Abstract
Purpose
To test the effects of segmental trunk support on seated postural and reaching control in children with cerebral palsy (CP).
Methods
Seventeen children (age range 2–15y, GMFCS levels III–V) were classified with the Segmental Assessment of Trunk Control into: mild (complete trunk control/lower-lumbar deficits), moderate (thoracic/upper lumbar deficits) and severe (cervical/upper thoracic deficits). Postural and arm kinematics were measured while reaching with trunk support at axillae, mid-ribs or pelvis.
Results
Children in the mild group did not display changes in posture or reaching across conditions. The moderately involved group showed decrements in postural and reaching performance with pelvic compared to higher supports (P < 0.01). Children in the severe group were unable to maintain posture with pelvic support and showed postural deficiencies with mid-ribs compared to axillae support (P < 0.01).
Conclusion
Children with CP and trunk dysfunction demonstrate improved motor performance when the external assistance matches their intrinsic level of trunk control.
Keywords: Segmental Cerebral Palsy, Trunk Control, Reaching, Posture, Kinematics
INTRODUCTION
Cerebral palsy (CP) is a non-progressive heterogeneous neurological condition, often associated with cognitive-attentional and visuomotor impairments. Motor dysfunction is considered the most characteristic clinical sign in these children due to their frequent problems in learning, planning and coordinating posture and voluntary movements.1,2 According to the Gross Motor Function Classification System (GMFCS), the most severe functional impairments are observed in children classified at levels IV and V, which are in fact, an understudied subpopulation.3
Children with CP show a delayed acquisition and dysregulation of automatic postural responses in both sitting and standing. Children with GMFCS level III may have the ability to walk short distances with external aids, but they still may require help for acquiring the sitting position at younger ages, and a hip support for maximizing hand function and keeping postural balance at older ages.4 Postural control is affected in those children with GMFCS levels III–V, whose sensorimotor experiences are substantially limited compared to healthy children.5,6 Lack of movement complexity and impoverished motor prognosis are also observed in children with CP without the ability to independently sit by the age of two, and when motor development plateaus early in life.3,7 The musculoskeletal and neural components of posture establish a stabilizing framework that supports the capacity to plan and generate skilled reaching and fine manipulation.8(195–222)–10 Even though postural and reaching abilities emerge early in infancy, fine control develops progressively throughout childhood due to the many degrees of freedom in the upper body that must be coordinated during reaching tasks.11
Research investigating postural control in children with CP has demonstrated ambiguous results in the type and amount of support that should be used to enhance posture and subsequent upper limb performance.12,13 The stabilization of the pelvis and/or trunk is a core biomechanical element to improve head stability, visual field orientation and hand manipulation across a wide range of children with CP14. An important goal in rehabilitation is to obtain the maximum degree of function, promoting volitional control of posture, and particularly, of hands and arms while providing the minimal extent of external assistance.15 Thus, providing intermediate levels of trunk stabilization would be a better solution than providing full support in children with CP. However, the optimal level of trunk support for a child with moderate-to-severe CP still remains unknown. This study aims to address this issue in rehabilitation.
Recent research in our lab supports that during development, sitting postural control is acquired following a cranial-caudal progression of the trunk segments, starting with the head, followed by the upper trunk, lower trunk and finally pelvic regions.16,17 Postural control and reaching performance are dependent on the extent of segmental trunk control acquired during sitting development.16 Children with CP (GMFCS III–V), without the ability to sit, display segmental deficits of the trunk, comparable to those observed during sitting development in typical infants. In fact, the extent of segmental trunk control acquired, measured with the Segmental Assessment of Trunk Control (SATCo), is related to the child’s functional disability.18,19 The SATCo evaluates trunk stability in static, reactive and active domains of balance at 7 different trunk segments – changing manual support progressively from the shoulders through the hips.18 The test helps clinicians identify the level of the trunk at which balance is lost.
With this concept in mind, we explored the effects of external trunk support located above and below the level of segmental deficit of the trunk, on posture and reaching performance in children with CP. Children differed on their level of segmental trunk control and thus, we supported the trunk at axillae, mid-ribs and pelvic levels. Children were classified according to their SATCo score as having mild, moderate or severe sitting postural impairments. It was hypothesized that children in the moderate and severe groups would display inefficient posture and reaching, depending on their intrinsic level of trunk control. Moderately affected children would perform worse with pelvic support due to deficits in thoracic-lumbar control; and participants in the severe group would perform worse with the external support at pelvic and mid-ribs levels due to their lack of control at cervical-thoracic segments. Effects of support would not be observed in the mild group, given their ability to sit independently. Thus, the mild group would serve to test the hypothesis that the optimal level of assistance depends on the child’s intrinsic trunk control.
METHODS
Participants
The sample included 17 children diagnosed with CP (12 males & 5 females) between 2 and 15 years. This age range was selected because studies on the developmental progression in children with CP demonstrate that gross motor skills, including independent sitting, plateau early in life, at the age of 3.5 and 3.7 years for children with GMFCS levels IV and III, respectively and at the age of 2.7 years for children with GMFCS level V.3 Thus, we selected 2 years as our lower limit since one-year olds did not comply with the experiment when doing pilot testing. Diagnosis and subtype of CP were confirmed by medical records. Families throughout Oregon were recruited and research was conducted at the University of Oregon. All procedures were approved by the Institutional Review Board for protection of human subjects.
As inclusion criteria, children with medical diagnosis of CP, classified as GMFCS III–V, with vision and ability to reach were considered for the experiment. Children with fixed structural vertebral deformities (scoliosis > 40° and/or kyphosis > 45°), spinal arthrodesis, or administration of chemodenervation in upper limb muscles three months prior to the experimental session were excluded. Functional gross motor ability was evaluated using the GMFM-66-Item-Set and then calculated with the GMAE-2 Gross Motor Ability Estimator (McMaster University, Canada). The ability to reach and grasp was further tested with the Quality of Upper Extremity Skills Test (QUEST). The child’s functional ability to manipulate was classified based on the Manual Ability Classification System (MACS). All children were grouped according to their level of segmental trunk impairment by using their SATCo score: severe (n = 4: lack of cervical/upper thoracic control); moderate (n = 7: lack of thoracic/upper lumbar control) and mild (n = 6: lack of lower lumbar control/complete trunk control) (Table 1).
Table 1.
Clinical Assessment and Demographic Data of the Study Sample
| Subject (Age) | CP Subtype | SATCo Score | Segmental Trunk Instability | SATCo Group | GMFCS | GMFM-66 (Average ± SE) | MACS | QUEST Score | N Reaches |
|---|---|---|---|---|---|---|---|---|---|
| 1 (8y 2mos) | Hypotonic Quadriplegia | 6 | Lower lumbar | Mild | IV | 47.9 (±1.1) | II | 32.33 | 21 |
| 2 (6y 8mos) | Spastic Diplegia | 6 | Lower lumbar | Mild | III | 51.1 (±1.2) | I | 85.20 | 40 |
| 3 (5y 3mos) | Spastic Diplegia | 6 | Lower lumbar | Mild | IV | 46.9 (±1.0) | II | 76.01 | 28 |
| 4 (5y 3mos) | Spastic Diplegia | 7 | Full trunk control | Mild | III | 51.9 (±1.2) | II | 94.15 | 24 |
| 5 (15y 1mos) | Spastic Diplegia | 7 | Full trunk control | Mild | III | 52.6 (±1.2) | I | 99.07 | 32 |
| 6 (2y 1mos) | Spastic Quadriplegia | 6 | Lower lumbar | Mild | IV | 25.3 (±1.9) | I | 55.02 | 34 |
| 7 (7y 4mos) | Dykinetic Quadriplegia | 5 | Upper Lumbar | Moderate | IV | 26.0 (±2.0) | II | 38.47 | 32 |
| 8 (2y 1mos) | Dyskinetic Quadriplegia | 4 | Lower thoracic | Moderate | III | 45.6 (±1.0) | II | 36.40 | 39 |
| 9 (3y 7mos) | Dyskinetic Quadriplegia | 4 | Lower thoracic | Moderate | IV | 30.0 (±1.9) | II | 39.79 | 15 |
| 10 (2y 1mos) | Spastic Quadriplegia | 5 | Upper lumbar | Moderate | IV | 36.0 (±1.5) | IV | 28.71 | 31 |
| 11 (15y 8mos) | Dyskinetic Quadriplegia | 4 | Lower thoracic | Moderate | IV | 50.1 (±1.2) | II | 45.73 | 38 |
| 12 (6y 8mos) | Spastic Quadriplegia | 3 | Mid-thoracic | Moderate | IV | 26.0 (±2.0) | V | 10.46 | 32 |
| 13 (10y 1mos) | Dykinetic Quadriplegia | 3 | Mid-thoracic | Moderate | IV | 41.6 (±1.1) | II | 34.45 | 22 |
| 14 (7y 4mos) | Hypotonic Quadriplegia | 1 | Cervical | Severe | V | 14.8 (±2.8) | V | 10.00 | 2 |
| 15 (7y 9mos) | Dyskinetic Quadriplegia | 2 | Upper Thoracic | Severe | V | 20.5 (±2.1) | IV | 12.53 | 21 |
| 16 (4y 3mos) | Spastic Quadriplegia | 1 | Cervical | Severe | V | 20.5 (±2.2) | IV | 5.11 | 14 |
| 17 (2y 5mos) | Hypotonic Quadriplegia | 1 | Cervical | Severe | V | 22.7 (±2.0) | V | 2.22 | 6 |
Abbreviations: CP, cerebral palsy; SATCo, segmental assessment of trunk control; GMFCS, gross motor function classification system; GMFM, gross motor function measure; MACS, manual ability classification system; QUEST, quality of upper extremity skills test.
Experimental Setup
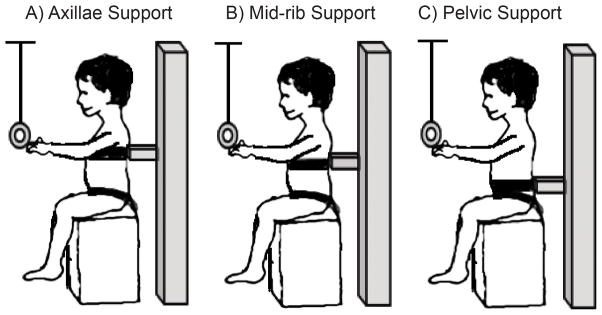
Children sat on a tall bench with feet off the floor to exclude postural reactions elicited by feet-ground contact. The pelvis and hips were firmly attached to the bench with non-elastic straps. A rigid U-shaped support placed behind the subject and a belt with a firm foam pad attached was used for restricting lateral and anterior displacement of the trunk at each level of support (Figure 1). The support was raised or lowered to allow evaluation of trunk segments above the support: axillae, surrounding armpits (T4–T5); mid-ribs, below the inferior angles of the scapulae (T7–T8); and pelvic, surrounding the waist (L3–L5). The order of trunk support was randomly assigned for each child.
Figure 1. Seated Reaching Task across Levels of Trunk Support.
Diagram representing the explicit level of trunk support provided during the reaching task with A) axillae support, B) mid-rib support and C) pelvic support.
During the reaching test, a colorful circular ring was used as the target toy. The toy was attached to a rigid rod which was presented from above, locked within a brace over the child’s head, to a point in front of the child’s suprasternal notch at his/her maximum reach length. This setup permitted a stationary and consistent presentation of the target along the vertical and anteroposterior axes. Children were instructed to rest their hands on their thighs at the beginning of each trial; however, this was not always possible due to their difficulties in maintaining balance (which required them to raise their arms) or in following instructions. Children were encouraged to reach and grasp the toy for 10–15 trials at each condition. The reaching task was video recorded (30Hz) and synchronized with kinematic data collection (84Hz).
Procedures
Data Collection
Magnetic tracking (Minibird system, Ascension Technology, Burlington, VT) was used to collect head, trunk and arm position. Tracking sensors were placed bilaterally on the two radial styloid processes, on the spinous process of 7th cervical vertebrae (C7) and on the center of the forehead. Aside from the tracking sensors, marker positions were digitized at: left and right tragus, suprasternal notch and the anteroposterior and mediolateral edges at the top of the external trunk support. These digitized points were used to estimate the location of the virtual center of the head (VHC) and trunk (VTC) and the center of the trunk support. The VHC location was computed by first calculating the midpoint of the vector created between the two tragus markers. The center between this point and the forehead sensor was estimated as being the VHC. The VTC location was estimated as the midpoint between the suprasternal notch and C7. For calculating the center of trunk support, the intersection between the vectors connecting the anteroposterior and mediolateral edges of the support were used. All position data were referenced to the center of the trunk support.
Video Coding
An open source, computerized video coding tool (http://www.datavyu.org/) was used to select intentional valid reaches when the child directed his/her gaze to the toy and contacted the toy with the hand. Trials in which children used other strategies for contacting the toy, like reaching with the head or mouth, were excluded. A primary coder scored for reach initiation and offset (ROFFSET), defined as beginning of arm movement and hand contact with object, respectively. The velocity profile of the reaching arm was used to refine the reach initiation for reach onset (RONSET). Inter-rater reliability of RONSET and ROFFSET were evaluated by having a second coder score 50% of each child’s data. The correlation coefficients for RONSET and ROFFSET were 0.94 (with an average coding error of 25ms) and 0.88 (with an average coding error of 145 ms), respectively.
Reaches were coded as unimanual or bimanual (when the child touched the object with both hands with an onset time difference less than 1000ms). For bimanual reaches, the reaching arm, defined as the first one contacting and manipulating the object, was used for further kinematic analysis.
Kinematic Analysis
Kinematics were analyzed offline with MATLAB (MathWorks, Inc., Boston, MA) and data were filtered with zero-lag fourth-order, low-pass Butterworth filter (cut-off frequency: 6Hz). Reaching variables included movement time (MT), the time between RONSET and ROFFSET, and arm path length (distance covered by the arm during the reach). Smoothness of the reach was described based on the spatial-temporal features of the arm trajectory. Straightness score assumes that a proficient reach follows a more or less uniform rectilinear trajectory; the number of movement units (MUs) represents the level of reaching coordination, with more MUs demonstrating uncoordinated patterns because of a change in speed or direction. Straightness score was computed as the ratio of the arm path length divided by the shortest path between RONSET and ROFFSET. The closer the score is to one, the straighter the reach. Increasing deviation from a straight line is represented by an increased ratio20. A MU was defined as the portion of the arm movement between two velocity minima (or speed valleys) with a peak velocity greater than 2.3cm/s.20,21
In order to analyze posture, the digitized markers were used to calculate the anatomical coordinate systems for head and trunk with their origins at VHC and VTC, respectively. The forehead and C7 sensors were used to track and update the head angular displacements relative to trunk and trunk angular displacements relative to the global space by using the following Euler’s sequence of rotations: X (flexion (+)/extension (−)), Y′ (right (+)/left (−) lateral flexion) and Z″ (right (+)/left (−) rotations).22,23 We computed angular displacement, absolute summation of angles, and angular orientation, defined as the average angular position of the head and trunk during the reach.
Statistical Analysis
A two-level Generalized Linear Mixed Model (GLMM) was applied using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). As fixed effects, we entered group classification (mild, moderate & severe), level of trunk support (axillae, mid-ribs and pelvic) and their interaction. Age was entered as a covariate. As random effects, we had intercepts for children and trials within children, accounting for child-to-child variability and trials-within-child variability in overall outcomes. Visual inspection of residual plots did not reveal obvious deviations from homoscedasticity and normality. We report all interaction effects, if there were no interactions then main effects were explored and reported. Post-hoc pairwise comparisons were performed in case of significant interactions. The P level was adjusted applying Bonferroni’s sequential procedure.
RESULTS
A total of 431 trials were analyzed. Children in the severe group were unable to perform more than 5 reaches on average, at each level of support; however, children in the moderate and mild groups performed at least 10 reaches per level of support. Number of reaches performed by each participant are shown in Table 1. All children performed unimanual reaches. However, eight children showed a combination of unimanual and bimanual reaches that were highly variable in number across levels of trunk support. In those cases, no significant differences were observed for the main reaching and postural variables between unimanual and bimanual reaches (straightness score: t(7) = 0.24, P = 0.82, and trunk displacement in the flexion-extension plane: t(7) = 0.57, P = 0.59). Thus, unimanual and bimanual reaches were pooled for kinematic analysis. We also found no effects of age on any of the kinematic parameters tested.
With pelvic support, children in the moderate and mild groups were able to maintain balance. Subjects in the severe group could not control their posture when provided with pelvic support and continuously lost balance. Therefore, reaches performed with axillae and mid-ribs supports were analyzed in the severe group.
Reaching Kinematics
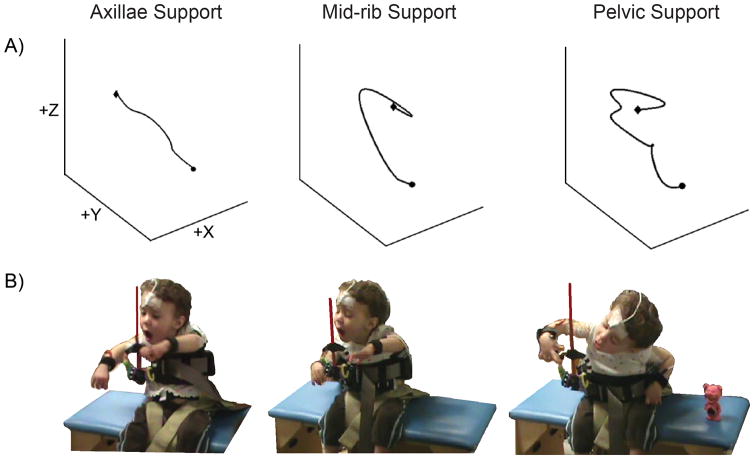
Figure 2 depicts the arm trajectories and corresponding photographic images of one child with moderate segmental trunk impairment across the three levels of trunk support. For the moderate group, impairments in reaching kinematics were observed when support was reduced to the pelvic level (see Video, Supplemental Digital Content 1, 2 & 3).
Figure 2. Three-dimensional Arm Trajectory and Reaching Task.
Panel A depicts the three-dimensional representation of the arm path with axillae, mid-rib and pelvic levels of support of one exemplar child in the moderate group. Circle shape indicates ReachingONSET and diamond shape indicates ReachingOFFSET.
Panel B shows the same child reaching across the three different external trunk conditions.
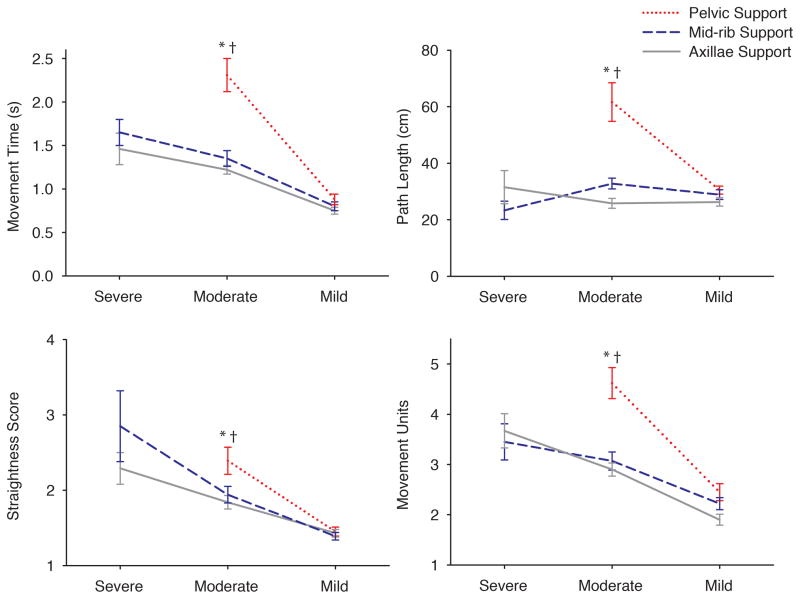
The interaction model was significant for MT, F(3, 418) = 11.33, P < 0.01, η2 = 0.08, path length, F(3, 415) = 10.31, P < 0.01, η2 = 0.07, straightness score, F(3, 417) = 2.89, P < 0.05, η2 = 0.02, and number of MUs, F(3, 416) = 5.39, P < 0.01, η2 = 0.04 (Figure 3).
Figure 3. Reaching Kinematics of Groups across Levels of Trunk Support.
Graphs representing the estimated means across groups for reaching kinematics with pelvic (dotted line), mid-rib (dashed line) and axillae (solid line) supports. Pelvic support is not shown for children with severe trunk dysfunction because they were unable to maintain sitting posture under this condition. Error bars, ± 1 SE. * = P < 0.01 between pelvic and mid-rib support; and † = P < 0.01 between pelvic and axillae support.
Children with moderate segmental trunk control demonstrated a significant increase in MT with pelvic support compared to both mid-ribs and axillae supports. In addition, the path covered by the arm during the reach was significantly increased with pelvic support in comparison to mid-ribs and axillae supports (Table 2a).
Table 2a.
Reaching Kinematics
| Group | Level of Trunk Support | Movement Time (ms) (95% CI) | Path Length (cm) (95% CI) | Straightness Score (95% CI) | Movement Units (95% CI) |
|---|---|---|---|---|---|
| Severe | Axillae | 155 (108 – 202) | 41.3 (23.0 – 59.6) | 2.76 (2.20 – 3.32) | 3.64 (2.81 – 4.47) |
| Mid-Rib | 167 (120 – 214) | 35.7 (17.3 – 54.1) | 2.22 (1.67 – 2.78) | 3.56 (2.75 – 4.38) | |
| Moderate | Axillae | 130 (101 – 160)† | 28.7 (16.4 – 41.0)† | 1.98 (1.64 – 2.32) † | 2.97 (2.48 – 3.45)† |
| Mid-Rib | 138 (107 – 168)* | 34.1 (21.6 – 46.6)* | 1.90 (1.55 – 2.25)* | 3.08 (2.57 – 3.58)* | |
| Pelvic | 230 (200 – 261) | 58.5 (45.9 – 71.5) | 2.45 (2.08 – 2.81) | 4.60 (4.08 – 5.12) | |
| Mild | Axillae | 77 (44 – 110) | 26.6 (13.0 – 40.2) | 1.42 (1.03 – 1.81) | 2.03 (1.47 – 2.59) |
| Mid-Rib | 79 (48 – 111) | 29.0 (15.7 – 42.3) | 1.45 (1.08 – 1.82) | 2.23 (1.71 – 2.75) | |
| Pelvic | 86 (54 – 119) | 30.1 (16.6 – 44.0) | 1.49 (1.10 – 1.87) | 2.44 (1.90 – 3.00) |
P < 0.01 between pelvic and mid-rib support;
P < 0.01 between pelvic and axillae support
Major changes were also observed in the moderate group for the level of reaching smoothness. Straightness score worsened when the support was lowered at pelvic level in comparison to mid-ribs and axillae supports. Pelvic support was also accompanied by an increased number of MUs in contrast to mid-ribs and axillae levels of support (Table 2a).
Trunk Kinematics
Angular Displacement
The interaction model was significant for trunk displacement in the flexion-extension plane, F(3, 387) = 7.76, P < 0.01, η2 = 0.06, the lateral flexion plane, F(3, 388) = 4.60, P < 0.01, η2 = 0.04, and the rotational plane, F(3, 389) = 9.82, P < 0.01, η2 = 0.08.
Children in the moderate group showed significantly increased angular trunk displacement in the three axes of motion with pelvic support compared to mid-ribs and axillae levels. Children with severe trunk dysfunction increased lateral displacement of the trunk while reaching with mid-ribs compared to axillae support. However, these children unexpectedly presented an exacerbated trunk rotation with axillae compared to mid-ribs support (Table 2b).
Table 2b.
Postural Kinematics
| Group | Level of External Trunk Support | Trunk Angular Displacement (95% CI) | Head | |
|---|---|---|---|---|
|
| ||||
| Angular Displacement (95% CI) | Angular Orientation (95% CI) | |||
| Severe | Axillae | F-E: 52.28 (37.53 – 67.04) | F-E: 44.82 (24.97 – 63.68) | F-E: −0.99 (−10.46 – 8.49) ## |
| Lat: 42.98 (22.97 – 62.98) # | Lat: 60.45 (31.64 – 89.26) | Lat: −2.56 (−17.53 – 12.41) | ||
| Rot: 52.02 (38.13 – 65.91) # | Rot: 44.41 (28.53–60.28) # | Rot: −1.77 (−13.96 – 10.41) | ||
|
| ||||
| Mid-Rib | F-E: 48.67 (34.34 – 62.99) | F-E: 58.99 (39.42 – 78.55) | F-E: −16.71 (−25.87 – −7.54) | |
| Lat: 64.83 (45.06 – 84.59) | Lat: 80.55 (52.70 – 108.41) | Lat: 17.67 (3.10 – 32.24) | ||
| Rot: 33.71 (20.19 – 47.24) | Rot: 64.48 (49.12 – 79.84)† | Rot: 2.38 (−9.62 – 14.39) | ||
|
| ||||
| Moderate | Axillae | F-E: 8.81 (0.54 – 17.08)† | F-E: 35.00 (23.04 – 46.95)† | F-E: 0.80 (−4.36 – 5.97) |
| Lat: 14.19 (1.95 – 26.44)† | Lat: 51.66 (36.19 – 67.12) | Lat: −3.14 (−10.74 – 4.47) | ||
| Rot: 9.24 (1.36 – 17.13)† | Rot: 30.24 (21.60 –38.87)† | Rot: 4.01 (−3.33 – 11.36) | ||
|
| ||||
| Mid-Rib | F-E: 11.84 (3.14 – 20.54)* | F-E: 31.52 (19.19 – 43.86)* | F-E: 5.58 (0.09 – 11.06) | |
| Lat: 12.17 (−0.39 – 24.73)* | Lat: 47.18 (30.62 – 63.73) | Lat: 3.30 (−5.10 – 11.69) | ||
| Rot: 10.84 (2.58 – 19.11)* | Rot: 28.86 (19.67–38.05)* | Rot: 3.61 (−3.97 – 11.18) | ||
|
| ||||
| Pelvic | F-E: 41.34 (32.22 – 50.46) | F-E: 71.53 (58.79 – 84.27) | F-E: 0.43 (−5.37 – 6.22) | |
| Lat: 39.22 (26.29 – 52.14) | Lat: 76.00 (58.46 – 93.53) | Lat: 3.89 (−5.11 – 12.90) | ||
| Rot: 38.88 (30.23 – 47.52) | Rot: 72.68 (62.97 – 82.38) | Rot: 2.38 (−5.44 – 10.19) | ||
|
| ||||
| Mild | Axillae | F-E: 5.21 (−5.59 – 16.01) | F-E: 11.28 (−3.80 – 26.36) | F-E: −0.44 (−7.29 – 6.42) |
| Lat: 10.00 (−5.29 – 25.30) | Lat: 20.68 (−0.06 – 41.43) | Lat: −2.62 (−13.24 – 8.01) | ||
| Rot: 5.36 (−4.87 – 15.59) | Rot: 12.55 (1.07 – 24.04) | Rot: −3.71 (−12.96 – 5.55) | ||
|
| ||||
| Mid-Rib | F-E: 7.43 (−2.29 – 17.14) | F-E: 11.48 (−2.60 – 25.56) | F-E: −2.81 (−8.86 – 3.24) | |
| Lat: 12.75 (−1.69 – 27.19) | Lat: 22.31 (4.18 – 40.43) | Lat: −2.66 (−11.56 – 6.25) | ||
| Rot: 6.93 (−2.34 – 16.88) | Rot: 11.19 (1.07 – 21.05) | Rot: −2.09 (−10.75 – 6.56) | ||
|
| ||||
| Pelvic | F-E: 7.11 (−3.19 – 17.40) | F-E: 11.16 (−3.44 – 25.76) | F-E: 0.59 (−5.90 – 7.09) | |
| Lat: 14.83 (−0.04 – 29.69) | Lat: 21.22 (1.64 – 40.81) | Lat: 2.13 (−7.80 – 12.06) | ||
| Rot: 7.09 (−2.69 – 16.88) | Rot: 10.18 (−0.69 – 21.05) | Rot: −2.05 (−11.02 – 6.91) | ||
Estimated means for postural kinematics and 95% confidence interval (CI) are represented. F-E, flexion-extension; Lat, lateroflexion; Rot, rotation.
P < 0.01 between pelvic and mid-rib support;
P < 0.01 between pelvic and axillae support;
P < 0.05 between mid-rib and axillae support;
P < 0.01 between mid-rib and axillae support.
Head Kinematics
Angular Displacement
The interaction model was significant for head displacement in the flexion-extension, F(3, 390) = 10.01, P < 0.01, η2 = 0.08 and rotational planes of motion, F(3, 387) = 14.04, P < 0.01, η2 = 0.11.
Trunk support had a significant impact on head displacement during reaching for the moderate group. Compared to mid-ribs and axillae supports, the angular motion of the head increased along flexion-extension and rotational planes with pelvic support. In the severe group, no significant differences between levels of support were observed for angular head displacement in the flexion-extension and lateral planes; however, head instability in the rotation plane was substantially reduced with mid-ribs support (Table 2b).
Angular Orientation
The interaction model was significant for head orientation in the flexion-extension plane, F(3, 385) = 3.86, P < 0.05, η2 = 0.03.
External trunk support affected head positioning in the flexion-extension plane during the reach in the severe group. With axillae support, the head angle was vertically oriented, but with mid-ribs support the head drifted to a more extended position while reaching (Table 2b).
DISCUSSION
This study investigated the effects of different levels of trunk support on posture and reaching in children with moderate-severe CP. Children differed in their extent of intrinsic trunk control, measured with the SATCo. The biomechanical analysis demonstrated that for children in the moderate group pelvic support was the level that had significant detrimental effects on postural and reaching control. Children with severe trunk impairments were unable to sustain posture for reaching with pelvic support and displayed drastic postural deficits during reaching with mid-ribs support. Children in the mild group did not display differences across levels of support, as hypothesized. Thus, external support below the level of the trunk at which postural deficiencies are observed has adverse effects on posture and reaching performance. Identifying the trunk segment at which stability is compromised in children with moderate-severe CP may guide clinicians toward implementation of optimal levels of trunk assistance during rehabilitation.
Children in the mild group did not present deficits in segmental trunk control at cervical, thoracic or upper lumbar regions. Thus, none of the provided trunk supports had an impact on their performance. Children with mild trunk dysfunction were able to sit independently and walk limited distances with the use of external aids. They would most likely have experienced deficiencies if we had provided an external support at, or below, the level of the hips, as has been previously described for sitting and standing positions.24,25 The stabilization of their trunk while sitting did not modify reaching proficiency in spite of the fact that they were classified as GMFCS-III and were restricted to the sitting position during most of their daily-life activities.26 This is a critical aspect that should be taken into consideration during assessment and treatment in children with mild trunk dysfunctions.
In the moderate group, children mainly presented quadriplegic forms of CP and most were classified as GMFCS-IV, showing difficulties in postural control while sitting. In addition, they displayed low reaching and manipulation skills, determined by the QUEST and MACS. In this group, postural and reaching skills were highly dependent on the level of support provided, given their segmental deficiencies in the thoracic-lumbar regions. No striking differences were observed when lowering the support from axillae to mid-ribs level; however, transitioning trunk support from mid-ribs to pelvis significantly impaired trunk stability and reaching control. Reaching time, the path covered by the arm and the degree of spatial-temporal deviation considerably increased. In addition, they demonstrated excessive head and trunk motion during reaching. Thus, according to these biomechanical outcomes, children with moderate trunk dysfunction do not benefit from support at the axillae due to the excessive restriction of posture. On the contrary, reaching and postural control are impaired when the trunk is completely free with pelvic support. Thus, external trunk support should be placed at mid-ribs to promote volitional control of posture and reaching. At this anatomical region, reaching ability is preserved while simultaneously allowing for optimal voluntary control of the greatest number of trunk segments in upright sitting.
These results are comparable to those observed in typically developing infants. We recently explored reaching performance across development of segmental trunk control.16 Infants who had not acquired control of the lumbar region demonstrated significant postural and reaching impairments with pelvic compared to mid-ribs support. However, once infants had acquired lumbar control, and thus, had the ability to sit independently, reaching differences between levels of support disappeared. This suggests that the acquisition of lumbar control leads both to an increased proficiency of posture and reaching skills, and is critical for achievement of independent sitting. Similarly, in our sample, all children in the mild group had independent sitting and showed no performance differences between support levels. In the case of the moderate-severe groups, the ability to independently sit was absent.
Previous studies reported that children classified as GMFCS-V manifest profound seated postural dysfunction for counter-balancing upper limb movements.6,27 In our current study, children in the severe group were all categorized as GMFCS-V. These children demonstrated extensive deficits in segmental trunk control that disrupted postural sway during static and active sitting. When trunk support was provided at pelvic level, children continuously collapsed over the level of support and were incapable of reaching. Nonetheless, when the external device was located at mid-ribs or axillae level, these children maintained posture in sitting and were able to generate a limited number of reaches. This observation mimics the exploratory and reaching behaviors observed in healthy newborns when the head is fixed in upright sitting, reinforcing the concept of posture being the foundation for reaching skills.28 The quality of the reach was not altered by support at axillae level compared to mid-ribs, suggesting that external support is not enough to promote reaching control in this group of children. However, they demonstrated remarkable improvements in postural kinematics with axillae compared to mid-ribs support. The compensatory lateral displacement of the trunk associated with the reaching arm was reduced with axillae support; however, it was accompanied by excessive trunk rotation. Also, the alignment of the head was more vertically oriented in the flexion-extension plane while reaching, a pre-requisite for visual stabilization during reaching tasks. It is well known that children with CP frequently display visual impairments associated with postural dysfunction.29 Results from our study indicate that axillae support could lead to clinical improvements in posture and also enhance eye-head coordination in children with severe CP.
LIMITATIONS
We note that cognitive impairments and visuomotor deficits in the severe group could have interfered with postural and reaching performance and could have contributed to reaching impairments. Also, recruiting children with CP who fit our inclusion criteria was challenging and resulted in unequal sample sizes among groups. Specifically, the low sample size of the severe group means that results should be interpreted with caution.
CONCLUSIONS
Postural dysfunction is one of the most limiting factors in the population with CP. It restricts reaching skills and in turn reduces participation in daily-life activities. The motivation underlying the current study was the lack of research and evidence-based therapies for children diagnosed with CP (GMFCS levels III–V). According to our results, children with the ability to independently sit would not require external trunk support above the pelvis. Training protocols with a goal of sitting without hip straps or restriction of the levels below the pelvis could be a plausible approach to train posture in these children. With respect to those lacking trunk control at thoracolumbar levels, we highlight the importance of using external support targeting the thoracic segment for training static and active balance control with the use of upper extremity activities. The results obtained in children with severe trunk control deficits indicate that postural performance could be maximized with external support at the axillae level. In future research, a multi-segmented approach to trunk control in children with CP could be investigated within the context of evidence-based training protocols of upper limbs, such as Constraint-Induced Movement Therapy or Hand-Arm Bilateral Intensive Therapy, expanding the use of these interventions to children with GMFCS levels III–V.30
Acknowledgments
GRANT SUPPORT
This work was supported by the NIH Grant 1R01HD062745-01, Marjorie Woollacott, principal investigator, and honorarium from the 2014 Evonuk Award, Human Physiology Department at University of Oregon, Victor Santamaria.
We thank Wayne Manselle for his indispensable technical support.
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflict of interest to disclosure in the current study.
References
- 1.Krageloh-Mann I, Bax M. Cerebral Palsy. In: Aicardi J, editor. Diseases of the Nervous System in Childhood. 3. England, London: Mc Keith Press; 2009. pp. 210–242. [Google Scholar]
- 2.Richards L, Malouin F. Cerebral Palsy: definition, assessment and rehabilitation. In: Aminoff M, Boller F, Swaab D, editors. Handbook of Clinical Neurology: Pediatric Neurology, Part I. Amsterdan, The Netherlands: Elsevier; 2013. pp. 169–176. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum PL, Walter SD, Hanna SE, et al. Prognosis for Gross Motor Function Creation of Motor Development Curves. JAMA. 2002;288(11):1357–1363. doi: 10.1001/jama.288.11.1357. [DOI] [PubMed] [Google Scholar]
- 4.Palisano R, Rosenbaum P, Bartlett D, Livingston M. GMFCS-E & R: Gross motor functional classification system, expanded and revised. CanChild Centre for Childhood Disability Research; Hamilton, ON: 2007. Retrieved from: http://www.canchild.ca/en/ [Google Scholar]
- 5.Woollacott MH, Burtner P, Jensen J, Jasiewicz J, Roncesvalles N, Sveistrup H. Development of postural responses during standing in healthy children and children with spastic diplegia. Neurosci Biobehav Rev. 1998;22(4):583–589. doi: 10.1016/s0149-7634(97)00048-1. [DOI] [PubMed] [Google Scholar]
- 6.Van der Heide JC, Begeer C, Fock JM, et al. Postural control during reaching in preterm children with cerebral palsy. Dev Med Child Neurol. 2004;46(4):253–266. doi: 10.1017/s0012162204000416. [DOI] [PubMed] [Google Scholar]
- 7.Wu YW, Day SM, Strauss DJ, Shavelle RM. Prognosis for ambulation in cerebral palsy: a population-based study. Pediatrics. 2004;114(5):1264–1271. doi: 10.1542/peds.2004-0114. [DOI] [PubMed] [Google Scholar]
- 8.Shumway-Cook A, Woollacott M. Development of postural control. In: Shumway-Cook A, Woollacott M, editors. Motor control: Translating research into clinical practice. Baltimore, MD: Lippincott Williams & Wilkins; 2012. pp. 195–222. [Google Scholar]
- 9.Shumway-Cook A, Woollacott M. The growth of stability: A postural control from a developmental perspective. J Mot Behav. 1985;17(2):131–147. doi: 10.1080/00222895.1985.10735341. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann M, Toni I, de Lange FP. Body posture modulates action perception. J Neurosci. 2013;33(14):5930–5938. doi: 10.1523/JNEUROSCI.5570-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneiberg S, Sveistrup H, McFadyen B, McKinley P, Levin MF. The development of coordination for reach-to-grasp movements in children. Exp Brain Res. 2002;146:142–154. doi: 10.1007/s00221-002-1156-z. [DOI] [PubMed] [Google Scholar]
- 12.McNamara L, Casey J. Seat inclinations affect the function of children with cerebral palsy: a review of the effect of different seat inclines. Disabil Rehabil Assist Technol. 2007;2(6):309–318. doi: 10.1080/17483100701661314. [DOI] [PubMed] [Google Scholar]
- 13.Myhr U, von Wendt L. Improvement of functional sitting position for children with cerebral palsy. Dev Med Child Neurol. 1991;33(3):246–256. doi: 10.1111/j.1469-8749.1991.tb05114.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HYK, Lien YJ, Yu YC, et al. The effect of lower body stabilization and different writing tools on writing biomechanics in children with cerebral palsy. Res Dev Disabil. 2013;34(4):1152–1159. doi: 10.1016/j.ridd.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Butler P. A preliminary report on the effectiveness of trunk targeting in achieving independent sitting balance in children with cerebral palsy. Clin Rehabil. 1998;12:281–293. doi: 10.1191/026921598667577442. [DOI] [PubMed] [Google Scholar]
- 16.Rachwani J, Santamaria V, Saavedra SL, Woollacott MH. The development of trunk control and its relation to reaching in infancy: a longitudinal study. Front Hum Neurosci. 2015;9:94. doi: 10.3389/fnhum.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saavedra SL, van Donkelaar P, Woollacott MH. Learning about gravity: segmental assessment of upright control as infants develop independent sitting. J Neurophysiol. 2012;108:2215–2229. doi: 10.1152/jn.01193.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler P, Saavedra S, Sofranac M, Jarvis S, Woollacott M. Refinement, Reliability and Validity of the Segmental Assessment of Trunk Control (SATCo) Pediatr Phys Ther. 2010;22(3):246–257. doi: 10.1097/PEP.0b013e3181e69490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis DJ, Butler P, Saavedra S, Bencke J, Kallemose T, Sonne-Holm S, Woollacott M. The central role of trunk control in the gross motor function of children with cerebral palsy: a retrospective cross-sectional study. Dev Med Child Neurol. 2015;57(4):351–357. doi: 10.1111/dmcn.12641. [DOI] [PubMed] [Google Scholar]
- 20.Fetters L, Todd J. Quantitive assessment of infant reaching movements. J Mot Behav. 1987;19(2):147–166. doi: 10.1080/00222895.1987.10735405. [DOI] [PubMed] [Google Scholar]
- 21.Grönqvist H, Strand Brodd K, von Hofsten C. Reaching strategies of very preterm infants at 8 months corrected age. Exp Brain Res. 2011;209:225–233. doi: 10.1007/s00221-011-2538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu G, van der Helm FCT, Veeger HE, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38:981–992. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 23.St-Onge N, Côté JN, Preuss R, Patenaude I, Fung J. Direction-dependent neck and trunk postural reactions during sitting. J Electromyogr Kinesiol. 2011;21(6):904–912. doi: 10.1016/j.jelekin.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Saavedra SL, Joshi A, van Donkeelar P, Woollacott M. Eye hand coordination in children with cerebral palsy. Exp Brain Res. 2009;201(2):155–165. doi: 10.1007/s00221-008-1549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heyrman L, Desloovere K, Molenaers G, et al. Clinical characteristics of impaired trunk control in children with spastic cerebral palsy. Res Dev Disabil. 2013;34(1):327–334. doi: 10.1016/j.ridd.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 27.Van der Heide JC, Fock JM, Otten B, Stremmelaar E, Hadders-Algra M. Kinematic characteristics of reaching movements in preterm children with cerebral palsy. Pediatr Res. 2005;57(6):883–889. doi: 10.1203/01.PDR.0000157771.20683.14. [DOI] [PubMed] [Google Scholar]
- 28.Amiel-Tison C, Grenier A. Expression of liberated motor activity (LMA) following manual immobilization of the head. In: Amiel-Tison C, Grenier A, editors. Neurological Evaluation of the Newborn and the Infant. New York, NY: Masson Publishing USA; 1983. pp. 87–109. [Google Scholar]
- 29.Porro G, van der Linden D, van Nieuwenhuizen O, Wittebol-Post D. Role of visual dysfunction in postural control in children with cerebral palsy. Neural plasticity. 2005;12(2–3):205–210. doi: 10.1155/NP.2005.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon AM, Hung YC, Brandao M, et al. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: a randomized trial. Neurorehabil Neural Rep. 2011;25(8):692–702. doi: 10.1177/1545968311402508. [DOI] [PubMed] [Google Scholar]