Abstract
Objective
To identify modifiable patient and provider factors associated with allopurinol adherence and the achievement of a serum urate (sUA) goal in gout.
Methods
We identified a retrospective cohort of gout patients, newly initiated on allopurinol. All patient data came from administrative datasets at a large integrated health delivery system. Patients were > 18 years old at time of initial allopurinol dispensing, and had 12 months or more of membership and drug eligibility prior to the index date. Allopurinol adherence was defined as a proportion of days covered ≥ 0.80, evaluated during the first 12 months of observation after the initial dispensing. Multivariable logistic regression was used to examine factors associated with allopurinol nonadherence and attaining a sUA concentration < 6.0 mg/dl.
Results
We identified 13,341 gout patients with incident allopurinol use (mean age 60 years, 78% male). Of these, 9,581 patients (72%) had sUA measured both at baseline and during follow-up. Only 3,078 patients (32%) attained sUA target of < 6.0 mg/dl during follow-up. Potentially modifiable factors associated with treatment adherence and obtaining sUA goal in the multivariable analysis included concomitant diuretic use, prescriber specialty, and allopurinol dosing practices. Adherent patients were 2.5-fold more likely than nonadherent patients to achieve a sUA < 6.0 mg/dl during observation.
Conclusion
Among gout patients initiating allopurinol in this study, 68% did not reach sUA goal and 57% of patients were nonadherent. Modifiable factors, including allopurinol dose escalation, treatment adherence, rheumatology referral, and concomitant medication use could be important factors to consider in efforts aimed at optimizing gout treatment outcomes.
Keywords: gout, allopurinol, adherence, incident
Given the central role of hyperuricemia in the etiology of gout, urate lowering therapy (ULT) has become the cornerstone treatment in chronic gout. A serum urate (sUA) level of < 6.0 mg/dl has been widely accepted as the therapeutic target for patients with gout. It is the primary endpoint in recent randomized controlled trials (1-4), and has been endorsed by all internationally recognized evidence-based gout management guidelines to date (5-8). Each of the published guidelines includes a treat-to-target strategy with gradual ULT titration until sUA levels reach < 6.0 mg/dl (or < 5.0 mg/dl in select circumstances). Studies have consistently demonstrated improvements in long term patient outcomes after achieving a target sUA < 6.0 including reduction in gouty flares (1-4, 9, 10), reduction in tophus size (1, 4, 10) and depletion of urate stores in synovial tissues (10).
Available for more than 40 years, allopurinol remains the most frequently prescribed ULT in all studies examining practice patterns in gout management (11-14), accounting for 97% of ULT prescriptions in at least one study (12). Allopurinol can be dosed once daily, is inexpensive, and is potentially effective and well tolerated in a vast majority of gout patients.
Despite its many advantages as a ULT, numerous studies indicate poor patient adherence to allopurinol therapy (11-17). In addition to medication adherence, quality of care in gout is far from optimal (17-21) and a limited number of prior studies have examined the direct link between ULT adherence and sUA goal achievement. None have simultaneously accounted for the many confounders that could impact this relationship. The primary objective of this study was to examine potentially modifiable patient and provider factors associated with allopurinol adherence and sUA goal attainment among gout patients initiating allopurinol treatment. Our goal from this study was to provide some basis for prioritization of factors amenable to future quality improvement initiatives focused on gout outcomes.
MATERIALS AND METHODS
Setting and Dataset
We examined data from Kaiser Permanente Southern California (KPSC), a large integrated healthcare delivery system with approximately 3.6 million members. Available administrative data included patient demographics, diagnoses, medication dispensing, laboratory results, medical and hospital encounters. KPSC had no known policies that would have directly affected the type of care provided to gout patients during the time of this study. Specifically, there are no restrictions or other disincentives for providers with regards to specialist referral or use of non-allopurinol ULT in gout patients. The health system membership currently represents 15% of the underlying population in the Southern California region and closely mirrors the area's demographic characteristics; it is racially diverse and includes the entire socioeconomic spectrum (22). The KPSC institutional review board approved this study.
Design and Study Population
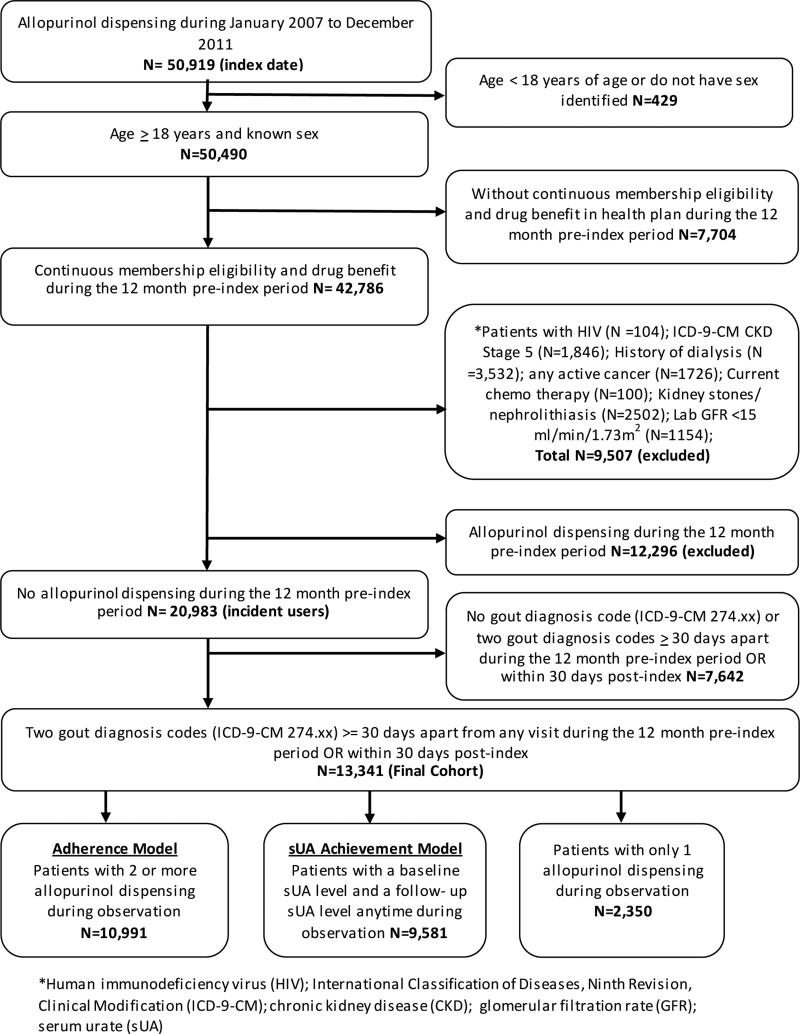
A retrospective cohort database analysis was conducted for the study enrollment period of January 1, 2007 through December 31, 2011. Patients were selected if they received a new allopurinol dispensing during the study enrollment period; were > 18 years of age at the time of initial allopurinol dispensing during the study period; and had at least 12 months of membership eligibility including drug benefits prior to the initial allopurinol dispensing. Enrollment gaps of ≤ 30 days were considered continuous enrollment. The index date was defined as the first allopurinol dispensing identified during the study enrollment period. Eligible patients were required to have two gout diagnoses ≥ 30 days apart coded at any outpatient or inpatient visit during the 12 month pre-index period and extending to 30 days post-index (International Classification of Diseases, Ninth Revision, Clinical Modification [1CD-9-CM] code 274.xx). Patients were excluded if, during the pre-index period, they had an allopurinol dispensing, history of human immunodeficiency virus (HIV), a diagnosis code for chronic kidney disease (CKD) stage 5 or an estimated glomerular filtration rate (eGFR) <15 ml/min/1.73m2, a history of dialysis, active cancer or on current chemotherapy; or kidney stones/ nephrolithiasis. Each exclusion criteria was included to limit the patient population to those whose primary indication for allopurinol was gout. Patients were followed from index allopurinol dispensing until disenrollment from the health plan or the end of the study time period (December 31, 2012), whichever came first (Figure 1). This observation period provided a minimum of 12 months for follow-up after the index allopurinol dispensing.
Figure 1. Sample Selection Flowchart.
Covariates and measures
Baseline characteristics such as age, sex, race, comorbid conditions, concomitant medication use, renal function, and prescriber specialty were evaluated 12 months prior to and including the index date. Prescription anti-inflammatory medication use (defined throughout as non-steroidals, colchicine or glucocorticoids) was evaluated over the 60 day period spanning 30 days pre-index and 30 days post-index. Baseline sUA levels were measured up to 12 months prior to the index date or within 30 days after the index date. For a majority of patients (82%), a baseline sUA level was obtained at least once during the 12 month period prior to the index date, and 18% had a sUA level obtained only during the 30 day post index period. For those patients with multiple potential baseline sUA values available, the measurement obtained most proximate to the index date was used. Allopurinol treatment information (changes in dose), adherence, and sUA goal attainment were captured post-index. Patients were considered to have had allopurinol dose escalation if the final observed daily dose was greater than the index dose. Conversely patients were considered to have had dose decreases if the final daily allopurinol dose was less than the initial dose.
Adherence measure
Medication adherence was summarized using proportion of days covered (PDC). The PDC was calculated as the number of days with allopurinol drug on hand divided by the number of days in the specified time interval (360 days). We evaluated the PDC within the first 12 months of initiating allopurinol. A uniform period of 360 days, representing four 90-day allopurinol dispensings, was used in our calculation to ensure comparable and sufficient data to characterize long term adherence behavior profiles for all patients. The 90-day period represents the most common number of days supplied for an allopurinol dispensing. The PDC was dichotomized for the multivariable analysis, with a PDC of < 80% considered as nonadherent and ≥ 80% considered adherent (11, 12, 14, 15). We first calculated the PDC for all patients including those patients receiving only a single allopurinol dispensing. To limit the effect of immediate discontinuation, we also calculated the PDC for gout patients receiving two or more allopurinol dispensings during the 12 months post-index period. Given its rare incidence (estimated at approximately 1 in 1,000 patient-years, based upon external literature) and imprecision in its identification, we did not examine the impact of severe cutaneous reactions (e.g. Stevens-Johnson syndrome) on allopurinol adherence or outcomes (23).
Attainment of serum urate goal
Attainment of sUA goal was achieved if the last follow-up sUA obtained more than 30 days after the index date had a value < 6.0 mg/dl. By this definition, a patient would not be considered to have attained sUA goal if the final observed sUA was ≥ 6.0 mg/dl even if any prior sUA more than 30 days after index was < 6.0 mg/dl. This outcome was evaluated only among patients that had both baseline and follow-up sUA levels available. Taking into account the 30-day buffer period, all patients had at least 11 months or longer of follow-up during which a sUA could be recorded.
Statistical analyses
Unadjusted descriptive statistics summarized patient characteristics of the study population, patients that were adherent versus nonadherent, and patients at sUA goal versus not at sUA goal. Two models were developed using the same dataset, one model for the nonadherence outcome and a second for the sUA goal achievement outcome. Each model had a different population subset created from the final cohort as shown in Figure 1. Differences between groups were tested using two-sided t-test for continuous variables and the chi-squared test for categorical variables. Multivariable logistic regression models were used to evaluate the association of factors, selected a priori, with nonadherence (PDC of < 80%) and the achievement of sUA <6.0 mg/dl. Factors including age, sex, race, selected comorbid conditions (hypertension, myocardial infarction, congestive heart failure, and diabetes), diuretic use, renal function (by eGFR), care by rheumatologist, anti-inflammatory medications, and treatment adherence (only for the model examining sUA goal achievement) were controlled for in the models. Based on the strong association between initial prescribing physician (rheumatologist vs. non-rheumatologist) and dose adjustment (no change vs. dose escalation vs. dose decrease), multivariable models include initial prescribing physician instead of dose adjustment. All data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC). P-values < 0.05 were considered to be statistically significant.
RESULTS
Patient population
There were 13,341 gout patients identified as incident allopurinol users (mean age 60 years, men 78%) using our selection criteria (Figure 1). Table 1 summarizes baseline characteristics of incident allopurinol users. Patients were predominantly Caucasian (41%), and the most common comorbidities were hypertension (71%) and diabetes (23%). The mean baseline GFR was 63.5 ml/min (SD ± 18.5), with a majority of patients categorized into either CKD stage 2 (46%: eGFR 60-89 mL/min) or CKD stage 3 (38%: eGFR 30-59 mL/min). Considering concomitant medications, 39% patients were on either a thiazide or loop diuretic and 69% of patients used a prescription anti-inflammatory medication (colchicine, non-steroidal, or glucocorticoid) within the period covering 30 days pre-index to 30 days post-index. A majority (87%) of the patients had at least one sUA level in the baseline period (mean sUA 8.58 mg/dl; SD ± 1.8 mg/dl).
Table 1.
Baseline Patient Characteristics for Newly Initiated Allopurinol Users
| Total Incident Allopurinol Users | N= 13,341 |
| Follow-up Duration, years (mean, SD) | 3.27±1.55 |
| Male, (n,%) | 10,410 (78.0) |
| Age, years (mean, SD) | 60.2±13.9 |
| BMI kg/m2 (mean, SD) | 31.5±6.6 |
| Race/Ethnicity (n,%) | N= 13,341 |
| Caucasian | 5,469 (41.0) |
| African American | 2,089 (15.7) |
| Hispanic | 2,608 (19.5) |
| Asian/Pacific Islander | 3,086 (23.1) |
| Other | 89 (0.7) |
| Comorbidities (n,%) | N= 13,341 |
| Hypertension | 9,449 (70.8) |
| Myocardial infarction | 905 (6.8) |
| Congestive heart failure | 1,047 (7.8) |
| Diabetes | 3,124 (23.4) |
| Laboratory Data | |
| Patients with a Baseline eGFR Lab Level (n,%) | N= 11,352 (85.1) |
| Baseline eGFR level, ml/min (mean, SD) | 63.5±18.5 |
| CKD Stage 1, eGFR ≥ 90 ml/min, (n, %) | 1,487 (13.1) |
| CKD Stage 2, eGFR 60-89 ml/min, (n, %) | 5,171 (45.6) |
| CKD Stage 3, eGFR 30-59 ml/min, (n, %) | 4,286 (37.8) |
| CKD Stage 4, eGFR 15-29 ml/min, (n, %) | 426 (3.8) |
| Patients with a Baseline sUAa (n,%) | N= 11,645 (87.3) |
| Baseline sUA, mg/dl (mean, SD) | 8.58±1.8 |
| Concomitant Medication Use with Initial Allopurinol | |
| Diuretic Use (n, %) | N= 5,212 (39.1) |
| Loop diuretics | 1,094 (8.2) |
| Thiazides diuretics | 3,750 (28.1) |
| Anti-inflammatory medications (n,%)b | |
| NSAID | 4,842 (36.3) |
| Corticosteroid | 2,816 (21.1) |
| Colchicine | 5,073 (38.0) |
| Any of above | 9,222 (69.1) |
Serum urate evaluated 12 months prior and up to 30 days after allopurinol dispensing
patients could be prescribed more than two anti-inflammatory medications; anti-inflammatory use was extracted from the period covering 30 days prior and up to 30 days after the index allopurinol dispensing.
Standard deviation (SD); body mass index (BMI); estimated glomerular filtration rate (GFR); chronic kidney disease (CKD); serum urate (sUA); Non-steroidal anti-inflammatory drug (NSAID).
Table 2 summarizes prescriber specialty, allopurinol initial dose and changes in dose, treatment adherence, and sUA levels during follow-up. Over 80% of physicians who prescribed the index allopurinol were primary care physicians, while 6% were rheumatologists. A majority of the patients started allopurinol at a dose of either 100 mg per day (48%) or 300 mg per day (37%). Based on pharmacy claims data, 82% of patients continued with two or more allopurinol dispensings with a mean duration of allopurinol therapy of 2.6 (SD ± 1.6) years. Of these, less than 2% (n = 202) switched to febuxostat during the 360 day follow-up. Among the remaining patients (18%) who received only a single allopurinol dispensing, the mean duration of therapy was 93 ± 21 days. Allopurinol doses were not changed for a majority (71%) of patients throughout observation; only 22% had a dose increase from their initial dispensing (Table 2). The mean PDC was 65% (SD ± 23.2%), and evaluating patients with two or more dispensings (N = 10,991), the mean PDC was slightly higher at 74% (SD ± 21.4%). Of gout patients with both baseline and follow-up sUA measurements available (n = 9,581), 75% had their last follow-up sUA obtained more than one year post-index (Table 2).
Table 2.
Treatment Information and Related Outcomes for New Allopurinol Users
| Prescriber Specialty Initiated Allopurinol Rx (n,%) | N=13,341 |
| Family medicine | 6,796 (50.9) |
| Internal medicine | 4,296 (32.2) |
| Rheumatology | 805 (6.0) |
| Other | 1,444 (10.8) |
| Starting Allopurinol Dose, mg/day (n,%) | N= 13,341 |
| 50 mg/day | 126 (0.9) |
| 100 mg/day | 6,382 (47.8) |
| >100 mg to <300 mg/day | 1,761 (13.2) |
| 300 mg/day | 4,981 (37.3) |
| >300 mg/day | 91 (0.7) |
| Allopurinol Treatment | N= 13,341 |
| Patients with only one dispensing, n (%) | 2,350 (17.6) |
| Duration of treatment for single Rx, mean days, SD | 93.3±21.0 |
| Patients receiving more than single dispensing, n (%) | 10,991 (82.4) |
| Duration of treatment, mean years, SD | 2.6±1.6 |
| Dose escalation during observation, n (%) | 2,994 (22.4) |
| Dose decrease during observation, n (%) | 817 (6.1) |
| No Dose Change during observation, n (%) | 9,530 (71.4) |
| Allopurinol Adherence (PDC), %*a | N= 13,341 |
| Proportion of Days Covered (PDC) mean, SD | 65.2±23.2 |
| Adherent (PDC ≥ 80%), n (%) | 4,454 (33.4) |
| Nonadherent (PDC < 80%), n (%) | 8,886 (66.6) |
| Allopurinol Adherence (PDC)* For Patients with ≥ 2 Dispensingsb | N= 10,991 |
| PDC mean, SD | 74.2±21.4 |
| Adherent (PDC ≥ 80%), n (%) | 4,656 (42.4) |
| Nonadherent (PDC < 80%), n (%) | 6,335 (57.6) |
| Serum Urate (sUA)C | N= 9,581 |
| sUA level at end of observation, mean, SD | 6.9±1.8 |
| Patients at sUA goal (<6mg/dl) at end of observation, n (%) | 3,078 (32.1) |
| Patients’ Last sUA Level, n (mean sUA ± SD)d | N= 9,581 |
| 31-90 days | 643, 6.5±1.7 |
| 91-180 days | 674, 6.9±1.9 |
| 181-270 days | 541, 6.9±1.7 |
| 271-365 days | 563, 6.7±1.8 |
| > 365 days | 7,160, 6.9±1.8 |
Calculated during 12 months post-index.
PDC calculation included patients with one or more allopurinol dispensing.
PDC calculation focused on only patients with 2 or more allopurinol dispensings.
Patients with a baseline sUA level and had one level during follow-up and was the last level before follow-up.
Categorized by days from baseline.
Standard deviation (SD); proportion of days covered (PDC); serum urate (sUA).
Allopurinol prescribing practices in rheumatologist and non-rheumatologists
In an additional analysis comparing rheumatology to non-rheumatology prescribers, there were striking differences in allopurinol use. Among gout patients treated by a rheumatologist, 98% received allopurinol dose escalation during their care, compared to just 5% of those treated by non-rheumatologists (p < 0.0001). Overall, only 11% of patients received a dose escalation. Rheumatologists were also more likely than non-rheumatologists to initiate allopurinol in daily doses of 100 mg or less (64% vs. 48%, p < 0.0001). Although more common in the context of rheumatology care, an ending daily dose of allopurinol > 300 mg/day was uncommon for both rheumatologists and non-rheumatologists (6.3% vs. 2.0%, p < 0.0001).
Factors associated with allopurinol adherence and serum urate goal attainment
Adherence was measured in the subgroup of patients that had at least two or more allopurinol dispensings during 12 months post index (N=10,991, 82% of total). Of these, 4,656 patients (42%) were adherent with a PDC ≥ 80% over the first year of allopurinol use (Table 3). In unadjusted analyses, factors significantly associated with allopurinol nonadherence included male sex, younger age, minority racial/ethnic status, the absence of select comorbid conditions, lower eGFR, higher initial allopurinol doses, a non-rheumatology prescriber, a lack of diuretic use, and the use of anti-inflammatory drugs (Table 3).
Table 3.
Unadjusted Comparisons of Patient Characteristics For New Allopurinol Users Stratified by Adherence
| Patient Characteristics | Total | Adherent (PDC ≥80%) | Non-adherent (PDC <80%) | P Value* |
|---|---|---|---|---|
| N=10,991a | N= 4,656 | N= 6,335 | ||
| Men (vs. women) n, (%) | 8,561 (77.9) | 3,504 (75.3) | 5,057 (79.8) | 0.002* |
| Patient Age Groups, years, n(%) | <.0001* | |||
| <55 | 4,007 (36.5) | 1,315 (28.2) | 2,692 (42.5) | |
| 55-64 | 3,588 (32.6) | 1,577 (33.9) | 2,011 (31.7) | |
| ≥65 | 3,394 (30.9) | 1,763 (37.9) | 1,631 (25.7) | |
| Race/ethnicity, n(%) | <.0001* | |||
| Caucasian | 4,540 (41.3) | 2,343 (50.3) | 2,197 (34.7) | |
| African American | 1,712 (15.6) | 620 (13.3) | 1,092 (17.2) | |
| Hispanic | 2,134 (19.4) | 728 (15.6) | 1,406 (22.2) | |
| Asian/Pacific Islander | 2,484 (22.6) | 884 (19) | 1,600 (25.3) | |
| Other | 121 (1.1) | 80 (1.7) | 41 (0.6) | |
| Comorbidities, n (%) | ||||
| Hypertension | 7,817 (71.1) | 3,695 (79.4) | 4,122 (65.1) | <.0001* |
| Myocardial infarction | 751 (6.8) | 387 (8.3) | 364 (5.7) | 0.0002* |
| Congestive heart failure | 875 (8) | 484 (10.4) | 391 (6.2) | <.0001* |
| Diabetes | 2,593 (23.6) | 1,332 (28.6) | 1,261 (19.9) | <.0001* |
| Other Covariates | ||||
| Baseline GFR, ml/min, mean SD | 63.6±18.7 | 67.5±18.3 | 54.6±18.5 | <.0001* |
| Diuretic use, n(%) | 4,329 (39.4) | 2,256 (48.5) | 2,073 (32.7) | 0.004* |
| Use of baseline anti-inflammatoryb, n(%) | 7,650 (69.6) | 2,894 (62.2) | 4,756 (75.1) | <.0001* |
| Rheumatologist as initial prescriber, n(%) | 656 (6.0) | 382 (8.2) | 274 (4.3) | <.0001* |
| Starting Allopurinol Dose, mg/day, n(%) | ||||
| ≤ 100 | 5,382 (49.0) | 2,533 (54.4) | 2,849 (45.0) | <.0001* |
| >100 to < 300 | 1,450 (13.2) | 609 (13.1) | 841 (13.3) | 0.18 |
| ≥ 300 | 4,158 (37.8) | 1,514 (32.5) | 2,644 (41.7) | 0.002* |
| Allopurinol Dose Adjustment | ||||
| Dose escalation | 2,994 (27.2) | 1393 (21.9) | 1601 (34.2) | 0.04* |
PDC was calculated for patients with 2 or more allopurinol dispensings during 12 months post-index (N=10,991).
anti-inflammatory drugs include non-steroidals, NSAIDs and glucocorticoids.
P value was set at <0.05 for statistical significance.
Proportion of days covered (PDC); glomerular filtration rate (GFR); standard deviation (SD).
Among gout patients with both a baseline and a follow-up sUA level available (N=9,581, 72% of total), approximately one in three (32%) patients attained a sUA < 6.0 mg/dl (Table 4). Unadjusted comparisons showed that male sex, younger age (<65), minority race/ethnicity, congestive heart failure, higher GFR, the use of anti-inflammatory agents, non-rheumatologist prescribers, lower initial allopurinol doses (≤100mg/day), and lower treatment adherence were more common among patients failing to achieve a target sUA < 6.0 mg/dl (Table 4).
Table 4.
Unadjusted Comparisons of Patient Characteristics For New Allopurinol Users Stratified by sUA Goal versus Not at sUA Goal
| Patient Characteristics | Total | sUA at Goal (<6mg/dl) | sUA not at Goal (≥6mg/dl) | P Value* |
|---|---|---|---|---|
| N= 9,581a | N= 3,078 | N= 6,503 | ||
| Male (vs. female) n, (%) | 7,218 (75.3) | 2,149 (69.8) | 5,069 (77.9) | <.0001* |
| Patient Age Groups, years, n(%) | <.0001* | |||
| <55 | 3,462 (36.1) | 782 (25.4) | 2,680 (41.2) | |
| 55-64 | 3,923 (40.9) | 1,086 (35.3) | 2,837 (43.6) | |
| ≥65 | 2,196 (22.9) | 1,209 (39.3) | 987 (15.2) | |
| Race/Ethnicity, n(%) | <.0001* | |||
| Caucasian | 4,762 (49.7) | 1,622 (52.7) | 3,140 (48.3) | |
| African American | 1,246 (13.0) | 442 (14.4) | 804 (12.4) | |
| Hispanic | 1,471 (15.4) | 406 (13.2) | 1,065 (16.4) | |
| Asian/Pacific Islander | 78 (0.8) | 12 (0.4) | 66 (1.0) | |
| Other | 2,024 (21.1) | 596 (19.4) | 1,428 (22.0) | |
| Comorbidities, n (%) | ||||
| Hypertension | 7,743 (80.8) | 2,505 (81.4) | 5,238 (80.5) | 0.78 |
| Myocardial infarction | 823 (8.6) | 219 (7.1) | 604 (9.3) | 0.77 |
| Congestive heart failure | 1,055 (11.0) | 254 (8.3) | 801 (12.3) | 0.0003* |
| Diabetes | 2,850 (29.7) | 858 (27.9) | 1,992 (30.6) | 0.07 |
| Other Covariates | ||||
| Baseline GFR, ml/min, mean SD | 60.5±18.0 | 61.6±15.9 | 55.2±18.8 | <.0001* |
| Diuretic use, n(%) | 4,798 (50.1) | 1,584 (51.5) | 3,214 (49.4) | 0.25 |
| Use of baseline anti-inflammatory, n(%) | 5,357 (55.9) | 1,272 (41.3) | 4,085 (62.8) | <.0001* |
| Rheumatologist as initial prescriber, n(%) | 757 (7.9) | 412 (13.4) | 345 (5.3) | <.0001* |
| Allopurinol Adherence (PDC ≥80) | 4,767 (49.8) | 2,001 (65) | 2,766 (42.5) | 0.004* |
| Starting Allopurinol Total Dose, mg/day, n(%) | ||||
| ≤ 100 | 5,070 (52.9) | 699 (22.7) | 4,371 (67.2) | <.0001* |
| >100 to < 300 | 1,995 (20.8) | 1,217 (39.5) | 778 (12.0) | <.0001* |
| ≥ 300 | 2,516 (26.3) | 1,162 (37.8) | 1,354 (20.8) | <.0001* |
| Allopurinol Dose Adjustment | ||||
| Dose escalation | 2992 (31.2) | 1797 (58.3) | 1195 (18.4) | <0.002* |
sUA was evaluated for patients that had a baseline and follow-up sUA level anytime during observation (N=9,581). The last sUA level was taken for each patient during follow-up period.
P value was set at <0.05 for statistical significance.
Serum urate (sUA); glomerular filtration rate (GFR); standard deviation (SD).
Using multivariable analyses, we subsequently identified factors that were independently associated with allopurinol nonadherence and the achievement of sUA goal (Table 5). For the multivariable nonadherence model, male sex was no longer significantly associated with nonadherence and allopurinol dose escalation was removed from the model due to high collinearity with rheumatologist as initial prescriber. All other significant associations from unadjusted analysis remained for the multivariable model of nonadherence.
Table 5.
Factors Associated With Nonadherence to Allopurinol and sUA Goal Attainment from Logistic Multivariable Regression in New Allopurinol Users
| Study Covariates | Nonadherence* To Allopurinol During First 12 Months Versus Adherence | Patients Achieving sUA Goal** versus Not Achieving Goal |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Male (vs. female) | 1.04 (0.88,1.20) | 0.60 (0.52, 0.67) |
| Patient Age Groups, years | ||
| <55 | 1.22 (1.09,1.34) | 0.82 (0.78,0.92) |
| 55-64 | 0.87 (0.65,1.06) | 0.91 (0.85,1.06) |
| ≥65 (reference) | 1.00 | 1.00 |
| Race/Ethnicity | ||
| Caucasian (reference) | 1.00 | 1.00 |
| African American | 1.46 (1.32,1.61) | 0.80 (0.78,0.89) |
| Hispanic | 1.42 (1.35,1.62) | 0.86 (0.75,0.97) |
| Asian/Pacific Islander | 1.31 (1.21,1.38) | 0.79 (0.70,0.93) |
| Other | 1.21 (0.82,1.64) | 0.95 (0.93,1.05) |
| Comorbidities | ||
| Hypertension | 0.87 (0.71,0.95) | 1.02 (0.91,1.17) |
| Myocardial infarction | 0.89 (0.82,0.98) | 1.03 (0.84,1.22) |
| Congestive heart failure | 0.85 (0.75,0.97) | 0.68 (0.57,0.89) |
| Diabetes | 0.74 (0.67,0.82) | 0.91 (0.79,1.11) |
| CKD Stages | ||
| CKD Stage 1 (reference) | 1.00 | 1.00 |
| CKD Stage 2 | 0.95 (0.91,1.07) | 0.98 (0.88,1.10) |
| CKD Stage 3 | 1.15 (1.20,1.60) | 0.72 (0.65,0.88) |
| CKD Stage 4 | 1.60 (1.38,1.98) | 0.62 (0.54,0.75) |
| Other Covariates | ||
| Diuretic use | 0.75 (0.71, 0.85) | 0.95 (0.79, 1.05) |
| Use of baseline anti-inflammatory | 1.25 (1.19,1.37) | 0.75 (0.64, 0.89) |
| Rheumatologist as initial prescriber | 0.80 (0.74,0.87) | 1.72 (1.45,1.85) |
| Allopurinol Adherence (PDC≥80) | - | 2.52 (2.41,3.01) |
| Starting Allopurinol Total Dose, mg/day | ||
| ≤ 100 (reference) | 1.00 | 1.00 |
| >100 to < 300 | 1.20 (1.13,1.24) | 1.92 (1.86,2.22) |
| ≥ 300 | 1.10 (1.05,1.32) | 2.12 (1.81,2.55) |
Model included PDC for patients that had 2 or more allopurinol dispensings during 12 months post-index (N=10,991).
sUA goal is <6mg/dl anytime during post-index and was evaluated for patients with baseline and follow-up sUA levels (N=9,581) during observation.
Bold numbers indicate statistical significance; allopurinol dose escalation not included in multivariable models due its strong association with provider type (rheumatologist vs. non-rheumatologist). Odds ratio (OR); confidence interval (CI); chronic kidney disease (CKD); proportion of days covered (PDC).
DISCUSSION
It has been widely reported that quality of care for gout is suboptimal (17-21). It has also been demonstrated that medication adherence in gout is exceedingly low (11-15, 17), ranking among the lowest observed of several chronic health conditions examined (16). In addition to supporting these earlier studies, our study identifies several modifiable factors associated with treatment adherence and outcomes in gout. To our knowledge, this is the first study to demonstrate an independent and robust association of allopurinol treatment adherence with sUA goal achievement after accounting for a rich array of confounding factors using a large generalizable population. Among the many patient and provider factors examined, allopurinol treatment adherence was the single strongest determinant of achieving sUA goal over follow-up with a corresponding odds ratio exceeding 2.5.
Quality improvement initiatives routinely target at-risk patient populations and our results indicate that a similar strategy may be considered in gout management. We found, for instance, that younger patients, males, and individuals reporting minority race/ethnicity may be at increased risk of nonadherence, even after adjustment for factors including comorbidities and CKD (21). Frequent comorbidity and polypharmacy in gout have drawn concerns that patient complexity may be an impediment to optimal care (24). However, as reported elsewhere (11-13, 15), we found that select comorbidities were associated with greater medication adherence. We also report a novel observation that current diuretic use is associated with greater allopurinol adherence. It is possible that patients with more comorbidities and concomitant medications such as diuretics have developed more effective self-management behaviors. It is noteworthy that diuretic use also increases urate retention, which may explain the simultaneously observed lower odds of sUA goal attainment among diuretic users.
Our study re-emphasizes the important association of more advanced CKD with lower sUA goal attainment (21) and extends the association to lower medication adherence. While residual confounding cannot be excluded with certainty, it is well known that CKD complicates the prescription of NSAIDs and colchicine for acute gout treatment and anti-inflammatory prophylaxis. If CKD limits effective prophylaxis, then resulting “rebound” gout flares may discourage ULT adherence. Although not examined in our study, both qualitative and retrospective cohort studies have demonstrated a detrimental impact of gout flares on medication use (11, 25, 26). Advanced CKD further limits sUA goal attainment in two ways. Higher serum urate levels are associated with more advanced CKD because uric acid removal is dependent on renal excretion. Moreover, dose titration may not occur owing to inappropriately rigid adherence to previous renal dosing recommendations (27, 28) that have been refuted in recent studies and evidence-based guidelines (5-7, 29, 30).
Provider prescribing practices also appear to influence treatment adherence and outcomes in gout. A rheumatologist as the initial prescriber was significantly associated with improved allopurinol adherence and sUA goal attainment after adjusting for medication adherence among other factors. Allopurinol dose escalation was very common among rheumatologists, but was extremely uncommon among other providers. It is well accepted that dose escalation is required for most patients to achieve sUA goal, including those with CKD (29). Our analysis suggests that dose escalation may help to explain the associations observed between provider specialty and sUA goal attainment.
Starting allopurinol dose and anti-inflammatory prophylaxis are increasingly important considerations for prescribing providers. As such, our results require careful review. In adjusted analysis, initial allopurinol doses above 100 mg/day were associated simultaneously with greater sUA goal attainment and decreased adherence. This could indicate that higher dose allopurinol is associated with a higher risk of gout attacks , which might result in patients prematurely discontinuing ULT. Indeed, randomized controlled trials (RCT) have demonstrated higher gout flare rates with correspondingly high dropout rates due to flares among users of higher dose febuxostat (1-4). Likewise, a limited post-hoc analysis of multiple RCTs indicated that a lower sUA after beginning treatment was significantly associated with the occurrence of gout flares (31). We show that when adjusting for adherence, a higher dose increases the likelihood of achieving sUA goal. This should not be construed to indicate that a higher starting dose is optimal. Instead, a low starting dose with escalation appears to balance the benefits of increased allopurinol adherence with the ultimate need for higher doses to achieve sUA goal. Anti-inflammatory prophylaxis was unexpectedly associated with decreased allopurinol adherence. However, in the experience of the authors some patients prefer to treat their gout acutely and broad access to anti-inflammatory medications, including over-the-counter preparations, may actually discourage patients’ adherence to long-term therapeutic options. There are other potential reasons that together might help to explain our results regarding starting dose and prescription anti-inflammatory prophylaxis.
Our study has strengths that distinguish it from prior efforts. For instance, we examined only incident allopurinol use by requiring no previous dispensings in the 12-month pre-index period. Inclusion of prevalent users in other studies likely overestimates medication adherence (11, 13, 14, 21). Additionally, poor adherence or treatment discontinuation among gout patients may lead to misclassification of patients as incident allopurinol users in studies requiring shorter pre-index eligibility periods (12, 17). We were able to further validate our measure of medication adherence given its robust and independent association with sUA goal achievement while several prior investigations failed to explore this important relationship (11-13, 15). Among the three previous studies examining factors associated with sUA goal attainment, two did not examine the role of ULT adherence (17, 21) and the other reported only a crude association between allopurinol adherence and sUA goal attainment stratified by time period (14).
Despite its significant strengths, this study also has limitations. Recognizing the diagnostic uncertainty with reliance on administrative data, we attempted to limit misclassification bias by requiring a gout diagnosis, incident allopurinol dispensing and exclusion of other potential reasons for allopurinol use. Gout severity could not be addressed in our analysis, limiting understanding of differential case mix among specialties. Our analysis also did not incorporate any measure of gout flares. While a limitation, flares often go unreported to the medical system and attempts to measure flares in the medical records are prone to significant underreporting. Finally, this study represents a large cohort from an integrated healthcare delivery system. While the findings of poor quality of care are broadly reported, the relative importance of different factors represented in our models may not universally apply to other healthcare systems.
Subpopulations of gout patients are at heightened risk for ULT nonadherence and failure to attain sUA goal. At-risk populations should be targeted for interventions to improve medication adherence and promote appropriate ULT dose titration. Our study demonstrates an unmet need for improvement in gout care and identifies potential factors to target.
Acknowledgments
This paper has no financial support or other benefits from commercial sources for the work reported on in the manuscript, or any other financial interests that any of the authors may have, which could create a potential conflict of interest with regard to the work. This work was supported by a grant from the NIH/NIAIMS 1P50AR060772-01A1. Dr. Curtis is supported by the Agency for Healthcare Research and Quality (R01HS018517) and the National Institutes of Health (AR060772).
References
- 1.Becker M, Schumacher H, Wortmann R, MacDonald P, Eustace D, Palo W, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–61. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher H, Becker M, Wortmann R, Macdonald P, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: A 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540–8. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 3.Becker M, Schumacher H, Espinoza L, Wells A, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: The CONFIRMS trial. Arthritis Res Ther. 2010:12. doi: 10.1186/ar2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundy J, Baraf H, Yood R, Edwards N, Gutierrez-Urena S, Treadwell E, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: Two randomized controlled trials. JAMA. 2011;306:711–20. doi: 10.1001/jama.2011.1169. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. part II: Management. report of a task force of the EULAR standing committee for international clinical studies including therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312–24. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan K, Cameron J, Snaith M, Zhang W, Doherty M, Seckl J, et al. British society for rheumatology and british health professionals in rheumatology guideline for the management of gout. Rheumatology. 2007;46:1372–4. doi: 10.1093/rheumatology/kem056a. [DOI] [PubMed] [Google Scholar]
- 7.Khanna D, Fitzgerald J, Khanna P, Bae S, Singh M, Neogi T, et al. 2012 american college of rheumatology guidelines for management of gout. part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthrit Care Res. 2012;64:1431–46. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manara M, Bortoluzzi A, Favero M, Prevete I, Scirè C, Bianchi G, et al. Italian society of rheumatology recommendations for the management of gout. Reumatismo. 2013;65:4–21. doi: 10.4081/reumatismo.2013.4. [DOI] [PubMed] [Google Scholar]
- 9.Sarawate C, Patel P, Schumacher H, Yang W, Brewer K, Bakst A. Serum urate levels and gout flares: Analysis from managed care data. J Clin Rheumatol. 2006;12:61–5. doi: 10.1097/01.rhu.0000209882.50228.9f. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Ruiz F, Frédéric Lioté. Lowering serum uric acid levels: What is the optimal target for improving clinical outcomes in gout? Arthritis Rheum. 2007;57:1324–8. doi: 10.1002/art.23007. [DOI] [PubMed] [Google Scholar]
- 11.Sarawate C, Brewer K, Yang W, Patel P, Schumacher H, Saag K, et al. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc. 2006;81:925–34. doi: 10.4065/81.7.925. [DOI] [PubMed] [Google Scholar]
- 12.Harrold L, Andrade S, Briesacher B, Raebel M, Fouayzi H, Yood R, et al. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009:11. doi: 10.1186/ar2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riedel A, Nelson M, Joseph-Ridge N, Wallace K, MacDonald P, Becker M. Compliance with allopurinol therapy among managed care enrollees with gout: A retrospective analysis of administrative claims. J Rheumatol. 2004;31:1575–81. [PubMed] [Google Scholar]
- 14.Halpern R, Mody R, Fuldeore M, Patel P, Mikuls T. Impact of noncompliance with urate-lowering drug on serum urate and gout-related healthcare costs: Administrative claims analysis. Curr Med Res Opin. 2009;25:1711–9. doi: 10.1185/03007990903017966. [DOI] [PubMed] [Google Scholar]
- 15.Solomon D, Avorn J, Levin R, Brookhart M. Uric acid lowering therapy: Prescribing patterns in a large cohort of older adults. Ann Rheum Dis. 2008;67:609–13. doi: 10.1136/ard.2007.076182. [DOI] [PubMed] [Google Scholar]
- 16.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008 Apr;28:437–43. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh J, Hodges J, Asch S. Opportunities for improving medication use and monitoring in gout. Ann Rheum Dis. 2009;68:1265–70. doi: 10.1136/ard.2008.092619. [DOI] [PubMed] [Google Scholar]
- 18.Singh JA, Hodges JS, Toscano JP, Asch SM. Quality of care for gout in the US needs improvement. Arthritis Rheum. 2007 Jun 15;57:822–9. doi: 10.1002/art.22767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikuls T, Curtis J, Allison J, Hicks R. Medication errors with the use of allopurinol and colchicine: A retrospective study of a national, anonymous internet-accessible error reporting system. J Rheumatol. 2006;33:562–6. [PubMed] [Google Scholar]
- 20.Mikuls T, Farrar J, Bilker W, Fernandes S, Saag K. Suboptimal physician adherence to quality indicators for the management of gout and asymptomatic hyperuricaemia: Results from the UK general practice research database (GPRD). Rheumatology. 2005;44:1038–42. doi: 10.1093/rheumatology/keh679. [DOI] [PubMed] [Google Scholar]
- 21.Pandya B, Riedel A, Swindle J, Becker L, Hariri A, Dabbous O, et al. Relationship between physician specialty and allopurinol prescribing patterns: A study of patients with gout in managed care settings. Curr Med Res Opin. 2011;27:737–44. doi: 10.1185/03007995.2011.552570. [DOI] [PubMed] [Google Scholar]
- 22.Koebnick C, Langer-Gould A, Gould M, Chao C, Iyer R, Smith N, et al. Sociodemographic characteristics of members of a large, integrated health care system: Comparison with US census bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Newcomb C, Margolis D, Roy J, Hennessy S. Severe cutaneous reactions requiring hospitalization in allopurinol initiators: A population-based cohort study. Arthrit Care Res. 2013;65:578–84. doi: 10.1002/acr.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalbeth N. Management of gout in primary care: Challenges and potential solutions. Rheumatology. 2013;52:1549–50. doi: 10.1093/rheumatology/ket215. [DOI] [PubMed] [Google Scholar]
- 25.Spencer K, Carr A, Doherty M. Patient and provider barriers to effective management of gout in general practice: A qualitative study. Ann Rheum Dis. 2012;71:1490–5. doi: 10.1136/annrheumdis-2011-200801. [DOI] [PubMed] [Google Scholar]
- 26.Harrold L, Mazor K, Velten S, Ockene I, Yood R. Patients and providers view gout differently: A qualitative study. Chronic Illn. 2010;6:263–71. doi: 10.1177/1742395310378761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hande K, Noone R, Stone W. Severe allopurinol toxicity. description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47–56. doi: 10.1016/0002-9343(84)90743-5. [DOI] [PubMed] [Google Scholar]
- 28.Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol. 2006;33:1646–50. [PubMed] [Google Scholar]
- 29.Stamp L, O'Donnell J, Zhang M, James J, Frampton C, Barclay M, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63:412–21. doi: 10.1002/art.30119. [DOI] [PubMed] [Google Scholar]
- 30.Vázquez-Mellado J, Morales E, Pacheco-Tena C, Burgos-Vargas R. Relation between adverse events associated with allopurinol and renal function in patients with gout. Ann Rheum Dis. 2001;60:981–3. doi: 10.1136/ard.60.10.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker M, MacDonald P, Hunt B, Lademacher C, Joseph-Ridge N. Determinants of the clinical outcomes of gout during the first year of urate-lowering therapy. Nucleos Nucleot Nucl. 2008;27:585–91. doi: 10.1080/15257770802136032. [DOI] [PubMed] [Google Scholar]