Abstract
Colorectal cancer (CRC) is a leading cause of cancer incidence and mortality in the U.S. Notwithstanding major improvements in the early detection and treatment of CRC, an important proportion of patients diagnosed with localized disease ultimately recur and die, underscoring the need of new therapeutic approaches. Vitamin D and physical activity (PA) have emerged as two potential interventions for both prevention and treatment of CRC. Plausible biological mechanisms have been described for the anti-neoplastic effects of vitamin D and PA, and a wealth of epidemiological evidence indicates that 25(OH)D (the main circulating form of vitamin D) and PA levels are inversely associated with CRC risk. Recent efforts have now focused on the role of vitamin D and PA as adjunct treatments after a CRC diagnosis. Observational studies evaluating pre- and post-diagnosis circulating 25(OH)D levels among patients with CRC of all stages have found that subjects with levels in the highest quantiles have improved overall and CRC-specific survival compared with those with levels in the lowest quantiles. Similarly, prospective studies of PA have found that higher levels of post-diagnosis PA are associated with lower overall and CRC-specific mortality in patients with non-metastatic CRC. Meta-analyses of the observational studies of 25(OH)D and post-diagnosis PA have confirmed significant protective associations against overall and CRC-specific mortality as well as significant dose-response relationships. No randomized, controlled trial of vitamin D or PA using survival outcomes as endpoints has been completed to date. Two randomized, placebo-controlled trials of vitamin D in patients with metastatic CRC assessing patient survival as an endpoint are underway: the first is a phase II trial comparing high-dose vitamin D3 (8000 IU day-1 for two weeks followed by 4000 IU day-1) versus standard dose (400 IU day-1), and the second is a phase I-II trial comparing customized oral doses of vitamin D3 titrated to raise serum 25(OH)D levels to 80-100 ng mL-1, versus 2000 IU day-1. For PA, the ongoing phase III Colon Health and Life-Long Exercise Change (CHALLENGE) study is the first randomized, controlled trial using survival as an endpoint among patients with stage II-III colon cancer. The results of these trials will pave the way to more conclusive phase III trials that will provide more definitive answers about the role of these interventions in the treatment of CRC. Lastly, the advent of genomic technologies will allow identifying molecular signatures in CRC associated with improved response to vitamin D and PA and will usher in a precision medicine approach to these therapies.
Keywords: Colorectal cancer, metastatic, vitamin D, physical activity, clinical trials
Colorectal cancer (CRC) is the fourth most common cancer in men and women combined in the U.S., with an estimated 134,490 new cases predicted to be diagnosed in 2016, and the second leading cause of cancer-related death in men and women, expected to cause approximately 49,190 deaths in 2016 (1). Improvements in early detection and treatment have contributed to a steady decline in CRC mortality in recent years (1). However, one fifth of CRC patients have metastatic disease at the time of diagnosis, with only 13% five-year survival (2), and a significant proportion of patients diagnosed with localized disease ultimately recur and die. Consequently, new treatment approaches are urgently needed to improve the prognosis of these patients.
Recent improvements in the treatment and supportive care of patients with metastatic disease have been considerable, and have allowed these patients to live on average more than two years after diagnosis (3). The combination of high incidence and improved survival has resulted in a large number of patients living chronically with CRC, along with a new set of challenges associated with cancer as a chronic illness. Cancer survivors have become more active in their care and often inquire what lifestyle changes they can implement to improve their outcomes. There is ample evidence that modifiable factors like obesity, physical exercise, smoking, a diet high in red and processed meats, and very low in whole-grain fiber, fruit, and vegetables increase the risk of developing CRC (1). Growing research efforts have more recently focused on the role of these factors after a patient has received a diagnosis of CRC, and whether they can impact recurrence rates and survival outcomes. In this review, we will discuss the current evidence supporting the role of vitamin D status and physical activity (PA) in CRC. We will focus predominantly on the influence of these two factors on the survival of patients diagnosed with CRC and their potential application as adjunctive treatments for patients with metastatic disease.
Vitamin D
Background
Vitamin D is a fat-soluble vitamin that can be obtained from multiple sources. It can be synthesized in the skin after exposure to ultraviolet (UV)-B radiation from sunlight, ingested from dietary sources, or obtained from vitamin D supplements. Vitamin D from these sources is in the form of vitamin D3 and undergoes enzymatic hydroxylation by 25-hydroxylase (CYP2R1) in the liver to form 25-hydroxyvitamin D (25[OH]D), the main circulating form of vitamin D and the best indicator of vitamin D status (4). 25(OH)D undergoes a second hydroxylation step in the kidney by 1α-hydroxylase (CYP27B1) to yield its active form 1,25-dihydroxyvitamin D (1,25[OH]2D) or calcitriol, which binds to the vitamin D receptor (VDR) in the cell nucleus. Bound VDR forms a heterodimer with the retinoic-acid receptor (RXR) and this complex binds to vitamin D response elements to regulate target gene expression.
The study of vitamin D has focused predominantly on its role in bone health as a regulator of calcium and phosphate metabolism. However, studies over the last two decades have observed that both 1α-hydroxylase and VDR can be found in virtually all human cells, (5) including CRC cells (6). In vitro and in vivo CRC models have demonstrated that vitamin D has important antineoplastic effects: it induces differentiation and inhibits growth in cell lines and xenografts (7,8), reduces the size of intestinal adenomas in ApcMin mice (9), and heightens cellular sensitivity to 5-FU (10). While the exact underlying mechanism by which vitamin D exerts these effects on CRC is not clear, several potential pathways have been proposed. There is evidence that vitamin D is an inductor of apoptosis (11), counteracts aberrant WNT-β catenin signaling (involved in the etiology of CRC) (12), has broad anti-inflammatory effects via down-regulation of nuclear factor (NF)-κB and inhibition of cyclooxygenase (COX) expression (13), and is an inhibitor of proliferation (14), angiogenesis (15), and metastatic potential (16). Recent studies have observed that vitamin D upregulates the expression of the calcium-sensing receptor (CaSR), which plays an important role in cell proliferation and has been proposed as a mediator of the antineoplastic effects of vitamin D (17).
The activity of vitamin D in the laboratory is largely compatible with epidemiological evidence in CRC. Several ecological and prospective observational studies have consistently revealed an inverse association between plasma 25(OH)D levels and risk of developing CRC (18-21). Conversely, the results of randomized, placebo-controlled trials of vitamin D supplementation have been conflicting. The Women's Health Initiative (WHI) trial (22), a study in healthy, post-menopausal women from Nebraska (23), and a trial among elderly men and women at risk for fractures in the United Kingdom (UK) (24) revealed contrasting results using vitamin D3 400 IU day-1, 1100 IU day-1, and 100,000 IU every four months, respectively. The Nebraska study (23) found a significant 60% decrease in all-cancer risk among participants in the vitamin D supplementation arm. Conversely, the WHI(22) and UK(24) studies obtained largely null results; nevertheless, it was observed that women with the highest baseline 25(OH)D levels in the WHI study experienced a significant 60% reduction in CRC risk (P trend = 0.02). These studies should be interpreted with caution because CRC incidence was not the primary endpoint. A trial of vitamin D3 1000 IU day-1 using recurrent adenoma as the primary endpoint was recently completed with null findings, (25) but it remains unclear what the clinical significance of recurrent diminutive adenoma is; moreover, the vitamin D3 dose and the duration of treatment and follow-up (3 to 5 years) might have been insufficient to see a meaningful effect. The Vitamin D and Omega-3 Trial (VITAL) phase III trial (ClinicalTrials.gov identifier NCT01169259) is an ongoing study using vitamin D3 2000 IU day-1 that will evaluate cardiovascular disease and different cancer types, including CRC, as primary endpoints. VITAL is due to be completed in late 2017.
An important methodological difference between these trials is the dose of vitamin D used in the study. Three out of every four Americans is currently vitamin D insufficient (26), and there is an ongoing debate about where the threshold for optimal level should be set and what dose of supplemental vitamin D is needed to reach it. The Institute of Medicine (IOM) and the Endocrine Society have issued their respective recommendations, with notable discrepancies (27,28). The IOM considers circulating 25(OH)D levels of 20 to 50 ng mL-1 as safe and effective (focused mostly on adequate levels for optimal bone health), while the Endocrine Society recommends levels ≥30 ng mL-1. As for vitamin D supplementation, the IOM recommends total vitamin D intake of 600 IU and 800 IU day-1 for people below and over age 70, respectively, while the Endocrine Society considers a daily intake of 1500-2000 IU day-1 to be adequate for all adults irrespective of age (27,28).
The wealth of experimental and epidemiological evidence supporting a protective effect of vitamin D against development of CRC has motivated observational studies and clinical trials to assess whether the antineoplastic effects of vitamin D translate into improved recurrence and survival outcomes among CRC survivors.
Observational studies
Several prospective studies have evaluated the role of pre- and post-diagnosis vitamin D status on the prognosis of CRC patients (4,29-37). The majority of these studies have used direct measurements of circulating 25(OH)D in the plasma or serum, while a few others have estimated vitamin D status based on known clinical predictors (Table 1). These studies vary greatly in size, disease stage, and timing of exposure assessment.
Table 1.
Prospective observational studies evaluating the influence of vitamin D status on survival among patients diagnosed with CRC.
| Number of CRC cases | Clinical stages | Exposure evaluated | Timing of exposure evaluation | All-cause mortality | CRC-specific mortality | |
|---|---|---|---|---|---|---|
| NHS/HPFS29 | 304 | I-IV | Plasma 25(OH)D | 6 years prior to CRC diagnosis (mean) | HR 0.52a (95% CI 0.29, 0.94) P trend = 0.02 | HR 0.61a (95% CI 0.31, 1.19) P trend = 0.23 |
| NHS/HPFS-score4 | 1017 | I-IV | Vitamin D scoreb | 1-4 years after CRC diagnosis | HR 0.62a (95% CI 0.42, 0.93) P trend = 0.002 | HR 0.50a (95% CI 0.26, 0.95) P trend = 0.02 |
| Japanese cohort30 | 257 | I-IV | Serum 25(OH)D | At the time of surgery | HR 0.16a (95% CI 0.04, 0.63) P = 0.0009 | - |
| NCCTG 9741 trial31 | 515 | IV | Plasma 25(OH)D | Before first-line chemotherapy | HR 0.94a (95% CI 0.72, 1.23) P trend = 0.55 | - |
| EPIC cohort32 | 1202 | I-IV | Serum 25(OH)D | 3.8 years prior to CRC diagnosis (mean) | HR 0.67a (95% CI 0.50, 0.88) P trend <0.01 | HR 0.69a (95% CI 0.50, 0.93) P trend = 0.04 |
| JANUS cohort33 | 52 | I-IV | Serum 25(OH)D | 37 days after CRC diagnosis (median) | HR 0.40a (95% CI 0.10, 1.60) P trend = 0.23 | HR 0.20a (95% CI 0.04, 1.10) P trend = 0.16 |
| SOCCS cohort34 | 1598 | I-III | Plasma 25(OH)D | 105 days after surgery for CRC (median) | HR 0.70a (95% CI 0.55, 0.89) P trend = 0.003 | HR 0.68a (95% CI 0.50, 0.90) P trend = 0.009 |
| CPS-II cohort35 | 2284 | I-III | Dietary plus supplemental vitamin D3 | Pre- and post-CRC diagnosis | RR 0.88a,c (95% CI 0.57, 1.35) P trend = 0.35 | RR 1.74a (95% CI 0.80, 3.77) P trend = 0.52 |
| CPRD cohort36 | 4122 | Not specified | Supplemental vitamin D3 | Within 5 years prior to CRC diagnosis | - | HR 0.90d (95% CI 0.78, 1.04) |
| CALGB 80405 trial37 | 1043 | IV | Plasma 25(OH)D | Before first-line chemotherapy | HR 0.67a (95% CI 0.53, 0.86) P trend = 0.002 | HR 0.80a,e (95% CI 0.64, 1.01) P trend = 0.02 |
Effect estimate comparing subjects in the highest versus the lowest quantile of the assessed exposure.
Calculated based on race, geographical region of residence, dietary and supplemental vitamin D intake.
Total post-diagnosis vitamin D (dietary plus supplemental).
Any number of vitamin D prescriptions versus no vitamin D prescriptions.
Progression-free survival
CRC=Colorectal cancer; HPFS=Health Professionals Follow-Up Study; EPIC=European Prospective Investigation into Cancer and Nutrition; SOCCS=Study of Colorectal Cancer in Scotland; NCCTG=North Central Cancer Treatment Group; CALGB=Cancer and Leukemia Group B; CPS II=Cancer Prevention Study II Nutrition Cohort; CPRD=UK Clinical Practice Research Datalink.
Among the two studies that measured circulating pre-diagnosis 25(OH)D directly (29,32), 25(OH)D levels in the highest quantiles were associated with a 33%-48% lower risk of all-cause mortality and 31%-39% lower risk of CRC-specific mortality (although the latter was statistically significant in only one study[32]) compared with patients with 25(OH)D levels in the lowest quantiles (29,32). The effect estimates vary more widely among the five studies with direct post-diagnostic measurements. Patients with post-diagnosis 25(OH)D levels in the highest quantiles had a 6%-84% lower risk of all-cause mortality (statistically significant in three of five studies) (30,34,37) and a 20%-80% lower risk of CRC-specific mortality (statistically significant in only one of three studies) (34) compared with patients with circulating levels in the lowest quantiles (30,31,33,34,37). A potential explanation for the inconsistent associations seen with pre- and post-diagnosis 25(OH)D assessments may lie in the heterogeneity of the study populations. In contrast with cohorts evaluating pre-diagnosis 25(OH)D levels, the patients in the studies of post-diagnosis 25(OH)D differ with regards to disease stage and treatment status. Two studies (31,37) were conducted in the setting of randomized, controlled trials of first-line chemotherapy for patients with metastatic CRC. Cohort studies nested within clinical trials offer several advantages over traditional prospective observational cohort studies: the baseline characteristics of the study population – including disease stage and treatment history –are more homogeneous at baseline; treatment regimens are controlled after randomization; and patients receive frequent and comprehensive follow-up evaluations that may include assessment of disease recurrence or progression. In both nested studies, plasma 25(OH)D levels were measured before the start of first-line chemotherapy and an inverse association was observed between higher levels of 25(OH)D and mortality, although this association was statistically significant in only one study (37), revealing a 33% lower risk of all-cause mortality and improved progression-free survival (PFS; HR 0.80, 95% CI 0.64-1.01, P trend = 0.02). Importantly, some of these studies (4,29,32,37) conducted sensitivity analyses excluding patients who died shortly after the 25(OH)D measurement to control for reverse causation, whereby worsening disease potentially causes a decrease in circulating 25(OH)D levels. The observed results remained largely unchanged after these exclusions.
Recent meta-analyses (21,38) of prospective studies using direct serum or plasma 25(OH)D measurements corroborate the inverse association between higher 25(OH)D and mortality risk. The first meta-analysis (21) included five of the studies discussed above (29-33) and yielded a pooled HR of 0.61, 95% CI 0.55-0.91 for all-cause mortality and HR 0.65, 95% CI 0.49-0.86 for CRC-specific mortality comparing the highest vs. lowest quantiles of 25(OH)D levels. A second meta-analysis (38) evaluated three of the discussed prospective studies (29,32,33), obtaining similar results (CRC-specific mortality HR 0.63, 95% CI 0.5-0.8, P <0.0001 comparing the highest vs. the lowest levels of circulating 25[OH]D). Both studies found an inverse dose-response relationship between increasing circulating 25(OH)D levels and CRC-specific mortality (21,38). Meta-analyses have the advantage of calculating a pooled summary effect estimate despite methodological differences in the individual studies (e.g. different categories of exposure classification) using a large number of subjects, thus increasing the precision of the results (21,38).
Given the limited data sets with available plasma to measure 25 (OH)D levels, a predictive vitamin D score was developed as an indirect marker of vitamin D status (39). The score incorporates factors that influence vitamin D levels in the body, including race (as a surrogate for skin pigmentation), geographic region of residence (as a surrogate for UV-B exposure), body mass index, PA, and dietary and supplemental vitamin D intake. Predicted vitamin D score after a diagnosis of stage I-IV CRC was associated with a significantly lower risk of all-cause and CRC-specific mortality (38% and 50% lower risk, respectively) (4).
Recent studies have also evaluated the association of dietary and supplemental vitamin D with survival outcomes in CRC. Yang et al (35) assessed the role of pre- and post-diagnosis dietary plus supplemental vitamin D intake among 2,284 patients with non-metastatic CRC, and Jeffreys et al (36) studied the effect of pre-diagnosis vitamin D supplements prescriptions in the United Kingdom (UK). Both studies failed to find a significant protective association. This discrepancy with the results observed in studies of circulating 25(OH)D may stem from several reasons. Dietary and supplemental intake of vitamin D are only one source of this vitamin and are unlikely to provide an accurate reflection of circulating 25(OH)D status. Moreover, the amount of vitamin D3 contained in the diet and multivitamins is most likely not sufficient to significantly increase serum levels – in the WHI study referred to previously, vitamin D3 400 IU day-1 increased plasma 25(OH)D levels by only 2-3 ng mL-1 among healthy women (22). On the other hand, the study from the UK (36) lacked information about vitamin D supplements bought over the counter, dose of the prescribed supplements, and treatment adherence, all of which could potentially bias the observed results.
A pilot study (40) in patients with stage II-IV CRC found that vitamin D supplementation can effectively raise serum 25(OH)D to levels ≥30 ng mL-1 after six months of treatment. Among 50 patients with mean baseline serum 25(OH)D of 17.5 ng mL-1, vitamin D3 2000 IU day-1 for six months increased serum levels by 16.2 ng mL-1 on average. Interestingly, the study noted that the response to vitamin D supplementation was diminished among patients undergoing chemotherapy. The reasons behind this discrepancy can be manifold, including impaired vitamin D3 absorption as a result of chemotherapy-related enteritis, increased catabolism of 25(OH)D, and an accentuated systemic inflammatory state (40). Nevertheless, it suggests that while a vitamin D3 dose of 2000 IU day-1 may restore sufficiency among CRC patients overall, patients receiving chemotherapy likely need higher doses to reach sufficiency. Rigorously designed randomized, controlled trials are needed to establish what the optimal dose and duration of treatment are in order to achieve vitamin D sufficiency among CRC patients, and whether supplementation and preservation of sufficiency can positively impact tumor recurrence and survival rates.
Clinical trials
No randomized, placebo-controlled trial of vitamin D among patients with CRC has been completed to date. Two randomized, placebo-controlled trials are currently evaluating the impact of vitamin D3 supplementation in patients with metastatic CRC. The first one is a double-blind, phase II trial (ClinicalTrials.gov identifier NCT01516216) (Figure 1) comparing high-dose vitamin D3 (8000 IU day-1 for two weeks, followed by 4000 IU day-1) versus standard dose (400 IU day-1) in combination with standard FOLFOX + bevacizumab chemotherapy for patients with previously untreated metastatic CRC. The primary endpoint is PFS, and secondary endpoints include OS, objective tumor response rate, safety, and incidence of vitamin D deficiency. This trial aims to enroll 140 participants and is actively recruiting patients in multiple centers across the US. The second trial is a phase I-II study taking place in Canada (ClinicalTrials.gov identifier NCT01150877) among patients with metastatic CRC. Unlike the trial discussed previously, this study does not use a fixed predetermined vitamin D3 supplemental dose and includes patients in all lines of therapy. Patients randomized to the experimental arm receive customized oral doses of vitamin D3 titrated to raise serum 25(OH)D levels to 80-100 ng/mL, while controls receive a standard dose of at least 2000 IU day-1. The primary goal is to evaluate the tolerability and toxicity of the intervention, and the secondary endpoint is the survival impact of the treatment; both will be assessed 16 months after the intervention, and then after 12 months of follow-up. This trial is designed to enroll 40 patients and is actively recruiting participants.
Figure 1.
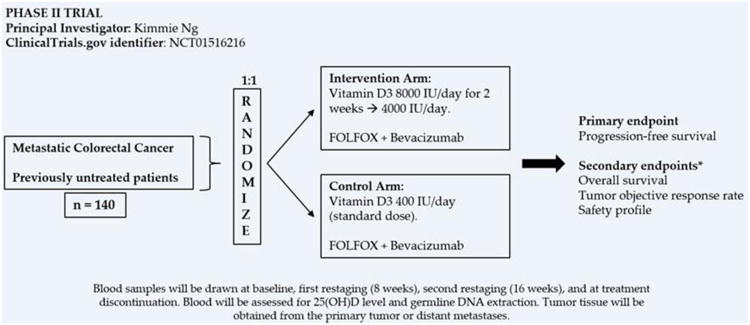
Phase II study of vitamin D in untreated metastatic colorectal cancer. *Among other secondary endpoints.
In addition to assessing the survival impact of vitamin D supplementation, it is critical to unveil the underlying biological mechanisms through which vitamin D exerts its antineoplastic effects on CRC. With this objective in mind, a pilot study being conducted as part of the Dana-Farber/Harvard Cancer Center SPORE in Gastrointestinal Cancer (ClinicalTrials.gov identifier NCT02172651) seeks to identify the transcriptional targets of vitamin D and VDR in CRC patients undergoing surgery for stage I-III colon cancer or resectable liver metastases. Participants will be randomized to receive high-dose vitamin D3 (50,000 IU day-1 for seven days, followed by 10,000 IU day-1 until the time of surgery) versus placebo for 14-28 days prior to surgery. VDR binding sites and vitamin D-responsive transcripts will then be determined from malignant and healthy colon and liver tissue harvested during surgery. The characterization of the key molecular pathways driving the antineoplastic effects of vitamin D is essential to understand the mechanisms of susceptibility and resistance to vitamin D, and will pave the way for the development of effective and individualized treatment modalities.
Physical Activity
Background
Physical inactivity is an established risk factor for the development of CRC (1). Active females and males have a 29% and 22% reduced risk of CRC based on a meta-analysis of 19 cohort studies, respectively (41). Moreover, PA has been shown to inhibit primary tumor growth in animal models of breast and prostate adenocarcinoma, sarcoma, and hepatoma (42). Several hypotheses have been proposed to explain the underlying protective mechanism. Metabolic changes associated with lower levels of insulin and insulin-like growth factor-1 (IGF-1), decreased systemic inflammation, a reduction of central adiposity, and changes in the gut flora are all potential mediators of the action of PA on CRC (43). Recent experimental evidence from in vitro and in vivo studies has shed new light on how PA may impact CRC biology. McClellan et al (44) observed that exercise led to a reduction in the number of large intestinal polyps and significantly influenced immune cell parameters in mucosal tissue of Apcmin/+ mice, decreasing the expression of macrophage and regulatory T cells markers, and increasing that of cytotoxic T cells. In another study, Aoi et al (45) identified a new myokin, secreted protein acidic and rich cysteine (SPARC) which expression is increased in skeletal muscle of both mice and humans in response to exercise and may represent a biological link between PA and inhibition of colon tumorigenesis. SPARC displayed anti-proliferative and pro-apoptotic effects in colon cancer cells in vitro. Moreover, regular exercise caused a significant reduction in the number of aberrant crypt foci and induced apoptosis of mucosal cells in wild-type mice, but not in SPARC-null mice, suggesting that SPARC is a mediator of the antineoplastic effects of PA in the colon.
Observational studies
The role of different levels of PA before and after a diagnosis of CRC on survival outcomes has been examined in several prospective cohorts. A recent meta-analysis (46) has found that both pre- and post-diagnosis PA is inversely associated with all-cause and CRC-specific mortality. Given that this review concentrates on potential therapeutic applications after a CRC diagnosis, we will focus our discussion on post-diagnosis PA, which can be acted upon by cancer survivors.
Between 2006 and 2015, seven prospective studies have assessed the impact of post-diagnosis PA levels on the prognosis of CRC survivors (43,47-52) (Table 2). All of these studies were limited to patients with non-metastatic disease in order to control for the negative impact of metastatic disease on PA. In contrast to observational studies of circulating 25(OH)D, all of these studies were fairly large, with each one enrolling more than 500 individuals. All studies demonstrated a statistically significant decrease in all-cause mortality comparing subjects with the highest levels of PA to those with the lowest PA levels. The reduction in risk of all-cause mortality ranged from 25% to 63%. Similarly, CRC-specific mortality was lower among those with higher levels of PA, and this was statistically significant in four of the six studies that reported it (47,48,50,52). Among three studies (43,47,48) using similar categories of PA measured in MET-hr/week, a total of ≥18 to ≥27 MET-hr/week (corresponding to ≥6 to ≥9 hours per week of walking at an average pace [43]) was associated with reduction in all-cause mortality risk. One of the biggest challenges with studies of PA and CRC is the potential for reverse causation: patients who are able to exercise may have more favorable disease and hence improved outcomes. To address this issue, all the observational studies discussed here adjusted for favorable prognostic factors (e.g. age, disease stage, tumor grade, treatment status, smoking history). In addition to post-diagnosis PA, two studies (47,49) also assessed pre-diagnosis PA and whether the change in PA levels before and after diagnosis had an impact on survival. The consideration of pre-diagnosis PA is highly relevant in these studies, given that patients with high levels of pre-diagnosis PA may develop less aggressive and more favorable tumors and thus be better able to exercise. However, neither study found a protective effect of pre-diagnosis PA, although changes in PA levels from pre-diagnosis to post-diagnosis were associated with improved outcomes in both cohorts. One study observed that subjects who increased their PA levels after CRC diagnosis had a significantly lower risk of all-cause (HR 0.48, 95% CI 0.24, 0.79) and CRC-specific mortality (HR 0.51, 95% CI 0.30, 0.85), and the benefit was similar among subjects with relatively low and high PA levels at baseline (47). Baade and colleagues (49) found that changes between pre-diagnosis PA and PA levels five months after diagnosis did not decrease mortality risk; however, an increase of PA by more than two hours between five and 12 months after diagnosis was significantly associated with lower all-cause (HR 0.69, 95% CI 0.50, 0.94) and CRC-specific mortality (HR 0.64, 95% CI 0.44, 0.93) compared with subjects whose PA levels did not change. The reason for changes in PA levels is unknown in both studies. It is possible that patients who increased their PA levels were healthier and hence more likely to exercise, and their improved prognosis was not a direct effect of PA. However, this is unlikely since both studies observed a survival benefit of increased PA level after adjusting for known predictors of survival like clinical stage, tumor differentiation, and treatment status (47,49).
Table 2.
Prospective observational studies assessing the effect of post-diagnosis physical activity on survival among CRC survivors.
| Number of CRC casesa | Clinical stages | Exposures evaluated | PA classification categories | Time of post-diagnosis PA assessment | All-cause mortalityb | CRC-specific mortalityb | |
|---|---|---|---|---|---|---|---|
| Meyerhardt et al CALGB 8980343 | 832 | III | Post-diagnosis | <3, 3-8.9, 9-17.9, 18-26.9, ≥27 MET-hour/week | 7.1 months after completion of adjuvant treatment (median) | HR 0.37, 95% CI 0.16, 0.82, P trend = 0.01 | HRd 0.55, 95% CI 0.33, 0.91, P trend = 0.01 |
| Meyerhardt et al NHS47 | 554 | I-III | Pre- and post-diagnosis | <3, 3-8.9, 9-17.9, ≥18 MET-hour/week | ≥1 year but ≤4 years after CRC diagnosis (median 22 months) | HR 0.43, 95% CI 0.25, 0.74, P trend = 0.003 | HR 0.39, 95% CI 0.18, 0.82, P trend = 0.008 |
| Meyerhardt et al HPFS48 | 661 | I-III | Post-diagnosis | ≤3, 3.1-9, 9.1-18, 18.1-27, >27 MET-hour/week | ≥6 months but ≤4 years after CRC diagnosis (median 15 months) | HR 0.59, 95% CI 0.41, 0.86, P trend <0.001 | HR 0.47, 95% CI 0.24, 0.92, P trend = 0.002 |
| Baade et al49 | 1,825 | I-III | Pre- and post-diagnosis | 0, 1-149, ≥150 minutes/week | 5 months after CRC diagnosis | HR 0.75, 95% CI 0.60, 0.94, P trend = 0.007 | HR 0.88, 95% CI 0.68, 1.15, P trend = 0.585 |
| Kuiper et al50 | 606 | I-III | Pre- and post-diagnosis | Quartiles of MET-hour/week | 1.5 years after CRC diagnosis (median) | HR 0.41, 95% CI 0.21, 0.81, P trend = 0.005 | HR 0.29, 95% CI 0.11, 0.77, P trend = 0.02 |
| Campbell et al51 | 1,932 | I-III | Pre- and post-diagnosis | <3.5, 3.5-8.74, ≥8.75 MET-hour/week | 1.4 years after CRC diagnosis (median) | HR 0.58, 95% CI 0.47, 0.71 | HR 0.87, 95% CI 0.61, 1.24 |
| Arem H et al52 | 1,759 | I-III | Pre- and post-diagnosis | 0, <1, 1-3.9, 4-6.9, ≥7 hours/week | 4.2 years after CRC diagnosis (median) | HR 0.69, 95% CI 0.49, 0.98, P trend = 0.006 | HR 0.53, 95% CI 0.27, 1.03, P trend = 0.041 |
Number of cases included in the post-diagnosis physical activity analysis.
Effect estimate comparing subjects in the highest versus the lowest category of the assessed exposure.
After completion of the post-exposure assessment.
Effect estimate assessing disease-free survival, defined as time free of tumor recurrence, occurrence of a new primary colon tumor, or death as a result of any cause.
CRC=Colorectal cancer; PA=Physical activity; MET: Metabolic Equivalent of Task; NHS=Nurses Health Study; CALGB=Cancer and Leukemia Group B; HPFS=Health Professionals Follow-Up Study.
A meta-analysis (53) of six of the above studies found that higher levels of post-diagnosis PA had a significant protective effect on all-cause and CRC-specific mortality. Compared with the lowest categories, higher levels of PA were associated with a 42% lower risk of all-cause mortality (RR 0.58, 95% CI 0.48, 0.70) and 39% lower risk of CRC-specific mortality (RR 0.61, 95% CI 0.40, 0.92). Furthermore, a dose-response association was observed whereby increases in post-diagnosis MET-h/week were associated with decreased risk of both all-cause and CRC-specific mortality.
Recent efforts have focused on identifying molecular and metabolic features of CRC that may influence the effects of PA on survival, which would provide strong evidence of a causal link. Observational studies in CRC patients from two prospective cohorts (Nurses' Health Study [NHS] and Health Professionals Follow-Up Study [HPFS]) have investigated the interactions of tumor molecular alterations (KRAS, PIK3CA, BRAF, CpG island methylator phenotype, and microsatellite instability) and protein expression patterns (fatty acid synthase, p53, p21, CDKN1B [p27], CTNNB1 [β-catenin], PTGS2 [COX-2], and insulin receptor substrate 1 [IRS1]) with post-diagnosis PA and its association with patient outcomes (54-57). The results of these studies suggest that p27, β-catenin, COX-2, and IRS1 expression significantly modifies the association of post-diagnosis PA with CRC-specific survival. Patients with high levels of post-diagnosis PA harboring tumors demonstrating positive expression of p27 and COX-2, or negative/low expression of β-catenin and IRS1 had significantly improved CRC-specific survival. None of the other protein or molecular markers significantly modified the impact of PA on survival outcomes. The relevance of these studies is enormous: in addition to shedding light on the mechanisms underlying the impact of PA on CRC biology, they represent the first steps towards a precision medicine approach to identify subsets of patients that will derive the greatest benefits from PA interventions using protein expression and molecular signatures.
Clinical trials
Nine pilot randomized clinical trials have been performed to assess the feasibility and efficacy of PA interventions among CRC survivors, though the endpoints have primarily been focused on quality of life and fitness (58-66). These studies vary widely in their sample size, type of intervention, and endpoints. Patients with all disease stages and at different points of treatment were included. Once an important concern for clinicians and patients, it has been shown that aerobic and resistance exercise is safe and does not increase the risk of adverse events during and after chemotherapy in cancer survivors (62,67). Notwithstanding the fact that none of these studies assessed the impact of PA on disease recurrence or survival, they have provided valuable insight about its effects among CRC survivors and laid the foundations for future trials focused on survival outcomes.
One of the main challenges of interventions focused on increasing PA levels is consistent and durable adherence (62). The adherence observed in the trials discussed here was excellent, ranging from 76-91% (the estimated adherence among cancer survivors in general lies between 60-70%) (61,68). This is remarkable, since it shows that these interventions are capable of engaging participants and keeping them motivated throughout the trial. In fact, one of the challenges encountered in these trials was “contamination” of the control group, whereby participants assigned to control arms of the study started to exercise. This was prominent in two trials (59,61) where slightly more than half of the controls were exercising. In addition to providing evidence of the safety and feasibility of PA interventions among CRC survivors, these trials revealed that PA is associated with improvements in cardiorespiratory fitness, physical functioning, mobility, fatigue, sleep quality, and circulating markers of insulin resistance.
Observational evidence suggests a link between increased levels of IGF-binding protein-3 (IGBBP-3) and improved outcomes in metastatic CRC, indicating potential response mediators and targets for intervention (69). An ongoing multicenter, randomized phase II trial of supervised PA and metformin in stage I-III CRC survivors that have completed standard adjuvant treatment will further elucidate the relationship between PA and the insulin and IGF axes in CRC (ClinicalTrials.gov identifier NCT01340300). Metformin is a biguanide drug used for the treatment of type II diabetes that reduces insulin levels through suppression of hepatic gluconeogenesis, and its use has been associated with decreased cancer-specific mortality (70). This trial aims to determine whether PA and metformin (alone or in combination) can decrease fasting insulin levels within three months of intervention; secondary endpoints include changes in additional insulin-related biomarkers, body mass index, and pro-inflammatory markers.
The first randomized, controlled trial designed primarily to assess the impact of PA on survival among colon cancer survivors is the Colon Health and Life-Long Exercise Change (CHALLENGE) study (ClinicalTrials.gov identifier NCT00819208) (Figure 2), sponsored by the National Cancer Institute of Canada Clinical Trials Group. It is a phase III trial comparing a PA program in combination with health education materials versus health education materials alone among stage II-III colon cancer (excluding rectal cancer) patients. The primary endpoint of CHALLENGE is disease-free survival (DFS); OS, quality of life, and physical fitness are secondary endpoints of this study; outcomes will be assessed over a 10-year time frame. This trial is actively recruiting participants and its goal enrollment is 962 patients.
Figure 2.
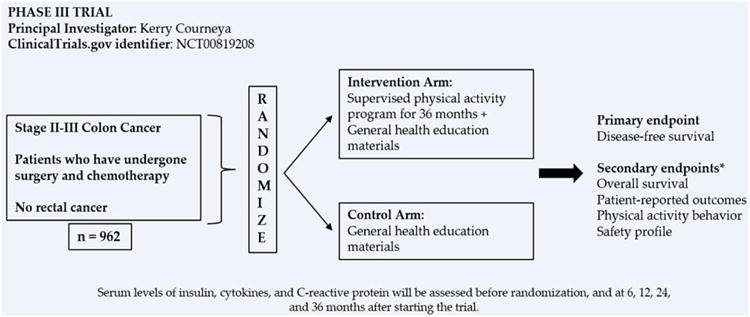
Phase III Colon Health and Life-Long Exercise Change (CHALLENGE) study. *Among other secondary endpoints.
Conclusions and Future Directions
A wealth of epidemiological evidence supports the notion that higher circulating 25(OH)D levels and post-diagnosis PA favorably impact survival outcomes among CRC patients. Nonetheless, we must be cautious before attempting to translate these observational results into more conclusive phase III trials or day-to-day clinical practice. While observational evidence has enormous value for generating hypotheses, it is not sufficient to prove a causal link. If our goal is to advance vitamin D and PA as standard interventions for CRC survivors, we must evaluate them with a rigor comparable to that used to assess new drug therapies. Plausible biological mechanisms have been proposed to explain the antineoplastic effects of vitamin D and PA, and there is strong preclinical evidence using in vitro and in vivo models to support them. Notwithstanding the fact that the safety profiles of both vitamin D supplementation and PA differ substantially from conventional chemotherapy, their tolerability still needs to be carefully assessed. The two ongoing vitamin D trials are using higher experimental doses than those recommended by the IOM and the Endocrine Society, and treatment tolerability is an endpoint in both studies. On the other hand, while the safety of PA among CRC survivors has been corroborated in several clinical trials, the evidence remains sparse for patients with metastatic disease.
The ongoing vitamin D and PA trials will pave the way to larger randomized phase III trials that will provide a more definitive answer about the role of these interventions as adjunctive treatments for patients with metastatic CRC. As we move forward and establish the biological plausibility of these therapies as well as their safety, there will be several more questions to answer and challenges to overcome. It remains unclear what vitamin D supplemental dose is required to achieve a protective benefit, and what the optimal serum 25(OH)D concentration in CRC patients is; moreover, it is equivocal whether we should focus on a fixed supplemental dose or rather on varying regimens designed to reach a target circulating level. Similar concerns exist in PA trials. Most studies to date have focused on self-reported total PA measured in MET-h/week, which may not provide an accurate reflection of the intensity of the exercise. Given that different exercise intensities evoke distinct biological responses, objective PA measurements that take exercise intensity into consideration are needed. Finally, we must cease to look at vitamin D and PA as one-size-fits-all interventions and appraise them through the prism of precision medicine. The advent of genomic technologies has allowed us to identify molecular signatures that predict response to novel targeted therapies in several cancer types – including CRC – leading to improved recurrence and survival outcomes. The identification of molecular markers of response to vitamin D and PA will guide a more refined patient selection and will lead to customized treatment regimens designed to fit each patient's needs.
References
- 1.Colon/Rectum Cancer. American Cancer Society. American Cancer Society, Inc.; 2006. [cited 2016 March 3]. Internet. Available from http://www.cancer.org/cancer/colonandrectumcancer/index. [Google Scholar]
- 2.Cancer of the Colon and Rectum. SEER Cancer Stat Fact Sheets. National Cancer Institute; [cited 2016 March 3]. Internet. Available from http://seer.cancer.gov/statfacts/html/colorect.html. [Google Scholar]
- 3.Venook AP, Niedzwiecki D, Lenz H, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients with KRAS wild-type untreated metastatic adenocarcinoma of the colon or rectum. J Clin Oncol. 2014;32:4s. suppl 15s; abstr LBA3. [Google Scholar]
- 4.Ng K, Wolpin BM, Meyerhardt JA, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101(6):916–923. doi: 10.1038/sj.bjc.6605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523(1):123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Meggouh F, Lointier P, Saez S. Sex steroid and 1,25-dihydroxyvitamin D3 receptors in human colorectal adenocarcinoma and normal mucosa. Cancer Res. 1991;51(4):1227–1233. [PubMed] [Google Scholar]
- 7.Shabahang M, Buras RR, Davoodi, et al. Growth inhibition of HT-29 human colon cancer cells by analogues 1,25-dihydroxyvitamin D3. Cancer Res. 1994;54(15):4057–4064. [PubMed] [Google Scholar]
- 8.Eisman JA, Barkla DH, Tutton PJ. Suppression of in vivo growth of human cancer solid tumor xenografts by 1,25-dihydroxyvitamin D3. Cancer Res. 1987;47(1):21–25. [PubMed] [Google Scholar]
- 9.Huerta S, Irwin RW, Heber D, et al. 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) mouse. Cancer Res. 2002;62(3):741–746. [PubMed] [Google Scholar]
- 10.Liu G, Hu X, Chakrabarty S. Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner: downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU. Int J Cancer. 2010;126(3):631–639. doi: 10.1002/ijc.24762. [DOI] [PubMed] [Google Scholar]
- 11.Diaz GD, Paraskeva CT, Binderup L, et al. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60(8):2304–2312. [PubMed] [Google Scholar]
- 12.Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8(4):174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 14.Scaglione-Sewell BA, Bissonnette M, Skarosi S, et al. A vitamin D3 analog induces a G1-phase arrest in CaCo-2 cells by inhibiting cdk2 and cdk6: roles of cyclin E, p21Waf1, and p27Kip1. Endocrinology. 2000;141(11):3931–3939. doi: 10.1210/endo.141.11.7782. [DOI] [PubMed] [Google Scholar]
- 15.Iseki K, Tatsuta M, Uehara H, et al. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-hydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer. 1999;821(5):730–733. doi: 10.1002/(sici)1097-0215(19990531)81:5<730::aid-ijc11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci. 2001;952:73–87. doi: 10.1111/j.1749-6632.2001.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal A, Hoebaus J, Tennakoon, et al. Active vitamin D potentiates the anti-neoplastic effects of calcium in the colon: a cross-talk through the calcium-sensing receptor. J Steroid Biochem Mol Biol. 2016;155(Pt B):231–238. doi: 10.1016/j.jsbmb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Braun MM, Helzlsouer KJ, Hollis BW, et al. Colon cancer and serum vitamin D metabolite levels 10-17 years prior to diagnosis. Am J Epidemiol. 1995;142(6):608–611. doi: 10.1093/oxfordjournals.aje.a117682. [DOI] [PubMed] [Google Scholar]
- 19.Grant WB. Ecological studies of the UVB-vitamin D-cancer hypothesis. Anticancer Res. 2012;32(1):223–236. [PubMed] [Google Scholar]
- 20.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1502–1508. [PubMed] [Google Scholar]
- 21.Maalmi H, Ordoñez-Mena JM, Schöttker B, et al. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer. 2014;50(8):1510–1521. doi: 10.1016/j.ejca.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Eng J Med. 2006;354(7):684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 23.Lappe J, Travers-Gustafson D, Daviers KM, et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469–472. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Eng J Med. 2015;373(16):1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 29.Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26(18):2984–2991. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 30.Mezawa H, Sugiura T, Watanabe M, et al. Serum vitamin D levels and survival of patients with colorectal cancer: post-hoc analysis of a prospective cohort study. BMC Cancer. 2010;10:347. doi: 10.1186/1471-2407-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng K, Sargent DJ, Goldberg RM, et al. Vitamin D status in patients with stage IV colorectal cancer: findings from intergroup trial N9741. J Clin Oncol. 2011;29(12):1599–1606. doi: 10.1200/JCO.2010.31.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fedirko V, Riboli E, Tjonneland, et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European populations. Cancer Epidemiol Biomarkers Prev. 2012;21(4):582–593. doi: 10.1158/1055-9965.EPI-11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tretli S, Schwartz GG, Torjesen PA, et al. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control. 2012;23(2):363–370. doi: 10.1007/s10552-011-9885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zgaga L, Theodoratou E, Farrington SM, et al. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J Clin Oncol. 2014;32(23):2430–2439. doi: 10.1200/JCO.2013.54.5947. [DOI] [PubMed] [Google Scholar]
- 35.Yang B, McCullough ML, Gapstur SM, et al. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: the cancer prevention study-II nutrition cohort. J Clin Oncol. 2014;32(22):2335–2343. doi: 10.1200/JCO.2014.55.3024. [DOI] [PubMed] [Google Scholar]
- 36.Jeffreys M, Redaniel MT, Martin RM. The effect of pre-diagnostic vitamin D supplementation on cancer survival in women: a cohort study within the UK Clinical Practice Research Datalink. BMC Cancer. 2015;15:670. doi: 10.1186/s12885-015-1684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng K, Venook AP, Sato K, et al. Vitamin D status and survival of metastatic colorectal cancer patients: results from CALGB/SWOG 80405 (Alliance) J Clin Oncol. 2015;33 suppl 3; abstr 507. [Google Scholar]
- 38.Mohr SB, Gorham ED, Kim J, et al. Could vitamin D sufficiency improve the survival of colorectal cancer patients? J Steroid Biochem Mol Biol. 2015;148:239–244. doi: 10.1016/j.jsbmb.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 40.Fakih MG, Andrews C, McMahon J, et al. A prospective clinical trial of cholecalciferol 2000 IU/day in colorectal cancer patients: evidence of a chemotherapy-response interaction. Anticancer Res. 2012;32(4):1333–1338. [PubMed] [Google Scholar]
- 41.Samad AK, Taylor RS, Marshall T, et al. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7(3):204–213. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 42.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. 2013;30:S75–S87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 44.McClellan JL, Steiner JL, Day SD, et al. Exercise effects on polyp burden and immune markers in the Apcmin/+ mouse model of intestinal tumorigenesis. Int J Oncol. 2014;45(2):861–868. doi: 10.3892/ijo.2014.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoi W, Naito Y, Takagi T, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62(6):882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 46.Je Y, Jeon JY, Giovannucci EL, et al. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2013;133(8):1905–1913. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- 47.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 48.Meyerhardt JA, Giovannuci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169(22):2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baade PD, Meng X, Youl PH, et al. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1410–1420. doi: 10.1158/1055-9965.EPI-11-0079. [DOI] [PubMed] [Google Scholar]
- 50.Kuiper JG, Phipps AI, Neuhouser ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012;23(12):1939–1948. doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell PT, Patel AV, Newton CC, et al. Associations of recreational physical activity and leisure time spent witting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 52.Arem H, Pfeiffer RM, Engels EA, et al. Pre- and postdiagnosis physical activity, television viewing, and mortality among patients with colorectal cancer in the National Institutes of Health-AARP diet and health study. J Clin Oncol. 2015;33(2):180–188. doi: 10.1200/JCO.2014.58.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 54.Meyerhardt JA, Ogino S, Kirkner GJ, et al. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009;15(18):5931–5936. doi: 10.1158/1078-0432.CCR-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morikawa T, Kuchiba A, Yamauchi M, et al. Associations of CTNNB1 (β-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305(16):1685–1694. doi: 10.1001/jama.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamauchi M, Lochhead P, Imamura Y, et al. Physical activity, tumor PTGS2 expression, and survival in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1142–1152. doi: 10.1158/1055-9965.EPI-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanyuda A, Kim SA, Martinez-Fernandez A, et al. Survival benefit of exercise differs by tumor IRS1 expression status in colorectal cancer. Ann Surg Oncol. 2015;23(3):908–917. doi: 10.1245/s10434-015-4967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ligibel JA, Meyerhardt J, Pierce JP, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res Treat. 2012;132(1):205–213. doi: 10.1007/s10549-011-1882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinto BM, Papandonatos GD, Goldstein MG, et al. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22(1):54–65. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 60.Lee DH, Kim JY, Lee MK, et al. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013;21(9):2537–2545. doi: 10.1007/s00520-013-1822-7. [DOI] [PubMed] [Google Scholar]
- 61.Courneya KS, Fredenreich CM, Quinney HA, et al. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care. 2003;12(4):347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 62.Backman M, Wengström Y, Johansson B, et al. A randomized pilot study with daily walking during adjuvant chemotherapy for patients with breast and colorectal cancer. Acta Oncol. 2014;53(4):510–520. doi: 10.3109/0284186X.2013.873820. [DOI] [PubMed] [Google Scholar]
- 63.Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;35(4):811–821. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Devin JL, Sax AT, Hughes GI, et al. The influence of high-intensity compared with moderate-intensity exercise training on cardiorespiratory fitness and body composition in colorectal cancer survivors: a randomised controlled trial. J Cancer Surviv. 2015 doi: 10.1007/s11764-015-0490-7. In press. [DOI] [PubMed] [Google Scholar]
- 65.Park JH, Lee J, Oh M, et al. The effect of oncologists' exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: a randomized controlled trial. Cancer. 2015;121(16):2740–2748. doi: 10.1002/cncr.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch BM, Courneya KS, Sethi P, et al. A randomized controlled trial of a multiple health behavior change intervention delivered to colorectal cancer survivors. Effects on sedentary behavior Cancer. 2014;120(17):2665–2672. doi: 10.1002/cncr.28773. [DOI] [PubMed] [Google Scholar]
- 67.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 68.Courneya KS, Friedenreich CM, Sela R, et al. Correlates of adherence and contamination in a randomized trial of exercise in cancer survivors: an application of the theory of planned behavior and the five factor model of personality. Ann Behav Med. 2002;24(4):257–268. doi: 10.1207/S15324796ABM2404_02. [DOI] [PubMed] [Google Scholar]
- 69.Fuchs CS, Goldberg RM, Sargent DJ, et al. Plasma insulin-like growth factors, insulin-like binding protein-3, and outcome in metastatic colorectal cancer: results from intergroup trial N9741. Clin Cancer Res. 2008;14(24):8263–8269. doi: 10.1158/1078-0432.CCR-08-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32(9):1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]


