Abstract
There is little data regarding the incidence, clinical manifestations, risk factors and outcomes of late acute GVHD (aGVHD). We evaluated patients with late aGVHD after allogeneic HCT between 2007 and 2012 and compared their outcomes to patients with early onset aGVHD. Of the 511 allogeneic HCT recipients, 75 developed late aGVHD (cumulative incidence: 14.7% (95% CI: 11.6-17.8) versus 248 with early onset aGVHD (cumulative incidence: 49% (95% CI: 45-53). Amongst those with late aGVHD, 52% had persistent, 39% recurrent and 9% de-novo late aGVHD. Advanced (grade III-IV) early onset aGVHD was associated with a higher risk of developing late aGVHD (HR 1.9, 95% CI: 1.2 −3.1, p= 0.01). 48% (95% CI: 36-60%) of late aGVHD versus only 31% (95% CI: 26-37%) of early onset aGVHD progressed to chronic GVHD by 2 years. Higher proportion of persistent (53%) as compared to recurrent (39%) and de-novo (46%) late aGVHD progressed to cGVHD at 2 years. The overall survival was 59% (95% CI: 49-72) in late aGVHD and 50% (95% CI: 44-57%) in early onset aGVHD. Persistent late aGVHD had worse OS and NRM (45% and 39%) as compared to recurrent (74% and 18%) and de-novo (83% and 0%) late aGVHD. Compared to HLA-identical sibling HCT, unrelated donor transplants were associated with a higher risk of mortality in patients developing late aGVHD (HR 6.1, 95% CI: 2.3-16.2, p<0.01). In a landmark analysis (evaluating 100 day survivors among early onset aGVHD), no difference was seen in late mortality (after 100 days) between early onset and late aGVHD (HR 0.96, 95% CI: 0.59-1.55, p= 0.85), however the risk of cGVHD was nearly doubled (HR 1.81, 95% CI: 1.16-2.82, p=0.01) in patients with late aGVHD. Conclusions: Late aGVHD is a relatively common complication after allogeneic HCT. Poorer outcomes in those with persistent late aGVHD imply need for more effective therapy in this group to improve transplant outcomes. A higher risk of subsequent chronic GVHD needs further evaluation and close monitoring.
Keywords: Late acute graft-versus-host-disease, early onset acute graft-versus-host disease, allogeneic hematopoietic stem cell transplant
Introduction
Late acute graft versus host disease (late aGVHD), is defined as presence of symptoms and signs of acute GVHD (aGVHD) after 100 days of allogeneic hematopoietic stem cell transplant (HCT).1 Several retrospective studies have evaluated reclassification of patients with chronic GVHD (cGVHD) into late aGVHD, classic cGVHD and overlap syndrome, and reported conflicting outcomes in those with late aGVHD.2-4 Insufficient data are available regarding onset, clinical presentation, response to treatment and major outcomes of late aGVHD.
We evaluated a retrospective cohort of patients with late aGVHD amongst adult allogeneic HCT recipients who underwent a HCT at the University of Minnesota between January 1st, 2007 and December 31st, 2012.
The main objectives of this study were to evaluate the outcomes in late aGVHD, compared with early onset aGVHD. Secondary objectives included identifying risk factors for onset of late aGVHD and risk factors for NRM in patients with late aGVHD.
Patients and Methods
All consecutive adult patients who underwent an allogeneic HCT between January 1st, 2007 and December 31st, 2012 (n=511), and were reported to have GVHD (acute and/or chronic) in the University of Minnesota BMT database, were retrospectively reviewed to identify patients with late aGVHD. Five patients were reclassified from cGVHD to late aGVHD. Overall, 75 patients with late aGVHD and 248 with early onset aGVHD were included in this study. All patients signed informed consent prior to the HCT and this retrospective analysis was approved by the Institutional Review Board at the University of Minnesota.
Definitions
Early onset aGVHD and late aGVHD were defined according to 2005 NIH consensus criteria,1 and confirmed in the 2014 NIH consensus conference.5 Early onset aGVHD was defined as features of aGVHD, in the absence of cGVHD, with onset before day +100 after HCT. Late aGVHD was defined as features of aGVHD observed beyond day +100. This was further classified as de-novo, if new onset of symptoms and signs of aGVHD were seen after day 100, without prior early onset aGVHD; recurrent, if there was recurrence of previously resolved aGVHD after day 100; or persistent, if persistent symptoms and signs of aGVHD were seen after day 100 without prior resolution. Acute GVHD was graded according to the established criteria.6 Grading of late aGVHD followed standard grading of aGVHD.6 Classic cGVHD was defined according to 2005 NIH consensus criteria.1 Overlap syndrome was included in the 2005 NIH consensus criteria1 as a sub-category of cGVHD with overlapping features of both acute and cGVHD, but this nomenclature was not retained in the 2014 NIH consensus conference.5 As both classic cGVHD and overlap syndrome manifest features of cGVHD, we combined both these categories into cGVHD for the purpose of this analysis.
The regimens for myeloablative (MA) conditioning consisted of cyclophosphamide and fractionated total body irradiation (TBI) or busulphan and cyclophosphamide, with or without anti-thymocyte globulin (ATG), or fludarabine based regimen, with or without ATG.7-10 MA umbilical cord blood (UCB) recipients also received fludarabine, with or without ATG.10 Reduced intensity conditioning (RIC) consisted of cyclophosphamide, fludarabine and low dose TBI, with or without ATG.7
GVHD prophylaxis consisted of cyclosporine (CSA) + mycophenolate mofetil (MMF) for all RIC and UCB HCT. Methotrexate (MTX) + either CSA or tacrolimus (Tac) were used for GVHD prophylaxis in MA HLA-identical sibling donor and URD HCT recipients. Patients diagnosed with aGVHD were treated with oral prednisone (2 mg/kg daily, or 60 mg/m2/day or methylprednisolone i.v. equivalent) as initial therapy for 2 weeks and tapered over the next 8 weeks. Patients diagnosed as cGVHD (and re-classified as late aGVHD) were treated as cGVHD. Response to treatment was assessed as previously described.11 Complete response (CR) was defined as complete resolution of aGVHD manifestations in all organs, without need for secondary GVHD therapy. Partial response (PR) was defined as improvement in GVHD stage in all initially affected organs, without resolution in all organs, worsening in any other GVHD target organs, or need for secondary GVHD therapy. No response was defined as the same severity of GVHD in any organ, or death, or the addition of secondary GVHD therapy before day 28. Patients who experienced a flare of aGVHD before day 28 and required therapy with increased steroids or additional GVHD therapy were also considered to have no response. Progression was defined as worsening GVHD in at least 1 organ with or without improvement in any other organ.11
Statistical analysis
Demographic and clinical characteristics were described using descriptive statistics. Cumulative incidences of late aGVHD, early onset aGVHD and cGVHD were estimated with non-GVHD deaths modeled as competing risks.12 Overall survival was calculated using Kaplan-Meier estimates, and 95% confidence intervals (CIs) were calculated using the Greenwood formula.13 Cumulative incidence of NRM was calculated using relapse or disease progression of the underlying malignancy as competing risk.12 Incidences of early onset and late aGVHD were calculated from time of HCT, and outcomes (OS, NRM, and cGVHD) following aGVHD were calculated from onset of early aGVHD for early outcomes or late aGVHD for late outcomes, using day +100 after HCT as the landmark for persistent late aGVHD.
Multiple regression was used to identify factors independently associated with risk of developing late aGVHD (among patients who developed early aGVHD), and risk of OS and NRM (among patients who developed late aGVHD). Cox regression was used for OS and Fine and Gray regression was used for other outcomes using the competing risks defined above.14,15 Factors tested in regression models included age, underlying disease, disease risk prior to HCT, conditioning regimen (MA vs RIC), donor/recipient CMV serostatus, donor-recipient gender match (female to male vs other), use of ATG in conditioning, stem cell source (marrow vs PBSC vs UCB) and maximum grade of prior early onset aGVHD (for late aGVHD). All statistical analyses were performed with SAS 9.3 (SAS Institute, Cary, NC) and R 3.0.2.
Results
Baseline Characteristics
Seventy five (n=75) patients with late aGVHD and 248 patients with early onset aGVHD were included in this study. Since 68 patients with early onset aGVHD developed recurrent or persistent late aGVHD, these groups are not mutually exclusive. Table 1 describes the demographics and transplant characteristics of patients. No major differences were seen in baseline and HCT characteristics of the two groups. The median age at HCT was 50.7 years (range: 19-72) in late aGVHD and 50.4 years (range: 18-75) in early onset aGVHD. The donor source included a fully HLA-matched sibling donor (MSD) in 41.3% vs 32%, an URD in 13.3% vs 12%, and UCB in 45.3% vs 56%, in late aGVHD and early onset aGVHD groups, respectively. Non-UCB graft sources included peripheral blood stem cell (PBSC) in 47% and 35%, and bone marrow in 8 and 9% in late aGVHD and early onset aGVHD, respectively. A majority of patients in both cohorts received RIC. The median follow up of survivors was 4.2 years.
Table 1. Baseline Demographics and Transplant characteristics.
| Late aGVHD (n=75) n (%) |
Early onset aGVHD (n=248) n (%) |
|
|---|---|---|
|
Age Median (range) |
50.7 (19-72) |
50.4 (18-75) |
| Gender | ||
| Male | 47 (63%) | 145 (58%) |
| Female | 28 (37%) | 103 (42%) |
| Diagnosis | ||
| Acute leukemia | 35 (47%) | 134 (54%) |
| Chronic leukemia | 8 (11%) | 25 (10%) |
| Lymphoma | 10 (13%) | 31 (12%) |
| MDS/MPN | 16 (21%) | 41 (17%) |
| Multiple Myeloma | 3 (4%) | 12 (5%) |
| Other | 3 (4%) | 5 (2%) |
| Donor Type | ||
| HLA - Matched sibling | 31 (41.3%) | 80 (32%) |
| URD | 10 (13.3%) | 31 (12%) |
| UCB | 34 (45.3%) | 139 (56%) |
| Graft type | ||
| Marrow | 6 (8%) | 22 (9%) |
| PBSC | 35 (47%) | 87 (35%) |
| UCB | 34 (45%) | 139 (56%) |
| Gender Match | ||
| Female donor to male recipient | 12 (16%) | 53 (21%) |
| Other | 63 (84%) | 195 (79%) |
| CMV Serostatus | ||
| Recipient positive | 46 (61%) | 155 (63%) |
| Recipient negative | 29 (39%) | 92 (37%) |
| Conditioning | ||
| Myeloablative | 27 (36%) | 94 (38%) |
| Reduced intensity | 48 (64%) | 154 (62%) |
| GVHD Prophylaxis | ||
| CSA/MMF | 64 (85%) | 200 (81%) |
| CSA or Tacrolimus+MTX | 10 (13%) | 35 (14%) |
| Ex vivo T cell depletion | 0 (0%) | 3 (1%) |
| Other | 1 (1%) | 10 (4%) |
| ATG | ||
| No | 55 (73%) | 192 (77%) |
| Yes | 20 (27%) | 56 (23%) |
MDS/MPN: myelodysplastic syndrome/myeloproliferative neoplasm, URD: adult unrelated donor, UCB: umbilical cord blood, PBSC: peripheral blood stem cells, CSA: cyclosporine, MMF: mycophenolate mofetil, MTX: methotrexate, ATG: anti-thymocyte globulin
Acute GVHD characteristics: Late vs. Early onset Acute GVHD
The cumulative incidence of late aGVHD at one year was 14.7% (95% CI: 11.6-17.8), and early onset aGVHD was 49% (95% CI: 45-53). Amongst the 75 patients with late aGVHD, 39 (52%) had persistent and 29 (39%) recurrent, while only 7 (9%) had de-novo late aGVHD.
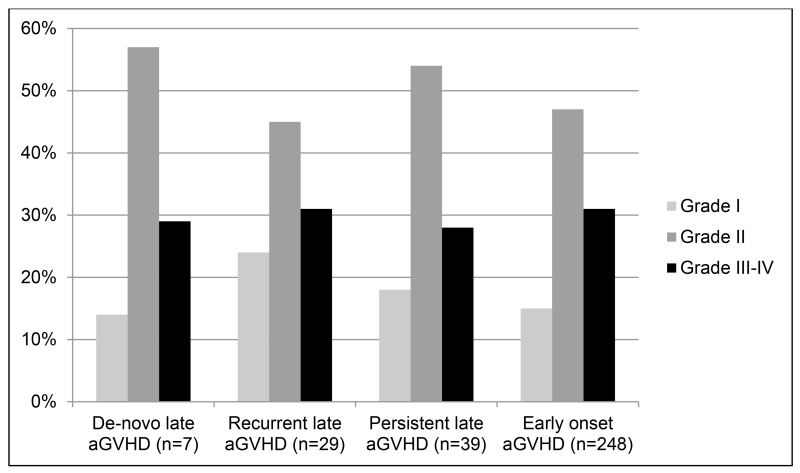
The median time from HCT to GVHD was 155 days (range: 100-262) for de-novo, 100 days for persistent, 143 days (range: 102-342) for recurrent late aGVHD compared to 34 days (range: 7-98 days) for early onset aGVHD. Figure 1A and 1B show the organ involvement and grade for each category of aGVHD. Skin involvement was less common in late aGVHD as compared to early onset aGVHD, whereas involvement of upper and lower GI tracts and liver was similar between the two cohorts. Most patients in each category developed grade II-IV aGVHD. Severe aGVHD (grade III-IV) was less common and was seen at a similar frequency in all categories. Among the 29 patients with recurrent late aGVHD, 4 patients developed the flare after being off all immune suppression (IS) for 2.3, 2.5, 3.5 and 6 weeks. Twelve patients had recurrence after being off systemic steroids (between 0.5 to 32 weeks). These 12 patients were still receiving CSA or Tac. Thirteen patients developed recurrent late aGVHD on tapering prednisone dosing ranging from 5mg every other day to 60mg daily.
Figure 1A. Organ involvement by Acute GVHD (any stage).
Figure 1B. Maximum Grade of Acute GVHD.
Risk Factors for Late Acute GVHD
In multivariable regression analysis, only the development of high grade (grade III-IV) early onset aGVHD was associated with higher risk of developing late aGVHD (HR:1.9, 95% CI: 1.2-3.1, p=0.01). Age, graft source or conditioning intensity had no independent effects on the risks of late aGVHD.
Response to GVHD therapy
Response was assessed at day 28 after initiation of steroids for de-novo and recurrent late aGVHD (n=36), and compared to response in a historical cohort of early onset aGVHD.16 Patients with persistent late aGVHD were excluded as response assessment from initiation of treatment could be prior to day +100 (at which point they were classified as persistent late aGVHD). Amongst 31 evaluable patients with de-novo or recurrent late aGVHD (3 had assessments outside the window period and 2 progressed to cGVHD), CR or PR was seen in 24 patients (77%) as compared to 65% in the historical cohort.16
With cGVHD, relapse and death as competing risks, 22 of the 75 late aGVHD patients had durable response to treatment without resumption of IS within 6 months of their discontinuation. The cumulative incidence of durable response was lowest in persistent (15%), followed by recurrent (41%) and de-novo (57%) late aGVHD.
Progression to Chronic GVHD
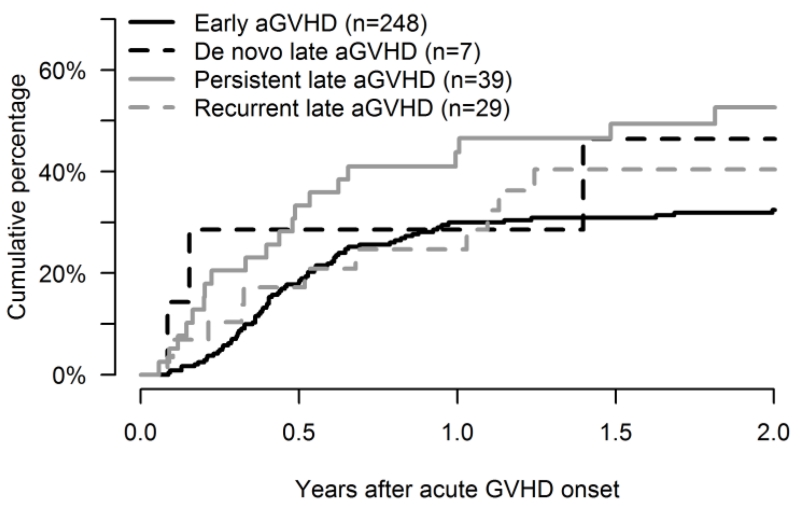
Overall, 48% (95% CI: 36%-60%) of patients with late aGVHD versus 31% (95% CI: 26-37) with early onset aGVHD developed cGVHD (Figure 2). Median time to onset of cGVHD was 175 days (range: 31-1192) after the onset of late aGVHD, and 158 days (range: 32-972) after early onset aGVHD. The cumulative incidence of cGVHD at 2 years after the onset of late aGVHD was the highest in persistent (53%, 95% CI: 36-70%), followed by 46% (95% CI: 5-91%) in de-novo, and 39% (95% CI: 20-58%) in recurrent late aGVHD (Table 2).
Figure 2. Progression to Chronic GVHD following onset of Acute GVHD.
Table 2. Outcomes 2 years following onset of Acute GVHD.
| De-novo Late aGVHD (n=7) |
Recurrent Late aGVHD (n=29) |
Persistent Late aGVHD (n=39) |
Early onset aGVHD (n=248) |
|
|---|---|---|---|---|
| Chronic GVHD (95% CI) | 46% (5-91) |
39% (20-58) |
53% (36-70) |
31% (26-37) |
|
Non-relapse mortality
(95%CI) |
0% | 18% (6-32) |
39% (21-57) |
29% (23-35) |
| Overall survival (95% CI) | 83% (27-97) |
74% (53-87) |
45% (28-60) |
50% (44-57) |
Survival and NRM
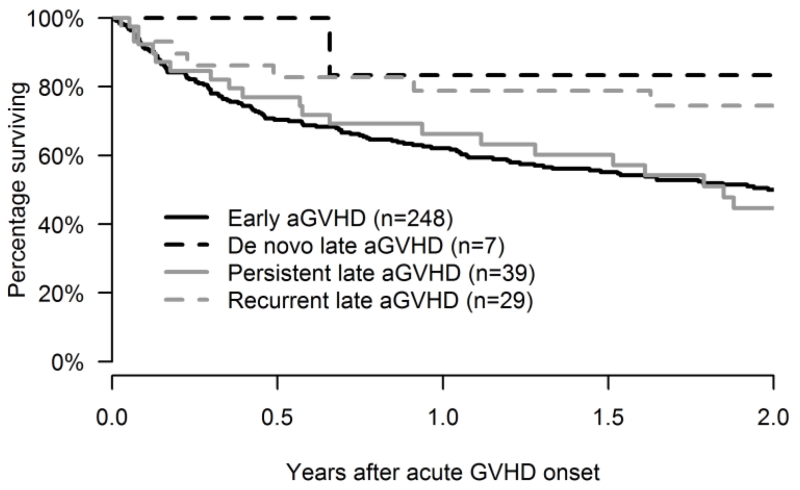
The probability of survival at 2 years after onset of late aGVHD was 59% (95% CI: 49%-72%) versus 50% (95% CI: 44%-57%) after onset of early onset aGVHD. The 2 year overall survival was the lowest in patients with persistent late aGVHD (45%, 95% CI: 28%-60%) and was notably better in patients with recurrent (74%, 95% CI: 53%-87%) and de-novo (83%, 95% CI: 27%-97%) late aGVHD (Table 2 and Figure 3).
Figure 3. Overall survival following onset of Acute GVHD.
The cumulative incidence of NRM at 2 years was 27% (95% CI: 16%-38%) in patients with late aGVHD versus 29% (95% CI: 23%-35%) in patients with early onset aGVHD. The cumulative incidence of NRM at 2 years was higher for persistent late aGVHD (39%, 95% CI: 21%-57%) followed by recurrent (18%, 95% CI: 6%-32%) and de-novo late aGVHD (0%) (Table 2). These outcomes were similar if only grades II-IV acute GVHD were analyzed.
Risk factors for NRM in Late Acute GVHD
Amongst patients with late aGVHD, compared to HLA-identical sibling donor HCT, the NRM was significantly higher with URD HCT (HR 6.1, 95% CI: 2.3-16.2, p<0.01), yet similar with UCB HCT (HR 1.0, 95% CI: 0.3-3.2, p=0.97). There was also a trend towards higher NRM in patients who developed grade III-IV versus less severe late aGVHD (HR: 2.0, 95% CI: 0.9-4.5, p=0.08). No other risk factors were associated with NRM.
Landmark analysis in +100 day survivors
To assess late mortality (after 100 days), we also performed a landmark analysis in late aGVHD versus early onset aGVHD. We evaluated 100 day survivors with early onset aGVHD (n=218) and included late aGVHD as a time dependent covariate in a proportional hazards regression. Among patients who developed early onset aGVHD and were alive at day 100, development of recurrent or persistent late aGVHD did not further increase their risk of death compared to early onset aGVHD patients who did not develop recurrent/persistent late aGVHD (HR: 0.96, 95% CI: 0.59-1.55, p= 0.85). However, in these 100 day survivors, the risk of cGVHD was nearly doubled (HR: 1.81, 95% CI: 1.16-2.82, p=0.01) following onset of late aGVHD.
To determine the effect of time of aGVHD onset from HCT on OS and NRM, we divided patients into quartiles (days 7-25, 26-34, 35-48 and >48 after HCT). Adjusting for age, type of conditioning, maximum grade of aGVHD and recipient CMV status, we found no association of time of aGVHD onset with OS, although there was a trend towards higher NRM with earlier onset (data not shown).
Discussion
Limited data are available regarding the clinical outcomes of late aGVHD following allogeneic HCT. Among our cohort, patients with persistent late aGVHD had the highest risk of progression to cGVHD, as well as a higher NRM and a poorer OS. Vigorito et al3 retrospectively looked at 740 myeloablative, mostly bone marrow transplant recipients, requiring systemic treatment for cGVHD and reclassified 352 as having late aGVHD according to 2005 NIH consensus criteria. No significant differences in terms of duration of systemic treatment, relapse, NRM or OS between classic cGVHD vs. late aGVHD was observed. As explained by the authors, this higher proportion of late aGVHD could possibly be explained by the higher number of patients with skin involvement being classified as late aGVHD - many of these patients were managed remotely from the HCT center (and hence could have missed recognition of subtle cGVHD skin changes). Our incidence of late aGVHD is slightly higher than recently reported by the cGVHD consortium (10%).17 This difference may be due to our inclusion of persistent late aGVHD which was not included in the consortium report.
We subcategorized our late aGVHD cohort into persistent, recurrent and de-novo subgroups and observed that the de-novo late aGVHD was infrequent in our cohort. This is, consistent with the recent cGVHD consortium report where amongst the 10% who developed late aGVHD, less than half developed de-novo late aGVHD.17 Our incidence of de-novo late aGVHD may also be slightly lower due to different patient populations. The consortium included more patients undergoing sibling and unrelated donor HCT, whereas our cohort included a higher proportion of patients undergoing UCB HCT.
In contrast to de-novo and recurrent late aGVHD, patients with persistent late aGVHD had more skin and lower GI tract involvement, similar to patients with early onset aGVHD. Our observed lower rate of durable responses (15%) in persistent late aGVHD is not unexpected, considering these are treatment refractory aGVHD patients, as opposed to the recurrent and de-novo categories. Among patients with late aGVHD, we observed a lower 2 year OS (45%) and higher 2 year NRM (39%) in the persistent late aGVHD cohort. This is in contrast to another study,2 which did not find worse OS, yet in a very small cohort- only 3 patients with persistent late aGVHD.
Progression to cGVHD was seen in a high proportion of our patients (48%). This is higher than reported recently by the cGVHD consortium.17 When evaluated in a landmark analysis, patients with late aGVHD in our cohort had a higher risk of cGVHD as compared to early onset aGVHD. This difference from the cGVHD consortium report is likely explained by the exclusion of persistent late aGVHD in the consortium study- a cohort with ongoing risks for cGVHD, as evidenced by a higher proportion of cGVHD in this subgroup in the current analysis.
Only advanced early onset aGVHD (grade III-IV) was associated with developing late aGVHD. This is similar to the cGVHD consortium report, which also identified no other risk factors for late aGVHD17 and differs from earlier reports of classic acute18-28 and cGVHD.19, 22-24, 26-31 Notably, nearly half of our patients were UCB recipients and the majority underwent RIC HCT. Due to concordance between graft source and assigned GVHD prophylaxis and conditioning, we could not separate the effect of GVHD prophylaxis on late aGVHD.
We found that late aGVHD contributes to post-transplant morbidity and mortality. Our data shows that de-novo and recurrent late aGVHD appear to be more responsive to therapy. On the other hand, high risk of progression to cGVHD and poorer survival in the persistent late aGVHD patients, warrant close monitoring and development of GVHD treatment trials targeting this group.
Footnotes
Authorship contributions
A.O and M.A proposed the study concept, analysis plan and wrote the manuscript; R.S performed statistical analysis and contributed to writing the manuscript; D.W, A.L, B.B, M.M, C.B and N.B contributed to data analysis and critical review of the manuscript.
Conflict of interest statement: No conflicts of interest to disclose.
Financial disclosure: The authors have nothing to disclose.
References
- 1.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Jagasia M, Giglia J, Chinratanalab W, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol Blood Marrow Transplant. 2007;13(10):1207–15. doi: 10.1016/j.bbmt.2007.07.001. See comment in PubMed Commons below. [DOI] [PubMed] [Google Scholar]
- 3.Vigorito AC, Campregher PV, Storer BE, et al. National Institutes of Health. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702–8. doi: 10.1182/blood-2009-03-208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora M, Nagaraj S, Witte J, et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone Marrow Transplant. 2009;43(2):149–53. doi: 10.1038/bmt.2008.305. [DOI] [PubMed] [Google Scholar]
- 5.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 7.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majhail NS, Brunstein CG, Shanley R, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant. 2012;47(4):494–8. doi: 10.1038/bmt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–7. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 11.MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761–7. doi: 10.1016/j.bbmt.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalbfleish JD, Prentice RL. The Statistical Analysis of Failure Time Data. Wiley; New York: 1980. [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 14.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society. 1972;34(2):187–220. [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16.MacMillan ML, DeFor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157(6):732–41. doi: 10.1111/j.1365-2141.2012.09114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora M, Cutler CS, Jagasia MH, et al. Late Acute and Chronic Graft-Versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015 Nov 2; doi: 10.1016/j.bbmt.2015.10.018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisner MD, August CS. Impact of donor and recipient characteristics on the development of acute and chronic graft-versus-host disease following pediatric bone marrow transplantation. Bone Marrow Transplant. 1995;15(5):663–668. [PubMed] [Google Scholar]
- 20.Hahn T, McCarthy PL, Jr., Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26(35):5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft versus host disease and for chronic graft versus host disease according to National Institute of Health consensus criteria. Blood. 2011;117(11):3214–9. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisdorf D, Hakke R, Blazar B, et al. Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation. 1991;51(6):1197–1203. doi: 10.1097/00007890-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graftversus-host disease after allogeneic peripheral blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19(16):3685–3691. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]
- 24.Flowers ME, Pepe MS, Longton G, et al. Previous donor pregnancy as a risk factor for acute graft versus-host disease in patients with aplastic anaemia treated by allogeneic marrow transplantation. Br J Haematol. 1990;74(4):492–496. doi: 10.1111/j.1365-2141.1990.tb06340.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 26.Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10(3):178–85. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Sorror ML, Maris MB, Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104(4):961–8. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- 28.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant. 2007;13(12):1461–1468. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SJ, Vogelsang G, Flowers ME. Chronic graft versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 30.Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT) Blood. 2000;95(12):3702–9. [PubMed] [Google Scholar]
- 31.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]