INTRODUCTION
Intracellular bacterial pathogens cause a wide range of diseases and significantly contribute to the morbidity and mortality associated with infectious diseases worldwide (1–16) (Table 1). These bacteria use several different strategies to replicate in host cells, and influence host processes such as membrane trafficking, signaling pathways, metabolism, cell death and survival (17–19). Broadly, intracellular bacteria colonize two topologically distinct regions of the host cell, and are divided into cytosolic and intravacuolar bacteria according to their intracellular lifestyle. However, most intracellular bacterial pathogens have unique intracellular life cycles with features strikingly different from one to the other (FIG. 1). It should also be noted that intravacuolar pathogens gain access to the host cytosol to some extent, and that cytosolic bacteria might spend an underestimated part on their intracellular life cycle within membrane-bound compartments (20–22).
Table 1.
Characteristics and diseases associated with intracellular pathogens that infect human myeloid cells
| Pathogen | Targeted myeloid cells | Secretion systema | Replication niche | Disease | Reference |
|---|---|---|---|---|---|
| Anaplasma phagocytophilum | Neutrophil | T4SS | Vacuole | Human granulocytic anaplasmosis | (3) |
| Brucella spp.b | Macrophage | T4SS | Vacuole | Brucellosis | (4) |
| Burkholderia pseudomallei | Macrophage | T3SS & T6SS | Cytosol | Melioidosis | (5) |
| Chlamydia pneumoniae | Macrophage | T3SS | Vacuole | Pneumonia and bronchitis | (6) |
| Citrobacter koseri | Macrophage | T3SS (putative) | Vacuole | Meningitis | (7) |
| Coxiella burnetii | Macrophage | T4SS | Vacuole | Q fever | (8) |
| Ehrlichia chaffeensis | Macrophage | T4SS | Vacuole | Ehrlichiosis | (9) |
| Francisella tularensis | Macrophage | T6SS | Cytosol | Tularemia | (10) |
| Legionella pneumophila | Macrophage | T4SS | Vacuole | Legionaire’s disease | (11) |
| Listeria monocytogenes | Macrophage | T2SS (Sec) | Cytosol | Listeriosis | (12) |
| Mycobacterium tuberculosis | Macrophage | T7SS | Vacuole | Tuberculosis | (13) |
| Rhodococcus equi | Macrophage | T2SS (Sec) | Vacuole | Pneumonia | (14) |
| Rickettsia rickettsii | Macrophage | T4SS (putative) | Cytosol | Rocky Mountain spotted fever | (15) |
| Salmonella enterica | Macrophage | T3SS & T6SS | Vacuole | Salmonellosis | (16) |
Bacterial secretion systems involved in pathogenesis.
Brucella abortus, B. canis, B. suis, and B. melitensis.
Figure 1.
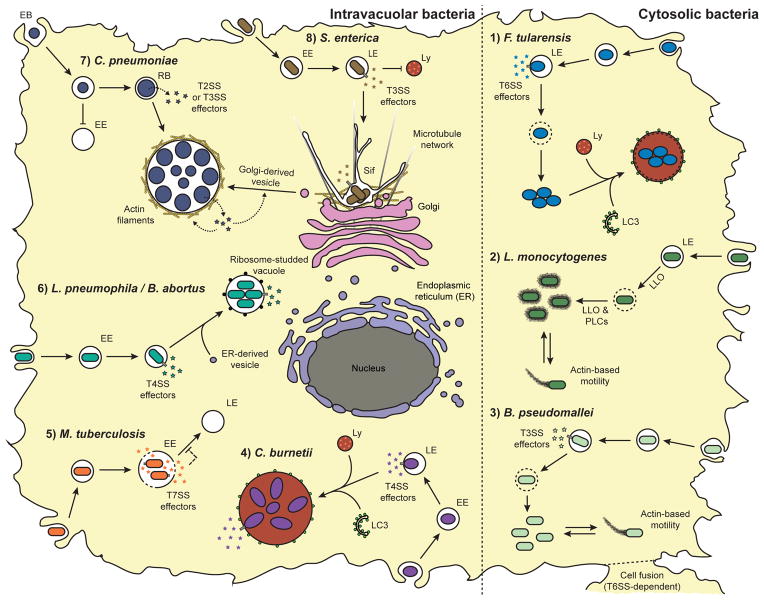
Lifestyles of intracellular bacterial pathogens. (1) F. tularensis escapes a late endosome (LE)-like vacuole in a T6SS-dependent manner. Following replication in the cytosol, F. tularensis may retranslocate to a membrane-bound compartment resembling an autolysosome. (2) L. monocytogenes escapes the phagolysosomal pathway using the T2SS (Sec) effectors LLO and PLCs. L. monocytogenes replicates rapidly in the cytosol and hijacks the host actin polymerization machinery to move within and between cells. (3) B. pseudomallei escapes into the cytosol in a T3SS-dependent manner. B. pseudomallei performs actin-based motility and promotes host cell fusion. (4) C. burnetii is adapted to the phagolysosomal pathway and resides in a spacious phagolysosomal-like compartment. The Dot/Icm system (T4SS) is required for recruiting the autophagosomal marker LC3 and for vacuole biogenesis. (5) M. tuberculosis arrests phagosome maturation at the early endosome (EE) stage in a T7SS-dependent manner. (6) L. pneumophila and B. abortus segregate from the endocytic route at the EE stage, recruit endoplasmic reticulum (ER)-derived vesicles, and form ribosome-studded specialized vacuoles in a T4SS-dependent manner. (7) C. pneumoniae segregates from the endocytic route and form a unique inclusion vacuole by recruiting Golgi-derived vesicles. C. pneumoniae effectors promote Golgi fragmentation and generate actin filaments around the inclusion. Chlamydia is found in two different forms: the non-replicating infectious elementary body (EB) and the intracytoplasmic replicative reticulate body (RB). T2SS and T3SS effectors are thought to be involved in the intracellular life cycle of Chlamydia. (8) S. enterica replicates in a late endosome (LE)-like compartment that excludes lysosomal degradation enzymes. The S. enterica containing vacuole migrates to the microtubule-organizing centre (MTOC) and forms Salmonella-induced filaments (Sif) along microtubules in a T3SS-dependent manner.
Cytosolic bacteria escape from the endocytic pathway and replicate in the host cytosol. The host cytosol indeed constitutes an attractive replicative niche for intracellular bacteria because this subcellular compartment provides an environment rich in nutrients. The cytoplasm also offers the distinct advantage of being separated from the extracellular environment, and thereby, may constitute an ideal hideout where pathogens can evade extracellular immune surveillance and killing.
Alternatively, intracellular bacterial pathogens reside and replicate within the host endomembrane system, which is comprised of an intricate network of membrane-bound organelles and vesicular trafficking intermediates. The replication of intracellular bacteria in these vesicular compartments is accompanied by concomitant vacuolar membrane expansion, which is driven by adaptive strategies from pathogens. Even though the vacuolar intracellular lifestyle requires complex host-pathogen interactions in order to maintain the unique membrane-bound replication niche, bacterial pathogens benefit from this lifestyle that provides protection from the host cytosolic innate immune defenses.
Most intracellular bacteria replicate in myeloid cells, especially in macrophages (2, 23). Macrophages are plastic cells characterized by their phenotypic diversity, and are involved in pathogen detection, antigen presentation, cytokine production, tissue reparation, and, more notoriously, microbial killing (24). These cells indeed possess an extensive antimicrobial arsenal, and are endowed with the ability to ingest and destroy microorganisms (25). The observation that most pathogenic bacteria preferentially replicate in macrophages thus constitutes a paradox (2, 17).
This chapter focuses on bacterial pathogens that have the ability to replicate in macrophages, and aims to provide an overview of the strategies deployed by these bacteria to grow intracellularly. A brief description of the defense mechanisms used by macrophages against these intracellular bacteria is provided, and the current knowledge about the pathogenic strategies specifically used by cytosolic and intravacuolar bacteria are reviewed.
DEFENSE MECHANISMS AGAINST INTRACELLULAR BACTERIA
Detection of intracellular bacterial infection by macrophages
Macrophages express a wide range of receptors that trigger innate immune responses and antimicrobial defenses upon bacterial infections (23, 26). These sensors are referred as to pattern recognition receptors (PRRs). PRRs recognized conserved microbial molecules referred to as pathogen-associated molecular patterns (PAMPs) as well as damage-associated molecular patterns (DAMPs) released in response to stress and tissue damage. There are two main classes of PRRs: the membrane-bound receptors (e.g. the Toll-like receptors (TLR)) and the cytosolic receptors (e.g. the NOD-like receptors (NLRs)).
TLRs are localized on the plasma membrane (e.g. TLR4) or on endosomal membrane compartments (e.g. TLR9), and recognize PAMPs such as lipoproteins, lipopolysaccharide (LPS), flagellin, or nucleic acids (27). Upon ligand recognition, TLRs activate signaling pathways and regulate downstream cytokine expression by interacting with adaptor proteins such as MyD88 (myeloid differentiation primary-response protein 88) and TRIF (TIR-domain-containing adaptor protein inducing interferon-β) (26).
In the cytosol, the NLR proteins NOD1 (nucleotide-binding oligomerization domain-containing protein 1) and NOD2 are triggered by the presence of peptidoglycan fragments, and activate NF-κB (28). Interestingly, it was shown that the activation of the NOD1 signaling pathway by peptidoglycan fragments is dependent on the small Rho GTPase RAC1 and, more broadly, that the manipulation of small Rho GTPases by pathogens is a process that can be detected by the host in a NOD1-dependent manner (29). Recognition of PAMPs or DAMPs by other cytosolic NLRs, such as NLRC4 (NLR family, CARD domain-containing 4), NLRP1 (NLR family, PYRIN domain-containing 1) and NLRP3, leads to the assembly of cytosolic multiprotein oligomers termed inflammasomes. In turn, inflammasomes activate caspase-1, induce the extracellular release of IL-1β and IL-18 and trigger a type of inflammatory cell death called pyroptosis (30, 31). The PYHIN member protein AIM2 (absent in melanoma 2) also activates an inflammasome in response to cytosolic DNA (32, 33) released from intracellular bacteria (34–39).
Besides AIM2, there are other PRRs that detect the presence of foreign nucleic acids in the cytosol, and trigger distinct immune responses (26). STING (stimulator of IFN genes) is important in the cytosolic response to nucleic acids (40, 41) such as DNA (42) and cyclic dinucleotides (CDNs) (43), and triggers a type I IFN response upon bacterial infection. STING directly binds to cyclic dinucleotides (CDNs), but not to DNA, and is then both a direct sensor and an adaptor molecule (44). The host cytosolic DNA-sensor cGAMP synthase (cGAS) is able to synthesis cyclic GMP-AMP (cGAMP), which is an endogenous secondary messenger that binds and activates STING (45, 46). Accordingly, cGAS is involved in the secretion of IFN-β following the detection of bacterial DNA in the host cytosol (47–49). It is noteworthy that other cytosolic PRRs also contribute to nucleic acids sensing and induce a type I IFN response including DDX41 (50, 51) and IFI16 (52).
Besides antiviral immune response, the role of type I IFN during bacterial infection is enigmatic. Many intracellular bacteria induce type I IFNs during infection, but, rather than being protective for the host, type I IFNs enhance the host susceptibility to intracellular pathogens such as Listeria monocytogenes (53, 54), Salmonella Typhimurium (55), Chlamydia trachomatis (56, 57) and Mycobacterium tuberculosis (58–61). The mechanisms by which type I IFN signaling increases host susceptibility to bacterial infection is an active area of study and is not well defined.
The phagolysosomal pathway is the front line defense against intracellular bacterial pathogens
Macrophages are proficient in the internalization and destruction of bacteria, and in promoting innate immune responses (25). Following phagocytosis, a series of coordinated fusion and fission events with specific compartments of the endocytic pathway ultimately leads to the generation of a phagolysosome. The phagolysosome possesses potent microbicidal features, and has a lumen that constitutes a highly acidic, oxidative, and degradative environment.
The acidification of the phagosome lumen is dependent on proton pumping by the V-ATPase, which is required for the optimal activity of lysosomal hydrolytic enzymes and interferes with bacterial growth by impairing the metabolism of some bacteria (25, 62). The generation of ROS (reactive oxygen species) and RNS (reactive nitrogen species) by the NOX2 NADPH oxidase (63, 64) and the inducible NOS2 nitric oxide synthase (65), respectively, are also important antimicrobial mechanisms of the phagolysosomal pathway. Limitation in the availability of essential nutrients may also impact the growth of bacteria in the phagosome. For instance, NRAMP1 (natural resistance-associated macrophage protein 1) interferes with housekeeping and antioxidative functions of some bacteria by extruding Fe2+, Zn2+ and Mn2+ from the phagosomal lumen (66). Molecules that directly compromise the integrity of bacteria such as defensins, cathelicidins, lyzozyme, endopeptidases, exopeptidases, as well as hydrolases targeting carbohydrates and lipids are also delivered to the phagosome lumen (25). The sum of these antimicrobial mechanisms defines the harsh environment that culminate in the phagolysosome, an organelle that intracellular bacteria need to avoid or adapt to in order to survive and proliferate.
Bacterial autophagy is a cell-autonomous defense mechanism
Macroautophagy (hereafter autophagy) involves the formation of a double-membrane vesicle (i.e., the autophagosome) that encloses and targets intracellular components for lysosomal degradation. During starvation, autophagy non-selectively targets parts of the cytoplasm in an attempt to maintain cellular homeostasis. Autophagy also selectively targets organelles (e.g. damaged mitochondria) and intracellular microbes. As such, autophagy contributes to innate immunity by restricting the intracellular growth of pathogens (67). Over 30 autophagy-related (ATG) proteins are involved in orchestrating autophagosome formation (68). However, some of these proteins have functions outside of the autophagy pathway and may affect host-pathogen interactions in an autophagy-independent manner (67, 69).
Initiation of autophagy is controlled by the availability of cellular nutrients and energy (70, 71), or by recognition of specific cargo (72). During autophagy, the production of phosphatidylinositol-3-phosphate (PI3P) is involved in the recruitment of ubiquitin-like conjugation systems that catalyze covalent binding of LC3 proteins to the isolation membrane (67, 72, 73). This isolation membrane is then recruited to and engulfs the cytosolic cargo. The resulting autophagosome matures into an autolysosome, in which the intraluminal content is digested (73). In the case of selective autophagy, cargo-specific receptors (74–76) and adaptor proteins containing LC3-interacting region (LIR) recruit the LC3-decorated autophagosome to the cargo (77). Ubiquitylation and ubiquitin-binding protein adaptors such as NBR1, NDP52, Optineurin and p62 are involved in the specific targeting of intracellular bacteria by autophagy (78–83).
Several alternative or non-canonical forms of autophagy do not require some of the core autophagy machinery components (84–86). Alternative autophagy mechanisms also play a role in cell-autonomous defense against intracellular microorganisms as exemplified for Toxoplasma gondii (87) and Staphylococcus aureus (88). In addition, under some circumstances, the autophagy machinery participates in a degradative process referred as to LC3-associated phagocytosis (LAP) (89–91). In contrast to canonical autophagy, LC3 is conjugated directly to the single-membrane phagosome and the ULK1 complex is dispensable during LAP (90, 91). In addition, the engagement of TLRs (89) and the NOX2-dependent production of ROS are involved in the formation of LC3-associated phagosomes (92). By promoting phagosome acidification and maturation, LAP restricts the intracellular growth of bacterial pathogens such as S. Typhimurium (92) and Burkholderia pseudomallei (93, 94).
Programmed cell death is a host defense mechanism that destroys the replication niche of intracellular bacteria
Programmed cell death plays an important role in innate immunity and is an effective way to eliminate infected cells and control infection. There are three major cell death pathways: apoptosis, programmed necrosis, and pyroptosis. These cell death pathways all contribute to the host defense against microbial infections (95, 96).
Apoptosis is a non-inflammatory form of cell death triggered by extrinsic (receptor-mediated) and intrinsic (mitochondria-mediated) pathways. Apoptosis is characterized by membrane blebbing, cell shrinkage, DNA fragmentation, increased mitochondrial permeability, and the eventual cell breakdown and release of apoptotic bodies (95, 96). Both external and internal stimuli trigger apoptosis through the activation of cysteine proteases termed caspases, which target intracellular components to induce cell death. Mitochondria are central in modulating host cell death and survival pathways, especially apoptosis (97). As such, mitochondria-dependent cell death pathways are important in host-bacteria interactions (95, 97).
Although necrosis was traditionally regarded as an accidental and uncontrolled cell death, it is now appreciated that some forms of necrosis, referred as to necroptosis, are genetically controlled (96, 98). Necrosis is a caspase-independent cell death pathway characterized by organelle damage, cell swelling, rupture of the plasma membrane, and release of the cellular content. Necrotic cell death is induced upon bacterial infection and physical damage, and is triggered by ROS, lysosomal permeabilization, calpain activation, and depletion of ATP. More specifically, necroptosis is initiated by the activation of RIP1 (receptor-interacting protein 1) and RIP3 kinases that activate downstream targets by phosphorylation and induce cell death, especially when caspase-8 is compromised (99, 100). Many proteins involved in the regulation of apoptosis also regulate necroptosis, highlighting the importance of crosstalk between these cell death pathways (101).
Pyroptosis is a non-apoptotic cell death pathway induced following inflammasome mediated caspase-1 activation. Pyroptosis is characterized by DNA fragmentation, loss of membrane integrity, and release of the cell content. In addition, pyroptosis is associated with the secretion of the pro-inflammatory cytokines IL-1β and IL-18 (30, 31). The induction of pyroptosis decreases the replication of intracellular bacteria within host cells and exposes them to the extracellular immune response (102).
Although some microbes block or delay host cell death to promote their intracellular replication at early times of infection, escape and dissemination may eventually require host cell lysis. In some cases, cell death pathways are co-opted by pathogens as a strategy of pathogenesis (103, 104). Furthermore, by inducing host cell death, bacterial pathogens can eliminate key immune cells and, consequently, evade host defenses (95).
INFECTION OF MACROPHAGES BY INTRAVACUOLAR BACTERIA
Remodeling host pathways through specialized secretion systems
During their intracellular life cycle, intravacuolar bacteria generally reside in a remodeled membrane-bound compartment. In order to successfully survive and replicate in this “sealed” environment, one of the main challenges encountered by intravacuolar pathogens is to exert actions beyond the vacuolar membrane. This is mainly accomplished using systems that secrete effectors that actively modify the host physiology to create an environment permissive to bacterial proliferation.
Protein secretion plays a central role in modulating the interactions of bacteria with their environment. This process is more complex for intravacuolar bacteria because secretion requires translocation across both the bacterial surface (plasma membrane and cell wall) and the host (plasma/vacuole) membrane. Four accessory secretion systems are known to play a role in establishing intravacuolar bacterial replication niches by delivering effector proteins into the host cytosol and by modulating a large variety of host cell functions, including vesicular trafficking and the host immune responses (Table 1).
The type III secretion system (T3SS), also called an injectisome, is found in Gram-negative bacteria that interact with both plant and animal hosts. The T3SS appears to have a common evolutionary origin with the flagellum (105). It is comprised of up to 25 proteins that form a series of rings that span the bacterial inner and outer membranes, and connect the bacterial and the host cytosol with a hollow filament (106). There are two T3SSs present in Salmonella enterica, each encoded in distinct genomic islands known as Salmonella pathogenicity island 1 (SPI-1) and SPI-2. SPI-1 confers the ability to invade nonphagocytic cells while SPI-2 allows Salmonella to survive within mammalian cells and spread to internal organs (105). In contrast, Chlamydia species possess one T3SS, which is active both at early and late phase of infection (107). Several putative Chlamydia T3SS substrates were identified using Salmonella (108), Shigella (109, 110), or Yersinia (111) as genetically tractable heterologous hosts.
Type IV secretion systems (T4SS) are extensively used by intravacuolar Gram-negative bacteria to colonize host cells. In comparison to other secretion systems, T4SS is unique in its ability to transport nucleic acid in addition to proteins into plant and animal cells, and is evolutionarily related to bacterial conjugation systems (112, 113). The T4SS can be divided into the canonical VirB (type IVA) and Dot/Icm (type IVB) secretion systems.
Both systems are multi-protein complexes that can span the bacterial surface and the host membrane. Essential roles for T4SS in pathogenesis are established in several important intravacuolar bacterial pathogens including Brucella suis (VirB) (114), Legionella pneumophila (Dot/Icm) (115, 116) and Coxiella burnetii (Dot/Icm) (117). In addition, other intravacuolar bacterial pathogens, including Anaplasma phagocytophilum and Ehrlichia chaffeensis, may also encode functional type IVA secretion systems that have a role in pathogenesis, as their putative T4SS genes were up-regulated during infection and several secreted effectors have been identified (118–120).
Type VI secretion systems (T6SS) are widely distributed in Gram-negative bacteria. The molecular mechanism by which T6SSs translocate effector proteins into target cells is similar to that of bacteriophage-like injection devices. Based on their function, T6SSs can be classified into two categories: eukaryotic cell targeting T6SS and competitor bacterial cell targeting T6SS (121, 122). For intravacuolar bacteria, the function of cell targeting is more relevant to their intracellular life cycle. Salmonella spp. harbour five phylogenetically distinct T6SSs, which are differentially distributed among serotypes (123). Two of them, SPI-6 and SPI-19, contribute to the pathogenesis of serotypes S. Typhimurium and S. Gallinarum in mouse and chicken macrophages, respectively (124, 125).
Mycobacteria are unique among intracellular bacteria due to the composition of their cell wall, which is heavily modified by lipids and termed the mycomembrane. Perhaps as a consequence of this special structure that forms an outer membrane bilayer (126), these species use a family of specialized secretion system named the type VII section systems (T7SS) (127). Putative T7SSs have been identified in some other Gram-positive organisms and are defined by the presence of two conserved elements, a membrane-bound ATPase EccC and a small secretion substrate EsxB (128, 129). M. tuberculosis encodes up to five T7SSs (ESX-1 to ESX-5), that do not functionally complement each other. The importance of these secretion systems is highlighted by the fact that loss of the ESX-1 T7SS in M. tuberculosis is the most important genetic difference between virulent strains and the attenuated vaccine strain BCG (Bacillus Calmette–Guérin) (130, 131). The detailed structure and function of T7SS are an active area of investigation (132). A current model suggests that the M. tuberculosis-containing vacuole is permeabilized by the T7SS effectors ESAT-6 and CFP-10, which allows bacterial products to access the cytosol (61, 127, 133).
Avoidance and adaptation to the phagolysosomal pathway
The majority of intravacuolar bacterial pathogens have mechanisms to avoid being trafficked to the terminal phagolysosome and generate specialized remodelled membrane-bounded compartments. Although the common function of these vacuolar compartments is to support the intracellular replication of these bacteria, the specific features of these compartments are determined in a pathogen-specific fashion. As such, intravacuolar bacteria exploit several strategies to generate their specialized vacuoles, leading to hybrid organelles that are biochemically and morphologically distinct from typical compartments found in uninfected cells (Figure 1).
Some intravacuolar bacteria actively evade the phagolysosomal pathway. Following entry, vacuoles containing Brucellae, Chlamydiae, and Legionellae rapidly diverge from the phagolysosomal pathway and fail to acquire late-phagosomal/lysosomal proteins (134). Both Legionella- and Brucella-containing vacuoles are ER-like membrane compartments and are associated with ribosomes (135, 136). Bacterial secretion systems are required for this divergent trafficking process (115, 116, 137). For example, L. pneumophila type IV effector proteins hijack the early secretory pathway and regulate vesicle traffic between the Golgi and the ER by targeting the host small GTPases Arf1 and Rab1, respectively (138–143). C. trachomatis also resides within a vacuole that is segregated from the phagolysosomal pathway. The Chlamydia-containing vacuole fuses with Golgi-derived vesicles and traffics to the Golgi area (144). The molecular mechanism of this process is still largely unknown, but the host GTPase Dynamin and Golgin-84 are involved in Chlamydia-mediated Golgi fragmentation, which is required for generating the Chlamydia replicative vacuoles (145, 146).
Mycobacteria and Salmonella exploit another strategy to avoid terminal phagolysosome formation and block phagosome maturation. Mycobacterial vacuoles are decorated with the early-endosomal marker Rab5 and exclude the late-endosomal GTPase Rab7, indicating an early arrest of the vacuole maturation (147). The type VII effector, EsxH, interacts with the host endosomal-sorting complex required for transport (ESCRT) and disrupts phagosome maturation (148). Moreover, the secreted M. tuberculosis protein tyrosine phosphatase (PtpA) prevents acidification of Mycobacterial vacuoles by binding and excluding the V-ATPase machinery from the phagosome membrane (149). Interestingly, Legionella also targets and inhibits the host V-ATPase using the type IV effector SidK (150). These suggest that targeting the vacuolar proton pump is a common strategy utilized by intravacuolar bacteria for the biogenesis of their specialized vacuoles.
In addition, the active exclusion of PI3P from the M. tuberculosis-containing vacuole (151, 152) represents another strategy of maturation arrest by removing the docking site for the Rab5 effectors EEA1 and Hrs (153–155). Conversely, S. Typhimurium increases PI3P levels on the vacuole to stimulate biogenesis of a unique compartment with properties of late endosomes, and characterized by transient acquisition of EEA1, gradual lumen acidification and acquisition of some lysosomal glycoproteins (156). However, unlike typical phagolysosomes, Salmonella-containing vacuoles are depleted of lysosomal degradation enzymes (157).
C. burnetii is a unique intravacuolar bacterium that does not follow this paradigm. C. burnetii resides in a vacuole that resembles a terminal phagolysosome and contains a variety of antimicrobial agents (158). However, instead of passively replicating in its vacuole, C. burnetii actively remodels it. For example, Coxiella is able to recruit the autophagy marker LC3 to its residing vacuole early after internalization and generate a specialized spacious vacuole in a T4SS-dependent manner (158–160).
Maintenance of vacuole integrity
The specialized vacuolar environment provides an ideal hideout from recognition by cytosolic innate immune sensors. Several studies suggest that damage to the bacterial vacuole membrane exposes the intravacuolar bacteria to the host cytosol, and compromises the ability of these bacteria to colonize host cells (161–166). In macrophages and epithelial cells, cytosolic galectins are able to bind β-galactoside-associated membrane remnants derived from ruptured vacuoles. This leads to the recruitment of autophagy adaptor proteins and targeting by the autophagy machinery (161, 162). In addition, the presence of bacterial components in the host cytosol may activate immune responses and trigger cell death (167). Therefore, maintenance of the integrity of bacteria-containing vacuoles is essential for intravacuolar bacterial pathogens. Accordingly, recent studies suggest that intravacuolar bacteria target multiple host components and signaling pathways to maintain the stability of their residing vacuoles (164, 165).
The formation of specialized bacteria-containing vacuoles largely depends on vesicle trafficking, which relies on the host cytoskeleton network. Thus, it is not surprising that intravacuolar bacterial pathogens exploit effector proteins to remodel the cytoskeleton network. Interestingly, in addition to their role as a physical and structural support for trafficking, the cytoskeleton and cytoskeletal motors also contribute to vacuole stability as exemplified by Salmonella and Chlamydia-containing vacuoles (168, 169). Interestingly, loss of vacuole integrity is observed during infection with a Chlamydia mutant lacking the bacterial protease CPAF, which digests intermediate filament proteins to form a filamentous structure around its containing vacuoles (170). Similarly, during Salmonella infection, the type III effector SifA regulates the interaction between cytoskeletal motors and the bacteria-containing vacuole (171, 172), and deletion of SifA leads to loss of vacuole integrity (165, 172, 173).
The formation of specialized bacteria-containing vacuoles is a highly ordered and tightly controlled process that requires the active modifications of the vacuole. The majority of these modifications involve the regulation of small GTPases by secreted bacterial effectors. Remarkably, in addition to their essential role in vacuole development, the timely acquisition of these small GTPases also contributes to vacuole stability (164). For example, one Legionella type IV effector, LidA, is involved in recruiting Rab1 and contributes to vacuole integrity (174). Comparatively, during S. Typhimurium infection, perturbation of GTPase activity by overexpressing dominant-positive Rab5 or dominant-negative Rab7 causes vacuole rupture and the release of bacteria into the cytosol (175).
A variety of bacterial pathogens affect the stability of the residing vacuoles by subverting their lipid composition for their own benefit. For example, certain phosphatidylinositol phosphates (PIPs) species can be either enriched or excluded from bacteria-containing vacuoles in order to regulate the binding of host signaling molecules and bacterial effectors (176). Nevertheless, instead of just providing a docking site for the recruitment of molecules, some vacuolar membrane lipids may play additional roles in vacuole stability. C. trachomatis recruits host sphingomyelin to its vacuole by incorporating lipid-droplets (111). Although the precise role of this molecule in vacuole stability is not yet clear, host sphingomyelin acquisition is absolutely required for vacuole expansion and prevents Chlamydia vacuole fragmentation (177, 178). S. Typhimurium and L. pneumophila are able to reduce cholesterol levels from their specialized vacuoles by secreting phospholipases SseJ and PlaA (179, 180), respectively. This process is thought to be beneficial to these intravacuolar bacteria by increasing membrane fluidity and inhibiting lipid rafts formation. However, exclusion of cholesterol from the vacuolar surface adversely affects its stability. S. Typhimurium and L. pneumophila use additional secreted effectors, SifA and SdhA, to counter this defect (164, 181, 182).
Autophagy is a double-edged sword for intravacuolar bacteria
Although vacuole integrity is critical for intravacuolar bacteria, interacting with the host cytosol requires some level of vacuole permeabilization. However, membrane-permeabilization leads to targeting by the selective autophagy pathway, as exemplified by M. tuberculosis (183). Exposure of M. tuberculosis DNA following vacuole permeabilization triggers ubiquitin-mediated autophagy through the cGAS/STING/TANK-binding kinase 1 (TBK1) pathway (49, 183). Similarly, S. Typhimurium accidently exposed to the cytosol are targeted by autophagy, as shown by the recruitment of LC3 and other ATG proteins to the bacteria. Adaptor proteins, such as NDP52 and optineurin, as a well as TBK1 have a role in recognizing ubiquitylated Salmonella in the cytosol (82, 184).
In order to survive and propagate in the face of host cell-autonomous defense mechanisms, intravacuolar bacteria such as M. tuberculosis and L. pneumophila antagonize the host autophagy machinery. The M. tuberculosis secreted redox regulator Eis inhibits autophagy by indirectly blocking JUN N-terminal kinase (JNK) activation (185). The suppression of the PI3-kinase VPS34 (151) and exclusion of PI3P from M. tuberculosis-containing vacuoles (152) could be another active strategy to avoid autophagy, as PI3P is required for autophagy initiation. In addition, a recent study suggests that M. tuberculosis inhibits autophagy flux in a T7SS-dependent manner, which suggests the existence of an uncharacterized autophagy modulator (186). Remarkably, the L. pneumophila effector RavZ directly and irreversibly uncouples ATG8 proteins attached to phosphatidylethanolamine on pre-autophagosomal structures (187).
In contrast, some bacteria, such as C. burnetii (159, 188, 189), B. abortus (190), and A. phagocytophilum (191, 192), subvert the autophagic pathway for the formation of their specialized vacuole. For example, pharmacological inhibition of autophagy flux arrests the biogenesis of Coxiella and Anaplasma-containing vacuoles, and impairs their growth in macrophages (189, 192). Both C. burnetii and A. phagocytophilum vacuoles are decorated with the autophagic protein beclin-1 (BECN1) (191, 193), a protein that forms a complex with VPS34 and is required for the initial steps of the autophagy pathway. Interestingly, C. burnetii infection also induces recruitment of the anti-apoptotic protein B cell lymphoma 2 (BCL-2) to its vacuole surface, and the interaction between BECN1 and BCL-2 inhibits host apoptosis (193). Therefore, some intravacuolar bacteria use the autophagy pathway to promote intracellular replication and to block apoptosis (194). It is also possible that the autophagy pathway plays an important role in providing nutrients to the specialized vacuoles of these pathogens (67, 194). One C. burnetii T4SS effector protein, Cig2, is involved in recruiting autophagosomes to Coxiella vacuoles (159). However, the detailed mechanism of how autophagosomes are recruited and incorporated into vacuoles still needs to be elucidated, although bacterial secretion systems and effectors are likely to play a pivotal role in these processes (67).
Manipulation of host cell death by intravacuolar bacteria
Even though intravacuolar bacteria reside in an environment that prevents host cytosolic sensing, the subversion of the host processes required to generate their specialized vacuoles can pose stresses to eukaryotic cells and trigger host cell death. Hence, intravacuolar bacterial pathogens have developed a myriad of strategies to modulate host cell death pathways (96, 195).
Mitochondria play a central role in modulating host cell death and survival. As such, many intravacuolar bacteria subvert mitochondria-dependent cell death pathways. L. pneumophila and C. burnetii secrete the type IV effector proteins SidF and AnkG into the host cytosol and target the proapoptotic factors BNIP3 and P32, respectively, thereby inhibiting mitochondria-mediated apoptotic signaling (196, 197). Similarly, C. trachomatis inhibits apoptosis by secreting the protease CPAF which degrades pro-apoptotic BH3-only proteins, including BIM, PUMA, and BAD (198, 199).
In addition to dampening pro-apoptotic pathways, activation of pro-survival pathways is another mechanism utilized by intravacuolar bacteria to prevent host cell death. The master regulators of the innate immune response NF-κB promotes cell survival, and the subversion of the NF-κB pathway also represents a common bacterial strategy to modulate host cell death. For example, the L. pneumophila effector LegK1 phosphorylates and inactivates the NF-κB inhibitor IκBα and p100, thus enhancing the activation of the NF-κB pro-survival pathway (200). Some intravacuolar bacteria alter the activity of host kinases that are linked to NF-κB activation. One S. Typhimurium effector, AvrA, has acetyltransferase activity that targets MAPKKs and strongly inhibits the JNK signaling pathway (201), which helps dampen inflammatory and cell death responses. Moreover, although the molecular mechanisms are still largely unknown, Akt activation and enhancement of cell survival have been observed during C. trachomatis (202), C. burnetii (203), and S. Typhimurium (204) infections.
Although intravacuolar bacteria are separated from most cytosolic immune sensing mechanisms, their specialized secretion systems poke holes in host membranes and can be detected by the host cell. It has been shown that the basal body rod component of the T3SS SPI-1 apparatus of S. Typhimurium (PrgJ) is detected by NLRC4 and triggers inflammasome activation. However, SsaI, the equivalent basal body rod component of the T3SS SPI-2, is not detected by NLRC4. This constitutes an evasion strategy required for S. Typhimurium virulence (205).
INFECTION OF MACROPHAGES BY CYTOSOLIC BACTERIA
General strategies deployed by cytosolic bacteria to infect macrophages
During their intracellular life cycle, B. pseudomallei, Francisella tularensis, and L. monocytogenes escape from a phagosome, replicate in the macrophage cytosol, and manipulate host immune responses (18). In contrast to F. tularensis, both B. pseudomallei and L. monocytogenes exploit the host actin polymerization machinery to move within the cytosol and spread from cell-to-cell (206). The intracellular life cycles of B. pseudomallei and F. tularensis also have unique features such as the formation of multinucleated giant cells (MNGC) (207, 208) and the translocation to a membrane-bound compartment subsequent to replication in the host cytosol (22), respectively. It is noteworthy that other cytosolic bacterial pathogens such as Mycobacterium marinum, Rickettsia spp. and Shigella flexneri are also known to infect macrophages (18). However, macrophages are not the primary cells targeted during Rickettsial infections (209), and Shigella spp. induce macrophage cell death instead of replicating intracellularly as a strategy of pathogenesis (210–212).
Cytosolic bacteria secrete virulence factors within the host cells in order to establish infection. In L. monocytogenes, the virulence factors involved in phagosomal escape and cell-to-cell spread are well characterized, and are mostly encoded on the Listeria pathogenicity island-1 (LIPI-1) (213, 214). L. monocytogenes mainly relies on the canonical Sec translocation system to secrete virulence factors (215, 216) while the translocation of effectors in B. pseudomallei and F. tularensis depends on specialized secretion systems. In B. pseudomallei, a genetic locus with similarity to the Salmonella and Shigella type III secretion system (T3SS) is required for replication in host cells (217, 218). A type VI secretion system (T6SS) also contributes to the cell-to-cell spread ability and the virulence of B. pseudomallei, but the effectors secreted by this system are not well characterized (219, 220). Interestingly, it was recently shown that the T6SS effector VrgG5 mediates host cell fusion (221, 222), which is suggested to be required for intercellular spread (223). Similarly, several virulence determinants involved in the intracellular life cycle of F. tularensis are encoded on the Francisella pathogenicity island (FPI) (224, 225). A number of the genes within this island share sequence homology to the Type VI secretion system gene clusters found in Vibrio cholerae and Pseudomonas aeruginosa (226, 227), and the products of at least two of these genes are translocated in the host cytosol during infection (228).
Mechanisms used by cytosolic bacteria to escape phagosomes
The ability to escape from vacuoles is crucial to cytosolic pathogens. Following internalization, cytosolic bacteria escape from a phagosomal compartment in as early as 5 minutes, which may reflect a need to escape the vacuole before fusion with the lysosome (18). Cytosolic bacteria that exploit actin-based motility to spread from cell-to-cell also encounter a double-membrane vesicle referred as to the secondary vacuole. Escape from the secondary vacuole seems to process in a similar manner to the escape from the primary vacuole, at least for L. monocytogenes and S. flexneri (18).
L. monocytogenes avoids fusion with lysosomes, and escapes an acidified compartment with features of a late endosome (229, 230). More specifically, L. monocytogenes escapes the phagosome using the cholesterol-dependent cytolysin (CDC) Listeriolysin O (LLO) (231), which forms pores in vacuoles, blocks the trafficking of the bacteria within the endosomal pathway (229, 230), and ultimately promotes membrane disruption (232–234). LLO activity is compartmentalized by its acidic pH optimun, which prevents damage to the host cell (235). In addition, the reduction of LLO by GILT (γ-Interferon-inducible lysosomal thiol reductase) (236) and the CFTR (Cystic fibrosis transmembrane conductance regulator)-dependent increase in chloride concentration (237) potentiate the activity of LLO within the phagosome. The escape of L. monocytogenes from vacuoles is also facilitated by a phosphatidylinositol-specific phospholipase C (PlcA) and a broad-range phospholipase C (PlcB) (238). Whereas Goldfine and colleagues showed that PlcA promotes phagosomal escape by activating the host PKCβ (239, 240), the involvement of PlcB in this process is attributed to a wide-spectrum activity again phospholipids and to an ability to mediate membrane fusion (241, 242). The interplay between LLO and the phospholipases C (PLCs) is not completely understood, but it is possible that these Listerial factors synergistically destabilize the membrane to promote optimal escape from the phagosome. It has also been suggested that PLCs translocate into the host cytosol through LLO pores (243).
The detailed molecular mechanisms involved in phagosomal escape are mostly uncharacterized in most other cytosolic bacterial pathogens. B. pseudomallei mutants lacking components of the T3SS and, more specifically, the T3SS effector BopA have a defect in the escape from the phagosome (93, 217). In F. tularensis, the secretion system encoded by the FPI is required for phagosomal escape, but the specific set of virulence factors involved is not well characterized (225, 228, 244–246). Interestingly, the Shigella effectors IpaB and IpaC, like LLO, form a pore complex that binds cholesterol and inserts into cell membranes (247, 248), and Rickettsia spp. produces a haemolysin and phospholipases that may play a role in escape from vacuoles (249–252).
Sensing of the vacuole to cytosol transition by cytosolic bacteria
Cytosolic bacteria are likely to exploit distinct sets of virulence factors at different stages of their intracellular life cycle. The sensing of the environmental changes encountered throughout their passage within the host cell is likely to be critical for the tight regulation of their virulence factors. However, although it is established that cytosolic bacteria induce the expression of specific sets of genes during intracellular infections (253–256), less is known about their spatial and temporal regulation within the intracellular niche. The intricacies of virulence factors regulation is exemplified by the on/off expression of the S. flexneri T3SS apparatus during cell infection, which is specifically activated during bacterial entry (257).
The Crp-family member transcription factor PrfA up-regulates virulence factors expression once L. monocytogenes reaches the host cytosol (258, 259). The activity of PrfA integrates both environmental and bacterial signals, and is regulated by temperature (260), a bacterial auto-repressor (261), and the availability of specific nutrients (262–268). Reniere et al. (269) recently identified that bacterial and host derived glutathione allosterically binds and activates PrfA in the host cytosol. Given that enhanced PrfA activation is dispensable for vacuole escape (270), the authors suggested a two-step activation mechanism based on the oxidation-reduction states of PrfA thiols and glutathione in the vacuole (oxidizing/repressing environment) and in the cytosol (reducing/activating environment). Although it is suggested that S. flexneri also takes advantage of an intracellular signal to activate the MxiE transcription factor during infection (271), the environmental cues and the bacterial regulatory machinery used to regulate virulence factors expression are still largely unknown in other cytosolic bacteria.
Manipulation of host sensing pathways by cytosolic bacteria
Although cytosolic bacteria are protected from the extracellular immune responses and escape the microbicidal lysosomal environment, their detection by cytosolic PRRs activates defense mechanisms including autophagy, host cell death pathways, and secretion of cytokines. However, under some instances, it should be noted that the detection by the host cell and the subsequent consequences might be advantageous for the pathogen. For example, as discussed above, there is increasing evidence that some intracellular bacteria benefit from IFN-β signaling (272).
The notion that cytosolic pathogens employ active mechanisms to delay or redirect the immune response is attractive, but is supported by few studies. L. monocytogenes might dampen the host immune response by secreting the virulence factor InlC and by interfering with the activation of NF-κB during intracellular infection (273), but it is unclear whether this has an impact on virulence (274). It is also speculated that the active modulation of the immune response by F. tularensis contributes to pathogenesis, although the mechanisms remain poorly understood (224, 275). The ability of L. monocytogenes to grow in host cells without inducing inflammasomes activation is quite striking, and might involved regulatory mechanisms that limit flagellin expression during infection in order to avoid detection by NAIP5/NLRC4 (30, 276, 277). It is also plausible that L. monocytogenes avoids autolysis in host cells to minimize detection by the AIM2 inflammasome. Sauer et al. (37) demonstrated that while L. monocytogenes mutants with cell wall defects lyse within host cells and induce the AIM2 inflammasome, wild-type bacteria showed very little intracellular bacteriolysis and induced low levels of pyroptosis. Conversely, the activation of the AIM2/ASC inflammasome plays a pivotal role in the innate immune response to F. tularensis, and leads to the control of bacterial replication both in macrophages and in vivo (34–36, 278). However, the F. tularensis protein encoded by FTL-0325 delays inflammasome activation during infection (279), possibly by contributing to the structural integrity of the bacterial surface rather than by actively repressing host inflammasome activation (280). Interestingly, it was recently shown that the S. flexneri T3SS effector OspC3 inhibits the induction of a non-canonical inflammasome by directly targeting caspase-4, the human ortholog of mouse caspase-11 (281). Nevertheless, it is not known whether this mechanism is active in myeloid cells or if other cytosolic pathogens use a similar strategy to inhibit inflammasome activation.
Interaction of cytosolic bacteria with the autophagy pathway
Infection of host cells by cytosolic pathogens such as L. monocytogenes and S. flexneri leads to induction of autophagy (282–284), and not surprisingly, mechanisms of autophagy evasion are described for most cytosolic bacterial pathogens. L. monocytogenes uses ActA and InlK to recruit host proteins (Arp2/3 and Ena/VASP, and the Major Vault Protein) to the bacterial surface and interfere with autophagic recognition (78, 285). L. monocytogenes also causes stalling of pre-autophagosomal structures by reducing the autophagic flux and PI3P levels in a PLCs-dependent manner (283). Accordingly, it was recently demonstrated that the inactivation of both ActA and PlcA drastically impairs the ability of L. monocytogenes to avoid autophagy and to grow within macrophages (286). S. flexneri also interferes with autophagic recognition by secreting IcsB, which competes with ATG5 for binding to the bacterial protein VirG/IcsA (287). Interestingly, the F. tularensis polysaccharidic O-antigen contributes to autophagy evasion by preventing ubiquitylation and the recruitment of the autophagy adaptor p62 (288). Overall, these studies clearly demonstrate that interference with autophagy is common among cytosolic bacteria.
Instead of being targeted by macroautophagy, B. pseudomallei is targeted by LAP (93). B. pseudomallei mutants lacking the T3SS effector BopA showed a defect in escaping single-membrane vacuoles and an increased colocalization with LC3 (93). Although the B. pseudomallei proteins BimA and BopA have high homology with the S. flexneri proteins IcsA and IcsB, it was suggested that they interfere with autophagy through different mechanisms (289, 290). However, recent data suggests that IcsB not only interferes with autophagic recognition of the bacterial surface, but also inhibits LAP or LC3 recruitment to vacuolar remnants early during S. flexneri infection (291). In addition, L. monocytogenes is also targeted by LAP early during macrophage infection (292). Overall, these studies suggest that the autophagy machinery can target cytosolic bacteria at the entry stage of their intracellular life cycle.
In most cases, it is assumed that the activity of the autophagy pathway is detrimental for cytosolic bacteria although it might not always be the case. For example, a proportion of F. tularensis re-enters a membrane-bound compartment with autolysosomal features subsequent to replication in the cytosol (22), but it is not clear whether this represents a mechanism of dissemination or a host cellular defense mechanism. Evidence also suggests that autophagy provides F. tularensis with the nutrients required for intracellular replication (293). It is thus plausible that cytosolic bacteria benefit from an increase in nutrient availability resulting from autophagy activation.
Manipulation of host cell death pathways by cytosolic bacteria
Although cytosolic bacteria may protect the intracellular replication niche by suppressing host cell death pathways, death of the infected host cell is inevitable because the host cell has multilayered mechanisms to induce cell death pathways in response to bacterial infections. For example, infection of macrophages with B. pseudomallei induces an early NLRC4-dependent pyroptosis, and late caspase-1-dependent and -independent cell death pathways (294). More specifically, induction of apoptosis was observed in caspase-1/11-deficicent macrophages infected with B. pseudomallei (294). Similarly, caspase-1-deficient macrophages infected with F. tularensis undergo AIM2/ASC-dependent caspase-8-mediated apoptosis (295). As already mentioned, F. tularensis delays inflammasome activation during the early stages of infection (279), but also uses TolC-secreted effectors to delay the activation of the intrinsic apoptotic pathway in order to preserve the host cell (296). This suggests that cytosolic bacteria have to interact with several host pathways in order to inhibit, or at least delay, cell death. Nevertheless, our understanding of the mechanisms used by cytosolic bacteria to inhibit host cell death pathways remains mostly superficial.
Another strategy used by cytosolic bacteria to maintain their replication niche is to induce host pro-survival pathways. For example, S. flexneri and R. rickettsii activate pro-survival pathways to dampen cell death signals in nonphagocytic cells (297–299), but it is unknown whether these pathways also promote macrophage survival during infection. Cytosolic bacteria such as L. monocytogenes damage host membranes during their intracellular life cycle. However, in the case of L. monocytogenes, LLO has several mechanisms to minimize damage to the host cell membranes (231). In addition, adaptive mechanisms are likely to be deployed by the host cell to withstand and repair the lesions, and thus promote survival (300). The induction of autophagy is a common host response to several bacterial toxins, and might constitute a host protection or detoxification mechanism (300, 301). Interestingly, a tight relationship exists between autophagy and apoptosis, and it is possible that bacterial pathogens target host components shared by both pathways to promote survival of the host cell (301).
Some cytosolic bacteria may benefit from inducing a specific host cell death pathway during infection. For example, it was suggested that the accumulation of apoptotic cell debris leads to alternative macrophage activation and decreased bacterial clearance during F. tularensis infection (302). It thus seems conceivable that F. tularensis prevents early cell death induction to allow intracellular bacterial replication (279, 296), but then specifically induces apoptosis to promote an anti-inflammatory immune response (302, 303). Interestingly, B. pseudomallei deliver a cycle-inhibiting factor homolog (CHBP) that triggers macrophage-specific apoptosis (304), but whether this leads to an anti-inflammatory immune response remains to be determined. Although macrophages infected with L. monocytogenes do not undergo apoptotic cell death (305), L. monocytogenes exploits the process of apoptotic cell death clearance by macrophages (i.e., efferocytosis) to facilitate cell-to-cell spread and dissemination (306). Overall, these studies suggest that cytosolic bacteria manipulate the host apoptotic pathway to their own advantage during intracellular infection.
CONCLUDING REMARKS
Macrophages, as major immune sentinel cells, play an essential role in sensing and destroying invading microorganisms, and release cytokines that shape the immune response of other cells. In comparison to extracellular bacterial pathogens, which are fully expose to all immune cells and components, intracellular bacteria hide and replicate within host cells. Paradoxically, many intracellular bacterial pathogens preferentially replicate within macrophages. Following long-term evolutionary selection, these highly adapted intracellular bacteria have selected a specific replication niche inside these host cells and have evolved sophisticated mechanisms that facilitate their intracellular replication (Fig. 1). The study of these bacterial pathogens has led to numerous mechanistic insights on basic host processes including endocytosis, vesicle trafficking and innate immune responses.
As reviewed here, intracellular bacteria have to deal with a variety of host defense mechanisms after being phagocytosed by macrophages (Table 2). In order to facilitate a comparative analysis of bacterial counter strategies, we exemplify some pathogen-host interactions that illustrate how these organisms use their effector arsenal to face the challenges encountered during an intracellular infection (Table 3). A delicate balance exists between the ability of the host cell to control invading microbes and the ability of pathogens to hide from or subvert these defenses. Due to the length of this review, we are not able to cover all aspects of host-pathogen interaction, but we highlight some of the most important and current topics in the field. Importantly, several interesting questions are still not fully understood. For example, both M. tuberculosis and M. marinum encode ESX-1 type VII secretion systems, however, they replicate in a vacuolar compartment and in the cytosol, respectively. It is puzzling why these genetically related bacteria that share common secretion systems replicate in distinct intracellular niches. Also, the function of type I interferon induction during intracellular bacterial infection is still elusive. Elucidating the molecular mechanisms underlying pathogen subversion strategies and how they specifically affect innate immune responses is critical to a comprehensive understanding of bacterial pathogenesis and the host response. In addition, the study of host-intracellular bacteria interactions may provide clues for advancing the development of novel therapeutics against pathogens and inflammatory diseases.
Table 2.
General strategies used by intravacuolar and cytosolic bacteria to deal with challenges encountered within host cells
| Challenge | Strategies utilized by intracellular bacteria | |
|---|---|---|
| Intravacuolar | Cytosolic | |
| Phagolysosomal pathway | Avoidance, blockage and adaptation | Escape into the cytosol |
| Access to nutrients | Hijack host vesicle trafficking to promote vacuole biogenesis and nutrient acquisition | Direct access to cytosolic nutrients |
| Microenvironment | Intravacuolar bacteria create and enlarge their replication space using a variety of effectors | The cytosol constitutes a spacious and favorable environments |
| Innate recognition | The vacuolar compartment provides a hideout from cytosolic innate sensinga | Cytosolic bacteria are directly exposed to the cytosolic sensing machinery, and need to down- regulate PAMP expression to delay recognition |
| Autophagy | Maintenance of vacuole integrity Inhibition of autophagy initiation Inhibition of autophagy flux |
Inhibition of autophagy initiation Inhibition of autophagy flux Prevention of autophagic recognition |
| Host cell death | Dampening of pro-apoptotic pathways Activation of pro-survival pathways |
Interference with inflammasone activation Dampening of pro-apoptotic pathways Activation of pro-survival pathways |
Some components of intravacuolar bacterial pathogens can be recognized by host cytosolic sensing mechanisms due to damage to the pathogen-containing vacuoles or effector translocation by specialized secretion systems. For example, DNA from M. tuberculosis, PrgJ from S. enterica and the flagellum of L. pneumophila are recognized by the host cytosolic sensing machinery.
Table 3.
Examples of factors used by intracellular bacteria to counteract host defense mechanisms
| Subverted host defense mechanism | Strategies | Pathogen | Example of bacterial factors |
|---|---|---|---|
| Immune detection | Actin-based cell-to-cell spread | B. pseudomallei | BimA |
| L. monocytogenes | ActA | ||
| Modification of the immune response | F. tularensis | T6SS effectors | |
| L. monocytogenes | InlC | ||
| Low flagellin expression | L. monocytogenes | MogR | |
| Maintenance of vacuole integrity | C. pneumoniae | CPAF | |
| L. pneumophila | LidA, PlaA, SdhA | ||
| S. Typhimurium | SseJ, SifA | ||
| Phagolysosomal pathway | Blockage of phagosome maturation | L. pneumophila | SidK |
| M. tuberculosis | EsxH, PtpA | ||
| S. Typhimurium | Unknown | ||
| Escape from the vacuole | B. pseudomallei | BopA | |
| F. tularensis | T6SS effector(s) | ||
| L. monocytogenes | LLO, PLCs | ||
| Evasion of the endocytic pathway | Brucella spp. | VirB(T4SS) effectors | |
| C. trachomatis | Unknown | ||
| L. pneumophila | Dot/Icm(T4SS) effectors | ||
| Phagosome permeabilization | M. tuberculosis | ESAT-6, CFP-10 | |
| Survival in a lysosome-like structure | C. burnetii | Dot/Icm(T4SS) effectors | |
| Autophagy | Escape from LAP | B. pseudomallei | BopA |
| Formation of a replication niche | A. phagocytophilum | Ats-1 | |
| Brucella spp. | VirB(T4SS) effectors | ||
| C. burnetii | Dot/Icm(T4SS) effectors | ||
| Interference with recognition | F. tularensis | O-antigen | |
| L. monocytogenes | ActA, InlK | ||
| Inhibition of autophagy initiation/flux | L. monocytogenes | PLCs | |
| L. pneumophila | RavZ | ||
| M. tuberculosis | Eis | ||
| Nutrients acquisition | F. tularensis | Unknown | |
| Cell death | Inhibition/delay of apoptosis | C. burnetii | AnkG |
| C. trachomatis | CPAF | ||
| F. tularensis | TolC-dependent | ||
| L. pneumophila | SidF | ||
| L. pneumophila | LegK1 | ||
| S. Typhimurium | AvrA | ||
| Induction of apoptosis | B. pseudomallei | CHBP | |
| Hijacking of efferocytosis | L. monocytogenes | LLO, ActA | |
| Evasion of inflammasome | S. Typhimurium | SsaI |
Acknowledgments
We thank Thomas P. Burke for the critical reading of this manuscript. This work was supported by National Institutes of Health grants 1PO1 AI63302 (D.A.P.) and 1R01 AI27655 (D.A.P.). GM was supported by fellowships from Fonds de recherche santé Québec (FRSQ), and the Natural Sciences and Engineering Research Council of Canada (NSERC). CC was supported by NRSA Institutional Training Grants 1T32AI100829. Daniel A. Portnoy has a consulting relationship with and a financial interest in Aduro Biotech, and both he and the company stand to benefit from the commercialization of the results of his research.
References
- 1.Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366:454–461. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- 2.Price JV, Vance RE. The macrophage paradox. Immunity. 2014;41:685–693. doi: 10.1016/j.immuni.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 5.Piggott JA, Hochholzer L. Human melioidosis. A histopathologic study of acute and chronic melioidosis. Arch Pathol. 1970;90:101–111. [PubMed] [Google Scholar]
- 6.Grayston JT, Aldous MB, Easton A, Wang SP, Kuo CC, Campbell LA, Altman J. Evidence that Chlamydia pneumoniae causes pneumonia and bronchitis. J Infect Dis. 1993;168:1231–1235. doi: 10.1093/infdis/168.5.1231. [DOI] [PubMed] [Google Scholar]
- 7.Gross RJ, Rowe B, Easton JA. Neonatal meningitis caused by Citrobacter koseri. J Clin Pathol. 1973;26:138–139. doi: 10.1136/jcp.26.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(Suppl 1):S45–51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- 10.Sjostedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci. 2007;1105:1–29. doi: 10.1196/annals.1409.009. [DOI] [PubMed] [Google Scholar]
- 11.Phin N, Parry-Ford F, Harrison T, Stagg HR, Zhang N, Kumar K, Lortholary O, Zumla A, Abubakar I. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect Dis. 2014;14:1011–1021. doi: 10.1016/S1473-3099(14)70713-3. [DOI] [PubMed] [Google Scholar]
- 12.Hof H. History and epidemiology of listeriosis. FEMS Immunol Med Microbiol. 2003;35:199–202. doi: 10.1016/S0928-8244(02)00471-6. [DOI] [PubMed] [Google Scholar]
- 13.Daniel TM. The history of tuberculosis. Respir Med. 2006;100:1862–1870. doi: 10.1016/j.rmed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Prescott JF. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991;4:20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrell GT. Rocky Mountain spotted fever. Medicine (Baltimore) 1949;28:333–370. doi: 10.1097/00005792-194912000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Blaser MJ, Newman LS. A review of human salmonellosis: I. Infective dose. Rev Infect Dis. 1982;4:1096–1106. doi: 10.1093/clinids/4.6.1096. [DOI] [PubMed] [Google Scholar]
- 17.Thi EP, Lambertz U, Reiner NE. Sleeping with the enemy: how intracellular pathogens cope with a macrophage lifestyle. PLoS Pathog. 2012;8:e1002551. doi: 10.1371/journal.ppat.1002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol. 2009;7:333–340. doi: 10.1038/nrmicro2112. [DOI] [PubMed] [Google Scholar]
- 19.Alix E, Mukherjee S, Roy CR. Subversion of membrane transport pathways by vacuolar pathogens. J Cell Biol. 2011;195:943–952. doi: 10.1083/jcb.201105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredlund J, Enninga J. Cytoplasmic access by intracellular bacterial pathogens. Trends Microbiol. 2014;22:128–137. doi: 10.1016/j.tim.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Birmingham CL, Canadien V, Kaniuk NA, Steinberg BE, Higgins DE, Brumell JH. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature. 2008;451:350–354. doi: 10.1038/nature06479. [DOI] [PubMed] [Google Scholar]
- 22.Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci U S A. 2006;103:14578–14583. doi: 10.1073/pnas.0601838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pluddemann A, Mukhopadhyay S, Gordon S. Innate immunity to intracellular pathogens: macrophage receptors and responses to microbial entry. Immunol Rev. 2011;240:11–24. doi: 10.1111/j.1600-065X.2010.00989.x. [DOI] [PubMed] [Google Scholar]
- 24.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 26.Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013;13:551–565. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 28.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 29.Keestra AM, Winter MG, Auburger JJ, Frassle SP, Xavier MN, Winter SE, Kim A, Poon V, Ravesloot MM, Waldenmaier JF, Tsolis RM, Eigenheer RA, Baumler AJ. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1. Nature. 2013;496:233–237. doi: 10.1038/nature12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Moltke J, Ayres JS, Kofoed EM, Chavarria-Smith J, Vance RE. Recognition of bacteria by inflammasomes. Annu Rev Immunol. 2013;31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 31.Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015 doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7:412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchiya K, Hara H, Kawamura I, Nomura T, Yamamoto T, Daim S, Dewamitta SR, Shen Y, Fang R, Mitsuyama M. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J Immunol. 2010;185:1186–1195. doi: 10.4049/jimmunol.1001058. [DOI] [PubMed] [Google Scholar]
- 39.Warren SE, Armstrong A, Hamilton MK, Mao DP, Leaf IA, Miao EA, Aderem A. Cutting edge: Cytosolic bacterial DNA activates the inflammasome via Aim2. J Immunol. 2010;185:818–821. doi: 10.4049/jimmunol.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu HB. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, Hyodo M, Hayakawa Y, Woodward JJ, Portnoy DA, Vance RE. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun. 2011;79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Yeruva L, Marinov A, Prantner D, Wyrick PB, Lupashin V, Nagarajan UM. The DNA sensor, cyclic GMP-AMP synthase, is essential for induction of IFN-β during Chlamydia trachomatis infection. J Immunol. 2014;193:2394–2404. doi: 10.4049/jimmunol.1302718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen K, Prabakaran T, Laustsen A, Jorgensen SE, Rahbaek SH, Jensen SB, Nielsen R, Leber JH, Decker T, Horan KA, Jakobsen MR, Paludan SR. Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 2014;33:1654–1666. doi: 10.15252/embj.201488029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS. cGAS detects Mycobacterium tuberculosis DNA to activate type I interferon and autophagy. Cell Host Microbe. doi: 10.1016/j.chom.2015.05.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, Zaver SA, Schenk M, Zeng S, Zhong W, Liu ZJ, Modlin RL, Liu YJ, Cheng G. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13:1155–1161. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, Miller JF, Cheng G. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagarajan UM, Prantner D, Sikes JD, Andrews CW, Jr, Goodwin AM, Nagarajan S, Darville T. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect Immun. 2008;76:4642–4648. doi: 10.1128/IAI.00629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu H, Fan Y, Joyee AG, Wang S, Han X, Bai H, Jiao L, Van Rooijen N, Yang X. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J Immunol. 2008;181:2092–2102. doi: 10.4049/jimmunol.181.3.2092. [DOI] [PubMed] [Google Scholar]
- 58.Desvignes L, Wolf AJ, Ernst JD. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J Immunol. 2012;188:6205–6215. doi: 10.4049/jimmunol.1200255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, Barry C, Kaplan G. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25:694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- 60.Dorhoi A, Yeremeev V, Nouailles G, Weiner J, 3rd, Jorg S, Heinemann E, Oberbeck-Muller D, Knaul JK, Vogelzang A, Reece ST, Hahnke K, Mollenkopf HJ, Brinkmann V, Kaufmann SH. Type I IFN signaling triggers immunopathology in tuberculosis-susceptible mice by modulating lung phagocyte dynamics. Eur J Immunol. 2014;44:2380–2393. doi: 10.1002/eji.201344219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe. 2012;11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huynh KK, Grinstein S. Regulation of vacuolar pH and its modulation by some microbial species. Microbiol Mol Biol Rev. 2007;71:452–462. doi: 10.1128/MMBR.00003-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 64.Minakami R, Sumimotoa H. Phagocytosis-coupled activation of the superoxide-producing phagocyte oxidase, a member of the NADPH oxidase (nox) family. Int J Hematol. 2006;84:193–198. doi: 10.1532/IJH97.06133. [DOI] [PubMed] [Google Scholar]
- 65.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 66.Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Huang J, Brumell JH. Bacteria-autophagy interplay: a battle for survival. Nat Rev Microbiol. 2014;12:101–114. doi: 10.1038/nrmicro3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 69.Bestebroer J, V’Kovski P, Mauthe M, Reggiori F. Hidden behind autophagy: the unconventional roles of ATG proteins. Traffic. 2013;14:1029–1041. doi: 10.1111/tra.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 71.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, Gustafsson AB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem. 2012;287:19094–19104. doi: 10.1074/jbc.M111.322933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, Huang L, Xue P, Li B, Wang X, Jin H, Wang J, Yang F, Liu P, Zhu Y, Sui S, Chen Q. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 76.Novak I, Kirkin V, McEwan DG, Zhang J, Wild P, Rozenknop A, Rogov V, Lohr F, Popovic D, Occhipinti A, Reichert AS, Terzic J, Dotsch V, Ney PA, Dikic I. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boyle KB, Randow F. The role of ‘eat-me’ signals and autophagy cargo receptors in innate immunity. Curr Opin Microbiol. 2013;16:339–348. doi: 10.1016/j.mib.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 78.Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M, Mimuro H, Nakagawa I, Yanagawa T, Ishii T, Kakizuka A, Sztul E, Chakraborty T, Sasakawa C. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat Cell Biol. 2009;11:1233–1240. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- 79.Mostowy S, Sancho-Shimizu V, Hamon MA, Simeone R, Brosch R, Johansen T, Cossart P. p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J Biol Chem. 2011;286:26987–26995. doi: 10.1074/jbc.M111.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J Immunol. 2009;183:5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]
- 81.von Muhlinen N, Thurston T, Ryzhakov G, Bloor S, Randow F. NDP52, a novel autophagy receptor for ubiquitin-decorated cytosolic bacteria. Autophagy. 2010;6:288–289. doi: 10.4161/auto.6.2.11118. [DOI] [PubMed] [Google Scholar]
- 82.Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dotsch V, Bumann D, Dikic I. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chong A, Wehrly TD, Child R, Hansen B, Hwang S, Virgin HW, Celli J. Cytosolic clearance of replication-deficient mutants reveals Francisella tularensis interactions with the autophagic pathway. Autophagy. 2012;8:1342–1356. doi: 10.4161/auto.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci U S A. 2011;108:11121–11126. doi: 10.1073/pnas.1107969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 86.Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy. 2011;7:1115–1131. doi: 10.4161/auto.7.10.16608. [DOI] [PubMed] [Google Scholar]
- 87.Choi J, Park S, Biering SB, Selleck E, Liu CY, Zhang X, Fujita N, Saitoh T, Akira S, Yoshimori T, Sibley LD, Hwang S, Virgin HW. The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity. 2014;40:924–935. doi: 10.1016/j.immuni.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mestre MB, Fader CM, Sola C, Colombo MI. Alpha-hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus-infected cells. Autophagy. 2010;6:110–125. doi: 10.4161/auto.6.1.10698. [DOI] [PubMed] [Google Scholar]
- 89.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 90.Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, Coyle AJ, Kolbeck R, Green DR, Sanjuan MA. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–997. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci U S A. 2011;108:17396–17401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, Glogauer M, Grinstein S, Brumell JH. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci U S A. 2009;106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong L, Cullinane M, Treerat P, Ramm G, Prescott M, Adler B, Boyce JD, Devenish RJ. The Burkholderia pseudomallei type III secretion system and BopA are required for evasion of LC3-associated phagocytosis. PLoS One. 2011;6:e17852. doi: 10.1371/journal.pone.0017852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cullinane M, Gong L, Li X, Lazar-Adler N, Tra T, Wolvetang E, Prescott M, Boyce JD, Devenish RJ, Adler B. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy. 2008;4:744–753. doi: 10.4161/auto.6246. [DOI] [PubMed] [Google Scholar]
- 95.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 96.Ashida H, Mimuro H, Ogawa M, Kobayashi T, Sanada T, Kim M, Sasakawa C. Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol. 2011;195:931–942. doi: 10.1083/jcb.201108081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rudel T, Kepp O, Kozjak-Pavlovic V. Interactions between bacterial pathogens and mitochondrial cell death pathways. Nat Rev Microbiol. 2010;8:693–705. doi: 10.1038/nrmicro2421. [DOI] [PubMed] [Google Scholar]
- 98.Sridharan H, Upton JW. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 2014;22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 99.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 101.Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2012;12:79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ross TM. Using death to one’s advantage: HIV modulation of apoptosis. Leukemia. 2001;15:332–341. doi: 10.1038/sj.leu.2402028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Early J, Fischer K, Bermudez LE. Mycobacterium avium uses apoptotic macrophages as tools for spreading. Microb Pathog. 2011;50:132–139. doi: 10.1016/j.micpath.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- 106.Blocker AJ, Deane JE, Veenendaal AK, Roversi P, Hodgkinson JL, Johnson S, Lea SM. What’s the point of the type III secretion system needle? Proc Natl Acad Sci U S A. 2008;105:6507–6513. doi: 10.1073/pnas.0708344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valdivia RH. Chlamydia effector proteins and new insights into chlamydial cellular microbiology. Curr Opin Microbiol. 2008;11:53–59. doi: 10.1016/j.mib.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 108.Ho TD, Starnbach MN. The Salmonella enterica serovar Typhimurium-encoded type III secretion systems can translocate Chlamydia trachomatis proteins into the cytosol of host cells. Infect Immun. 2005;73:905–911. doi: 10.1128/IAI.73.2.905-911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Subtil A, Delevoye C, Balana ME, Tastevin L, Perrinet S, Dautry-Varsat A. A directed screen for chlamydial proteins secreted by a type III mechanism identifies a translocated protein and numerous other new candidates. Mol Microbiol. 2005;56:1636–1647. doi: 10.1111/j.1365-2958.2005.04647.x. [DOI] [PubMed] [Google Scholar]
- 110.Pennini ME, Perrinet S, Dautry-Varsat A, Subtil A. Histone methylation by NUE, a novel nuclear effector of the intracellular pathogen Chlamydia trachomatis. PLoS Pathog. 2010;6:e1000995. doi: 10.1371/journal.ppat.1000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.da Cunha M, Milho C, Almeida F, Pais SV, Borges V, Mauricio R, Borrego MJ, Gomes JP, Mota LJ. Identification of type III secretion substrates of Chlamydia trachomatis using Yersinia enterocolitica as a heterologous system. BMC Microbiol. 2014;14:40. doi: 10.1186/1471-2180-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christie PJ, Cascales E. Structural and dynamic properties of bacterial type IV secretion systems (review) Mol Membr Biol. 2005;22:51–61. doi: 10.1080/09687860500063316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 114.O’Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 115.Sadosky AB, Wiater LA, Shuman HA. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berger KH, Merriam JJ, Isberg RR. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 117.Carey KL, Newton HJ, Luhrmann A, Roy CR. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog. 2011;7:e1002056. doi: 10.1371/journal.ppat.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu H, Bao W, Lin M, Niu H, Rikihisa Y. Ehrlichia type IV secretion effector ECH0825 is translocated to mitochondria and curbs ROS and apoptosis by upregulating host MnSOD. Cell Microbiol. 2012;14:1037–1050. doi: 10.1111/j.1462-5822.2012.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin M, den Dulk-Ras A, Hooykaas PJ, Rikihisa Y. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cell Microbiol. 2007;9:2644–2657. doi: 10.1111/j.1462-5822.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 120.Rikihisa Y, Lin M. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr Opin Microbiol. 2010;13:59–66. doi: 10.1016/j.mib.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kudryashev M, Wang RY, Brackmann M, Scherer S, Maier T, Baker D, DiMaio F, Stahlberg H, Egelman EH, Basler M. Structure of the type VI secretion system contractile sheath. Cell. 2015;160:952–962. doi: 10.1016/j.cell.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Coulthurst SJ. The Type VI secretion system - a widespread and versatile cell targeting system. Res Microbiol. 2013;164:640–654. doi: 10.1016/j.resmic.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 123.Pezoa D, Blondel CJ, Silva CA, Yang HJ, Andrews-Polymenis H, Santiviago CA, Contreras I. Only one of the two type VI secretion systems encoded in the Salmonella enterica serotype Dublin genome is involved in colonization of the avian and murine hosts. Vet Res. 2014;45:2. doi: 10.1186/1297-9716-45-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mulder DT, Cooper CA, Coombes BK. Type VI secretion system-associated gene clusters contribute to pathogenesis of Salmonella enterica serovar Typhimurium. Infect Immun. 2012;80:1996–2007. doi: 10.1128/IAI.06205-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blondel CJ, Jimenez JC, Leiva LE, Alvarez SA, Pinto BI, Contreras F, Pezoa D, Santiviago CA, Contreras I. The type VI secretion system encoded in Salmonella pathogenicity island 19 is required for Salmonella enterica serotype Gallinarum survival within infected macrophages. Infect Immun. 2013;81:1207–1220. doi: 10.1128/IAI.01165-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bansal-Mutalik R, Nikaido H. Mycobacterial outer membrane is a lipid bilayer and the inner membrane is unusually rich in diacyl phosphatidylinositol dimannosides. Proc Natl Acad Sci U S A. 2014;111:4958–4963. doi: 10.1073/pnas.1403078111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. Type VII secretion--mycobacteria show the way. Nat Rev Microbiol. 2007;5:883–891. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- 128.Bitter W, Houben EN, Bottai D, Brodin P, Brown EJ, Cox JS, Derbyshire K, Fortune SM, Gao LY, Liu J, Gey van Pittius NC, Pym AS, Rubin EJ, Sherman DR, Cole ST, Brosch R. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 2009;5:e1000507. doi: 10.1371/journal.ppat.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pallen MJ. The ESAT-6/WXG100 superfamily -- and a new Gram-positive secretion system? Trends Microbiol. 2002;10:209–212. doi: 10.1016/s0966-842x(02)02345-4. [DOI] [PubMed] [Google Scholar]
- 130.Brodin P, Majlessi L, Marsollier L, de Jonge MI, Bottai D, Demangel C, Hinds J, Neyrolles O, Butcher PD, Leclerc C, Cole ST, Brosch R. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect Immun. 2006;74:88–98. doi: 10.1128/IAI.74.1.88-98.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9:533–539. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- 132.Ekiert DC, Cox JS. Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc Natl Acad Sci U S A. 2014;111:14758–14763. doi: 10.1073/pnas.1409345111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 134.Meresse S, Steele-Mortimer O, Moreno E, Desjardins M, Finlay B, Gorvel JP. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat Cell Biol. 1999;1:E183–188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- 135.Vogel JP, Isberg RR. Cell biology of Legionella pneumophila. Curr Opin Microbiol. 1999;2:30–34. doi: 10.1016/s1369-5274(99)80005-8. [DOI] [PubMed] [Google Scholar]
- 136.Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel JP. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Franco IS, Shuman HA, Charpentier X. The perplexing functions and surprising origins of Legionella pneumophila type IV secretion effectors. Cell Microbiol. 2009;11:1435–1443. doi: 10.1111/j.1462-5822.2009.01351.x. [DOI] [PubMed] [Google Scholar]
- 139.Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- 140.Neunuebel MR, Chen Y, Gaspar AH, Backlund PS, Yergey A, Machner MP. De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science. 2011;333:453–456. doi: 10.1126/science.1207193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neunuebel MR, Machner MP. The taming of a Rab GTPase by Legionella pneumophila. Small GTPases. 2012;3:28–33. doi: 10.4161/sgtp.18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Derre I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun. 2004;72:3048–3053. doi: 10.1128/IAI.72.5.3048-3053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kagan JC, Roy CR. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat Cell Biol. 2002;4:945–954. doi: 10.1038/ncb883. [DOI] [PubMed] [Google Scholar]
- 144.Scidmore MA, Fischer ER, Hackstadt T. Sphingolipids and glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J Cell Biol. 1996;134:363–374. doi: 10.1083/jcb.134.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Heuer D, Rejman Lipinski A, Machuy N, Karlas A, Wehrens A, Siedler F, Brinkmann V, Meyer TF. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–735. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- 146.Boleti H, Benmerah A, Ojcius DM, Cerf-Bensussan N, Dautry-Varsat A. Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: clathrin-independent entry into cells and dynamin-dependent productive growth. J Cell Sci. 1999;112 (Pt 10):1487–1496. doi: 10.1242/jcs.112.10.1487. [DOI] [PubMed] [Google Scholar]
- 147.Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 148.Mehra A, Zahra A, Thompson V, Sirisaengtaksin N, Wells A, Porto M, Koster S, Penberthy K, Kubota Y, Dricot A, Rogan D, Vidal M, Hill DE, Bean AJ, Philips JA. Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog. 2013;9:e1003734. doi: 10.1371/journal.ppat.1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wong D, Bach H, Sun J, Hmama Z, Av-Gay Y. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc Natl Acad Sci U S A. 2011;108:19371–19376. doi: 10.1073/pnas.1109201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu L, Shen X, Bryan A, Banga S, Swanson MS, Luo ZQ. Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector. PLoS Pathog. 2010;6:e1000822. doi: 10.1371/journal.ppat.1000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci U S A. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol. 2001;154:631–644. doi: 10.1083/jcb.200106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Philips JA. Mycobacterial manipulation of vacuolar sorting. Cell Microbiol. 2008;10:2408–2415. doi: 10.1111/j.1462-5822.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- 155.Alix E, Mukherjee S, Roy CR. Subversion of membrane transport pathways by vacuolar pathogens. J Cell Biol. 2011;195:943–952. doi: 10.1083/jcb.201105019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Steele-Mortimer O, Meresse S, Gorvel JP, Toh BH, Finlay BB. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1999;1:33–49. doi: 10.1046/j.1462-5822.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 157.Taraska T, Ward DM, Ajioka RS, Wyrick PB, Davis-Kaplan SR, Davis CH, Kaplan J. The late chlamydial inclusion membrane is not derived from the endocytic pathway and is relatively deficient in host proteins. Infect Immun. 1996;64:3713–3727. doi: 10.1128/iai.64.9.3713-3727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat Rev Microbiol. 2013;11:561–573. doi: 10.1038/nrmicro3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Newton HJ, Kohler LJ, McDonough JA, Temoche-Diaz M, Crabill E, Hartland EL, Roy CR. A screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog. 2014;10:e1004286. doi: 10.1371/journal.ppat.1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Larson CL, Beare PA, Voth DE, Howe D, Cockrell DC, Bastidas RJ, Valdivia RH, Heinzen RA. Coxiella burnetii effector proteins that localize to the parasitophorous vacuole membrane promote intracellular replication. Infect Immun. 2015;83:661–670. doi: 10.1128/IAI.02763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 2012;482:414–418. doi: 10.1038/nature10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, Leffler H, Poirier F, Prevost MC, Lafont F, Sansonetti P. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol. 2010;12:530–544. doi: 10.1111/j.1462-5822.2009.01415.x. [DOI] [PubMed] [Google Scholar]
- 163.Perrin AJ, Jiang X, Birmingham CL, So NS, Brumell JH. Recognition of bacteria in the cytosol of Mammalian cells by the ubiquitin system. Curr Biol. 2004;14:806–811. doi: 10.1016/j.cub.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 164.Creasey EA, Isberg RR. Maintenance of vacuole integrity by bacterial pathogens. Curr Opin Microbiol. 2014;17:46–52. doi: 10.1016/j.mib.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kumar Y, Valdivia RH. Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe. 2009;5:593–601. doi: 10.1016/j.chom.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Radtke AL, O’Riordan MX. Homeostatic maintenance of pathogen-containing vacuoles requires TBK1-dependent regulation of aquaporin-1. Cell Microbiol. 2008;10:2197–2207. doi: 10.1111/j.1462-5822.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 167.Meunier E, Dick MS, Dreier RF, Schürmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, Yamamoto M, Broz P. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 168.Kumar Y, Valdivia RH. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe. 2008;4:159–169. doi: 10.1016/j.chom.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Meresse S, Unsworth KE, Habermann A, Griffiths G, Fang F, Martinez-Lorenzo MJ, Waterman SR, Gorvel JP, Holden DW. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell Microbiol. 2001;3:567–577. doi: 10.1046/j.1462-5822.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 170.Jorgensen I, Bednar MM, Amin V, Davis BK, Ting JP, McCafferty DG, Valdivia RH. The Chlamydia protease CPAF regulates host and bacterial proteins to maintain pathogen vacuole integrity and promote virulence. Cell Host Microbe. 2011;10:21–32. doi: 10.1016/j.chom.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wasylnka JA, Bakowski MA, Szeto J, Ohlson MB, Trimble WS, Miller SI, Brumell JH. Role for myosin II in regulating positioning of Salmonella-containing vacuoles and intracellular replication. Infect Immun. 2008;76:2722–2735. doi: 10.1128/IAI.00152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dumont A, Boucrot E, Drevensek S, Daire V, Gorvel JP, Pous C, Holden DW, Meresse S. SKIP, the host target of the Salmonella virulence factor SifA, promotes kinesin-1-dependent vacuolar membrane exchanges. Traffic. 2010;11:899–911. doi: 10.1111/j.1600-0854.2010.01069.x. [DOI] [PubMed] [Google Scholar]
- 173.Beuzon CR, Meresse S, Unsworth KE, Ruiz-Albert J, Garvis S, Waterman SR, Ryder TA, Boucrot E, Holden DW. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheng W, Yin K, Lu D, Li B, Zhu D, Chen Y, Zhang H, Xu S, Chai J, Gu L. Structural insights into a unique Legionella pneumophila effector LidA recognizing both GDP and GTP bound Rab1 in their active state. PLoS Pathog. 2012;8:e1002528. doi: 10.1371/journal.ppat.1002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brumell JH, Tang P, Zaharik ML, Finlay BB. Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar Typhimurium in the cytosol of epithelial cells. Infect Immun. 2002;70:3264–3270. doi: 10.1128/IAI.70.6.3264-3270.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hilbi H, Weber S, Finsel I. Anchors for effectors: subversion of phosphoinositide lipids by Legionella. Front Microbiol. 2011;2:91. doi: 10.3389/fmicb.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Robertson DK, Gu L, Rowe RK, Beatty WL. Inclusion biogenesis and reactivation of persistent Chlamydia trachomatis requires host cell sphingolipid biosynthesis. PLoS Pathog. 2009;5:e1000664. doi: 10.1371/journal.ppat.1000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Elwell CA, Jiang S, Kim JH, Lee A, Wittmann T, Hanada K, Melancon P, Engel JN. Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog. 2011;7:e1002198. doi: 10.1371/journal.ppat.1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nawabi P, Catron DM, Haldar K. Esterification of cholesterol by a type III secretion effector during intracellular Salmonella infection. Mol Microbiol. 2008;68:173–185. doi: 10.1111/j.1365-2958.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 180.Flieger A, Neumeister B, Cianciotto NP. Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect Immun. 2002;70:6094–6106. doi: 10.1128/IAI.70.11.6094-6106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Creasey EA, Isberg RR. The protein SdhA maintains the integrity of the Legionella-containing vacuole. Proc Natl Acad Sci U S A. 2012;109:3481–3486. doi: 10.1073/pnas.1121286109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ruiz-Albert J, Yu XJ, Beuzon CR, Blakey AN, Galyov EE, Holden DW. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol Microbiol. 2002;44:645–661. doi: 10.1046/j.1365-2958.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 183.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol. 2009;10:1215–1221. doi: 10.1038/ni.1800. [DOI] [PubMed] [Google Scholar]
- 185.Shin DM, Jeon BY, Lee HM, Jin HS, Yuk JM, Song CH, Lee SH, Lee ZW, Cho SN, Kim JM, Friedman RL, Jo EK. Mycobacterium tuberculosis Eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog. 2010;6:e1001230. doi: 10.1371/journal.ppat.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, Corazzari M, Falasca L, Goletti D, Gafa V, Simeone R, Delogu G, Piacentini M, Brosch R, Fimia GM, Coccia EM. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012;8:1357–1370. doi: 10.4161/auto.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Choy A, Dancourt J, Mugo B, O’Connor TJ, Isberg RR, Melia TJ, Roy CR. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science. 2012;338:1072–1076. doi: 10.1126/science.1227026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, Colombo MI. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell Microbiol. 2005;7:981–993. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- 189.Beron W, Gutierrez MG, Rabinovitch M, Colombo MI. Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect Immun. 2002;70:5816–5821. doi: 10.1128/IAI.70.10.5816-5821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Starr T, Child R, Wehrly TD, Hansen B, Hwang S, Lopez-Otin C, Virgin HW, Celli J. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe. 2012;11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Niu H, Xiong Q, Yamamoto A, Hayashi-Nishino M, Rikihisa Y. Autophagosomes induced by a bacterial Beclin 1 binding protein facilitate obligatory intracellular infection. Proc Natl Acad Sci U S A. 2012;109:20800–20807. doi: 10.1073/pnas.1218674109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Niu H, Yamaguchi M, Rikihisa Y. Subversion of cellular autophagy by Anaplasma phagocytophilum. Cell Microbiol. 2008;10:593–605. doi: 10.1111/j.1462-5822.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 193.Vazquez CL, Colombo MI. Coxiella burnetii modulates Beclin 1 and Bcl-2, preventing host cell apoptosis to generate a persistent bacterial infection. Cell Death Differ. 2010;17:421–438. doi: 10.1038/cdd.2009.129. [DOI] [PubMed] [Google Scholar]
- 194.Campoy E, Colombo MI. Autophagy in intracellular bacterial infection. Biochim Biophys Acta. 2009;1793:1465–1477. doi: 10.1016/j.bbamcr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 195.Luo ZQ. Striking a balance: modulation of host cell death pathways by Legionella pneumophila. Front Microbiol. 2011;2:36. doi: 10.3389/fmicb.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Banga S, Gao P, Shen X, Fiscus V, Zong WX, Chen L, Luo ZQ. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc Natl Acad Sci U S A. 2007;104:5121–5126. doi: 10.1073/pnas.0611030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Luhrmann A, Nogueira CV, Carey KL, Roy CR. Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc Natl Acad Sci U S A. 2010;107:18997–19001. doi: 10.1073/pnas.1004380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fischer SF, Vier J, Kirschnek S, Klos A, Hess S, Ying S, Hacker G. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J Exp Med. 2004;200:905–916. doi: 10.1084/jem.20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ying S, Seiffert BM, Hacker G, Fischer SF. Broad degradation of proapoptotic proteins with the conserved Bcl-2 homology domain 3 during infection with Chlamydia trachomatis. Infect Immun. 2005;73:1399–1403. doi: 10.1128/IAI.73.3.1399-1403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ge J, Xu H, Li T, Zhou Y, Zhang Z, Li S, Liu L, Shao F. A Legionella type IV effector activates the NF-kappaB pathway by phosphorylating the IkappaB family of inhibitors. Proc Natl Acad Sci U S A. 2009;106:13725–13730. doi: 10.1073/pnas.0907200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jones RM, Wu H, Wentworth C, Luo L, Collier-Hyams L, Neish AS. Salmonella AvrA Coordinates Suppression of Host Immune and Apoptotic Defenses via JNK Pathway Blockade. Cell Host Microbe. 2008;3:233–244. doi: 10.1016/j.chom.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 202.Verbeke P, Welter-Stahl L, Ying S, Hansen J, Hacker G, Darville T, Ojcius DM. Recruitment of BAD by the Chlamydia trachomatis vacuole correlates with host-cell survival. PLoS Pathog. 2006;2:e45. doi: 10.1371/journal.ppat.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Voth DE, Heinzen RA. Sustained activation of Akt and Erk1/2 is required for Coxiella burnetii antiapoptotic activity. Infect Immun. 2009;77:205–213. doi: 10.1128/IAI.01124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Knodler LA, Finlay BB, Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280:9058–9064. doi: 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- 205.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ireton K. Molecular mechanisms of cell-cell spread of intracellular bacterial pathogens. Open Biol. 2013;3:130079. doi: 10.1098/rsob.130079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kespichayawattana W, Rattanachetkul S, Wanun T, Utaisincharoen P, Sirisinha S. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect Immun. 2000;68:5377–5384. doi: 10.1128/iai.68.9.5377-5384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Harley VS, Dance DA, Drasar BS, Tovey G. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios. 1998;96:71–93. [PubMed] [Google Scholar]
- 209.Sahni SK, Narra HP, Sahni A, Walker DH. Recent molecular insights into rickettsial pathogenesis and immunity. Future Microbiol. 2013;8:1265–1288. doi: 10.2217/fmb.13.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Suzuki T, Nakanishi K, Tsutsui H, Iwai H, Akira S, Inohara N, Chamaillard M, Nunez G, Sasakawa C. A novel caspase-1/toll-like receptor 4-independent pathway of cell death induced by cytosolic Shigella in infected macrophages. J Biol Chem. 2005;280:14042–14050. doi: 10.1074/jbc.M414671200. [DOI] [PubMed] [Google Scholar]
- 211.Schroeder GN, Hilbi H. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev. 2008;21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cunha LD, Zamboni DS. Subversion of inflammasome activation and pyroptosis by pathogenic bacteria. Front Cell Infect Microbiol. 2013;3:76. doi: 10.3389/fcimb.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cossart P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci U S A. 2011;108:19484–19491. doi: 10.1073/pnas.1112371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Burg-Golani T, Pozniak Y, Rabinovich L, Sigal N, Nir Paz R, Herskovits AA. Membrane chaperone SecDF plays a role in the secretion of Listeria monocytogenes major virulence factors. J Bacteriol. 2013;195:5262–5272. doi: 10.1128/JB.00697-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Desvaux M, Hebraud M. The protein secretion systems in Listeria: inside out bacterial virulence. FEMS Microbiol Rev. 2006;30:774–805. doi: 10.1111/j.1574-6976.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- 217.Stevens MP, Wood MW, Taylor LA, Monaghan P, Hawes P, Jones PW, Wallis TS, Galyov EE. An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen. Mol Microbiol. 2002;46:649–659. doi: 10.1046/j.1365-2958.2002.03190.x. [DOI] [PubMed] [Google Scholar]
- 218.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 219.Pilatz S, Breitbach K, Hein N, Fehlhaber B, Schulze J, Brenneke B, Eberl L, Steinmetz I. Identification of Burkholderia pseudomallei genes required for the intracellular life cycle and in vivo virulence. Infect Immun. 2006;74:3576–3586. doi: 10.1128/IAI.01262-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shalom G, Shaw JG, Thomas MS. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology. 2007;153:2689–2699. doi: 10.1099/mic.0.2007/006585-0. [DOI] [PubMed] [Google Scholar]
- 221.Schwarz S, Singh P, Robertson JD, LeRoux M, Skerrett SJ, Goodlett DR, West TE, Mougous JD. VgrG-5 is a Burkholderia type VI secretion system-exported protein required for multinucleated giant cell formation and virulence. Infect Immun. 2014;82:1445–1452. doi: 10.1128/IAI.01368-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Toesca IJ, French CT, Miller JF. The Type VI secretion system spike protein VgrG5 mediates membrane fusion during intercellular spread by pseudomallei group Burkholderia species. Infect Immun. 2014;82:1436–1444. doi: 10.1128/IAI.01367-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.French CT, Toesca IJ, Wu TH, Teslaa T, Beaty SM, Wong W, Liu M, Schroder I, Chiou PY, Teitell MA, Miller JF. Dissection of the Burkholderia intracellular life cycle using a photothermal nanoblade. Proc Natl Acad Sci U S A. 2011;108:12095–12100. doi: 10.1073/pnas.1107183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Steiner DJ, Furuya Y, Metzger DW. Host-pathogen interactions and immune evasion strategies in Francisella tularensis pathogenicity. Infect Drug Resist. 2014;7:239–251. doi: 10.2147/IDR.S53700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nano FE, Schmerk C. The Francisella pathogenicity island. Ann N Y Acad Sci. 2007;1105:122–137. doi: 10.1196/annals.1409.000. [DOI] [PubMed] [Google Scholar]
- 226.de Bruin OM, Duplantis BN, Ludu JS, Hare RF, Nix EB, Schmerk CL, Robb CS, Boraston AB, Hueffer K, Nano FE. The biochemical properties of the Francisella pathogenicity island (FPI)-encoded proteins IglA, IglB, IglC, PdpB and DotU suggest roles in type VI secretion. Microbiology. 2011;157:3483–3491. doi: 10.1099/mic.0.052308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Broms JE, Meyer L, Lavander M, Larsson P, Sjostedt A. DotU and VgrG, core components of type VI secretion systems, are essential for Francisella LVS pathogenicity. PLoS One. 2012;7:e34639. doi: 10.1371/journal.pone.0034639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Barker JR, Chong A, Wehrly TD, Yu JJ, Rodriguez SA, Liu J, Celli J, Arulanandam BP, Klose KE. The Francisella tularensis pathogenicity island encodes a secretion system that is required for phagosome escape and virulence. Mol Microbiol. 2009;74:1459–1470. doi: 10.1111/j.1365-2958.2009.06947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Henry R, Shaughnessy L, Loessner MJ, Alberti-Segui C, Higgins DE, Swanson JA. Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell Microbiol. 2006;8:107–119. doi: 10.1111/j.1462-5822.2005.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol. 2006;8:781–792. doi: 10.1111/j.1462-5822.2005.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schnupf P, Portnoy DA. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 2007;9:1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 232.Beauregard KE, Lee KD, Collier RJ, Swanson JA. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186:1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bielecki J, Youngman P, Connelly P, Portnoy DA. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 234.Portnoy DA, Tweten RK, Kehoe M, Bielecki J. Capacity of listeriolysin O, streptolysin O, and perfringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect Immun. 1992;60:2710–2717. doi: 10.1128/iai.60.7.2710-2717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol. 2002;156:1029–1038. doi: 10.1083/jcb.200201081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Singh R, Jamieson A, Cresswell P. GILT is a critical host factor for Listeria monocytogenes infection. Nature. 2008;455:1244–1247. doi: 10.1038/nature07344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Radtke AL, Anderson KL, Davis MJ, DiMagno MJ, Swanson JA, O’Riordan MX. Listeria monocytogenes exploits cystic fibrosis transmembrane conductance regulator (CFTR) to escape the phagosome. Proc Natl Acad Sci U S A. 2011;108:1633–1638. doi: 10.1073/pnas.1013262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Poussin MA, Leitges M, Goldfine H. The ability of Listeria monocytogenes PI-PLC to facilitate escape from the macrophage phagosome is dependent on host PKCbeta. Microb Pathog. 2009;46:1–5. doi: 10.1016/j.micpath.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Poussin MA, Goldfine H. Involvement of Listeria monocytogenes phosphatidylinositol-specific phospholipase C and host protein kinase C in permeabilization of the macrophage phagosome. Infect Immun. 2005;73:4410–4413. doi: 10.1128/IAI.73.7.4410-4413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Montes LR, Goni FM, Johnston NC, Goldfine H, Alonso A. Membrane fusion induced by the catalytic activity of a phospholipase C/sphingomyelinase from Listeria monocytogenes. Biochemistry. 2004;43:3688–3695. doi: 10.1021/bi0352522. [DOI] [PubMed] [Google Scholar]
- 242.Alberti-Segui C, Goeden KR, Higgins DE. Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell Microbiol. 2007;9:179–195. doi: 10.1111/j.1462-5822.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 243.Sibelius U, Chakraborty T, Krogel B, Wolf J, Rose F, Schmidt R, Wehland J, Seeger W, Grimminger F. The listerial exotoxins listeriolysin and phosphatidylinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J Immunol. 1996;157:4055–4060. [PubMed] [Google Scholar]
- 244.Barker JR, Klose KE. Molecular and genetic basis of pathogenesis in Francisella tularensis. Ann N Y Acad Sci. 2007;1105:138–159. doi: 10.1196/annals.1409.010. [DOI] [PubMed] [Google Scholar]
- 245.de Bruin OM, Ludu JS, Nano FE. The Francisella pathogenicity island protein IglA localizes to the bacterial cytoplasm and is needed for intracellular growth. BMC Microbiol. 2007;7:1. doi: 10.1186/1471-2180-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Buchan BW, McCaffrey RL, Lindemann SR, Allen LA, Jones BD. Identification of migR, a regulatory element of the Francisella tularensis live vaccine strain iglABCD virulence operon required for normal replication and trafficking in macrophages. Infect Immun. 2009;77:2517–2529. doi: 10.1128/IAI.00229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hayward RD, Cain RJ, McGhie EJ, Phillips N, Garner MJ, Koronakis V. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Mol Microbiol. 2005;56:590–603. doi: 10.1111/j.1365-2958.2005.04568.x. [DOI] [PubMed] [Google Scholar]
- 248.Picking WL, Nishioka H, Hearn PD, Baxter MA, Harrington AT, Blocker A, Picking WD. IpaD of Shigella flexneri is independently required for regulation of Ipa protein secretion and efficient insertion of IpaB and IpaC into host membranes. Infect Immun. 2005;73:1432–1440. doi: 10.1128/IAI.73.3.1432-1440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Whitworth T, Popov VL, Yu XJ, Walker DH, Bouyer DH. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica serovar Typhimurium mediates phagosomal escape. Infect Immun. 2005;73:6668–6673. doi: 10.1128/IAI.73.10.6668-6673.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Renesto P, Dehoux P, Gouin E, Touqui L, Cossart P, Raoult D. Identification and characterization of a phospholipase D-superfamily gene in rickettsiae. J Infect Dis. 2003;188:1276–1283. doi: 10.1086/379080. [DOI] [PubMed] [Google Scholar]
- 251.Silverman DJ, Santucci LA, Meyers N, Sekeyova Z. Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infect Immun. 1992;60:2733–2740. doi: 10.1128/iai.60.7.2733-2740.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Winkler HH, Miller ET. Phospholipase A and the interaction of Rickettsia prowazekii and mouse fibroblasts (L-929 cells) Infect Immun. 1982;38:109–113. doi: 10.1128/iai.38.1.109-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lucchini S, Liu H, Jin Q, Hinton JC, Yu J. Transcriptional adaptation of Shigella flexneri during infection of macrophages and epithelial cells: insights into the strategies of a cytosolic bacterial pathogen. Infect Immun. 2005;73:88–102. doi: 10.1128/IAI.73.1.88-102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chieng S, Carreto L, Nathan S. Burkholderia pseudomallei transcriptional adaptation in macrophages. BMC Genomics. 2012;13:328. doi: 10.1186/1471-2164-13-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wehrly TD, Chong A, Virtaneva K, Sturdevant DE, Child R, Edwards JA, Brouwer D, Nair V, Fischer ER, Wicke L, Curda AJ, Kupko JJ, 3rd, Martens C, Crane DD, Bosio CM, Porcella SF, Celli J. Intracellular biology and virulence determinants of Francisella tularensis revealed by transcriptional profiling inside macrophages. Cell Microbiol. 2009;11:1128–1150. doi: 10.1111/j.1462-5822.2009.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chatterjee SS, Hossain H, Otten S, Kuenne C, Kuchmina K, Machata S, Domann E, Chakraborty T, Hain T. Intracellular gene expression profile of Listeria monocytogenes. Infect Immun. 2006;74:1323–1338. doi: 10.1128/IAI.74.2.1323-1338.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Campbell-Valois FX, Schnupf P, Nigro G, Sachse M, Sansonetti PJ, Parsot C. A fluorescent reporter reveals on/off regulation of the Shigella type III secretion apparatus during entry and cell-to-cell spread. Cell Host Microbe. 2014;15:177–189. doi: 10.1016/j.chom.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 258.Scortti M, Monzo HJ, Lacharme-Lora L, Lewis DA, Vazquez-Boland JA. The PrfA virulence regulon. Microbes Infect. 2007;9:1196–1207. doi: 10.1016/j.micinf.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 259.Chakraborty T, Leimeister-Wachter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Johansson J, Mandin P, Renzoni A, Chiaruttini C, Springer M, Cossart P. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell. 2002;110:551–561. doi: 10.1016/s0092-8674(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 261.Ermolaeva S, Novella S, Vega Y, Ripio MT, Scortti M, Vazquez-Boland JA. Negative control of Listeria monocytogenes virulence genes by a diffusible autorepressor. Mol Microbiol. 2004;52:601–611. doi: 10.1111/j.1365-2958.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- 262.Milenbachs AA, Brown DP, Moors M, Youngman P. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol Microbiol. 1997;23:1075–1085. doi: 10.1046/j.1365-2958.1997.2711634.x. [DOI] [PubMed] [Google Scholar]
- 263.Brehm K, Ripio MT, Kreft J, Vazquez-Boland JA. The bvr locus of Listeria monocytogenes mediates virulence gene repression by beta-glucosides. J Bacteriol. 1999;181:5024–5032. doi: 10.1128/jb.181.16.5024-5032.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ripio MT, Brehm K, Lara M, Suarez M, Vazquez-Boland JA. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J Bacteriol. 1997;179:7174–7180. doi: 10.1128/jb.179.22.7174-7180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Herro R, Poncet S, Cossart P, Buchrieser C, Gouin E, Glaser P, Deutscher J. How seryl-phosphorylated HPr inhibits PrfA, a transcription activator of Listeria monocytogenes virulence genes. J Mol Microbiol Biotechnol. 2005;9:224–234. doi: 10.1159/000089650. [DOI] [PubMed] [Google Scholar]
- 266.Park SF, Kroll RG. Expression of listeriolysin and phosphatidylinositol-specific phospholipase C is repressed by the plant-derived molecule cellobiose in Listeria monocytogenes. Mol Microbiol. 1993;8:653–661. doi: 10.1111/j.1365-2958.1993.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 267.Lobel L, Sigal N, Borovok I, Belitsky BR, Sonenshein AL, Herskovits AA. The metabolic regulator CodY links Listeria monocytogenes metabolism to virulence by directly activating the virulence regulatory gene prfA. Mol Microbiol. 2015;95:624–644. doi: 10.1111/mmi.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lobel L, Sigal N, Borovok I, Ruppin E, Herskovits AA. Integrative genomic analysis identifies isoleucine and CodY as regulators of Listeria monocytogenes virulence. PLoS Genet. 2012;8:e1002887. doi: 10.1371/journal.pgen.1002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Reniere ML, Whiteley AT, Hamilton KL, John SM, Lauer P, Brennan RG, Portnoy DA. Glutathione activates virulence gene expression of an intracellular pathogen. Nature. 2015;517:170–173. doi: 10.1038/nature14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Deshayes C, Bielecka MK, Cain RJ, Scortti M, de las Heras A, Pietras Z, Luisi BF, Nunez Miguel R, Vazquez-Boland JA. Allosteric mutants show that PrfA activation is dispensable for vacuole escape but required for efficient spread and Listeria survival in vivo. Mol Microbiol. 2012;85:461–477. doi: 10.1111/j.1365-2958.2012.08121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kane CD, Schuch R, Day WA, Jr, Maurelli AT. MxiE regulates intracellular expression of factors secreted by the Shigella flexneri 2a type III secretion system. J Bacteriol. 2002;184:4409–4419. doi: 10.1128/JB.184.16.4409-4419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Dhariwala MO, Anderson DM. Bacterial programming of host responses: coordination between type I interferon and cell death. Front Microbiol. 2014;5:545. doi: 10.3389/fmicb.2014.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gouin E, Adib-Conquy M, Balestrino D, Nahori MA, Villiers V, Colland F, Dramsi S, Dussurget O, Cossart P. The Listeria monocytogenes InlC protein interferes with innate immune responses by targeting the IκB kinase subunit IKKα. Proc Natl Acad Sci U S A. 2010;107:17333–17338. doi: 10.1073/pnas.1007765107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Leung N, Gianfelice A, Gray-Owen SD, Ireton K. Impact of the Listeria monocytogenes protein InlC on infection in mice. Infect Immun. 2013;81:1334–1340. doi: 10.1128/IAI.01377-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jones BD, Faron M, Rasmussen JA, Fletcher JR. Uncovering the components of the Francisella tularensis virulence stealth strategy. Front Cell Infect Microbiol. 2014;4:32. doi: 10.3389/fcimb.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Dons L, Rasmussen OF, Olsen JE. Cloning and characterization of a gene encoding flagellin of Listeria monocytogenes. Mol Microbiol. 1992;6:2919–2929. doi: 10.1111/j.1365-2958.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 277.Grundling A, Burrack LS, Bouwer HG, Higgins DE. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc Natl Acad Sci U S A. 2004;101:12318–12323. doi: 10.1073/pnas.0404924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Dotson RJ, Rabadi SM, Westcott EL, Bradley S, Catlett SV, Banik S, Harton JA, Bakshi CS, Malik M. Repression of inflammasome by Francisella tularensis during early stages of infection. J Biol Chem. 2013;288:23844–23857. doi: 10.1074/jbc.M113.490086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Robertson GT, Case ED, Dobbs N, Ingle C, Balaban M, Celli J, Norgard MV. FTT0831c/FTL_0325 contributes to Francisella tularensis cell division, maintenance of cell shape, and structural integrity. Infect Immun. 2014;82:2935–2948. doi: 10.1128/IAI.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kobayashi T, Ogawa M, Sanada T, Mimuro H, Kim M, Ashida H, Akakura R, Yoshida M, Kawalec M, Reichhart JM, Mizushima T, Sasakawa C. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe. 2013;13:570–583. doi: 10.1016/j.chom.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 282.Tattoli I, Sorbara MT, Vuckovic D, Ling A, Soares F, Carneiro LA, Yang C, Emili A, Philpott DJ, Girardin SE. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe. 2012;11:563–575. doi: 10.1016/j.chom.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 283.Tattoli I, Sorbara MT, Yang C, Tooze SA, Philpott DJ, Girardin SE. Listeria phospholipases subvert host autophagic defenses by stalling pre-autophagosomal structures. EMBO J. 2013;32:3066–3078. doi: 10.1038/emboj.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Meyer-Morse N, Robbins JR, Rae CS, Mochegova SN, Swanson MS, Zhao Z, Virgin HW, Portnoy D. Listeriolysin O is necessary and sufficient to induce autophagy during Listeria monocytogenes infection. PLoS One. 2010;5:e8610. doi: 10.1371/journal.pone.0008610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Dortet L, Mostowy S, Samba-Louaka A, Gouin E, Nahori MA, Wiemer EA, Dussurget O, Cossart P. Recruitment of the major vault protein by InlK: a Listeria monocytogenes strategy to avoid autophagy. PLoS Pathog. 2011;7:e1002168. doi: 10.1371/journal.ppat.1002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mitchell G, Ge L, Huang Q, Chen C, Kianian S, Roberts MF, Schekman R, Portnoy DA. Avoidance of autophagy mediated by PlcA or ActA is required for Listeria monocytogenes growth in macrophages. Infect Immun. 2015;83:2175–2184. doi: 10.1128/IAI.00110-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- 288.Case ED, Chong A, Wehrly TD, Hansen B, Child R, Hwang S, Virgin HW, Celli J. The Francisella O-antigen mediates survival in the macrophage cytosol via autophagy avoidance. Cell Microbiol. 2014;16:862–877. doi: 10.1111/cmi.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Devenish RJ, Lai SC. Autophagy and Burkholderia. Immunol Cell Biol. 2015;93:18–24. doi: 10.1038/icb.2014.87. [DOI] [PubMed] [Google Scholar]
- 290.Galyov EE, Brett PJ, DeShazer D. Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annu Rev Microbiol. 2010;64:495–517. doi: 10.1146/annurev.micro.112408.134030. [DOI] [PubMed] [Google Scholar]
- 291.Baxt LA, Goldberg MB. Host and bacterial proteins that repress recruitment of LC3 to Shigella early during infection. PLoS One. 2014;9:e94653. doi: 10.1371/journal.pone.0094653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lam GY, Cemma M, Muise AM, Higgins DE, Brumell JH. Host and bacterial factors that regulate LC3 recruitment to Listeria monocytogenes during the early stages of macrophage infection. Autophagy. 2013;9:985–995. doi: 10.4161/auto.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Steele S, Brunton J, Ziehr B, Taft-Benz S, Moorman N, Kawula T. Francisella tularensis harvests nutrients derived via ATG5-independent autophagy to support intracellular growth. PLoS Pathog. 2013;9:e1003562. doi: 10.1371/journal.ppat.1003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bast A, Krause K, Schmidt IH, Pudla M, Brakopp S, Hopf V, Breitbach K, Steinmetz I. Caspase-1-dependent and -independent cell death pathways in Burkholderia pseudomallei infection of macrophages. PLoS Pathog. 2014;10:e1003986. doi: 10.1371/journal.ppat.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 2012;19:1709–1721. doi: 10.1038/cdd.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Doyle CR, Pan JA, Mena P, Zong WX, Thanassi DG. TolC-dependent modulation of host cell death by the Francisella tularensis live vaccine strain. Infect Immun. 2014;82:2068–2078. doi: 10.1128/IAI.00044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Carneiro LA, Travassos LH, Soares F, Tattoli I, Magalhaes JG, Bozza MT, Plotkowski MC, Sansonetti PJ, Molkentin JD, Philpott DJ, Girardin SE. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host Microbe. 2009;5:123–136. doi: 10.1016/j.chom.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 298.Joshi SG, Francis CW, Silverman DJ, Sahni SK. NF-kappaB activation suppresses host cell apoptosis during Rickettsia rickettsii infection via regulatory effects on intracellular localization or levels of apoptogenic and anti-apoptotic proteins. FEMS Microbiol Lett. 2004;234:333–341. doi: 10.1016/j.femsle.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 299.Joshi SG, Francis CW, Silverman DJ, Sahni SK. Nuclear factor kappa B protects against host cell apoptosis during Rickettsia rickettsii infection by inhibiting activation of apical and effector caspases and maintaining mitochondrial integrity. Infect Immun. 2003;71:4127–4136. doi: 10.1128/IAI.71.7.4127-4136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Cassidy SK, O’Riordan MX. More than a pore: the cellular response to cholesterol-dependent cytolysins. Toxins (Basel) 2013;5:618–636. doi: 10.3390/toxins5040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Mestre MB, Colombo MI. Autophagy and toxins: a matter of life or death. Curr Mol Med. 2013;13:241–251. doi: 10.2174/156652413804810790. [DOI] [PubMed] [Google Scholar]
- 302.Mares CA, Sharma J, Li Q, Rangel EL, Morris EG, Enriquez MI, Teale JM. Defect in efferocytosis leads to alternative activation of macrophages in Francisella infections. Immunol Cell Biol. 2011;89:167–172. doi: 10.1038/icb.2010.81. [DOI] [PubMed] [Google Scholar]
- 303.Lai XH, Golovliov I, Sjostedt A. Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect Immun. 2001;69:4691–4694. doi: 10.1128/IAI.69.7.4691-4694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yao Q, Cui J, Wang J, Li T, Wan X, Luo T, Gong YN, Xu Y, Huang N, Shao F. Structural mechanism of ubiquitin and NEDD8 deamidation catalyzed by bacterial effectors that induce macrophage-specific apoptosis. Proc Natl Acad Sci U S A. 2012;109:20395–20400. doi: 10.1073/pnas.1210831109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Cassidy SK, Hagar JA, Kanneganti TD, Franchi L, Nunez G, O’Riordan MX. Membrane damage during Listeria monocytogenes infection triggers a caspase-7 dependent cytoprotective response. PLoS Pathog. 2012;8:e1002628. doi: 10.1371/journal.ppat.1002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Czuczman MA, Fattouh R, van Rijn JM, Canadien V, Osborne S, Muise AM, Kuchroo VK, Higgins DE, Brumell JH. Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread. Nature. 2014;509:230–234. doi: 10.1038/nature13168. [DOI] [PMC free article] [PubMed] [Google Scholar]