Abstract
Degeneracy of respiratory network function would imply that anatomically discrete aspects of the brain stem are capable of producing respiratory rhythm. To test this theory we a priori transected brain stem preparations before reperfusion and reoxygenation at 4 rostrocaudal levels: 1.5 mm caudal to obex (n = 5), at obex (n = 5), and 1.5 (n = 7) and 3 mm (n = 6) rostral to obex. The respiratory activity of these preparations was assessed via recordings of phrenic and vagal nerves and lumbar spinal expiratory motor output. Preparations with a priori transection at level of the caudal brain stem did not produce stable rhythmic respiratory bursting, even when the arterial chemoreceptors were stimulated with sodium cyanide (NaCN). Reperfusion of brain stems that preserved the pre-Bötzinger complex (pre-BötC) showed spontaneous and sustained rhythmic respiratory bursting at low phrenic nerve activity (PNA) amplitude that occurred simultaneously in all respiratory motor outputs. We refer to this rhythm as the pre-BötC burstlet-type rhythm. Conserving circuitry up to the pontomedullary junction consistently produced robust high-amplitude PNA at lower burst rates, whereas sequential motor patterning across the respiratory motor outputs remained absent. Some of the rostrally transected preparations expressed both burstlet-type and regular PNA amplitude rhythms. Further analysis showed that the burstlet-type rhythm and high-amplitude PNA had 1:2 quantal relation, with burstlets appearing to trigger high-amplitude bursts. We conclude that no degenerate rhythmogenic circuits are located in the caudal medulla oblongata and confirm the pre-BötC as the primary rhythmogenic kernel. The absence of sequential motor patterning in a priori transected preparations suggests that pontine circuits govern respiratory pattern formation.
Keywords: respiratory rhythm generation, breathing, medulla oblongata, neural circuits, respiratory pattern
one of the great challenges posed when studying respiratory network function is to identify its anatomical and functional foundations. A pontomedullary network within the mammalian brain stem generates the sequential motor pattern of respiration and thus is considered to be the primary respiratory central pattern generator (rCPG). During the last century the primary network organization of the pontomedullary column of respiratory neurons and centers has been characterized, initially with transection (Lumsden 1923; Markwald 1888) and lesion experiments. With time these experimental techniques became progressively more refined, utilizing anatomical, pharmacological, and electrophysiological tools, and we are currently seeing techniques revolutionized by recent advances in genetics and molecular biology, with the latter allowing for the investigation of respiratory network function in behaving animals (Burke et al. 2015; Gray et al. 2001; McKay and Feldman 2008). Such recent advances have identified functionally and anatomically discrete modules of the respiratory network that may operate within a larger pontomedullary rCPG (Feldman et al. 2013; Ramirez et al. 2012; Smith et al. 2013). The most prominent modules of the respiratory network are the pre-Bötzinger complex (pre-BötC), marked as the kernel for inspiratory rhythm generation (Johnson et al. 1994, 2001; Ramirez et al. 1998; Schwarzacher et al. 1995; Smith et al. 1991), and the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG). The RTN/pFRG was initially characterized as a preinspiratory rhythmogenic region (Onimaru et al. 1997; Onimaru and Homma 2003) but more recently was suggested to be the location of the oscillator responsible for active expiration (Abbott et al. 2011; Abdala et al. 2009; Janczewski and Feldman 2006). The RTN/pFRG was also characterized as a region with primary central CO2 chemosensitivity (Guyenet 2012; Guyenet et al. 2012; Mulkey et al. 2004; Wang et al. 2013).
Such technological advances have been invaluable in furthering our understanding into the respiratory network and have allowed a consensus to be reached on the functional importance of anatomically defined modules of the rCPG. However, the precise synaptic mechanisms for respiratory rhythm generation and pattern formation are not fully understood. Currently, three major working hypotheses exist: 1) the pre-BötC, as the kernel of the respiratory network, is responsible for the genesis of respiratory rhythm via intrinsic bursting properties, and its signals are transformed into a physiological sequential motor pattern by the larger rCPG (Feldman and Del Negro 2006; Ramirez et al. 2002; Smith et al. 2013); 2) both respiratory rhythm and pattern are generated by synaptic oscillation in distributed networks across the entire rCPG (Bianchi et al. 1995; Richter 1982; St-John 1998; St John 2009; Voneuler 1983); and 3) there are anatomically identifiable oscillators for specific phases of the respiratory motor cycle (Baghdadwala et al. 2016). When considering the first hypothesis, that the pre-BötC is the kernel of rhythm generation, it stands to reason that in the absence of an intact pre-BötC, respiratory rhythm should be eliminated. However, it was shown in previous studies that ablation of the pre-BötC neurons in conscious animals does not necessarily result in the complete arrest of the breathing rhythm (Forster et al. 2014; Gray et al. 2001; McKay and Feldman 2008; Wang et al. 2002). These data suggested that respiratory rhythm can still be generated by undetermined structures, or in other words, by a redundant or degenerate circuit (see below).
A prior study from our research group suggested a putative autorhythmic region caudal to the obex in the in situ interarterially perfused preparation in rat (Jones et al. 2012); however, an extensive follow-up study within a functionally intact rCPG failed to prove the existence of rhythmogenic capacity of the caudal medulla (Jones et al. 2015). In the present study we extended our efforts in looking for an autorhythmic brain stem region external and caudal to the pre-BötC, hypothesizing the presence of a rhythmogenic degenerate rhythmogenic circuit for respiration. Degeneracy is defined as the phenomenon whereby structurally distinct components are able to perform the same role under certain conditions and can be observed throughout the increasing levels of complexity within biological systems, from degeneracy within the genetic code to degeneracy within network systems (Edelman and Gally 2001; Leonardo 2005). Redundant circuitry is also commonly found in neural networks, with multiple components capable of performing the same task. The difference between a redundant and degenerate neural network is that a degenerate circuit is able to change function, depending on the network state, and is not limited to one function. Furthermore, a redundant circuit will utilize the same mechanism as the circuit of interest to produce function, whereas a degenerate circuit is mechanistically different (Leonardo 2005). Degeneracy within biological systems is evolutionarily advantageous because it provides an inherent robustness to the system, protecting against system failure. The functioning of the rCPG is essential to maintain life, and so it is intuitive to hypothesize that degeneracy exists within the rCPG network (Mellen 2010).
In the current study we tested the hypothesis that a preparation lacking essential circuit components for respiratory rhythm generation will gain the capacity to elicit rhythmic respiratory function from a degenerate neural substrate that is located in the brain stem caudal to the pre-BötC. To do so, we use the in situ perfused brain stem preparation. Contrary to standard experimental protocols for the in situ perfused brain stem preparation (Paton 1996) that only use precollicular decerebration, we performed additional medullary brain stem transections prior to the reperfusion and oxygenation of the remaining brain tissue. This allows resurrection of reduced network circuitry containing brain stem circuits caudal to the pre-BötC. To further investigate the rhythmogenic capacity of the entire medullary rCPG circuits, we performed additional experiments that also contained the pre-BötC or tissue up to the pontomedullary junction.
MATERIALS AND METHODS
Sprague-Dawley rats were maintained on a 12:12-h light-dark cycle with food and water available ad libitum. All experiments followed protocols approved by the Florey Institute of Neuroscience and Mental Health Animal Ethics Committee (AEC 12-005) and performed in accordance with the Australian National Health and Medical Research Council Code of Practice for the Use of Animals for Scientific Purposes.
In Situ Perfused Brain Stem-Spinal Cord Preparation
Surgical procedure.
All experiments were performed using the interarterially perfused brain stem-spinal cord preparation of juvenile rat (Sprague-Dawley rats of both sex, postnatal day 18–23, n = 23). Initial surgical procedures were performed in accordance with previously published studies (Dutschmann et al. 2009; Paton 1996). To summarize, rats were induced into a state of deep anesthesia via inhalation of isoflurane. When its breathing rate was depressed and the animal failed to elicit a hind paw withdrawal reflex in response to noxious pinch, it was bisected below the diaphragm. The caudal portion was discarded and the rostral portion submerged in an ice-cold (5°C) artificial cerebrospinal fluid solution (aCSF) containing (in mM) 125 NaCl, 24 NaHCO3, 2.5 CaCl2, 1.25 MgSO4, 4 KCl, 1.25 KH2PO4, and 10 d-glucose and bubbled with carbogen (95% O2-5% CO2). Removal of the occipital and atlas bones and cerebellectomy allowed for optimum visualization of the dorsal surface of caudal brain stem and upper cervical spinal cord. Before arterial reperfusion of the preparation, the brain stem was transected at one of four different rostrocaudal levels with measurements taken relative to the obex, an anatomical landmark clearly visible on the dorsal surface of the brain stem. Complete transverse transections were made with a single knife cut, and all brain tissue rostral to the cut was discarded to ensure integrity only of the circuitry caudal to the transection level. Transections were performed at the following levels: level 1 at the caudal ventral respiratory group (cVRG), 1.5 mm caudal to obex; level 2 at the obex; level 3 just rostral to the pre-Bötzinger complex, 1.5 mm rostral to obex; and level 4, 3 mm rostral to obex at the pontomedullary junction. Further preparations (n = 9) were perfused after precollicular transection that left the entire pontomedullary rCPG intact. These preparations served as reference for the respiratory activity observed in a priori transected preparations. In all preparations the heart was tied off anterior to the aortic arch and removed to avoid mechanical and electrical artifacts from the cardiac tissue (see Dutschmann et al. 2009).
Nerve recordings.
The right thoracic phrenic nerve (PN), the left iliohypogastric branch of the abdominal nerve (L1), and the right cervical vagus nerve (VN) were isolated and cut distally with activity recorded from cut nerve ends using suction electrodes; signals were amplified (differential amplifier DP-311; Warner Instruments, Hamden, CT), bandpass filtered from 100 Hz to 5 kHz, digitized (PowerLab/16SP; ADInstruments, Sydney, Australia), and then viewed and recorded with LabChart version 7.0 software (ADInstruments). Additional post hoc analysis included application of a high-pass digital filter at a cutoff frequency of 50 Hz to stabilize the baseline recording and eliminate movement artifacts.
Standardized perfusion protocol.
After transfer of preparation to the recording chamber, the descending aorta was cannulated and retrogradely perfused via a peristaltic pump (Watson and Marlow) using warmed (31°C) aCSF (composition as described earlier plus 12 mM sucrose) equilibrated with carbogen (95% O2-5% CO2). After oxygenation, aCSF pH equaled 7.35 ± 0.05. Following the protocol described in Fiamma et al. (2013), sucrose was used in the perfusate in the place of Ficoll, which has traditionally been used (Paton 1996). The use of sucrose resulted in no change in osmotic pressure and yielded no deleterious effects to the quality or longevity of the preparations. Cannulation of the descending aorta was always made distal to the first lumbar spinal cord segment to ensure adequate perfusion of abdominal motor neurons. From experience over the past decade, we initially perfused the transected preparation at a flow rate of 20 ml/min, the flow rate where the vast majority of intact pontomedullary brain stem preparations display the characteristic eupnea-like motor pattern (Bautista and Dutschmann 2014; Fiamma et al. 2013). The current study involved the perfusion of an anatomically reduced pontomedullary rCPG. The more caudal transection levels, levels 1 and 2, did not produce continuous respiratory bursting (see results). Therefore, to ensure that the lack of respiratory activity was not related to under- or overperfusion of the brain stem tissue, we always administered a bolus injection of NaCN (0.2%; 0.1–0.3 ml) 5 min after the target flow rate was reached. The NaCN evoked chemoreceptor reflex triggered motor responses in all experiments, indicating adequate brain stem perfusion; however, reliable respiratory motor bursting was only observed in the rostrally transected brain stem preparations, i.e., those made at levels 3 and 4. Finally, to further verify the absence or presence of rhythmic or sporadic respiratory motor bursting, we varied the flow rate with stepwise increases or decreases for up to 1 h. Importantly, the variation of flow rates was also used to determine whether the respiratory motor pattern could be further transformed, for example, shortening or lengthening of the respiratory burst durations.
Data analysis.
For data analysis we took PNA as the lead motor output. In general, the maximum duration of an apneustic respiratory burst is considered to be ∼4 s, and so for the purpose of these analyses, PNA activity with a duration of >4 s was considered to be nonrespiratory and was described in a qualitative manner only (see results and Table 1). In cases where burst duration was <4 s we analyzed the bursts over a 5-min period deemed representative of the preparations rhythmogenic potential. Respiratory parameters were measured using phrenic nerve output and included duration of inspiration (Ti), respiratory cycle duration (Ttot), and integrated burst amplitude relative to baseline (Amp). All values are means ± SE. To compare rhythmicity, irregularity scores (IS) were calculated on a cycle-by-cycle basis using the formula [abs(Pn − Pn−1)/Pn−1] and then averaged over the 5-min window analyzed in each preparation. In this calculation, P = Ttot, or respiratory cycle length, and abs indicates the absolute value; hence, an IS value is produced, with values close to 0 reflective of highly regular rhythm and values close to 1 representing a highly arrhythmic pattern. As a visual representation of the IS, Ttot values were combined for each transection level and Poincaré plots were produced by plotting Ttot n against Ttot n+1 for each transection level. For the purpose of this article, “rhythmic” activity refers to activity whereby the standard deviation of Ttot is less than two times the mean Ttot, both within individual preparations and averaged over experimental groups. We describe the degree of rhythmicity based on the IS value: IS < 0.2 = highly rhythmic, 0.2 < IS < 0.4 = rhythmic, 0.4 < IS < 0.6 = modestly rhythmic, 0.6 < IS < 0.8 = weak, irregular rhythm, and IS > 0.8 = sporadic bursting.
Table 1.
Qualitatively described nerve activity for preparations not included in quantitative analyses
| Experiment No. | Bursting Initial Minutes Following Reperfusion | Response to NaCN Bolus Injection | Baseline Activity in L1 | Baseline Activity in PN | Baseline Activity in VN |
|---|---|---|---|---|---|
| SJ164 | PN | Tonic PN, VN, L1 + 4–5 rhythmic bursts PN, VN, L1 | Slowly oscillating from high to low level of tonic activity | Sporadic single-unit firing | Sporadic single-unit firing |
| SJ185 | VN PN | 7–8 rhythmic bursts in PN, VN, 2–3 rhythmic bursts in L1 | No baseline activity | Sporadic single-unit firing | No baseline activity |
| SJ170 | PN VN | Tonic PN, VN, L1 + 1 burst PN, VN, L1 | Robust activity, duration 6–10 s (2/min) | Sporadic single-unit firing | Sporadic low-amplitude firing, duration 0-5-2.5 s; occasionally synched with L1 (1–2/min) |
| SJ162 | VN PN | Tonic PN, VN, L1 + 4–5 rhythmic bursts PN, VN, L1 | Occasional slow wave-like activity, duration 18–29 s (2–3/h) | Occasional low-amplitude bursts (3–4/h), duration <4 s; occasional sporadic activity (2–3/h), duratoin 5–19 s | Occasional low-amplitude bursts, duration <4 s (2/h); occasional activity (2–4/h), duration 25–38 s |
| SJ163 | PN L1 VN | Tonic PN, VN, L1 + 3–4 rhythmic bursts VN, L1 | Mainly single-unit firing, 1 burst synched with VN burst | Slowly oscillating from high to low level of tonic activity | Sporadic single-unit firing; occasional bursts (3/h), 1 burst synched with L1 |
| SJ177 | PN VN | Tonic | No baseline activity | No baseline activity | No baseline activity |
| SJ176 | PN VN L1 | Tonic | Long, slow, robust bursts, duration 7–15 s (8/h) | Sporadic single-unit firing | Sporadic single-unit firing |
Data are qualitative descriptions of nerve activity for preparations that did not exhibit rhythmic respiratory motor output (as defined in materials and methods) and therefore were not included in quantitative analyses. Activity is described in phrenic nerve (PN), vagus nerve (VN), and abdominal nerve (L1).
For each 5-min sample analyzed, we counted the number of bursts that were synchronized in their onset throughout all three motor outputs, as well as the number of bursts that were only present in one or more of the recorded motor outputs, in all possible iterations. In a subset of preparations transected at a rostral level, two different and clearly identifiable burst patterns existed. In such instances the latency between the lower amplitude bursts (burstlet type) that preceded the larger amplitude bursts was measured. These latency values were also compiled into Poincaré plots, with latencyn plotted against latencyn+1.
Statistical Analysis
Comparison of Ttot, Ti, PNA amplitude, and IS was performed with a one-way ANOVA (GraphPad Prism) restricted to comparison of the values of the intact pontomedullary preparations. A P value <0.05 was considered to be significant. For comparison of statistical differences of respiratory parameters between individual transection levels 1–4, we used a two-tailed unequal variance t-test (Excel).
Histological Verification of Transection Levels
After nerve recordings had ended and arterial perfusion of the preparation ceased, the remaining brain stems were dissected out of the preparation up to cervical segment 2, postfixed for 48 h in 0.1 M phosphate buffer (pH 7.35) containing 4% paraformaldehyde and 0.2% picric acid, and then transferred to 0.1 M phosphate buffer (pH 7.4) containing 10% sucrose for a further 48 h. The brain stems were then blocked and frozen using compressed CO2, and a cryostat (Leica Biosystems, Richmond, IL) was used to cut 50-μm free-floating coronal sections from the rostral surface of the brain stem block; i.e., the first intact coronal section caudal to the transection cut was collected. These first intact coronal sections were mounted, counterstained with 0.25% cresyl violet (Sigma-Aldrich), rinsed through graded ethanol solutions, and coverslipped with DPX (Sigma-Aldrich).
Criteria for a Degenerate Rhythm Generator
In the present study we were looking for a degenerate rhythmogenic circuit capable of producing a respiratory rhythm that would be sufficient to maintain lung ventilation necessary for survival. Thus, to be classified as a rhythmogenic degenerate circuit, the respiratory activity has to fulfill two criteria: an IS value <0.4, an average baseline respiratory cycle length no longer than two times Ttot under intact conditions (see results).
RESULTS
Precollicular Transections: Intact Pontomedullary Brain Stem
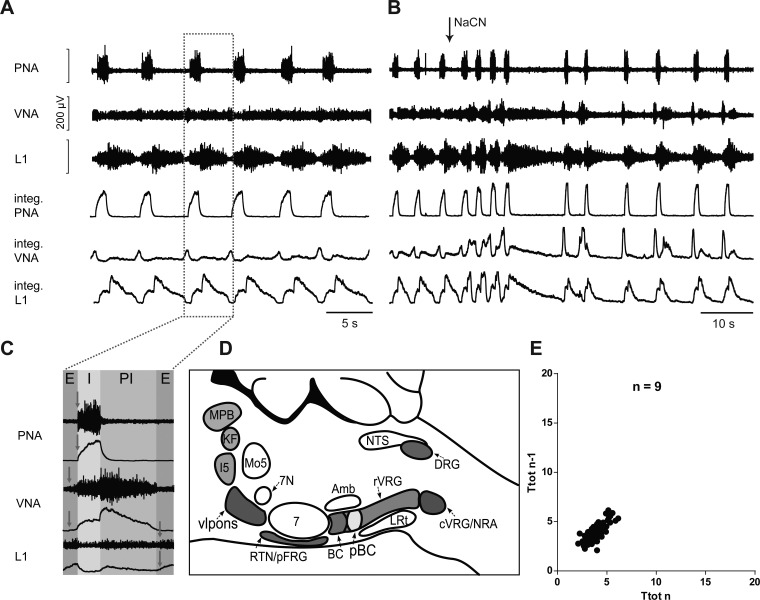
In the intact in situ perfused brain stem preparation, nerve discharge recorded from VN, PN, and L1 is reminiscent of a sequential eupneic respiratory motor output in vivo in terms of both rhythm and pattern. All preparations expressed a three-phase sequential motor pattern: inspiration, postinspiration, and active expiration. The sequential phases are expressed in their specific motor output (Fig. 1). The respiratory motor pattern evolves after reperfusion and reoxygenation and subsequent functional resurrection of the fully conserved pontomedullary respiratory network. In this study nine fully intact preparations were perfused and analyzed for means of comparison with experimentally reduced preparations. In the intact condition, mean Ttot = 3.89 ± 0.26 s, Ti = 1.01 ± 0.07 s, mean PNA amplitude = 237 ± 54.5 μV, and mean IS = 0.10 ± 0.01 as analyzed and averaged over representative 5-min samples in each preparation (Fig. 1).
Fig. 1.
A: original recordings of phrenic (PNA), vagal (VNA), and lumbar iliohypogastric nerve (L1) activity from the intact in situ perfused brain stem preparation, including the physiological response to sodium cyanide (NaCN) bolus injection of the intact preparation (B). integ., Integrated burst amplitude. C: enlarged single burst illustrates nerve discharge during phases of inspiration (I), postinspiration (PI), and expiration (E) in the intact preparation. Arrows indicate, for each nerve, the onset of nerve discharge within the respiratory cycle. D: schematic illustration of pontomedullary brain stem circuitry remaining intact following precollicular decerebration. E: Poincaré plots of respiratory cycle length (Ttot) averaged over 5 min from 9 intact preparations. Amb, nucleus ambiguus; BC, Bötzinger complex; c-rVRG, caudal and rostral ventral respiratory group; DRG, dorsal respiratory group; I5, intermediate reticular nucleus; LRt, lateral reticular nucleus; Mo5, motor trigeminal nucleus; MPB, medial parabrachial nucleus; NRA, nucleus retroambiguus; NTS, nucleus tractus solitarii; pBC, pre-Bötzinger complex; vlpons, ventrolateral pons; 7N, facial nerve.
A Priori Transected Brain Stem Preparations
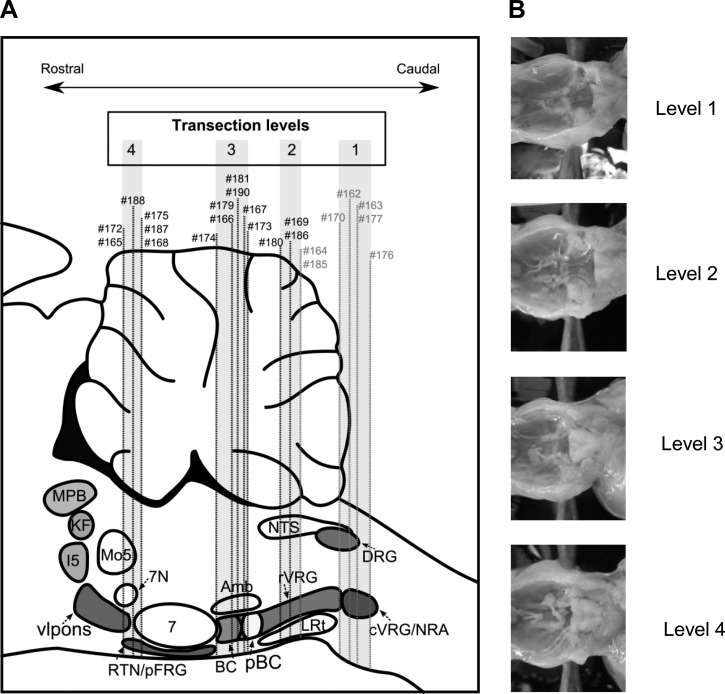
To test the hypothesis of degeneracy of respiratory network function, we transected the brain stem before reperfusion and reoxygenation (a priori transection) at four distinct rostrocaudal levels of the pontomedullary brain stem (Fig. 2) to investigate which network elements are capable of generating rhythmic respiratory motor output. A priori transverse transections were performed at the following coordinates (see Fig. 2A): 1.5 mm caudal to obex (level 1), at the level of the obex (level 2), 1.5 mm rostral to obex (level 3), and 3 mm rostral to obex at the pontomedullary junction (level 4). The transection levels made in each preparation were verified histologically post hoc and are marked on the schematic representation in Fig. 2A, and examples of a priori brain stem preparations for each level are illustrated in Fig. 2B. Of a total of 23 a priori transected brain stem preparations, 13 preparations produced spontaneous rhythmic activity after reperfusion and 7 preparations did not exhibit rhythmic respiratory activity, whereas 3 preparations did not develop spontaneous rhythmic bursting but had periods of rhythmic activity during and transiently after stimulation of arterial chemoreceptors with NaCN.
Fig. 2.
A: illustration of the anatomical location of the a priori transection at 4 distinct levels (level 1, 1.5 mm caudal to obex; level 2, obex level and rostral to obex; level 3, 1.5 mm rostral to obex; and level 4, 3 mm rostral to obex) in a schematic drawing of a sagittal section through the pontomedullary brain stem. Examples of respiratory activity and histology of individual preparations (#) are illustrated in subsequent figures. B: photographs showing examples of a priori transected brain stems.
Transections 1.5 mm caudal to obex (level 1).
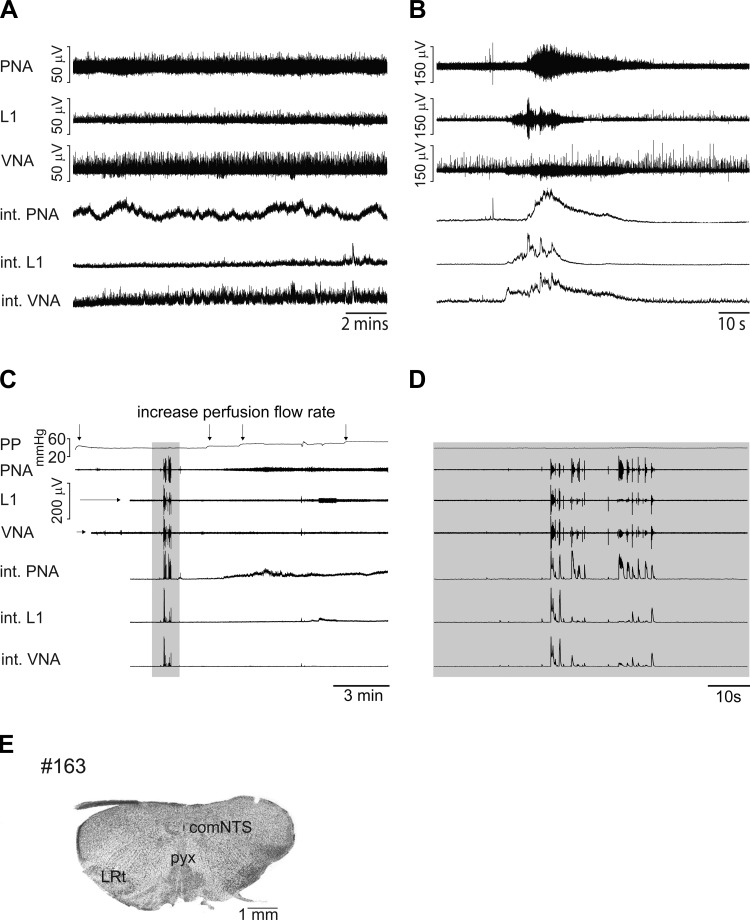
Five transections were made at level 1, targeted at the level of the cVRG/nucleus retroambiguus (NRA) 1.5 mm caudal to the obex. Post hoc histological verification showed the most caudal transection to be at the level of the pyramidal decussation and the most rostral to be at the most caudal tip of the lateral reticular nucleus (LRt). None of the preparations made at this level produced any stable rhythmic respiratory activity between 15 min and 2 h following reperfusion. PNA, VNA, and L1 showed tonic activity and sporadic bursting; however, none of the recorded motor outputs had rhythmic bursting (see Table 1). The most prominent activity was observed in recordings of spinal motor outputs (PNA and/or L1 iliohypogastric nerve recordings). Long-duration tonic activity waves above baseline activity (ranging from 10 to 40 s) were observed in a total of four preparations (see Table 1), and an example of such activity in the PNA is illustrated in Fig. 3A.
Fig. 3.
A: original recordings of phrenic (PNA), vagal (VNA), and lumbar iliohypogastric nerve (L1) activity from an a priori transected brain stem preparation at level 1 (1.5 mm caudal to obex). B: respiratory response to carotid body stimulation via intra-arterial bolus injection of sodium cyanide (NaCN). C: recording of transient and robust respiratory motor bursting (shaded area) during early stages of the reperfusion protocol. D: expanded view of initial motor bursting. E: photograph of the first intact coronal section cut from the a priori transected brain stem preparation. comNTS, commissural nucleus of the nucleus tractus solitarii; LRt, lateral reticular nucleus; pyx: pyramidal decussation.
In preparations transected caudal to the obex, stimulation of the arterial chemoreceptors with a bolus injection of NaCN (0.2%) triggered tonic drive throughout PNA, VNA, and L1 (Fig. 3B). In three of the five preparations, two to eight short bursts (0.8–3 s) that were synchronized across all three recorded motor outputs could be observed immediately following the NaCN bolus injection. Such NaCN-induced bursts were transient and sometimes obscured by tonic activity (see Fig. 3B).
An interesting observation in a priori transected preparations at level 1 (n = 5/5) and in the more caudally transected preparations at level 2 (preparations #164 and #185 in Fig. 2A) was a transient onset of robust bursting activity immediately after cannulation at initial low-flow perfusion (Fig. 3, C and D). This activity was only observed in caudally transected preparations and was not observed in any other type of a priori transected preparations or in the intact preparations. However, this activity was restricted to the initial minutes following cannulation and reperfusion, before the tissue was fully oxygenated, and never stabilized or reemerged upon full oxygenation of the caudal brain stem tissue. We speculate that this activity may relate to spinal cord-mediated respiratory bursting, which can be observed under experimental conditions of severe anoxia (see discussion).
Transections at obex (level 2).
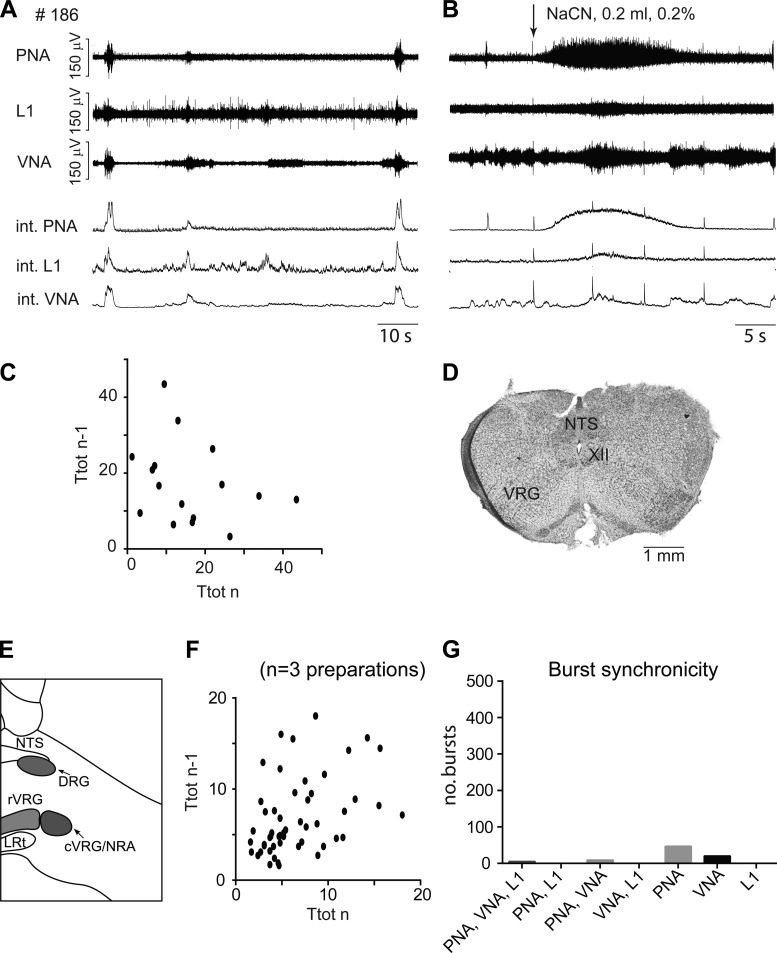
A priori transections targeted at level 2, transected at the level of the obex (n = 2; see preparations #164 and #185 in Fig. 2A) or slightly more rostral through the rostral ventral respiratory group (rVRG; n = 3; see preparations #169, #180, and #186 in Fig. 2A). The preparations transected at obex level behaved in a similar manner to preparations transected at level 1; they did not produce any rhythmic activity and are included in the qualitative description of the nonrhythmic preparations (see Table 1). The remaining three preparations with transection through the rVRG caudal to pre-BötC exhibited transient periods of sporadic respiratory bursting (IS = 1.10 ± 0.54; Fig. 4). From these preparations, mean Ti = 2.29 ± 0.23 s, Ttot = 9.47 ± 1.24 s, and the integrated burst amplitude relative to baseline (Amp) = 95.2 ± 16.0 μV. Compared with intact preparations, Ttot was highly significantly prolonged (P < 0.0001) and IS was significantly higher (P < 0.01; Table 2). Although respiratory bursts were sporadic according to the IS score, Ti and PNA amplitudes were not significantly different compared with the values in intact brain stem conditions.
Fig. 4.
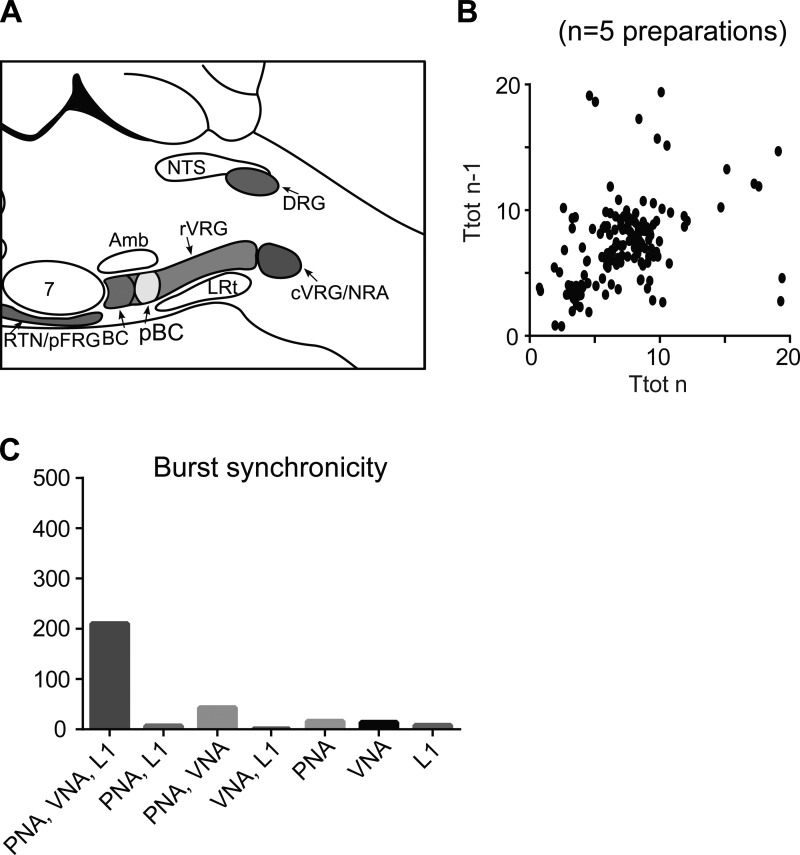
A: original recordings of phrenic (PNA), vagal (VNA), and lumbar iliohypogastric nerve (L1) activity from a priori transected brain stem preparation #186 at level 2 (obex). B: respiratory response to carotid body stimulation via intra-arterial bolus injection of sodium cyanide (NaCN). C: Poincaré plots of respiratory cycle length (Ttot) during the most rhythmic 5-min period of preparation #186. D: photograph of the first intact coronal section cut from the a priori transected brain stem preparation. E: schematic illustration of the transection level. F: Poincaré plots of Ttot from weakly rhythmic preparations that were a priori transected at level 2. G: bar diagram illustrating the synchronicity of bursting activity across the 3 recorded motor outputs. c-rVRG, caudal and rostral ventral respiratory group; DRG, dorsal respiratory group; LRt: lateral reticular nucleus; NRA, nucleus retroambiguus; NTS, nucleus tractus solitarii; XII, hypoglossal motor nucleus.
Table 2.
Statistical comparisons of variables at each transection level
Symbols indicate statistical comparisions of variables at each transection level with values for the intact preparation. Ttot, respiratory cycle length; Ti, inspiratory duration; PNA Amp, phrenic nerve activity amplitude; IS, irregularity score. P values were calculated using 1-way ANOVA test for multiple comparisons.
P < 0.011;
P < 0.001;
P < 0.0001; ns, not significant.
Over the total pooled 15 min of recordings in 3 preparations, 64 PNA bursts could be identified and analyzed, averaging 4.5 bursts/min (Fig. 4F). The respiratory activity produced, in addition to being irregular in terms of rhythm, never showed sequential motor patterning of the respiratory motor outputs with the numbers of synchronized bursts (onset of burst discharge simultaneously expressed in PNA, VNA, and L1, shown in Fig. 4G). Overall, 6% of bursts analyzed were synchronous across all three motor outputs, and 11% synchronized in phrenic and vagus nerves. The majority (58%) of bursts were present only in the phrenic nerve, and a further 25% were present only in the vagus nerve. Transections made at level 2 displayed mainly tonic rather than rhythmic bursting of respiratory motor output(s) in response to NaCN (see Fig. 4B).
Transections rostral to obex (level 3).
A priori transected brain stem preparations (n = 7) 2 mm rostral to the obex showed stable, rhythmic, low-amplitude respiratory motor bursting. Histological verification of each transection level revealed that the most caudal transection intersected the rostral edge of the pre-BötC and that the most rostral transection intersected the rostral edge of the Bötzinger complex (BötC). All 7 preparations produced periods of stable rhythmic respiratory bursting, with mean IS = 0.39 ± 0.12 after flow and pressure of the perfusion were adjusted. An example of the rhythmic motor activity of an a priori transected perfused brain stem preparation is illustrated in Fig. 5. Mean Ti = 0.92 ± 0.02 s, mean Ttot = 3.75 ± 0.12 s, and mean PNA amplitude = 16.8 ± 0.8 μV. Since the bursts were at low amplitudes, we refer to the bursting following level-3 transections as burstlet-like rhythmic activity. The analysis included a total of 541 bursts pooled from a 5-min recording period of all 7 preparations, giving an overall average of 15.5 bursts/min. However, in 1/7 preparations (preparation #179), the rhythmic activity was periodic and was interrupted for periods of silent motor discharge ranging from 49 s to 1.07 min. Of all the transections in the current study, those made at level 3 were most rhythmically stable as reflected in the relatively tight scatter shown in Poincaré plots for level-3 pooled Ttot data (see Fig. 5, C and F) and the relatively low IS score of 0.39 ± 0.12. Compared with measurements in intact preparations, only PNA amplitudes were significantly lower (P < 0.01).
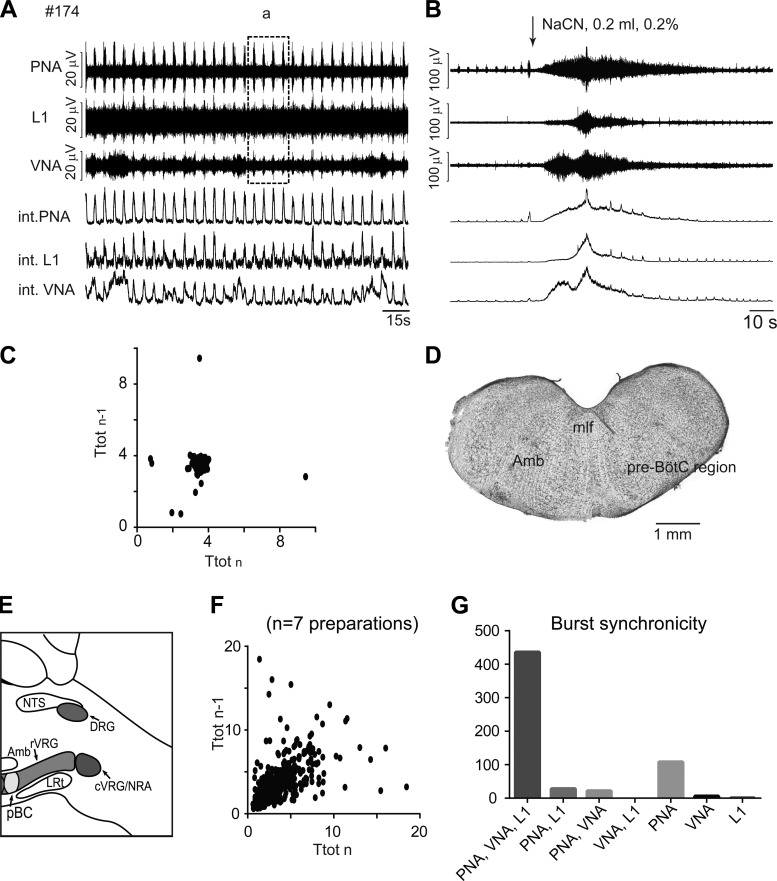
Fig. 5.
A: original recordings of phrenic (PNA), vagal (VNA), and lumbar iliohypogastric nerve (L1) activity from a priori transected brain stem preparation #174 at level 3 (1.5 mm rostral to obex). Note that recording area in box a is used for comparison with the activity from more rostrally transected preparations (see Fig. 7). B: respiratory response to carotid body stimulation via intra-arterial bolus injection of sodium cyanide (NaCN). C: Poincaré plots of respiratory cycle length (Ttot) from a rhythmic 5-min period of preparation #174. D: photograph of the first intact coronal section cut from the a priori transected brain stem preparation. E: schematic illustration of the transection level. F: Poincaré plots of Ttot from all rhythmic preparations a priori transected at level 3. G: bar diagram illustrating the synchronicity of bursting activity across the 3 recorded motor outputs. Amb, nucleus ambiguus; c-rVRG, caudal and rostral ventral respiratory group; DRG, dorsal respiratory group; LRt, lateral reticular nucleus; NRA, nucleus retroambiguus; mlf, medial longitudinal fasciculus; NTS, nucleus tractus solitarii; pre-BötC/pBC, pre-Bötzinger complex; XII, hypoglossal motor nucleus.
A priori transected brain stem preparations at level 3 showed the highest numbers of synchronized bursts expressed in all three motor outputs (PNA, VNA, and L1; see Fig. 5G). Seventy-three percent of the respiratory bursts generated in all 7 preparations were fully synchronized within all recorded motor outputs, a further 18% were present in the phrenic nerve alone, and ≤5% of bursts were expressed in any other nerve combination.
Preparations that included the pre-BötC (level 3) typically showed increased tonic activity across all three nerves in the augmentation phase in response to NaCN bolus injections. The strong tonic activity was often masking respiratory bursting, but typically two to five PNA bursts could be detected. The overall impact of the NaCN stimulation on the quality of the baseline rhythmic bursting was variable. In three of the seven preparations, baseline respiratory rhythm and amplitude remained unchanged after stimulation; in two of the seven preparations, baseline frequency and amplitude of rhythmic bursting were transiently reduced (3–5 min); and in two of the seven preparations, the amplitude of bursting and rhythmicity were transiently improved.
Transections 3 mm rostral to obex (level 4).
Transections (n = 6) were targeted at level 4, at the level of the pontomedullary junction, 3 mm rostral to the obex. Histological verification of transection sites revealed three of the preparations were transected at the level of the facial nerve, slightly rostral to the pontomedullary boundary of the brain stem. The other three preparations were transected at the rostral tip of the facial motor nucleus. All six preparations produced high-amplitude rhythmic respiratory activity. However, the mean IS of the high-amplitude bursting was modest at 0.56 ± 0.15. In four of the six preparations, rhythmic activity was sustained. In two preparations, however, rhythmic motor bursting was transiently interrupted by periods of absent motor bursting ranging from 48 s to 3 min 48 s. In general, the motor bursting was now, for the first time, characterized by high-amplitude bursting of PNA. The mean PNA amplitude was significantly higher (197.0 ± 19.0 μV) compared with that of PNA bursts recorded after transections at level 3 (16.8 ± 0.8 μV; t-test, P < 0.001). The high-amplitude PNA bursts recorded in 6/6 preparations had a mean Ti = 2.16 ± 0.18 s and mean Ttot = 7.39 ± 0.42 s, both significantly prolonged compared with values in intact control preparations (P < 0.01 and 0.001, respectively; Table 2). The analysis includes 244 respiratory bursts from the 5-min sample periods from 6 preparations. Preparations a priori transected at level 4 showed high-amplitude bursting activity of moderate rhythmicity, as indicated by the Poincare plots and IS value (Figs. 6B and 7C). The high-amplitude bursts were again highly synchronously expressed (70%) in all three recorded motor outputs (see Fig. 6C). Fourteen percent of the remaining bursts were synchronized in PNA and VNA, with the rest split between various nerve combinations, none exceeding 5%.
Fig. 6.
Analysis of high-amplitude bursts observed after transections at level 4. A: schematic illustration of the transection level. B: Poincaré plots of Ttot for high-amplitude phrenic nerve activity (PNA) of preparation a priori transected at level 4. C: bar diagram illustrating the synchronicity of burst activity across the 3 recorded motor outputs. Amb, nucleus ambiguus; BC, Bötzinger complex; c-rVRG, caudal and rostral ventral respiratory group; DRG, dorsal respiratory group; LRt, lateral reticular nucleus; NRA, nucleus retroambiguus; NTS, nucleus tractus solitarii; RTN/pFRG, retrotrapezoid nucleus/parafacial respiratory group.
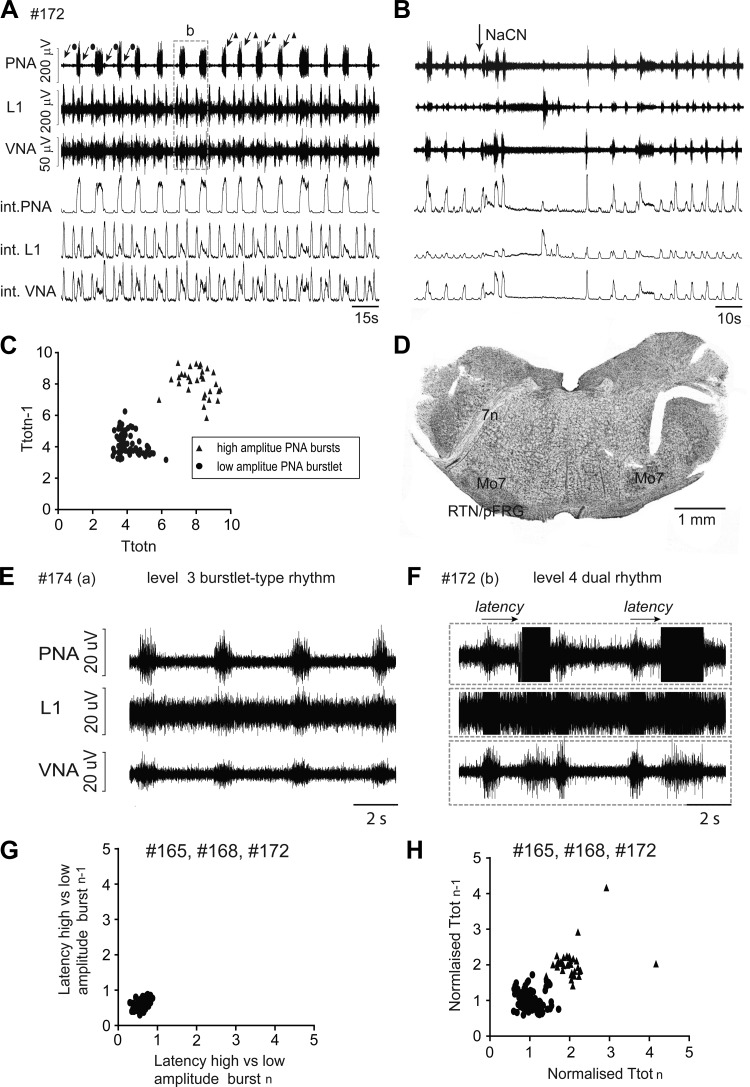
Fig. 7.
Original recordings of phrenic (PNA), vagal (VNA), and lumbar iliohypogastric nerve (L1) activity from a priori transected brain stem preparation #172 at level 4 (3 mm rostral to obex). Note the simultaneous expression of 2 distinct bursting rhythms with high (triangles) and low (circles) amplitudes in this preparation. B: respiratory response to carotid body stimulation via intra-arterial bolus injection of sodium cyanide (NaCN). C: Poincaré plots of respiratory cycle length (Ttot) of high- and low-amplitude bursts during 5-min recording period of preparation #172. D: photograph of the first intact coronal section cut from the a priori transected brain stem preparation. E and F: recordings show the similarity of low-amplitude burstlet-type rhythm observed after transection at level 3 (E) and level 4 (F). G: Poincaré plots of the latency between onset of burstlet-type activity and high-amplitude burst from all preparations that expressed dual rhythmic activity. H: Poincaré plots of Ttot from all rhythmic preparations that expressed dual bursting rhythms after a priori transection at level 4. 7n, facial nerve; Mo7, facial motor neurons; RTN/pFRG, retrotrapezoid nucleus/parafacial respiratory group.
In response to bolus injection of 0.2% NaCN, the most rostral transections (level 4) typically showed an initial brief augmentation of respiratory bursting (2–5 bursts), whereby the amplitude and frequency of bursts were increased, followed by a silent period of PNA before returning to baseline activity. NaCN failed to evoke the expression of sequential motor patterning during and after the burst frequency augmentation in all preparations.
Two distinct rhythms seen in preparations transected at level 4, rostral to pFRG.
In a subset of experiments with transection performed at level 4 (n = 3), two separate and easily identifiable bursting rhythms with low- and high-amplitude bursting in PNA were observed (Fig. 7). The bursting pattern in L1 and VN motor output was variable but tended to be higher amplitude during low-amplitude PN bursts compared with the high-amplitude PN bursts. The low-amplitude PN bursts showed short duration and thus had an identical discharge profiles to the burstlet-type pre-BötC activity observed in intact level-3 transected preparations, as well as exhibiting a similar level of stable rhythmicity (IS of burstlets = 0.37 ± 0.08 compared with level-3 IS = 0.39 ± 0.12; not significantly different). In level-4 preparations, the burstlet-type activity had a mean Ti = 1.00 ± 0.016 s (mean level-3 Ti = 0.92 ± 0.02 s), a mean Ttot = 3.93 ± 0.09 s (mean level-3 Ttot = 3.75 ± 0.12 s), mean Amp = 10.6 ± 0.54 μV (mean level-3 Amp = 16.8 ± 0.8 μV). All analyzed respiratory parameters were not significantly different compared with values obtained in preparations with level-3 transections.
Analysis of the relation between the dual bursting rhythms shows that Ttot values of the majority of the high-amplitude PNA were twice as long compared with those of low-amplitude burstlet-type rhythm (mean Ttot burstlet = 3.93 ± 0.09 s vs. mean Ttot high-amplitude bursts = 8.70 ± 0.59 s; P < 0.05), suggesting a 2:1 quantal relation of Ttot of burstlet-type activity and high-amplitude PNA. Finally, the mean latency between burstlets that preceded a large-amplitude PNA was highly consistent (0.64 ± 0.067 s; IS for latency = 0.17 ± 0.037), suggesting that burstlets may trigger a subsequent high-amplitude PNA burst.
DISCUSSION
In the present study we tested the theory of circuit degeneracy within the mammalian respiratory network. To do so, we used a priori transected arterially perfused brain stem preparations (Paton 1996), which were transected before reperfusion and resuscitation of respiratory network function. Our data support the hypothesis that the pre-Bötzinger complex (pre-BötC) located in the rostral medulla oblongata serves as a kernel for respiratory rhythm generation. Neural substrates caudal to the pre-BötC did not reveal any significant rhythmogenic capacity. Whereas the perfused medullary brain stem that included the pre-BötC generated low-amplitude rhythmic bursting (burstlet-type rhythm), only a priori transections made just rostral to the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) triggered sustained robust bursting of phrenic nerve activity (PNA). Although some of these experiments clearly show that a pre-BötC low-amplitude burstlet-type rhythm can trigger the robust PNA bursting in these preparations, physiological sequential motor patterning was never displayed. The latter observation indicates that the circuitry required for eupnea-like motor pattern formation may reside in more rostral pontine areas.
Rhythmogenic Capacity of the Caudal Medulla Oblongata
All preparations that were a priori transected 1 mm caudal to the obex failed to produce any stable rhythmic respiratory activity following sufficient reperfusion of the caudal medulla oblongata. Only nonrhythmic modulation of tonic neural activity in any of the recorded motor outputs was observed. The most caudal medullary tissue blocks contain the nucleus retroambiguus (NRA) as well as the spinal cord up to lumbar segment 3. According to previous publications, the expectation would be that at least some rhythmogenic activity could be generated by either the spinal cord (Aoki et al. 1980; Coglianese et al. 1977; Ford et al. 2016; Ghali and Marchenko 2015; Oku et al. 2008) or the NRA and surrounding caudal medullary area (Jones et al. 2012; Oku et al. 2008). In accordance with the present study, it was recently demonstrated that the dorsal aspects of most caudal medullary areas appear to have a role in respiratory pattern formation (Jones et al. 2015). However, no intrinsic rhythmogenic capacity of the most caudal medullary brain stem was identified (Jones et al. 2015). Preparations with a priori transections at level 2 that contained larger parts of VRG displayed periods of weak rhythmic respiratory motor activity. The observed bursting pattern of respiratory motor outputs did not match what would be considered normal, eupnea-like respiratory motor activity of an intact perfused brain stem preparation [see Fig. 1; i.e., presence of sequential inspiratory (∼1 s) and postinspiratory (∼2–4 s) motor phases (St-John and Paton 2003)]. The wide scatter of respiratory cycle lengths (Ttot) illustrated in the Poincare plots and IS from preparations transected at level 2 highlights the weak, irregular respiratory rhythmicity of the cVRG. Moreover, respiratory bursts were often expressed only in single channels of recorded respiratory motor outputs (see Fig. 4G), indicating that the observed sporadic respiratory bursting is not consistently transmitted to all recorded respiratory motor outputs. Our attempt to show a general capacity of the caudal aspects of the medulla oblongata for respiratory rhythm generation failed even after we provided strong excitatory drive to the reduced respiratory network via arterial chemoreceptor stimulation (NaCN bolus injection). NaCN consistently evoked only stimulus-dependent tonic discharge in the primary respiratory motor output (the phrenic nerve), and only occasional bursting activity was observed in the VN or L1 following NaCN administration.
In summary, the caudal medullary areas that include the dorsal respiratory group (Bautista and Dutschmann 2014; Jones et al. 2015), the NRA (Jones et al. 2015; Merrill 1970, 1974), and parts of the VRG (McCrimmon et al. 2000a; Monnier et al. 2003), which are all seen anatomically as integral parts of the rCPG (see Alheid et al. 2004), showed no intrinsic capacity for respiratory rhythm generation and pattern formation under the present experimental conditions.
Transient Anoxic/Hypoxic Respiratory Bursting in Caudally Transected Preparations
In preparations a priori transected caudal to the obex and in some preparations transected at obex level (see transection levels marked in gray, Fig. 2A), we consistently observed transient and robust respiratory bursting in phrenic nerve recordings during the initial minutes following brain stem reperfusion. At this stage of the experimental protocol the brain tissue is in severe anoxia (see Dutschmann et al. 2000; Wilson et al. 2001). The initial robust bursting pattern never stabilized into any persistent rhythmic activity at later stages of the experimental protocol when the brain stem tissue blocks become well oxygenated. The fact that such initial respiratory bursting could not be maintained or reactivated under conditions of sufficient caudal brain stem perfusion, and thus oxygenation, further renders the possibility that there may be burster neurons located in the most caudal aspects of the medulla oblongata. An alternative explanation for the initial reperfusion respiratory bursting could be linked to past and recent observations that the isolated cervical-thoracic spinal cord can trigger respiratory bursting in the absence of any brain structure (Ford et al. 2016; Ghali and Marchenko 2015; Wilson et al. 2015). In line with recent findings, respiratory bursting was only observed under extreme hypoxic conditions and stopped immediately upon reoxygenation (Wilson et al. 2015). Thus we suggest that the initial bursting is potentially generated by circuits within spinal cord.
Rhythmogenic Capacity of the Rostral Medulla Oblongata and Caudal Pons
A priori preparations transected at level 3 (1.5 mm rostral to obex) anatomically contain an intact pre-BötC. In accordance with previous data that strongly suggested the pre-BötC to be the rhythmogenic kernel of the mammalian respiratory network (Connelly et al. 1992; Feldman and Del Negro 2006; Feldman et al. 2013; Gray et al. 2001; Johnson et al. 2001; McCrimmon et al. 2000b; Ramirez et al. 2012; Richter and Smith 2014; Smith et al. 1991), these preparations displayed stable rhythmic activity. Although our experiments support the function of the pre-BötC as a rhythmogenic kernel, our data also provide some new insights. The cellular mechanisms underlying respiratory rhythm generation are usually investigated in neonatal slice preparations (Ballanyi and Ruangkittisakul 2009; Lieske et al. 2000; Ruangkittisakul et al. 2009; Smith et al. 1991) or en bloc brain stem spinal cord preparations (Onimaru et al. 1997; Onimaru and Homma 1992; Suzue 1984), often under the nonphysiological condition of elevated excitatory drive (e.g., high K+). Nevertheless, the physiological importance of the pre-BötC as the respiratory rhythm generator also has been verified in adult awake behaving animal preparations (Gray et al. 2001; McKay and Feldman 2008). In the present study, the new experimental approach using a priori transections now shows that the pre-BötC generates a low-amplitude burstlet-like rhythm (see Kam et al. 2013) that was expressed synchronously in phrenic, vagal, and iliohypogastric nerve recordings under conditions of physiological K+ concentrations (3 mM) in juvenile rat preparations. The synchronous motor activity implies that the primary pre-BötC-generated rhythm is broadcasted to the bulbospinal and propriospinal projection neurons located in the medulla oblongata (Tan et al. 2010) and from there into all primary respiratory motor pools. The frequency of the rhythmic burstlets initiated by the pre-BötC was remarkably close to the respiratory frequencies reported for the perfused preparation with a fully intact pontomedullary brain stem circuitry, both compared with control intact preparations in this study and compared with the baseline breathing frequencies experienced by other researchers using the same experimental preparation (Bautista and Dutschmann 2015; Paton et al. 2006; Pickering and Paton 2006; Smith et al. 2007).
The hypothesis that the rhythmic bursting of pre-BötC is converted into a robust respiratory motor output by a larger pontomedullary network (Alheid et al. 2004; Feldman and Del Negro 2006; Feldman et al. 2013; Ramirez et al. 2012; Smith et al. 2007) is supported by the finding that a priori transected preparations preserved the medullary brain stem, including at least parts of the caudal pontine RTN/pFRG. Some preparations with a priori transections that preserved the caudal pons expressed a clearly identifiable dual rhythmic activity. In this case the pre-BötC burstlet-like rhythm triggered the robust respiratory motor output with a remarkably constant latency (see Fig. 7). Although these data provide further support for the pre-BötC as a rhythmogenic kernel, the bursting activity remained synchronized across all recorded motor outputs and no sequential respiratory motor patterning emerged. Even stimulation of the arterial chemoreceptors with NaCN failed to force sequential motor patterning under the present experimental conditions. According to previous publications regarding dual oscillators, the expectation would be to see at least oscillations of inspiratory PNA with expiratory activity of the abdominal spinal cord motor output (Bautista and Dutschmann 2015; Janczewski and Feldman 2006; Molkov et al. 2013; Smith et al. 2013; Smith et al. 2007). However, even with stimulation of arterial chemoreceptors, no sequential motor pattern could be elicited, although previous studies demonstrated that at least the activation of chemosensitive pathways should have triggered transient abdominal expiratory motor activity (Abdala et al. 2009; Guyenet et al. 2012). The reason for the absence of spontaneous or evoked expiratory motor activity under the present experimental conditions remains to be clarified. Whereas physical damage of neurons (injured dendrites and axons) that belong to the proposed expiratory oscillator (Guyenet 2012; Janczewski and Feldman 2006; Onimaru and Homma 2003) of the caudal pons is one explanation, the results of the present study also suggest that synaptic interactions with more rostral pontine aspects of the rCPG are required for the generation of a sequential respiratory motor pattern.
Respiratory Rhythm Generation Independent of Fast Synaptic Inhibition?
The evident lack of sequential motor patterning we observed in our a priori transected brain stem preparations, which traditionally depends on synaptic inhibition (Grillner and El Manira 2015; Grillner and Wallen 1985; Marder and Bucher 2001; Marder and Calabrese 1996; Richter and Spyer 2001), supports the hypothesis that primary respiratory rhythm generation occurs independently of synaptic inhibition (Feldman and Del Negro 2006; Ramirez et al. 1996). Interestingly, the pre-BötC burstlets were terminated in the absence of synaptically driven Kölliker-Fuse nuclei (KF)-mediated inspiratory off-switching (Dutschmann and Dick 2012; Dutschmann and Herbert 2006; Mörschel and Dutschmann 2009; Rybak et al. 2008; Rybak et al. 2004; Smith et al. 2007) but showed burst durations around the 1-s margin that are normal for the perfused brain stem preparation. This suggests the presence of pre-BötC intrinsic mechanisms for burst termination (Del Negro et al. 2001, 2002; Kottick and Del Negro 2015) independent of pontine inputs. Previous experiments demonstrated that genetic knockout or pharmacological block of synaptic inhibition did not entirely disrupt respiratory rhythm generation but triggered simultaneous discharge of inspiratory and postinspiratory neurons and motor outputs (Busselberg et al. 2001a, 2001b; Dutschmann and Paton 2002a, 2002b, 2002c). Thus the observed synchronized respiratory bursting in functionally different motor outputs may reflect a lack of synaptic inhibition. Although such synchronized motor output may also correspond to the motor pattern of hypoxic gasping (Paton et al. 2006; St-John and Paton 2002), the burstlet-type rhythm observed in this study matches neither the expected motor amplitude nor the frequency of gasping.
The lack of sequential respiratory motor patterning of a priori transected preparations that preserved the pre-BötC and the RTN/pFRG suggests that critical synaptic inputs required for the formation of the respiratory motor pattern reside in the pontine aspects of rCPG. According to present knowledge, the KF could be one the pontine structures that is capable of providing excitatory drive for inhibitory interneurons, i.e., glycinergic inspiratory and postinspiratory neurons (Ellenberger and Feldman 1994; Ezure et al. 2003; Janczewski et al. 2013; Rahman et al. 2015; Sherman et al. 2015). Excitatory drive and synaptic gating of these inhibitory medullary neuron populations is critical for the generation of neural oscillation, and thus respiratory pattern formation may therefore depend on pontine inputs (Dutschmann and Dick 2012; Rybak et al. 2004; Smith et al. 2007).
The observation that phrenic nerve activity displayed prolonged durations (>2 s) in the most rostrally a priori transected preparations, compared with discharge duration observed in preparations with intact pontomedullary circuits (see Bautista and Dutschmann 2015; Farmer et al. 2014; Paton et al. 2006; Paton 1996; Smith et al. 2007), reflects the absence of KF-mediated pontomedullary synaptic interactions required for a proper inspiratory off switch. However, other pontine sources (see Dutschmann and Dick 2012) such as the intertrigeminal region (Chamberlin 2004) or the A5 (Jodkowski et al. 1997) may also contribute to respiratory pattern formation but require further investigation.
Future Directions of A Priori Transected Perfused Brain Stem Preparations
This study has been the first to use an a priori transection approach to reduce the neural network in the in situ perfused brain stem preparation before the start of reperfusion and thus network resurrection. The results from our first experimental series have failed to provide compelling evidence for a degenerate circuit in the caudal brain stem with the capacity to produce a respiratory rhythm. However, these basic transection techniques could be refined to nuclei-specific lesioning with the use of genetically targeted ablations of rhythmogenic cell types such as DBX1 neurons of the pre-BötC region (Bouvier et al. 2010; Wang et al. 2014). Such techniques could be used to further advance our understanding of respiratory network function. For instance, it is interesting to reconsider the previous studies mentioned earlier that have found breathing to persist following ablation of the pre-BötC (Forster et al. 2014; Gray et al. 2001; Krause et al. 2009; McKay and Feldman 2008; Wang et al. 2002) and therefore still support the idea of degeneracy existing within the respiratory network. To reconcile our results with such studies, we suggest the possible presence of a degenerate circuit in the pons, rostral to the pre-BötC. We suggest cell-specific lesions of the pre-BötC neurons in conjunction with reperfusion of a priori transected brain stem preparations at varying levels through the pontomedullary brain stem. This could identify potential neurodegenerate circuits in the pons that can generate rhythmic motor output in the absence of functional pre-BötC rhythm generator (St-John and Bledsoe 1985).
Conclusions
Our experimental approach of resurrecting a priori transected brain stem tissue, instead of the acute lesioning or ablation of functional network components, provides new insights into the hierarchical organization of the respiratory pattern generator in mammals. The results from our a priori transected preparations reveal that the neural circuitry preserved in caudal brain stem transections do not possess degenerate neural substrates that are capable of generating rhythmic respiratory bursting that would be sufficient for the survival of an organism. All rhythmic activity of the caudally transected preparations (levels 1 and 2) was too irregular and too slow to translate into sufficient lung ventilation. The initial bursting seen during the onset of reperfusion of the most caudally transected preparations only occurred under anoxic to hypoxic conditions and ceased immediately with increasing perfusion, and thus increasing oxygenation, of the tissue. Such activity could provide a last line of defense under extreme hypoxic conditions but would not have the capacity to compensate for a failure of the primary rhythm generator. The only motor activity that was deemed sufficient to translate into proper lung ventilation was observed in the most rostrally transected preparations, those which contained the entire medullary aspect of the respiratory pattern generator, including the RTN/pFRG in the caudal pons (level 4). Importantly, we show for the first time that rhythmic and sufficient respiratory bursting in the phrenic nerve is triggered by a pre-BötC-mediated burstlet-type rhythm. The isolated pre-BötC rhythm would not be sufficient to ventilate the lungs either, because the motor drive to respiratory muscles would fail to generate enough force. This shows that although the pre-BötC is the primary rhythm generator, a larger network is required to convert the priming burstlets of the pre-BötC into a motor output capable of supporting ventilation. Finally, the observation that none of our a priori transected preparations displayed the normal sequential motor pattern (inspiration, postinspiration, expiration) as it is seen in perfused brain stem preparations with a fully intact pontomedullary rCPG, which strongly suggests that respiratory pattern formation depends on pontomedullary synaptic interactions. In summary, our data provide new supporting evidence for the accepted hypothesis of the pre-BötC as the primary rhythm generator. However, larger networks are required to convert the pre-BötC activity into a vital motor rhythm, and even larger pontomedullary networks are necessary to generate the sequential motor pattern required for the adaptation of breathing to behavior, cognition, and emotion. Despite failure to identify a caudal degenerate circuitry capable of producing and sustaining a ventilator breathing pattern, we are still supportive of the idea that the brain stem networks contain degenerate rhythmogenic respiratory circuits, whether they are located more rostrally in the pons or in close proximity to the core rhythm generator of the pre-BötC.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.J. and M.D. conception and design of research; S.E.J. and M.D. performed experiments; S.E.J. and M.D. analyzed data; S.E.J. and M.D. interpreted results of experiments; S.E.J. and M.D. prepared figures; S.E.J. and M.D. drafted manuscript; S.E.J. and M.D. edited and revised manuscript; S.E.J. and M.D. approved final version of manuscript.
REFERENCES
- Abbott SB, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci 31: 16410–16422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol 587: 3539–3559, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol 143: 105–114, 2004. [DOI] [PubMed] [Google Scholar]
- Aoki M, Mori S, Kawahara K, Watanabe H, Ebata N. Generation of spontaneous respiratory rhythm in high spinal cats. Brain Res 202: 51–63, 1980. [PubMed] [Google Scholar]
- Baghdadwala MI, Duchcherer M, Trask WM, Gray PA, Wilson RJ. Diving into the mammalian swamp of respiratory rhythm generation with the bullfrog. Respir Physiol Neurobiol 14: 37–51, 2016. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Ruangkittisakul A. Structure-function analysis of rhythmogenic inspiratory pre-Bötzinger complex networks in “calibrated” newborn rat brainstem slices. Respir Physiol Neurobiol 168: 158–178, 2009. [DOI] [PubMed] [Google Scholar]
- Bautista TG, Dutschmann M. Ponto-medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. J Physiol 592: 2605–2623, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista TG, Dutschmann M. The role of the Kölliker-Fuse nuclei in the determination of abdominal motor output in a perfused brainstem preparation of juvenile rat. Respir Physiol Neurobiol. First published August 5, 2015; doi: 10.1016/j.resp.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavitsaubie M, Champagnat J. Central control of breathing in mammals–neuronal circuitry, membrane-properties, and neurotransmitters. Physiol Rev 75: 1–45, 1995. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chédotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci 13: 1066–1074, 2010. [DOI] [PubMed] [Google Scholar]
- Burke PG, Kanbar R, Basting TM, Hodges WM, Viar KE, Stornetta RL, Guyenet PG. State-dependent control of breathing by the retrotrapezoid nucleus. J Physiol 593: 2909–2926, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busselberg D, Bischoff AM, Becker K, Becker CM, Richter DW. The respiratory rhythm in mutant oscillator mice. Neurosci Lett 316: 99–102, 2001a. [DOI] [PubMed] [Google Scholar]
- Busselberg D, Bischoff AM, Paton JF, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflügers Arch 441: 444–449, 2001b. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol 143: 115–125, 2004. [DOI] [PubMed] [Google Scholar]
- Coglianese CJ, Peiss CN, Wurster RD. Rhythmic phrenic-nerve activity and respiratory activity in spinal dogs. Respir Physiol 29: 247–254, 1977. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Dobbins EG, Feldman JL. Pre-Bötzinger complex in cats: respiratory neuronal discharge patterns. Brain Res 590: 337–340, 1992. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Johnson SM, Butera RJ, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. III. Experimental tests of model predictions. J Neurophysiol 86: 59–74, 2001. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Bötzinger complex inspiratory neurons in vitro. J Neurophysiol 88: 2242–2250, 2002. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol 2: 2443–2469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci 24: 1071–1084, 2006. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Mörschel M, Rybak IA, Dick TE. Learning to breathe: control of the inspiratory-expiratory phase transition shifts from sensory-to central-dominated during postnatal development in rats. J Physiol 587: 4931–4948, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Paton JF. Glycinergic inhibition is essential for co-ordinating cranial and spinal respiratory motor outputs in the neonatal rat. J Physiol 543: 643–653, 2002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Paton JF. Trigeminal reflex regulation of the glottis depends on central glycinergic inhibition in the rat. Am J Physiol Regul Integr Comp Physiol 282: R999–R1005, 2002b. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Paton JF. Inhibitory synaptic mechanisms regulating upper airway patency. Respir Physiol Neurobiol 131: 57–63, 2002c. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Wilson RJ, Paton JF. Respiratory activity in neonatal rats. Auton Neurosci 84: 19–29, 2000. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Gally JA. Degeneracy and complexity in biological systems. Proc Natl Acad Sci USA 98: 13763–13768, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Origins of excitatory drive within the respiratory network: anatomical localization. Neuroreport 5: 1933–1936, 1994. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Saito Y. Brainstem and spinal projections of augmenting expiratory neurons in the rat. Neurosci Res 45: 41–51, 2003. [DOI] [PubMed] [Google Scholar]
- Farmer DG, Bautista TG, Jones SE, Stanic D, Dutschmann M. The midbrain periaqueductal grey has no role in the generation of the respiratory motor pattern, but provides command function for the modulation of respiratory activity. Respir Physiol Neurobiol 204: 14–20, 2014. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–242, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75: 423–452, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiamma MN, O'Connor ET, Roy A, Zuna I, Wilson RJ. The essential role of peripheral respiratory chemoreceptor inputs in maintaining breathing revealed when CO2 stimulation of central chemoreceptors is diminished. J Physiol 591: 1507–1521, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford TW, Anissimova NP, Meehan CF, Kirkwood PA. Functional plasticity in the respiratory drive to thoracic motoneurons in the segment above a chronic lateral spinal cord lesion. J Neurophysiol 115: 554–567, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster H, Bonis J, Krause K, Wenninger J, Neumueller S, Hodges M, Pan L. Contributions of the pre-Bötzinger complex and the Kölliker-Fuse nuclei to respiratory rhythm and pattern generation in awake and sleeping goats. Prog Brain Res 209: 73–89, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghali MG, Marchenko V. Patterns of phrenic nerve discharge after complete high cervical spinal cord injury in the decerebrate rat. J Neurotrauma. First published October 23, 2015; doi: 10.1089/neu.2015.4034. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, El Manira A. The intrinsic operation of the networks that make us locomote. Curr Opin Neurobiol 31: 244–249, 2015. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallen P. Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci 8: 233–261, 1985. [DOI] [PubMed] [Google Scholar]
- Guyenet P. How does CO2 activate the neurons of the retrotrapezoid nucleus? J Physiol 590: 2183–2184, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Kanbar R. The retrotrapezoid nucleus and breathing. Adv Exp Med Biol 758: 115–122, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci 33: 5454–5465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodkowski JS, Coles SK, Dick TE. Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol 82: 377–381, 1997. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Koshiya N, Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Bötzinger complex “island”. J Neurophysiol 85: 1772–1776, 2001. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Funk GD, Feldman JL. Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. J Neurophysiol 72: 2598–2608, 1994. [DOI] [PubMed] [Google Scholar]
- Jones SE, Saad M, Lewis DI, Subramanian HH, Dutschmann M. The nucleus retroambiguus as possible site for inspiratory rhythm generation caudal to obex. Respir Physiol Neurobiol 180: 305–310, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Stanić D, Dutschmann M. Dorsal and ventral aspects of the most caudal medullary reticular formation have differential roles in modulation and formation of the respiratory motor pattern in rat. Brain Struct Funct. First published December 11, 2015; doi: 10.1007/s00429-015-1165-x. [DOI] [PubMed] [Google Scholar]
- Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. J Neurosci 33: 9235–9245, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottick A, Del Negro CA. Synaptic depression influences inspiratory-expiratory phase transition in Dbx1 interneurons of the preBötzinger complex in neonatal mice. J Neurosci 35: 11606–11611, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Bötzinger complex and the surrounding region. J Appl Physiol 106: 605–619, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A. Degenerate coding in neural systems. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 995–1010, 2005. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci 3: 600–607, 2000. [DOI] [PubMed] [Google Scholar]
- Lumsden T. Observations on the respiratory centres in the cat. J Physiol 57: 153–160, 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986–R996, 2001. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev 76: 687–717, 1996. [DOI] [PubMed] [Google Scholar]
- Markwald M. The Movements of Respiration. London: Blackie and Son, 1888. [Google Scholar]
- McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol 27: 126–131, 2000a. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Ramirez JM, Alford S, Zuperku EJ. Unraveling the mechanism for respiratory rhythm generation. Bioessays 22: 6–9, 2000b. [DOI] [PubMed] [Google Scholar]
- McKay LC, Feldman JL. Unilateral ablation of pre-Bötzinger complex disrupts breathing during sleep but not wakefulness. Am J Respir Crit Care Med 178: 89–95, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM. Degeneracy as a substrate for respiratory regulation. Respir Physiol Neurobiol 172: 1–7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG. Finding a respiratory function for the medullary respiratory neurons. In: Essays on the Nervous System, edited by Bellairs R, Gray EG. Oxford, UK: Clarendon, 1974, p. 451–486. [Google Scholar]
- Merrill EG. The lateral respiratory neurones of the medulla: their associations with nucleus ambiguus, nucleus retroambiguus, spinal accessory nucleus and the spinal cord. Brain Res 24: 11–28, 1970. [DOI] [PubMed] [Google Scholar]
- Molkov YI, Bacak BJ, Dick TE, Rybak IA. Control of breathing by interacting pontine and pulmonary feedback loops. Front Neural Circuits 7: 16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol 548: 859–874, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos Trans R Soc Lond B Biol Sci 364: 2517–2526, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. [DOI] [PubMed] [Google Scholar]
- Oku Y, Okabe A, Hayakawa T, Okada Y. Respiratory neuron group in the high cervical spinal cord discovered by optical imaging. Neuroreport 19: 1739–1743, 2008. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation. Jpn J Physiol 47: 385–403, 1997. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23: 1478–1486, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Whole cell recordings from respiratory neurons in the medulla of brain-stem-spinal cord preparations isolated from newborn rats. Pflügers Arch 420: 399–406, 1992. [DOI] [PubMed] [Google Scholar]
- Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci 65: 63–68, 1996. [DOI] [PubMed] [Google Scholar]
- Paton JF, Abdala APL, Koizumi H, Smith JC, St John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci 9: 311–313, 2006. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Paton JF. A decerebrate, artificially-perfused in situ preparation of rat: utility for the study of autonomic and nociceptive processing. J Neurosci Methods 155: 260–271, 2006. [DOI] [PubMed] [Google Scholar]
- Rahman J, Besser S, Schnell C, Eulenburg V, Hirrlinger J, Wojcik SM, Hulsmann S. Genetic ablation of VIAAT in glycinergic neurons causes a severe respiratory phenotype and perinatal death. Brain Struct Funct 220: 2835–2849, 2015. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Doi A, Garcia AJ 3rd, Elson FP, Koch H, Wei AD. The cellular building blocks of breathing. Compr Physiol 2: 2683–2731, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol 491: 799–812, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. J Physiol 507: 895–907, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Zuperku EJ, Alheid GF, Lieske SP, Ptak K, McCrimmon DR. Respiratory rhythm generation: converging concepts from in vitro and in vivo approaches? Respir Physiol Neurobiol 131: 43–56, 2002. [DOI] [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol 100: 93–107, 1982. [DOI] [PubMed] [Google Scholar]
- Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology 29: 58–71, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci 24: 464–472, 2001. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Okada Y, Oku Y, Koshiya N, Ballanyi K. Fluorescence imaging of active respiratory networks. Respir Physiol Neurobiol 168: 26–38, 2009. [DOI] [PubMed] [Google Scholar]
- Rybak IA, O'Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 100: 1770–1799, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Paton JF, Dick TE, St-John WM, Mörschel M, Dutschmann M. Modeling the ponto-medullary respiratory network. Respir Physiol Neurobiol 143: 307–319, 2004. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol 73: 1452–1461, 1995. [DOI] [PubMed] [Google Scholar]
- Sherman D, Worrell JW, Cui Y, Feldman JL. Optogenetic perturbation of preBötzinger complex inhibitory neurons modulates respiratory pattern. Nat Neurosci 18: 408–414, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci 36: 152–162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John WM. Noeud vital for breathing in the brainstem: gasping–yes, eupnoea–doubtful. Philos Trans R Soc Lond B Biol Sci 364: 2625–2633, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM. Neurogenesis of patterns of automatic ventilatory activity. Prog Neurobiol 56: 97–117, 1998. [DOI] [PubMed] [Google Scholar]
- St John WM, Bledsoe TA. Genesis of rhythmic respiratory activity in pons independent of medulla. J Appl Physiol 59: 684–690, 1985. [DOI] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Neurogenesis of gasping does not require inhibitory transmission using GABAA or glycine receptors. Respir Physiol Neurobiol 132: 265–277, 2002. [DOI] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Defining eupnea. Respir Physiol Neurobiol 139: 97–103, 2003. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol 354: 173–183, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of preBötzinger complex neurons in adult rats. J Comp Neurol 518: 1862–1878, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voneuler C. On the central pattern generator for the basic breathing rhythmicity. J Appl Physiol 55: 1647–1659, 1983. [DOI] [PubMed] [Google Scholar]
- Wang H, Germanson TP, Guyenet PG. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci 22: 3755–3764, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J Neurosci 33: 7756–7761, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hayes JA, Revill AL, Song H, Kottick A, Vann NC, LaMar MD, Picardo MC, Akins VT, Funk GD, Del Negro CA. Laser ablation of Dbx1 neurons in the pre-Bötzinger complex stops inspiratory rhythm and impairs output in neonatal mice. Elife 3: e03427, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Derakhshan F, Roy A, Baghdadwala M, McDonald F, Scheibli E, Harris M, Dutschmann M. Spinal oxygen sensors (SOS) drive sympathetic activity that precedes, predicts and outlives phrenic gasps during hypoxia in the absence of the brainstem (Abstract). FASEB J 29: 859.7, 2015. [Google Scholar]
- Wilson RJ, Remmers JE, Paton JF. Brain stem Po2 and pH of the working heart-brain stem preparation during vascular perfusion with aqueous medium. Am J Physiol Regul Integr Comp Physiol 281: R528–R538, 2001. [DOI] [PubMed] [Google Scholar]