Abstract
Typically developing children understand and predict others’ behavior by extracting and processing relevant information such as the logic of their actions within the situational constraints and the intentions conveyed by their gaze direction and emotional expressions. Children with autism have difficulties understanding and predicting others’ actions. With the use of eye tracking and behavioral measures, we investigated action understanding mechanisms used by 18 children with autism and a well-matched group of 18 typically developing children. Results showed that children with autism (a) consider situational constraints in order to understand the logic of an agent’s action and (b) show typical usage of the agent’s emotional expressions to infer his or her intentions. We found (c) subtle atypicalities in the way children with autism respond to an agent’s direct gaze and (d) marked impairments in their ability to attend to and interpret referential cues such as a head turn for understanding an agent’s intentions.
Keywords: action understanding, autism, emotions, theory of mind, social cognition, eye tracking
Understanding and predicting others’ actions are crucial abilities that underlie cognitive development, social learning, and everyday life interactions (Bruner, 1990; Tomasello, 1999; Vygotsky, 1978). Current evidence indicates that infants, rather than perceiving a “great blooming, buzzing confusion” (James, 1890, p. 462), enter the world well prepared to make sense of the physical and social environment (Spelke & Kinzler, 2007; Tomasello, Carpenter, Call, Behne, & Moll, 2005). Children’s understanding of other people’s actions beyond the here and now of perception appears to be supported by the ability to identify and integrate relevant information in addition to the action itself, such as the agent’s gaze direction, his or her emotional expressions, and relevant aspects of the environment in which the action occurs. Children’s knowledge of the relevant information to be considered when interpreting actions seems to be based on several assumptions about the behavior of social agents. We refer to such assumptions using the term social expectations.
Children expect agents’ actions to be directed to a goal and to be the most functional way to achieve the goal within the constraints of the situation. This expectation can be characterized as “Agents will choose the most direct way to achieve their goals.” Infants, children, and adults, when observing, interpreting, predicting, and imitating actions, consider the context in which the action occurs and the way the physical constraints in the environment lead agents to use a specific action to achieve a goal (Baker, Saxe, & Tenenbaum, 2009; Gergely & Csibra, 2003; Phillips & Wellman, 2005). They show surprise when a goal is not achieved by using the most efficient available means, and at some ages, they tend not to imitate actions unless they are the most efficient and rational way to achieve a specific goal given the constraints of the situation (Bekkering, Wohlschläger, & Gattis, 2000; Csibra, 2003; Gergely, Bekkering, & Király, 2002; Hamlin, Hallinan, & Woodward, 2008; but also see Rogers, Young, Cook, Giolzetti, & Ozonoff, 2010; Whiten, McGuigan, Marshall-Pescini, & Hopper, 2009). Assuming that actions are rational requires connecting an action to an end state and to the environment in which it occurs, an ability that children seem to begin to acquire during the first year of life (Csibra, 2008; Csibra, Gergely, Biro, Koos, & Brockbank, 1999).
Another complex assumption, which develops during the second year of life, is that mental states such as intentions and desires underlie others’ actions and that these can be inferred on the basis of the agent’s gaze behavior and emotional expressions. This assumption is built on the ability to grasp predictable relations between overt behavior and inner mental states (Flavell, 1999). Infants, children, and adults demonstrate understanding of goals, intentions, and the relations of these mental states to gaze behavior (Gazzaniga, 2008; Tomasello et al., 2005). For example, they expect people to act upon objects that they are looking at rather than objects that they are ignoring (Phillips, Wellman, & Spelke, 2002). This expectation can be characterized as “Where others’ eyes go, their behavior follows” (see Mundy & Newell, 2007). Moreover, children as young as 18 months use emotional markers to determine an agent’s intention and predict his or her behavior. For example, they expect an agent to pick up an object that he or she is looking at with a happy or satisfied expression, rather than an object that he or she is looking at with a disgusted expression. This expectation can be characterized as “Agents act according to their emotional states” (Barna & Legerstee, 208005; Phillips et al., 2002; Repacholi & Gopnik, 1997).
Children also assume that an agent’s direct gaze at them signals a communicative intention (Senju & Johnson, 2009). Children’s attention is captured by direct gaze as early as a few days after birth (Batki, Baron-Cohen, Wheelright, Connellan, & Ahluwalia, 2000; Csibra, 2010; Farroni, Csibra, Simion, & Johnson, 2002; Frischen, Bayliss, & Tipper, 2007). From infancy and throughout development, children interpret a direct gaze as the agent’s deliberate intention to communicate about something of relevance to them, and they respond promptly to such a signal by establishing eye contact with the agent (Csibra, 2010; Southgate, Chevallier, & Csibra, 2009). This expectation can be characterized as “If an agent is looking at me, he or she is about to communicate with me.” The ability to prioritize the direct gaze over other information and to respond to it by establishing a mutual gaze is a crucial component in the development of communicative and social abilities and, in Western cultures, plays a major role in regulating social interactions (Farroni, Menon, & Johnson, 2006; C. D. Frith & Frith, 1999; Senju, Tojo, Yaguchi, & Hasegawa, 2005).
In summary, when understanding others’ actions, children are influenced by referential, emotional, and communicative cues conveyed by the agent’s face, body, and eyes and by the context in which the action occurs (e.g., the presence of physical events and constraints in the environment). Children’s tendency to select, appreciate, and integrate such information is documented very early in development. It is still debated whether these assumptions on how to read agents’ behavior reflect cognitive biases “hard-wired” in the infant’s brain or learned outcomes from patterns observed in social behavior during the first months of life (Csibra, 2008). In any case, these social expectations seem to provide an efficient infrastructure for understanding others’ actions, anticipating people’s behavior, reasoning about their mental states (i.e., having a “theory of mind”), and ultimately, learning from and taking advantage of the knowledge of other people (Baker et al., 2009; Bandura, 1971; Baron-Cohen, 1995; Carpenter, Nagell, & Tomasello, 1998; Dennett, 1996; Emery, 2000).
Difficulties in understanding and predicting others’ actions, as well as difficulties in theory of mind and social-cognitive skills, are frequently documented in children with autism (Baron-Cohen, 1995; Boria et al., 2009; Cattaneo et al., 2007; Zalla, Labruyère, Clément, & Georgieff, 2010), a neurodevelopmental disorder characterized by multiple deficits in the areas of social communication and reciprocity and by behavioral rigidity (Kanner, 1943; Rutter, 1978; Volkmar, Lord, Bailey, Schultz, & Klin, 2004). Social-cognitive studies have focused for many years on understanding these deficits in autism. Although earlier studies aimed at defining single psychological constraints in a top-down approach (e.g., a deficit in theory of mind, Baron-Cohen, 1995; a deficit in self–other mapping, Rogers & Pennington, 1991), current studies are using new tools to look in detail at information processing in autism and are constructing bottom-up theories (e.g., deficiency in the dorsal visual pathway, Pellicano & Gibson, 2008; deficits in the mirror neuron system, Rizzolatti & Fabbri-Destro, 2010). Bottom-up theories of autism seek to understand autism-specific patterns at foundational levels of processing and integration of information that might differentially affect some skills.
We suggest that in autism, failure to rely on the social expectations described above would impair interpretation of people’s actions. Such failure might originate from basic atypicalities in selecting, differentially attending to, and/or integrating relevant information. At this point, relatively little is known about the assumptions that children with autism hold and the processes they use to interpret others’ actions. Studies related to the assumption of rationality (the expectation that “agents choose the most direct way to achieve their goals”) have used imitation of goal-directed actions to ask this question and have reported that preschoolers as well as preadolescents with autism imitate rational acts more accurately than nonrational acts (Hamilton, Brindley, & Frith, 2007; Rogers et al., 2010). Several studies suggest a preserved ability to understand and reproduce an action’s goals across age ranges in those with autism (Avikainen, Wohlschläger, Liuhanen, Hanninen, & Hari, 2003; Hamilton, 2009). However, in another recent study, a group of preschoolers with autism, unlike typically developing controls, tended to imitate the acts that were relevant to achieving the goal as well as the irrelevant “accidental” acts performed by the demonstrator, thus failing to select the goal-directed and most rational acts in the stream of the demonstrator’s behavior (D’Entremont & Yazbek, 2007). Another study, involving a group of adolescents, documented an autism-specific difficulty in organizing pictures illustrating sequences of actions on objects, suggesting a difficulty in means–ends analysis in this population (Zalla, Labruyere, & Georgieff, 2006). Overall, available data are contradictory with regard to the assumptions that children with autism hold about the rationality of an agent’s actions within situational constraints; additional investigation is needed to resolve the inconsistencies.
Unlike the rationality assumption, a more consistent picture has emerged from research about the assumption that other peoples’ gaze patterns provide information about their intentions (“Where others’ eyes go, their behavior follows.”). Several studies show that preschoolers as well as older children with autism, unlike typically developing peers, have difficulties in understanding intentions on the basis of an agent’s eye gaze (Baron-Cohen, Campbell, Karmiloff-Smith, Grant, & Walker, 1995; Pierno, Mari, Glover, Georgiou, & Castiello, 2006). Moreover, it is unclear to what extent children with autism accurately process other visual cues to understand and predict others’ actions. Two studies have shown that toddlers and young children with autism, just like typically developing controls, can infer and reproduce an agent’s intention when observing his or her failed attempt to perform an action (Aldridge, Stone, Sweeney, & Bower, 2000; Carpenter, Pennington, & Rogers, 2001). However, a recent study found that school-aged children with autism, unlike those with typical development, fail to recognize an agent’s actions on objects when the agent’s intentions are not a match with the standard use of the objects (Boria et al., 2009). Overall, these studies are inconclusive on whether children with autism assume actions to reflect an agent’s intention and leave open the question of whether or not they attend to relevant social stimuli, such as the agent’s gaze, to detect intentionality.
No study, so far, has investigated whether children with autism are able, like their typically developing peers, to infer an agent’s intention on the basis of his or her emotions. Extensive research has been conducted on emotion understanding in children and adults with autism with varying results. Some studies found that individuals with autism across age ranges have a deficit in recognizing and understanding basic and complex emotional expressions (Ashwin, Chapman, Colle, & Baron-Cohen, 2006; Braverman, Fein, Lucci, & Waterhouse, 1989; Hobson, Ouston, & Lee, 1989; Macdonald et al., 1989; Tantam, Monaghan, Nicholson, & Stirling, 1989). However, there is also extensive evidence that basic emotion processing remains intact in those with autism (Back, Ropar, & Mitchell, 2007; Hubert, Wicker, Monfardini, & Deruelle, 2009; Lacroix, Guidetti, Roge, & Reilly, 2009; Loveland et al., 1997; Ozonoff, Pennington, & Rogers, 1990; Ponnet, Roeyers, Buysse, De Clercq, & Van der Heyden, 2004; Rieffe, Meerum Terwogt, & Stockmann, 2000; Wright et al., 2008). The ability to read emotions and use emotional information to make judgments has been documented best with regard to basic emotions, such as happiness and anger, in individuals with autism at all ages (Hubert et al., 2009; Lacroix et al., 2009; Ozonoff et al. 1990; Ponnet et al., 2004). A recent study has also found that children and adolescents with autism can accurately identify emotions with limited exposure to the stimulus (Tracy, Robins, Schriber, & Solomon, 2010). More research is needed to determine the extent to which children with autism are able to “read” emotional expressions and use such cues to infer an agent’s intention.
Finally, it is not clear whether children with autism expect a direct gaze to signal a communicative intent. Difficulties in understanding verbal and nonverbal communication are well documented in autism across age ranges (Landa, Holman, & Garrett-Mayer, 2007; Mundy, Sigman, Ungerer, & Sherman, 1986). A recent study found that school-age children with autism pay less attention to an agent’s face displaying a direct gaze than do typically developing children (Vivanti, Nadig, Ozonoff, & Rogers, 2008). However, another study showed that children with autism in the same age range, like their typically developing peers, increase their attention to an agent’s face when his or her gaze is direct (Senju, Kikuchi, Hasegawa, Tojo, & Osanai, 2008), although a direct gaze did not cue attention better than a nonsocial cue (Senju, Tojo, Dairoku, & Hasegawa, 2004). Moreover, children with autism, unlike typically developing children, may exhibit increased arousal while looking at faces directly gazing at them than while looking at faces whose gazes are averted (Kylliäinen & Hietanen, 2006). Thus, current evidence suggests atypicalities in both communication understanding and processing of direct gaze in autism. However, it is not known to what extent children with autism expect that a direct gaze signals communicative intent.
Understanding an agent’s behavior involves both selective attention to relevant information and an appreciation of the meaning of such information. For example, in order to understand an agent’s intention on the basis of his or her gaze direction, one has to (a) look at the agent’s gaze directionality, (b) determine the target of the gaze, and (c) assume that looks to an object signal the agent’s intention to act on that object. Evidence reveals atypicalities in all three of these processes in individuals with autism across age ranges and functioning levels. In some studies, children with autism were prone to focus on aspects of the environment different from those focused on by typically developing children (Klin, Jones, Schultz, & Volkmar, 2003; Vivanti et al., 2008). In other studies, children with autism attended to the same stimuli as typically developing children but processed the information in a different way (Dapretto et al., 2005). Both kinds of differences may disrupt the development of social expectations that ground the development of social cognition.
In conclusion, whereas developmental researchers have confirmed the presence of a variety of early emerging social expectations supporting action understanding in typical development, our understanding of social expectations and action processing in those with autism is piecemeal and contradictory. There are reasons to believe that children with autism might not rely on such expectations to interpret and predict people’s actions. The lack of infant-level understanding of others’ behavior would likely compromise understanding of more complex social behaviors.
Aims of the Present Study
The present study had two main aims: The first was to test social expectations that children with autism use to interpret and predict others’ actions and to compare these expectations with those of typically developing children. The second, if differences emerged, was to determine whether such differences were associated with differences in visual analysis of relevant stimuli.
Our working hypotheses were that, compared with children with typical development, children with autism, as a group,
would not assume agents to use rational means to achieve a goal,
would not predict an agent’s intention on the basis of gaze direction,
would not predict an agent’s intention on the basis of emotions, and
would not interpret the agent’s direct gaze as a communicative signal.
For each of these predictions, we tested two possible explanations: (a) Group differences are due to differences in gaze patterns to the salient stimuli, as measured with eye tracking, or (b) group differences are due to differences in cognitive processing of the observed actions, as tested by behavioral measures.
General Method
Participants
The participants were 18 children and adolescents with high-functioning autism (i.e., children who met full criteria for autism and whose Verbal IQs were in the normal range at the time of participation in the study; 16 boys and 2 girls; mean age = 13 years, Verbal IQ = 109, SD = 15) and 18 children and adolescents with typical development (15 boys and 3 girls; mean age = 12.2. years, Verbal IQ = 112, SD = 12). The two groups did not differ significantly in gender ratio, chronological age, language level, or Performance IQ. Participants were recruited from the subject database and from other research and clinical participation at the MIND Institute, University of California, Davis. Language level was assessed by either the Clinical Evaluation of Language Fundamentals— Fourth Edition (Semel, Wiig, & Secord, 2004) or the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Performance IQ was assessed using the WASI. The diagnosis of autism was previously made by various health care professionals and was confirmed by lab staff using the Autism Diagnostic Observation Schedule—Module 3 (Lord, Rutter, DiLavore, & Risi, 1999). All participants met full DSM–IV (Diagnostic and Statistical Manual of Mental Disorders, 4th edition; American Psychiatric Association, 1994) criteria for autistic disorder. Social abilities were assessed via the Socialization subscale of the Vineland Adaptive Behavior Scales—II (Sparrow, Cicchetti, & Balla, 2005) and the Social Communication Questionnaire— Lifetime (Rutter, Bailey, & Lord, 2003). Exclusionary criteria for the autism group included the presence of a genetic or metabolic disorder known to cause autistic-like features (e.g., fragile X syndrome or tuberous sclerosis), presence of a major medical problem or physical disability, or language level below the average range on Verbal IQ (< 85). Exclusion criteria for the typically developing comparison group included the presence or history of psychiatric or major medical conditions, history of developmental problems or delay, presence of first- or second-degree relatives with an autism spectrum disorder, or language below the average range, as determined by their Verbal IQ. Sample characteristics are presented in Table 1.
Table 1.
Characteristics of High-Functioning Participants With Autism and Typically Developing Participants
| Variable | High-functioning autism (n = 18) | Typically developing (n = 18) | p (t test) |
|---|---|---|---|
| Chronological age | .24 | ||
| M (years) | 13 | 12.2 | |
| SD (months) | 17 | 24 | |
| Range (years) | 10–16 | 9–15 | |
| Language level (CELF-4 or WASI) | .49 | ||
| M | 109 | 112 | |
| SD | 15 | 12 | |
| Range | 85–134 | 88–128 | |
| Performance IQ (WASI) | .82 | ||
| M | 112 | 114 | |
| SD | 14 | 17 | |
| Range | 81–133 | 85–148 | |
| Socialization (Vineland–II) | <.001 | ||
| M | 61.50 | 98.36 | |
| SD | 9.38 | 6.60 | |
| Range | 50–78 | 91–108 | |
| SCQ | <.001 | ||
| M | 25.69 | 2.27 | |
| SD | 7.4 | 2.49 | |
| Range | 18–36 | 0–7 | |
| ADOS-3 | 18 met criteria for autism | ||
| Gender | |||
| Male | 16 | 15 | |
| Female | 2 | 3 | |
| Ethnicity | |||
| Caucasian | 13 | 17 | |
| African American/Caucasian | 1 | ||
| African American/Latino | 1 | ||
| Asian/Caucasian | 1 | ||
| Asian | 3 | ||
Note. There were no significant differences between groups with respect to chronological age, language level, or Performance IQ. CELF–4 = Clinical Evaluation of Language Fundamentals—Fourth Edition; WASI = Wechsler Abbreviated Scale of Intelligence; Vineland–II = Vineland Adaptive Behavior Scales—II; SCQ = Social Communication Questionnaire—Lifetime; ADOS–3 = Autism Diagnostic Observation Schedule—Module 3.
Procedures
General procedures
The experimental protocol was approved by the Institutional Review Board at the University of California, Davis (UC Davis) Medical Center, and informed assent and consent were obtained from all participants and their parents on the day of the first visit. The subjects participated in all four studies reported in this article during the same visit. The average length of the experimental session was 45 min. Developmental and diagnostic evaluations were conducted on a separate visit as part of subjects’ participation in other research projects at the UC Davis MIND Institute.
Apparatus and eye-tracking procedure
All experimental video stimuli were viewed on a 17-in. (43-cm) 60-Hz Tobii 1750 binocular eye-tracker monitor with an imbedded camera (768 × 1,024 pixels resolution, average precision of 0.5° of visual angle). Data were analyzed using frame-by-frame defined areas of interest (AOIs) using ClearView analysis software (Version 2.7.0). Fixation criteria were set to ClearView defaults of a 30-pixel dispersion threshold for 100 ms. Participants were seated in a comfortable chair 23 in. (58 cm) from the monitor in front of a small table for materials. All participants were instructed that they would watch some videos and play some games. The session began with a 5-point calibration that was saved and used for the entire protocol. Data were not used if too few eye-tracking data were recorded (i.e., less than 10% of the time in one or more trials for each experiment) either for technical reasons or because the children did not cooperate. Such exclusion criteria resulted in variable numbers for different experiments (with similar numbers of subjects in both groups excluded). Even with exclusions, the two groups were still equivalently matched in terms of Performance IQ, language, and chronological age in all experiments.
Study 1
In Study 1 we used a violation of expectancy paradigm (Baillargeon, Spelke, & Wasserman, 1985) to investigate whether children with and without autism expect agents to achieve goals rationally. Participants viewed video clips with an actor performing a goal-directed action (e.g., switching on a light or closing a drawer) in an unconventional way (e.g., with a shoulder or an elbow) in two different conditions. In Condition 1 (the hands-occupied condition), the unconventional action made sense because the agent was holding a box. In Condition 2 (the hands-free condition), the same action was performed, but the box was on a chair and the actor was not holding any object. Literature suggests that children, from infancy, look longer at a person’s face when the person displays an unexpected behavior than when the person’s behavior is expected (Striano & Vaish, 2006). We hypothesized that participants in the control group, but not those in the autism group, would look more at the agent’s face in the hands-free condition, demonstrating that they did not expect the agent’s behavior in that condition.
Method
Stimuli
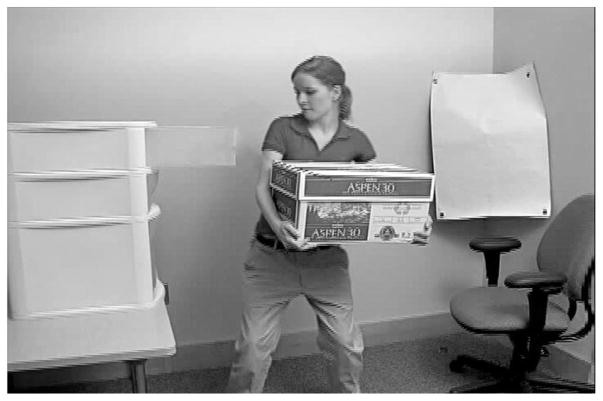
All videos were 4-s clips of the same female actor performing a goal-directed action in an unconventional way. The stimuli consisted of video clips with two conditions, each with three trials. In Condition 1 (the hands-occupied condition; see Figure 1), the actor held a box as she used a portion of her upper body other than her hands to perform a simple action (e.g., turning on a light switch or closing a drawer). In Condition 2 (the hands-free condition; see Figure 2), she performed the same unconventional action, but the box was on the floor and her hands were free.
Figure 1.
Study 1: the hands-occupied condition. The agent performs the action while holding the box.
Figure 2.
Study 1: the hands-free condition. The agent performs the same action as in Figure 1 but without holding the box.
Procedure
Participants were asked to observe the videos on the computer screen. While they viewed the videos, participants’ eye movements were digitally recorded by the Tobii eye-tracking system. The stimuli were presented in two different fixed random orders.
Analyses
Three subjects in the autism group and three in the control group were excluded because too few data were recorded for them. The eye-tracking variable included in the analyses was number of fixations, where a fixation was defined as any data point within a 30-pixel radius for a minimum duration of 100 ms. Such a measure is considered to be a reliable index of what elements in a scene are actually captured and processed (Poole & Ball, 2006). We analyzed the number of fixations to the box and to the agent’s face during the last portion of the videos, in which the agent achieved the goal (1,500 ms). We used a generalized estimating equations (GEE) approach to analyze the data. GEE is able to handle data sets with non-normally distributed discrete data in which correlations might exist among repeated observations (Hardin & Hilbe, 2003). We used a negative binomial distribution with a logit link function and assumed an exchangeable working correlation matrix for all the eye-tracking analyses.
Results
As a test of the groups’ assumption of rational behavior, we compared the number of fixations to the agent’s face in the last 1,500 ms of the videos in the hands-occupied condition and in the hands-free condition. We used GEE to model a repeated measures design, using number of fixations to the agent’s face as the dependent variable and group and condition as predictors. There was a main effect of condition, Wald χ2 = 4.18, p < .05; both groups looked more at the agent’s face in the hands-free condition, that is, when the action was performed in an “irrational” way. There was no effect for group and no group by condition interaction. In the hands-occupied condition, the average number of fixations was 1.9 (SD = 1.7) in the autism group and 2.6 (SD = 1.8) in the control group. In the hands-free condition, the average number of fixations was 2.86 (SD = 3.66) in the autism group and 3.53 (SD = 2.55) in the control group. The average increase in looking time to the face across conditions was 1 fixation.
We used the same design to investigate the effects of group and condition on number of fixations to the agent’s action. There was a main effect of condition, Wald χ2 = 56.41, p < .001; both groups looked more at the agent’s action in the hands-occupied condition, that is, when the action was performed in a “rational” way. There was no effect for group and no group by condition interaction. In the hands-occupied condition, the average number of fixations was 13.9 (SD = 3.7) in the autism group and 13.1 (SD = 5.2) in the control group. In the hands-free condition, the average number of fixations was 11.7 (SD = 3.3) in the autism group and 12.1 (SD = 5.5) in the control group.
We also analyzed whether the groups differed in terms of the number of fixations to the box through a GEE repeated measures design, using number of fixations to the box as the dependent variable and group and condition as predictors. There was a main effect of condition, Wald χ2 = 63.06, p < .001; both groups looked more at the box in the hands-occupied condition. There was no effect of group and no group by condition interaction.
Discussion
In this experiment, both groups increased their attention to the agent’s face and decreased their attention to her action when she performed an action in an “irrational” way versus a “rational” way. In order to understand the logic of the agent’s behavior, the observer had to frame the agent’s action in the context of the physical constraints in the environment in which the action occurred. We had hypothesized that children with autism would be unable to interpret the agent’s rational action by evaluating its logic within the constraints of the situation, perhaps because of difficulties in shifting attention (Landry & Bryson, 2004), in integration of multiple information (U. Frith, 2003), and in goal understanding (D’Entremont & Yazbek, 2007; Zalla et al., 2010), all of which have been reported in autism. Our findings do not support our initial hypothesis: Children with autism, like typically developing controls, considered the situational constraints when evaluating whether or not the agent’s action was an efficient means to her goal (they looked at the box as much as typically developing children did). Moreover, they looked at the agent’s face more often when her action was not rational than when it was. This behavior might reflect children’s tendency to seek an explanation for an agent’s behavior when the agent’s action is irrational (Carpenter & Call, 2007; Striano & Vaish, 2006).
Study 2
The purpose of this study was to examine whether children with autism expect that a person’s visual attention toward a specific object reflects his or her intention to act upon that object. To test this hypothesis, we had each participant view video clips inspired by Meltzoff’s unfulfilled intentions paradigm (Meltzoff, 1995). In these clips, the actor began to perform an action, but the clip ended before the end state of the action was completed. Participants were then asked to (a) imitate what they saw and (b) complete the action as the model would have. The way participants completed the observed action was interpreted to reveal their expectations of the agent’s behavior. In this task, we manipulated the agent’s gaze behavior so that in half of the trials her intention could be inferred only by considering the direction of her gaze. We hypothesized that children with autism, as a group, would not register such information and would fail to complete the action when the agent’s intention was conveyed only by her gaze direction.
Method
Stimuli
The stimuli consisted of an agent conducting an 8-s video demonstration of an incomplete action. All videos were created with the same female actor seated at a table. The test stimuli had two conditions, each with two trials. The two trials in Condition 1 (the neutral condition) consisted of the actor stacking blue and red Lego blocks in an alternating color pattern (see Figure 3). In this condition, the actor conducted the demonstration without any social cues: Her head remained still and she expressed no emotion during the clip. By watching the video clips in Condition 1, one would likely infer that she would continue stacking the Lego blocks according to the color pattern she had been using. In Condition 2 (the head-turning condition), the video was exactly the same as in the neutral condition except that toward the end of the video, the actor turned her head toward a specific block two times (see Figure 4). The head turn was meant to suggest that the actor intended to pick up and stack the block to which she turned and looked, which would change the color pattern of the stack. The overall duration of the head-turning episodes in Condition 2 was 2 s.
Figure 3.

Study 2: the neutral condition. The agent begins to stack blocks following an alternating color pattern. Her head remains still.
Figure 4.

Study 2: the head-turning condition. The agent begins to stack blocks following an alternating color pattern. Then she turns her head toward the block that discontinues the pattern.
Procedure
All participants were instructed to watch the demonstrator in the video clip, imitate her, and then act out what they thought would happen next. Participants were given two practice trials to ensure comprehension of the task: The video for the first trial showed the actor putting some CDs in a standard CD container (five CDs were on the table, and the video stopped after she put two of them in the container); in the second trial, the actor was taking some blocks out of a container and put them on the table (five blocks were in the container, and the video stopped after she put two blocks on the table). All participants were successful at the practice task; that is, they completed the demonstrator’s actions by putting all the CDs in the container and all the blocks on the table. Materials necessary for the reenactment were placed in front of the participant before the beginning of each trial. The materials were exactly the same as those in the video and were placed in the same arrangement as was displayed on the screen. Once the participant finished the reenactment, the materials for the following trial were placed on the table and the next trial was presented. The participant’s performance was recorded for later scoring. While participants viewed videos, their eye movements were digitally recorded by the Tobii eye-tracking system. The trials were presented in one of two fixed random orders.
Analyses and coding system
All videos were coded by a research assistant who was blind to the diagnosis of participants and to the study hypotheses. Participants’ performance was coded according to a simple pass/fail criterion. In Condition 1, a score of 1 was assigned when participants reenacted the agent’s action by continuing her pattern. A score of 0 was given if participants failed to continue the color stacking pattern demonstrated by the agent. In Condition 2, a score of 1 was assigned when participants broke the color pattern by choosing the block that the agent turned and looked at. A score of 0 was given if the participant picked any other block or used the materials in a different way. Interrater reliability between the first author and the trained research assistant for the coding of performance data was calculated on 20% of the entire data set. Cohen’s kappa was .95 for both Condition 1 and Condition 2. For each condition, participants’ total score was calculated by summing the scores obtained in each trial.
Two AOI regions were defined for each clip: the agent’s face and the area of the action, which also included the materials used by the agent. The eye-tracking measures included in the analyses were number of fixations to the AOIs and number of fixations to the agent’s face during the head-turning episodes. One subject from the comparison group and two subjects in the autism group were excluded because too few data were collected for them (less than 10% for each trial). Data were analyzed with a GEE model. For the eye-tracking analyses, we used a negative binomial distribution with a log link function and assumed an exchangeable working correlation matrix.
Results
We first analyzed whether the groups differed in terms of their attention to the agent’s face, using a GEE repeated measures model in which number of fixations to the agent’s face was the dependent variable and group and condition were the predictors. There was a main effect of group, Wald χ2 = 7.39, p < .01; no significant effect for condition; and no group by condition interaction. In the neutral condition, the average number of fixations to the face was 1.3 (SD = 1.7) in the autism group and 3 (SD = 3.1) in the control group. In the head-turning condition, the average number of fixations was 1.3 (SD = 1.1) in the autism group and 3.1 (SD = 2.5) in the control group. Children with autism made, on average, approximately 1.5 fewer fixations to the agent’s face than did typically developing children in both conditions.
The next measure of interest was how the groups differed in terms of number of fixations to the agent’s face when she was turning her head. To do so, we compared participants’ attention to the agent’s face during the head-turning episodes in the head-turning condition and during a portion of the same duration in the neutral condition clip. In the neutral condition, the average number of fixations was 0.5 (SD = 0.7) in the autism group and 1.2 (SD = 1.4) in the control group. In the head-turning condition, the average number of fixations was 0.4 (SD = 0.7) in the autism group and 1.2 (SD = 0.7) in the control group. Using a GEE model in which number of fixations to the agent’s face was the dependent variable and group and condition were the predictors, we found a main effect of group, Wald χ2 = 8.73, p < .005; no effect for condition; and no group by condition interaction. We found no significant effects of group or condition and no group by condition interaction with respect to the number of fixations to the agent’s action.
To test whether children with autism used the head turn to predict the agent’s action, we used GEE to model a repeated measures design, using performance in the action prediction task as an ordinal dependent variable (range = 0–2) and group and condition as independent predictors. We used a cumulative logit link function and an exchangeable working correlation matrix. There was a main effect for condition, Wald χ2 = 25.89, p < .001, and a group by condition interaction, Wald χ2 = 4.38, p < .05. In the neutral condition, the average performance was 1.9 (SD = 1.2) in the autism group and 1.7 (SD = 0.5) in the control group. In the head-turning condition, the average performance was 0.1 (SD = 0.5) in the autism group and 0.8 (SD = 0.8) in the control group. The results show (a) that both groups completed the task more accurately in the neutral condition than in the head-turning condition and (b) that the group of children with autism had significantly lower scores in the head-turning condition than did the group of typically developing children. In this condition, children with autism, as a group, performed four times worse than typically developing children.
We then explored the relationship between attention to the head turn and performance accuracy. Using the same ordinal regression model as above, we next added gaze to head-turning episodes as a covariate term and tested for the main effect and the interactions with condition and with group. There was a significant Gaze × Condition × Group interaction, Wald χ2 = 6.03, p < .05, indicating that in the head-turning condition, performance in the autism group was significantly affected by the number of fixations to the agent’s head during the head turning. The correlation (ρ) between number of fixations to the actor’s face during the head-turning episodes and performance scores on the task for the autism group was .51 (p < .05).
Discussion
In this experiment, we found that children with autism were as able as typically developing children to predict the actor’s intention by attending to her acts on the objects; however, they failed to predict the actor’s intention on the basis of her head turn. Indeed, even though both groups had more difficulties in the head-turning condition than in the neutral condition, whereas the majority of typically developing children in the sample (10 of 17) passed at least one trial in the head-turning condition, only 2 of 16 participants with autism passed at least one trial, and only one passed both. The vast majority of children in the autism group systematically chose the wrong response, using the block that continued the pattern rather than the one at which the agent was looking. We also found an important group difference with regard to the points of fixations. Our data suggest that participants in the autism group possibly failed to use the agent’s head turn to predict her behavior because, unlike typically developing control participants, they did not shift their gaze from objects to her head turn when she turned and gazed at the object. It also appears that those children in the autism group who did attend to the head turn performed the task more accurately and thus predicted her intention.
Study 3
In Study 3 we investigated whether children with and without autism have social expectancies concerning the relationship between emotional expressions and intentions. As in Study 2, the stimuli for this experiment were inspired by Meltzoff’s (1995) unfulfilled intentions paradigm. The participants viewed an actor sorting objects that varied along two dimensions into two containers. Emotional cues consisting of a smile and head nod or a frown and head shake indicated her intention to place an object into one or the other container. We predicted that the participants with autism would differ as a group from those with typical development in their use of emotional cues to establish the intention of the actor as measured by their enactment of the sorting activity.
Method
Stimuli
The stimuli were two 10-s video clips with a female actor seated at a table. In both videos, the actor sorted objects that had two main characteristics, although one was less obvious. In one trial, there were three red boxes and three green boxes. Three of these boxes (two red and one green) were empty, and three (two green and one red) contained an object. The actor tested the objects for the less obvious characteristic (e.g., shook the box to ascertain if it contained an object) and then sorted them accordingly. As she sorted, she was pairing either a negative or a positive emotion with the outcome of her test. In this trial, she showed a happy expression after shaking a box and realizing that it was full and a dissatisfied expression after shaking a box and realizing that it was empty. After shaking the boxes to test whether they were full or empty, she would place the empty ones in a container on her right and the full ones in a container on her left. In the videos, both the more obvious and the less obvious characteristics followed the same pattern (e.g., all the empty boxes were red) until the last item. On the last item demonstrated in the video, the patterns diverged (e.g., the full box was red) but the actor’s emotion was still paired with the less obvious characteristic (in this case, she was showing for the first time a happy facial expression when shaking up a red box, because the box was full). The video ended as the actor reached out to sort the item. Participants could understand that she was sorting the boxes according to whether they were full or empty rather than according to their color only if they noticed her emotional expression. The second trial involved the actor sorting pens and pencils; in this case, the emotional cues indicated that she was sorting according to writing ability as opposed to type of writing utensil (see Figure 5). The duration of the emotional component of the overall episode was 2.5 s. At the end of the video, the participant was asked to complete the sort as the model would have.
Figure 5.

Study 3: The agent begins to sort writing utensils. Her facial expression indicates that she is sorting according to whether they work, not according to the type of writing utensil.
Procedures
Procedures were the same as those in Study 2.
Analyses and coding system
The AOI regions were the actor’s face and the area of the sorting action. Number of fixations to the agent’s face and number of fixations to the agent’s action were the eye-tracking measures used in the analyses. A simple pass/fail coding scheme was used to code participants’ performance. A score of 1 was assigned when participants sorted the objects according to the criterion suggested by the actor’s emotional expression. A score of 0 was assigned when participants sorted objects using any other criterion. Interrater reliability between the first author and a trained research assistant for the coding of performance data was calculated on 20% of the entire data set. Cohen’s kappa was 1.00. For each condition, each participant’s total score was calculated by summing the score obtained in each trial. None of the subjects was excluded in this study. Data were analyzed with a GEE model. For the eye-tracking analyses, we used a negative binomial distribution with a log link function and assumed an exchangeable working correlation matrix.
Results
Using a GEE model in which number of fixations to the agent’s face was the dependent variable and group was the predictor, we found no effect of group, Wald χ2 = 0.70, p = .40. The average number of fixations to the agent’s face was 3.3 (SD = 0.7) in the autism group and 4.1 (SD = 0.6) in the control group. Similarly, there was no effect of group in the number of fixations to the agent’s action, Wald χ2 = 0, p = .98. Using a GEE model similar to that used in Study 3, we did not find any effect of group in the task performance, Wald χ2 = 0.38, p = .75. The average performance was 1.5 (SD = 0.7) in the autism group and 1.4 (SD = 0.7) in the control group. The majority of participants in both groups passed both trials.
Discussion
We hypothesized that children with autism would have more difficulties than typically developing controls in predicting an agent’s behavior on the basis of the agent’s emotions. Difficulties in understanding emotions (Hobson, 2005), in understanding intentions (Baron-Cohen, 1995), and in inhibiting a prepotent response (such as sorting the objects according to the most obvious criterion; Turner, 1999) as well as reduced attention to faces (Klin et al., 2003) should have concurred in making this task particularly arduous for children with autism. We observed the opposite. Children with autism, just like typically developing controls, systematically sorted the objects according to the intentions conveyed by the actor’s emotional states, rather than by the object’s most obvious properties. In Study 2, when the intentions expressed by the actor’s referential cues were competing with the pattern suggested by the material’s characteristics, children with autism tended to ignore the referential cues and to base their predictions on the characteristics of the materials. In Study 3, when the actor’s intentions, conveyed this time by emotional rather than referential cues, were competing with the action suggested by the material’s characteristics, children with autism shifted gaze to the face and relied on the agent’s emotions to solve the task. Moreover, unlike in Study 2, the number of fixations to the actor’s face did not differ between the two groups. These data suggest that emotional cues, unlike visual referential cues, trigger attention to the agent’s face in children with autism and are used successfully by children with autism to predict an agent’s behavior.
Study 4
In this experiment, we tested whether children with autism interpret direct gaze as a signal conveying an agent’s communicative intention toward them. To test this, we analyzed whether participants in the study switched their gaze to the agent’s face when she was gazing directly at them. Subjects were instructed to attend to and imitate actions that were demonstrated by an actor in a series of video clips. The actions were associated with the agent’s direct or averted gaze. We predicted that typically developing children, even if they were instructed to focus on the action, would switch their gaze to the agent’s face in the direct-gaze condition. We also predicted that children with autism, as a group, might not pay special attention to the actor’s face in the direct-gaze condition compared with the averted-gaze condition, because they would not appreciate the communicative value of such a signal.
Method
Stimuli
The stimuli consisted of six trials of a 7-s video demonstration of actions on objects. The same actor was used for all trials, and three different sets of materials were used in two trials each. During the video demonstration, the agent performed a simple action on the objects on the table in front of her while sometimes looking at her own action the whole time (the averted-gaze condition; see Figure 6) and sometimes looking straight at the camera (the direct-gaze condition; see Figure 7).
Figure 6.
Study 4: the averted-gaze condition. The agent performs an action with an object while her gaze is on her own action.
Figure 7.

Study 4: the direct-gaze condition. The agent performs an action with an object while looking directly at the camera.
Procedure
All participants were instructed to watch the demonstrator in the video clip and then imitate the action that was performed. Participants were given two practice trials to ensure comprehension of the task. The experimenter offered encouragement and coaching during these trials as needed. Once comprehension was confirmed, the testing phase started. Materials necessary for the imitation of each trial were placed in front of the participant before the beginning of the trial. The materials were exactly the same as those in the video and were placed in the same arrangement as displayed in the video. Once the participant observed and then imitated the action, the materials for the following trial were placed on the table and the next trial was presented. The trials were presented in one of two fixed random orders. The participant’s performance was video-recorded for later scoring.
Analyses
The AOI regions were the demonstrator’s face and the action area. Number of fixations was used in the eye-tracking analyses. We were also interested in exploring whether the two conditions would influence the imitative performance in the two groups. The coding system to assess the imitation performance involved a score of 0, 1, or 2. A score of 0 was given when participants did not imitate the actor’s action at all. A score of 1 was given when participants reproduced the observed action but made some errors. Participants obtained a score of 2 for an accurate reproduction of the observed action. All videos were coded by a trained research assistant who was blind to the diagnosis of participants and to the study hypotheses. Interrater reliability between the first author and the research assistant for the coding of performance data was calculated on 20% of the entire data set. Cohen’s kappa was .93. For each condition, participants’ total score was calculated by summing the scores obtained in each trial. Two subjects in the autism group and three subjects in the comparison group were excluded because too few data were collected for them. Data were analyzed with a GEE model. For the eye-tracking analyses, we used a negative binomial distribution with a log link function and assumed an exchangeable working correlation matrix.
Results
To test whether participants paid special attention to the agent’s direct gaze, we used GEE to model a repeated measures design, using number of fixations to the agent’s face as the dependent variable and group and condition as predictors. There was a trend toward significance for group, Wald χ2 = 3.11, p = .07, and a main effect of condition, Wald χ2 = 43.14, p < .001. In the averted-gaze condition, the average number of fixations was 2.2 (SD = 2.5) in the autism group and 4 (SD = 3.1) in the control group. In the direct-gaze condition, the average number of fixations was 5.5 (SD = 4.9) in the autism group and 8.1 (SD = 5.2) in the control group. The results show that both groups increased the number of fixations to the agent’s face in the direct-gaze condition (the average increase across groups was 2.5 fixations).
With respect to number of fixations to the agent’s action, we found a main effect of condition, Wald χ2 = 6.35, p = .01; no effect of group; but a group by condition interaction, Wald χ2 = 4.78, p < .05. In the averted-gaze condition, the average number of fixations to the agent’s action was 15.3 (SD = 3.4) in the autism group and 14.4 (SD = 4.2) in the control group. In the direct-gaze condition, the average number of fixations was 11 (SD = 3.5) in the autism group and 14 (SD = 4.6) in the control group. Children with autism made, on average, approximately three fewer fixations to the agent’s action than typically developing children in the direct-gaze condition.
Finally, we tested the effects of group and condition in the imitative task, using performance as an ordinal dependent variable and group and condition as independent factors. We used a cumulative logit link function and an exchangeable working correlation matrix. The results showed no main effects of group or condition but a significant condition by group interaction, Wald χ2 = 4.07, p < .05. In the averted-gaze condition, the average performance was 9.1 (SD = 1.4) in the autism group and 9.2 (SD = 1.7) in the control group. In the direct-gaze condition, the average number of fixations was 8.3 (SD = 1.5) in the autism group and 9.5 (SD = 1.2) in the control group. Whereas the two groups performed equally well in the averted-gaze condition, in the direct-gaze condition the control group performed better than the autism group. We then examined the correlations between number of fixations and the imitation score to determine whether looking patterns affected imitation. We found no relation between the number of fixations to the agent’s face or to the agent’s action and the imitation performance of either group.
Discussion
Both children with autism and children without autism promptly detected the direct gaze and looked more to the agent’s face when she was looking at them. Thus, children with autism were sensitive to direct gaze and responded to it with increased gaze; however, compared with controls, they tended to look less at the agent’s face in both the direct-gaze and averted-gaze conditions. Surprisingly, we also found that children with autism performed poorly, compared with participants in the control group, in the direct-gaze condition but not in the averted-gaze condition. Similarly, they looked less than the typically developing group to the agent’s action in the direct-gaze condition but not in the averted-gaze condition. This result was not expected on the basis of our working hypotheses. It is possible that the agent’s direct gaze disorganized the behavior of children with autism, as suggested by their poorer imitative performance and reduction in number of fixations to the model’s task performance compared with the typically developing group only in the direct-gaze condition. However, more research is needed to support this interpretation.
General Discussion
Understanding others’ actions, just like understanding language (Sperber & Wilson, 1986), is supported by a specific set of attentional and cognitive biases. From infancy on, children know where to look to extract relevant information and what to expect when observing others’ behavior. In this article, we described a series of experiments that tested whether children with autism benefit from the same social expectations that typically developing children exploit to understand and predict people’s behavior. The results depict a distinctive profile of impaired and preserved mechanisms of action understanding, involving atypicalities of both an attentional and a cognitive nature.
Our data show that children with autism, like typically developing controls, take into account the environment in which the action occurs and respond by looking longer at the agent when he or she does not use the most efficient means to achieve a goal within the constraints of the situation. This finding is consistent with previous literature showing that in autism there is an intact ability to understand actions’ outcomes and to selectively imitate rational means to achieve goals (Hamilton, 2009; Hamilton et al., 2007). In order to read the agent’s action as rational, the child has to attend to and consider information about both the agent’s behavior and the constraints of the situation. Our eye-tracking data show that children with autism are able to do so. Previous studies showed that gaze patterns in autism do not always diverge from those of typically developing individuals (Fletcher-Watson, Leekam, Findlay, & Stanton, 2008) and that the ability to shift attention between stimuli is not impaired in every circumstance (Todd, Mills, Wilson, Plumb, & Mon-Williams, 2009). The assumption that agents will act “rationally” to achieve goals is present from infancy on in typical development (Gergely & Csibra, 2003), and it has been documented in nonhuman species such as primates and dogs as well (Buttelmann, Carpenter, Call, & Tomasello, 2007; Range, Viranyi, & Huber, 2007). This expectation provides a rudimentary foundation for goal anticipation, behavior predictions, and the development of a theory of mind (Csibra & Gergely, 2007). Our data suggest that this basic cognitive platform supporting the development of understanding, predicting, and learning about other people’s actions is present and unaffected in high-functioning children with autism of this age range, as a group.
However, when we tested the ability to infer an agent’s intention on the basis of the agent’s gaze, rather than his or her action, we found a significant group difference. A person’s gaze informs us about his or her future behavior (Itier & Batty, 2009). In Study 2, we found that children with autism, unlike typically developing control children, did not follow the agent’s gaze shift and, as a consequence, did not predict her behavior by viewing the target of her gaze. Instead, they tended to rely on the characteristics of the objects to predict the course of the action. Previous findings showed intact ability to predict intentions in autism (Aldridge et al., 2000; Carpenter et al., 2001). Our data suggest that this intact ability might be explained by the tendency, in at least a subgroup of individuals with autism, to rely on the objects’ characteristics and standard use, rather than on facial cues indicating the agent’s intentions, in order to understand and anticipate actions. The relatively stronger ability in autism to imitate actions on objects than to imitate intransitive gestures (Rogers, Hepburn, Stackhouse, & Wehner, 2003; Vivanti et al., 2008) might also reflect such a compensatory strategy. Indeed, a recent study found that children with autism had difficulties understanding what an agent intended to do with an object only when the agent’s intentions did not match the conventional use of the object (Boria et al., 2009). Our data suggest that such phenomena originate from very important differences in one of the biases that support action understanding in typical development, namely, the sensitivity to the agent’s head and gaze direction. Children without autism promptly looked at the agent’s face when she was turning her head, whereas children with autism did not.
Insight into the nature of such differences is provided by the eye-tracking data in the control condition of this experiment (Study 2), when the agent’s face was neutral. In this condition, typically developing children tended to look at the agent’s face more than did children with autism. Even if no useful information was provided by the agent’s face, typically developing children appeared to be monitoring her face to catch a head turn, a gaze shift, or other referential cues. This continuous monitoring of eyes and head direction might reflect the action of a specialized or dedicated cognitive network or system (Baron-Cohen, 1995), and it is possible that the failure to readily detect changes in the agent’s gaze and head position leads children with autism to fail to use social cues to predict people’s behavior.
Another possible explanation for these results, however, is that a special interest in visual patterns (e.g., placing alternating colors of blocks in the stack; see Mottron, Dawson, & Soulières, 2009) drove the poor performance of the children with autism on the task regardless of whether they paid attention to the actor’s cues.
The results of Study 3 dismiss both of these explanations. In Study 3, children had to rely on the agent’s emotional expressions and disregard the action suggested by the material characteristics of the objects in order to understand her intention and predict her action. In this study, children with autism looked at the agent’s face as much as controls did; moreover, they systematically ignored the action suggested by the objects (sorting the objects according to a predictable visual pattern) in favor of the action suggested by the agent’s emotional expression. Provocatively, we can say that they choose to empathize rather than systemize. These data suggest that emotional expressions have a powerful effect in driving the gaze of children with autism to the agent’s face. The same children who ignored a very dramatic movement such as a head turn promptly looked at the agent’s face when she was smiling or displaying a disapproving expression. These results led us to rework our previous hypothesis, and we suggest that a lack of sensitivity specific to referential cues (such as a head turn) affects the ability of children with autism to read intentionality and predict people’s behavior. However, children with autism seem to assume that people act according to their emotional states and seem to successfully exploit emotional cues to predict people’s behavior. These findings look surprising, as difficulties in the processing of emotional information are considered by some authors to be a core feature of autism (Baron-Cohen, 1991; Grèzes, Wicker, Berthoz, & de Gelder, 2009; Hobson, Chidambi, Lee, & Meyer, 2006). However, a recent study that investigated the ability to interpret mental states from emotional expression found no differences between children with autism and healthy controls (Back et al., 2007). Moreover, a number of studies documented intact empathic responses in autism as well as an ability to explain and label emotional states (Hubert et al., 2009; Lacroix et al., 2009; Loveland et al., 1997; Ozonoff et al., 1990; Ponnet et al., 2004; Rieffe et al., 2000; Wright et al., 2008). A reduced sensitivity to referential cues such as a head turn, instead, is more reliably documented in autism (Charman, 2003; Kasari, Freeman, & Paparella, 2006; Mundy, Sigman, & Kasari, 1990). We believe that our data reflect a genuine dissociation between sensitivity to referential cues and emotional cues in autism (or at least in a significant subset of this population). This is one of the most important findings of this study.
Understanding communicative intentions plays an important role in social-cognitive development: Ostensive communicative cues—in particular, eye contact—induce the recipient to assume that the agent will show some new and relevant information to him or her (C. D. Frith, 2008). Our data in Study 4 suggest that children with autism are unimpaired in their sensitivity to an agent’s attention toward them (see also McCormick, Young, Herrera, Oden, & Rogers, 2010). We found that children with autism, compared with participants in the control group, tended to look less at the agent’s face when she was showing a direct gaze. However, they looked less even when she was showing an averted gaze. Moreover, like typically developing children, they looked more at the agent’s face in the direct-gaze condition. These results suggest that children with autism tend to look less frequently at a person’s face regardless of whether he or she is looking at them. Nevertheless, like typically developing children, they respond to direct gaze by looking more at the agent’s face. Despite slight quantitative differences related to the number of fixations, our data suggest that the ability to detect and respond to the communicative intent of a direct gaze is qualitatively intact in high-functioning children with autism.
We also found that the children with autism in our sample showed less accurate imitation of the agent’s action when her gaze was direct, whereas their imitative performance was similar to that of the control group in the averted-gaze condition. We found a similar pattern with regard to the number of fixations to the agent’s action. Previous data have shown that direct gaze may not modulate social information processing in autism (Senju & Johnson, 2009), whereas recent evidence suggests that observing faces with a direct gaze might have a disorganizing effect in children with autism. In a recent study that used skin conductance response (SCR) as a measure of arousal, Joseph, Ehrman, McNally, and Keehn (2008) reported that children with autism showed abnormally increased SCR in response to faces with directed versus averted gaze. Moreover, their performance in a face recognition task was inversely correlated with the amplitude of SRC, which suggests that looking at direct gaze was an arousing experience for children with autism (Joseph et al., 2008). It is possible that looking at the direct gaze negatively affected the performance of the children with autism in our study; however, we did not find a correlation between performance and number of fixations to the face. Clearly, more empirical work is necessary to explore this phenomenon. However, this finding points to the relevance of the demonstrator’s gaze direction in imitative tasks, and it might be one explanation for why findings regarding the impairment in autism of imitation of actions on objects are inconsistent across studies (Rogers & Williams, 2006).
In this article we examined several social expectations that, from infancy on, help children to interpret others’ actions and behavior vis à vis the others’ goals and social and communicative intentions. By using such social expectations, children can go beyond the “literal” information available in the environment (see Bruner, Goodnow, & Austin, 1962). The ability to go beyond the literal information given and to interpret others’ mental states is disrupted in autism. Children with autism do much better on tasks that are “self-explaining” (i.e., tasks in which the materials are organized and structured so that the meaning of the task is immediately understandable; Mesibov, Shea, & Schopler, 2004) and in closed-domain rather than open-domain activities and systems (i.e., activities and systems ruled by a determined set of explicit rules and operations that lead to predictable end states; Baron-Cohen, 2009; Klin et al., 2003). It might be the case that such phenomena reflect the difficulties that children with autism have in using some of the social expectations that help typically developing children make sense of others’ actions. Such difficulties may lead children with autism over time to develop expertise in tasks and processes that are more straightforward and require less social understanding.
We found that children with autism show a distinctive profile of preserved and diminished expectations in interpreting others’ actions. This profile involves difficulties in using the agent’s gaze direction to infer his or her intentions and subtle atypicalities in processing direct gaze alongside an intact ability to rely on the agent’s actions and emotions and to consider nonsocial cues such as the materials’ characteristics and the situational constraints to make sense of the agent’s action. Although a fine-grained investigation of face processing is beyond the scope of our study, our results suggests that atypicalities in the way children with autism scan the agent’s face are modulated by specific features of the agent’s facial expressions and actions, so that across different conditions, gaze patterns to the face in this group vary from very atypical (such as in Study 2) to moderately different (such as in Study 4) to perfectly normal (such as in Study 1). This variation is consistent with previous findings (Anderson, Colombo, & Jill Shaddy, 2006; Vivanti et al., 2008). Interestingly, we did not find any correlations between the results of the different experimental tasks, suggesting that our results reflect distinct action understanding processes. Moreover, we did not find any correlation between clinical and experimental measures.
In conclusion, our data suggest that despite specific atypicalities in gaze patterns and in the use of referential cues, children with autism have some basic social expectations for the rationality of agents’ behavior and also have social expectations concerning the meaning of agents’ emotional states, meaning that they can interpret in order to predict another’s actions. In other words, they have some clues that help them make sense of people’s behavior, but their deficits might render such an operation more effortful, more prone to errors, and less efficient than it is in typical development. Teaching strategies aimed at learning to compensate for these deficits might help children with autism better understand other people’s actions, and, consequently, more fully experience the social world.
Limitations of this study include the possibility that despite our preventative efforts, characteristics other than the actor’s action and gaze influenced the children’s gaze patterns. Another limitation is that our sample represented only a very select and high-functioning subgroup of children with autism and involved older children. It is possible that more fundamental atypicalities in action understanding are masked by experience and education in older children with high-functioning autism. Future work should address these questions in a more representative sample and possibly focus on younger children. Moreover, future research should investigate the effect of systematic variations in the duration and salience of social cues on children’s ability to capture and process such signals. The study of clinical comparison groups with specific impairments in attention, imitation, or action understanding would be productive in investigating questions left unresolved by this study, and future studies should provide a more fine-grained investigation of which specific features and areas of the agent’s face are attended to and considered by children with autism when predicting the agent’s intentions. Finally, the dissociation in autism between the ability to process referential cues and emotional and communicative cues conveyed by the eyes suggests that constructs such as “understanding intentions” and “face processing” might be better characterized as multifactorial constructs. Further studies using larger samples and more specific statistical techniques are needed to explicitly test the factor structure and components of the ability to understand intentions through the face.
Acknowledgments
This research was funded by a postdoctoral fellowship from the MIND Institute to Giacomo Vivanti. Special thanks are extended to Colleen Phillips, Marcella Agozzino, Rashmi Risbud, and Mary Ngo. We grateful to the families who participated in the study.
Contributor Information
Giacomo Vivanti, La Trobe University.
Carolyn McCormick, University of California, Davis.
Gregory S. Young, University of California, Davis
Floridette Abucayan, University of California, Davis.
Naomi Hatt, University of California, Davis.
Aparna Nadig, McGill University.
Sally Ozonoff, University of California, Davis.
Sally J. Rogers, University of California, Davis
References
- Aldridge MA, Stone KR, Sweeney MH, Bower TGR. Preverbal children with autism understand the intentions of others. Developmental Science. 2000;3(3):294–301. doi: 10.1111/1467-7687.00123. [DOI] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Anderson CJ, Colombo J, Jill Shaddy D. Visual scanning and pupillary responses in young children with autism spectrum disorder. Journal of Clinical and Experimental Neuropsychology. 2006;28:1238–1256. doi: 10.1080/13803390500376790. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Chapman E, Colle L, Baron-Cohen S. Impaired recognition of negative basic emotions in autism: A test of the amygdala theory. Social Neuroscience. 2006;1(3–4):349–363. doi: 10.1080/17470910601040772. [DOI] [PubMed] [Google Scholar]
- Avikainen S, Wohlschläger A, Liuhanen S, Hanninen R, Hari R. Impaired mirror-image imitation in Asperger and high-functioning autistic subjects. Current Biology. 2003;13(4):339–341. doi: 10.1016/S0960-9822(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Back E, Ropar D, Mitchell P. Do the eyes have it? Inferring mental states from animated faces in autism. Child Development. 2007;78(2):397–411. doi: 10.1111/j.1467-8624.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Baillargeon R, Spelke ES, Wasserman S. Object permanence in five-month-old infants. Cognition. 1985;20(3):191–208. doi: 10.1016/0010-0277(85)90008-3. [DOI] [PubMed] [Google Scholar]
- Baker CL, Saxe R, Tenenbaum JB. Action understanding as inverse planning. Cognition. 2009;113(3):329–349. doi: 10.1016/j.cognition.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social learning theory. New York, NY: General Learning Press; 1971. [Google Scholar]
- Barna J, Legerstee M. Nine- and twelve-month-old infants relate emotions to people’s actions. Cognition & Emotion. 2005;19(1):53–67. doi: 10.1080/02699930341000021. [DOI] [Google Scholar]
- Baron-Cohen S. Do people with autism understand what causes emotion? Child Development. 1991;62(2):385–395. doi: 10.2307/1131011. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Baron-Cohen S. Autism: The empathizing-systemizing (E-S) theory. Annals of the New York Academy of Science. 2009;1156:68–80. doi: 10.1111/j.1749-6632.2009.04467.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J, Walker J. Are children with autism blind to the mentalistic significance of the eyes? British Journal of Developmental Psychology. 1995;13:379–398. [Google Scholar]
- Batki A, Baron-Cohen S, Wheelright S, Connellan J, Ahluwalia J. Is there an innate gaze module? Evidence from human neonates. Infant Behavior & Development. 2000;23(2):223–229. doi: 10.1016/S0163-6383(01)00037-6. [DOI] [Google Scholar]
- Bekkering H, Wohlschläger A, Gattis M. Imitation of gestures in children is goal-directed. Quarterly Journal of Experimental Psychology. 2000;53:153–164. doi: 10.1080/027249800390718. [DOI] [PubMed] [Google Scholar]
- Boria S, Fabbri-Destro M, Cattaneo L, Sparaci L, Sinigaglia C, Santelli E, … Rizzolatti G. Intention understanding in autism. PLoS One. 2009;4(5):e5596. doi: 10.1371/journal.pone.0005596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman M, Fein D, Lucci D, Waterhouse L. Affect comprehension in children with pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1989;19:301–316. doi: 10.1007/BF02211848. [DOI] [PubMed] [Google Scholar]
- Bruner JS. Acts of meaning. Cambridge, MA: Harvard University Press; 1990. [Google Scholar]
- Bruner JS, Goodnow JJ, Austin GA. A study of thinking. New York, NY: Science Editions; 1962. [Google Scholar]
- Buttelmann D, Carpenter M, Call J, Tomasello M. Enculturated chimpanzees imitate rationally. Developmental Science. 2007;10(4):F31–F38. doi: 10.1111/j.1467-7687.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Call J. The question of ‘what to imitate’: Inferring goals and intentions from demonstrations. In: Dautenhahn K, Nehaniv C, editors. Imitation and social learning in robots, humans and animals: Behavioural, social and communicative dimensions. Cambridge, England: Cambridge University Press; 2007. pp. 135–151. [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development. 1998;63(4) doi: 10.2307/1166214. Serial No. 255. [DOI] [PubMed] [Google Scholar]
- Carpenter M, Pennington BF, Rogers SJ. Understanding of others’ intentions in children with autism. Journal of Autism and Developmental Disorders. 2001;31(6):589–599. doi: 10.1023/A:1013251112392. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Fabbri-Destro M, Boria S, Pieraccini C, Monti A, Cossu G, Rizzolatti G. Impairment of actions chains in autism and its possible role in intention understanding. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(45):17825–17830. doi: 10.1073/pnas.0706273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T. Why is joint attention a pivotal skill in autism? Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2003;358(1430):315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G. Teleological and referential understanding of action in infancy. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2003;358(1431):447–458. doi: 10.1098/rstb.2002.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G. Goal attribution to inanimate agents by 6.5-month-old infants. Cognition. 2008;107(2):705–717. doi: 10.1016/j.cognition.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Csibra G. Recognizing communicative intentions in infancy. Mind & Language. 2010;25:141–168. doi: 10.1111/j.1468-0017.2009.01384.x. [DOI] [Google Scholar]
- Csibra G, Gergely G. ‘Obsessed with goals’: Functions and mechanisms of teleological interpretation of actions in humans. Acta Psychologica. 2007;124(1):60–78. doi: 10.1016/j.actpsy.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Csibra G, Gergely G, Biro S, Koos O, Brockbank M. Goal attribution without agency cues: The perception of ‘pure reason’ in infancy. Cognition. 1999;72(3):237–267. doi: 10.1016/S0010-0277(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2005;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennett DC. Kinds of minds: Toward an understanding of consciousness. 1. New York, NY: Basic Books; 1996. [Google Scholar]
- D’Entremont B, Yazbek A. Imitation of intentional and accidental actions by children with autism. Journal of Autism and Developmental Disorders. 2007;37(9):1665–1678. doi: 10.1007/s10803-006-0291-y. [DOI] [PubMed] [Google Scholar]
- Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neuroscience and Biobehavioral Reviews. 2000;24(6):581–604. doi: 10.1016/S0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(14):9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni T, Menon E, Johnson MH. Factors influencing newborns’ preference for faces with eye contact. Journal of Experimental Child Psychology. 2006;95(4):298–308. doi: 10.1016/j.jecp.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Flavell JH. Cognitive development: Children’s knowledge about the mind. Annual Review of Psychology. 1999;50:21–45. doi: 10.1146/annurev.psych.50.1.21. [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S, Leekam SR, Findlay JM, Stanton EC. Brief report: Young adults with autism spectrum disorder show normal attention to eye-gaze information—Evidence from a new change blindness paradigm. Journal of Autism and Developmental Disorders. 2008;38:1785–1790. doi: 10.1007/s10803-008-0548-8. [DOI] [PubMed] [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133(4):694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. Social cognition. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363(1499):2033–2039. doi: 10.1098/rstb.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds—A biological basis. Science. 1999 Nov 26;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the enigma. 2. New York, NY: Blackwell; 2003. [Google Scholar]
- Gazzaniga M. Human: The science behind what makes us unique. New York, NY: HarperCollins; 2008. [Google Scholar]
- Gergely G, Bekkering H, Király I. Developmental psychology: Rational imitation in preverbal infants. Nature. 2002 Feb 14;415:755. doi: 10.1038/415755a. [DOI] [PubMed] [Google Scholar]
- Gergely G, Csibra G. Teleological reasoning in infancy: The naïve theory of rational action. Trends in Cognitive Sciences. 2003;7(7):287–292. doi: 10.1016/S1364-6613(03)00128-1. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Wicker B, Berthoz S, de Gelder B. A failure to grasp the affective meaning of actions in autism spectrum disorder subjects. Neuropsychologia. 2009;47(8–9):1816–1825. doi: 10.1016/j.neuropsychologia.2009.02.021. [DOI] [PubMed] [Google Scholar]
- de Hamilton AFC. Goals, intentions and mental states: Challenges for theories of autism. Journal of Child Psychology and Psychiatry. 2009;50(8):881–892. doi: 10.1111/j.1469-7610.2009.02098.x. [DOI] [PubMed] [Google Scholar]
- de Hamilton AFC, Brindley RM, Frith U. Imitation and action understanding in autistic spectrum disorders: How valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45(8):1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Hamlin JK, Hallinan EV, Woodward AL. Do as I do: 7-month-old infants selectively reproduce others’ goals. Developmental Science. 2008;11(4):487–494. doi: 10.1111/j.1467-7687.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW, Hilbe J. Generalized estimating equations. Boca Raton, FL: Chapman & Hall/CRC; 2003. [Google Scholar]
- Hobson P. Autism and emotion. In: Volkmar FR, Paul R, Klin A, Cohen D, editors. Handbook of autism and pervasive developmental disorders. 3. New York, NY: Wiley; 2005. pp. 406–422. [Google Scholar]
- Hobson PR, Chidambi G, Lee A, Meyer J. Foundations for self-awareness: An exploration through autism. Monographs of the Society for Research in Child Development. 2006;71(2):vii–166. doi: 10.1111/j.1540-5834.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Ouston J, Lee A. Naming emotion in faces and voices: Abilities and disabilities in autism and mental retardation. British Journal of Developmental Psychology. 1989;7:237–250. [Google Scholar]
- Hubert BE, Wicker B, Monfardini E, Deruelle C. Electrodermal reactivity to emotion processing in adults with autistic spectrum disorders. Autism. 2009;13(1):9–19. doi: 10.1177/1362361308091649. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: The core of social cognition. Neuroscience and Biobehavioral Reviews. 2009;33(6):843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The principles of psychology. New York, NY: Holt; 1890. [Google Scholar]
- Joseph RM, Ehrman K, McNally R, Keehn B. Affective response to eye contact and face recognition ability in children with ASD. Journal of the International Neuropsychological Society. 2008;14(6):947–955. doi: 10.1017/S1355617708081344. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kasari C, Freeman S, Paparella T. Joint attention and symbolic play in young children with autism: A randomized controlled intervention study. Journal of Child Psychology and Psychiatry. 2006;47(6):611–620. doi: 10.1111/j.1469-7610.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: Lessons from autism. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2003;358(1430):345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylliäinen A, Hietanen JK. Skin conductance responses to another person’s gaze in children with autism. Journal of Autism and Developmental Disorders. 2006;36(4):517–525. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Lacroix A, Guidetti M, Roge B, Reilly J. Recognition of emotional and nonemotional facial expressions: A comparison between Williams syndrome and autism. Research in Developmental Disabilities. 2009;30(5):976–985. doi: 10.1016/j.ridd.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Loveland KA, Tunali-Kotoski B, Chen YR, Ortegon J, Pearson DA, Brelsford KA, Gibbs MC. Emotion recognition in autism: Verbal and nonverbal information. Development and Psychopathology. 1997;9(3):579–593. doi: 10.1017/S0954579497001351. [DOI] [PubMed] [Google Scholar]
- Macdonald H, Rutter M, Howlin P, Rios P, Le Conteur A, Evered C, Folstein S. Recognition and expression of emotional cues by autistic and normal adults. Journal of Child Psychology and Psychiatry. 1989;30:865–877. doi: 10.1111/j.1469-7610.1989.tb00288.x. [DOI] [PubMed] [Google Scholar]
- McCormick C, Young GS, Herrera A, Oden T, Rogers S. Social responses of children with autism to attention and imitation. Paper presented at the International Meeting for Autism Research; Philadelphia, PA. 2010. May 20, [Google Scholar]
- Meltzoff A. Understanding the intentions of others: Re-enactment of intended acts by 18-month-old children. Developmental Psychology. 1995;31:838–850. doi: 10.1037/0012-1649.31.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesibov GB, Shea V, Schopler E. The TEACCH approach to autism spectrum disorders. New York, NY: Springer; 2004. [Google Scholar]
- Mottron L, Dawson M, Soulières I. Enhanced perception in savant syndrome: Patterns, structure and creativity. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2009;364(1522):1385–1391. doi: 10.1098/rstb.2008.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Newell L. Attention, joint attention and social cognition. Current Directions in Psychological Science. 2007;16:269–274. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders. 1990;20(1):115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27(5):657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Are there emotion perception deficits in young autistic children? Journal of Child Psychology and Psychiatry. 1990;31(3):343–361. doi: 10.1111/j.1469-7610.1990.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Gibson LY. Investigating the functional integrity of the dorsal visual pathway in autism and dyslexia. Neuropsychologia. 2008;46(10):2593–2596. doi: 10.1016/j.neuropsychologia.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Phillips AT, Wellman HM. Infants’ understanding of object-directed action. Cognition. 2005;98:137–155. doi: 10.1016/j.cognition.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Phillips AT, Wellman HM, Spelke ES. Infants’ ability, to connect gaze and emotional expression to intentional action. Cognition. 2002;85(1):53–78. doi: 10.1016/S0010-0277(02)00073-2. [DOI] [PubMed] [Google Scholar]
- Pierno AC, Mari M, Glover S, Georgiou I, Castiello U. Failure to read motor intentions from gaze in children with autism. Neuropsychologia. 2006;44(8):1483–1488. doi: 10.1016/j.neuropsychologia.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Ponnet KS, Roeyers H, Buysse A, De Clercq A, Van der Heyden E. Advanced mind-reading in adults with Asperger syndrome. Autism. 2004;8(3):249–266. doi: 10.1177/1362361304045214. [DOI] [PubMed] [Google Scholar]
- Poole A, Ball LJ. Eye tracking in human-computer interaction and usability research: Current status and future prospects. In: Ghaoui C, editor. Encyclopedia of human computer interaction. Hershey, PA: Idea Group; 2006. pp. 211–219. [DOI] [Google Scholar]
- Range F, Viranyi Z, Huber L. Selective imitation in domestic dogs. Current Biology. 2007;17(10):868–872. doi: 10.1016/j.cub.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Repacholi BM, Gopnik A. Early reasoning about desires: Evidence from 14- and 18-month-olds. Developmental Psychology. 1997;33(1):12–21. doi: 10.1037/0012-1649.33.1.12. [DOI] [PubMed] [Google Scholar]
- Rieffe C, Meerum Terwogt M, Stockmann L. Understanding atypical emotions among children with autism. Journal of Autism and Developmental Disorders. 2000;30(3):195–203. doi: 10.1023/A:1005540417877. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. Mirror neurons: From discovery to autism. Experimental Brain Research. 2010;200(3–4):223–237. doi: 10.1007/s00221-009-2002-3. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry. 2003;44(5):763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Pennington BF. A theoretical approach to the deficits in infantile autism. Development and Psychopathology. 1991;3:137–162. doi: 10.1017/S0954579400000043. [DOI] [Google Scholar]
- Rogers SJ, Williams JHG. Imitation and the social mind: Autism and typical development. New York, NY: Guilford Press; 2006. [Google Scholar]
- Rogers SJ, Young GS, Cook I, Giolzetti A, Ozonoff S. Imitating actions on objects in early-onset and regressive autism: Effects and implications of task characteristics on performance. Development and Psychopathology. 2010;22(1):71–85. doi: 10.1017/S0954579409990277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Diagnosis and definition of childhood autism. Journal of Autism and Childhood Schizophrenia. 1978;8(2):139–161. doi: 10.1007/BF01537863. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Semel EM, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals 4 (CELF-4) San Antonio, TX: Harcourt Assessment; 2004. [Google Scholar]
- Senju A, Johnson MH. The eye contact effect: Mechanisms and development. Trends in Cognitive Sciences. 2009;13(3):127–134. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Senju A, Kikuchi Y, Hasegawa T, Tojo Y, Osanai H. Is anyone looking at me? Direct gaze detection in children with and without autism. Brain and Cognition. 2008;67(2):127–139. doi: 10.1016/j.bandc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Dairoku H, Hasegawa T. Reflexive orienting in response to eye gaze and an arrow in children with and without autism. Journal of Child Psychology and Psychiatry. 2004;45(3):445–458. doi: 10.1111/j.1469-7610.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Yaguchi K, Hasegawa T. Deviant gaze processing in children with autism: An ERP study. Neuropsychologia. 2005;43(9):1297–1306. doi: 10.1016/j.neuropsychologia.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Southgate V, Chevallier C, Csibra G. Sensitivity to communicative relevance tells young children what to imitate. Developmental Science. 2009;12(6):1013–1019. doi: 10.1111/j.1467-7687.2009.00861.x. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, Balla DA. Vineland Adaptive Behavior Scales, second edition. San Antonio, TX: Pearson Assessments; 2005. [Google Scholar]
- Spelke ES, Kinzler KD. Core knowledge. Developmental Science. 2007;10(1):89–96. doi: 10.1111/j.1467-7687.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- Sperber D, Wilson D. Relevance: Communication and cognition. Oxford, England: Blackwell; 1986. [Google Scholar]
- Striano T, Vaish A. Seven- to 9-month-old infants use facial expressions to interpret others’ actions. British Journal of Developmental Psychology. 2006;24(4):753–760. doi: 10.1348/026151005X70319. [DOI] [Google Scholar]
- Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children’s ability to interpret faces: A research note. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1989;30(4):623–630. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Todd J, Mills C, Wilson AD, Plumb MS, Mon-Williams MA. Slow motor responses to visual stimuli of low salience in autism. Journal of Motor Behavior. 2009;41(5):419–426. doi: 10.3200/35-08-042. [DOI] [PubMed] [Google Scholar]
- Tomasello M. The cultural origins of human cognition. Cambridge, MA: Harvard University Press; 1999. [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behavioral and Brain Sciences. 2005;28(5):675–691. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Tracy JL, Robins RW, Schriber RA, Solomon M. Is emotion recognition impaired in individuals with autism spectrum disorders? Journal of Autism and Developmental Disorders. 2010;41:102–109. doi: 10.1007/s10803-010-1030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. Annotation: Repetitive behaviour in autism: A review of psychological research. Journal of Child Psychology and Psychiatry. 1999;40(6):839–849. doi: 10.1111/1469-7610.00502. [DOI] [PubMed] [Google Scholar]
- Vivanti G, Nadig A, Ozonoff S, Rogers SJ. What do children with autism attend to during imitation tasks? Journal of Experimental Child Psychology. 2008;101(3):186–205. doi: 10.1016/j.jecp.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. Journal of Child Psychology and Psychiatry. 2004;45(1):135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- Vygotsky . Mind in society: The development of higher psychological processes. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Whiten A, McGuigan N, Marshall-Pescini S, Hopper LM. Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2009;364(1528):2417–2428. doi: 10.1098/rstb.2009.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B, Clarke N, Jordan J, Young AW, Clarke P, Miles J, … Williams C. Emotion recognition in faces and the use of visual context in young people with high-functioning autism spectrum disorders. Autism. 2008;12(6):607–626. doi: 10.1177/1362361308097118. [DOI] [PubMed] [Google Scholar]
- Zalla T, Labruyère N, Clément A, Georgieff N. Predicting ensuing actions in children and adolescents with autism spectrum disorders. Experimental Brain Research. 2010;201(4):809–819. doi: 10.1007/s00221-009-2096-7. [DOI] [PubMed] [Google Scholar]
- Zalla T, Labruyere N, Georgieff N. Goal-directed action representation in autism. Journal of Autism and Developmental Disorders. 2006;36(4):527–540. doi: 10.1007/s10803-006-0092-3. [DOI] [PubMed] [Google Scholar]