Although plasma HDL-cholesterol levels correlate inversely with the incidence of cardiovascular disease (CVD),1 the causative nature of this relationship has been called into question by Mendelian randomization studies2 as well as several failed clinical trials involving HDL cholesterol raising drugs.3 Studies in humans have indicated that the macrophage cholesterol efflux capacity of HDL is a strong inverse predictor of subclinical atherosclerosis and CVD, and remains highly statistically significant after correction for HDL-cholesterol levels,4 suggesting that HDL cholesterol levels may be a poor surrogate for key functions of HDL mediating anti-atherogenic effects. In this issue of Circulation Research, Monette et al 5 measured acetylcholine-induced coronary artery vasodilation, an indicator of endothelial NO bioavailability, in subjects undergoing coronary angiography, and showed that the cholesterol efflux capacity of HDL correlated inversely with coronary endothelial dysfunction (ED), a key event in early atherogenesis. In contrast, HDL and LDL cholesterol levels did not correlate with coronary ED.5 However, the HDL particle concentration, as assessed by ion mobility analysis, did correlate with HDL’s cholesterol efflux capacity and inversely correlated with coronary ED, leading to the conclusion that both HDL cholesterol efflux capacity and HDL particle concentration might provide clinically useful information on ED and coronary risk5 and further supporting that HDL-mediated cholesterol efflux is directly related to suppression of atherogenesis in humans.
While the macrophage foam cell has recently received the lion’s share of attention in cholesterol efflux studies, this new human investigation spotlights the importance of endothelial cell cholesterol homeostasis, maintained by HDL and ATP Binding Cassette (ABC) transporter-mediated cholesterol efflux pathways, in the maintenance of coronary vasomotor function. Cholesterol efflux capacity of HDL was measured using a standard macrophage assay, in which the cellular ABC Transporters A1 and G1 (ABCA1 and ABCG1) promote efflux to smaller or larger HDL species, respectively.6 The same cholesterol efflux pathways have been implicated in endothelial cells.7 Interestingly, patients’ medium and large, but not small HDL particles correlated positively with cholesterol efflux capacity and inversely with ED,5 suggesting that cholesterol efflux mediated by ABCG1 could be important in maintaining coronary endothelial function. ABCG1, but not ABCA1, is highly expressed in cultured human aortic endothelial cells,7 and in mice, ABCG1 as well as Scavenger Receptor BI (SR-BI) have been shown to interact with HDL to maintain the activity of endothelial NO synthase (eNOS), preserving endothelial function.7, 8 In endothelium of the murine thoracic aorta both Abca1 and Abcg1 are induced by laminar blood flow.9 Moreover, heterozygous loss-of-function mutations of ABCA1 have been associated with decreased endothelium-dependent vasorelaxation in human forearm blood flow studies, and this was restored by infusions of cholesterol-poor reconstituted HDL particles.10 Finally, a recent study using an endothelial-specific knockout of the transporters in mice has shown that Abca1 and Abcg1 contribute independently and additively to protection from atherogenesis.11 Thus it is plausible that both ABCA1 and ABCG1 have an important role in cholesterol efflux from endothelium to HDL in both mice and humans contributing to protection from atherogenesis.
Several mechanisms linking preservation of endothelial function by HDL and cholesterol efflux have been proposed. Cholesterol efflux mediated by both ABCA1 and ABCG1 decreases the cholesterol content of caveolae, relieving the inhibitory interaction of eNOS with caveolin-1.12 ABCG1 mediates 7-ketocholesterol efflux to HDL, suppressing accumulation of reactive oxygen species that induce uncoupling of eNOS into inactive monomers.7 SR-BI mediates the HDL induced phosphorylation of eNOS at Ser1177, stimulating eNOS activation, possibly depending on cholesterol efflux.13 Several sterol efflux dependent mechanisms thus account for HDL’s capacity to preserve eNOS activity (Figure). HDL also stimulates EC NO production independent of cholesterol efflux.14 Somewhat inconsistent with the present study, it has been reported that HDL from CAD patients has a similar capacity to induce ABCA1 and ABCG1 mediated cholesterol efflux as compared to HDL from healthy subjects, but fails to stimulate eNOS activity in human aortic endothelial cells.14 HDL from CAD patients failed to induce eNOS phosphorylation at its activation site Ser1177, but induced eNOS phosphorylation at an inhibitory site Thr495.14 This was suggested to be partly the result of reduced HDL-associated paraoxonase activity, which increased malondialdehyde formation in HDL, thus activating endothelial lectin-like oxidized LDL-receptor 1 and PKCβII.14 The endothelial preserving effects of HDL and eNOS may go beyond its role in controlling coronary vasomotor function, by suppressing cytokine induced expression of vascular adhesion molecules and endothelial inflammation.14, 15 Endothelial ABCA1 and ABCG1 cholesterol efflux pathways decrease the tumor necrosis factor-α and lipopolysaccharide induced expression of vascular and intracellular adhesion molecules,11 potentially due to sustained NO production, but also likely as a consequence of decreased Toll like receptor cell surface expression in lipid rafts, similar to macrophages.16
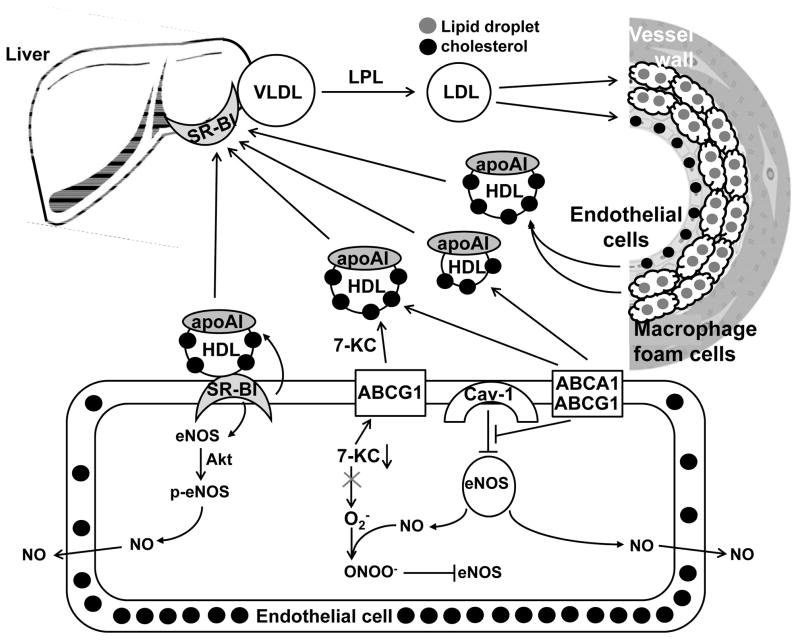
Figure. Both cholesterol efflux from macrophages and endothelial cells initiates reverse cholesterol transport, and endothelial cholesterol efflux pathways contribute to the HDL mediated preservation of eNOS activity.
Top: VLDL is produced by the liver, converted into LDL by lipoprotein lipase (LPL), and modified LDL is taken up by macrophages and endothelial cells in the vessel wall, leading to the formation of atherosclerotic plaques. Small and large-sized HDL mediate cholesterol efflux from macrophages and endothelial cells and HDL-cholesterol and cholesteryl esters are subsequently taken up by SR-BI in the liver. Bottom: magnification of an aortic endothelial cell. Left: SR-BI mediates the HDL induced phosphorylation of eNOS by Akt, stimulating eNOS activity, depending on cholesterol efflux. Middle: Large HDL induces efflux of 7-ketocholesterol (7-KC) mediated by ABCG1, preventing 7-KC accumulation, and formation of superoxide leading to eNOS uncoupling. Right: ABCA1 and ABCG1 mediate cholesterol efflux to small and large HDL, respectively, resulting in eNOS dissociating from caveolin-1 (cav-1), required for its activity.
As mentioned above, the Mendelian randomization approach has been used to argue that HDL is not in the causal pathway of atherosclerosis and thus that therapeutic approaches directed at HDL are bound to fail.2, 17 This generalization appears to be refuted by results of cholesterol efflux studies on HDL such as the present work by Monette et al,5 earlier macrophage efflux studies,4, 18, 19 as well as by numerous findings in preclinical models.20 Moreover, a recent study identified a rare loss of function variant of SCARB1, the gene encoding SR-BI.21 This variant was associated with increased plasma HDL-C and increased risk of CHD, recapitulating the findings in Scarb1−/− mice22 and indicating the key importance of HDL-mediated reverse cholesterol transport in suppressing atherogenesis.21 Together the evidence indicates that cholesterol efflux from both endothelium and macrophage foam cells, mediated by HDL and Apolipoprotein A-1 plays an important role in the suppression of atherogenesis. Challenges for the future include the development and further validation of clinically useful tests to evaluate HDL function, for example involving cholesterol efflux or HDL particle number, and the refinement of therapeutic approaches that increase cholesterol efflux and reverse cholesterol transport, rather than simply increasing HDL cholesterol levels.
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health grants HL107653 and HL87123 (to A.R. Tall). M. Westerterp was supported by VIDI grant 91715350 from the Netherlands Organization of Sciences and a Rosalind Franklin Fellowship from the University Medical Center Groningen.
Footnotes
Disclosures
A.R. Tall is a consultant to Amgen, Arisaph, and CSL. The other authors report no conflicts.
References
- 1.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The framingham study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma hdl cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenson RS. The high-density lipoprotein puzzle: Why classic epidemiology, genetic epidemiology, and clinical trials conflict? Arterioscler Thromb Vasc Biol. 2016;36:777–782. doi: 10.1161/ATVBAHA.116.307024. [DOI] [PubMed] [Google Scholar]
- 4.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monette JS, Hutchins PM, Ronsein GE, Wimberger J, Irwin AD, Tang C, Sara JD, Shao B, Vaisar T, Lerman A, Heinecke JW. Patients with coronary endothelial dysfunction have impaired cholesterol efflux capacity and reduced hdl particle concentration. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.308357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, Tall AR. Abcg1 and hdl protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008;118:3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-bi activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 9.Zhu M, Fu Y, Hou Y, Wang N, Guan Y, Tang C, Shyy JY, Zhu Y. Laminar shear stress regulates liver x receptor in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:527–533. doi: 10.1161/ATVBAHA.107.143487. [DOI] [PubMed] [Google Scholar]
- 10.Bisoendial RJ, Hovingh GK, Levels JH, Lerch PG, Andresen I, Hayden MR, Kastelein JJ, Stroes ES. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 11.Westerterp M, Tsuchiya K, Tattersall IW, Fotakis P, Bochem AE, Molusky MM, Ntonga V, Abramowicz S, Parks JS, Welch CL, Kitajewski J, Accili D, Tall AR. Deficiency of atp-binding cassette transporters a1 and g1 in endothelial cells accelerates atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.115.306670. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terasaka N, Westerterp M, Koetsveld J, Fernandez-Hernando C, Yvan-Charvet L, Wang N, Sessa WC, Tall AR. Atp-binding cassette transporter g1 and high-density lipoprotein promote endothelial no synthesis through a decrease in the interaction of caveolin-1 and endothelial no synthase. Arterioscler Thromb Vasc Biol. 2010;30:2219–2225. doi: 10.1161/ATVBAHA.110.213215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assanasen C, Mineo C, Seetharam D, Yuhanna IS, Marcel YL, Connelly MA, Williams DL, de la Llera-Moya M, Shaul PW, Silver DL. Cholesterol binding, efflux, and a pdz-interacting domain of scavenger receptor-bi mediate hdl-initiated signaling. J Clin Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besler C, Heinrich K, Rohrer L, et al. Mechanisms underlying adverse effects of hdl on enos-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121:2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in abc transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frikke-Schmidt R, Nordestgaard BG, Stene MC, Sethi AA, Remaley AT, Schnohr P, Grande P, Tybjaerg-Hansen A. Association of loss-of-function mutations in the abca1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299:2524–2532. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 18.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. Hdl cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleheen D, Scott R, Javad S, et al. Association of hdl cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes & Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westerterp M, Bochem AE, Yvan-Charvet L, Murphy AJ, Wang N, Tall AR. Atp-binding cassette transporters, atherosclerosis, and inflammation. Circ Res. 2014;114:157–170. doi: 10.1161/CIRCRESAHA.114.300738. [DOI] [PubMed] [Google Scholar]
- 21.Zanoni P, Khetarpal SA, Larach DB, et al. Rare variant in scavenger receptor bi raises hdl cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M. Influence of the high density lipoprotein receptor sr-bi on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci USA. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]