Abstract
Theta-burst stimulation (TBS) over human primary motor cortex evokes plasticity and metaplasticity, the latter contributing to the homeostatic balance of excitation and inhibition. Our knowledge of TBS-induced effects on primary somatosensory cortex (SI) is limited, and it is unknown whether TBS induces metaplasticity within human SI. Sixteen right-handed participants (6 females, mean age 23 yr) received two TBS protocols [continuous TBS (cTBS) and intermittent TBS (iTBS)] delivered in six different combinations over SI in separate sessions. TBS protocols were delivered at 30 Hz and were as follows: a single cTBS protocol, a single iTBS protocol, cTBS followed by cTBS, iTBS followed by iTBS, cTBS followed by iTBS, and iTBS followed by cTBS. Measures included the amplitudes of the first and second somatosensory evoked potentials (SEPs) via median nerve stimulation, their paired-pulse ratio (PPR), and temporal order judgment (TOJ). Dependent measures were obtained before TBS and at 5, 25, 50, and 90 min following stimulation. Results indicate similar effects following cTBS and iTBS; increased amplitudes of the second SEP and PPR without amplitude changes to SEP 1, and impairments in TOJ. Metaplasticity was observed such that TOJ impairments following a single cTBS protocol were abolished following consecutive cTBS protocols. Additionally, consecutive iTBS protocols altered the time course of effects when compared with a single iTBS protocol. In conclusion, 30-Hz cTBS and iTBS protocols delivered in isolation induce effects consistent with a TBS-induced reduction in intracortical inhibition within SI. Furthermore, cTBS- and iTBS-induced metaplasticity appear to follow homeostatic and nonhomeostatic rules, respectively.
Keywords: neural plasticity, paired pulse, somatosensory evoked potential, temporal order judgment, primary somatosensory cortex
neural plasticity is itself governed by the mechanisms of plasticity, an effect called metaplasticity whereby synaptic activity influences the direction and amplitude of forthcoming plasticity (Abraham and Bear 1996). Long-term potentiation (LTP) increases the threshold for Ca2+ entry thereby promoting the subsequent induction of long-term depression (LTD) (Abraham 2008; Abraham and Bear 1996; Bienenstock et al. 1982; Frey et al. 1995). In contrast, LTD at a synapse decreases the Ca2+ threshold and will therefore promote subsequent LTP (Abraham 2008; Abraham and Bear 1996; Bienenstock et al. 1982). Metaplasticity is considered to have an essential function of limiting excessive LTP and/or LTD that may otherwise damage cells (Abraham 2008) and simultaneously balance the levels of excitation and inhibition to allow for task-relevant synaptic plasticity (Abraham 2008; Murakami et al. 2012).
In humans, transcranial magnetic stimulation (TMS) plasticity protocols such as theta-burst stimulation (TBS) induce plasticity and metaplasticity within primary motor cortex (M1) such that exposure to one protocol facilitates or depresses neural responses to subsequent stimulation (Doeltgen and Ridding 2011; Gamboa et al. 2011; Goldsworthy et al. 2012a; Mastroeni et al. 2013; Muller-Dahlhaus and Ziemann 2015; Murakami et al. 2012; Todd et al. 2009). TBS delivered in continuous (cTBS) and intermittent (iTBS) patterns may evoke opposite effects such that motor evoked potentials (MEPs) are decreased (Goldsworthy et al. 2012b; Huang et al. 2005; Ishikawa et al. 2007; Jacobs et al. 2013; Talelli et al. 2007; Wu et al. 2012; Zafar et al. 2008) and increased (Huang et al. 2005; Zafar et al. 2008), respectively. However, this relationship is complicated by variability in stimulus parameters and intersubject variability, and opposite effects of cTBS and iTBS are not consistently reported (Fung and Robinson 2014; Gentner et al. 2008; Goldsworthy et al. 2012b; Hamada et al. 2013; Wu et al. 2012). When identical TBS protocols are applied consecutively over M1, responses evoked by cTBS and iTBS protocols are opposite such that cTBS followed by cTBS evokes LTP-like increases in MEP amplitude (Gamboa et al. 2011; Goldsworthy et al. 2012a), whereas iTBS followed by iTBS evokes LTD-like decreases in MEP amplitude (Gamboa et al. 2011; Mastroeni et al. 2013). Metaplasticity may therefore participate in human M1 by promoting a balance of excitation and inhibition and limiting excessive plasticity in either direction.
It is unclear whether metaplasticity principles derived from studies in human M1 apply to neighboring somatosensory cortex (SI). Electrophysiological and neurochemical studies in rat SI indicate that both cTBS and iTBS increase the amplitude of somatosensory evoked potentials (SEPs) and decrease the number of inhibitory cells containing parvalbumin (PV) and calbindin (CB) calcium-binding proteins (Benali et al. 2011; Funke and Benali 2011; Labedi et al. 2014). Collectively, these reports indicate a TBS-induced reduction in inhibitory circuits within SI. For iTBS, such reductions are suggested to be mediated via LTD at PV-expressing cells that consequently disinhibit pyramidal cell output (Benali et al. 2011). Furthermore, in rats, the effects of cTBS and to a greater extent iTBS are reduced when followed by a tactile discrimination learning task, indicating complex metaplasticity interactions within SI (Mix et al. 2010).
In humans, iTBS and cTBS over SI have been shown to increase, decrease, or not change the amplitude of SEPs (Ishikawa et al. 2007; Katayama et al. 2010; Katayama and Rothwell 2007; Meehan et al. 2011; Premji et al. 2010; Ragert et al. 2008). Measures of tactile perception reveal cTBS-induced impairments in both spatial (Rai et al. 2012) and temporal (Lee et al. 2013; Rai et al. 2012) acuity. To date, there are few studies in humans that examine TBS-induced effects on SI physiology and touch perception (Ragert et al. 2008), and there are no studies that investigate TBS-induced metaplasticity within SI. The present study examined the physiological and psychophysical effects of single and paired TBS protocols over SI. Of specific interest was the comparison of a single protocol of cTBS to iTBS to investigate their similarities and differences and dual protocols of cTBS and iTBS to investigate their metaplasticity effects. Our data indicate that cTBS and iTBS have similar effects such that measures of intracortical inhibition and touch perception are altered without changes to the first SEP, bearing strong similarity to the effects that follow intermittent high-frequency tactile stimulation, 5 Hz repetitive TMS (rTMS), or their consecutive combination (Gatica Tossi et al. 2013b). Additionally, consecutive identical TBS protocols evoke metaplasticity in measures of tactile perception only, suggesting that metaplasticity operates via changes in intracortical inhibition rather than changes in excitatory mechanisms within SI.
METHODS
Participants.
Sixteen healthy adults participated (6 females, mean age = 23 ± 5.2 yr). All participants were right-hand dominant and were screened using a modified version of the Edinburgh Handedness Scale (Oldfield 1971). Individuals participated in six sessions separated by a minimum of 1 wk. All sessions were held at approximately the same time of day to minimize cortisol-related excitability changes that may occur throughout the day (Sale et al. 2007, 2008). All subjects provided written informed consent before participation. The study was approved by the McMaster Research Ethics Board and conformed to the Declaration of Helsinki.
Electromyography recording.
Electromyography (EMG) was recorded using surface electrodes (9-mm-diameter Ag-AgCl) placed over the bilateral abductor pollicis brevis (APB) muscle and the first dorsal interosseus (FDI) muscle in a belly-tendon montage. Right APB was the target muscle for the M1 hotspot used for obtaining motor threshold as described below. The purpose of recording EMG over right FDI and left APB and FDI was to ensure neighboring muscles were relaxed during testing. EMG signals were band-passed filtered between 20 Hz and 2.5 kHz, amplified 1,000× (Intronix Technologies model 2024F with Signal Conditioning; Intronix Technologies, Bolton, Canada), and subsequently digitized at 5 kHz by an analog-to-digital interface (Power1401; Cambridge Electronics Design, Cambridge, UK). All EMG data were collected using Signal software (version 6.02; Cambridge Electronic Designs).
Transcranial magnetic stimulation and neuronavigation.
Single-pulse TMS was applied over left M1 using a 70-mm-inner diameter figure-of-eight air-cooled coil attached to a Magstim Super Rapid2 Stimulator (Magstim, Whitland, UK). The coil handle was oriented at a 45° angle to the midsagittal line to induce an initial anterior and medially directed current in M1. The motor hotspot was identified as the optimal site for eliciting a consistent MEP in the relaxed right APB muscle and was digitally marked using Brainsight Neuronavigation Software (Rogue Research, Montreal, Canada). Resting motor threshold (RMT) was obtained at the motor hotspot and defined as the minimum stimulation intensity required to elicit MEPs >50 μV in 5 out of 10 consecutive trials (Rossi et al. 2009). TBS was delivered to SI at the location of the C3′ electrode (Jacobs et al. 2013) as defined by the International 10–20 System. The location of C3′ was digitally marked for each participant using Brainsight Neuronavigation to maintain the same coil placement for each of the subsequent sessions. The coil was manually held by the experimenter and oriented over SI to induce a current directed anterior and medial (analogous to the direction used for RMT calculation) during the initial phase of the biphasic pulse (see Fig. 1C), an orientation commonly used to stimulate SI (Ishikawa et al. 2007; Katayama et al. 2010; Jacobs et al. 2013; Tsang et al. 2014), that selectively yields increases in SEPs following 50 Hz iTBS (Katayama and Rothwell 2007). cTBS was delivered at a frequency of 30 Hz with bursts of 3 pulses repeating at 6 Hz for a total of 612 pulses. iTBS was delivered at 30 Hz and consisted of a 2-s train of 3 pulse bursts (6-Hz burst frequency) repeated at 10-s intervals for a total of 612 pulses (modified from Goldsworthy et al. 2012b; Huang et al. 2005). Relative to other TBS frequencies, 30 Hz was selected because it demonstrates less interindividual variability (Goldsworthy et al. 2012b) and creates short-term plasticity when delivered over SI (Jacobs et al. 2013; Tsang et al. 2014), results in MEP suppression when delivered as cTBS over M1 (Tsang et al. 2014; Wu et al. 2012), and MEP facilitation when delivered as iTBS over M1 (Wu et al. 2012). All TBS protocols were applied at an intensity of 70% RMT as opposed to AMT to avoid muscle contraction before TBS, which can itself induce metaplasticity effects (Gentner et al. 2008; Goldsworthy et al. 2012a). The intensity of 70% RMT was chosen since it has been shown to alter motor cortical excitability measured by changes in MEPs when delivered over M1 (Goldsworthy et al. 2012a; Tsang et al. 2014) and also when delivered over SI (Tsang et al. 2014).
Fig. 1.
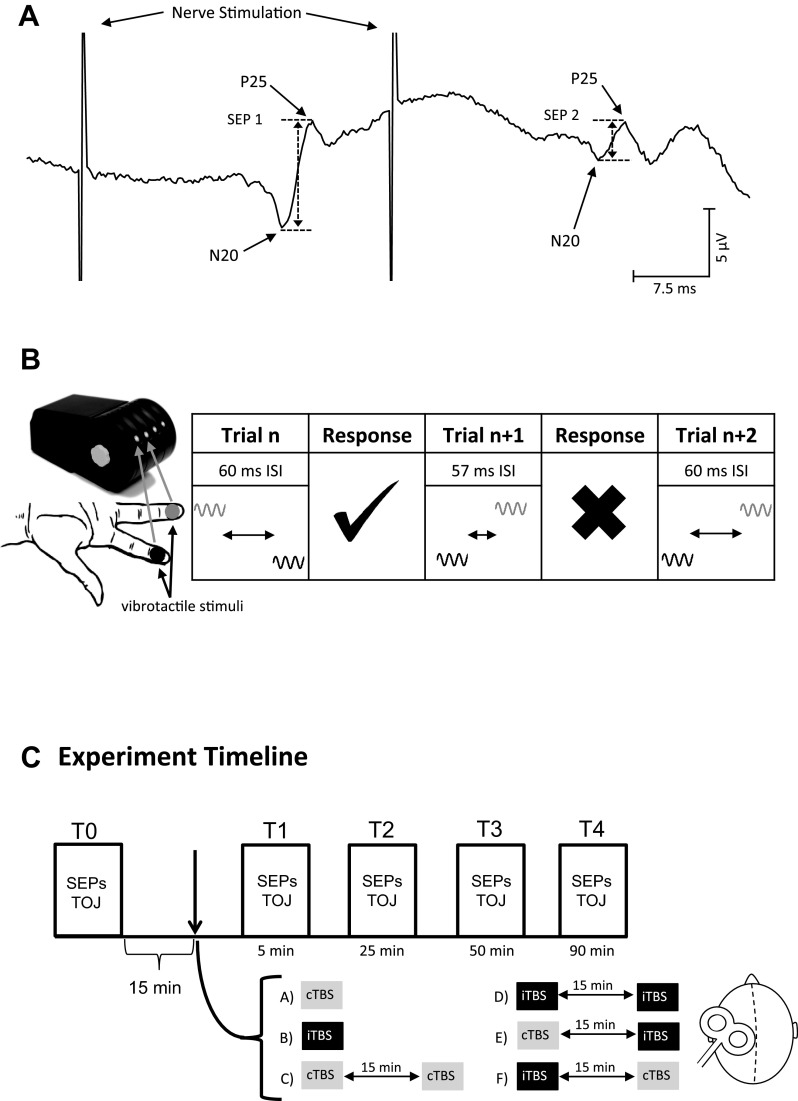
Experimental protocol. A: depiction of an average somatosensory evoked potential (SEP) trace from one participant. SEP 1 was calculated as the N20–P25 amplitude after the first stimulation, and SEP 2 was calculated as the N20–P25 amplitude after the second stimulation. Paired-pulse ratio (PPR) was calculated as the ratio of the 2 measures (i.e., SEP 2/SEP 1). B: temporal order judgement (TOJ) task. The Cortical Metrics Device was used to consecutively vibrate the volar surface of digits 2 (black) and 3 (gray). The amplitude of the two stimulations was 300 μm, and the interstimulus interval (ISI) between the two stimulations began at 150 ms for the first trial. Participants were queried to report the digit that experienced the first stimulation within the pair. A correction response would automatically reduce the ISI by 5%. An incorrect response would increase the ISI to match the previous trial. Thirty-one total trials were collected, and the last trial (trial 31) was defined as the TOJ threshold. C: experiment timeline. Five time blocks were collected. T0 indicates baseline measurements before theta-burst stimulation (TBS). Upon completing T0 measures, 15 min elapsed before the experimental protocol. Six protocols were completed in different sessions using continuous TBS (cTBS, gray) or intermittent TBS (iTBS, black) or metaplasticity protocols using combinations of cTBS and iTBS. Measures were acquired following the cessation of the TBS protocol at 5, 25, 50, and 90 min. Measures within each time block were completed within 15 min.
SEPs and paired-pulse ratio.
SEPs were recorded from SI using the International 10–20 System with the active electrode placed at C3′ and referenced to Fz (Nuwer et al. 1994). A ground electrode was placed over the left clavicle. Signals were band-passed filtered between 2 Hz and 2.5 kHz, amplified 10,000× (Intronix Technologies model 2024F with Signal Conditioning; Intronix Technologies), and digitized at 5 kHz by an analog-to-digital interface (Power1401; Cambridge Electronics Design). The active EEG lead was removed during application of the TBS protocol and was replaced immediately following TBS as performed elsewhere (Premji et al. 2010; Ragert et al. 2008). To ensure accurate replacement of the electrode, the location of C3′ was digitally marked using Brainsight Neuronavigation in addition to being marked directly on the scalp with nonpermanent marker. Electrode impedance was tested before and at every time block (described below) after TBS to maintain an impedance <5 kΩ (UFI Checktrode, model 1089 Mk III; UFI). The median nerve at the wrist was stimulated using a surface bar electrode with metal contacts that delivered pairs of stimuli (a pair consisted of two consecutive 200-μs pulses with a 30-ms interstimulus interval) at a frequency of 1 Hz (DS7A; Digitimer Research Instruments, Hertfordshire, UK). The electrode was taped in place and remained in this position during the entire session. The intensity of nerve stimulation was set at motor threshold, defined as the minimum intensity required to evoke a visible twitch in the APB muscle. A total of 500 pulse pairs were delivered during each time block. Changes in SI excitability were assessed via the amplitude of the first N20–P25 (SEP 1), and changes in SI intracortical inhibition were assessed via the amplitude of the second N20–P25 (SEP 2) that is typically inhibited relative to SEP 1. The ratio of SEP 2/SEP1 amplitude [i.e., paired-pulse ratio (PPR)] was assessed as a measure of intracortical inhibition since it has been shown to be altered following iTBS (Ragert et al. 2008) and 5 Hz rTMS over SI (Gatica Tossi et al. 2013b; Ragert et al. 2004) (see Fig. 1A).
Temporal order judgment.
Temporal order judgment (TOJ) was assessed using the Cortical Metrics Device version 6.0 (see Fig. 1B; Cortical Metrics). The right hand was placed on the device with each digit positioned in the individual finger grooves. The task delivered vibrotactile stimuli (1,000 ms, 25 Hz, 300 μm) to the volar pad of digits 2 and 3 with stimulus onset of the two stimuli separated by an interstimulus interval (ISI) that was defined by the ongoing performance. The first trial always began with the ISI set to 150 ms. Participants were queried to report the identity of the digit that received the first stimulus. A correct response resulted in a reduction of the ISI by 5% (i.e., the task became more difficult), and an incorrect response resulted in an increase in the ISI by 5% (i.e., task became easier). The intertrial interval was set to 4 s. A total of 31 trials were performed in each time block, and the order of digit presentation (i.e., digit 2 or digit 3) was randomized across trials. No visual or auditory feedback was provided to participants during or following the TOJ task. Before each time block, participants were reacquainted with the task and completed three practice trials wherein visual feedback was provided. In the case where a trial was answered incorrectly, the participant would repeat all three trials until all stimuli were correctly identified.
Experiment timeline.
Each experimental session consisted of a single or two consecutive TBS protocols (Fig. 1C). The order of TBS protocol delivery was pseudorandomized across participants. Measurements were collected before TBS (T0) and following TBS at 5 (T1), 25 (T2), 50 (T3), and 90 (T4) min. The order of SEPs and TOJ collections was counterbalanced across participants. A 15-min wait time was imposed following the collection of the T0 data and before the delivery of the first TBS. A 15-min time delay was imposed between consecutive TBS protocols, a timeframe used elsewhere (Fricke et al. 2011; Monte-Silva et al. 2010; Murakami et al. 2012), and dependent measures were not acquired during this delay. This is an important consideration since the dependent measures themselves may interfere with the metaplastic effects of TBS. Participants wore earplugs (29 dB) and sat upright with their eyes closed and head and neck supported in a headrest during acquisition of all dependent measures.
Data analysis.
The peak-to-peak amplitudes of SEP 1 and SEP 2 were calculated from the time-locked average of up to 500 trials for each individual at each time block. Trials were not included in the average if they contained excessive noise or eye-blink artifacts. The amplitude of SEP 1 was defined as the peak-to-peak amplitude between the negative N20 minimum and the positive P25 peak (see Fig. 1A). SEP 2 was measured as the peak-to-peak amplitude based on the latencies of the N20 and P25 of SEP 1, plus an additional 30 ms added to each to account for the time interval between the first and second peripheral nerve stimulus. PPR was calculated as the peak-to-peak amplitude of SEP 2 divided by the peak-to-peak amplitude of SEP 1 as performed elsewhere (David-Jurgens and Dinse 2010; Gatica Tossi et al. 2013a, 2013b; Höffken et al. 2007; Lenz et al. 2012; Ragert et al. 2004, 2008). However, other approaches to PPR calculation involving linear subtraction also exist (Höffken et al. 2010, 2013). TOJ threshold was defined as the ISI of the last trial in each time block. Statistical analyses were performed as follows. First, an outlier analysis was performed for all data at T0 (baseline). This analysis included all interventions and participants and was intended to identify individuals in whom the baseline data deviated >1.5 times the interquartile range for a given intervention. In the event an outlier was detected, the data (T0–T4) for that individual were removed for that particular intervention only, except in the case of direct comparisons between two interventions, where the data were removed from both interventions. Analyses were performed on normalized data (i.e., T1/T0, T2/T0, etc.) when T0 data were statistically different between the interventions being compared. A two-way repeated-measures ANOVA on normalized data using within-subject factors time (4 levels; T1, T2, T3, and T4) and intervention (6 levels; cTBS, iTBS, cTBS-cTBS, iTBS-iTBS, cTBS-iTBS, and iTBS-cTBS) was performed. Subsequent two-way repeated-measures ANOVAs were performed using within-subject factors time and intervention to specifically compare 1) iTBS vs. cTBS and 2) homogeneous protocols (cTBS vs. cTBS-cTBS; iTBS vs. iTBS-iTBS) to test for metaplasticity effects. In the event that data did not meet the assumption of sphericity, the Greenhouse-Geisser method was used to correct the P value. Post hoc Tukey's tests were used to further investigate significant differences. Two-tailed paired t-tests were additionally used for post hoc comparisons in the event that Tukey's test did not reveal differences between levels of a significant main effect. Effect size was calculated using Cohen's d. Significance was set at P ≤ 0.05.
RESULTS
Based on the outlier analysis at T0, the following data were removed. For SEP 1, participant 2 was removed from cTBS-cTBS. For SEP 2 and PPR data, participants 2 and 11 were removed from cTBS-cTBS and participant 2 from cTBS, iTBS, and iTBS-iTBS. For TOJ, participants 6 and 16 were removed from cTBS. The group-averaged RMT was 62.3 ± 10.6% of maximum stimulator output and was not different across interventions [F(5,95) = 0.12, P = 0.99, cTBS = 60.9%, iTBS = 63.0%, cTBS-cTBS = 61.4%, iTBS-iTBS = 62.4%, cTBS-iTBS = 62.5%, iTBS-cTBS = 63.4%]. Similarly, the TBS intensity was not different between interventions [F(5,95) = 0.15, P = 0.98, cTBS = 42.6%, iTBS = 44.3%, cTBS-cTBS = 43.0%, iTBS-iTBS = 43.9%, cTBS-iTBS = 43.9%, iTBS-cTBS = 44.5%]. Table 1 displays the results of all statistical analyses. Two-way ANOVA examining all protocols revealed an effect of time without an intervention effect or time × intervention interaction. We observed no notable effects using the nonhomologous consecutive TBS protocols (cTBS-iTBS and iTBS-cTBS) and therefore focused our analysis on the specific questions posed. Table 2 displays all group-averaged means and SE for each intervention at each time block for each dependent measure.
Table 1.
Statistical results of two-way ANOVAs for each dependent measure
| Dependent Measure |
||||
|---|---|---|---|---|
| Two-way ANOVA | SEP 1 | SEP 2 | PPR | TOJ Threshold |
| All 6 interventions | Time(3,45) = 7.25 P = 0.001* T1 < T4 T2 < T4 | Time(3,45) = 2.80 P = 0.051* T1 < T3 (**P = 0.051) | Time(3,45) = 2.44 P = 0.077 | Time(3,45) = 1.45 P = 0.241 |
| Interven(5,74) = 0.790 P = 0.558 | Interven(5,70) = 0.820 P = 0.541 | Interven(5,70) = 0.56 P = 0.732 | Interven(5,73) = 1.36 P = 0.248 | |
| Time × Interven(15,220) = 0.530 P = 0.921 | Time × Interven(15,210) = 0.890 P = 0.573 | Time × Interven(15,210) = 1.13 P = 0.327 | Time × Interven(15,219) = 1.32 P = 0.193 | |
| cTBS vs. iTBS | Time(3,45) = 0.99 P = 0.407 | Time(3,42) = 3.183 P = 0.034* T1 < T3 | Time(3,42) = 2.759 P = 0.054* T1 < T2 (**P = 0.01) | Time(4,52) = 3.424 P = 0.015* T0 < T2 T1 < T2 |
| Interven(1,15) = 0.04 P = 0.850 | Interven(1,14) = 0.056 P = 0.817 | Interven(1,14) = 0.297 P = 0.594 | Interven(1,13) = 0.133 P = 0.722 | |
| Time × Interven(3,43) = 0.56 P = 0.646 | Time × Interven(3,42) = 2.625 P = 0.063 | Time × Interven(3,42) = 1.827 P = 0.157 | Time × Interven(4,52) = 0.343 P = 0.847 | |
| cTBS vs. cTBS-cTBS | Time(3,42) = 1.648 P = 0.193 | Time(3,39) = 2.87 P = 0.048** T1 < T2 (**P = 0.026) T1 < T3 (**P = 0.013) | Time(3,39) = 3.008 P = 0.066 | Time(3,39) = 0.943 P = 0.429 |
| Interven(1,14) = 1.829 P = 0.198 | Interven(1,13) = 1.22 P = 0.289 | Interven(1,13) = 0.067 P = 0.800 | Interven(1,13) = 7.270 P = 0.018* | |
| Time × Interven(3,45) = 0.647 P = 0.589 | Time × Interven(3,39) = 0.74 P = 0.534 | Time × Interven(3,39) = 1.038 P = 0.387 | Time × Interven(3,39) = 0.845 P = 0.478 | |
| iTBS vs. iTBS-iTBS | Time(3,45) = 1.27 P = 0.296 | Time(3,42) = 3.067 P = 0.038* T1 < T4 | Time(3,42) = 1.072 P = 0.371 | Time(4,60) = 2.313 P = 0.106 |
| Interven(1,15) = 0.04 P = 0.847 | Interven(1,14) = 0.049 P = 0.828 | Interven(1,14) = 0.081 P = 0.781 | Interven(1,15) = 0.038 P = 0.849 | |
| Time × Interven(3,45) = 0.86 P = 0.469 | Time × Interven(3,42) = 0.096 P = 0.962 | Time × Interven(3,42) = 0.431 P = 0.732 | Interven × Time(4,60) = 2.842 P = 0 0.0317* iTBS: T0 < T2 II: T0 < T3 I vs. II: iTBS T3 < IIT3 | |
SEP, somatosensory evoked potentials; PPR, paried-pulse rato; TOJ, temporal order judgement; cTBS, coninuous theta-burst stimulation; iTBS, intermittent theta-burst stimulation. I, intermittent theta-burst stimulation; II, intermittent followed by intermittent theta-burst stimulation.
Significance at P ≤ 0.05.
Significant post hoc two-tailed t-test.
Table 2.
Group-averaged means (with SE) for SEPs and TOJ
| T0 (baseline) | T1 (5 min) | T2 (25 min) | T3 (50 min) | T4 (90 min) | |
|---|---|---|---|---|---|
| cTBS | |||||
| SEP 1 | 3.85 ± 0.508 | 3.98 ± 0.535 | 4.03 ± 0.592 | 3.74 ± 0.536 | 3.86 ± 0.511 |
| SEP 2 | 1.42 ± 0.170 | 1.44 ± 0.203 | 1.72 ± 0.208 | 1.66 ± 0.194 | 1.50 ± 0.196 |
| PPR | 0.431 ± 0.047 | 0.4217 ± 0.041 | 0.530 ± 0.063 | 0.503 ± 0.053 | 0.433 ± 0.048 |
| TOJ | 48.761 ± 3.834 | 50.9013 ± 4.103 | 55.835 ± 6.566 | 52.447 ± 4.373 | 54.857 ± 5.918 |
| iTBS | |||||
| SEP 1 | 3.91 ± 0.473 | 3.96 ± 0.454 | 3.93 ± 0.473 | 4.14 ± 0.497 | 4.05 ± 0.474 |
| SEP 2 | 1.45 ± 0.161 | 1.50 ± 0.186 | 1.58 ± 0.180 | 1.59 ± 0.200 | 1.62 ± 0.211 |
| PPR | 0.458 ± 0.063 | 0.4344 ± 0.047 | 0.472 ± 0.054 | 0.452 ± 0.053 | 0.467 ± 0.054 |
| TOJ | 55.169 ± 5.567 | 55.3707 ± 5.633 | 67.734 ± 8.405 | 54.165 ± 5.161 | 61.178 ± 6.112 |
| cTBS-cTBS | |||||
| SEP 1 | 3.85 ± 0.505 | 4.06 ± 0.444 | 3.94 ± 0.514 | 4.08 ± 0.487 | 4.17 ± 0.475 |
| SEP 2 | 1.31 ± 0.180 | 1.49 ± 0.169 | 1.58 ± 0.175 | 1.60 ± 0.212 | 1.55 ± 0.183 |
| PPR | 0.394 ± 0.049 | 0.4143 ± 0.046 | 0.466 ± 0.047 | 0.438 ± 0.058 | 0.405 ± 0.051 |
| TOJ | 58.373 ± 4.677 | 54.4131 ± 5.414 | 58.509 ± 6.196 | 56.516 ± 6.036 | 52.884 ± 5.600 |
| iTBS-iTBS | |||||
| SEP 1 | 4.21 ± 0.482 | 4.25 ± 0.494 | 4.47 ± 0.550 | 4.48 ± 0.560 | 4.51 ± 0.589 |
| SEP 2 | 1.56 ± 0.186 | 1.59 ± 0.236 | 1.67 ± 0.236 | 1.72 ± 0.239 | 1.81 ± 0.261 |
| PPR | 0.413 ± 0.039 | 0.4169 ± 0.058 | 0.425 ± 0.060 | 0.428 ± 0.049 | 0.446 ± 0.053 |
| TOJ | 52.947 ± 4.868 | 56.5651 ± 5.850 | 58.452 ± 5.709 | 66.890 ± 10.611 | 56.651 ± 6.197 |
| cTBS-iTBS | |||||
| SEP 1 | 3.78 ± 0.537 | 3.93 ± 0.626 | 4.04 ± 0.622 | 4.02 ± 0.580 | 4.02 ± 0.574 |
| SEP 2 | 1.59 ± 0.195 | 1.78 ± 0.286 | 1.90 ± 0.281 | 1.88 ± 0.289 | 2.01 ± 0.323 |
| PPR | 0.483 ± 0.050 | 0.4953 ± 0.050 | 0.5323 ± 0.059 | 0.501 ± 0.049 | 0.509 ± 0.058 |
| TOJ | 54.834 ± 3.545 | 54.9465 ± 4.396 | 61.750 ± 6.214 | 59.191 ± 5.717 | 55.594 ± 5.743 |
| iTBS-cTBS | |||||
| SEP 1 | 3.91 ± 0.505 | 4.09 ± 0.532 | 4.29 ± 0.602 | 4.27 ± 0.602 | 4.52 ± 0.616 |
| SEP 2 | 1.78 ± 0.226 | 1.88 ± 0.294 | 1.84 ± 0.263 | 1.95 ± 0.273 | 1.94 ± 0.240 |
| PPR | 0.491 ± 0.050 | 0.4743 ± 0.051 | 0.453 ± 0.050 | 0.538 ± 0.073 | 0.468 ± 0.045 |
| TOJ | 52.184 ± 3.460 | 61.7957 ± 7.405 | 54.357 ± 5.754 | 57.714 ± 6.626 | 55.063 ± 5.296 |
Values are means ± SE. Units for SEP are μV. Units for TOJ are ms.
cTBS vs. iTBS.
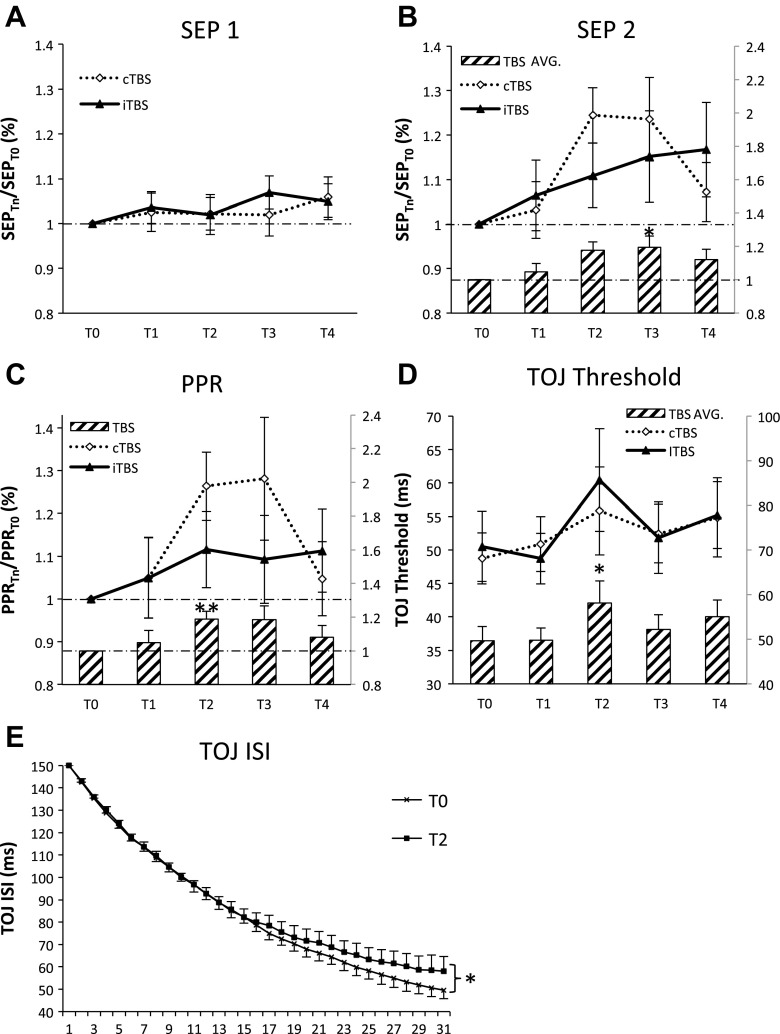
The effects of cTBS and iTBS were similar for all measures of SEP physiology and tactile perception (Fig. 2). CTBS and iTBS did not alter SEP 1 (Fig. 2A) yet increased the amplitude of SEP 2 at T3 (50 min) (Cohen's d = 0.60; Fig. 2B). At an individual level, SEP 2 increased in 11 and decreased in 4 participants following cTBS, whereas iTBS increased SEP 2 in 9, decreased in 4, and had no effect in 2 individuals. For PPR, a main effect of time was also observed with increases at T2 (25 min) (Cohen's d = 0.60; Fig. 2C). Both cTBS and iTBS increased PPR in nine participants and decreased or did not change responses in six individuals. For TOJ thresholds, the main effect of time revealed decrements in performance at T2 (25 min) by ∼15% for cTBS and ∼20% for iTBS (Cohen's d = 0.12; Fig. 2D). Following cTBS, TOJ increased in eight, decreased in four, and had no effect in two participants. TOJ following iTBS increased in nine participants, decreased in three, and did not change responses in two individuals. Figure 2E plots this effect on TOJ performance (averaged for cTBS and iTBS) at T0 and T2 as a function of trial number. These data indicate that decrements in TOJ emerge as performance approaches threshold (i.e., at about trial 25) and not at suprathreshold levels. Collectively, these data indicate that single protocols of 30 Hz cTBS and iTBS exert similar effects on SI physiology and TOJ performance. Furthermore, we note that no trends were observed to categorize individuals as “responders” or “nonresponders” since the TBS protocols had similar or even opposite effects in a given participant, and these effects varied across SEP 2-, PPR-, and TOJ-dependent measures.
Fig. 2.
Plasticity effects of cTBS vs. iTBS. All somatosensory cortex (SI) physiology data were normalized at each post-TBS time block to T0 except for TOJ where T0 data were not statistically different between cTBS and iTBS. Values above the horizontal broken line correspond to an increase in the amplitude (SEP 1 and SEP 2) or increase in the PPR compared with baseline values. A: group-averaged SEP 1 (with SE) (N = 16). B: group-averaged SEP 2 (with SE) (N = 15). Histogram depicts the main effect of time (average of cTBS and iTBS) with an increase in SEP 2 amplitude at T3. C: group-averaged PPR (with SE) (N = 15). Post hoc 2-tailed t-tests revealed increased PPR at T2. D: group-averaged TOJ (with SE) (N = 14). Histogram depicts the main effect of time showing a significant increase in TOJ threshold at T2. E: TOJ averaged over cTBS and iTBS (with SE) for T0 and T2 as a function of trial number (N = 14). Impairments occur as the participant reaches threshold, but not at suprathreshold ISIs. Asterisks indicate significant post hoc Tukey's honest significant difference (HSD, *) and 2-tailed t-tests (**).
Metaplasticity within SI.
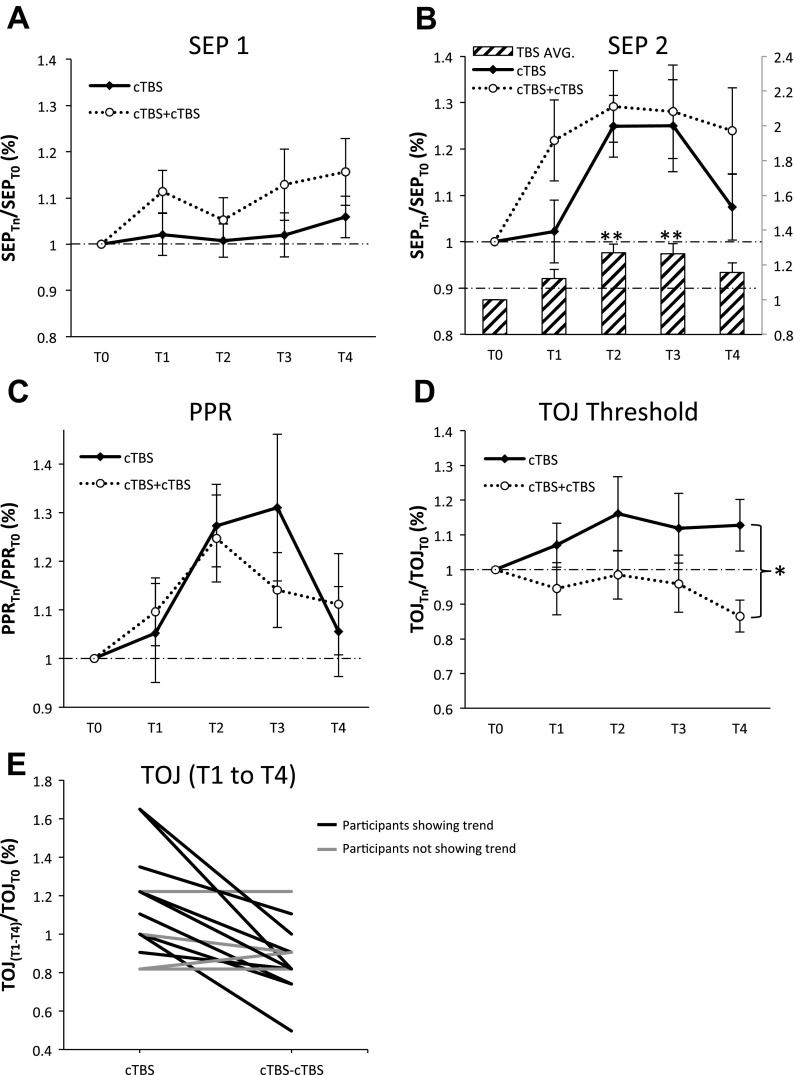
Figure 3 displays the group-averaged physiology and psychophysical data investigating metaplasticity effects of cTBS. No differences were observed between cTBS and cTBS-cTBS for SEP 1, SEP 2, and PPR (Fig. 3, A–C). For TOJ, there was a significant effect of intervention (Table 1) indicating that thresholds were significantly elevated following cTBS compared with cTBS-cTBS across all levels of time (Cohen's d = 0.62). Furthermore, the cTBS-cTBS protocol demonstrated a trend toward improvements in TOJ performance at T4 (Fig. 3D), an effect observed in 10 out of 14 individuals (Fig. 3E). In summary, these data indicate that cTBS-cTBS demonstrates metaplasticity for the measure of TOJ only.
Fig. 3.
Metaplastic effects of cTBS. All data were normalized at each post-TBS time block to the baseline measurement T0. A: group-averaged SEP 1 (with SE) (N = 15). B: group-averaged SEP 2 (with SE) (N = 14). A significant effect of time was revealed, and post hoc analysis revealed significant facilitation of SEP 2 at T2 and T3 compared with T1. C: group-averaged PPR (with SE) (N = 14). D: group-averaged TOJ (with SE) (N = 14). *Significant effect of intervention. E: TOJ data (average of T1–T4) from individual participants for cTBS and cTBS-cTBS depicting trend for improvement in performance after cTBS-cTBS. Asterisks indicate significant post hoc Tukey's HSD (*) and 2-tailed t-tests (**).
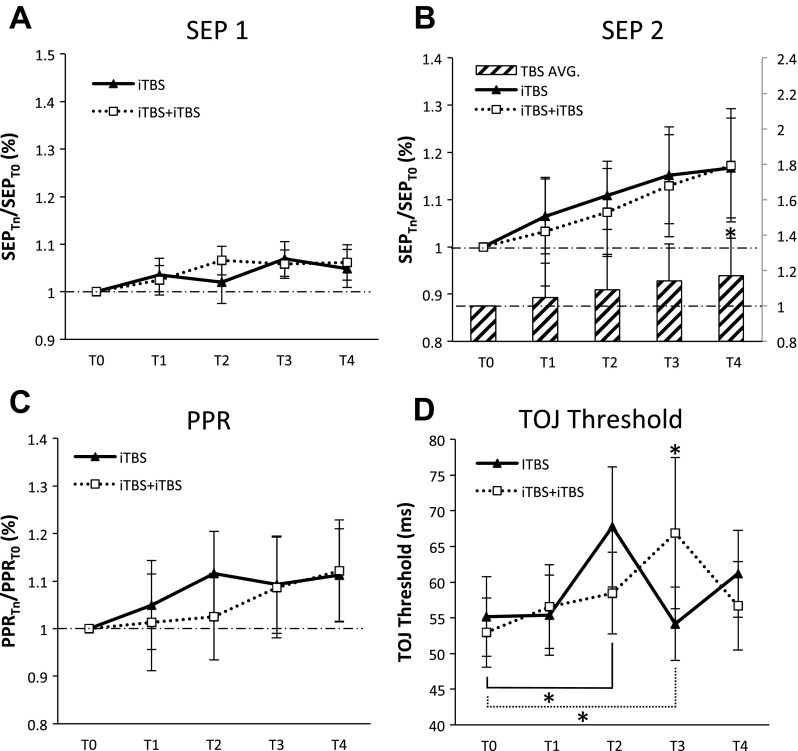
Figure 4 plots the group-averaged data investigating the metaplasticity effects of iTBS. Metaplasticity effects were not observed for measures of SEP 1, SEP 2 (showing facilitation at T4), or PPR (Fig. 4, A–C). Evidence of metaplasticity was observed for TOJ (Fig. 4D) with a significant interaction at T3 (50 min) whereby TOJ thresholds were elevated following iTBS-iTBS compared with iTBS (Cohen's d = 0.38). Furthermore, both protocols induced significant decrements in performance at different time blocks (iTBS at T2, Cohen's d = 0.44 and iTBS-iTBS at T3, Cohen's d = 0.42) (Table 1). These data indicate that iTBS protocols demonstrate metaplasticity not as a change in the direction of effects (i.e., both iTBS and iTBS-iTBS impair TOJ) but rather as a change in the temporal evolution of these effects.
Fig. 4.
Metaplastic effects of iTBS. All data for SEP 1, SEP 2, and PPR were normalized to T0 when T0 was different between iTBS and iTBS-iTBS. A: group-averaged SEP 1 (with SE) (N = 16). B: group-averaged SEP 2 (with SE) (N = 15). A significant effect of time revealed differences between T1 and T4 (main effect shown in histogram). C: group-averaged PPR (with SE) (N = 15). D: group-averaged TOJ (with SE) (N = 16). Significant interaction revealed as increase in TOJ at T3 following iTBS-iTBS compared with iTBS and impairment in TOJ following iTBS at T2 and iTBS-iTBS at T3. *Significant post hoc Tukeys.
DISCUSSION
Three novel findings were revealed. First, cTBS and iTBS over SI yielded similar effects on SEP physiology and tactile perception. Second, cTBS- and iTBS-induced metaplasticity was observed for measures of tactile perception but not physiology. Third, the metaplasticity effects of cTBS and iTBS differed with respect to the nature of induced changes. These data suggest that metaplasticity effects of consecutive cTBS and iTBS protocols may promote homeostatic and nonhomeostatic changes, respectively.
CTBS and iTBS modulate SI physiology and perception similarly.
Our findings indicate that cTBS and iTBS increase the amplitude of SEP 2 (23.5 and 15.2%, respectively) and do not significantly alter SEP 1. A similar lack of change in SEP 1 is observed following 50 Hz cTBS over SI (Katayama et al. 2010; Murakami et al. 2008) and 50 Hz iTBS over SI (Murakami et al. 2008) although iTBS is also reported to facilitate the N20–P25 (Katayama and Rothwell 2007). The effects on SEP 2 emerge at 25 min following stimulation yet follow a different time course for each TBS protocol. For cTBS, the maximal effects occur between 25 and 50 min and subsequently return to baseline levels. For iTBS, SEP 2 continues to increase over time, with maximal effects occurring at 90 min following stimulation. Previous literature demonstrates the time course of SI iTBS effects to be ∼15–30 min following stimulation although additional time points were not obtained (Katayama and Rothwell 2007; Katayama et al. 2010; Premji et al. 2010). We also observed changes in PPR, a result of the increase in the amplitude of SEP 2 with little to no contribution from changes in SEP 1. These data support the iTBS-induced decreases in paired-pulse inhibition reported elsewhere (Ragert et al. 2008). Last, both cTBS and iTBS impaired the ability to perform TOJ (at ∼15 and 20%, respectively) at ∼25 min following stimulation, corresponding to the timing of the SEP 2 changes. A similar decrement in TOJ (∼18%) follows 50 Hz cTBS (Lee et al. 2013). Our results indicate that 30 Hz cTBS and iTBS act similarly in terms of their net effects on SI physiology and tactile perception. Similarities in iTBS and cTBS effects are also observed in rat models where both protocols decrease the expression of glutamic acid decarboxylase 67 and increase glutamic acid decarboxylase 65, enzymes that are responsible for GABA synthesis (Trippe et al. 2009).
Collectively, the lack of change in SEP 1 and the observed effects in SEP 2, PPR, and TOJ suggest that cTBS and iTBS target cortical inhibitory circuits within SI. Evidence from animal literature suggests that, following previous activation of the pyramidal cell, CB-expressing inhibitory interneurons act to inhibit pyramidal cells within the microcolumn through synapses on superficial dendrites (Gulysr and Freundl 1996). Additionally, the pyramidal cells synapse on PV-expressing inhibitory interneurons, via N-methyl-d-aspartate receptors, located within layers IV/V of SI (Labedi et al. 2014). These PV cells synapse perisomatically on the pyramidal cell itself while also synapsing with basal dendrites of pyramidal cells in neighboring macrocolumns (Blatow et al. 2003; Freund 2003; Howard et al. 2005; Kawaguchi and Kubota 1998; Labedi et al. 2014; Markram et al. 2004). The inhibition provided by the CB-expressing cells, perisomatic PV-expressing cells, and lateral PV-expressing cells may account for the overall inhibition of the pyramidal cells and thus contribute to the reduced amplitude of SEP 2 compared with SEP 1. The action of TBS may therefore function to disrupt the normal sequence of direct and recurrent inhibition acting on the pyramidal cells, therefore producing less inhibition and resulting in an increase in SEP 2 and PPR (i.e., less inhibition).
TOJ requires us to distinguish between stimuli in neighboring receptive fields, a task that relies on GABAergic inhibition acting via long-range inhibitory projections that can operate between cortical columns. Some evidence alluding to the involvement of GABAergic inhibition in TOJ is derived from studies revealing impaired TOJ in individuals with autism (Tommerdahl et al. 2008), a disorder associated with alterations in GABAergic inhibition (Puts et al. 2014; Tavassoli et al. 2012; Tommerdahl et al. 2007). Our data suggest that 30-Hz TBS protocols, which we suggest reduce inhibition within SI, alter TOJ by reducing the lateral inhibition necessary to create the spatial contrast between the cortical columns receiving inputs from digit 2 from those receiving inputs from digit 3, and also reduce the recurrent inhibition responsible for the typical reduction in SEP 2 amplitude.
Collectively, our data suggest that TBS acts to reduce activity in inhibitory circuits. Although the origin remains speculative, the generation of somatosensory-evoked high-frequency oscillations (HFOs) is hypothesized to stem from GABAergic inhibitory interneurons within lamina IV of area 3b (Hashimoto et al. 1996). In humans, cTBS reduces late HFOs (Katayama et al. 2010; Murakami et al. 2008) and short-interval intracortical inhibition (SICI) (Huang et al. 2005; Murakami et al. 2008; Suppa et al. 2008) while not altering the amplitude of the N20–P25 (Katayama et al. 2010; Murakami et al. 2008). Furthermore, magnetic resonance spectroscopy reveals an increase in GABA in human sensorimotor cortexes that follows cTBS, which may result from hypoactivity within the PV- and CB-expressing neurons, leading to an increase in GABA concentration in presynaptic stores (Stagg et al. 2009). In further support of this suggestion, in the rat model, TBS over SI generates LTD on PV- and CB-expressing interneurons, thereby reducing their activity and leading to an overall disinhibition within SI cortex (Benali et al. 2011).
Metaplasticity via homeostatic vs. nonhomeostatic mechanisms.
A fundamental comparison in this study was the evaluation of metaplasticity effects following two consecutive identical TBS protocols where effects were observed for TOJ only. We therefore suggest that metaplasticity effects are likely occurring at synapses between long-range lateral inhibitory projections and pyramidal cells in neighboring macrocolumns responsible for TOJ. As suggested elsewhere, if the term “homeostatic” applies to metaplasticity that demonstrates an effect opposite to the priming protocol (Karabanov et al. 2015), we suggest that cTBS-induced metaplasticity follows homeostatic rules such that the direction of cTBS-cTBS effects is opposite of that induced by a single protocol of cTBS. This finding bears similarity to the homeostatic metaplasticity for measures of SICI that follows consecutive identical TBS protocols when delivered over M1 (Murakami et al. 2012). Evidence for homeostatic metaplasticity is also observed in SI when high-frequency tactile stimulation is preceded by paired associated stimulation (PAS)N20-2.5 and PASN20-15 (Bliem et al. 2008). In that study, PASN20-2.5 followed by high-frequency tactile stimulation reduced the N20–P25 albeit with data only trending toward opposite effects when replaced with PASN20-15. Similar observations were made for performance on a tactile orientation grating task (Bliem et al. 2008). In contrast, our data indicate that iTBS-induced metaplasticity appears to follow nonhomeostatic metaplasticity since both single and consecutive iTBS protocols yield impairments in TOJ (i.e., same direction) although the time course of effects induced by the two protocols differs. In Gatica Tossi et al. (2013), rTMS followed by high-frequency tactile stimulation increases PPR (i.e., reduces inhibition), and similar but larger effects are observed following rTMS only. According to recent suggestions (Karabanov et al. 2015), such changes might be regarded as nonhomeostatic metaplasticity, as we suggest of our iTBS-induced metaplasticity. Irrespective of the metaplasticity type, Gatica Tossi et al. (2013) demonstrate overall reductions in inhibition as measured by an increase in SEP 2, an increase in PPR, and changes in tactile acuity with no changes in SEP 1, very similar to the overall findings we present with TBS. Last, the fact that cTBS- and iTBS-induced metaplasticity effects differ implies that subtle differences exist for 30 Hz iTBS and cTBS, although these differences are not exposed when delivered as a single protocol.
We tested the combination of cTBS and iTBS delivered in succession with its nonidentical TBS protocol to examine whether the effect of either would be amplified in terms of its amplitude or duration of effects. CTBS-iTBS delivered to M1 led to greater MEPs than iTBS alone (Murakami et al. 2012). In the reverse scenario, however, iTBS-cTBS produced no change compared with the effects of a single cTBS protocol (Murakami et al. 2012). Our data targeting SI did not show this trend. This may be due to the observation that cTBS did not operate to reduce SI excitability as it did in the aforementioned study focused on M1.
Methodological considerations.
We imposed a 15-min wait period between consecutive TBS protocols to be consistent with the timing needed to reverse effects following TBS over M1 (Murakami et al. 2012). However, altering the timing between two consecutive TBS protocols has been demonstrated to yield very different effects on cortical physiology (Gamboa et al. 2011; Mastroeni et al. 2013; Murakami et al. 2012), and it is possible that a different delay would impact the results. We opted to use a 30-Hz TBS protocol to reduce the intersubject variability associated with TBS delivery over M1 (Goldsworthy et al. 2012b). However, simulations have demonstrated that reducing the frequency of TBS may promote the induction of depression (Fung and Robinson 2014). We note that 30 Hz cTBS and iTBS over M1 suppress and facilitate MEPs, respectively, similar to the 50-Hz protocol (Wu et al. 2012). Furthermore, we measured PPR using an approach that does not consider the contribution of the later SEP components elicited by the first nerve stimulus. For example, the first nerve stimulus may evoke later components such as the N35 and P45 components (see Fig. 3 in Lorenz et al. 1996). As we show in the SEP example in Fig. 1A, the EEG signal immediately before SEP 2 is larger than that before SEP 1, and, as such, we cannot exclude the possibility that changes in SEP 2 and PPR are contributed by variations that occur in the later SEP components evoked by the first nerve stimulus. Also, because both cTBS and iTBS induced similar effects on all dependent measures, we cannot exclude the possibility that an unspecific effect of time contributed to these findings. However, given the time course of effects such that dependent measures were modified by ∼25 min following stimulation and subsequently returned toward pre-TBS values, this possibility seems unlikely. Finally, we did not collect SEP or psychophysical data in the 15-min interval between consecutive TBS protocols. In our view, this was an important consideration to minimize the contribution of our dependent measure on the effects of metaplasticity.
In conclusion, our data indicate a similar effect of cTBS and iTBS on SI physiology and tactile perception. Metaplasticity is observed as a change in the TOJ-induced impairments following cTBS-cTBS and iTBS-iTBS. Our data also suggest that cTBS and iTBS operate differently in their metaplasticity effects, with changes in perception occurring as a reversal of effects after cTBS-cTBS and a change in the time course of effects that follow iTBS-iTBS. In particular, the metaplasticity effect of consecutive cTBS protocols may provide one opportunity to improve tactile perception and is a fundamental point of progress that has potential for clinical applications.
GRANTS
We thank the Natural Sciences and Engineering Research Council of Canada for operating funds to A. J. Nelson.
DISCLOSURES
Mark Tommerdahl is a co-founder of the company that has a license from the University of North Carolina to distribute the Cortical Metrics Version 6.0 Device. More than 5 percent equity.
AUTHOR CONTRIBUTIONS
C.B.J., T.L., A.Z.B., T.N.M., and A.J.N. conception and design of research; C.B.J., T.L., A.Z.B., T.N.M., and A.J.N. performed experiments; C.B.J., T.L., A.Z.B., and A.J.N. analyzed data; C.B.J., T.L., A.Z.B., T.N.M., Y.Q.M., M.T., and A.J.N. interpreted results of experiments; C.B.J., A.Z.B., and A.J.N. prepared figures; C.B.J., A.Z.B., T.N.M., Y.Q.M., and A.J.N. drafted manuscript; C.B.J., T.L., A.Z.B., T.N.M., Y.Q.M., M.T., and A.J.N. edited and revised manuscript; C.B.J., T.L., A.Z.B., T.N.M., Y.Q.M., M.T., and A.J.N. approved final version of manuscript.
REFERENCES
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130, 1996. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 9: 387, 2008. [DOI] [PubMed] [Google Scholar]
- Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, Eysel UT, Erdmann R, Funke K. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci 31: 1193–1203, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci 2: 32–48, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex multipolar bursting cells constitute a novel expression of EGFP in transgenic mice expressing this. Neuron 38: 805–817, 2003. [DOI] [PubMed] [Google Scholar]
- David-Jurgens M, Dinse HR. Effects of aging on paired-pulse behavior of rat somatosensory cortical neurons. Cereb Cortex 20: 1208–1216, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeltgen SH, Ridding MC. Modulation of cortical motor networks following primed theta burst transcranial magnetic stimulation. Exp Brain Res 215: 199–206, 2011. [DOI] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci 26: 489–495, 2003. [DOI] [PubMed] [Google Scholar]
- Frey U, Schollmeier K, Reymann KG, Seidenbecher T. Asymptotic hippocampal long-term potentiation in rats does not preclude additional potentiation at later phases. Neuroscience 67: 799–807, 1995. [DOI] [PubMed] [Google Scholar]
- Fricke K, Seeber a a Thirugnanasambandam N, Paulus W, Nitsche a M, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol 105: 1141–1149, 2011. [DOI] [PubMed] [Google Scholar]
- Fung PK, Robinson PA. Neural field theory of synaptic metaplasticity with applications to theta burst stimulation. J Theor Biol 340: 164–176, 2014. [DOI] [PubMed] [Google Scholar]
- Funke K, Benali A. Modulation of cortical inhibition by rTMS - findings obtained from animal models. J Physiol 589: 4423–4435, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa OL, Antal A, Laczo B, Moliadze V, Nitsche a M., Paulus W. Impact of repetitive theta burst stimulation on motor cortex excitability. Brain Stimul 4: 145–151, 2011. [DOI] [PubMed] [Google Scholar]
- Gatica Tossi a M, Lillemeier AS, Dinse HR. Influence of stimulation intensity on paired-pulse suppression of human median nerve somatosensory evoked potentials. Neuroreport 24: 451–456, 2013a. [DOI] [PubMed] [Google Scholar]
- Gatica Tossi MA, Stude P, Schwenkreis P, Tegenthoff M, Dinse HR. Behavioural and neurophysiological markers reveal differential sensitivity to homeostatic interactions between centrally and peripherally applied passive stimulation. Eur J Neurosci 38: 2893–2901, 2013b. [DOI] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex 18: 2046–2053, 2008. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Pitcher JB, Ridding MC. The application of spaced theta burst protocols induces long-lasting neuroplastic changes in the human motor cortex. Eur J Neurosci 35: 125–134, 2012a. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Pitcher JB, Ridding MC. A comparison of two different continuous theta burst stimulation paradigms applied to the human primary motor cortex. Clin Neurophysiol 123: 2256–2263, 2012b. [DOI] [PubMed] [Google Scholar]
- Gulysr AI, Freundl F. Pyramidal cell dendrites are the primary targets of calbindin D28k-immunoreactive interneurons in the hippocampus. Hippocampus 534, 1996. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex 23: 1593–1605, 2013. [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Mashiko T, Imada T. Somatic evoked high-frequency magnetic oscillations reflect activity of inhibitory interneurons in the human somatosensory cortex. Electroencephalogr Clin Neurophysiol 100: 189–203, 1996. [DOI] [PubMed] [Google Scholar]
- Höffken O, Lenz M, Tegenthoff M, Schwenkreis P. Multichannel SEP-recording after paired median nerve stimulation suggests origin of paired-pulse inhibition rostral of the brainstem. Neurosci Lett 468: 308–311, 2010. [DOI] [PubMed] [Google Scholar]
- Höffken O, Tannwitz J, Lenz M, Sczesny-Kaiser M, Tegenthoff M, Schwenkreis P. Influence of parameter settings on paired-pulse-suppression in somatosensory evoked potentials: a systematic analysis. Clin Neurophysiol 124: 574–580, 2013. [DOI] [PubMed] [Google Scholar]
- Höffken O, Veit M, Knossalla F, Lissek S, Bliem B, Ragert P, Dinse HR, Tegenthoff M. Sustained increase of somatosensory cortex excitability by tactile coactivation studied by paired median nerve stimulation in humans correlates with perceptual gain. J Physiol 584: 463–471, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci 28: 310–316, 2005. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 45: 201–206, 2005. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Matsunaga K, Nakanishi R, Kawahira K, Murayama N, Tsuji S, Huang YZ, Rothwell JC. Effect of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin Neurophysiol 118: 1033–1043, 2007. [DOI] [PubMed] [Google Scholar]
- Jacobs MF, Tsang P, Lee KG, Asmussen MJ, Zapallow CM, Nelson AJ. 30 Hz theta-burst stimulation over primary somatosensory cortex modulates corticospinal output to the hand. Brain Stimul 7: 269–274, 2013. [DOI] [PubMed] [Google Scholar]
- Karabanov A, Ziemann U, Hamada M, George MS, Classen J, Massimini M, Rothwell J, Roman H. Consensus paper: probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul 8: 442–454, 2015. [DOI] [PubMed] [Google Scholar]
- Katayama T, Rothwell JC. Modulation of somatosensory evoked potentials using transcranial magnetic intermittent theta burst stimulation. Clin Neurophysiol 118: 2506–2511, 2007. [DOI] [PubMed] [Google Scholar]
- Katayama T, Suppa A, Rothwell JC. Somatosensory evoked potentials and high frequency oscillations are differently modulated by theta burst stimulation over primary somatosensory cortex in humans. Clin Neurophysiol 121: 2097–2103, 2010. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience 85: 677–701, 1998. [DOI] [PubMed] [Google Scholar]
- Labedi A, Benali A, Mix A, Neubacher U, Funke K. Modulation of inhibitory activity markers by intermittent theta-burst stimulation in rat cortex is NMDA-receptor dependent. Brain Stimul 7: 394–400, 2014. [DOI] [PubMed] [Google Scholar]
- Lee KG, Jacobs MF, Asmussen MJ, Zapallow CM, Tommerdahl M, Nelson AJ. Continuous theta-burst stimulation modulates tactile synchronization. BMC Neurosci 14: 89, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz M, Tegenthoff M, Kohlhaas K, Stude P, Hoffken O, Gatica Tossi MA, Kalisch T, Dinse HR. Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J Neurosci 32: 1811–1816, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Hansen HC, Kunze K, Bromm B. Sensory deficits of a nerve root lesion can be objectively documented by somatosensory evoked potentials elicited by painful infrared laser stimulations: a case study. J Neurol Neurosurg Psychiatry 61: 107–110, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-rodriguez M, Wang Y, Gupta A. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004. [DOI] [PubMed] [Google Scholar]
- Mastroeni C, Bergmann TO, Rizzo V, Ritter C, Klein C, Pohlmann I, Brueggemann N, Quartarone A, Siebner HR. Brain-derived neurotrophic factor–a major player in stimulation-induced homeostatic metaplasticity of human motor cortex? PLoS One 8: e57957, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan SK, Linsdell MA, Handy TC, Boyd LA. Interhemispheric enhancement of somatosensory cortical excitability through contralateral repetitive transcranial magnetic stimulation. Clin Neurophysiol 122: 1637–1644, 2011. [DOI] [PubMed] [Google Scholar]
- Mix A, Benali A, Eysel UT, Funke K. Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur J Neurosci 32: 1575–1586, 2010. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Liebetanz D, Paulus W, Nitsche a M. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). J Neurophysiol 103: 1735–1740, 2010. [DOI] [PubMed] [Google Scholar]
- Muller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. Neuroscience 21: 185–202, 2015. [DOI] [PubMed] [Google Scholar]
- Murakami T, Müller-Dahlhaus F, Lu MK, Ziemann U. Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J Physiol 590: 5765–5781, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Sakuma K, Nomura T, Nakashima K, Hashimoto I. High-frequency oscillations change in parallel with short-interval intracortical inhibition after theta burst magnetic stimulation. Clin Neurophysiol 119: 301–308, 2008. [DOI] [PubMed] [Google Scholar]
- Nuwer MR, Aminoff M, Desmedt J, Eisen AA, Matsuoka S, Mauguiere F, Shibasaki H, Sutherling W, Vibert JF. IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 6–11, 1994. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Premji A, Ziluk A, Nelson AJ. Bilateral somatosensory evoked potentials following intermittent theta-burst repetitive transcranial magnetic stimulation. BMC Neurosci 11: 91, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts JN, Wodka EL, Tommerdahl M, Mostofsky SH, Edden ER. Impaired tactile processing in children with autism spectrum disorder. J Neurophysiol 111: 1803–1811, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Becker M, Tegenthoff M, Pleger B, Dinse HR. Sustained increase of somatosensory cortex excitability by 5 Hz repetitive transcranial magnetic stimulation studied by paired median nerve stimulation in humans. Neurosci Lett 356: 91–94, 2004. [DOI] [PubMed] [Google Scholar]
- Ragert P, Franzkowiak S, Schwenkreis P, Tegenthoff M, Dinse HR. Improvement of tactile perception and enhancement of cortical excitability through intermittent theta burst rTMS over human primary somatosensory cortex. Exp Brain Res 184: 1–11, 2008. [DOI] [PubMed] [Google Scholar]
- Rai N, Premji A, Tommerdahl M, Nelson J. Continuous theta-burst rTMS over primary somatosensory cortex modulates tactile perception on the hand. Clin Neurophysiol 123: 1226–1233, 2012. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom M. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci 28: 8285–8293, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Ridding MC, Nordstrom M. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res 181: 615–626, 2007. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol 101: 2872–2877, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppa A, Ortu E, Zafar N, Deriu F, Paulus W, Berardelli A, Rothwell JC. Theta burst stimulation induces after-effects on contralateral primary motor cortex excitability in humans. J Physiol 586: 4489–4500, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Exploring Theta Burst Stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol 118: 333–342, 2007. [DOI] [PubMed] [Google Scholar]
- Tavassoli T, Auyeung B, Murphy LC, Baron-Cohen S, Chakrabarti B. Variation in the autism candidate gene GABRB3 modulates tactile sensitivity in typically developing children. Mol Autism 3: 1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Flavel SC, Ridding MC. Priming theta-burst repetitive transcranial magnetic stimulation with low- and high-frequency stimulation. Exp Brain Res 195: 307–315, 2009. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL. Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Res 1154: 116–123, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Holden JK, Baranek GT. Absence of stimulus-driven synchronization effects on sensory perception in autism: evidence for local underconnectivity? Behav Brain Funct 4: 19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. Theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res 199: 411–421, 2009. [DOI] [PubMed] [Google Scholar]
- Tsang P, Jacobs MF, Lee KGH, Asmussen MJ, Zapallow CM, Nelson AJ. Continuous theta-burst stimulation over primary somatosensory cortex modulates short-latency afferent inhibition. Clin Neurophysiol 125: 2253–2259, 2014. [DOI] [PubMed] [Google Scholar]
- Wu SW, Shahana N, Huddleston D, Gilbert DL. Effects of 30Hz theta burst transcranial magnetic stimulation on the primary motor cortex. J Neurosci Methods 208: 161–164, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar N, Paulus W, Sommer M. Comparative assessment of best conventional with best theta burst repetitive transcranial magnetic stimulation protocols on human motor cortex excitability. Clin Neurophysiol 119: 1393–1399, 2008. [DOI] [PubMed] [Google Scholar]