was conducted by binning the trajectories falling in the same grids (20 × 20 pixels or 2.7 cm square). We computed various statistics per grid point, such as the number of times animals revisited each grid location. We calculated the mean distance between successive interpulse intervals (IPIs) per grid point to determine the sampling density, defined as the average number of sensory samples per unit distance traveled (no. of EOD pulses/cm). We pooled data from four animals belonging to the same group unless otherwise stated. The significance tests were conducted in a manner appropriate to each data set: two-sample t-test (two-tailed), the nonparametric Kolmogorov-Smirnov (KS) test, two-proportion z-test, or Kruskal-Wallis test, as noted in each case; corrections for multiple comparisons were made where appropriate. The standard errors (SE of the mean) were determined by bootstrap sampling for independently sampled data.
In the learning curve analysis, the total distance traveled per trial measures the path length of the head trajectory from the entrance into the arena to within 1 cm of the food, and the task duration was computed as the associated time interval. At this distance, the fish always clearly detected the food, as shown by a large increase in EODR and subsequently eating the mealworm. Early trials are defined as the first two sessions when the fish was unfamiliar with the tank spatial layout (32 trials from 4 animals); and Late trials are defined as the late six sessions (96 trials from 4 animals) when performance had reached asymptotic values. Transition periods between the Early and Late trials were not analyzed.
Since each of the four animals entered the test arena from different gates, the landmarks were arranged symmetrically to produce identical relative positions with respect to the gate for each animal. This was done by rotating the four different landmarks 0, 90, 180, or 270° for each animal, with the gate always located near the top right corner of the active zone. The landmarks were repositioned before starting trials for each animal. This procedure enabled pooling the trajectories across the four animals to generate pseudo-color maps, such as Fig. 3, B and D.
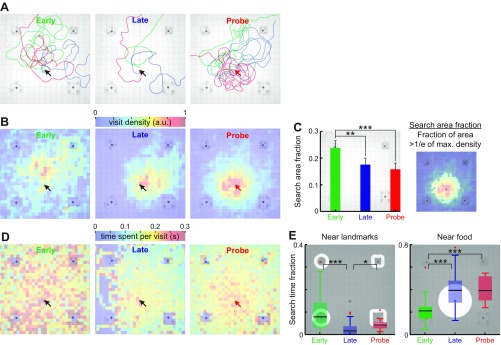
Fig. 3.
Trajectory patterns change with learning. A: three example trajectories from the Early, Late and Probe trials. No food was placed during Probe trials. Trajectories are plotted once the fish has left the edge of the tank. Note that, even during Late trials, the fish will take a curved route to the food, and that its final angle of approach to the food is very variable. The trajectories during Probe trials are clearly centered on, or near, the food location of the previous trial. Blue box indicates one of the landmarks. Throughout this figure, black arrows indicate the food location, and the red arrow indicates the missing food location. B: visit density maps quantify the number of times animals visit each grid location and are plotted here for the Early, Late, and Probe trials. The pseudo-colors indicate the visit count density per grid location (2.7 × 2.7 cm2 square) and was normalized by the maximum count value across all grid locations after pooling trajectories from all animals. C: the search area fraction is defined as the fraction of area above threshold in the visit density map (right). The search area fraction was computed for each visit density map (mean ± SE) and compared between Early, Late and Probe trials. D: pseudo-colors represent average time spent in each grid location per visit for the Early, Late and Probe trials. Black arrows indicate the food location, and a red arrow indicates a missing food location. E: search time fraction is defined as fraction of time spent in a region of interest (unmasked image portion). Search time fractions were plotted near all landmarks (within 3 cm from the object edges) or near food (within 15 cm from the food center). Box plot represents the median, interquartile range and extrema (red crosses indicate outliers). *P < 0.05. **P < 0.01. ***P < 0.001.
It was necessary to exclude outlier trials in the Early and Late learning conditions to obtain meaningful statistics. In particular, the initial direction of the fish was very variable in the Early trials. If the fish initially headed straight forward, it might encounter the food quickly; if it initially followed the wall of the tank, it might never find the food. We thus eliminated those trials (top 1/8th and bottom 1/8th of the distance outliers). The region-based statistics were not strongly affected by excluding the outlier trials, since the animals stayed near the wall (outside of the active zone) in the majority of the time during the top outlier trials. Subsequent analyses were performed within the active zone (80-cm-square area in the center), which is at least 18 cm away from the wall. Likewise, excluding the bottom outlier trials did not strongly affect our conclusion since they typically lasted <5 s and lead straight to the food location. All of the subsequent analysis excludes these outlier trials.
During the Late learning condition, animals completed the tasks ∼2.7× faster than the Early learning condition, thus we pooled 3× more number trials to obtain spatial statistics. Our conclusions did not significantly change with a varying number of sampling size. For example, randomly dropping 10% of trials did not significantly change the visit density (see Fig. 3C) compared with the original results (n = 10, P < 0.05, two-proportion z-test with Bonferroni correction).
We could not use the intermediate trials (3rd to 5th sessions) because it was clear from preliminary inspection of the data that there was a great deal of variability within and across fish. Some fish learned very rapidly, but, when this occurred, it varied across fish. Thus the data were clearly nonstationary and, therefore, not suitable for statistical analyses. This set the first two sessions for the “Early learning” condition. We set six sessions after the intermediate trials for the “Late learning” to be absolutely certain that our fish had reached asymptotic behavior.
We wanted to limit the total experimental time for practical reasons: these experiments took a very long time, and the fish kept growing over this time. We performed four Probe trials per animal as a compromise because it did limit the overall experiment duration, and our preliminary analyses showed us that we got very consistent results over these sessions.
RESULTS
Our laboratory has previously demonstrated that, in the dark, Gymotus sp. alternates between periods when it is moving vs. resting motionless. We refer to these periods as Up and Down states respectively; the fish's EODR is ∼12 pulses/s higher than during the Down states (Jun et al. 2014b). Our studies were carried out during Up states.
In this paper, we first demonstrate that Gymnotus sp. can learn the location of food in the dark. We then go on to analyze how active sensing, both electrosensing and scanning movements, might relate to spatial learning. Finally, we investigate the potential role of landmarks in spatial learning that utilizes a range-limited sense.
Evidence of Spatial Learning in Gymnotus sp.
Spatial learning curves.
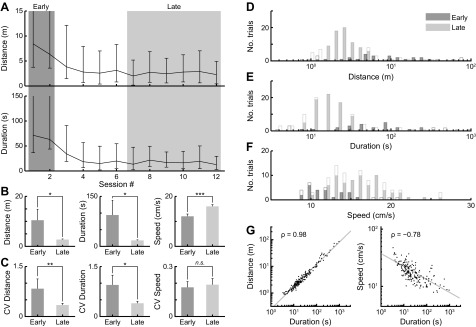
All animals (n = 4; 192 trials total) reached stable performance measured by the distance traveled and duration taken to find food (Fig. 2A). Asymptotic performance was reached after four sessions of four trials each. We compared task performance between the Early and Late trials (see materials and methods). Consistent with the learning curve (Fig. 2A), the distance traveled and the task duration significantly decreased from the Early to Late trials (Fig. 2B). To prevent outliers from skewing the results, all of the subsequent analyses excluded trials falling in the extreme ranges (below 12.5th or above 87.5th percentiles) in the distance distribution (Fig. 2D). The total number of excluded trials, out of a total of 32 trials, were 8 for fish A, 3 for fish B, 14 for fish C, and 7 for fish D. The average swim-speed (distance/duration) significantly increased with learning (Fig. 2B, right), while both the distance and duration decreased. The three behavioral measures (distance, duration and speed) exhibited high pairwise correlations (Fig. 2G) and agreed closely with each other; we assume that these measures are indicative of spatial learning and, therefore, use them for quantifying the spatial learning performances. We chose the distance-traveled measure to represent learning performance, since it did not directly depend on the swim speed. The variability of task performances (distance and duration), quantified by the coefficient of variation (CV), also decreased with learning (Fig. 2C), indicative of more consistent and reliable task performance after learning.
Fig. 2.
Performance improves with learning in the food searching tasks. A: animals learned to retrieve food quickly after about four sessions (16 trials), as indicated by the distance traveled (top) and the task duration plots (bottom). These plots (median ± interquartile range) indicate improvements in task performance over learning until the animal reaches asymptotic performance during the Late learning trials. Each session contains four trials/animal, and four animals were pooled to compute the mean (as in all the figures below). The first two sessions are defined as Early trials and the last six sessions as Late trials. B: mean task performances (distance, duration, and speed) are compared between Early and Late trials, indicating significant changes after learning (pairwise KS test). C: coefficient of variation (CV, mean/SD) of the task performances are compared between Early and Late trials. Error bars indicate bootstrapped SE of the mean. *P < 0.05. **P < 0.01. ***P < 0.001. D–F: distributions of the task performances from all trials are indicated as open bars (Early trials: darker shade, Late trials: lighter shade). We also discarded outlier trials based on the distance traveled (below 12.5th or above 87.5th percentiles), and the remaining trials are indicated as solid bars. G: the strong correlations between task performances (distance vs. duration on left, and speed vs. duration on right) indicate large consistencies between different learning performance measures. Gray lines indicate the best fit, and ρ indicates correlation coefficients.
These results demonstrate that Gymnotus sp. can be trained to reliably perform spatial tasks; after learning they find the food by swimming faster and over shorter travel times and trajectories. We argue below (see discussion) that these fish learn a spatial map mainly based on short-range electroreception.
Visit density changes with spatial learning.
We analyzed the swim trajectories (head position, see materials and methods) from each trial to describe the location-specific exploratory behaviors. Figure 3A shows three example trajectories at various stages of learning (Early, Late and Probe trials). The example trajectories for the Early and Late trials are shown from the moment animals entered the active zone (80-cm-square zone shown in the background, see materials and methods) until the animals found food. Although the Probe trials lasted 5 min, only the first 20 s were shown as examples for visual clarity and to illustrate the initial search locations. The search trajectories in the Early trials appeared indirect and random, but the Late trials exhibited shorter trajectories toward the food location, and the distance traveled was more consistent across trials (Fig. 2, B and C).
After the learning performances stabilized (Late trials), Probe trials (no food) were conducted. During the Probe trials, animals frequently crossed where the food had been previously located. This spatial preference is what accounts for the decreased search area fraction (Fig. 3, B and C) and increased time spent near the prior food location (Fig. 3D), thus strongly implying a spatial memory of the expected food location. The trajectories from four animals were pooled to reveal the frequency of visiting various locations (visit density). Figure 3B illustrates visit density maps at various stages of learning, and the pseudo-colors indicate the number of times animals visited each grid location (2.7-cm-square per grid). The visit density measure is insensitive to the variations in swim speeds, since each grid passing was counted only once per visit, regardless of the speed.
The visit density maps in Fig. 3B are consistent with the example trajectories in Fig. 3A. During Early trials, the visit density was initially broad, but it became more concentrated near the food location during the Late and Probe trials. The visit density during the Probe trials (n = 16) is more centered around the missing food location compared with the Late trials (n = 96), since the trajectory tracking was terminated as soon as animals found food in the Late trials, whereas, in the Probe trials, animals repeatedly visited the missing food location for the 5-min search period allowed.
Search area fraction measures the spatial spread of the visit density (Fig. 3C, right), by calculating a fraction of the number of grid points having visit density values above the threshold (defined as 1/e of the maximum visit density across all grid points). The Late and Probe trials were not significantly different and, therefore, were pooled for further statistical comparison. Early trials (n = 32) exhibited a large search area fraction (Fig. 3C, left) that significantly decreased (P < 0.01, two-proportion z-test with Bonferroni correction) after learning (96 Late and 16 Probe trials).
In summary, the visit patterns during food search became more confined after learning; they were also more sharply defined and were well centered on the expected food location (see below for quantification). The decreased distance and time to find food (Fig. 2, A and B) is, therefore, presumably due to more precise food location memory.
Search time spent at various locations changes with spatial learning.
Animals tend to slow down near the food location, so we quantified the average visit duration at various locations in Fig. 3D. The pseudo-colors represent the average time animals spent per visiting each grid location. The time per visit measure increases when an animal slows down while passing by a grid location. During the Early trials, the time per visit was generally higher than the later trials and especially high near the landmarks (Fig. 3D, left). The time per visit during the Late trials decreased globally across the map (Fig. 3D, middle), which is consistent with the increasing swim speed after learning (Fig. 2B, right).
Location-specific variations in the time per visit measure were quantified by the search time fraction, which divides the time spent near the landmarks (within 3 cm) or food (within 15 cm) by the total time spent in the active zone. The region of interest extends 3 cm from the landmark edges, because the animals began to increase their sensory sampling at this distance (see Fig. 5C). The search time fraction near the landmarks (Fig. 3E, left) significantly decreased (P < 10−3, 4 animals pooled, Kruskal-Wallis test with two-tailed Dunn's multiple-comparison post hoc test) after learning (from Early to Late). In contrast, the search time fraction near the food location (15 cm from the center of food) significantly increased (P < 10−4, 4 animals pooled, Kruskal-Wallis test with two-tailed Dunn's multiple-comparison post hoc test) after learning (Fig. 3E, right). The region of interest extending 15 cm from the food was sufficiently away from the detection range of the landmarks and captured most trajectories of near misses during food-searching attempts.
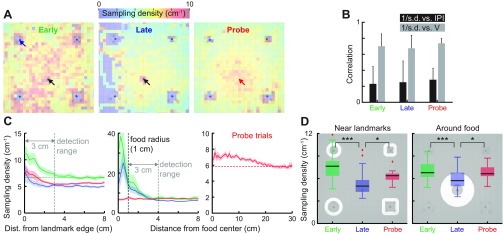
Fig. 5.
Sampling density becomes location dependent after learning. A: sampling density quantifies the number of EOD pulses per unit distance traveled, and plotted in pseudo-colors as a function of location for Early, Late and Probe trials. Black arrows indicate the food location, and a red arrow indicates the missing food location. B: Pearson correlation coefficient between the distances traveled between successive IPIs (= sampling density−1) vs. IPI or swim-speed. Distance/IPI is the reciprocal of the sampling density (#IPI/distance) and is used here to obtain positive correlations for easier interpretation (mean ± SD). C: sampling density is plotted as a function of distance (median ± SE) from the landmark edges (left) and the center of food location (middle and right) for three learning phases (Early: green; Late: blue; Probe: red). Green horizontal dashed line (left) indicates the mean baseline sampling density in Early trials at distant locations where the landmarks and food could not be sensed. Gray vertical dashed line (left) indicates the edge of the detection range (Caputi et al. 2013). Gray vertical dashed line (middle) indicates the edge of food. The high sampling density within the food detection radius are feeding related. Sampling density for Probe trials (right) is plotted at a greater distance scale to reveal a trend of increasing sampling as the animals approached a missing food location. D: the sampling density is compared between three learning phases near the landmarks (left) and food (right), as indicated by the background images. The sampling density significantly decreased from Early to Late trials, and also significantly increased in Probe trials. To rule out feeding-related increase in the EOD rate, the area within the food detection range is excluded (<4 cm from the center). Box plot represents the median, interquartile range and extrema (red crosses indicate outliers). *P < 0.05. ***P < 0.001.
These results demonstrate that the animals spent less time near the landmarks after they learned the food location but, instead, spent more time searching at the expected food location.
Heading direction error decreases with spatial learning.
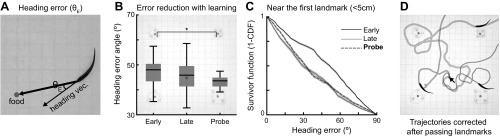
In addition to tracking learning-dependent changes in the locations and durations of visits, we examined how the direction of swimming with respect to the food location changed after learning. The heading error measures the acute angle between the heading direction (velocity vector of the head) and the direction toward the food location (Fig. 4A). The heading error ranges between 0 and 90°, and a zero heading error means fish swimming in parallel to the direction toward food. The heading errors decreased with learning (Fig. 4B), and the Probe trials (n = 16) exhibited significantly lower heading errors compared with the Early (P < 0.05, n = 32) trials (4 animals pooled, Kruskal-Wallis test with two-tailed Dunn's multiple-comparison post hoc test). The improvements in the direction of approach toward the expected food location might be explained by assuming that, after learning, the fish had improved spatial memory of the relation between its own location and that of the food. In support of this assumption, Fig. 4C shows that the heading errors were greatly reduced when the animals were near (<5 cm) the landmark located nearest to the entrance. Animals generally encountered this landmark first after entering the arena; thus we refer to it as the “first landmark.” We hypothesize that the reduction in heading error near the first landmark is due to the fish sensing this landmark, remembering its location relative to the food and changing its direction accordingly. Consistent with this hypothesis, we found that animals frequently changed their heading directions after encountering landmarks, as shown by three example trajectories (Fig. 4D). The region of interest extends 5 cm from the first landmark edges to account for delays between the initial landmark detection (3-cm detection range) and subsequent trajectory corrections.
Fig. 4.
Heading direction error is reduced after learning. A: heading error angle (θE) is defined as the acute angle between the heading vector and food vector and ranges between 0 and 90°. B: averaged heading error angle in the active zone (background image) was decreased after learning. This indicates that animals learned to approach the food location more accurately. Box plot represents the median, interquartile range and extrema. *P < 0.05. C: the heading error angle distribution near (<5 cm) the first landmark (i.e., nearest to the corner compartment from which the fish enters) is used to compute the survivor function via its cumulative density function (CDF). The survivor function shows an overall reduction of heading angle error in the late phases of learning. Narrow shaded bands represent bootstrapped SE of the mean (95% confidence intervals). D: three example trajectories are shown where the animals corrected their heading directions after encountering the landmarks.
These results strongly suggest that the animals learned the location of the food relative to the landmarks and so were able to use landmarks to determine their heading direction toward food. The fact that the fish were able to improve their heading angle from any direction further implies that, while navigating, they continuously update a representation of their location with respect to the landmark(s) and food; presumably, this occurs via a path integration mechanism (see discussion).
Active Sensing During Spatial Learning in Gymnotus sp.
Sampling density changes with spatial learning.
Above, we described learning-dependent changes in the visit locations based on movement tracking. We now analyze how the animals modulate their electrosensory sampling rates at various locations based on EOD recordings synchronized with movement tracking. For a moving-pulse WEF, there are two ways to acquire more electrosensory information at a given location: by slowing down while maintaining a constant EODR, or by increasing their EODR. The combination of the two results in shorter distance traveled between sequential EOD pulses. We, therefore, determined the number of sampling events (EOD pulses) per unit distance traveled, which we define as the sampling density. The sampling density is calculated by taking a reciprocal of the average distances per IPI and measures EODR normalized by the swim speed.
Factors contributing to the sampling density.
The sampling density maps (Fig. 5A) closely resembled the time per visit maps (Fig. 3D). This is expected since the sampling density is inversely proportional to the speed, thus proportional to the average time spent visiting each grid location. However, the EODRs also contribute to the sampling density. We thus examined how much the swim speed and IPI duration contributed to the sampling density. Figure 5B shows that both factors were positively correlated with the reciprocal of the sampling density (distance/IPI) in all animals and in all trials; but the swim speed had a higher correlation than the IPIs due to a greater dynamic range of the swim speed (CV = 20%) than that of the IPI (CV = 10%). The EODR always increased up to 100–200 Hz during food ingestion (Black-Cleworth 1970) even in the Early trials. This response was, therefore, at least partly a reflex response to food. Therefore, we separated these responses from those that vary with spatial learning. We conclude that the fish increase their sampling density by both spending more time near important locations (i.e., swimming more slowly near landmarks) and increasing their EODR at the same locations.
Figure 5A shows the map of sampling density at various learning stages (32 Early, 96 Late and 16 Probe trials pooled from 4 animals). The sampling density underwent location-dependent changes after learning. The sampling density during Early trials was generally higher than for later trials (Late and Probe trials), and it was especially high near landmarks. In comparison, Late trials show much reduced sampling density throughout, except at the food location where feeding-associated increases in the EODR occur (Black-Cleworth 1970). During Probe trials, animals increased their sampling density at the missing food location, which again suggests that the electrosensory sampling in Gymnotus sp. is influenced by a memory of the expected food location. The overall sampling density in the active zone significantly decreased after learning (P < 10−4, pairwise KS test), and the sampling density significantly increased from Late to Probe trials (P < 0.003, pairwise KS test).
The sampling density is plotted as a function of distance from the landmarks, food and the missing food location (Fig. 5C). Animals increased their sampling density within 3 cm from the edges of all landmarks (data pooled), suggesting a detection limit consistent with the estimated spatial range of electroreception of gymnotiform fish, including Gymnotus (Caputi et al. 2013; Snyder et al. 2007). Animals greatly increased the sampling density as they approached within the contact distance from food (<1 cm) during Early and Late trials (Fig. 5C, middle). Such increases were absent during Probe trials due to absence of food, but Probe trials still showed gradual increases in the sampling density as the fish approached the missing food, although over a greater distance scale with the increase clearly apparent at 15 cm from the food (Fig. 5C, right). The sampling density near the detection limit of landmarks (<3 cm) significantly decreased with learning (from Early to Late trials, P < 10−6, 96 trials from 4 animals), but significantly increased again in Probe trials (P < 10−4, 88 trials from 4 animals) (Fig. 5D, left). Similar differences were observed around the food location (4–15 cm from the food center); the sampling density significantly decreased from the Early to Late trials (Fig. 5D, right). The analysis excluded locations within the food detection limit (<3 cm from the edge of food) to exclude drastic increases in the EODRs during feeding. During Probe trials, the sampling density significantly increased (P < 10−3, 88 trials from 4 animals) around the missing food location (Fig. 5D, right), suggesting an enhanced sensory sampling at the expected food location.
In summary, during learning, electrosensory sampling behavior is concentrated near landmarks and food, but habituates after repeated trials; sampling behavior at both locations increases when the animals failed to locate food during Probe trials. In addition, the sampling density increases gradually (from 15 cm) as the animal approaches the missing food location. In agreement with our analysis of B-scans, this data strongly suggest that spatial memory can drive active electrosensing.
Locations of bidirectional swimming and spatial learning.
WEF often exhibit a stereotyped swimming pattern near novel objects (Hofmann et al. 2014; Lannoo and Lannoo 1992; MacIver et al. 2001; Toerring and Moller 1984): they swim forward, then backward and then forward again (va-et-vient movements) and sometimes repeat this pattern two or more times. These movements are believed to be an active electrosensory scanning behavior because the objects pass two or more times across their body's electroreceptor array; we have, therefore, used bidirectional scanning (B-scan) to refer to this form of active sensing. We note that, in the backward phase of a B-scan, the fish brings the object toward its head. The head of WEFs has the highest electroreceptor density and is considered to be functionally equivalent to the retinal fovea (Bacelo et al. 2008; Carr et al. 1982; Castello et al. 2000; Pusch et al. 2008); the backward phase of B-scans can, therefore, be considered as equivalent to foveation (see below).
We characterized the probability of B-scans at various stages of learning. Pseudo-color plots in Fig. 6A represent the B-scan probability per grid location in the active zone. B-scans occurred more frequently and broadly during Early trials, but the frequency and distribution of B-scans both diminished after learning (Late trials). During the Probe trials (n = 16), the animals exhibited B-scans near the missing food location and near the landmarks. The probability of B-scans significantly decreased near the landmarks (<3 cm) in Late trials (P < 10−3, 32 Early trials and 96 Late trials, 4 animals, Kruskal-Wallis test with two-tailed Dunn's multiple-comparison post hoc test) (Fig. 6B, left). However, relative to Late trials, the probability of B-scans significantly increased in Probe trials near the landmarks (<3 cm, Fig. 6B, left) and near the food (4–15 cm from the center, Fig. 6B, right). The area immediately near the food (<4 cm from the center) was excluded from the analysis due to feeding-associated B-scans.
Fig. 6.
Bidirectional swimming (B-scans) is location specific and dependent on learning. A: probability of B-scans was computed for each grid location and plotted for Early, Late and Probe trials. The locations of B-scans decreased from the Early to Late trials, but then increased markedly during Probe trials. Black arrows indicate the food location, and a red arrow indicates the missing food location. B: the probability of B-scans is plotted (mean ± SE) near all landmarks (left, <3 cm from the edges) or near the food location (right, 4–15 cm from the food center), as indicated by the masked background images. This probability was computed as an average of the backward swimming probability in the regions of interest. The probability of B-scans near landmarks significantly decreased after learning (from Early to Late). The probability of B-scans significantly increased in the Probe trials, indicating repeated scanning near the landmarks and the missing food location. C: the sampling density was significantly higher during backward swimming compared with the forward swimming in all three trial types (mean ± SE). D and E: the increase in the sampling density in A was due to simultaneous decreases in the swim-speed (D) and increase in the EOD rate (E). The same pattern was observed in all animals tested during all trial types. *P < 0.05. ***P < 0.001.
These observations suggest that the animals do not need extensive active sampling of the landmarks once they have learned the spatial relationship between landmarks and food. Furthermore, failures of finding food during Probe trials led to increased B-scans near the landmarks; we speculate that the animal is probing the landmark to determine where it might have gone wrong. Finally, increased B-scans near the missing food location are again strong evidence for the existence of spatial memory in Gymnotus sp. and for increased active sensing when there is a mismatch between sense input and a memory-based expectation. Since there is no worm present, spatial memory must be driving B-scans.
Enhanced sampling density during B-scans.
We now compare the sampling density during the back-swimming phase of B-scans. Figure 6C shows that the sampling density during backward phase of B-scans was significantly higher than during forward swimming (P < 0.05, 96 trials pooled from 4 animals, pairwise KS test). This was due to a simultaneous decrease in the swim speed (Fig. 6D) and increase in the EODR (Fig. 6E) during backward swimming. The same pattern was observed during all phases of learning (Early, Late and Probe trials), showing that animals always increased sensory sampling whenever they swam backward by significantly increasing the EODR and decreasing the swim speed. This is consistent with the idea that the backward-swimming phase of a B-scan is analogous to visual foveation of an object.
We conclude that there is dynamic coupling of two types of active sensing. The backward phase of B-scans and EOD sampling density are coupled, but EODR increases can also occur during forward swimming near landmarks and while approaching food.
EOD pulse bursts (E-scans) change with learning.
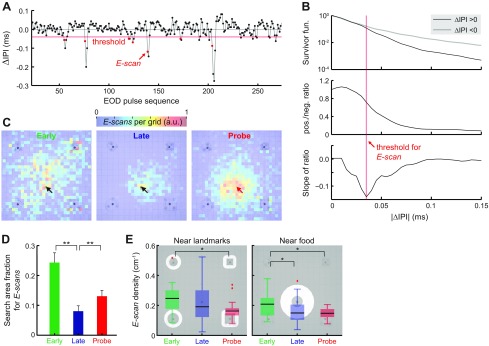
Freely swimming Gymnotus sp. spontaneously exhibit transient IPI shortenings (2–4 IPIs; ∼0.1–0.5 s) that are emitted in bursts (Fig. 7A). Since these bursts occurred in the context of active exploration, we define these events as electrosensory scans (E-scans). A threshold was defined to detect transient shortening in the IPIs based on the asymmetry in the ΔIPI (= IPIk − IPIk−1) distribution. The ΔIPI distribution exhibited a longer tail in the negative domain (Fig. 7B, top), as expected from the rapid IPI decreases followed by slower increases. The survivor functions of the positive and negative ΔIPI were initially similar, but they deviated at further ranges. The ΔIPI value is shown in Fig. 7B when the ratio between the positive-to-negative survivor functions decreased most rapidly according to the slope of the ratio curve (Fig. 7B, bottom). Since this value remained stable across individuals and over multiple days, it was used as a threshold to detect E-scan events. The analysis excluded threshold-crossing events that only lasted one cycle below the threshold to remove the false positives (Fig. 7A).
Fig. 7.
Locations and frequency of the E-scan events during spatial learning. A: E-scan events (red circles) are defined as transient shortenings of the EOD interpulse intervals (IPIs), i.e., they represent bursts in electrosensory sampling. The cycle-to-cycle changes in the IPIs (ΔIPIs) are plotted as the ordinate, and the IPI sequence order (IPI #) as the abscissa. EOD pulse events are indicated by black circles. E-scan events are indicated by red circles and were detected as onsets of falling below the threshold (red horizontal line) and remaining below it for at least two cycles. B: method of determining the E-scan threshold. The survivor functions (1 − cumulative density function) for the positive (black curve) and negative (gray curve) ΔIPIs are plotted in a semilogarithmic scale (top). Ratio between the positive and negative survivor functions (pos./neg. ratio) is plotted as a function of ΔIPI (middle). Slope of the pos./neg. ratio is plotted as a function of ΔIPI (bottom). The ΔIPI value minimizing this slope defines the detection threshold for E-scan events (red vertical line). C: E-scan maps (number of E-scans per grid) are plotted for the Early, Late and Probe trials. Black arrows indicate the food location, and a red arrow indicates the missing food location. D: the search area fractions for E-scans were plotted for the E-scan maps using the method illustrated in Fig. 3C. The fraction of areas having high E-scan rates (>1/e of the peak) significantly decreased with learning, but the area again widened during the Probe trials. E: E-scan density quantifies the number of E-scans per unit distance traveled. The E-scan density significantly decreased from the Early to Probe trials near the landmarks (<3 cm from the edges) and the food (4–15 cm from the center). The background images indicate the regions of interest. Box plot represents the median, interquartile range and extrema (red crosses indicate outliers). *P < 0.05. **P < 0.01.
Similar to the way sampling density is defined, distances between successive E-scan events were measured to compute the E-scan density, the number of E-scan events per unit distance traveled. Figure 7C shows that E-scans initially occurred throughout the active zone; after learning, they occurred almost exclusively near the food. The E-scan density during the Probe trials gradually increased toward the missing food location (Fig. 7C, right). This means that the E-scan density increased along each trajectory toward food. Similarly to the sampling density results (see above), this suggests that animals increased their electrosensory sampling at the location where they expected to find food. The fraction of areas having elevated E-scans per grid (>1/e of the peak) significantly decreased between Early to Late trials, but increased again during Probe trials (Fig. 7D, two-proportion z-test with Bonferroni correction). Figure 7E shows the E-scan densities (E-scans/cm) near the landmarks (<3 cm from the edges) and food (4–15 cm from the food center); they show significant decreases between Early and Probe trials (Kruskal-Wallis test with two-tailed Tukey's honestly significant difference post hoc test).
In summary, both measures of the electrosensory sampling (sampling density, E-scan density) were significantly reduced after spatial learning and also had a more focused spatial distribution near the food location. Furthermore, during Probe trials, both sampling and E-scans occurred over an expanded area compared with the Late learning trials. For both measures, sampling increased along trajectories toward the missing food location (Probe trials). We can again conclude that both forms of active electrosensory sampling are driven in a coordinated manner by spatial memory. However, there is a clear disconnect between E-scans and both sampling density and B-scans. Both latter measures increase at landmarks and food during Probe trials (compared with Late learning), whereas E-scans do not. We conclude that there must be at least partially independent control of these active sampling behaviors.
Landmarks Improve Animals' Estimation of Food Location
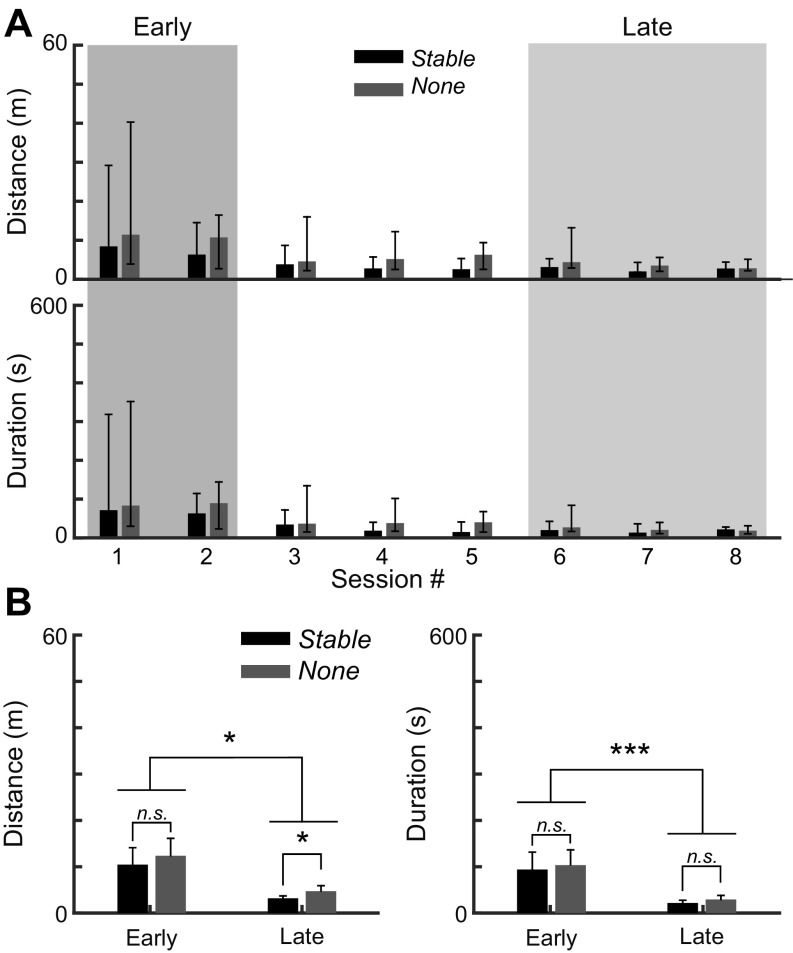
In the previous sections, we reported an increased visit and sampling density near the stable landmarks in the Early and Probe trials, when animals were less certain about their location relative to the food. To directly test whether stable landmarks play a role in spatial learning, we compared the landmark-trained group (Landmark group) with a control group trained as before with food in a stable location but without any within-tank landmarks (None group). We note that there are always landmarks at the tank boundary, but that these are further from the food than the within-tank landmarks. Four control animals (128 trials total) were trained, and the within-group data pooled. Robust learning occurred in both animal groups (Fig. 8A). In both Early and Late trials, the Stable group performed slightly better than the None group; this difference was significant for distance traveled, but not for the duration of search (Fig. 8B, Kruskal-Wallis test with two-tailed Dunn's multiple-comparison post hoc test).
Fig. 8.
Comparison of learning performances between animals trained with stable landmarks or without. A: animals trained with landmarks (Landmark group) demonstrated better learning performances than the group trained without landmarks (None group) in all sessions. Bar plots (median ± interquartile range) indicate the total distance traveled or the associated total duration of the food-searching task. B: Early training phase (first two sessions, 32 trials) was compared against the Late phase (last three sessions, 48 trials). Bar plots (mean ± SE) indicate significant improvements in the food-searching performance after learning in both groups (4 animals per group). The None group traveled significantly greater distance than the Landmark group in the Late phase. *P < 0.05. ***P < 0.001. n.s., Not significant.
Quantifying food location estimates.
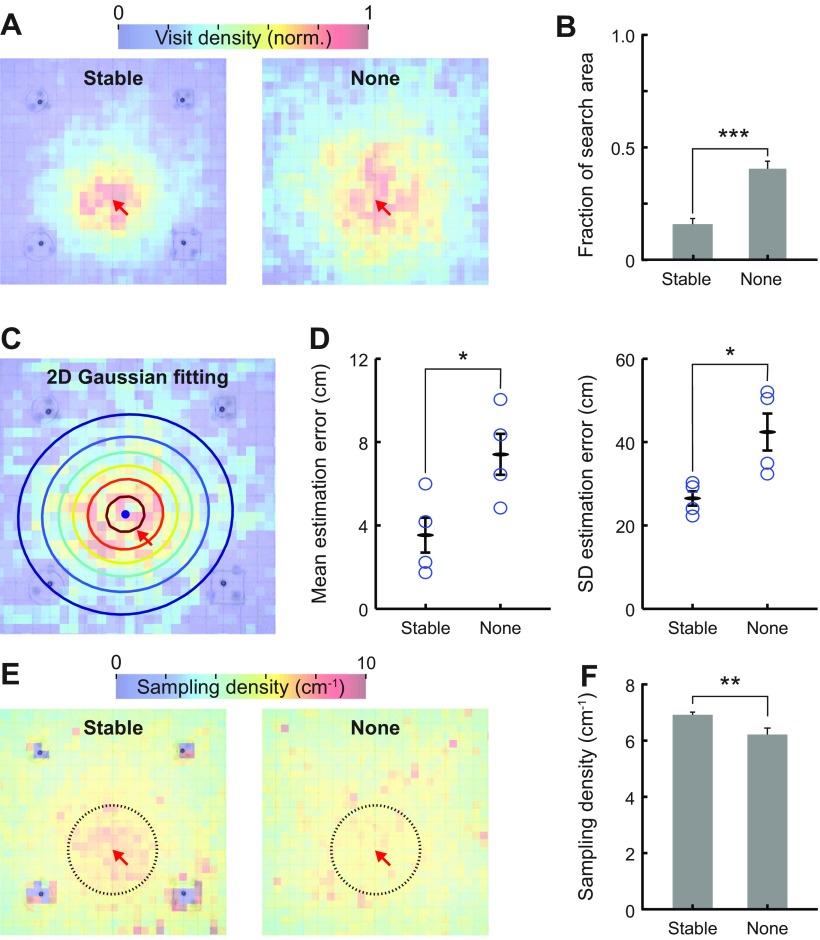
We were puzzled by the minimal differences in learning rates between the Landmark and None groups, given that we had shown that the fish sample at high rates near landmarks (Early learning, Figs. 5 and 6) and can reorient themselves toward food when they pass landmarks (Fig. 4, C and D). We, therefore, evaluated the visit density maps (Fig. 9A) during Probe trials. The control (None) group exhibited significantly greater fraction of search area than the Landmark group (two-proportion z-test, Fig. 9B), despite the lack of a difference in the learning curves (Fig. 8B). We next compared the accuracy of the remembered food location between the Landmark and None groups for these Probe trials. We fitted a two-dimensional Gaussian function described by six parameters (μx, μy, σx, σy, θ, A) to the visit density maps for each animal. Figure 9C shows an example of the two-dimensional Gaussian function fitted to a visit density map, which shows the center of all locations visited (a black dot), and the spread of the spatial distribution shown as a contour plot. Mean estimation error (bias) is defined as the distance between the center of locations visited and the actual location of missing food. The mean estimation errors were significantly lower (P = 0.041, two-sample t-test, 4 animals/group) in the Landmark group than the None group (Fig. 9D, left). Similarly, the spread of visit density [√(σx2 + σy2)] was significantly higher (P = 0.027, two-sample t-test, 8 animals) in the None group (Fig. 9D, right). The visit density spread can be considered as a measure of how precisely the fishes can estimate the food location; clearly, the fish have a better estimate in the Landmark vs. None condition. This analysis confirms that landmarks indeed aided animals to estimate food location more accurately and with less bias compared with the fish that did not have access to within-tank landmarks. This improvement likely did not show up in the learning curves because of high between-fish variability.
Fig. 9.
Landmarks promote efficient spatial search and sensory sampling. A: the visit density maps indicate that the Landmark group searched closer to the missing food location during Probe trials than the None group. Landmark group animals were trained with four landmarks presented at fixed locations; None group animals were trained without any landmarks. Four animals were pooled in each group. The normalization was done separately in each group. B: the search area fraction demonstrates that the group trained with stable landmarks has the most focused search area around the food location. C: two-dimensional (2D) Gaussian function (indicated by the contour plot) was fitted to the visit density map to estimate the center (blue circle) and spread of the search locations. D: the mean estimation error quantifies the distance between the missing food location and the center of the 2D Gaussian fitting. Landmark group showed lower estimation errors than the None group. The spread of search is quantified by the SD of the 2D Gaussian fit, and this was also lower in the Landmark group (circles indicate different individuals). E: sampling density maps indicate that the Landmark group increased their sampling density near the missing-food location, while the None group did not. F: bar plot (mean ± SE) indicates the sampling density near the missing-food location (<15 cm, indicated by the black circles in E). Landmark group had significantly higher sampling density than the None group in Probe trials. *P < 0.05. **P < 0.01. ***P < 0.001.
Comparison of sampling density.
Figure 9E shows the sampling density maps during Probe trials from the two groups. Landmark group showed a clear increase in sampling density near the expected food location, while the None group did not. To quantify this visually apparent difference, the mean sampling density throughout the active zone is compared between the groups in Fig. 9F. Landmark group exhibited significantly greater average sampling density compared with the None group (P < 0.01, 16 trials per group, two-sample t-test).
These results suggest that landmarks were used by the fish to accurately estimate the location of the food and then increase their active sampling (sampling density, E-scans, B-scans) at the expected food location. It appears that, when lacking within-tank landmarks, animals may have relied instead on edge landmarks and a stochastic search strategy within the active zone.
DISCUSSION
We have demonstrated that Gymnotus sp. is adept at spatial learning: they rapidly and accurately learn a food location after repeated exposures to an environment containing landmarks. The evidence for spatial memory includes efficient food searching (distance traveled and duration of trials, Fig. 2, A and B), focused search locations (Fig. 3, A–E), and accurate heading direction toward the food location (Fig. 4, B and C). Behavior of animals during Probe trials (absence of food) revealed important signatures of spatial memory, including repeated visits and accurate heading direction toward the missing food location.
Spatial learning and memory have been demonstrated in teleost (Fukumori et al. 2010; Reese 1989; Rodrigues et al. 1994; Warburton 1990), as well as cartilaginous fish (Schluessel and Bleckmann 2005); in these studies spatial cues (landmarks) sensed by the long-range visual system were available. Our experiments take place in the dark, and the Probe trials demonstrate that olfaction is not essential for retrieval of spatial memories. The immense numerical dominance of the EOD-responsive tuberous electroreceptors over passive ampullary electroreceptors and lateral line receptors (Carr et al. 1982), as well as the increased EODR and E-scans when approaching landmarks and the missing food, strongly suggest that it is the most important sense for spatial learning. We cannot, however, rule out a contribution of lateral line receptors in signaling the approach to the large landmarks (Windsor et al. 2010) or of ampullary electroreceptors signaling the presence of live food (Lonneke et al. 2008). In any case, all of these senses have a restricted spatial range (∼3 cm range) (Nelson et al. 2002). In all of the experiments, we took extensive measures to eliminate possible chemical cues from the food (mealworm, see materials and methods), and we were especially vigilant in this regard before Probe (no food) trials. We, therefore, conclude that the fish's trajectories on Probe trials cannot be attributed to chemical cues.
Given its limited range, electrosensory spatial learning is best compared with the active vibrissae sense of rodents. Vibrissae extend out to a similar distance (1.5–5.0 cm) (Towal et al. 2011) and are actively moved for sensing; their use in active sensing is also modified by spatial learning in a manner similar to that described above for active electrosensing (Gordon et al. 2014; Sofroniew and Svoboda 2015). In blind rats, the vibrissae sense supports spatial navigation and the formation of place fields in hippocampal cells (Save et al. 1998). Unlike the visual system, the vibrissae- and electro-sense must “piece together” multiple local (<3 cm) sense inputs to support navigation on a much larger scale. Below we suggest that, as is the case in blind rats, the WEF combine egocentric (path integration) and allocentric (landmark) input during learning, and that memory-based path integration becomes dominant after learning.
Spatial Navigation in the Dark: Landmarks and Path Integration
Extensive studies (Collett et al. 1986; Etienne et al. 1996, 1998; McNaughton et al. 2006) have shown that spatial learning in rodents typically depends on both path integration and visual acquisition of landmarks. Path integration requires that the animal depend on the memory of idiothetic input (e.g., vestibular, proprioceptive) (Kay 1993; Stackman et al. 2002; Terrazas et al. 2005; Wallace et al. 2002) to guide them. Errors accumulate during path integration, making it unreliable over long distances. Once the location of landmarks has been learned, it can correct path integration and, therefore, improve localization of important environmental features.
Spatial learning in WEFs can be readily understood in this framework. However, given the short range of the electrosense, even the location of landmarks must be learned via path integration. In the None control, the fish has features at the tank boundaries as landmarks; since its electrosense only extends ∼3 cm, it must, from memory, estimate the food location (direction and distance) over a distance of at least 80 cm and traverse this distance by path integration. With landmarks present, the distance between the tank boundary and landmarks drops to 35–51 cm and from landmarks to food to 24–39 cm. We hypothesize that this is the reason that fish with landmarks do better at estimating the food location than fish without landmarks. Finding food can be considered a problem in statistical estimation (Kay 1993); from this point of view, the fish with landmarks have far lower mean estimation error and far lower estimation variance (Fig. 9D). We hypothesize that this is because the path integration errors are lower since the landmarks permit recalibration after only a short distance from the tank boundaries. This view is reinforced by the frequent observation of fish choosing an accurate food-directed heading angle after encountering a landmark (Fig. 4D).
We further hypothesize that this is the basis of the increased active sensing (B-scans swimming, increased sampling density, increased E-scans) that is especially prevalent near landmarks and food in the early stage of learning. The fish are, we hypothesize, calibrating their path integration mechanisms by sampling their routes from the tank boundary to landmarks and from landmarks to food (Collett and Graham 2004; Etienne et al. 1996). Once such calibration is complete, sampling can be reduced as the fish can more efficiently navigate primarily using path integration and its memory of the food location. During Probe trials, active sensing again increases. In our view, this is again readily understood as an attempt by the fish to recalibrate its path integration mechanism by increased sampling of the landmarks.
Why Does Gymnotus sp. Need Three Types of Active Sensing When Learning to Locate Food?
While learning food location, Gymnotus sp. will use increased sampling density, E-scans and B-scans at the landmark and food locations. While there are linkages between, e.g., the occurrence of B-scans (backward phase) and increased sampling density, there are also clear examples of independence, e.g., sampling density and E-scans. We conclude that there are at least partially independent neural controls for these types of active sensing, and, in tandem, there is likely different processing of the resulting electrosensory reafferent input.
The increase in sampling density may simply be due to the need for increased electrosensory input at sites associated with food; this would be analogous to a human directing multiple saccades toward an expected food location. But this does not explain the additional need for B- and E-scans. We propose, based on three lines of evidence, that B-scans are specifically required for better spatial localization of food and landmarks. 1) A computational study in a related gymnotiform fish has demonstrated that the fish's motion allows objects to “pop out” of the background and so be more readily detected (Babineau et al. 2007). 2) The longitudinal motion during B-scans is known to activate an excitatory direct feedback pathway to the first-order electrosensory lobe (Bratton and Bastian 1990), and it has been hypothesized that the dynamics of this feedback would greatly potentiate the response of electrosensory lobe neurons to such motion (Berman and Maler 1999; Oswald et al. 2002). 3) The increase in sampling density during the back-swimming phase of a B-scan will increase sampling as the object being scanned moves toward the head foveal region (Carr et al. 1982; Castello et al. 2000), as often occurs during prey capture (Nelson et al. 2002; Nelson and MacIver 1999). Taken together, this strongly suggests that B-scans are utilized for the precise spatial localization of landmarks and food. In contrast, we propose that the brief E-scans serve to precisely time stamp the location of detected objects. Such a time stamp would be useful for a path integration mechanism because it would, e.g., signal the duration of the trajectory from landmark to food.
Rats also learn about their spatial environment by engaging in exploratory behavior (Gordon et al. 2014; Poucet and Herrmann 2001). Several types of active sensing occur upon encountering novel objects during exploration, including head scanning (Drai et al. 2001) and rearing (Lever et al. 2006). In the dark, whisking (Gordon et al. 2014; Sofroniew and Svoboda 2015) and tightly coupled sniffing (Moore et al. 2013) may dominate during exploration. Presumably active sensing provides the input required for rats to generate spatial maps (Poucet and Herrmann 2001), but the specific information provided by each type of active sensing is not known. It is possible to directly connect scanning to spatial learning (see below), but the role of the other types of active sensing in learning has not been studied in detail.
Active Sensing and Spatial Learning
We quantified the EOD pulses and analyzed the learning-dependent changes in their sampling density and pattern (E-scans). The sampling and E-scan densities and B-scans all decreased after repeated exposures to a stable spatial environment (Figs. 5, C and D, 6, A–C, and 7, C–E). The sampling and E-scan density measures of sampling selectively increased near the expected food location (Probe trials) after learning (Figs. 5, C and D, and 7, C–E), which is consistent with their use as quantifiers of food-searching efficiency. These measures reflected the spatial knowledge of the animals; enhanced sampling occurred in the early phase of learning and also during the Probe trials when animals were less certain about the food location (Fig. 5A).
Stable landmarks played a critical role in accurately locating the food using the electrosense. Sampling density and B-scans were initially high near landmarks, emphasizing their likely importance in spatial learning for efficient navigation. This conclusion is reinforced by the None control group (Figs. 8, A and B, and 9, A–D). Animals trained without landmarks exhibited significantly lower sampling density near the missing food, which is indicative of less reliance on active electrosensing (Fig. 9, E and F). Our results support our initial hypothesis that Gymnotus sp. adapt their electrosensory sampling in an optimal manner by using past experiences and landmarks for more efficient navigation and spatial search.
A recent paper has shown that, in the rat, there is a direct connection between head scanning and the neural substrate of spatial maps-hippocampal place cell activity (Monaco et al. 2014). The animals exhibited head scanning at locations with discrepancies between the expected location of local and distant spatial cues. Neural activity of hippocampal neurons during this scanning behavior led to the strengthening or the formation of new place fields of the cells active at this location. The Monaco et al. paper provides the critical direct connection between one form of active sensing and spatial learning in rats.
Our results demonstrate that active sensing is closely associated with spatial learning in WEFs. We hypothesize that, as is the case in mammals, the neural link is in the dorsal telencephalon (pallium). In fish, the dorsolateral pallium (DL) has been shown to be necessary for spatial learning (Broglio et al. 2010; Duran et al. 2010; Rodriguez et al. 2002). DL receives electrosensory tectal input related to object motion (Bastian 1982; Giassi et al. 2012) and, via dorso-central pallium (DC), can drive tectal cells that control movement, including saccades (Giassi et al. 2012; Luque et al. 2005). DL has a dense local recurrent circuitry appropriate for memory storage (Trinh et al. 2015) and is believed to be homologous to mammalian hippocampus and/or cortex (Harvey-Girard et al. 2012; Trinh et al. 2015). DL is connected to a dorsal pallial region that appears to respond to novel stimuli (Elliott and Maler 2015; Harvey-Girard et al. 2010; Trinh et al. 2015) and may, therefore, be activated during active-sensing episodes. Lastly, there has been a preliminary description of a place cell in the goldfish pallium at what appears to be the boundary of DL and DC (Canfield and Mizumori 2004). We, therefore, hypothesize that the core neural circuitry for spatial learning is similar in teleosts and mammals and that, for all vertebrates, spatial learning will prove to be tightly linked to active sensing.
Active Sensing as an Indicator of Attention and the Connection to Spatial Learning
A vast amount of information reaches the vertebrate central nervous system from the various sensory systems. The role of attention is to select “important” or “interesting” input, e.g., a spatial location in the case of spatial attention, for intense processing (Itti and Koch 2001; Knudsen 2007). Selection can be based on the salience of a sense input (bottom-up), a memory trace that some sense input or location is important (top-down) or some combination of these two selection criteria (Itti and Koch 2001; Knudsen 2007). Following a lucid exposition by Knudsen (2007), attention requires at least three fundamental components: 1) filters that enhance the neural response to strong or unexpected stimuli (salience filters) or memories (e.g., locations) that are especially relevant because of learning (memory filter); 2) a selection process that determines which filter output will be intensively processed; and 3) working memory, i.e., a short-term memory that stores the selected filter inputs for detailed analysis with respect to relevant long-term memories. Most work on attention has focused on the primate visual system (Itti and Koch 2001), but, more recently, the same mechanisms were hypothesized to apply to the avian visual system (Knudsen 2007) and to the rodent vibrissae sense (Mitchinson and Prescott 2013). A key point for our arguments below is that an observable element of spatial attention is the motor outputs that enhance particular inputs: saccadic eye movements for vision (Itti and Koch 2001; Knudsen 2007), and directed whisking for the vibrissae sense (Mitchinson and Prescott 2013). In the case of both mammals and birds, vast pallial territories are important for attention, but the final decision on what to attend may reside in the tectum/superior colliculus (Itti and Koch 2001; Knudsen 2007; Krauzlis et al. 2013; Mitchinson and Prescott 2013), i.e., the behavioral manifestation of attention is superior colliculus-initiated saccadic eye movements or directed whisking.
To present our arguments within the above framework, we discuss changes in electrosensory sampling as bottom-up driven (novelty response), top-down driven (food location in Probe trials) and mixed driven (landmark in Probe trials).
The novelty response of gymnotiform pulse WEFs is a stimulus-evoked, stereotyped, short-latency and transient increase in EODR. The stimulus can be electrosensory (Caputi et al. 2003; Grau 1985; Heiligenberg 1980), visual (Grau 1985) or acoustic (Grau 1985; Jun et al. 2014b). Latencies of the novelty response depend on the baseline inter-EOD interval and can be as low as ∼20 ms for electrosensory stimuli (Caputi et al. 2003) and ∼30 ms for acoustic stimuli (Jun et al. 2014b). The sound-evoked novelty response is likely due to brain stem circuitry (Comas and Borde 2010). These observations are consistent with the novelty response being a simple reflex response unrelated to attention. However, the novelty responses to electric or acoustic stimuli habituate for stimuli presented at rates of only once per 10–20 s (Grau 1985). Detailed physiological studies revealed that habituation occurred at a central nervous system level “higher” than the midbrain (Grau and Bastian 1986). This implies that the presentation of a novel stimulus triggers activity in some “higher” brain region that persists for >10 s. It is, therefore, possible that novel stimuli, in addition to evoking a reflex novelty response, also activate a low-level salience filter to induce a high-level attentional state. This possibility is certainly not proven by existing experiments, and we return below to a possible experimental approach to this question.
We hypothesize that, for Probe trials, the active sensing measures, sampling density, E-scans, B-scans, focused at the location of the missing food are, like directed saccades, the behavioral indicators of top-down spatial attention. In Probe trials, there is no stimulus present, and active sensing is memory driven. Since spatial memories are presumably stored in DL, this further implies that pallium is driving all types of spatially focused active sensing. Short-term memory has been demonstrated in a related gymnotiform fish and is likely also mediated by DL and associated pallial regions (Harvey-Girard et al. 2010). While we do not know whether WEFs have the neural substrates for working memory, the strong and focused active sensing at the missing food location suggests that there is increased activity in DL and connected pallial regions.
We hypothesize that, for Probe trials, the increase in sampling density and B-scans near landmarks represents a mixed spatial attention process. This increased active sampling is stimulus (landmark) driven (bottom-up), but the stimulus is presumably significant because the fish remembers the spatial connection between landmarks and food (top-down). As above, DL is implicated in such spatial memory, and again the focused active sensing implies increased activity in DL.
Lastly, these arguments suggest an experimental approach to the issue raised above: does a salient novel stimulus elicit, in addition to a reflex novelty response, bottom-up attention? We propose that, if this claim is correct, then the salient stimulus, e.g., a loud sound, will induce a reverberatory spike discharge in DL neurons that outlasts the brief (1 s) stimulus by 10–20 s.
We have hypothesized that pulse gymnotiform fish may have an attentional mechanism that is, at a computational level, analogous to spatial attention in mammals and birds. Whether this mechanism is mediated by neural structures homologous to amniote pallium (i.e., cortex, hippocampus) will depend on a deeper characterization of teleost pallial circuitry, gene expression and development (Harvey-Girard et al. 2007, 2012; Karten 2015; Trinh et al. 2015).
Conclusion
WEFs can rapidly learn to locate food in the dark, and learning is most efficient when stable landmarks are available. They use short-range active electrosensing (sampling density, E-scans) in combination with directed movements (B-scans) to learn the location of food and landmarks. Once they have learned the food location, active sampling diminishes at landmarks but remains focused at the food site, even when the food is missing. We hypothesize that, as in other animals, WEFs combine landmark/food (electrosensory) and path integration (idiothetic) input to generate spatial maps. This permits WEFs to rapidly find food based on trajectories computed from memory, and the requirement for slow perceptual computations is diminished.
Volition and attention are typically studied in primates, especially humans, and have been associated with vast cortical territories (Buschman and Kastner 2015; Haggard 2008). Our laboratory has previously suggested that pulse gymnotiform fish exhibit behaviors consistent with volition (Jun et al. 2014b). Here we suggest that they also exhibit behaviors consistent with attention. We hypothesize that volition and attention may be core cognitive processes of all gnathostomes and already realized in the circuitry of the simpler and smaller teleost pallium.
GRANTS
This project was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC PGS scholarship to J. J. Jun; Grant 121891-2009 to A. Longtin), and by the Canadian Institutes of Health Research (Grant 49510 to L. Maler and A. Longtin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.J.J. and L.M. conception and design of research; J.J.J. performed experiments; J.J.J. analyzed data; J.J.J., A.L., and L.M. interpreted results of experiments; J.J.J. prepared figures; J.J.J. drafted manuscript; J.J.J., A.L., and L.M. edited and revised manuscript; J.J.J., A.L., and L.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors express sincere gratitude to Lee Hunton and Declan Lu for help setting up the experiments, Etienne Benard Seguin and Chris Anderson for help in data collection, and Aida Ahrari for support in the data analysis.
Present address of J. J. Jun: HHMI, Janelia Research Campus, 2C.150, Ashburn, VA 20147.
REFERENCES
- Alvernhe A, Sargolini F, Poucet B. Rats build and update topological representations through exploration. Anim Cogn 15: 359–368, 2012. [DOI] [PubMed] [Google Scholar]
- Ardanaz JL, Silva A, Macadar O. Temperature sensitivity of the electric organ discharge waveform in Gymnotus carapo. J Comp Physiol A 187: 853–864, 2001. [DOI] [PubMed] [Google Scholar]
- Babineau D, Lewis JE, Longtin A. Spatial acuity and prey detection in weakly electric fish. PLoS Comput Biol 3: e38, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacelo J, Engelmann J, Hollmann M, von der Emde G, Grant K. Functional foveae in an electrosensory system. J Comp Neurol 511: 342–359, 2008. [DOI] [PubMed] [Google Scholar]
- Bastian J. Vision and electroreception. Integration of sensory information in the optic tectum of the weakly electric fish Apteronotus leptorhynchus. J Comp Physiol A 147: 287–297, 1982. [Google Scholar]
- Berman NJ, Maler L. Neural architecture of the electrosensory lateral line lobe: adaptations for coincidence detection, a sensory searchlight and frequency-dependent adaptive filtering. J Exp Biol 202: 1243–1253, 1999. [DOI] [PubMed] [Google Scholar]
- Black-Cleworth P. The role of electrical discharges in the non-reproductive social behaviour of Gymnotus carapo (Gymnoidae, Pisces). In: Animal Behavior Monographs. London: Bailliere, Tindall & Cassell, 1970, vol. 3, pt. 1, p. 1–77. [Google Scholar]
- Bratton B, Bastian J. Descending control of electroreception. II. Properties of nucleus praeeminentialis neurons projecting directly to the electrosensory lateral line lobe. J Neurosci 10: 1241–1253, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio C, Rodriguez F, Gomez A, Arias JL, Salas C. Selective involvement of the goldfish lateral pallium in spatial memory. Behav Brain Res 210: 191–201, 2010. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Kastner S. From behavior to neural dynamics: an integrated theory of attention. Neuron 88: 127–144, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield JG, Mizumori SJ. Methods for chronic neural recording in the telencephalon of freely behaving fish. J Neurosci Methods 133: 127–134, 2004. [DOI] [PubMed] [Google Scholar]
- Caputi AA, Aguilera PA, Carolina Pereira A, Rodriguez-Cattaneo A. On the haptic nature of the active electric sense of fish. Brain Res 1536: 27–43, 2013. [DOI] [PubMed] [Google Scholar]
- Caputi AA, Aguilera PA, Castello ME. Probability and amplitude of novelty responses as a function of the change in contrast of the reafferent image in G carapo. J Exp Biol 206: 999–1010, 2003. [DOI] [PubMed] [Google Scholar]
- Caputi AA, Castello ME, Aguilera PA, Pereira C, Nogueira J, Rodriguez-Cattaneo A, Lezcano C. Active electroreception in Gymnotus omari: imaging, object discrimination, and early processing of actively generated signals. J Physiol (Paris) 102: 256–271, 2008. [DOI] [PubMed] [Google Scholar]
- Carr CE, Maler L, Sas E. Peripheral organization and central projections of the electrosensory organs in gymnotiform fish. J Comp Neurol 211: 139–153, 1982. [DOI] [PubMed] [Google Scholar]
- Castello ME, Aguilera PA, Trujillo-Cenoz O, Caputi AA. Electroreception in Gymnotus carapo: pre-receptor processing and the distribution of electroreceptor types. J Exp Biol 203: 3279–3287, 2000. [DOI] [PubMed] [Google Scholar]
- Clarke SE, Longtin A, Maler L. The neural dynamics of sensory focus. Nat Commun 6: 8764, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett TS, Cartwright BA, Smith BA. Landmark learning and visuo-spatial memories in gerbils. J Comp Physiol A 158: 835–851, 1986. [DOI] [PubMed] [Google Scholar]
- Collett TS, Graham P. Animal navigation: path integration, visual landmarks and cognitive maps. Curr Biol 14: R475–R477, 2004. [DOI] [PubMed] [Google Scholar]
- Comas V, Borde M. Neural substrate of an increase in sensory sampling triggered by a motor command in a gymnotid fish. J Neurophysiol 104: 2147–2157, 2010. [DOI] [PubMed] [Google Scholar]
- Douglas RH, Hawryshyn CW. Behavioral studies of fish vision: an analysis of visual capabilities. In: The Visual System of Fish, edited by Douglas R, and Djamgoz M. London: Chapman and Hall, 1990, p. 373–418. [Google Scholar]
- Drai D, Kafkafi N, Benjamini Y, Elmer G, Golani I. Rats and mice share common ethologically relevant parameters of exploratory behavior. Behav Brain Res 125: 133–140, 2001. [DOI] [PubMed] [Google Scholar]
- Duran E, Ocana FM, Broglio C, Rodriguez F, Salas C. Lateral but not medial telencephalic pallium ablation impairs the use of goldfish spatial allocentric strategies in a “hole-board” task. Behav Brain Res 214: 480–487, 2010. [DOI] [PubMed] [Google Scholar]
- Elliott SB, Maler L. Stimulus induced up states in the dorsal pallium of a weakly electric fish. J Neurophysiol 114: 2071–2076, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne AS, Maurer R, Berlie J, Reverdin B, Rowe T, Georgakopoulos J, Seguinot V. Navigation through vector addition. Nature 396: 161–164, 1998. [DOI] [PubMed] [Google Scholar]
- Etienne AS, Maurer R, Seguinot V. Path integration in mammals and its interaction with visual landmarks. J Exp Biol 199: 201–209, 1996. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Aquatic adaptations in fish eyes. In: Sensory Biology of Aquatic Animals, edited by Fay RR, Popper AN, and Tavolga WN. New York: Springer, 1988, p. 435–466. [Google Scholar]
- Fonio E, Benjamini Y, Golani I. Freedom of movement and the stability of its unfolding in free exploration of mice. Proc Natl Acad Sci U S A 106: 21335–21340, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori K, Okuda N, Yamaoka K, Yanagisawa Y. Remarkable spatial memory in a migratory cardinalfish. Anim Cogn 13: 385–389, 2010. [DOI] [PubMed] [Google Scholar]
- Giassi AC, Duarte TT, Ellis W, Maler L. The organization of the Gymnotiform fish pallium in relation to learning and memory. II. Extrinsic connections. J Comp Neurol 520: 3338–3368, 2012. [DOI] [PubMed] [Google Scholar]
- Gordon G, Fonio E, Ahissar E. Emergent exploration via novelty management. J Neurosci 34: 12646–12661, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau HJ. The Novelty Response of Pulse Type Weakly Electric Fish: A Neuroethological Study (PhD Thesis). Norman, OK: University of Oklahoma, 1985. [Google Scholar]
- Grau HJ, Bastian J. Neural correlates of novelty detection in pulse-type electric fish. J Comp Physiol A 159: 191–200, 1986. [DOI] [PubMed] [Google Scholar]
- Haggard P. Human volition: towards a neuroscience of will. Nat Rev Neurosci 9: 934–946, 2008. [DOI] [PubMed] [Google Scholar]
- Harvey-Girard E, Dunn RJ, Maler L. Regulated expression of N-methyl-d-aspartate receptors and associated proteins in teleost electrosensory system and telencephalon. J Comp Neurol 505: 644–668, 2007. [DOI] [PubMed] [Google Scholar]
- Harvey-Girard E, Giassi AC, Ellis W, Maler L. The organization of the gymnotiform fish pallium in relation to learning and memory. IV. Expression of conserved transcription factors and implications for the evolution of dorsal telencephalon. J Comp Neurol 520: 3395–3413, 2012. [DOI] [PubMed] [Google Scholar]
- Harvey-Girard E, Tweedle J, Ironstone J, Cuddy M, Ellis W, Maler L. Long-term recognition memory of individual conspecifics is associated with telencephalic expression of Egr-1 in the electric fish Apteronotus leptorhynchus. J Comp Neurol 518: 2666–2692, 2010. [DOI] [PubMed] [Google Scholar]
- Heiligenberg W. The evaluation of electroreceptive feedback in a gymnotid fish. J Comp Physiol A 138: 173–185, 1980. [Google Scholar]
- Hofmann V, Geurten BR, Sanguinetti-Scheck JI, Gomez-Sena L, Engelmann J. Motor patterns during active electrosensory acquisition. Front Behav Neurosci 8: 186, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci 2: 194–203, 2001. [DOI] [PubMed] [Google Scholar]
- Jun JJ, Longtin A, Maler L. Precision measurement of electric organ discharge timing from freely moving weakly electric fish. J Neurophysiol 107: 1996–2007, 2012. [DOI] [PubMed] [Google Scholar]
- Jun JJ, Longtin A, Maler L. Real-time localization of moving dipole sources for tracking multiple free-swimming weakly electric fish. PLoS One 8: e66596, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JJ, Longtin A, Maler L. Long-term behavioral tracking of freely swimming weakly electric fish. J Vis Exp 85: e50962, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JJ, Longtin A, Maler L. Enhanced sensory sampling precedes self-initiated locomotion in an electric fish. J Exp Biol 217: 3615–3628, 2014b. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Vertebrate brains and evolutionary connectomics: on the origins of the mammalian “neocortex”. Philos Trans R Soc Lond B Biol Sci 370: 1684, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S. Fundamentals of Statistical Signal Processing. Estimation Theory. Upper Saddle City, NJ: Prentice Hall, 1993, vol. 1. [Google Scholar]
- Knudsen EI. Spatial aspects of electric fields generated by weakly electric fish. J Comp Physiol 99: 103–118, 1975. [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci 30: 57–78, 2007. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci 36: 165–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoo MJ, Lannoo SJ. Why do electric fish swim backwards? An hypothesis based on gymnotiform behavior, interpreted through sensory constraints. Environ Biol Fishes 36: 157–165, 1992. [Google Scholar]
- Lever C, Burton S, O'Keefe J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev Neurosci 17: 111–133, 2006. [DOI] [PubMed] [Google Scholar]
- Lonneke B, Peters RC, Bretschneider F. Behavioural relevance of AC and DC in prey detection by the brown bullhead, Ameirus nebulosus. Anim Biol Leiden Neth 58: 312–336, 2008. [Google Scholar]
- Luque M, Perez-Perez M, Herrero L, Torres B. Involvement of the optic tectum and mesencephalic reticular formation in the generation of saccadic eye movements in goldfish. Brain Res Rev 49: 388–397, 2005. [DOI] [PubMed] [Google Scholar]
- MacIver MA, Sharabash NM, Nelson ME. Prey capture behavior in gymnotid electric fish: motion analysis and effects of water conductivity. J Exp Biol 204: 543–557, 2001. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the “cognitive map”. Nat Rev Neurosci 7: 663–678, 2006. [DOI] [PubMed] [Google Scholar]
- Mitchinson B, Prescott TJ. Whisker movements reveal spatial attention: a unified computational model of active sensing control in the rat. PLoS Comput Biol 9: e1003236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco JD, Rao G, Roth ED, Knierim JJ. Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat Neurosci 17: 725–731, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Deschenes M, Furuta T, Huber D, Smear MC, Demers M, Kleinfeld D. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature 497: 205–210, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, MacIver MA. Prey capture in the weakly electric fish Apteronotus leptorhynchus: sensory acquisition strategies and electrosensory consequences. J Exp Biol 202: 1195–1203, 1999. [DOI] [PubMed] [Google Scholar]
- Nelson ME, MacIver MA, Coombs S. Modeling electrosensory and mechanosensory images during the predatory behavior of weakly electric fish. Brain Behav Evol 59: 199–210, 2002. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Lewis JE, Maler L. Dynamically interacting processes underlie synaptic plasticity in a feedback pathway. J Neurophysiol 87: 2450–2463, 2002. [DOI] [PubMed] [Google Scholar]
- Poucet B, Herrmann T. Exploratory patterns of rats on a complex maze provide evidence for topological coding. Behav Processes 53: 155–162, 2001. [DOI] [PubMed] [Google Scholar]
- Pusch R, von der Emde G, Hollmann M, Bacelo J, Nobel S, Grant K, Engelmann J. Active sensing in a mormyrid fish: electric images and peripheral modifications of the signal carrier give evidence of dual foveation. J Exp Biol 211: 921–934, 2008. [DOI] [PubMed] [Google Scholar]
- Reese ES. Orientation behavior of butterflyfishes (family Chaetodontidae) on coral reefs: spatial learning of route specific landmarks and cognitive maps. Environ Biol Fishes 25: 79–86, 1989. [Google Scholar]
- Rodrigues F, Duran E, Vargas JP, Torres B, Salas C. Performance of goldfish trained in allocentric and egocentric maze procedures suggests the presence of a cognitive mapping system in fishes. Anim Learn Behav 22: 409–420, 1994. [Google Scholar]
- Rodriguez F, Lopez JC, Vargas JP, Gomez Y, Broglio C, Salas C. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J Neurosci 22: 2894–2903, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Cressant A, Thinus-Blanc C, Poucet B. Spatial firing of hippocampal place cells in blind rats. J Neurosci 18: 1818–1826, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluessel V, Bleckmann H. Spatial memory and orientation strategies in the elasmobranch Potamotrygon motoro. J Comp Physiol A 191: 695–706, 2005. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P. Dynamics of active sensing and perceptual selection. Curr Opin Neurobiol 20: 172–176, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JB, Nelson ME, Burdick JW, Maciver MA. Omnidirectional sensory and motor volumes in electric fish. PLoS Biol 5: e301, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew NJ, Svoboda K. Whisking. Curr Biol 25: R137–R140, 2015. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus 12: 291–303, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper SA, Roth E, Cowan NJ, Fortune ES. Active sensing via movement shapes spatiotemporal patterns of sensory feedback. J Exp Biol 215: 1567–1574, 2012. [DOI] [PubMed] [Google Scholar]
- Terrazas A, Krause M, Lipa P, Gothard KM, Barnes CA, McNaughton BL. Self-motion and the hippocampal spatial metric. J Neurosci 25: 8085–8096, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toerring MJ, Moller P. Locomotor and electric displays associated with electrolocation during exploratory behavior in mormyrid fish. Behav Brain Res 12: 291–306, 1984. [DOI] [PubMed] [Google Scholar]
- Towal RB, Quist BW, Gopal V, Solomon JH, Hartmann MJ. The morphology of the rat vibrissal array: a model for quantifying spatiotemporal patterns of whisker-object contact. PLoS Comput Biol 7: e1001120, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh AT, Harvey-Girard E, Teixeira F, Maler L. Cryptic laminar and columnar organization in the dorsolateral pallium of a weakly electric fish. J Comp Neurol 524: 408–428, 2015. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Hines DJ, Pellis SM, Whishaw IQ. Vestibular information is required for dead reckoning in the rat. J Neurosci 22: 10009–10017, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton K. The use of local landmarks by foraging goldfish. Anim Behav 40: 500–505, 1990. [Google Scholar]
- Windsor SP, Norris SE, Cameron SM, Mallinson GD, Montgomery JC. The flow fields involved in hydrodynamic imaging by blind Mexican cave fish (Astyanax fasciatus). I. Open water and heading towards a wall. J Exp Biol 213: 3819–3831, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.