Abstract
After Ca2+ influx, mitochondria can sequester Ca2+ and subsequently release it back into the cytosol. This form of Ca2+-induced Ca2+ release (CICR) prolongs Ca2+ signaling and can potentially mediate activity-dependent plasticity. As Ca2+ is required for its subsequent release, Ca2+ removal systems, like the plasma membrane Ca2+-ATPase (PMCA), could impact CICR. Here we examine such a role for the PMCA in the bag cell neurons of Aplysia californica. CICR is triggered in these neurons during an afterdischarge and is implicated in sustaining membrane excitability and peptide secretion. Somatic Ca2+ was measured from fura-PE3-loaded cultured bag cell neurons recorded under whole cell voltage clamp. Voltage-gated Ca2+ influx was elicited with a 5-Hz, 1-min train, which mimics the fast phase of the afterdischarge. PMCA inhibition with carboxyeosin or extracellular alkalization augmented the effectiveness of Ca2+ influx in eliciting mitochondrial CICR. A Ca2+ compartment model recapitulated these findings and indicated that disrupting PMCA-dependent Ca2+ removal increases CICR by enhancing mitochondrial Ca2+ loading. Indeed, carboxyeosin augmented train-evoked mitochondrial Ca2+ uptake. Consistent with their role on Ca2+ dynamics, cell labeling revealed that the PMCA and mitochondria overlap with Ca2+ entry sites. Finally, PMCA-dependent Ca2+ extrusion did not impact endoplasmic reticulum-dependent Ca2+ removal or release, despite the organelle residing near Ca2+ entry sites. Our results demonstrate that Ca2+ removal by the PMCA influences the propensity for stimulus-evoked CICR by adjusting the amount of Ca2+ available for mitochondrial Ca2+ uptake. This study highlights a mechanism by which the PMCA could impact activity-dependent plasticity in the bag cell neurons.
Keywords: Aplysia californica, Ca2+-induced Ca2+ release, neuroendocrine cells, voltage-gated Ca2+ channels, Ca2+ buffering
intracellular ca2+ is a fundamental biochemical messenger that controls numerous processes in neurons including transmitter/peptide release, ion channel activity, gene expression, and aerobic metabolism (Berridge 1998; Chouhan et al. 2012; Clapham 2007). Changes to cytosolic Ca2+ are derived principally from plasma membrane voltage-gated Ca2+ channels but can also be provided by Ca2+-induced Ca2+ release (CICR) from intracellular organelles (Berridge 2002). Although CICR has classically been described as arising from the endoplasmic reticulum (ER), an analogous mechanism can also be mediated by the mitochondria and exists in a variety of vertebrate and invertebrate neuronal types (Colegrove et al. 2000a; Friel and Tsien 1994; Geiger and Magoski 2008; Groten et al. 2013; Lee et al. 2007; Tang and Zucker 1997; Werth and Thayer 1994). This process is initiated when Ca2+ derived from voltage-gated Ca2+ channels diffuses into the mitochondria through a Ca2+-selective ion channel on the inner mitochondrial membrane—the mitochondrial Ca2+ uniporter (MCU) (Baughman et al. 2011; Kirichok et al. 2004). Subsequently, Ca2+ is slowly released from the mitochondria into the cytosol by a Na+/Ca2+ and/or H+/Ca2+ exchanger (Carafoli et al. 1974; Palty et al. 2010, 2012).
As CICR transduces brief periods of Ca2+ influx into prolonged Ca2+ signals, it can mediate several forms of activity-dependent plasticity, including posttetanic potentiation of synaptic transmission (Garcia-Chacon et al. 2006; Lee et al. 2007; Tang and Zucker 1997), depolarizing afterpotentials (Partridge and Valenzuela 1999), and afterhyperpolarizations (Davies et al. 1996; Jobling et al. 1993). Considering the significance of these events, it is of central importance to discern the systems that dictate whether a given stimulus will elicit CICR. To date, most research has focused on the role of Ca2+ influx through voltage-gated Ca2+ channels (Colegrove et al. 2000a, 2000b; D'Arco et al. 2015; Friel and Tsien 1994). Alternatively, the propensity for CICR could be influenced by Ca2+ removal systems, such as the plasma membrane Ca2+-ATPase (PMCA) and Na+/Ca2+ exchanger (Clapham 2007). Because Ca2+ is required for initiating CICR, these plasma membrane systems, which operate alongside the mitochondria to remove Ca2+ from voltage-gated Ca2+ channels, would be expected to also impact CICR itself. In the present study, we examined such a role for the PMCA in the bag cell neurons of Aplysia californica.
Upon brief stimulation, these neuroendocrine cells undergo a prolonged (∼30 min) period of action potential firing called the afterdischarge (Kupfermann 1967; Kupfermann and Kandel 1970). This burst begins with a fast phase of action potential firing (∼5 Hz for ∼1 min) followed by a slow phase of firing (∼1 Hz for ∼30 min) (Kaczmarek et al. 1982). During this time, egg-laying hormone is released to initiate the high-priority fixed action pattern of egg laying behavior (Arch 1972; Loechner et al. 1990; Pinsker and Dudek 1977; Wayne et al. 1998). Prior work has established that the mitochondria contribute prominently to both Ca2+ removal and CICR after voltage-gated Ca2+ influx in cultured bag cell neurons (Geiger and Magoski 2008; Groten et al. 2013). Consequently, the mitochondria are implicated in sustaining peptide release and membrane excitability during the afterdischarge (Fisher et al. 1994; Hickey et al. 2010, 2013; Michel and Wayne 2002; Wayne et al. 1998).
Here we demonstrate that, in the presence of an exogenous intracellular Ca2+ buffer, Ca2+ extrusion by the PMCA limits both the amount of Ca2+ taken up by the mitochondria and the ability of Ca2+ influx to elicit Ca2+ release from this organelle. The influence of the PMCA is specific to mitochondrial Ca2+ signaling, as a similar phenomenon does not occur with ER Ca2+ uptake or release. This Ca2+ interplay could represent a mechanism by which Ca2+ removal systems impact activity-dependent Ca2+ signaling and possibly neuronal plasticity. For Aplysia, our findings suggest that changes in the function and/or expression of the PMCA could impact afterdischarge production and reproductive behavior.
MATERIALS AND METHODS
Animals and Cell Culture
Adult A. californica (a hermaphrodite) weighing 150–500 g were obtained from Marinus (Long Beach, CA), housed in an ∼300-liter aquarium containing continuously circulating, aerated seawater (Instant Ocean; Aquarium Systems, Mentor, OH) at 14–16°C on a 12:12-h light-dark cycle, and fed romaine lettuce five times per week. All experiments were approved by the Queen's University Animal Care Committee (Protocols Magoski-100323 and Magoski-100845). For primary cultures of isolated bag cell neurons, animals were anesthetized by an injection of isotonic MgCl2 (∼50% of body wt) and the abdominal ganglion was removed and treated with Dispase II (13.3 mg/ml; 165859, Roche Diagnostics, Indianapolis, IN) dissolved in tissue culture artificial seawater (tcASW) [composition in mM: 460 NaCl, 10.4 KCl, 11 CaCl2, 55 MgCl2, and 15 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), with 1 mg/ml glucose, 100 U/ml penicillin, and 0.1 mg/ml streptomycin, pH 7.8 with NaOH] for 18 h at 22°C. The ganglion was then rinsed in tcASW for 1 h, and the bag cell neuron clusters were dissected from their surrounding connective tissue. With the use of a fire-polished glass Pasteur pipette and gentle trituration, neurons were dissociated and dispersed in tcASW onto 35 × 10-mm polystyrene tissue culture dishes (353001, Falcon Becton-Dickinson, Franklin Lakes, NJ). Cultures were maintained in a 14°C incubator in tcASW and used for experimentation within 1–3 days. Salts were obtained from Fisher Scientific (Ottawa, ON, Canada) or Sigma-Aldrich (St. Louis, MO).
Whole Cell Voltage-Clamp Recording
Voltage-clamp recordings were made with an EPC-8 amplifier (HEKA Electronics, Mahone Bay, NS, Canada) and the tight-seal whole cell method. Microelectrodes were pulled from 1.5-mm-external-diameter, 1.2-mm-internal-diameter borosilicate glass capillaries (TW150F-4, World Precision Instruments, Sarasota, FL) and had a resistance of 1–2 MΩ when filled with intracellular saline (see below). For recording pipette junction potentials were nulled, and subsequent to seal formation pipette capacitive currents were canceled and the series resistance (2–5 MΩ) was compensated to 70–80% while the neuronal capacitance current was canceled. Current was filtered at 1 kHz by the EPC-8 built-in Bessel filter and sampled at 2 kHz with an IBM-compatible personal computer and a Digidata 1322A analog-to-digital converter and the Clampex acquisition program of pCLAMP software (v10.2, Molecular Devices, Sunnyvale, CA). Clampex was also used to set the holding and command potentials. Recordings were performed in Ca2+-Cs+-tetraethylammonium (TEA) ASW, as per tcASW but with the NaCl and KCl replaced by TEA-Cl and CsCl, respectively, and the glucose and antibiotics omitted (composition in mM: 460 TEA-Cl, 10.4 CsCl, 55 MgCl2, 11 CaCl2, 15 HEPES, pH 7.8 with CsOH). In some cases, the Ca-Cs-TEA external solution was alkalized with CsOH to produce a high-pH external solution (pH 8.8). Whole cell recordings used a Cs+-aspartate-based intracellular saline [composition in mM: 70 CsCl, 10 HEPES, 11 glucose, 10 glutathione, 5 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 500 aspartic acid, 5 adenosine 5′-triphosphate 2Na·H2O (ATP; A3377, Sigma-Aldrich), and 0.1 guanosine 5′-triphosphate Na·H2O (GTP; G8877, Sigma-Aldrich), pH 7.3 with CsOH]. In one set of recordings, EGTA was excluded from the intracellular solution. To image Ca2+ (see Calcium Imaging) under whole cell voltage clamp, the intracellular saline was supplemented with 1 mM fura-PE3 (0110, TEFLabs, Austin, TX).
Calcium Imaging
For Ca2+ imaging, 1 mM fura-PE3 was introduced by dialysis via the whole cell pipette during voltage-clamp recordings. Ca2+ imaging was performed with a Nikon TS100-F inverted microscope (Nikon, Mississauga, ON, Canada) equipped with a Nikon Plan Fluor ×20 [numerical aperture (NA): 0.5]. The light source was a 75-W Xe arc lamp and a multi-wavelength DeltaRAM V monochromatic illuminator (Photon Technology International, London, ON, Canada) coupled to the microscope with a UV-grade liquid-light guide. Excitation wavelengths were 340 and 380 nm. The excitation illumination was controlled by a shutter, which along with the excitation wavelength was controlled by the computer, a Photon Technology International computer interface, and EasyRatio Pro software (v1.10, Photon Technology International). To allow for continuous image acquisition during experiments, the shutter was left open. Emitted light passed through a 400-nm long-pass dichroic mirror and a 510/40-nm emission barrier filter before being detected by a Photometrics Cool SNAP HQ2 charge-coupled device camera (Photometrics, Tucson, AZ). The ratio of the emission following 340- and 380-nm excitation (340/380) was taken to reflect free intracellular Ca2+ (Grynkiewicz et al. 1985) and saved for subsequent analysis. Image acquisition, emitted light sampling, and ratio calculations were performed with EasyRatio Pro. Ca2+ measurements were acquired from a somatic region of interest (ROI) at approximately the midpoint of the vertical focal plane and one-half to three-quarters of the cell diameter. Camera gain was maximized, pixel binning was set at 2, and exposure time at each wavelength was fixed to 1 s. For presentation of ratiometric images in Fig. 7A, the background noise in the 340-nm channel was eliminated by applying an arbitrary threshold of 250 fluorescence units.
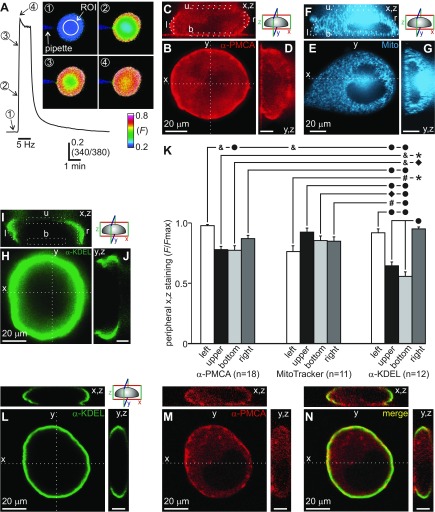
Fig. 7.
The PMCA, mitochondria, and ER are present near regions of voltage-gated Ca2+ influx in the soma. A: intracellular Ca2+ of a cultured bag cell neuron during the 5-Hz, 1-min train. Insets: ratiometric fura images of a neuron obtained with conventional fluorescence microscopy from the midpoint of the vertical somatic focal plane; the recording pipette and the region of interest (ROI) for data acquisition are indicated. Images are from the time points numbered on the sample trace. Inset 1, before stimulation, intracellular Ca2+ is low and homogeneous throughout the soma. Inset 2, shortly after the train onset, a rise in intracellular Ca2+ is apparent at the soma periphery. Insets 3 and 4, as the stimulus progresses, intracellular Ca2+ appears to propagate from the periphery toward the middle of the soma. B: representative 1-μm horizontal optical section (x-, y-axes) gathered by confocal microscopy at the midpoint of the vertical (z-axis) focal plane shows PMCA immunolabeling (1:200 anti-PMCA 1°; 1:200 Alexa Fluor 594 2°) of a fixed cultured bag cell neuron. PMCA immunolabeling is most abundant in association with the plasma membrane. Scale bar also applies to C. C and D: vertical (z-axis) cross sections dissect the cell into slices along the x (C) and y (D) planes. PMCA immunolabeling is present in most near-membrane regions, including the left (l), right (r), upper (u; away from the glass coverslip), and bottom (b; toward the glass coverslip) portions of the soma. Outlined regions denote the ROI used to quantify peripheral staining (see Live-Cell Staining, Immunocytochemistry, and Immunohistochemistry for details) Scale bar, 20 μm. E: a live cultured bag cell neuron labeled with MitoTracker Red (500 nM in DMSO) to stain mitochondria. Confocal images are pseudocolored blue to avoid confusion with the red PMCA immunolabeling in B–D. Representative 1-μm horizontal optical section (x-, y-axes) from halfway up the z-axis shows mitochondrial labeling. Mitochondria are distributed peripherally, near the plasma membrane, and in the cytosol surrounding the nucleus. Scale bar also applies to F. F and G: vertical (z-axis) cross sections dissect the cell along the x (F) and y (G) planes. Mitochondria are present throughout the cell and form a dense core above the nucleus. Scale bar, 20 μm. H: a fixed cultured bag cell neuron immunolabeled with α-KDEL to visualize the ER (1:200 anti-KDEL 1°; 1:200 Alexa Fluor 488 2°). Confocal image shows a 2-μm horizontal (x,y plane) cross section of ER staining at mid-z-axis. The ER is present in greatest proportion near the soma periphery. Scale bar also applies to I. I and J: vertical (z-axis) cross sections dissect the cell into x (I) and y (J) planes. ER is highly polarized toward the left and right regions, with substantially less in the upper and bottom portions. Scale bar, 20 μm. K: summary data showing the relative PMCA immunolabeling, mitochondrial MitoTracker staining, and KDEL/ER immunolabeling in the upper, bottom, left, and right regions in the x,z plane of the soma periphery. Fluorescence is normalized by dividing each ROI by the ROI with the greatest fluorescence in a given neuron (F/Fmax). Differences in labeling are apparent within and between the 3 groups (F11,156 = 13.88, P < 0.0001, 1-way ANOVA; *P < 0.02, ⧫P < 0.01, #P < 0.005, ▲P < 0.004, &P < 0.001, ●P < 0.0001, Tukey's post hoc test adjusted for multiple comparisons; see Supplemental Table S1 for the details of all statistical comparisons).1 PMCA labeling in the left region of the soma is significantly greater than in the upper and bottom regions but not the right. Any differences in mitochondrial labeling between regions fail to reach significance; however, the left region labeled for mitochondria is significantly less than the left region of PMCA labeling. ER labeling reveals that the left and right regions, while not significantly different from one another, are significantly greater than the upper and bottom regions. Also, labeling of ER in the upper region is significantly less than in the left and right regions of PMCA labeling, along with the upper, bottom, and right regions of mitochondrial labeling. Furthermore, ER labeling in the bottom region is significantly less compared with all regions of PMCA or mitochondria labeling. Finally, the right region of ER labeling is significantly greater than the upper and bottom regions of PMCA labeling, along with the left region of mitochondrial labeling. L and M: horizontal (1-μm width, x,y) and vertical (x,z and y,z) confocal sections of a cultured bag cell neuron double-labeled with anti-KDEL and anti-PMCA. N: overlay of the horizontal optical sections for anti-KDEL and anti-PMCA shows similarity in the distribution of the ER and PMCA at the soma periphery. Vertical cross sections in the x,z and y,z planes show that the ER and PMCA are both found in the middle portions of the soma, but this similarity is less obvious at the upper and bottom poles, where the PMCA, but not the ER, is more abundant.
Live-Cell Staining, Immunocytochemistry, and Immunohistochemistry
For all fluorescence microscopy other than Ca2+ imaging, bag cell neurons were prepared as described in Animals and Cell Culture, with the exception that cells were plated onto glass coverslips (no. 1, 12-542-B, Fisher Scientific) coated with 1 μg/ml poly-l-lysine hydrobromide (mol wt 300,000, P1534-25MG, Sigma-Aldrich) and glued with Sylgard silicone elastomer (SYLG184, World Precision Instruments) to holes drilled out of the bottom of the tissue culture dish. To visualize the mitochondria, cultured cells were stained with 500 nM MitoTracker Red CMXRos (M-7512, Invitrogen, Eugene, OR) in dimethyl sulfoxide (DMSO; BP231, Fisher Scientific) for 30 min and then washed with nASW (composition as per tcASW but with the antibiotics and glucose omitted). Because MitoTracker Red staining was not well preserved in the soma after fixation, mitochondria were imaged in living cells. On the other hand, fixed neurons were used to determine the ER distribution with immunocytochemistry and a rat monoclonal antibody against the highly conserved ER-retention signal, KDEL (Lys-Asp-Glu-Leu) (Munro and Pelham 1987) (ab50601, Abcam, Cambridge, MA). This antibody has been used successfully for this purpose in a range of animal species, including Aplysia (Lyles et al. 2006; O'Sullivan et al. 2012; Pierrot et al. 2013; Veiga-da-Cunha et al. 2010; Zhang and Forscher 2009). Similarly, PMCA immunocytochemistry was also performed on fixed neurons but with a mouse monoclonal antibody against the purified human erythrocyte PMCA (MA3-914, Thermo Scientific). The antibody recognizes an epitope between residues 724 and 783 of the human PMCA. At the amino acid level, this epitope is 70% identical and 93% homologous with a putative Aplysia PMCA homolog, which we identified in silico from the University of California Santa Cruz Sea Hare Genome Browser (http://genome.ucsc.edu/cgi-bin/hgGateway?hgsid=446561943_I0RJ4dOl771LBFSkiuH95nQ2cXBK&clade=other&org=Sea+hare&db=0).
For immunocytochemistry, culture dishes were first drained of all fluid except for the contents of the glass-bottomed well and new solutions were delivered by Pasteur pipette directly onto the cells. Neurons were then fixed for 25 min with 4% (wt/vol) paraformaldehyde (04042, Fisher Scientific) in 400 mM sucrose-nASW, pH 7.5 with NaOH. They were then permeabilized for 5 min with 0.3% (wt/vol) Triton X-100 (BP151, Fisher Scientific) in fix and washed twice with PBS (composition in mM: 137 NaCl, 2.7 KCl, 4.3 Na2HPO4, 1.5 KH2PO4, pH 7.0 with NaOH). Neurons were blocked for 60 min in a blocking solution of 5% (vol/vol) goat serum (G9023, Sigma-Aldrich) in PBS. For single-antibody labeling experiments, the primary antibody, either rat anti-KDEL IgG or mouse anti-PMCA IgG, was applied at 1:200 in blocking solution. Neurons were incubated in the primary antibody in the dark for 1 h and subsequently washed four times with PBS. The secondary antibody against either anti-KDEL (goat anti-rat IgG conjugated to Alexa Fluor 488; A-11006, Invitrogen) or anti-PMCA (goat anti-mouse IgG conjugated to Alexa Fluor 594; A-11005, Invitrogen) was applied at 1:200 in blocking solution and incubated in the dark for 2 h. Neurons were then washed four times with PBS, and the wells were filled with mounting solution [26% (wt/vol) glycerol (BP2291, Fisher Scientific), 11% (wt/vol) Mowiol 4-88 (17951, Polysciences, Warrington, PA), and 110 mM Tris, pH 8.5] and covered with a glass coverslip. For double-labeling experiments, cultured neurons were first processed for PMCA immunolabeling as described above. Subsequently, neurons were washed four times with PBS and then processed for ER immunocytochemistry with the KDEL antibody. Neurons were then washed four times with PBS, and the wells were filled with mounting solution and covered with a glass coverslip.
To visualize cultured bag cell neurons with a single label (MitoTracker Red, anti-KDEL, or anti-PMCA), a Quorum Wave FX-X1 spinning disk confocal system (Quorum Technologies, Guelph, ON, Canada) equipped with a ×40 (NA = 0.95) objective was used. Excitation light was provided by a laser line, and emitted light was passed through a Yokogawa CSU-X1 spinning disk head (Yokogawa, Calgary, AB, Canada) and an emission filter wheel before detection with a Hamamatsu Orca EM-CCD camera (model 09100-13, Hamamatsu Photonics, Bridgewater, NJ) operated with Metamorph imaging software (v1.0.2, Molecular Devices). The camera exposure time was set to 250 ms with a gain of 200 and laser power usually set to 30%. To visualize the ER, fixed neurons immunolabeled for KDEL were excited with light provided by a 491-nm laser line while the emitted light was passed through a 502- to 537-nm band-pass emission filter. MitoTracker Red and the PMCA immunolabeling (Alexa 594) were visualized by exciting neurons with a 568-nm laser and emission light passed through a 590- to 650-nm band-pass emission filter. Images of double-labeled cultured neurons were acquired with a Leica TCS SP2 multiphoton laser scanning confocal microscope (Heidelberg, Germany) equipped with a ×40 (NA = 0.75) Leica objective. Excitation light was provided by a laser line, and emitted light passed through a prism and was collected by photomultiplier tubes. For each focal plane, PMCA and KDEL immunolabeling were measured. To visualize the PMCA, neurons were excited with light provided by a 543-nm laser line, while the emitted light was collected from a bandwidth range between 575 and 615 nm. Conversely, KDEL immunolabeling was assessed by excitation with a 488-nm argon laser, while fluorescent emission was collected from a bandwidth range between 515 and 535 nm.
Confocal imaging stacks of ∼30–40 horizontal optical sections of 1- to 2-μm thickness were acquired along the entire vertical axis and saved for off-line analysis in ImageJ (v1.43; http://rsbweb.nih.gov/ij/). Images used for presentation were taken from the central portion of the soma in either the horizontal (x,y axes) or vertical (x,z and y,z) planes. A horizontal section divided the soma into upper (furthest from the glass bottom) and bottom (nearest the glass substrate) sections. Conversely, a vertical section divided the soma into left and right halves when viewing the dish perpendicular to the glass coverslip. Images presented as vertical cross sections were reconstructed with ImageJ by taking fluorescence measurements in the middle of the soma along the x- or y-axis of a given horizontal optical section. The measurements from each horizontal section in the entire image stack were then compiled to form a vertical optical section of the soma. Each horizontal optical section constituted 1 pixel (1–2 μm/pixel) in the z-axis of the vertical optical section, while the pixel density in the horizontal plane (x- or y-axis) was 0.227 μm/pixel. Thus, unlike horizontal sections, pixels in the scaled vertical cross sections were rectangular, not square.
Immunohistochemistry for KDEL was also performed on sections of bag cell neuron clusters from the abdominal ganglion. Abdominal ganglia were dissected from the animal (see Animals and Cell Culture) and fixed overnight at room temperature in 4% paraformaldehyde with 30% sucrose in 0.1 M sodium phosphate buffer (0.1 M NaHPO4·H2O and 0.1 M NaHPO4·7H2O) pH 7.3 with NaOH. The next day, ganglia were rinsed four times, 10 min each, at room temperature in 30% sucrose in sodium phosphate buffer. Fixed ganglia were tamped dry, mounted in Optimal Cutting Temperature embedding medium (4583, Miles, Elkhart, IN), frozen at −80°C, and cut into 6-μm coronal sections with a cryostat microtome. Sections were mounted on Superfrost Plus slides (12-550-15, Fisher Scientific) and stored at −20°C until being used.
Tissue sections were permeabilized for 10 min at room temperature with 2% (wt/vol) Triton X-100 in PBS (composition in mM: 137 NaCl, 2.7 KCl, 4.3 Na2HPO4, pH 7.0 with NaOH). After being washed four times, 10 min each, with PBS, sections were blocked for 30 min at room temperature in a blocking solution of 5% (vol/vol) goat serum in PBS. Rat anti-KDEL primary was applied at 1:200 in blocking solution, and sections were incubated overnight at 4°C in the dark. Sections were then washed four times, 10 min each, with PBS and incubated for 1 h in the dark at room temperature in 1:200 secondary goat anti-rabbit IgG conjugated to Alexa Fluor 488. Sections were then washed four times, 10 min each, with PBS, mounted in mounting medium, and covered with a glass coverslip.
Stained abdominal ganglion tissue sections were imaged with a Nikon TS100-F inverted microscope equipped with Nikon Plan Fluor ×10 (NA = 0.3) or Nikon Plan Fluor ×20 oil-immersion (NA = 0.75) objectives. Neurons were excited with a 50-W Hg lamp and a 480/15-nm band-pass filter. Fluorescence was emitted to the eyepiece or camera through a 505-nm dichroic mirror and a 520-nm barrier filter. For KDEL imaging (Alexa Fluor 488), neurons were excited with light supplied by a 50-W Hg lamp that was first passed through a 480/15-nm band-pass filter. Fluorescence was emitted to the eyepiece or camera through a 505-nm dichroic mirror and a 520-nm barrier filter. Images were acquired at the focal plane of either the neurites or soma with a Pixelfly USB camera (PCO-TECH, Photon Technology International) and the Micro-Manager (v1.4.5 http://micro-manager.org) plug-in for ImageJ with 100- to 2,000-ms exposure times.
Reagents and Drug Application
Solution exchanges were accomplished by manual perfusion using a calibrated transfer pipette to first exchange the bath (tissue culture dish) solution. In most cases where a drug was applied, a small volume (2–10 μl) of concentrated stock solution was mixed with a larger volume of saline (100–150 μl) that was initially removed from the bath, and this mixture was then pipetted back into the bath. Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP; 21857, Sigma-Aldrich), carboxyeosin (C-22803, Invitrogen), ryanodine (559276, Calbiochem, San Diego, CA), and cyclopiazonic acid (CPA; C1530, Sigma-Aldrich or 239805, Calbiochem) all required DMSO as a vehicle. The maximal final concentration of DMSO was 0.5% (vol/vol), which in control experiments as well as prior work from our laboratory had no effect on membrane potential, various macroscopic or single-channel currents, resting intracellular Ca2+, or Ca2+ transients evoked by a train of action potentials (Gardam et al. 2008; Geiger and Magoski 2008; Hickey et al. 2010; Hung and Magoski 2007; Kachoei et al. 2006; Lupinsky and Magoski 2006; Tam et al. 2009, 2011). Tetraphenylphosphonium chloride (TPP; 218790, Sigma-Aldrich) was prepared in water.
Analysis
Origin (v7, OriginLab, Northampton, MA) was used to import and plot ImageMaster Pro files as line graphs. Analysis of intracellular Ca2+ usually compared the steady-state value of the baseline 340/380 with the ratio from regions that had reached a peak or new steady state. Measurements of the baseline and peak regions were determined by eye or with five-point adjacent-averaging in Origin. CICR magnitude and the extent of Ca2+ removal after a stimulus train were quantified with measurements of posttrain area, but over different time periods. During circumstances where CICR was present, area was calculated by integrating the region above the prestimulus baseline from 1 to 11 min after the train. Measurements began at 1 min after the train to capture peak CICR and avoid including the initial recovery from Ca2+ influx. Under circumstances where CICR was eliminated with TPP, which inhibits mitochondrial Ca2+ exchangers (Karadjov et al. 1986; Wingrove and Gunter 1986), the efficacy of posttrain Ca2+ removal was quantified by measuring the area above baseline between immediately after the train to 10 min later. This timespan captures both the initial and late periods of posttrain Ca2+ decay. To plot the rate of Ca2+ removal as a function of intracellular Ca2+, the slope [(340/380)/t] of the posttrain decay period was determined at sequential time points with Excel (v14, Microsoft, Redmond, WA). A fitted slope was measured from the initial point of posttrain Ca2+ decay over the next five sequential time points, while incrementally shifting the start time until the end of the decay period. From this, a plot of Ca2+ decay rate vs. 340/380 was produced and fit with a polynomial function in Excel. The mitochondrial rate component (Rmit) was discerned by subtracting the rate of decay measured in FCCP (RFCCP) from the rate of decay in the absence of FCCP (Rcntl).
The staining distribution of MitoTracker Red and α-KDEL was quantified in ImageJ from vertical cross sections (x,z plane) at the midpoint of the soma. Mean fluorescence intensity was assessed from regions of interest (ROIs) in the left, right, upper, and bottom domains of the soma periphery. ROIs in the left and right poles consisted of polygons that outlined a portion near the apparent plasma membrane and spanned to a vertical boundary 5 μm from the leftmost or rightmost region of the cell (see Fig. 7, C, F, and I). For the upper and bottom poles, ROIs captured the region spanning between the apparent membrane edge and 5 μm in the intracellular direction. The upper and bottom ROIs were 50% of the maximum width of the soma and were centered at its midpoint (see Fig. 7, C, F, and I). For analysis, the mean fluorescence intensity of the left, right, upper, and bottom areas of the soma were normalized to the maximum fluorescence of the four regions in a given neuron.
Summary data are presented as bar graphs and error bars representing means and SE, respectively. Statistics were performed with InStat (version 3.0, GraphPad Software, San Diego, CA). The Kolmogorov-Smirnov method was used to test data sets for normality. If the data were normal, Student's paired or unpaired t-test (with Welch correction or Bonferroni correction as required) was used to test for differences between two means. If the data were not normally distributed, the Mann-Whitney U-test was used. Comparison between multiple means was performed with an ordinary one-way analysis of variance (ANOVA) followed by Tukey's multiple-comparisons post hoc test, where each P value was adjusted for multiple comparisons. Unless stated otherwise, all statistical comparisons were two-tailed, and means were considered significantly different if the P value was <0.05.
Model Development
A compartment model of intracellular Ca2+ dynamics was constructed to examine the interaction between voltage-gated Ca2+ entry, plasma membrane Ca2+ extrusion by the PMCA, and mitochondrial Ca2+ fluxes. The model was adapted from the functions and parameters that describe a similar phenomenon involving stimulus-evoked Ca2+ dynamics and mitochondrial Ca2+ release in bullfrog sympathetic neurons (Colegrove et al. 2000a, 2000b).
Plasma membrane Ca2+ flux.
| (1) |
| (2) |
| (3) |
| (4) |
where Jinflux is the rate of Ca2+ influx across the plasma membrane, kinflux refers to the Ca2+ permeability of the membrane, and [Ca2+]i and [Ca2+]e are the intracellular and extracellular Ca2+ concentrations, respectively. To produce Ca2+ influx in the model, kinflux was transiently increased and then reduced manually. JPMCA is the rate of plasma membrane efflux by the PMCA, Vmax,PMCA is the maximal rate of efflux by the PMCA, EC50,PMCA is the Ca2+ concentration at which JPMCA is half of Vmax,PMCA, and n,PMCA is the Hill coefficient that controls the sensitivity of JPMCA to changes in cytosolic Ca2+. In some of our experimental conditions, the PMCA is disrupted with carboxyeosin (a PMCA inhibitor) but CICR still recovers to prestimulus levels, indicating that a residual extrusion system must be involved. Thus we included a residual extrusion system to fulfill this function in the model and termed it Jextru—the rate of plasma membrane Ca2+ extrusion by residual Ca2+ removal systems. Vmax,extru is the maximal rate of extrusion, EC50,extru is the Ca2+ concentration at which Jextru is half of Vmax,extru, and n,extru is the Hill coefficient. Jpm is the net plasma membrane Ca2+ flux and is determined by the combined function of plasma membrane Ca2+ influx (Jinflux) and efflux (JPMCA and Jextru).
Mitochondrial Ca2+ dynamics.
| (5) |
| (6) |
| (7) |
| (8) |
where Juptake is the rate of mitochondrial Ca2+ sequestration, kmax,uptake is the mitochondrial uptake rate constant and represents the limiting slope at high cytosolic Ca2+, EC50,uptake describes the Ca2+ concentration at which uptake is half-maximal, and n,uptake is the Hill coefficient. The factor δ([Ca2+]i) describes the inhibition of mitochondrial Ca2+ extrusion by cytosolic Ca2+. Kinhib is the Ca2+ concentration at which inhibition of Jrelease is half-maximal, and n,inhib describes the sensitivity of inhibition to cytosolic Ca2+. Vmax,release is the maximal rate of Ca2+ release from the mitochondria, and EC50,release is the concentration of mitochondrial Ca2+ ([Ca2+]m) at which efflux rate is half of Vmax,release. Jmito is the net Ca2+ flux of the mitochondria.
Exogenous Ca2+ buffers.
| (9) |
where JEGTA is the rate of free cytosolic Ca2+ removal by EGTA (Nowycky and Pinter 1993), koff and kon are the reverse and forward reaction constants, respectively, [CaB] is the concentration of the Ca2+-EGTA complex, [Ca2+]i is the concentration of cytosolic Ca2+, and [B] is the concentration of free EGTA. Values for koff and kon (Table 1) were taken from Naraghi (1997), whereas [CaB] and [B] were calculated from the total EGTA concentration with MaxChelator (http://maxchelator.stanford.edu/CaEGTA-NIST.htm).
Table 1.
Parameter values used in the compartment model of intracellular Ca2+
| Definition | Model Variable | Value |
|---|---|---|
| Rate constant for plasma membrane Ca2+ influx | kinflux | 5 × 10−6 s−1 |
| Extracellular Ca2+ concentration | [Ca2+]e | 11 mM |
| [Ca2+]i at half-maximal rate of PMCA extrusion | EC50,PMCA | 378.8 nM |
| Hill coefficient for PMCA extrusion | n,PMCA | 1.8 |
| Maximal rate of PMCA extrusion | Vmax,PMCA | 28 nM/s |
| [Ca2+]i at half-maximal rate of residual extrusion | EC50,extru | 378.8 nM |
| Hill coefficient for residual extrusion | n,extru | 1.8 |
| Maximal rate of residual extrusion | Vmax,extru | 7 nM/s |
| [Ca2+]i at half-maximal rate of mitochondrial uptake | EC50,uptake | 10 μM |
| Hill coefficient for mitochondrial Ca2+ uptake | n,uptake | 2 |
| Rate constant for mitochondrial Ca2+ uptake | kmax,uptake | 10.3 s−1 |
| [Ca2+]m at half-maximal rate of release | EC50,release | 307 nM |
| Maximal rate of mitochondrial Ca2+ release | Vmax,release | 24.8 nM/s |
| Mitochondrial-to-cytosolic effective volume ratio | γ | 2 |
| [Ca2+]i at half-maximal release inhibition | Kinhib | 500 nM |
| Hill coefficient for release inhibition | n,inhib | 6 |
| Dissociation constant of EGTA | Kd,EGTA | 180 nM |
| Forward rate constant of EGTA | kon | 2.7 × 106 M−1 · s−1 |
| Reverse rate constant of EGTA | koff | 0.5 s−1 |
Collective Ca2+ dynamics.
| (10) |
| (11) |
where d[Ca2+]i/dt is the rate of change in cytosolic Ca2+, d[Ca2+]m/dt is the rate of change of mitochondrial Ca2+, and γ is the ratio of effective mitochondrial and cytoplasmic volumes. The γ value was taken from estimates in bullfrog sympathetic neurons (Colegrove et al. 2000b). The components describing mitochondrial Ca2+ uptake (EC50,uptake and n,uptake) were made in accordance with measurements from isolated mitochondria (Colegrove et al. 2000b; Gunter and Gunter 1994; Gunter and Pfeiffer 1990). All other parameter values (see Table 1) were within an order of magnitude of those estimated from the bag cell neurons or bullfrog sympathetic neurons (Colegrove et al. 2000b; Groten et al. 2013). The only time-dependent component of the model was the transient change in plasma membrane Ca2+ permeability (kinflux) to produce Ca2+ influx; all other parameters were active throughout each simulation. Differential equations were solved numerically with Euler's method written in MATLAB (v7.6; MathWorks, Natick, MA) with a time step of 75 ms to produce graphical outputs of total cytosolic and mitochondrial Ca2+ over time.
RESULTS
Inhibiting Ca2+ Extrusion by Plasma Membrane Ca2+-ATPase Unveils Large Ca2+ Plateau After Fast-Phase-Like Stimulus
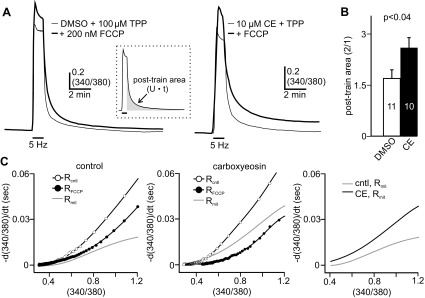
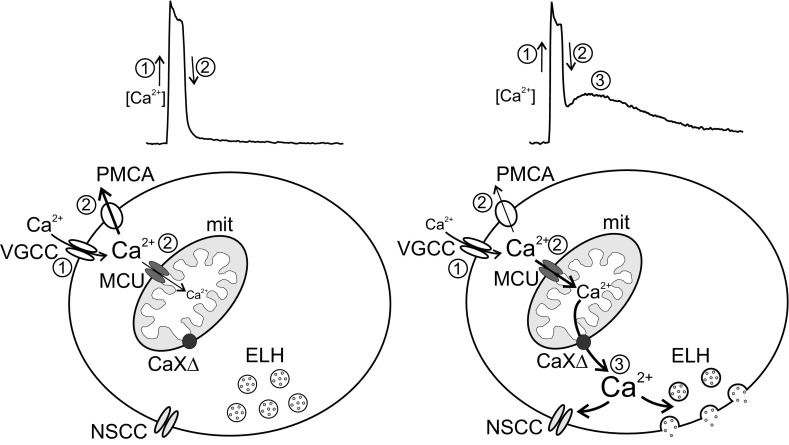
In many neurons, the presence of CICR is highly dependent on the magnitude of Ca2+ influx (Colegrove et al. 2000a; Llano et al. 1994; Richter et al. 2005; Shmigol et al. 1995; Usachev and Thayer 1997). Likewise, sharp-electrode recordings from bag cell neurons have shown that CICR is only elicited by prolonged action potential firing (Geiger and Magoski 2008). Moreover, when whole cell recordings of bag cell neurons are performed with an intracellular solution containing the exogenous Ca2+ chelator EGTA, prolonged stimulation is insufficient to elicit CICR (Groten et al. 2013). To demonstrate this here, intracellular Ca2+ dynamics were measured in fura-PE3-loaded cultured bag cell neurons under whole cell voltage clamp (Fig. 1A, inset). Voltage-gated Ca2+ influx was initiated by delivering a 5-Hz, 1-min train of 75-ms depolarizing pulses from −80 to 0 mV, which mimics the fast phase of the afterdischarge (Kaczmarek et al. 1982; Kupfermann and Kandel 1970). Except for one set of experiments (see below), all neurons were recorded with a Cs+-based intracellular solution and a Ca-Cs-TEA external solution to isolate Ca2+ currents. Cs+ and TEA+ were substituted for K+ and Na+, respectively (see Whole Cell Voltage-Clamp Recording for details). Although these conditions prevent the activity of the Na+/Ca2+ exchanger (Blaustein and Lederer 1999; Knox et al. 1996), our prior work has shown that this has no impact on voltage-gated Ca2+ influx or its subsequent removal (Groten et al. 2013). In neurons treated with DMSO (the vehicle) and recorded with the standard EGTA-containing intracellular solution, the train caused a transient rise in intracellular Ca2+, followed by an exponential return to prestimulus baseline levels within ∼5 min (n = 10) (Fig. 1A, left).
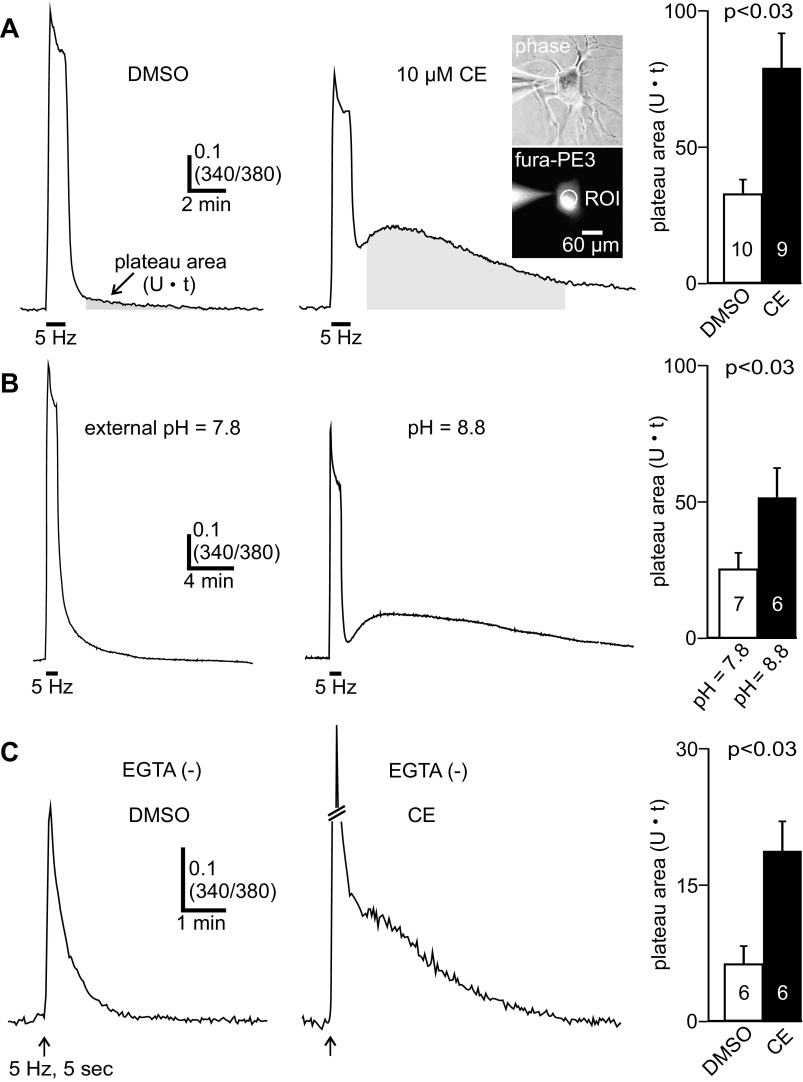
Fig. 1.
Inhibiting the plasma membrane Ca2+-ATPase (PMCA) unveils a large Ca2+ plateau following a fast-phase-like stimulus. A: ratiometric fluorescence measurements (340/380 nm) of intracellular Ca2+ from the soma of fura-PE3-loaded cultured bag cell neurons recorded under whole cell voltage clamp with our standard Cs+-based intracellular solution containing the exogenous Ca2+ chelator EGTA. Inset, phase (top) and fura fluorescence (bottom) images of a bag cell neuron; the whole cell recording pipette, and the somatic region of interest (ROI) used for analysis are indicated. Left: a 5-Hz, 1-min train of 75-ms depolarizing steps to 0 mV from a holding potential of −80 mV activates voltage-gated Ca2+ channels and elicits a large rise in intracellular Ca2+, as indicated by an increase in the fluorescence ratio. After the train, intracellular Ca2+ decays exponentially to basal levels within ∼5 min because of Ca2+ removal systems. Center: in a different neuron treated for ∼10 min with 10 μM carboxyeosin (CE) to disrupt the PMCA, the train elicits a transient rise in Ca2+; subsequently, the Ca2+ partially recovers to baseline but then transitions into a prolonged elevation that long outlasts the initial stimulus duration. The posttrain Ca2+ signal peaks within ∼2 min and returns to basal levels after 10–20 min. For analysis, we measured the plateau area [340/380 units (U)·s (t)] above prestimulus baseline between 1 and 11 min after the train. This timespan was used because it avoids the initial recovery period following influx and largely captures the posttrain Ca2+ response in most cells (see shaded gray region). Right: summary data showing the posttrain plateau area. Compared with control, carboxyeosin significantly augments the plateau area (unpaired Mann-Whitney U-test). For this and all subsequent bar graphs, data are means ± SE, with the number of neurons (n) indicated within or just above the bars. B, left: in normal external pH (pH = 7.8), the 5-Hz, 1-min train produces Ca2+ influx and a subsequent exponential decay to baseline after stimulation. Center: in contrast, cells bathed in high external pH (pH = 8.8) to inhibit the PMCA display an initial Ca2+ influx during the train that is followed by a sustained Ca2+ elevation. Right: compared with normal external pH, high external pH significantly increases the posttrain plateau area (unpaired 1-tailed Mann-Whitney U-test). C: ratiometric Ca2+ measurements acquired from cells recorded with an EGTA-free (−) intracellular solution. Left: applying a brief, 5-Hz, 5-s train to a DMSO-exposed neuron produces a transient Ca2+ response that rapidly returns to baseline. Center: another neuron, given carboxyeosin to inhibit the PMCA, shows a pronounced Ca2+ plateau in response to the 5-Hz, 5-s train stimulus. Right: for cells in EGTA-free conditions, the posttrain plateau area is significantly increased by carboxyeosin (unpaired Student's t-test).
We next examined the influence of PMCA function on posttrain Ca2+ dynamics by exposing bag cell neurons for ∼10 min to carboxyeosin, a PMCA inhibitor (Gatto et al. 1995; Gatto and Milanick 1993; Shmigol et al. 1998). Carboxyeosin had no significant effect on the prestimulus Ca2+ concentration (340/380 DMSO: 0.34 ± 0.01, n = 10; 340/380 carboxyeosin: 0.32 ± 0.02, n = 9; unpaired Student's t-test, P > 0.05). Delivering the 5-Hz, 1-min train produced a peak Ca2+ rise in carboxyeosin-treated neurons that was only slightly smaller (Fig. 1A, center) and not statistically different from cells in control conditions (DMSO peak Δ340/380: 0.69 ± 0.07, n = 10; carboxyeosin: 0.58 ± 0.07, n = 9; unpaired Student's t-test, P > 0.05). However, with carboxyeosin, the Ca2+ dynamics after the stimulus were temporally more complex and long outlasted the duration of the initial stimulus. After an early posttrain recovery, intracellular Ca2+ progressively increased to a plateau or second peak by 1–3 min, and then slowly returned to baseline over 10–20 min (n = 9) (Fig. 1A, center). The kinetics and magnitude of this response are strikingly similar to CICR (Geiger and Magoski 2008; Groten et al. 2013). The Ca2+ plateau size and duration were quantified by measuring the area above baseline between 1 and 11 min after the train. Measurements began at 1 min after the train to capture the peak posttrain Ca2+ response and avoided including the initial decay period. Relative to control, carboxyeosin exposure significantly increased the plateau area above baseline (Fig. 1A, right).
We sought to confirm the influence of Ca2+ extrusion by the PMCA, but with a nonpharmacological method of inhibition. Ca2+ export by the PMCA requires the concomitant import of protons and can be disrupted by extracellular alkalization (Niggli et al. 1982; Thomas 2011). This is a standard technique to prevent Ca2+ removal by the PMCA and has been successfully employed in other neuronal preparations (Shutov et al. 2013; Usachev et al. 2002). Here, the impact of increasing extracellular pH (from pH 7.8 to 8.8) was examined on train-evoked Ca2+ signals. As with carboxyeosin, increasing extracellular pH from pH 7.8 to 8.8 had no significant effect on the prestimulus Ca2+ concentration (resting 340/380 normal pH: 0.28 ± 0.01, n = 7; high pH: 0.32 ± 0.02, n = 6; unpaired Mann-Whitney U-test, P > 0.05). Cells in normal-pH external solution showed a transient surge in Ca2+ during the 1-min train and then a standard recovery to baseline (n = 7) (Fig. 1B, left). Conversely, neurons immersed in high-pH external solution, to hinder Ca2+ extrusion by the PMCA, presented a robust and protracted posttrain Ca2+ elevation that was indistinguishable from that seen in carboxyeosin (n = 6) (Fig. 1B, center). The plateau area above baseline between 1 min and 11 min after the train was significantly increased in high external pH relative to normal external pH (Fig. 1B, right). Like carboxyeosin, high external pH did not significantly alter the peak train Ca2+ response (DMSO peak Δ340/380: 0.76 ± 0.06, n = 7; carboxyeosin: 0.72 ± 0.04, n = 6; unpaired Student's t-test, P > 0.05).
Considering that our standard recording conditions contain EGTA, which influences Ca2+ dynamics, we examined whether protracted posttrain Ca2+ elevation could also be observed in the absence of this exogenous Ca2+ chelator. With an EGTA-free intracellular solution, cells were stimulated with a 5-Hz, 5-s train of depolarizing steps, which is a stimulus that should be subthreshold for eliciting CICR under these conditions (Geiger and Magoski 2008). In response to the 5-s train, there was a transient increase in Ca2+, followed by a rapid recovery to baseline (n = 6) (Fig. 1C, left). In contrast, cells exposed to 10 μM carboxyeosin presented a pronounced posttrain Ca2+ plateau that required more time to recover to prestimulus baseline (n = 6) (Fig. 1C, center). This is reflected in the summary data, showing that carboxyeosin significantly increased the plateau area above baseline between 1 min and 11 min after the train (Fig. 1C, right). This response is qualitatively similar to the 1-min train-evoked Ca2+ dynamics seen in carboxyeosin-treated neurons recorded with EGTA-containing intracellular saline. In the EGTA-free recording conditions, neurons given carboxyeosin showed slightly greater resting Ca2+ levels and peak train Ca2+ responses relative to untreated controls. However, these differences did not reach statistical significance [resting 340/380 DMSO: 0.38 ± 0.023 (n = 6), carboxyeosin: 0.45 ± 0.06 (n = 6), unpaired Student's t-test, P > 0.05; DMSO peak Δ340/380: 0.31 ± 0.03 (n = 6), carboxyeosin: 0.54 ± 0.11 (n = 6), unpaired Welch corrected Student's t-test, P > 0.05].
The Posttrain Ca2+ Plateau That Occurs in Absence of PMCA Activity Is Mediated by Ca2+ Release from Mitochondria but Not ER
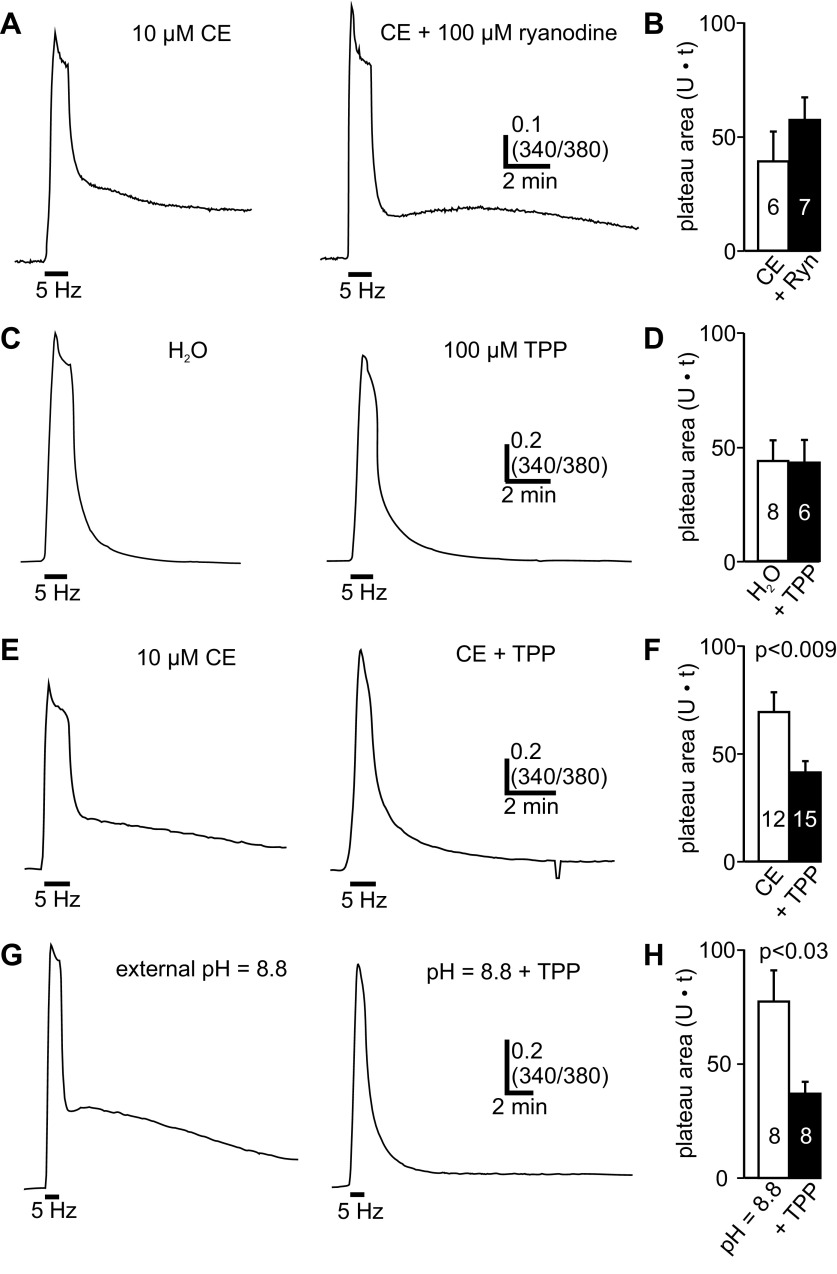
The delayed Ca2+ peak and prolonged Ca2+ elevation that is unveiled in the absence of PMCA activity may represent intracellular Ca2+ release (Geiger and Magoski 2008; Groten et al. 2013). However, the prolonged Ca2+ elevation could be mediated by other processes, such as reduced removal of cytosolic Ca2+. Thus we examined the contribution of intracellular Ca2+ release to the 1-min train-evoked Ca2+ plateau seen in carboxyeosin. All subsequent recordings were performed with our standard EGTA-containing Cs+-based intracellular solution. In many other types of neurons, CICR occurs when voltage-gated Ca2+ influx triggers Ca2+ release through ryanodine receptors on the ER membrane (Verkhratsky 2005). To test whether this was the case for bag cell neurons, 100 μM ryanodine, a dose that inhibits ryanodine receptor-derived Ca2+ release (Meissner 1985; Verkhratsky 2005), was used. Compared with 10 μM carboxyeosin alone (n = 6), a 10-min treatment with 100 μM ryanodine in carboxyeosin (n = 7) had no obvious effect on the Ca2+ plateau area after the 1-min train, with no significant difference between the group data (Fig. 2, A and B).
Fig. 2.
The carboxyeosin-dependent posttrain Ca2+ plateau is mediated by Ca2+ release from the mitochondria. A, left: in the presence of carboxyeosin (CE), the 5-Hz, 1-min train produces a prolonged poststimulus Ca2+ plateau. This and all subsequent recordings were performed with our standard EGTA-containing intracellular solution. Right: pretreating neurons with high-concentration (100 μM) ryanodine does not appreciably alter the posttrain Ca2+ dynamics in a neuron exposed to 10 μM carboxyeosin. B: ryanodine does not significantly alter the posttrain plateau area (1–11 min after train) seen in the presence of carboxyeosin (unpaired Student's t-test). C, left: in control conditions (H2O), a neuron shows a rapid exponential recovery to baseline after the 5-Hz, 1-min train. Right: another neuron, exposed to 100 μM TPP for 30 min to inhibit mitochondrial Ca2+ exchangers, presents a peak-train Ca2+ response and posttrain Ca2+ decay that is indistinguishable from control. D: with the PMCA active, TPP does not significantly alter the posttrain Ca2+ plateau area relative to control (unpaired Student's t-test). E, left: in the presence of carboxyeosin, the train evokes a large posttrain Ca2+ elevation. Right: in TPP + carboxyeosin, there is a similar train-evoked Ca2+ influx, but subsequently Ca2+ returns quickly to prestimulus baseline without an ensuing Ca2+ elevation. The transient decrease in the 340-to-380 ratio in the latter portion of the recording is an artifact caused by a brief, incidental closure of the light shutter. F: in the presence of carboxyeosin, the Ca2+ plateau area is significantly reduced by TPP (unpaired Student's t-test). G: disrupting the PMCA with high external pH (pH = 8.8) results in a posttrain Ca2+ elevation that is eliminated by TPP. H: in high-pH external, TPP significantly reduces the plateau area compared with high pH alone (Welch corrected unpaired Student's t-test).
Given the negative ryanodine result, we next investigated a role for mitochondrial Ca2+ release. Mitochondrial CICR can be prevented by inhibiting mitochondrial Ca2+ exchangers with TPP (Geiger and Magoski 2008; Groten et al. 2013; Lee et al. 2013). As a control, we first discerned the impact of TPP on Ca2+ influx and removal in the absence of carboxyeosin, when PMCA activity is not altered. As there is no apparent Ca2+ release under these conditions, TPP would not be expected to influence Ca2+ dynamics. Relative to H2O (the vehicle), TPP did not significantly impact resting Ca2+ levels (resting 340/380 H2O: 0.26 ± 0.01, n = 8; TPP: 0.26 ± 0.02, n = 6; unpaired Student's t-test, P > 0.05) or peak train Ca2+ influx (peak Δ340/380 H2O: 1.03 ± 0.076, n = 8; Δ340/380 TPP: 0.878 ± 0.11, n = 6; unpaired Student's t-test, P > 0.05). Moreover, in response to the 5-Hz, 1-min train, TPP-treated neurons (n = 6) did not present a significantly different posttrain plateau area vs. controls (n = 8) (Fig. 2, C and D).
Subsequently, the effect of TPP on train-evoked Ca2+ dynamics was examined when the PMCA was blocked. Again, in 10 μM carboxyeosin, the train resulted in a delayed Ca2+ plateau after the Ca2+ influx signal (n = 12) (Fig. 2E, left). Conversely, delivering a train to neurons given both carboxyeosin and 100 μM TPP resulted in a Ca2+ response that decayed exponentially to baseline after the train (n = 15) (Fig. 2E, right). The posttrain Ca2+ elevation in carboxyeosin was reduced under these conditions, as indicated by the significantly smaller plateau area in TPP-treated neurons (Fig. 2F). We also examined the influence of TPP on the posttrain Ca2+ plateau produced when PMCA activity was prevented with extracellular alkalization. As before, in high-pH external solution, the train elicited a robust posttrain Ca2+ plateau (n = 8) (Fig. 2G, left). In contrast, cells bathed in high-pH external solution with TPP showed no posttrain Ca2+ elevation (n = 8) (Fig. 2G, right). This was confirmed by the summary data, showing that TPP significantly reduced the Ca2+ plateau in high external pH (Fig. 2H).
Model of Intracellular Ca2+ Dynamics Recapitulates Effect of PMCA Disruption on Posttrain Ca2+ Dynamics
Our results suggested that Ca2+ extrusion by the PMCA influenced the ability of stimulation to elicit CICR from the mitochondria. The precise mechanism of Ca2+ interplay between the PMCA and the mitochondria is unclear. In bag cell neurons, intracellular Ca2+ is governed by the concerted action of multiple Ca2+ sources and Ca2+ extrusion/buffering systems (Groten et al. 2013; Kachoei et al. 2006; Knox et al. 1996). Therefore, we employed a simple rate model of Ca2+ dynamics, consisting of these primary components, in an attempt to recapitulate our findings and provide information on underlying mechanisms. Such models have proven helpful for explaining stimulus-evoked changes in cytosolic Ca2+ and CICR in the other systems (Colegrove et al. 2000b; Friel and Tsien 1994; Gabso et al. 1997).
The model consisted of extracellular, intracellular, and mitochondrial compartments (Fig. 3A, inset) (see Model Development for details). Ca2+ influx across the plasma membrane was mediated by Jinflux, while plasma membrane Ca2+ efflux was controlled by JPMCA and Jextru, reflecting the function of the PMCA and residual Ca2+ extrusion systems, respectively. The movement of Ca2+ into and out of the mitochondrial compartment are represented by Juptake and Jrelease, respectively. As the ER does not contribute to Ca2+ removal following voltage-gated Ca2+ influx (Geiger and Magoski 2008; Groten et al. 2013), it was not included in the model. Finally, Ca2+ buffering by the exogenous Ca2+ chelator EGTA was contributed by JEGTA.
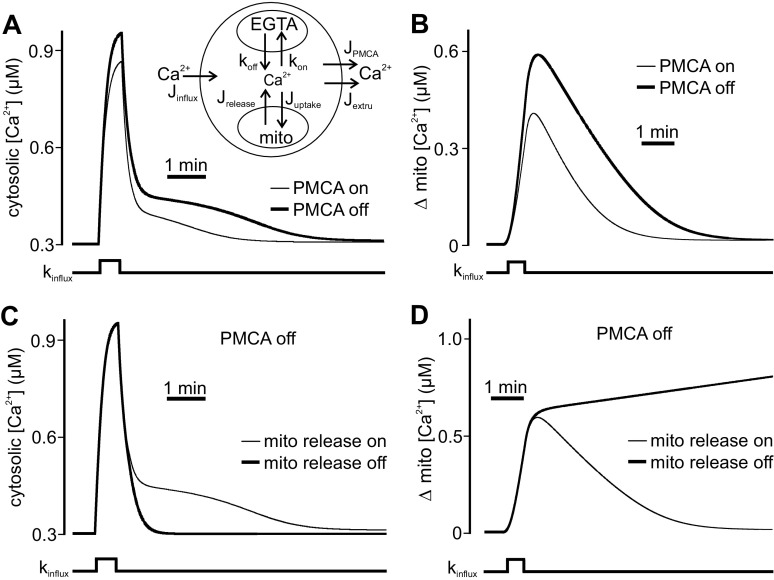
Fig. 3.
A compartment model of Ca2+ dynamics recapitulates the influence of PMCA activity on CICR. A: the 3-component model of intracellular Ca2+ dynamics consists of extracellular, cytosolic, and mitochondrial compartments (inset). Jinflux corresponds to plasma membrane Ca2+ influx, while JPMCA and Jextru represent the PMCA and residual plasma membrane Ca2+ extrusion systems, respectively. Juptake denotes mitochondrial Ca2+ uptake, and Jrelease signifies Ca2+ release into the cytosol by the mitochondrial Ca2+ exchangers. Cytosolic Ca2+ buffering by the exogenous Ca2+ chelator EGTA is determined by its forward (kon) and reverse (koff) rate constants. All model presentations were derived with functions and parameter values (Table 1) consistent with the relevant literature on this topic (see Model Development for details). Train-induced voltage-gated Ca2+ influx is produced in the model by increasing the plasma membrane Ca2+ influx rate constant (kinflux). With JPMCA functional (light trace), a transient change in kinflux elicits a rise in cytosolic Ca2+ and an ensuing response that contains a small Ca2+ shoulder. In another simulation, where JPMCA was eliminated (dark trace), triggering the same kinflux produces a slightly larger cytosolic Ca2+ rise and a prominent Ca2+ plateau, similar to that seen physiologically during carboxyeosin treatment. B: changes in mitochondrial Ca2+ concentration occurring in response to cytosolic Ca2+ influx in the presence and absence of JPMCA. Compared with simulations when all components are functional (light trace), eliminating JPMCA (dark trace) leads to a greater increase in mitochondrial Ca2+ loading by Ca2+ influx. C: cytosolic Ca2+ influx and release when the PMCA (JPMCA) is not functional and the rate of mitochondrial Ca2+ release (Jrelease) is varied to mimic the effect of TPP. Relative to when it is active (light trace), eliminating Jrelease (dark trace) prevents the Ca2+ plateau seen when JPMCA is off. D: corresponding changes in mitochondrial Ca2+ in the absence of JPMCA function but with altered mitochondrial Ca2+ release (Jrelease). The decay of mitochondrial Ca2+ that occurs when the Jrelease is functional (light trace) is prevented after the latter is inactive (dark trace).
Figure 3, A and B, show simulated changes in cytosolic and mitochondrial Ca2+ resulting from a transient increase in the plasma membrane Ca2+ permeability coefficient (kinflux). This is meant to reflect the opening of voltage-gated Ca2+ channels during a train stimulus. When all components of the model were active, turning on kinflux produced a rise in cytosolic Ca2+, followed by a rapid Ca2+ removal (Fig. 3A). In parallel, the cytosolic Ca2+ elevation due to influx caused mitochondrial Ca2+ concentration to grow, after which it returned to basal levels because of the function of the Ca2+ release parameter (Jrelease) (Fig. 3B). The extruded mitochondrial Ca2+ produced only a slight cytosolic Ca2+ response when JPMCA was functional. The impact of blocking the PMCA with carboxyeosin or extracellular alkalization was modeled by repeating the same simulations but with JPMCA eliminated. Under these conditions, transiently activating kinflux resulted in a large cytosolic Ca2+ influx, followed by a more substantial poststimulus Ca2+ elevation (Fig. 3A). Simulated mitochondrial Ca2+ concentration showed that eliminating PMCA function produced greater Ca2+ loading of the store by Ca2+ influx (Fig. 3B). The contribution of mitochondrial Ca2+ release to cytosolic Ca2+ was demonstrated by repeating the simulation with Jrelease off. Eliminating mitochondrial Ca2+ release (Fig. 3D) had no influence on the initial increase in mitochondrial Ca2+ but prevented its subsequent reduction vs. intact Ca2+ release (Fig. 3D). Furthermore, the posttrain Ca2+ plateau, normally seen in the absence of JPMCA, was eliminated without Ca2+ release from the mitochondria (Fig. 3C).
Inhibiting Ca2+ Extrusion by PMCA Enhances Involvement of Mitochondrial Ca2+ Uptake in Response to Voltage-Gated Ca2+ Influx
The compartment model of intracellular Ca2+ dynamics suggested that disrupting plasma membrane Ca2+ extrusion by the PMCA could facilitate CICR by making Ca2+ more available for mitochondrial Ca2+ uptake during stimulation. Thus we examined whether the relative contribution of the mitochondria to the removal of Ca2+ derived from voltage-gated Ca2+ channels is augmented when Ca2+ extrusion by the PMCA is prevented. If this occurs, then carboxyeosin should increase the sensitivity of posttrain Ca2+ decay to the disruption of mitochondrial Ca2+ sequestration with FCCP. This protonophore reduces the mitochondrial proton motive force and thereby collapses the inner mitochondrial membrane potential that is required for Ca2+ uptake into the matrix (Babcock et al. 1997; Heytler and Prichard 1962). In the following experiments, posttrain Ca2+ removal was studied in isolation from CICR by treating neurons with 100 μM TPP. A low FCCP concentration (200 nM) was used, to avoid the enhanced rundown of voltage-gated Ca2+ current that occurs with higher amounts (μM range) (Groten et al. 2013). In addition, our earlier work showed that mitochondrial Ca2+ liberated by FCCP can depolarize bag cell neurons by opening a nonselective cation channel (Hickey et al. 2010). However, all of the present Ca2+ measurements were carried out under voltage clamp at −80 mV, meaning that FCCP could not have depolarized the membrane potential or caused erroneous voltage-gated Ca2+ influx.
DMSO-treated neurons were first given the 5-Hz, 1-min train as a control. After full recovery of Ca2+ to baseline, cells were exposed to 200 nM FCCP for ∼10 min and then received a second 1-min train (n = 11). As displayed in Fig. 4A, left, posttrain Ca2+ removal occurred more slowly after FCCP treatment. These results are consistent with our prior work that showed a similar, albeit larger, effect of FCCP on posttrain Ca2+ decay at higher concentrations (20 μM) (Geiger and Magoski 2008; Groten et al. 2013). Next, we repeated the experiment but with cells bathed in 10 μM carboxyeosin, to disrupt Ca2+ removal by the PMCA. The first train produced robust Ca2+ influx and an exponential recovery to baseline (Fig. 4A, right). Delivering the second train, after FCCP delivery, produced prominent Ca2+ influx, but the Ca2+ removal was substantially slowed (n = 10) (Fig. 4A, right). We compared the involvement of the mitochondria in posttrain Ca2+ removal between DMSO- and carboxyeosin-treated neurons by measuring the extent to which FCCP enhanced the posttrain area in each condition. The time frame used for this analysis spanned from the end of the train to 10 min after the train and was well-suited to capturing the initial and later periods of posttrain Ca2+ recovery (Fig. 4A, inset). The ratio of posttrain area between before and after FCCP was significantly larger in cells exposed to carboxyeosin (Fig. 4B).
Fig. 4.
Disrupting the PMCA enhances mitochondrial involvement in the removal of Ca2+ from voltage-gated influx after a train. A, left: applying the 5-Hz, 1-min train to a DMSO-exposed cell elicits an increase in cytosolic Ca2+ followed by an exponential return to baseline (light trace). After disruption of mitochondrial Ca2+ uptake with 200 nM FCCP for ∼10 min, a second train produces a similarly sized Ca2+ influx signal as in the absence of FCCP, but the posttrain Ca2+ recovery is slowed (dark trace). All experiments are performed in 100 μM TPP to isolate the Ca2+ removal process from any potential CICR. Inset: the efficacy of posttrain Ca2+ removal was determined by measuring the area [340/380 units (U)·s (t)] above baseline between the end of the train and 10 min later (10 min total) (shaded region). Right: in another cell, continuously bathed in 10 μM carboxyeosin (CE), a train produces Ca2+ influx and a subsequent recovery (light trace). After the addition of 200 nM FCCP (still in carboxyeosin), a second train evokes Ca2+ influx that recovers to baseline more slowly (dark trace). Note that the effect of FCCP on posttrain Ca2+ removal is more substantial in the presence of carboxyeosin (right) than in DMSO (left). B: the ratio (2/1) of posttrain area between the second train (2) in FCCP and the first train (1) in DMSO is significantly enhanced by carboxyeosin (unpaired Student's t-test). C: Ca2+ clearance rate (R), acquired from the decay period of Ca2+ transients shown in A, as a function of 340-to-380 ratio. Fitted polynomial functions are plotted over the data points. The difference between control (Rcntl) and FCCP (RFCCP) is used to produce the estimated mitochondrial uptake rate (Rmit) (see Analysis for details). Left: in the absence of carboxyeosin (control), the rate of Ca2+ clearance [d(340/380)/dt] has a moderate Rmit component over the range of Ca2+ measured. Center: in carboxyeosin, Rmit constitutes a large fraction of the total Ca2+ clearance rate over the entire Ca2+ range. Right: in carboxyeosin, Rmit is larger than DMSO control (cntl) and increases more steeply with changes in intracellular Ca2+ (340/380). Rmit curves are replotted from left and center.
Using the decay of the train Ca2+ responses, we also evaluated the influence of carboxyeosin on the apparent rate of mitochondrial Ca2+ uptake (Rmit) as a function of intracellular Ca2+ (see Analysis for details). Rmit (i.e., the FCCP-sensitive rate component) was derived by subtracting the residual removal rate in FCCP (RFCCP) from the removal rate in control conditions (Rcntl). Figure 4C displays the relationship between these rate components and intracellular Ca2+ in the absence (Fig. 4C, left) and presence (Fig. 4C, center) of carboxyeosin. In DMSO, mitochondrial Ca2+ uptake occurred throughout the range of Ca2+ measured and increased moderately with intracellular Ca2+ (Fig. 4C, left). In carboxyeosin, the mitochondrial Ca2+ uptake rate was larger and increased more steeply to changes in intracellular Ca2+ (Fig. 4C, center and right).
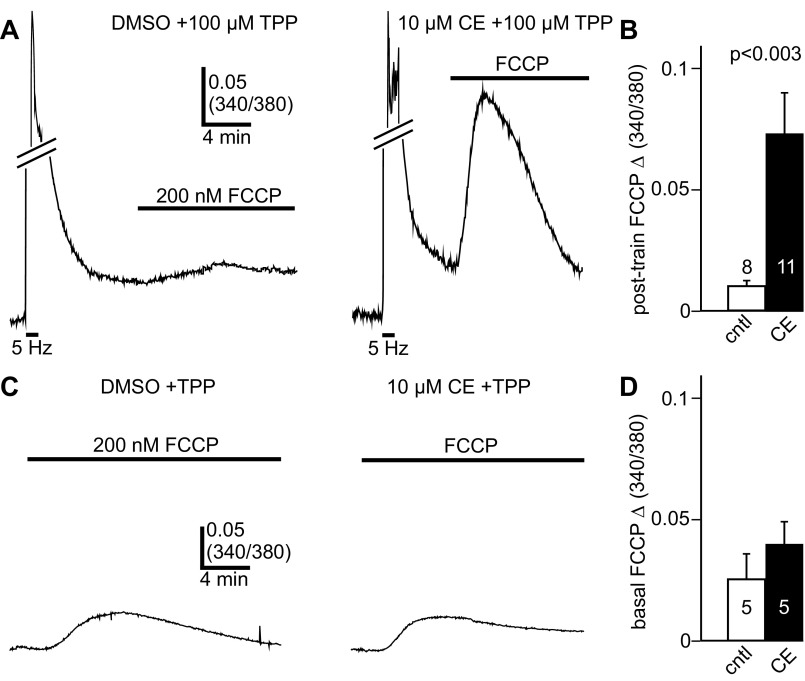
Hindering Ca2+ Extrusion by PMCA Enhances Amount of Ca2+ Stored in Mitochondria After Train Stimulus
Our results suggested that preventing Ca2+ handling by the PMCA enhanced the contribution of the mitochondria to the removal of Ca2+ derived from voltage-gated Ca2+ channels. Consequently, carboxyeosin should increase the level of Ca2+ in the mitochondria after stimulation. In addition to preventing Ca2+ uptake, FCCP causes the liberation of stored mitochondrial Ca2+ (Friel and Tsien 1994; Geiger and Magoski 2008; Groten et al. 2013; Zenisek and Matthews 2000). Therefore, we assessed the amount of mitochondrial Ca2+ loading by treating bag cell neurons with FCCP, subsequent to the 5-Hz, 1-min train. All neurons were given 100 μM TPP to ensure that mitochondrial Ca2+ was not released via Ca2+ exchangers after stimulation. In cells exposed only to DMSO, delivering 200 nM FCCP after the train produced a small, slow-rising Ca2+ signal (n = 8) (Fig. 5A, left). Conversely, in neurons that were continuously bathed in 10 μM carboxyeosin, applying 200 nM FCCP after the train resulted in markedly greater Ca2+ liberation (n = 11) (Fig. 5A, right). Indeed, compared with DMSO control, the peak FCCP-induced Ca2+ response was significantly augmented by carboxyeosin (Fig. 5B).
Fig. 5.
Blocking the PMCA increases the loading of the mitochondrial Ca2+ store by voltage-gated Ca2+ influx. A, left: after recovery from the 5-Hz, 1-min train in DMSO, application of 200 nM FCCP produces a small Ca2+ response due to the liberation of stored mitochondrial Ca2+. Right: in 10 μM carboxyeosin (CE), delivering FCCP after a train elicits a comparatively larger Ca2+ signal. TPP (100 μM) is present in both conditions to ensure that the mitochondrial Ca2+ load was not reduced by posttrain Ca2+ release. Line breaks in both traces omit a portion of the Ca2+ influx signal during the train to emphasize the Ca2+ liberation by FCCP. B: compared with DMSO controls, the peak change in FCCP-induced Ca2+ release following the train is significantly larger in cells given carboxyeosin (Welch corrected unpaired Student's t-test with Bonferroni's correction for multiple comparisons; threshold for significance P < 0.025). C, left: delivering 200 nM FCCP to a DMSO-exposed neuron, without a prior train stimulus, produces a relatively small Ca2+ increase. Right: in another cell exposed to carboxyeosin, FCCP elicits a Ca2+ signal that is not substantially different in magnitude from control. As per A and B, TPP is included in both experimental conditions. D: the peak change in Ca2+ to FCCP is not significantly different between DMSO and carboxyeosin when a prior train stimulus is not given (unpaired Student's t-test with Bonferroni's correction for multiple comparisons; threshold for significance P < 0.025).
Carboxyeosin appears to facilitate the extent of mitochondrial Ca2+ loading by voltage-gated Ca2+ influx. However, it is possible that it alters the FCCP-dependent Ca2+ signal by other means. For example, disrupting Ca2+ removal by the PMCA could directly escalate the Ca2+ response by preventing its extrusion from the cytosol. Alternatively, carboxyeosin might augment mitochondrial Ca2+ loading under basal conditions, without voltage-gated Ca2+ influx. Thus, as a control, FCCP-evoked Ca2+ release was measured in the presence and absence of carboxyeosin, without prior train stimulation. Cells were also given 100 μM TPP to maintain consistency with prior experiments. In carboxyeosin (n = 5), the Ca2+ release signal to FCCP was not significantly different from control (n = 5) (Fig. 5, C and D).
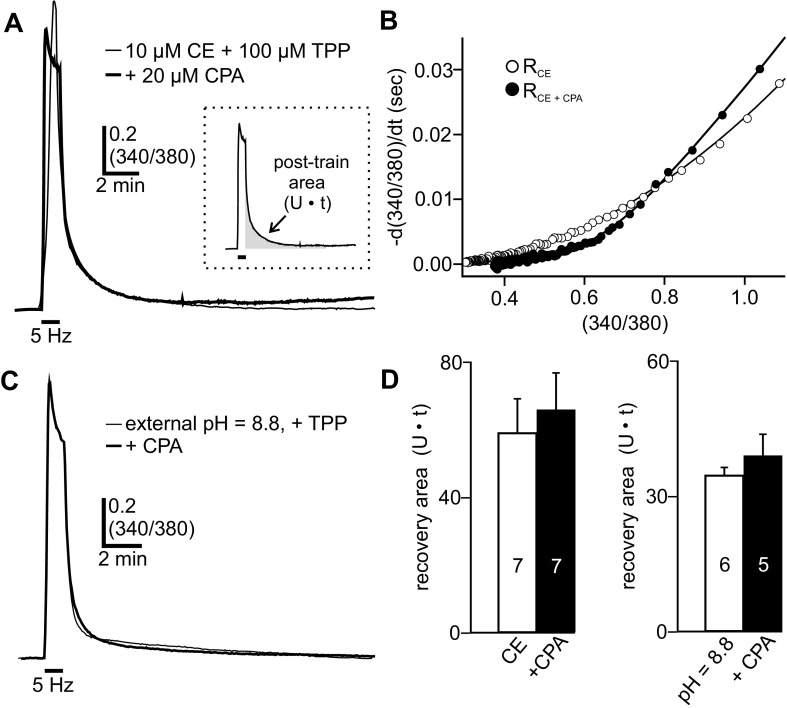
Disrupting Ca2+ Removal by PMCA Does Not Change Contribution of ER to Removal of Ca2+ from Voltage-Gated Influx
Ca2+ handling by the PMCA appears to influence the amount of Ca2+ available for mitochondrial Ca2+ uptake in response to voltage-gated Ca2+ influx. Whether an analogous Ca2+ interplay occurs between the PMCA and other intracellular Ca2+ stores, such as the ER, is unclear. Ca2+ sequestration by the ER occurs through the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) (Berridge 2002) and in the bag cell neurons has a lesser role in the handling of Ca2+ derived from voltage-gated Ca2+ influx (Geiger and Magoski 2008; Groten et al. 2013). We tested whether inhibiting PMCA Ca2+ extrusion with carboxyeosin could enhance the sensitivity of posttrain Ca2+ removal to CPA—an inhibitor of SERCA activity (Seidler et al. 1989). For these experiments, neurons were bathed in 100 μM TPP to prevent CICR from the mitochondria and isolate posttrain Ca2+ removal. As seen previously, in the presence of 10 μM carboxyeosin the 5-Hz, 1-min train elicited voltage-gated Ca2+ influx and a subsequent recovery to baseline (n = 7) (Fig. 6A). In other neurons, given both carboxyeosin and 20 μM CPA (n = 7), prestimulus Ca2+ levels were significantly increased relative to carboxyeosin alone (resting 340/380, carboxyeosin: 0.26 ± 0.01, n = 7; carboxyeosin + CPA: 0.36 ± 0.03, n = 7; Welch corrected unpaired Student's t-test, P < 0.02). The higher basal Ca2+ under these conditions is likely due to the release of stored ER Ca2+ by CPA (Groten et al. 2013; Kachoei et al. 2006). Despite this, the train elicited Ca2+ influx and a subsequent Ca2+ recovery was indistinguishable from that seen in the absence of CPA (Fig. 6A). This was reflected in the summary data, showing that the posttrain area was not significantly different between carboxyeosin alone and carboxyeosin plus CPA (Fig. 6D, left). Next, the relationship between the rate of Ca2+ removal and intracellular Ca2+ was evaluated using the posttrain Ca2+ decay periods of the data presented in Fig. 6A. The rate of Ca2+ removal across the range of Ca2+ measured was not substantially different between carboxyeosin (RCE) and carboxyeosin plus CPA (RCE+CPA) (Fig. 6B). Finally, extracellular alkalization was used to inhibit Ca2+ removal via the PMCA; again, 100 μM TPP was present to prevent CICR. Cells exposed to both high external pH and 20 μM CPA (n = 5) showed a posttrain Ca2+ decay period that was comparable to high external pH alone (n = 6) (Fig. 6C). In high external pH, CPA did not significantly change the posttrain area above baseline (Fig. 6D, right).
Fig. 6.
PMCA disruption does not change the involvement of ER Ca2+ uptake during posttrain Ca2+ removal. A: compared with 10 μM carboxyeosin (CE) alone (light trace), a neuron exposed to both carboxyeosin and 20 μM CPA (to prevent Ca2+ uptake into the ER by the SERCA) shows very similar Ca2+ influx and Ca2+ removal in response to the 5-Hz, 1-min train. TPP (100 μM) is present in both conditions to prevent CICR and isolate the posttrain Ca2+ removal. Inset: posttrain Ca2+ decay was quantified by measuring the area [340/380 units (U)·s (t)] above baseline between the end of the train to 10 min later (shaded region). B: rate of Ca2+ removal [d(340/380)/dt] plotted as a function of intracellular Ca2+ (340/380) for the Ca2+ responses in A and fitted with polynomial functions. The total Ca2+ removal rate in carboxyeosin (RCE) is not substantially different from the Ca2+ removal rate in carboxyeosin + CPA (RCE+CPA) across the range of Ca2+ measured. C: sample trace showing train-evoked Ca2+ influx and removal with high external pH (pH = 8.8) to inhibit the PMCA (light trace). In high external pH, CPA does not change the peak Ca2+ influx or Ca2+ removal elicited by the train (dark trace). TPP is included in both experimental conditions. D: in the presence of carboxyeosin (left) or high external pH (pH = 8.8) (right), CPA does not significantly alter the posttrain area (unpaired Student's t-test for both).
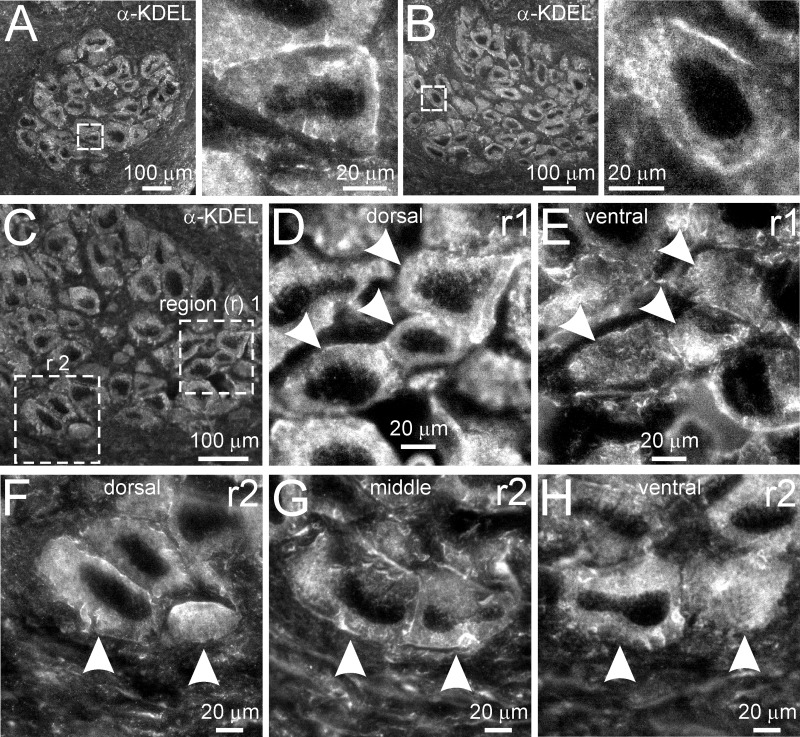
PMCA, Mitochondria, and ER Localize to Regions of Voltage-Gated Ca2+ Influx in Soma
That the PMCA and mitochondria influence activity-dependent Ca2+ dynamics in the bag cell neurons suggests that these Ca2+ handling systems are prevalent near sites of voltage-gated Ca2+ entry in the soma. To address this, we examined the spatial pattern of voltage-gated Ca2+ influx and compared it with the somatic distribution of the PMCA and mitochondria in cultured bag cell neurons. Initially, the spatial pattern of voltage-gated Ca2+ entry was assessed by inspecting ratiometric Ca2+ images before and during the 5-Hz, 1-min train from H2O- or DMSO-treated control neurons presented in Fig. 2D and Fig. 4B. In all neurons (n = 19), images from the midpoint of the vertical somatic focal plane showed that intracellular Ca2+ was low and homogeneous throughout the soma at rest (Fig. 7A, inset 1). Shortly after the stimulus onset, Ca2+ increased first at the soma periphery (Fig. 7A, inset 2). As the stimulus progressed, Ca2+ in the center of the soma increased to similar levels as the periphery, likely as a result of Ca2+ diffusion from the plasma membrane (Fig. 7A, insets 3 and 4).
The distribution of the PMCA was then determined by immunolabeling fixed cultured bag cell neurons (n = 18) with a mouse antibody raised against an epitope of the human PMCA (1:200 mouse anti-PMCA; 1:200 goat anti-mouse Alexa Fluor 594). This epitope is strongly conserved in a putative Aplysia PMCA homologue (see Live-Cell Staining, Immunocytochemistry, and Immunohistochemistry for details). Considering the influence of the PMCA on the handling of Ca2+ from voltage-gated Ca2+ channels, we expected abundant membrane staining. To discern this, confocal microscopy was used to view PMCA immunolabeling in the midsomatic focal plane. Horizontal and vertical optical sections through the center of the soma revealed that, although fluorescent signal was certainly present throughout, the near-membrane region contained the greatest immunolabeling intensity, consistent with the plasma membrane localization of the PMCA (Fig. 7, B–D). Vertical optical sections also showed PMCA immunolabeling in most portions at or near the somatic membrane, with slightly more abundance in the left and right portions than the upper and bottom poles (Fig. 7, C and D).
To label and view the distribution of mitochondria in living cultured cells, we stained with MitoTracker Red (500 nM in DMSO), a vital dye used previously for this purpose in bag cell neurons (Groten and Magoski 2015; White and Kaczmarek 1997) (n = 11). Horizontal and vertical confocal microscopy optical sections through the center of the soma revealed that the mitochondria were present both peripherally, near the plasma membrane, and in central cytosolic regions (Fig. 7, E–G). The staining pattern in the soma often appeared reticular in arrangement. This morphology bears likeness to the “mitochondrial networks” present in other cell types (MacAskill and Kittler 2010; Rizzuto et al. 1998). Furthermore, in 7 of 11 cells, vertical optical sections revealed a large, dense core of mitochondrial labeling spanning from the perinuclear region to the upper portion of the cytosol above the nucleus (Fig. 7, F and G).
The present study, as well as our prior work (Groten et al. 2013), suggests that, unlike the mitochondria, the ER is seemingly not involved in the removal of Ca2+ from voltage-gated influx. A possible explanation for this is a difference in the distribution or abundance of the ER relative to sources of voltage-gated Ca2+ entry. To address this, a rat anti-KDEL antibody (1:200 anti-KDEL; 1:200 goat anti-rat Alexa Fluor 488) was used to immunolabel the ER in fixed cultured neurons (n = 12). KDEL (Lys-Asp-Glu-Leu) is a highly conserved ER-retention signal found in the COOH terminus of proteins localized to the ER (Munro and Pelham 1987), including those of Aplysia (Kennedy et al. 1992). Also, this antibody has been employed previously to identify ER in cultured Aplysia sensory neurons and bag cell neurons (Lyles et al. 2006; Zhang and Forscher 2009). Interestingly, confocal microscopy horizontal optical sections revealed that the ER was abundant near the soma periphery, where voltage-gated Ca2+ influx is observed (Fig. 7H). However, this positioning at the membrane was not consistent throughout all portions of the soma. In contrast to the mitochondria, vertical optical sections revealed that the ER was polarized to select divisions of the soma. Specifically, the upper and bottom poles of the somatic membrane region, where mitochondria are prevalent, were comparatively devoid of ER (Fig. 7, I and J vs. F and G).
To assess regional differences in their distribution, PMCA, mitochondria, and ER fluorescence intensities were quantified from regions of interest in the left, right, upper, and bottom poles of the soma (see Live-Cell Staining, Immunocytochemistry, and Immunohistochemistry for details) (Fig. 7K). Although PMCA immunolabeling was present in the upper and bottom regions, it was less abundant here compared with the left, but not the right, region. Mitochondrial staining was essentially the same across all regions of the soma periphery; furthermore, there were no differences in the distribution of the PMCA vs. the mitochondria, except for the left region of PMCA labeling being greater than the left region of mitochondrial staining. Immunolabeling of the ER was not different between the left and right regions or the upper and bottom regions; however, these two groups were collectively different from each other. In addition, the signal from the upper and bottom regions for ER was less than the majority of the regions labeled for the PMCA or mitochondria. Specifically, immunolabeling of the ER in the upper region was smaller than the left and right regions of PMCA immunolabeling, along with the upper, bottom, and right regions of mitochondrial staining, while signal from the bottom region for ER was less compared with all regions of PMCA or mitochondria labeling. Finally, the right region of ER immunolabeling was greater than the upper and bottom regions of PMCA immunolabeling, as well as the left region of mitochondrial staining.
Even with competition for Ca2+ removal by the PMCA disrupted, the ER was not shown to influence the removal of Ca2+ from voltage-gated Ca2+ channels. This may suggest that the areas of the plasma membrane containing the PMCA are not associated with regions of the ER that approach the somatic plasma membrane. We examined this by assessing the colocalization between peripheral ER and PMCA in single cultured neurons with double labeling with anti-PMCA and anti-KDEL (n = 15). Horizontal confocal optical sections revealed that the ER (x,y image of Fig. 7L) and PMCA (x,y image of Fig. 7M) are present in similar regions of the periphery, particularly in the left and right portions of the soma. However, vertical cross sections revealed that this regional similarity was less apparent at the upper and bottom poles, where the PMCA, but not the ER, is present (x,z and y,z images of Fig. 7L vs. Fig. 7M). Merging the KDEL and PMCA immunocytochemical images distinctly showed a more restricted ER labeling compared with the PMCA (x,z and y,z images of Fig. 7N).
As culture conditions can influence the morphology of bag cell neurons (Gruenbaum and Carew 1999), it is possible that the distribution of the ER in the soma is altered in vitro relative to the cluster in the abdominal ganglion. This may explain why we found that the distribution of the ER differed in relation to the site of cell attachment on the glass coverslip. To examine this prospect, cryostat horizontal sections of bag cell neuron clusters in the abdominal ganglion were immunolabeled with anti-KDEL to stain the ER (n = 6 clusters from 3 animals). Unsurprisingly, the ER was present in bag cell neurons within the cluster (Fig. 8, A, left, and B, left). For individual neurons, the ER was abundant throughout the soma, and like the in vitro conditions it frequently formed dense appositions near the plasma membrane (Fig. 8A, right, and B, right). That stated, the markedly heterogeneous distribution seen in cultured bag cell neurons, where the ER was concentrated along the sides of the cell, was less pronounced in the cluster (compare to Fig. 7, H–J and L). To explore this in detail, we examined the ER distribution in individual bag cell neurons that were clearly identifiable across neighboring cyrosections. In six different cases, one or more immunolabeled neurons could be recognized in consecutive serial sections; two examples are provided in Fig. 8, C–H. These instances revealed that while the ER was evident near the plasma membrane it was also distributed within the cytoplasm along the dorso-ventral axis of single bag cell neurons in the cluster.
Fig. 8.
In the bag cell neuron cluster, the ER is particularly abundant at the soma periphery but is also present in other cellular regions. A: images of ER immunolabeling (α-KDEL 1° at 1:200; Alexa Fluor 488 2° at 1:200) in 6-μm thick tissue sections of the abdominal ganglion (see Live-Cell Staining, Immunocytochemistry, and Immunohistochemistry for details). Left: photomicrograph of a cluster of bag cell neurons and surrounding tissue. White dashed box outlines the region magnified at right. Right: higher-magnification image showing ER labeling in the soma of a single bag cell neuron. The ER is present throughout the soma but is particularly abundant near the membrane. B: another bag cell neuron cluster (left), where strong peripheral labeling is apparent in the soma of the outlined cell (magnified at right). C: serial neighboring tissue sections (6 μm thick) from a single bag cell neuron cluster along the dorso-ventral axis. Rectangles delineate 2 regions (r1 and r2) of the cluster where neurons were identifiable across neighboring tissue sections. D and E: magnified images of region r1 from C. Arrowheads point to 2 neurons that are identifiable in serial neighboring sections. D and E are dorsal and ventral regions of the abdominal ganglion, respectively, and ER labeling is apparent in neurons from both sections. F–H: magnified images of region r2 from C. Arrowheads point to 3 neurons that are identifiable across 3 neighboring sections in the dorso-ventral axis: dorsal (F), middle (G), and ventral (H). In all 3 sections, the ER is abundant throughout each bag cell neuron.
DISCUSSION
The present study demonstrates that Ca2+ removal by the PMCA influences the threshold of stimulation required to elicit CICR. Evidence for this comes from our finding that inhibiting Ca2+ removal by the PMCA rescues train-evoked CICR in cells recorded with EGTA-containing intracellular solution. That stated, we realize that the increased Ca2+ buffering capacity provided by EGTA definitely impacts the ability of PMCA inhibition to bring back CICR. Nevertheless, even in the absence of intracellular EGTA, antagonizing the PMCA with carboxyeosin allows for apparent CICR following a stimulus that is typically subthreshold for initiating the response. This is consistent with other neuronal types, where a steep relationship between the extent of Ca2+ influx and the magnitude of intracellular Ca2+ release has been identified. For example, in bullfrog sympathetic neurons, CICR grows dramatically with longer depolarizing stimuli (Colegrove et al. 2000a, 2000b; Friel and Tsien 1994). Likewise, in dorsal root ganglion neurons, augmented Ca2+ influx, mediated by the insertion of additional plasma membrane Ca2+ channels, causes the appearance of mitochondrial CICR to a normally ineffective depolarizing stimulus (D'Arco et al. 2015).
The strong dependence of CICR on intracellular Ca2+ has been attributed to the properties of the MCU, which underlies mitochondrial Ca2+ uptake and, in turn, the level of subsequent Ca2+ release. Although the uniporter only opens after intracellular Ca2+ is elevated considerably by voltage-gated influx, once engaged uptake occurs with great capacity over a large range of Ca2+ concentrations (Herrington et al. 1996; Kirichok et al. 2004). Importantly, the passage of Ca2+ through the mitochondrial uniporter increases in a nonlinear fashion with cytosolic Ca2+ (Gunter and Pfeiffer 1990; Herrington et al. 1996; Kirichok et al. 2004; Mallilankaraman et al. 2012; Patron et al. 2014; Perocchi et al. 2010). Consequently, slight changes in cytosolic Ca2+ can profoundly alter both the scale of Ca2+ uptake and ensuing Ca2+ release.
In this respect, the magnitude of Ca2+ efflux by the PMCA could in effect gate CICR by influencing the availability of Ca2+ for uptake by the mitochondria (Fig. 9). Carboxyeosin heightens the sensitivity of posttrain Ca2+ removal to FCCP, a finding consistent with an augmented role of the mitochondria in the removal of Ca2+ following voltage-gated Ca2+ influx. Furthermore, our physiological data and a Ca2+ compartment model demonstrate that the extent to which the mitochondria take up the Ca2+ entering through voltage-gated channels is increased when the PMCA is inhibited. The influence of Ca2+ removal by the PMCA on mitochondrial Ca2+ uptake and release is consistent with their apparent distribution in the bag cell neurons. Immunocytochemistry and live cell staining suggest that at the somatic plasma membrane, where voltage-gated Ca2+ influx occurs, the PMCA and mitochondria are relatively abundant with essentially similar distributions (although mitochondria are most concentrated in the central regions of the soma). Consequently, the PMCA appears ideally positioned to remove Ca2+ entry across the plasma membrane and limit the availability of Ca2+ for uptake by the mitochondria.
Fig. 9.
The interplay between the PMCA and mitochondria determines the removal of Ca2+ from voltage-gated influx and the presence of CICR in the bag cell neurons: conceptual model of activity-dependent Ca2+ dynamics in the bag cell neurons under whole cell recording conditions based on the present study and prior work by our laboratory and others (Geiger and Magoski 2008; Groten and Magoski 2015; Hickey et al. 2010, 2013; Hung and Magoski 2007; Michel and Wayne 2002; Wayne et al. 1998). Left: sample trace (top) of intracellular Ca2+ in response to a fast-phase period of activity when the PMCA is fully active. Numbers correspond to the chronology portrayed in the illustration: 1, voltage-gated Ca2+ channels (VGCC) open and allow Ca2+ to enter the cytosol; 2, subsequently, intracellular Ca2+ is removed by plasma membrane Ca2+ extrusion with the PMCA and mitochondrial Ca2+ uptake via the mitochondrial Ca2+ uniporter (MCU). The PMCA limits the Ca2+ available for uptake into the mitochondria and, in turn, prevents CICR from the mitochondria. Right: sample trace (top) showing intracellular Ca2+ during fast-phase activity when Ca2+ extrusion by the PMCA is diminished: 1, the activation of voltage-gated Ca2+ channels causes Ca2+ influx into the cytosol; 2, reduced Ca2+ extrusion by the PMCA increases the availability of Ca2+ for uptake by the mitochondria; 3, the greater Ca2+ load results in Ca2+ extrusion out of the mitochondria (CaXΔ) and thereby prolongs the initial influx signal. Such Ca2+ release could elicit egg-laying hormone (ELH) secretion and activate Ca2+-dependent nonselective cation channels (NSCC) to increase membrane excitability.
An interplay between Ca2+ removal systems and mitochondrial Ca2+ uptake has been characterized in other neurons. In the synaptic terminals of goldfish retinal bipolar neurons, the PMCA is the principal handling system for Ca2+ arising from voltage-gated influx, while the mitochondria are typically uninvolved (Zenisek and Matthews 2000). However, after disrupting the PMCA, the mitochondria then participate in the removal of Ca2+ from voltage-gated Ca2+ channels (Zenisek and Matthews 2000). Likewise, in the rat calyx of Held, Ca2+ removal by the plasma membrane Na+/Ca2+ exchanger influences the amount of mitochondrial Ca2+ uptake (Kim et al. 2005). In doing so, it was shown that developmental changes in Na+/Ca2+ exchanger expression at this synapse dictate the appearance of posttetanic mitochondrial Ca2+ release (Lee et al. 2013). Interestingly, compared with these other neuronal systems, bag cell neuron CICR occurs as a result of augmented mitochondrial Ca2+ uptake and is much more apparent. This may be explained by the fact that Ca2+ removal in the bag cell neurons has a greater dependence on mitochondrial Ca2+ uptake than in other neurons, as indicated by the sensitivity of posttrain Ca2+ handling to FCCP even when the PMCA is active (Geiger and Magoski 2008; Groten et al. 2013).
It appears that the PMCA does not play a large role in handling Ca2+ entering at rest, given that we found no difference in basal Ca2+ levels between control and carboxyeosin or high external pH. A contributing factor might be the relatively small resting Ca2+ influx across the bag cell neuron plasma membrane (Geiger et al. 2009). With a small Ca2+ leak, there may be little Ca2+ for the PMCA to handle at rest, and as a result any effect of inhibiting the PMCA on resting Ca2+ would not be easily detected. Also, any Ca2+ that becomes available after PMCA inhibition may be quickly removed by other buffering systems, such as the mitochondria.
Although our results indicate that the influence of Ca2+ handling by the PMCA on Ca2+ uptake and release occurs primarily through its effects on cytosolic Ca2+, different mechanisms must also be considered. In other neurons, reducing PMCA activity has been shown to prevent the transient extracellular alkalization and intracellular acidification that arise from its proton transport ability (Makani and Chesler 2010; Niggli et al. 1982; Thomas 2011). Such changes can modulate the function of several ion channels (Chen and Chesler 2015), including voltage-gated Ca2+ channels (Zhou and Jones 1996), or influence the pH-dependent processes required for mitochondrial Ca2+ uptake and H+/Ca2+ exchanger-mediated Ca2+ release (Jiang et al. 2009; Santo-Domingo and Demaurex 2010). That stated, it is doubtful that the activation of the PMCA during voltage-gated Ca2+ influx has a substantial effect on intracellular pH in the present study, as prior work has shown that Ca2+ derived from other channels does not alter the acidity of the cytosol (Knox et al. 2004). Even if such changes occurred, it is unlikely that disrupting proton transport by the PMCA would enhance bag cell neuron CICR by modulating voltage-gated Ca2+ channels or mitochondrial Ca2+ release. Carboxyeosin should preclude extracellular alkalization by the PMCA (Makani and Chesler 2010), a property that would prevent any alkaline shift-dependent enhancement of Ca2+ current (Zhou and Jones 1996). Indeed, our measurements of intracellular Ca2+ show that train-evoked Ca2+ influx was slightly smaller in carboxyeosin and high external pH. Both mitochondrial Ca2+ uptake and H+/Ca2+-mediated release increase with intracellular acidification (Jiang et al. 2009; Santo-Domingo and Demaurex 2010). Thus carboxyeosin or high external pH, which would prevent any potential PMCA-dependent intracellular acidification, should decrease, not increase, mitochondrial Ca2+ uptake and release. Consequently, the effects of the PMCA on stimulus-evoked Ca2+ dynamics can be most parsimoniously attributed to its influence on Ca2+ transport across the plasma membrane.
A noteworthy result from our study was that, unlike the mitochondria, the ER appears uninvolved in the removal of Ca2+ from voltage-gated influx, even when Ca2+ handling by the PMCA is disrupted. This contrasts with some neurons in other species, where both the ER and mitochondria have a role in Ca2+ removal (Fierro et al. 1998; Kim et al. 2003; Wheeler et al. 2012). Like other neurons (McDonough et al. 2000; Wheeler et al. 2012), we find that the ER in bag cell neurons forms dense appositions near the soma periphery both in vitro and in the cluster. These structures may represent subsurface cisternae, stacks of densely packed ER membrane that function in highly compartmentalized Ca2+ signaling (Berridge 1998). Indeed, freeze-fracture sections of bag cell neuron clusters reveal an array of ER membrane, resembling subsurface cisternae, that is often opposed to the somatic plasma membrane (Kaczmarek et al. 1979). Considering that the ER in the soma periphery is closely associated with sites of voltage-gated Ca2+ influx and the PMCA, it is surprising that the organelle does not contribute to Ca2+ removal, even when the PMCA is inhibited. However, given that our Ca2+ measurements reflect the average Ca2+ signal from the entire soma, and considering that the mitochondria, but not the ER, are found throughout the cell, it may be reasonable to expect that Ca2+ handling by the mitochondria would be more readily detected than that by the ER. Furthermore, the SERCA is known to be comparatively high-affinity, low-capacity relative to the mitochondria, while the MCU is often considered a low-affinity, high-capacity uptake system (Berridge 1998; Herrington et al. 1996; Werth and Thayer 1994). Consequently, it is likely that the ER contributes only in a small manner to remove Ca2+ from voltage-gated influx, and this is largely undetectable over the time periods we observed.
The relative abundance of the ER and mitochondria in the soma may contribute to the preeminence of mitochondrial Ca2+ handling. In cultured neurons, the localization of the ER is polarized, with less of a concentration in the lower and upper regions of the soma periphery compared with both the left and right regions, as well as the majority of regions for both the PMCA and the mitochondria. Also, a greater prevalence of mitochondria in the center of the soma may confer an advantage to these organelles in removing Ca2+ entering via voltage-gated channels. However, it should be noted that the apparent lack of ER involvement could be a by-product of the cell culture conditions, as the organelle is more homogeneously distributed in bag cell neurons in the cluster than in vitro. This may reflect the influence of cell-to-cell and cell-to-extracellular matrix interactions on how the ER is localized in the cluster compared with in vitro. Also, for cultured neurons the attachment to the culture dish, as well as the lack of attachment at the top of the cell, could restrict the ER from those areas. Finally, there are some apparent differences in somatic distribution that may not be all that biologically meaningful, for example, the disparity between the ER in the right region and mitochondria in the left region, or the amount of PMCA vs. mitochondria in the left regions. Ostensibly, these findings are largely the function of the left mitochondrial staining being, inexplicably, slightly low.
A growing body of evidence has established that plasma membrane Ca2+ extrusion by either the PMCA or Na+/Ca2+ exchanger can modify activity-dependent processes, including short-term presynaptic plasticity, long-term potentiation, and afterhyperpolarizations (Empson et al. 2007; Ghosh et al. 2011; Jensen et al. 2007; Jeon et al. 2003). These findings have been primarily attributed to the ability of Ca2+ handling systems to control the rate at which Ca2+ from voltage-gated influx is removed. In many neurons, intracellular Ca2+ release promotes activity-dependent changes in excitability and synaptic transmission (Garcia-Chacon et al. 2006; Lee et al. 2007; Tang and Zucker 1997). Consequently, our research indicates that Ca2+ removal systems could control neuronal plasticity by regulating CICR. Evidence for this comes from the calyx of Held, where changes in Na+/Ca2+ exchanger expression are shown to gate the presence of posttetanic potentiation by controlling the magnitude of mitochondrial Ca2+ uptake and release (Kim et al. 2005; Lee et al. 2013).
In this respect, the ability of Ca2+ removal by the PMCA to influence CICR may have important consequences for bag cell neuron afterdischarge and reproductive behavior (Fig. 9). CICR is initiated during the bag cell neuron in the intact cluster (Fisher et al. 1994) and is implicated in sustaining peptide secretion, as some prior studies show that a substantial amount of egg-laying hormone release during the afterdischarge occurs independent of extracellular Ca2+ (Michel and Wayne 2002; Wayne et al. 1998). Because the mitochondrial Ca2+ store is a principal source of CICR in the bag cell neurons, it may contribute to peptide secretion during the afterdischarge (Geiger and Magoski 2008; Groten et al. 2013). Aside from secretion, mitochondrial Ca2+ release also activates nonselective cation currents (Hickey et al. 2010), which provide the depolarizing drive that sustains the afterdischarge (Hung and Magoski 2007; Wilson et al. 1996).
Mitochondrial Ca2+ release is implicated in several crucial events during the afterdischarge; thus our present findings suggest that the modulation of PMCA activity could potently change the propensity for CICR and its downstream effectors. However, because the effect of the PMCA on CICR was demonstrated pharmacologically, it is not clear whether this occurs in response to physiological changes in PMCA function. Interestingly, in other preparations, the contribution of plasma membrane Ca2+ extrusion by the PMCA can be modified by pretranslational mechanisms, as well as protein kinases known to be engaged during the afterdischarge (Ghosh et al. 2011; Kaczmarek et al. 1978; Lee et al. 2013; Magoski and Kaczmarek 2005; Usachev et al. 2002; Wang et al. 1992; Wayne et al. 1999; Zacharias and Strehler 1996). It may be that physiological changes in PMCA function occur in the bag cell neurons to influence activity-dependent Ca2+ dynamics.
GRANTS
This work was supported by a Canadian Institutes of Health Research operating grant to N. S. Magoski. C. J. Groten held an National Sciences and Engineering Research Council Post-graduate Doctoral Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.J.G., J.T.R., G.B., and N.S.M. conception and design of research; C.J.G., J.T.R., H.M.H., and A.K.C. performed experiments; C.J.G., J.T.R., and H.M.H. analyzed data; C.J.G., J.T.R., G.B., and N.S.M. interpreted results of experiments; C.J.G. and J.T.R. prepared figures; C.J.G. drafted manuscript; C.J.G. and N.S.M. edited and revised manuscript; C.J.G., J.T.R., H.M.H., A.K.C., G.B., and N.S.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. C. J. Carter for the in silico identification of the Aplysia californica PMCA and Dr. K. Ding for advice on statistical analysis.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- Arch S. Polypeptide secretion from the isolated parietovisceral ganglion of Aplysia californica. J Gen Physiol 59: 47–59, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol 136: 833–844, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron 21: 13–26, 1998. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32: 235–249, 2002. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Genazzani A, Guerini D. Calcium controls the transcription of its own transporters and channels in developing neurons. Biochem Biophys Res Commun 266: 624–632, 1999. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Tiozzo R, Lugli G, Crovetti F, Kratzing C. The release of calcium from heart mitochondria by sodium. J Mol Cell Cardiol 6: 361–371, 1974. [DOI] [PubMed] [Google Scholar]
- Chen HY, Chesler M. Autocrine boost of NMDAR current in hippocampal CA1 pyramidal neurons by a PMCA-dependent, perisynaptic, extracellular pH shift. J Neurosci 35: 873–877, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouhan AK, Ivannikov MV, Lu Z, Sugimori M, Llinas RR, Macleod GT. Cytosolic calcium coordinates mitochondrial energy metabolism with presynaptic activity. J Neurosci 32: 1233–1243, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell 131: 1047–1058, 2007. [DOI] [PubMed] [Google Scholar]
- Colegrove SL, Albrecht MA, Friel DD. Dissection of mitochondrial Ca2+ uptake and release fluxes in situ after depolarization-evoked [Ca2+]i elevations in sympathetic neurons. J Gen Physiol 115: 351–370, 2000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegrove SL, Albrecht MA, Friel DD. Quantitative analysis of mitochondrial Ca2+ uptake and release pathways in sympathetic neurons. Reconstruction of the recovery after depolarization-evoked [Ca2+]i elevations. J Gen Physiol 115: 371–388, 2000b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arco M, Margas W, Cassidy JS, Dolphin AC. The upregulation of α2δ-1 subunit modulates activity-dependent Ca2+ signals in sensory neurons. J Neurosci 35: 5891–5903, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ, Ireland DR, McLachlan EM. Sources of Ca2+ for different Ca2+-activated K+ conductances in neurones of the rat superior cervical ganglion. J Physiol 495: 353–366, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empson RM, Garside ML, Knopfel T. Plasma membrane Ca2+ ATPase 2 contributes to short-term synapse plasticity at the parallel fiber to Purkinje neuron synapse. J Neurosci 27: 3753–3758, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L, DiPolo R, Llano I. Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices. J Physiol 510: 499–512, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink LA, Connor JA, Kaczmarek LK. Inositol trisphosphate releases intracellularly stored calcium and modulates ion channels in molluscan neurons. J Neurosci 8: 2544–2555, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TE, Levy S, Kaczmarek LK. Transient changes in intracellular calcium associated with a prolonged increase in excitability in neurons of Aplysia californica. J Neurophysiol 71: 1254–1257, 1994. [DOI] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci 14: 4007–4024, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabso M, Neher E, Spira ME. Low mobility of the Ca2+ buffers in axons of cultured Aplysia neurons. Neuron 18: 473–481, 1997. [DOI] [PubMed] [Google Scholar]
- Garcia-Chacon LE, Nguyen KT, David G, Barrett EF. Extrusion of Ca2+ from mouse motor terminal mitochondria via a Na+-Ca2+ exchanger increases post-tetanic evoked release. J Physiol 574: 663–675, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardam KE, Geiger JE, Hickey CM, Hung AY, Magoski NS. Flufenamic acid affects multiple currents and causes intracellular Ca2+ release in Aplysia bag cell neurons. J Neurophysiol 100: 38–49, 2008. [DOI] [PubMed] [Google Scholar]
- Gatto C, Hale CC, Xu W, Milanick MA. Eosin, a potent inhibitor of the plasma membrane Ca pump, does not inhibit the cardiac Na-Ca exchanger. Biochemistry 34: 965–972, 1995. [DOI] [PubMed] [Google Scholar]
- Gatto C, Milanick MA. Inhibition of the red blood cell calcium pump by eosin and other fluorescein analogues. Am J Physiol Cell Physiol 264: C1577–C1586, 1993. [DOI] [PubMed] [Google Scholar]
- Geiger JE, Hickey CM, Magoski NS. Ca2+ entry through a non-selective cation channel in Aplysia bag cell neurons. Neuroscience 162: 1023–1038, 2009. [DOI] [PubMed] [Google Scholar]
- Geiger JE, Magoski NS. Ca2+-induced Ca2+ release in Aplysia bag cell neurons requires interaction between mitochondrial and endoplasmic reticulum stores. J Neurophysiol 100: 24–37, 2008. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Li Y, Thayer SA. Inhibition of the plasma membrane Ca2+ pump by CD44 receptor activation of tyrosine kinases increases the action potential afterhyperpolarization in sensory neurons. J Neurosci 31: 2361–2370, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groten CJ, Magoski NS. PKC enhances the capacity for secretion by rapidly recruiting covert voltage-gated Ca2+ channels to the membrane. J Neurosci 35: 2747–2765, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groten CJ, Rebane JT, Blohm G, Magoski NS. Separate Ca2+ sources are buffered by distinct Ca2+ handling systems in Aplysia neuroendocrine cells. J Neurosci 33: 6476–6491, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum LM, Carew TJ. Growth factor modulation of substrate-specific morphological patterns in Aplysia bag cell neurons. Learn Mem 6: 292–306, 1999. [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- Gunter KK, Gunter TE. Transport of calcium by mitochondria. J Bioenerg Biomembr 26: 471–485, 1994. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol Cell Physiol 258: C755–C786, 1990. [DOI] [PubMed] [Google Scholar]
- Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron 16: 219–228, 1996. [DOI] [PubMed] [Google Scholar]
- Heytler PG, Prichard WW. A new class of uncoupling agents—carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun 7: 272–275, 1962. [DOI] [PubMed] [Google Scholar]
- Hickey CM, Geiger JE, Groten CJ, Magoski NS. Mitochondrial Ca2+ activates a cation current in Aplysia bag cell neurons. J Neurophysiol 103: 1543–1556, 2010. [DOI] [PubMed] [Google Scholar]
- Hickey CM, Groten CJ, Sham L, Carter CJ, Magoski NS. Voltage-gated Ca2+ influx and mitochondrial Ca2+ initiate secretion from Aplysia neuroendocrine cells. Neuroscience 250: 755–772, 2013. [DOI] [PubMed] [Google Scholar]
- Hung AY, Magoski NS. Activity-dependent initiation of a prolonged depolarization in Aplysia bag cell neurons: role for a cation channel. J Neurophysiol 97: 2465–2479, 2007. [DOI] [PubMed] [Google Scholar]
- Jensen TP, Filoteo AG, Knopfel T, Empson RM. Presynaptic plasma membrane Ca2+ ATPase isoform 2a regulates excitatory synaptic transmission in rat hippocampal CA3. J Physiol 579: 85–99, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Yang YM, Jeong MJ, Philipson KD, Rhim H, Shin HS. Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron 38: 965–976, 2003. [DOI] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326: 144–147, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling P, McLachlan EM, Sah P. Calcium induced calcium release is involved in the afterhyperpolarization in one class of guinea pig sympathetic neurone. J Auton Nerv Syst 42: 251–257, 1993. [DOI] [PubMed] [Google Scholar]
- Jonas EA, Knox RJ, Smith TC, Wayne NL, Connor JA, Kaczmarek LK. Regulation by insulin of a unique neuronal Ca2+ pool and of neuropeptide secretion. Nature 385: 343–346, 1997. [DOI] [PubMed] [Google Scholar]
- Kachoei BA, Knox RJ, Uthuza D, Levy S, Kaczmarek LK, Magoski NS. A store-operated Ca2+ influx pathway in the bag cell neurons of Aplysia. J Neurophysiol 96: 2688–2698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK, Finbow M, Revel JP, Strumwasser F. The morphology and coupling of Aplysia bag cells within the abdominal ganglion and in cell culture. J Neurobiol 10: 535–550, 1979. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Jennings K, Strumwasser F. Neurotransmitter modulation, phosphodiesterase inhibitor effects, and cyclic AMP correlates of afterdischarge in peptidergic neurites. Proc Natl Acad Sci USA 77: 7487–7491, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK, Jennings KR, Strumwasser F. An early sodium and a late calcium phase in the afterdischarge of peptide-secreting neurons of Aplysia. Brain Res 238: 105–115, 1982. [DOI] [PubMed] [Google Scholar]
- Karadjov JS, Kudzina LY, Zinchenko VP. TPP+ inhibits Na+-stimulated Ca2+ efflux from brain mitochondria. Cell Calcium 7: 115–119, 1986. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Kuhl D, Barzilai A, Sweatt JD, Kandel ER. Long-term sensitization training in Aplysia leads to an increase in calreticulin, a major presynaptic calcium-binding protein. Neuron 9: 1013–1024, 1992. [DOI] [PubMed] [Google Scholar]
- Kim MH, Korogod N, Schneggenburger R, Ho WK, Lee SH. Interplay between Na+/Ca2+ exchangers and mitochondria in Ca2+ clearance at the calyx of Held. J Neurosci 25: 6057–6065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Lee SH, Park KH, Ho WK, Lee SH. Distribution of K+-dependent Na+/Ca2+ exchangers in the rat supraoptic magnocellular neuron is polarized to axon terminals. J Neurosci 23: 11673–11680, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364, 2004. [DOI] [PubMed] [Google Scholar]
- Knox RJ, Jonas EA, Kao LS, Smith PJ, Connor JA, Kaczmarek LK. Ca2+ influx and activation of a cation current are coupled to intracellular Ca2+ release in peptidergic neurons of Aplysia californica. J Physiol 494.3: 627–639, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox RJ, Magoski NS, Wing D, Barbee SJ, Kaczmarek LK. Activation of a calcium entry pathway by sodium pyrithione in the bag cell neurons of Aplysia. J Neurobiol 60: 411–423, 2004. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Stimulation of egg laying: possible neuroendocrine function of bag cells of abdominal ganglion of Aplysia californica. Nature 216: 814–815, 1967. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Kandel ER. Electrophysiological properties and functional interconnections of two symmetrical neurosecretory clusters (bag cells) in abdominal ganglion of Aplysia. J Neurophysiol 33: 865–876, 1970. [DOI] [PubMed] [Google Scholar]
- Lee D, Lee KH, Ho WK, Lee SH. Target cell-specific involvement of presynaptic mitochondria in post-tetanic potentiation at hippocampal mossy fiber synapses. J Neurosci 27: 13603–13613, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim MH, Ho WK, Lee SH. Developmental upregulation of presynaptic NCKX underlies the decrease of mitochondria-dependent posttetanic potentiation at the rat calyx of Held synapse. J Neurophysiol 109: 1724–1734, 2013. [DOI] [PubMed] [Google Scholar]
- Llano I, DiPolo R, Marty A. Calcium-induced calcium release in cerebellar Purkinje cells. Neuron 12: 663–673, 1994. [DOI] [PubMed] [Google Scholar]
- Loechner KJ, Azhderian EM, Dreyer R, Kaczmarek LK. Progressive potentiation of peptide release during a neuronal discharge. J Neurophysiol 63: 738–744, 1990. [DOI] [PubMed] [Google Scholar]
- Lupinsky DA, Magoski NS. Ca2+-dependent regulation of a non-selective cation channel from Aplysia bag cell neurones. J Physiol 575: 491–506, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles V, Zhao Y, Martin KC. Synapse formation and mRNA localization in cultured Aplysia neurons. Neuron 49: 349–356, 2006. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol 20: 102–112, 2010. [DOI] [PubMed] [Google Scholar]
- Magoski NS, Kaczmarek LK. Association/dissociation of a channel-kinase complex underlies state-dependent modulation. J Neurosci 25: 8037–8047, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani S, Chesler M. Rapid rise of extracellular pH evoked by neural activity is generated by the plasma membrane calcium ATPase. J Neurophysiol 103: 667–676, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, Madesh M. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 14: 1336–1343, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough SI, Cseresnyes Z, Schneider MF. Origin sites of calcium release and calcium oscillations in frog sympathetic neurons. J Neurosci 20: 9059–9070, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem 261: 6300–6306, 1985. [PubMed] [Google Scholar]
- Michel S, Wayne NL. Neurohormone secretion persists after post-afterdischarge membrane depolarization and cytosolic calcium elevation in peptidergic neurons in intact nervous tissue. J Neurosci 22: 9063–9069, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell 48: 899–907, 1987. [DOI] [PubMed] [Google Scholar]
- Naraghi M. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium 22: 255–268, 1997. [DOI] [PubMed] [Google Scholar]
- Niggli V, Sigel E, Carafoli E. The purified Ca2+ pump of human erythrocyte membranes catalyzes an electroneutral Ca2+-H+ exchange in reconstituted liposomal systems. J Biol Chem 257: 2350–2356, 1982. [PubMed] [Google Scholar]
- Nowycky MC, Pinter MJ. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys J 64: 77–91, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan NC, Jahn TR, Reid E, O'Kane CJ. Reticulon-like-1, the Drosophila orthologue of the hereditary spastic paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons. Hum Mol Genet 21: 3356–3365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R, Hershfinkel M, Sekler I. Molecular identity and functional properties of the mitochondrial Na+/Ca2+ exchanger. J Biol Chem 287: 31650–31657, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA 107: 436–441, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF. Ca2+ store-dependent potentiation of Ca2+-activated non-selective cation channels in rat hippocampal neurones in vitro. J Physiol 521: 617–627, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio RD, Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D, Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell 53: 726–737, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467: 291–296, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot N, Tyteca D, D'auria L, Dewachter I, Gailly P, Hendrickx A, Tasiaux B, Haylani LE, Muls N, N'kuli F, Laquerriere A, Demoulin JB, Campion D, Brion JP, Courtoy PJ, Kienlen-Campard P, Octave JN. Amyloid precursor protein controls cholesterol turnover needed for neuronal activity. EMBO Mol Med 5: 608–625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker HM, Dudek FE. Bag cell control of egg laying in freely behaving Aplysia. Science 197: 490–493, 1977. [DOI] [PubMed] [Google Scholar]
- Pivovarova NB, Hongpaisan J, Andrews SB, Friel DD. Depolarization-induced mitochondrial Ca2+ accumulation in sympathetic neurons: spatial and temporal characteristics. J Neurosci 19: 6372–6384, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter TA, Kolaj M, Renaud LP. Low voltage-activated Ca2+ channels are coupled to Ca2+-induced Ca2+ release in rat thalamic midline neurons. J Neurosci 25: 8267–8271, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766, 1998. [DOI] [PubMed] [Google Scholar]
- Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta 1797: 907–912, 2010. [DOI] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem 264: 17816–17823, 1989. [PubMed] [Google Scholar]
- Shmigol A, Eisner DA, Wray S. Carboxyeosin decreases the rate of decay of the [Ca2+]i transient in uterine smooth muscle cells isolated from pregnant rats. Pflügers Arch 437: 158–160, 1998. [DOI] [PubMed] [Google Scholar]
- Shmigol A, Verkhratsky A, Isenberg G. Calcium-induced calcium release in rat sensory neurons. J Physiol 489: 627–636, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutov LP, Kim MS, Houlihan PR, Medvedeva YV, Usachev YM. Mitochondria and plasma membrane Ca2+-ATPase control presynaptic Ca2+ clearance in capsaicin-sensitive rat sensory neurons. J Physiol 591: 2443–2462, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam AK, Gardam KE, Lamb S, Kachoei BA, Magoski NS. Role for protein kinase C in controlling Aplysia bag cell neuron excitability. Neuroscience 179: 41–55, 2011. [DOI] [PubMed] [Google Scholar]
- Tam AK, Geiger JE, Hung AY, Groten CJ, Magoski NS. Persistent Ca2+ current contributes to a prolonged depolarization in Aplysia bag cell neurons. J Neurophysiol 102: 3753–3765, 2009. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron 18: 483–491, 1997. [DOI] [PubMed] [Google Scholar]
- Thomas RC. The Ca2+:H+ coupling ratio of the plasma membrane calcium ATPase in neurones is little sensitive to changes in external or internal pH. Cell Calcium 49: 357–364, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usachev YM, DeMarco SJ, Campbell C, Strehler EE, Thayer SA. Bradykinin and ATP accelerate Ca2+ efflux from rat sensory neurons via protein kinase C and the plasma membrane Ca2+ pump isoform 4. Neuron 33: 113–122, 2002. [DOI] [PubMed] [Google Scholar]
- Usachev YM, Thayer SA. All-or-none Ca2+ release from intracellular stores triggered by Ca2+ influx through voltage-gated Ca2+ channels in rat sensory neurons. J Neurosci 17: 7404–7414, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-da-Cunha M, Tyteca D, Stroobant V, Courtoy PJ, Opperdoes FR, Van SE. Molecular identification of NAT8 as the enzyme that acetylates cysteine S-conjugates to mercapturic acids. J Biol Chem 285: 18888–18898, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85: 201–279, 2005. [DOI] [PubMed] [Google Scholar]
- Wang KK, Villalobo A, Roufogalis BD. The plasma membrane calcium pump: a multiregulated transporter. Trends Cell Biol 2: 46–52, 1992. [DOI] [PubMed] [Google Scholar]
- Wayne NL, Kim J, Lee E. Prolonged hormone secretion from neuroendocrine cells of Aplysia is independent of extracellular calcium. J Neuroendocrinol 10: 529–537, 1998. [DOI] [PubMed] [Google Scholar]
- Wayne NL, Lee W, Kim YJ. Persistent activation of calcium-activated and calcium-independent protein kinase C in response to electrical afterdischarge from peptidergic neurons of Aplysia. Brain Res 834: 211–213, 1999. [DOI] [PubMed] [Google Scholar]
- Werth JL, Thayer SA. Mitochondria buffer physiological calcium loads in cultured dorsal root ganglion neurons. J Neurosci 14: 348–356, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW. CaV1 and CaV2 channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell 149: 1112–1124, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BH, Kaczmarek LK. Identification of a vesicular pool of calcium channels in the bag cell neurons of Aplysia californica. J Neurosci 17: 1582–1595, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GF, Richardson FC, Fisher TE, Olivera BM, Kaczmarek LK. Identification and characterization of a Ca2+-sensitive nonspecific cation channel underlying prolonged repetitive firing in Aplysia neurons. J Neurosci 16: 3661–3671, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove DE, Gunter TE. Kinetics of mitochondrial calcium transport. II. A kinetic description of the sodium-dependent calcium efflux mechanism of liver mitochondria and inhibition by ruthenium red and by tetraphenylphosphonium. J Biol Chem 261: 15166–15171, 1986. [PubMed] [Google Scholar]
- Zacharias DA, Strehler EE. Change in plasma membrane Ca2+-ATPase splice-variant expression in response to a rise in intracellular Ca2+. Curr Biol 6: 1642–1652, 1996. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron 25: 229–237, 2000. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Forscher P. Rac1 modulates stimulus-evoked Ca2+ release in neuronal growth cones via parallel effects on microtubule/endoplasmic reticulum dynamics and reactive oxygen species production. Mol Biol Cell 20: 3700–3712, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Jones SW. The effects of external pH on calcium channel currents in bullfrog sympathetic neurons. Biophys J 70: 1326–1334, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.