Abstract
Skeletal muscle fibers hypertrophy in response to strength training, with type II fibers generally demonstrating the greatest plasticity in regards to cross-sectional area (CSA). However, assessing fiber type-specific CSA in humans requires invasive muscle biopsies. With advancements in the decomposition of surface electromyographic (sEMG) signals recorded using multichannel electrode arrays, the firing properties of individual motor units (MUs) can now be detected noninvasively. Since action potential amplitude (APSIZE) has a documented relationship with muscle fiber size, as well as with its parent MU's recruitment threshold (RT) force, our purpose was to examine if MU APSIZE, as a function of its RT (i.e., the size principle), could potentially be used as a longitudinal indicator of MU-specific hypertrophy. By decomposing the sEMG signals from the vastus lateralis muscle of 10 subjects during maximal voluntary knee extensions, we noninvasively assessed the relationship between MU APSIZE and RT before and immediately after an 8-wk strength training intervention. In addition to significant increases in muscle size and strength (P < 0.02), our data show that training elicited an increase in MU APSIZE of high-threshold MUs. Additionally, a large portion of the variance (83.6%) in the change in each individual's relationship between MU APSIZE and RT was explained by training-induced changes in whole muscle CSA (obtained via ultrasonography). Our findings suggest that the noninvasive, electrophysiological assessment of longitudinal changes to MU APSIZE appears to reflect hypertrophy specific to MUs across the RT continuum.
Keywords: size principle, surface EMG decomposition, strength training, skeletal muscle fiber type
skeletal muscle fibers demonstrate a marked plasticity in response to external stimuli. For instance, evidence from cross-sectional (Schantz et al. 1981) and longitudinal (Hather et al. 1991) investigations suggests that chronic resistance exercise (i.e., strength training) elicits increases in the cross-sectional area (CSA) of muscle fibers. This presumably contributes to the increase in whole muscle CSA (DeFreitas et al. 2011), although training-induced increases in CSA are variable between individuals (Hubal et al. 2005).
To better understand the adaptations to strength training, various approaches involving invasive and painful biopsies have been used to separate muscle fibers into “types” (Scott et al. 2001). However, the tendency of muscle fibers is to display more often a continuum of responses, rather than distinct groupings (Enoka and Duchateau 2015). Although the prevailing finding suggests that type II muscle fibers display a greater capacity for growth than their type I counterparts (Aagaard et al. 2001; Hather et al. 1991; Houston et al. 1983; McCall et al. 1996; Thorstensson et al. 1976), this response does not appear to be uniform within, or across, muscle fiber types (McCall et al. 1996).
In humans, force production is believed to be accomplished through the orderly activation of progressively larger motor units (MUs) in response to a net increase in excitatory synaptic drive to the MU pool (De Luca and Erim 1994). Activated at greater recruitment threshold force (RT) levels, these larger (high-threshold) MUs are also characterized as having greater action potential amplitude (APSIZE), twitch tension, and faster contraction times and are highly fatigable compared with the smaller (low-threshold) MUs (Milner-Brown et al. 1973; Stephens and Usherwood 1977; Tanji and Kato 1973). Although it has not been demonstrated directly in humans, the apparent alignment of functional characteristics regarding voluntary MU recruitment order and muscle fiber type are generally considered as evidence suggesting that low-threshold MUs are associated with type I muscle fibers, while high-threshold MUs are associated with type II muscle fibers.
With the recent technological advancements in the automated algorithms used for MU decomposition, surface electromyographic (sEMG) signals recorded using multichannel electrode arrays can now be used to discriminate MU action potential waveforms and their firing instances from a large population of individual MUs (De Luca et al. 2006; Nawab et al. 2010). Across large force ranges, numerous MU characteristics can now be simultaneously examined. Recently, Hu et al. (2013b) showed that sEMG can be used to demonstrate a strong relationship between a MU's APSIZE and its RT. In other words, sEMG decomposition can be used to examine the size principle (Henneman et al. 1965), thereby indicating the relative size of MUs across the entire force spectrum. Considering this, in conjunction with the documented relationship between APSIZE and muscle fiber circumference (i.e., larger fibers have larger amplitude action potentials in vitro) (Hakansson 1956), could MU APSIZE be used longitudinally as a noninvasive indicator of hypertrophy specific to MUs of different RT populations?
In attempt to answer this, our investigation provided 8 wk of strength training intended to induce muscle hypertrophy and increase strength. Both before and following the training intervention, we assessed MU APSIZE as a function of RT (MU APSIZE vs. RT) on the individual and group levels. The purpose of this study was twofold: 1) to examine if this noninvasive measure is longitudinally responsive to training, and 2) to evaluate if each individual's change in MU APSIZE was due to a respective change in whole muscle CSA. Evidence suggests that the plasticity of type II muscle fibers is greater than type I (Häkkinen et al. 1981a), which may explain why short-term (8–10 wk) strength training research studies observed hypertrophy of only type II muscle fibers (Houston et al. 1983; Thorstensson et al. 1976). Presuming that type II muscle fibers are likely constituents of high-threshold MUs, we hypothesized that the slope of the MU APSIZE vs. RT relationship would become steeper due to a increase in the APSIZE of the high-threshold MUs (compared to low-threshold MUs). Additionally, we hypothesized that the extent of change to an individual's MU APSIZE vs. RT relationship would be dependent on the extent of their training-induced muscle hypertrophy.
METHODS
Study design and experimental procedures.
Twenty young, recreationally trained males (mean ± SD: age = 22.2 ± 2.6 yr; body mass = 85.3 ± 14.8 kg; height = 1.8 ± 0.1 m; and resistance training experience ≥6 mo) performed 8 wk of high-intensity strength training (3 training sessions/wk). The training sessions consisted of 3 sets of 10 repetitions for 7 dynamic, resistance exercises (3 of which targeted the knee extensors) with a load roughly corresponding to each participant's 10 repetition maximum. Ultrasound images (US) and MU recordings were obtained from the vastus lateralis (VL) muscle of the dominant leg before (PRE) and after (POST) the training intervention. All participants were informed of the experimental procedures, risks, and their ability to withdraw from the study without penalty before signing consent. This investigation was conducted in accordance with the Declaration of Helsinki and was approved by the Oklahoma State University Institutional Review Board.
MU recordings and processing.
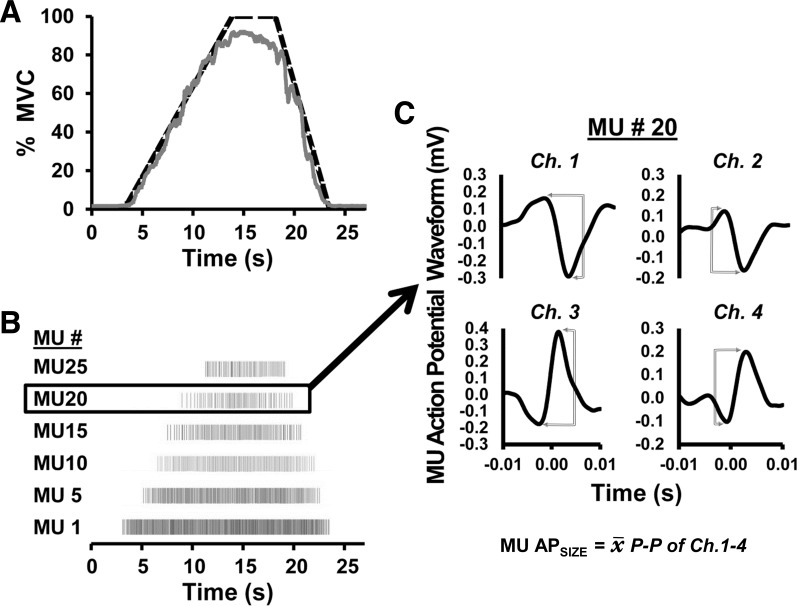
Following a brief warm up, three separate isometric maximal voluntary contractions (MVC) were performed using a force transducer attached to a cuff around the ankle (Model SSM-AJ-500; Interface, Scottsdale, AZ) with the participants seated upright and restrained (the hip and knee angles were ∼110 and 90°, respectively). Following the three MVC trials, each participant performed a MVC ramp contraction (MVCRamp) using a trapezoidal-shaped force trajectory. Illustrated in Fig. 1A, the trajectory increased linearly at a rate of 10% of MVC/s, was held at the maximal sustainable force level for 5 s, and followed by a linear return to zero (−20% MVC/s). Each participant was familiarized and practiced several submaximal versions of the trapezoidal-shaped ramp contractions during the warm-up using real-time visual feedback for accurate force trajectory replication. Sufficient rest was allotted between all trials to avoid fatigue. sEMG was recorded during the MVCRamp contractions using a five-pin surface electrode array and a 16-channel Bagnoli acquisition system (Delsys, Boston, MA). Following thorough, recommended skin preparation procedures, the specialized sensor was securely fastened over the VL muscle with hypoallergenic tape at approximately two-thirds the distance between the center of the muscle belly toward the distal tendon (Zaheer et al. 2012) and a reference electrode (Dermatrode; American Imex, Irvine, CA) was placed on the spinous process of the C7 vertebrae. The four channels of raw sEMG from the 5-pin sensor were decomposed into their constituent MU action potential trains (see Fig. 1B) using the Precision Decomposition III algorithm described by De Luca et al. (2006) and improved upon by Nawab et al. (2010). This algorithm has been shown to reliably discriminate the discharge characteristics of a large number of individual MUs up to maximal force levels (Nawab et al. 2010). The accuracy and validity of the decomposition process, as described by De Luca and Contessa (2012), have recently been independently validated using both simulated and naturally occurring signals (Hu et al. 2013a,b, 2014). Furthermore, the decomposition output provides four unique action potential template waveforms (1 from each sEMG channel) for each individual MU (see Fig. 1C, for example). The shape and size of the action potential waveforms have been shown to compare favorably with those derived using spike-triggered averaging (Hu et al. 2013b). The decomposition algorithm involves separate complex stages to identify, match, and update each MU's action potential waveform template. Figure 1C shows the resulting four MU action potentials templates from a single MU. These four waveforms represent a weighted average action potential shape and amplitude throughout the contraction from each of the four different sEMG channels. From each these four waveforms, the peak-to-peak amplitude (mV) was determined before averaging the four peak-to-peak amplitude values to calculate a single APSIZE representing that individual MU.
Fig. 1.
A: example of a maximal trapezoidal isometric contraction performed with a template (dashed line) and real-time force feedback (solid line). B: individual motor unit (MU) spike trains generated from the decomposition of the surface electromyographic (sEMG) signals. C: 4 unique action potential template waveforms (1 from each sEMG channel) from the decomposition output. The decomposition algorithm involves separate stages to first identify, match, and update each MU's action potential waveform template. The resulting 4 MU action potentials templates represent a weighted average action potential shape and amplitude throughout the contraction. Note that these 4 potentials all represent the same MU recorded from each of the 4 different sEMG channels. From each of these channels, MU peak-to-peak (P-P) amplitude (expressed as mV) was determined before averaging the 4 values to obtain a single MU APSIZE value (MU APSIZE). MVC, maximal voluntary contraction; Ch. 1–4, sEMG channels 1–4.
MU exclusion criteria and analysis.
All MUs that did not demonstrate at least 90.0% accuracy assessed using the Decompose-Synthesize-Decompose-Compare test (De Luca and Contessa 2012) were eliminated from all subsequent analyses. For the remaining MUs, custom-written software (using LabVIEW 2012; National Instruments, Austin, TX) was used to calculate the following properties for each identified MU; 1) RT, defined as the relative force level (%MVC) at the instance of the MU's first discharge; and 2) MU APSIZE, calculated by taking the average of the peak-to-peak amplitudes (mV) from each of the four action potential waveforms. Considering the regression analyses required to establish a sufficient relationship (see Statistical analysis) for MU APSIZE vs. RT, a RT range ≥25% of MVC was required (for both PRE and POST) for each participant to be included in any analysis (i.e., the MUs detected could not be clustered together across a small RT range). In such a case, the regression equation could be strongly affected by minimal changes to the limited range of data.
Ultrasonography.
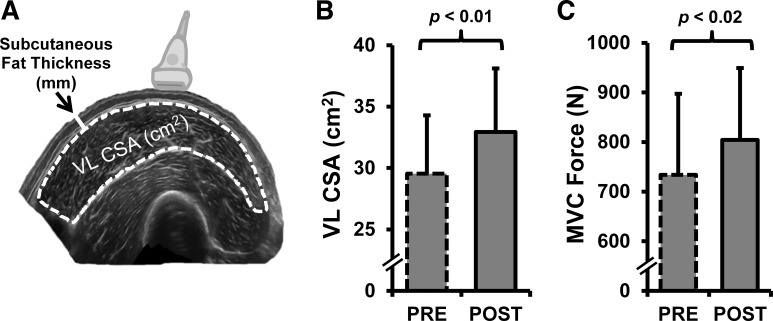
Transverse US images were obtained of each participant's VL muscle using procedures similar to those described previously (Ahtiainen et al. 2010). As demonstrated in Fig. 2A, real-time panoramic cross-sectional images were generated using B-mode US imaging with LogicView software (GE Logiq S8) and a linear-array probe (Model ML-615; 12 MHz). Equipment settings were optimized for image quality by a trained sonographer and held constant across visits. For each laboratory visit, whole muscle CSA (cm2) and subcutaneous fat thickness were measured for each of the US images using Adobe Photoshop (Version 12.1 × 32; Adobe Systems, San Jose, CA). CSA was measured by carefully selecting as much of the muscle as possible while avoiding the outer fascia. Subcutaneous fat thickness was measured as the linear distance (mm) between the VL's outer fascia and the dermis where the sEMG sensor array was to be attached to confirm that changes in MU APSIZE were not attributable to altered subcutaneous fat thickness. From the multiple US images obtained during each individual visit, the closest two were averaged to obtain a single CSA and subcutaneous fat thickness for PRE and POST, respectively.
Fig. 2.
A: cross-sectional ultrasound image of the vastus lateralis (VL). The dashed white line illustrates the assessment of cross-sectional area (CSA; expressed as cm2) and the solid white line demonstrates the measurement of subcutaneous fat thickness (taken at the location where the electrode array was to be attached). The group VL CSA and knee extension maximal voluntary contraction force (MVC; expressed as N) means from before (PRE) and after (POST) training are shown in B and C, respectively.
Statistical analysis.
All statistical analyses were administered using SPSS software (version 21, Chicago, IL). Paired-samples t-tests were conducted to compare the group means between PRE to POST using an α-level of P < 0.05 for statistical significance. Regression analyses were performed separately for PRE and POST MU data to determine the effect of our strength training intervention on the MU APSIZE vs. RT relationship. Recruitment threshold bin widths of 10% (e.g., 0–10, 10–20%, etc.) were utilized to condense the data. The average for each bin was used in the regression analyses. The group's PRE and POST data were assessed using polynomial regression analysis to determine the best fit model for the relationship (i.e., linear vs. quadratic vs. cubic). The statistical significance for the increment in the proportion of the variance that would be accounted for by a higher degree polynomial (i.e., F-test for R2-change) was determined using the F-test described by Pedhazur (1997a).
To test the hypothesis that high-threshold MUs would predominantly be affected by strength training, linear regression was applied separately to the low- and high-threshold MUs for a comparison between PRE and POST. MUs activated at <30% of MVC were classified as low threshold, while ≥30% of MVC was considered high threshold in the VL muscle as described previously (DeFreitas et al. 2014). Two tests for differences in linear slope coefficients (as described by Pedhazur 1997b) for the MU APSIZE vs. RT relationships were performed to separately compare the PRE and POST slopes of the low- and high-threshold MUs.
Linear regression was also used for each individual's PRE and POST training MU data to examine the change in slope coefficient for their MU APSIZE vs. RT relationship (AP-RTSLOPE; expressed as mV/%MVC). Lastly, each individual's change (PRE vs. POST) in AP-RTSLOPE and change in VL CSA were used in a linear regression model to determine what percentage of the variance in AP-RTSLOPE could be accounted for by the variance in whole muscle CSA, as assessed by the coefficient of determination (R2).
RESULTS
MU exclusion analysis.
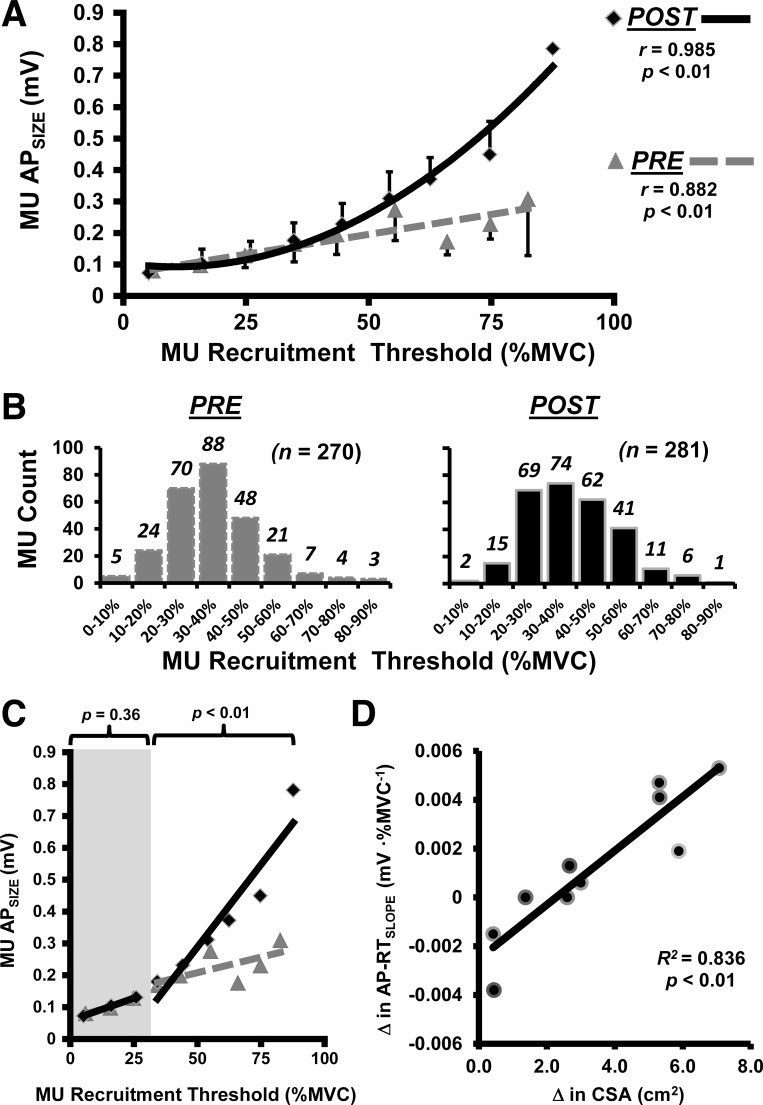
Of the original 20 participants, 10 did not meet the established MU criteria (i.e., a RT range < 25.0% of MVC for either PRE or POST) and were eliminated from all further analyses. From the remaining 10 participants, 632 MUs were initially detected (PRE = 299/POST = 327). Of these MUs, 81 were discarded due to poor decomposition accuracy (i.e., < 90.0%). The final number of MUs used for statistical analyses (PRE = 270 / POST = 281), as well as their distribution across RTs, can be seen in Fig. 3B. The MU numbers used and discarded (in parentheses) for each individual are shown in Table 1.
Fig. 3.
A: pooled group data from before (PRE; dashed gray regression line with triangle data points) and after (POST; solid black regression line with diamond data points) training. The data were assessed separately using polynomial regression to determine the best fit model for the relationship between motor unit (MU) action potential amplitude (MU APSIZE; expressed in mV) and recruitment threshold force [RT; expressed as a %maximal voluntary contraction (MVC)]. The vertical bars represent the SD within each bin (note the bars extend above for the POST data points and below for the PRE data points). B: final number of MUs used for statistical analyses, as well as their distribution across RTs. C: when the slope coefficients were assessed separately using linear regression, only the high-threshold (RT >30% of MVC) MUs experienced a significant increase with training. D: relationship observed between each individual's change (Δ) in cross-sectional area (CSA) and change in linear slope coefficient for their relationship between MU APSIZE vs. RT (AP-RTSLOPE).
Table 1.
Individual responses and grouped means for each variable from before and following 8 wk of strength training
| Before Training (PRE) |
After Training (POST) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | MUs | RT Range | MU APSIZE Range | AP-RTSLOPE | r | MVC | CSA | MUs | RT Range | MU APSIZE Range | AP-RTSLOPE | r | MVC | CSA |
| 1 | 28 (2) | 11.4–83.8 | 0.059–0.228 | 0.0018 | 0.965 | 688.4 | 29.38 | 29 (9) | 9.8–58.3 | 0.045–0.154 | 0.0018 | 0.961 | 798.6 | 30.75 |
| 2 | 28 (0) | 13.0–59.9 | 0.104–0.428 | 0.0067 | 0.973 | 553.8 | 25.39 | 31 (1) | 0.7–54.2 | 0.080–0.294 | 0.0029 | 0.855 | 598.7 | 25.83 |
| 3 | 40 (2) | 10.2–46.6 | 0.087–0.320 | 0.0077 | 0.991 | 827.2 | 37.97 | 35 (1) | 21.6–70.1 | 0.108–0.506 | 0.0062 | 0.898 | 961.8 | 38.39 |
| 4 | 38 (0) | 17.6–75.7 | 0.080–0.299 | 0.0032 | 0.945 | 598.7 | 27.86 | 28 (5) | 30.6–57.7 | 0.108–0.259 | 0.0045 | 0.998 | 643.6 | 30.53 |
| 5 | 18 (9) | 24.7–58.2 | 0.143–0.431 | 0.0036 | 0.953 | 998.5 | 30.83 | 31 (7) | 29.0–76.6 | 0.172–0.556 | 0.0077 | 0.987 | 970.0 | 36.15 |
| 6 | 25 (4) | 16.7–42.1 | 0.055–0.201 | 0.0040 | 0.923 | 480.4 | 30.62 | 23 (0) | 21.3–73.2 | 0.069–0.331 | 0.0040 | 0.977 | 594.6 | 33.22 |
| 7 | 19 (5) | 3.2–47.9 | 0.086–0.252 | 0.0030 | 0.949 | 876.1 | 26.41 | 33 (0) | 24.6–87.5 | 0.170–0.786 | 0.0083 | 0.906 | 884.3 | 33.48 |
| 8 | 29 (1) | 23.3–70.7 | 0.090–0.267 | 0.0030 | 0.962 | 855.7 | 36.05 | 21 (0) | 15.4–63.6 | 0.099–0.482 | 0.0077 | 0.982 | 921.0 | 41.36 |
| 9 | 26 (2) | 6.2–76.4 | 0.064–0.224 | 0.0020 | 0.980 | 659.9 | 28.85 | 29 (5) | 15.9–74.5 | 0.076–0.307 | 0.0039 | 0.980 | 868.0 | 34.73 |
| 10 | 19 (6) | 10.5–80.8 | 0.102–0.514 | 0.0062 | 0.984 | 798.6 | 21.96 | 21 (14) | 32.2–60.1 | 0.181–0.464 | 0.0068 | 0.845 | 802.7 | 24.96 |
| Mean | 27 (3.1) | 13.7–64.2 | 0.087–0.316 | 0.0041 | 0.963 | 733.7 | 29.53 | 28.1 (4.2) | 20.1–67.6 | 0.111–0.414 | 0.0054 | 0.939 | 804.3 | 32.94 |
| Mean change from PRE to POST | +0.0013 | +70.6 | +3.41 | |||||||||||
Data are the number of motor units (MU) analyzed with the number discarded due to inaccuracy in parenthesis, recruitment threshold (RT) range [expressed as a %maximal voluntary contraction force (MVC)], MU action potential amplitude (MU APSIZE) range (expressed in mV), slope coefficient (AP-RTSLOPE) from the MU APSIZE vs. recruitment threshold force (RT) linear regression (expressed as mV/% MVC), regression r value, MVC (expressed as N), and cross-sectional area (CSA) of the vastus lateralis (expressed as cm2) of each participant from before (PRE) and following (POST) 8 wk of strength training. The group means, as well as the mean change from PRE to POST training, are shown in the bottom row.
Ultrasonography.
Following our strength training intervention, our results revealed no significant change (P = 0.12) in subcutaneous fat thickness (PRE = 77.8 ± 18.5 mm; POST = 79.3 ± 19.7 mm). However, a significant (P < 0.01) increase of 13.7% was observed for VL CSA. The PRE and POST group means are illustrated in Fig. 2B. Each individual's PRE and POST CSA values are shown in Table 1.
MU APSIZE vs. recruitment threshold.
Following training, knee extension MVC force significantly increased (P < 0.02) by 9.6%. The PRE and POST group means are illustrated in Fig. 2C. The RT range and APSIZE range of the MUs analyzed for each participant (PRE and POST) can be seen in Table 1. The pooled PRE and POST group MU APSIZE vs. RT data were assessed separately using polynomial regression analysis to determine the best fit model for the relationship (Pedhazur 1997a). Figure 3A illustrates that the PRE data were best fit with a linear function (y = 0.0025x + 0.0692; r = 0.882; P < 0.01). However, the POST data were best fit with a quadratic function (y = 0.0001x2 −0.0023x + 0.1048; r = 0.985; P < 0.01). A qualitative examination of Fig. 3A seemingly indicates that the APSIZE of MUs with higher RTs experienced the largest increases following our strength training intervention. We further examined this hypothesis using linear regression (MU APSIZE vs. RT) applied separately to the low- and high-threshold MUs to test for significant changes to the linear slope coefficients (Pedhazur 1997b). As seen in Fig. 3C, there was no significant difference (P = 0.36) between PRE and POST for the low-threshold MUs. The high-threshold MUs, however, did show a significant increase (P < 0.01) in slope.
For each individual participant, linear regression analyses were performed to obtain AP-RTSLOPE for comparison (PRE vs. POST). Positive AP-RTSLOPE values (i.e., orderly recruitment of MUs of increasing APSIZE with increased excitation/force) were observed for every participant during every trial. Table 1 shows each participant's AP-RTSLOPEs, the group AP-RTSLOPE means, as well as the mean change from PRE to POST training. Despite a 31.7% increase (PRE = 0.0041 ± 0.0020 mV/%MVC; POST = 0.0054 ± 0.0023 mV/%MVC) in the group means, there was no significant difference (P = 0.20) in AP-RTSLOPEs following training; likely due to the considerable variability. However, VL CSA also demonstrated considerable variability (see Table 1). More importantly, as seen in Fig. 3D, the relationship observed between each individual's change in VL CSA and AP-RTSLOPE was strong (R2 = 0.836).
DISCUSSION
The purpose of this study was twofold: 1) to examine if a noninvasive measure of MU APSIZE is longitudinally responsive to 8 wk of strength training, and 2) to evaluate if each individual's change in MU APSIZE was due to a respective change in whole muscle CSA (obtained via ultrasonography). Despite some anticipated variability between participants (Hubal et al. 2005), there was a significant increase in knee extension MVC force and VL CSA with training. The change we observed in the MU APSIZE vs. RT relationship indicates an increase in the MU APSIZE of the higher threshold MUs following the training intervention. Furthermore, the change in this relationship was highly correlated with changes occurring in whole muscle CSA. Following a discussion of some potential physiological mechanisms, we will summarize some unique aspects of our approach, potential applications, as well as the current limitations that require methodological refinement before any widespread use.
During our investigation, MUs were recruited in accord with Henneman's “size principle” (i.e., increasing APSIZE with increasing excitation/force suggests that the smaller MUs were recruited before the larger ones) (Henneman and Olson 1965; Hu et al. 2013b; Olson et al. 1968; Tanji and Kato 1973) throughout the entire force spectrum. As previously demonstrated, this orderly recruitment was evident before and was not affected by training (Van Cutsem et al. 1998). The change we observed in the MU APSIZE vs. RT relationship indicates that training elicited an increase in MU APSIZE of predominantly the high-threshold MUs. Speculatively, this observation offers support to the presumed relationship between high-threshold MUs and type II muscle fibers in human muscles with mixed fiber composition. It is important to note, however, that fiber type distribution is variable between individuals (Staron et al. 2000) and the direct relationship with MU distribution across RTs requires further investigation. Häkkinen et al. (1998b) reported that the percentage values for type I muscle fibers were inversely correlated (r = −0.56) with training-induced whole muscle hypertrophy. Perhaps the ratio of muscle fiber types may have provided some explanation for the susceptibility for whole muscle hypertrophy reported in the current and previous research investigations (Hubal et al. 2005).
Our regression analysis revealed a strong correlation (R2 = 0.836) between each individuals' change in the slope coefficient of the MU APSIZE vs. RT relationship (AP-RTSLOPE) and change in muscle CSA (see Fig. 3D). Previous investigations have used muscle biopsies with traditional fiber typing techniques to investigate how the extent of change in fiber CSA contributes to change in muscle CSA, but these results have been incongruent. For example, McCall et al. (1996) found little correlation when comparing change in muscle CSA: vs. mean fiber CSA (R2 = 0.037), vs. type I area (R2 = 0.039), or vs. type II area (R2 = 0.125). Yet, Aagaard et al. (2001) found a considerably different relationship between the training-induced increase in muscle CSA vs. mean fiber CSA (R2 = 0.336). Conversely, Narici et al. (1996) found no change in mean fiber CSA, despite a muscle CSA increase of 19%. The substantial improvement in the variance explained by our approach may be the added benefit of expressing RT as a continuous variable across the continuum from 0 to 100% of MVC.
Aside from any presumed relationship regarding muscle fiber type, the adaptation we observed in the high-threshold MUs may be an artifact inherent to the size principle itself. That is, a MU's RT is positively related to its de-recruitment threshold and inversely related to its firing rate in vivo (De Luca and Contessa 2012). This hierarchal arrangement allows force to be produced in an efficient, controlled manner at low to moderate force levels (by MUs with low to moderate RTs), while high-threshold, fatigable, stronger MUs remain dormant until the force level requires their activation (De Luca and Contessa 2015). As a consequence, there exists a discrepancy in the nature of impulse activity conveyed by motor neurons to their respective muscle units. The uniformity of the muscle fibers comprising a single MU and evidence from cross-innervation research speak to the neural influence of impulse activity on muscle fiber adaptations (Dubowitz 1967). The high-force production required during our strength training intervention likely increased the impulse activity of these high-threshold MUs and subsequently may have elicited a number of intracellular responses/adaptations, ultimately requiring that sarcolemma fiber area increase to accommodate (i.e., fiber hypertrophy). With increased fiber hypertrophy, APSIZE likely increased because the current generated by an action potential is related to membrane area (Hakansson 1956).
The methodological approach we used exploits a foundational, unifying theory of MU organization and behavior (i.e., the size principle). Our data suggest that high-threshold MUs were predominantly affected by 8 wk of strength training. However, we only made the distinction between low and high threshold to statistically test the hypothesis of high-threshold MU hypertrophy and to discuss our findings in the context of previous research. With that said, we and others (Enoka and Duchateau 2015) stress the importance of moving away from simplified nomenclature as this impedes progress in the understanding of MU properties. Figure 3A illustrates the potential application of our approach to indicate MU-specific hypertrophy across the RT continuum.
We are not suggesting our approach replace the use of muscle biopsies. Instead, we feel that a combination of techniques should be employed to overcome the lack of “resolution” in describing MU attributes. Examining the size principle (as well as the corresponding MU firing rates) in conjunction with muscle biopsy, spike-triggered force averaging, and/or MU number estimation techniques could provide considerable insight and enhance our understanding of MU physiology. Alternatively, the noninvasive nature of our approach may be desirable for clinicians, investigations involving frequent sampling, and certain populations (e.g., children, individuals with trypanophobia).
Limitations.
The present study had a number of limitations that should be considered by future research. Within our investigation, 10 participants were excluded because they did not meet the recommended (i.e., 25% of MVC) RT range during both the PRE and POST testing sessions (see MU exclusion criteria and analysis). Unfortunately, the MUs successfully and accurately decomposed cannot be controlled. Another limitation that is important to acknowledge is that MU yield varied considerably between bins. As seen in Fig. 3B, both the very low- and very high-threshold MUs were underrepresented. Consequently, the regression analysis can be strongly affected by these limited data points. The high force levels we used limit the number of very low-threshold MUs extractable from the decomposition algorithm. Also, the physiological prevalence of very high-threshold MUs within a muscle can present an additional challenge. Accordingly, we recommend future research aim to best ensure that these populations of MUs are well represented. For example, the MU yield accurately detected and the RT range used for analysis may plausibly be improved by including an additional contraction(s) (i.e., pooling MUs from multiple contractions for each participant). However, this would first require a critical evaluation. Additionally, longitudinal investigations should use intermediate time points and control groups for validation. Furthermore, while the individuals that showed the largest increases in CSA also showed the largest increases in AP-RTSLOPE, the individuals that showed small increases in CSA did not necessarily exhibit small increases in AP-RTSLOPE (see Fig. 3D). This suggests our current approach may not be sensitive enough for detecting very small hypertrophic changes. More research is required for a more comprehensive understanding of the utility of this measure as a tool (e.g., reliability, sensitivity to change, additional potential applications, and threats to its validity).
Conclusions.
Our findings suggest that the noninvasive, electrophysiological assessment of the size principle (i.e., MU APSIZE as a function of RT) is longitudinally sensitive to change and may be a useful indicator of MU-specific hypertrophy. Using this approach, we demonstrate that 8 wk of strength training elicited an increase in the MU APSIZE of high-threshold MUs. There was also a strong positive relationship (R2 = 0.836) between the subject's change in the slope of MU APSIZE vs. RT relationship and their change in whole muscle CSA. This new assessment also explains a substantially larger percentage of the variance (83.6%) in whole muscle hypertrophy compared with the invasive biopsy studies that examined fiber size by type (only 3.7–33.6% of the variance explained, respectfully). An important advantage to our approach is that RT is a continuous variable that can fall anywhere on a continuum from 0 to 100% of MVC.
GRANTS
This research was made possible in total or in part by funding through the award for the project number HR-14-023 from the Oklahoma Center for the Advancement of Science and Technology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Z.K.P. and J.M.D. conception and design of research; Z.K.P., G.M.H., and F.M.B. performed experiments; Z.K.P., G.M.H., and F.M.B. analyzed data; Z.K.P. and J.M.D. interpreted results of experiments; Z.K.P. prepared figures; Z.K.P. drafted manuscript; Z.K.P., G.M.H., F.M.B., and J.M.D. approved final version of manuscript; J.M.D. edited and revised manuscript.
REFERENCES
- Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol 534: 613–623, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahtiainen JP, Hoffren M, Hulmi JJ, Pietikäinen M, Mero AA, Avela J, Häkkinen K. Panoramic ultrasonography is a valid method to measure changes in skeletal muscle cross-sectional area. Eur J Appl Physiol 108: 273–279, 2010. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Adam A, Wotiz R, Gilmore LD, Nawab SH. Decomposition of surface EMG signals. J Neurophysiol 96: 1646–1657, 2006. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Contessa P. Biomechanical benefits of the onion-skin motor unit control scheme. J Biomech 48: 195–203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Contessa P. Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107: 178–195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends Neurosci 17: 299–305, 1994. [DOI] [PubMed] [Google Scholar]
- DeFreitas JM, Beck TW, Stock MS, Dillon MA, Kasishke PR 2nd. An examination of the time course of training-induced skeletal muscle hypertrophy. Eur J Appl Physiol 111: 2785–2790, 2011. [DOI] [PubMed] [Google Scholar]
- DeFreitas JM, Beck TW, Ye X, Stock MS. Synchronization of low- and high-threshold motor units. Muscle Nerve 49: 575–583, 2014. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. Cross-innervated mammalian skeletal muscle: histochemical, physiological and biochemical observations. J Physiol 193: 481–496, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. Inappropriate interpretation of surface EMG signals and muscle fiber characteristics impedes progress on understanding the control of neuromuscular function. J Appl Physiol 119: 1516–1518, 2015. [DOI] [PubMed] [Google Scholar]
- Hakansson C. Conduction velocity and amplitude of the action potential as related to circumference in the isolated fibre of frog muscle. Acta Physiol Scand 37: 14–34, 1956. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Komi P, Tesch P. Effects of combined concentric and eccentric strength training and detraining on force-time, muscle fiber and metabolic characteristics of leg extensor muscles. Scand J Sports Sci 3: 50–58, 1981a. [Google Scholar]
- Häkkinen K, Newton RU, Gordon SE, McCormick M, Volek JS, Nindl BC, Gotshalk LA, Campbell WW, Evans WJ, Häkkinen A, Humphries BJ, Kraemer WJ. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol A Biol Sci Med Sci 53A: B415–B423, 1998b. [DOI] [PubMed] [Google Scholar]
- Hather BM, Tesch PA, Buchanan P, Dudley GA. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand 143: 177–185, 1991. [DOI] [PubMed] [Google Scholar]
- Henneman E, Olson CB. Relations between structure and function in the design of skeletal muscles. J Neurophysiol 28: 581–598, 1965. [DOI] [PubMed] [Google Scholar]
- Houston M, Froese E, Valeriote SP, Green H, Ranney D. Muscle performance, morphology and metabolic capacity during strength training and detraining: a one leg model. Eur J Appl Physiol Occup Physiol 51: 25–35, 1983. [DOI] [PubMed] [Google Scholar]
- Hu X, Rymer WZ, Suresh NL. Accuracy assessment of a surface electromyogram decomposition system in human first dorsal interosseus muscle. J Neural Eng 11: 026007, 2014. [DOI] [PubMed] [Google Scholar]
- Hu X, Rymer WZ, Suresh NL. Assessment of validity of a high-yield surface electromyogram decomposition. J Neuroeng Rehabil 10: 99, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Rymer WZ, Suresh NL. Motor unit pool organization examined via spike-triggered averaging of the surface electromyogram. J Neurophysiol 110: 1205–1220, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Siep RL, Clarkson PM. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 37: 964–972, 2005. [PubMed] [Google Scholar]
- McCall G, Byrnes W, Dickinson A, Pattany P, Fleck S. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol 81: 2004–2012, 1996. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol 230: 359–370, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Hoppeler H, Kayser B, Landoni L, Claassen H, Gavardi C, Conti M, Cerretelli P. Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiol Scand 157: 175–186, 1996. [DOI] [PubMed] [Google Scholar]
- Nawab SH, Chang SS, De Luca CJ. High-yield decomposition of surface EMG signals. Clin Neurophysiol 121: 1602–1615, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CB, Carpenter DO, Henneman E. Orderly recruitment of muscle action potentials: motor unit threshold and EMG amplitude. Arch Neurol 19: 591–597, 1968. [DOI] [PubMed] [Google Scholar]
- Pedhazur EJ. Curvilinear regression analysis. In: Multiple Regression in Behavioral Research: Explanation and Prediction. Fort Worth, TX: Harcourt Brace, 1997a, chapt. 13, p. 513–559. [Google Scholar]
- Pedhazur EJ. Continuous and categorical independent variables–I: attribute-treatment interaction; comparing regression equations. In: Multiple Regression in Behavioral Research: Explanation and Prediction. Fort Worth, TX: Harcourt Brace, 1997b, chapt. 14, 560–627. [Google Scholar]
- Schantz P, Fox E, Norgren P, Tydén A. The relationship between the mean muscle fibre area and the muscle cross-sectional area of the thigh in subjects with large differences in thigh girth. Acta Physiol Scand 113: 537–539, 1981. [DOI] [PubMed] [Google Scholar]
- Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther 81: 1810–1816, 2001. [PubMed] [Google Scholar]
- Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48: 623–629, 2000. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Usherwood TP. The mechanical properties of human motor units with special reference to their fatiguability and recruitment threshold. Brain Res 125: 91–97, 1977. [DOI] [PubMed] [Google Scholar]
- Tanji J, Kato M. Recruitment of motor units in voluntary contraction of a finger muscle in man. Exp Neurol 40: 759–770, 1973. [DOI] [PubMed] [Google Scholar]
- Thorstensson A, Karlsson J, Viitasalo JT, Luhtanen P, Komi PV. Effect of strength training on EMG of human skeletal muscle. Acta Physiol Scand 98: 232–236, 1976. [DOI] [PubMed] [Google Scholar]
- Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol 513: 295, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer F, Roy SH, De Luca CJ. Preferred sensor sites for surface EMG signal decomposition. Physiol Meas 33: 195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]