Abstract
Copper promotes a toxic buildup of amyloid-beta (Aβ) and neurofibrillary tangle pathology in the brain, and its exposure may increase the risk for Alzheimer’s disease (AD). However, underlying molecular mechanisms by which copper triggers such pathological changes remain largely unknown. We hypothesized that the copper exposure perturbs brain inflammatory responses, leading to impairment of Aβ clearance from the brain parenchyma. Here, we investigated whether copper attenuated Aβ clearance by microglial phagocytosis or by low-density lipoprotein-related receptor protein-1 (LRP1) dependent transcytosis in both in vitro and in vivo. When murine monocyte BV2 cells were exposed to copper, their phagocytic activation induced by fibrillar Aβ or LPS was significantly reduced, while the secretion of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, were increased. Interestingly, not only copper itself but also IL-1β, IL-6, or TNF-α were capable of markedly reducing the expression of LRP1 in human microvascular endothelial cells (MVECs) in a concentration-dependent manner. While copper-mediated downregulation of LRP1 was proteasome-dependent, the cytokine-induced loss of LRP1 was proteasome- or lysosome-independent. In the mouse model, copper exposure also significantly elevated neuroinflammation and downregulated LRP1 in the brain, consistent with our in vitro results. Taken together, our findings support the pathological impact of copper on inflammatory responses and Aβ clearance in the brain, which could serve as key mechanisms to explain, in part, the copper exposure as an environmental risk factor for AD.
Keywords: Alzheimer’s disease, phagocytosis, microglia, copper, inflammation, cytokines, LRP1
Copper is an essential transition metal serving as an important cofactor for various enzymatic reactions and physiological activities. However, its absorption, distribution in the body, metabolism and excretion have to be properly regulated, as the systemic copper dyshomeostasis due to genetic mutations is known to cause neurological and psychiatric abnormalities, represented by Wilson’s disease and Menkes disease (Burkhead et al., 2011; Kaler, 2011). Even through dietary intake, the excessive copper, together with high fat diet, has been reported to significantly accelerate cognitive decline among elderly population (Morris et al., 2006). Large-scale meta-analysis and prospective follow-up studies have unveiled a strong positive correlation between free copper levels in serum and poor cognitive performance, as well as shortening the conversion period from mild cognitive impairment (MCI) to Alzheimer’s disease (AD) (Brewer et al., 2010; Park et al., 2013; Squitti et al., 2006, 2014; Ventriglia et al., 2012). These epidemiological studies support that chronic copper exposure through diet, drinking water or air pollution may result in elevated levels of free copper in the circulation and increase the risk for developing AD in later life.
Pre-clinical studies using animal models and cell culture models have revealed several possible mechanisms by which copper exhibits neurotoxicity and exacerbates a pathological buildup of Aβ and tau. Copper is capable of producing reactive free radicals by Fenton reaction (Greenough et al., 2013; Marlatt et al., 2004; Multhaup et al., 2002) and directly interacting with Aβ and amyloid precursor protein (APP) to promote the production and aggregation of Aβ (Kitazawa et al., 2009; Multhaup et al., 1998; Sparks et al., 2006; Sparks and Schreurs, 2003; Wang et al., 2012). However, in animal models, mixed results—both beneficial and detrimental effects of copper - have been reported (Kitazawa et al., 2009; Singh et al., 2013; Sparks et al., 2006; Sparks and Schreurs, 2003). Bayer and colleagues demonstrated that chronic high-dose copper in drinking water reduces Aβ pathology and free radical production by stabilizing superoxide dismutase activity in mice (Bayer et al., 2003). On the other hand, we have applied the same exposure to 3xTg-AD mouse model of AD and found it significantly exacerbated both Aβ and tau pathologies (Kitazawa et al., 2009). Recent study reveals these pathological changes are accompanied by cognitive decline (Yu et al., 2015). Remarkably, copper-induced Aβ pathology is detected even in a trace amount of copper in drinking water (0.12 ppm) (Sparks et al., 2006; Sparks and Schreurs, 2003). Likewise, Deane and colleagues report that chronic exposure of 0.12 ppm copper in drinking water alone significantly exacerbated Aβ pathology in the brain via mechanism mediated by reduction of low-density lipoprotein receptor-related protein-1 (LRP1) expression in brain capillaries (Singh et al., 2013). Although the contribution of brain LRP1 to the clearance of Aβ in AD has long been controversial (Ito et al., 2010; Nazer et al., 2008; Xu et al., 2012), a recent in vivo study clearly supports the transcytotic clearance of Aβ from the brain parenchyma via capillary endothelial LRP1 (Storck et al., 2016). In human, the LRP1 expression in endothelial cells is decreased with age, potentially increasing the risk for AD (Donahue et al., 2006; Silverberg et al., 2010). Thus, these findings suggest that chronic copper exposure increases the risk of impairing the brain’s Aβ clearance mechanisms.
In this study, we sought to further investigate whether copper exposure mediates a pathological buildup of Aβ by perturbing neuroinflammatory responses as numerous epidemiological, genome-wide association studies (GWAS), in vitro and in vivo studies suggest inflammation as one of the early changes in AD [reviewed in (Heppner et al., 2015; McGeer and McGeer, 2007)]. Here, we found that copper significantly reduced phagocytic clearance of Aβ and exacerbated Aβ-elicited pro-inflammatory cytokine release. Elevated levels of pro-inflammatory cytokines, IL-1β, TNF-α, and IL-6 alone significantly down-regulated LRP1 in microvascular endothelial cells, and this proinflammatory cytokine-mediated downregulation of LRP1 was not proteasome- or lysosome-dependent. These findings provide further mechanistic insights into our current understanding of copper neurotoxicity, its potential synergistic effects on both phagocytosis- and LRP1 transcytosis-mediated clearance of Aβ, and underlying cellular mechanisms by which the environmental copper exposure increases the risk for AD.
MATERIALS AND METHODS
Fibrillar Aβ preparation. Synthetic Aβ1-42 peptides were purchased from the American Peptide (Sunnyvale, California). One mg of Aβ1-42 peptides was reconstituted in 1 ml sterile water and incubated at 37 °C for 6 days with continuous stirring. Resulting fibrillar Aβ (fAβ) was aliquoted and stored at −80 °C.
Cell culture and treatment. Murine macrophage/microglia-like BV2 cells were maintained in DMEM supplemented with 10% FBS and penicillin and streptomycin. Cells were grown and treated in humidified incubator with 5% CO2 in atmospheric air and a temperature of 37 °C. Cells were plated onto 96-well or 6-well with initial seeding density of 20 000 cells/well or 300 000 cell/well, respectively. For cytotoxicity assay, cells were incubated in serum-free DMEM for 24 h to reduce background activation of BV2 cells (Koenigsknecht-Talboo and Landreth, 2005; Pan et al., 2011), then 0.01 to 5 µM of fAβ (final concentration) was added to the media and incubated for additional 1 h. For 3-h copper followed by 1-h fAβ exposure, after 24 h of serum-free DMEM, copper sulfate solution was added to the media to reach the final concentration of 0.01–5 µM copper and incubate additional 3 h. Then, media were replaced with fAβ-containing serum-free DMEM and incubated for 1 h. For 24-h copper followed by 1-h fAβ exposure, cells were incubated with copper in serum-free DMEM for 24 h, then media were replaced with fAβ-containing serum-free DMEM and incubated for 1 h.
Human primary microvascular endothelial cells (MVECs) were purchased from Cell Systems (Kirkland, Washington, cat#: ACBRI 376). MVECs were grown and maintained in the Complete Classic Media (Cell Systems) as recommended by the vendor. Cells were plated onto six-well with initial seeding density of 300 000 cell/well. Copper sulfate (0.5–10 µM), human recombinant IL-1β, IL-6 or TNF-α (0.001–1 ng/ml) was added to the media with or without proteosomal inhibitors, MG-132 (0.5 µM), lactacystin (5 µM) or lysosomal inhibitor, chloroquine (CHQ, 50 µM) for 24 h. All were obtained from Sigma-Aldrich and prepared according to the manufacturer’s instructions.
Condition media were collected from cells at the end of exposure period, briefly centrifuged to remove any cells and debris, and stored in −80 °C. For protein extraction, cells were rinsed with ice-cold PBS, then M-PER (ThermoFisher Scientific) supplemented with protease inhibitor and phosphatase inhibitor cocktails (Sigma-Aldrich) was added for complete lysis. Cell lysates were centrifuged at 100 000×g for 1 h at 4 °C, and supernatants were collected as total cell homogenates. Protein concentration was determined by Bio-Rad Bradford protein assay dye, and homogenates were stored in −80 °C until analysis. For RNA extraction, cells were lysed in TRI-reagent (Molecular Research) to purify total RNA. RNA was quantified by nano-drop (ThermoFisher Scientific) and stored in −80 °C until analysis.
Brain tissues from copper-exposed mice. Previously collected brain tissues from 3xTg-AD mice (Thy1.2-APPswe, Thy1.2-TauP301L, PS1M146V-KI) exposed to 250 ppm copper sulfate (CuSO4) in the drinking water for a period of 3 or 12 months were used (Kitazawa et al., 2009). Brain homogenates for biochemical analysis were stored in −80 °C, and brain sections for histological analysis were stored in 4 °C.
MTT cytotoxicity assay. After the exposure, cells were washed once with PBS, then 100 µl of serum-free DMEM with 250 µg/ml of MTT was added. Cells were incubated in the humidified incubator for 3 h, allowing buildup of insoluble formazan. After the incubation, 100 µL of isopropanol–HCl solution (isopropanol:HCl = 100:1) was added and mixed well to completely dissolve formazan. Absorbance at 570 nm and reference absorbance at 630 nm were measured by the Spectromax plate reader (Molecular Devices).
Phagocytosis assay. pHydro Red E. coli BioParticle (Life Technologies) was reconstituted in serum-free DMEM at a concentration of 2 mg/ml and sonicated for 5 min until all particles were dispersed evenly. The solution was further diluted to working concentration of 200 µg/ml with serum-free DMEM, and 100 µl of the working solution was applied directly onto the cells after the exposure. Cells with BioParticles were incubated at 37ºC (ambient air) for 1–4 h. The fluorescent reading at excitation 560 nm and emission 585 nm was recorded every hour.
BV2 cells were plated onto a poly lysine-coated coverslip at an initial seeding density of 10 000 per coverslip (12–15 mm diameter). Cells were treated with or without 0.5 µM copper in serum-free DMEM for 24 h, then the media were replaced to serum-free DMEM containing 0.5 µM fAβ. Cells were further incubated for 1 h, and caboxylated fluorospheres (1 µm diameter, Life Technologies) in PBS and 1 mg/ml BSA was added to the media at a final concentration of 1 × 107 beads/ml. Thirty minutes after the addition of the beads, cells were washed well to remove all non-phagocytosed beads and fixed with 4% paraformaldehyde, then stained with Iba1 (1:500) and DAPI. The number of beads phagocytosed in the cell was counted under the fluorescent microscope.
Cytokine assays. Brain cytokines were quantitatively measured by ELISA (ThermoFisher Scientific) or Bio-Plex (Bio-Rad Laboratories), respectively, as described previously (Kitazawa et al., 2011). Briefly, 50 µl of brain homogenates were used to determine IL-1β, IL-6 and TNF-α levels by Endogen ELISA kits. For Bio-Plex, we used a custom 8-plex detection kit, which measured IL-1α, IL-1β, IL-4, IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1), TNFα, and interferon-γ (IFNγ). We strictly followed the manufacturer’s instructions. Briefly, 10 µl of plasma was mixed with 40 µl of standard diluent and incubated with a mixture of antibodies conjugated with fluorescent beads. Following the detection antibody and streptavidin-PE treatments, levels of each cytokine were measured using the Bio-Plex 200 System (Bio-Rad Laboratories). Concentrations were calculated by standard curve and expressed in pg/ml.
Immunoblot analysis. The equal amounts of protein from cells or brain homogenates were resolved in 4–12% gradient gel, transferred onto PVDF membrane and blotted with anti-LRP1 antibody (1:25, Santa Cruz Biotechnologies), anti-SYK antibody (1:1000, Cell Signaling Technology), anti-phospho-SYK antibody (1:1000, Cell Signaling Technology), anti-transferrin receptor (TfR) antibody (1:3000, R&D Systems), anti-ubiquitin antibody (1:3000, Enzo Biochem), and anti-LC3 antibody (1:3000, Abnova). Tubulin (1:20000, Sigma-Aldrich) or GAPDH (1:5000, Santa Cruz Biotechnologies) was used as a loading control to normalize the protein band for each sample. All immunoblot images were captured and analyzed by Li-Cor Odyssey and Image Station software (Li-Cor).
Immunostaining analysis. Each half brain was cut into 50 μm slices using a Vibratome and stored in TBS with 0.01% sodium azide solution. Brain sections from each animal were stained with Iba1 (1:500, Wako Chemicals) to detect microglia and 6E10 (1:500, Covance) to detect Aβ plaques. Briefly, sections were pre-treated with 90% formic acid for 7 min followed by washing with TBS/0.1% Triton X-100, and overnight incubation with primary antibodies at 4ºC. To visualize co-localization, sections were then incubated for 1 hour with goat antimouse Alexafluor 488 and goat anti-rabbit Alexfluor 555, with TOTO3 (1:200) for nuclear staining. Fluorescent images were captured and analyzed by confocal microscopy.
Real-time quantitative PCR. One microgram of total RNA isolated from cells or brain tissues were used to prepare cDNA template using SuperScript III cDNA synthesis kit (Life Technologies) for qRT-PCR as described previously (Kitazawa 2011, Kitazawa 2005). Briefly, 1 µl of cDNA was subject to qRT-PCR using iQ SYBR master mix (Bio-Rad Laboratories) to detect IL-1β (fwd: AAATGCCTCGTGCTGTCTGACC, rev: CTGCTTGAGAGGTGCTGATGTACC), IL-6 (fwd: AGTTGCCTTCTTGGGACTGA, rev: TCCACGATTTCCCAGAGAAC), TNFα (fwd: CTGGGACAGTGACCTGGACT, rev: GCACCTCAGGGAAGAGTCTG), YM-1 (fwd: CTGGAATTGGTGCCCCTACA, rev: CAAGCATGGTGGTTTTACAGGA) and Arg-1 (fwd: CATTGGCTTGCGAGACGTAGAC, rev: GCTGAAGGTCTCTTCCATCACC). Threshold cycle (Ct) was calculated with MyiQ software (Bio-Rad Laboratories), and fold changes were determined and normalized to GAPDH (fwd: AACTTTGGCATTGTGGAAGG, rev: ACACATTGGGGGTAGGAACA).
Statistical analysis. All data were quantitatively analyzed using Li-Cor Image Station software and/or Image J software. Statistics were carried out using 1-way ANOVA with post-hoc tests or unpaired t-test, and P < 0.05 was considered to be significant.
RESULTS
Copper Exposure Inhibits the Activation of Phagocytosis in BV2 Cells
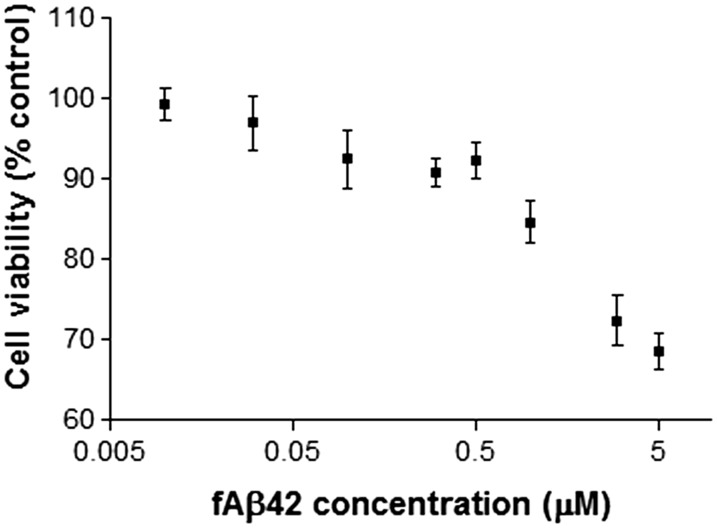
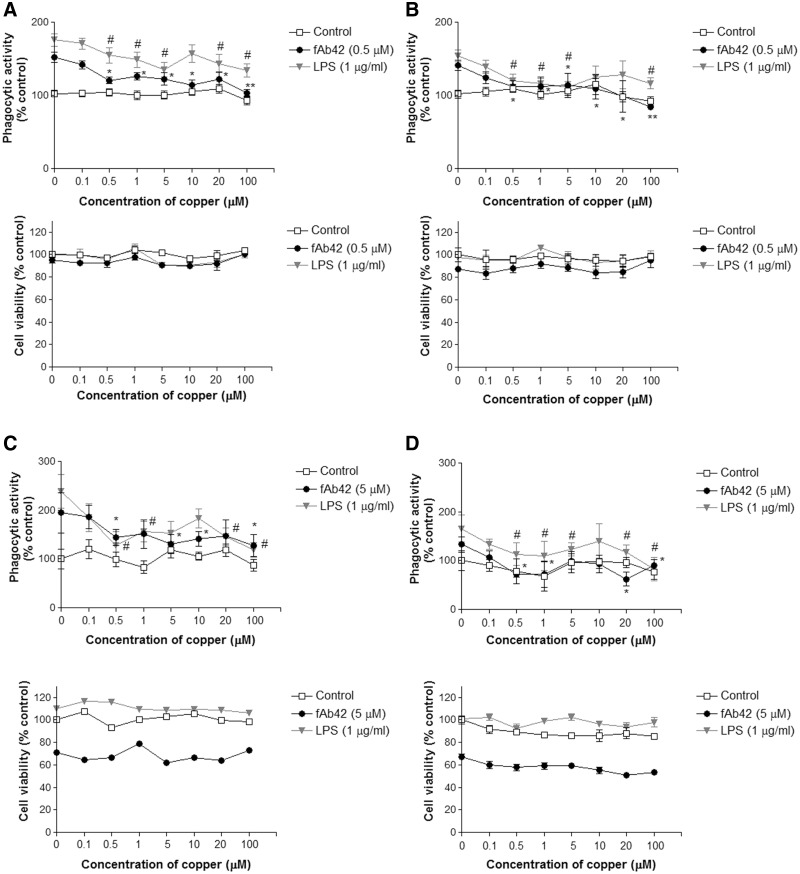
We first examined the acute cytotoxicity of synthetic fibrillar Aβ1-42 (fAβ) on macrophage/microglia-like murine BV2 cells to determine the subtoxic concentration to be used to stimulate phagocytosis. MTT assay showed a concentration-dependent reduction of mitochondrial activity, an indirect indicator of cytotoxicity and cell death, between 0.01 and 5 µM fAβ in 1 h of exposure (Figure 1). Reverse 42-1 Aβ peptide at 5 µM concentration did not show any significant reduction in MTT assay (data not shown). We selected 2 concentrations, 0.5 and 5 µM, to test its ability to activate phagocytosis and how copper exposure modulated it. In the 3-h copper exposure, BV2 cells were exposed to copper (0, 0.1, 0.5, 1, 5, 10, 20, and 100 µM) 3 h prior to the 1-h exposure to 0.5 or 5 µM fAβ. The pre-exposure to copper significantly inhibited BV2’s ability to activate phagocytosis by fAβ stimulation at a concentration of 0.5 µM or higher (Figures 2A and C). Similarly, copper exposure inhibited LPS-stimulated phagocytosis while copper exposure by itself had no effect on the basal phagocytosis (Figures 2A and C), suggesting that copper attenuated the ability of BV2 to activate inflammatory pathways. The significant reduction of phagocytosis was not due to a loss of BV2 cells because MTT assay showed the copper exposure did not induce significant cytotoxicity in combination with fAβ or LPS (Figures 2A and C). Higher concentration of fAβ (5 µM) exposure resulted in 22–39% reduction in mitochondrial activity, and 0.5 µM fAβ exposure caused 10% reduction compared to control groups (Figure 2C). In another study, we exposed BV2 cells to copper (0, 0.1, 0.5, 1, 5, 10, 20, and 100 µM) in serum-free media for 24 h prior to the 1-h exposure to fAβ or LPS. We found that the 24-h copper exposure also significantly inhibited phagocytic activation of BV2 cells at a concentration of 0.5 µM or higher (Figure 2B and D).
FIG. 1.
Fibrillar Aβ induces acute cytotoxicity to BV2 cells. BV2 cells were exposed to a range of concentrations (0–5 µM) of synthetic fibrillar Aβ1-42 (fAβ) for 1 h in a serum-free condition. Acute fAβ toxicity was determined by the MTT assay. Each plot represents mean ± SEM of 3–4 separate experiments in triplicates (n = 9–12).
FIG. 2.
Copper exposure significantly inhibits fAβ-induced phagocytosis by BV2 cells. BV2 cells were exposed to various concentrations (0, 0.1, 0.5, 1, 5, 10, 20, 100 µM) of copper for 3 or 24 h followed by 1-h exposure to 0.5 or 5 µM fAβ or 1 µg/ml LPS to stimulate phagocytosis. A, Phagocytic activation of BV2 cells and toxicity assay following the 3-h copper exposure and 0.5 µM fAβ stimulation. B, Phagocytic activation of BV2 cells and toxicity assay following the 24-h copper exposure and 0.5 µM fAβ stimulation. C, Phagocytic activation of BV2 cells and toxicity assay following the 3-h copper exposure and 5 µM fAβ stimulation. D, Phagocytic activation of BV2 cells and toxicity assay following the 24-h copper exposure and 5 µM fAβ stimulation. Each graph represents mean ± SEM of 3 separate experiments in triplicates (n = 9). Veh = vehicle control (no stimulation). *P < 0.05 or **P < 0.01 compared to the corresponding stimulating agent (fAβ or LPS) alone.
We also employed a different phagocytosis assay using fluorescent microbeads and confirmed the effect of copper on inhibiting fAβ-stimulated phagocytic activation of BV2 cells. Copper exposure (0.5 µM) for 24 h significantly reduced the number of BV2 cells that phagocytosed microbeads following the stimulation by 0.5 µM fAβ (Figure 3A). The expressions of YM-1 and arginase-1, markers for phagocytic activation (Latta et al., 2015; Medeiros et al., 2013), were also reduced in the copper/fAβ-exposed BV2 cells (Figure 3B). In addition, the activation of spleen tyrosine kinase (SYK) and subsequent tyrosine phosphorylation have been reported in immune cells during phagocytic activation, and pharmacological inhibition of SYK or Src tyrosine kinases significantly impairs Aβ-elicited phagocytic activation (Greenberg et al., 1993; Koenigsknecht and Landreth, 2004). The exposure of fAβ significantly increased phosphorylation of SYK while the pre-exposure to copper in 2 concentrations (0.5 and 1 µM) significantly inhibited the activation of SYK (Figure 3C). Copper alone was not different from the control level (data not shown). Collectively, these data strongly supported that copper interfered with the fAβ-induced phagocytic activation pathway in BV2 cells.
FIG. 3.
Copper exposure suppresses phagocytic markers and inactivates SYK in BV2 cells. A, Phagocytic assay by fluorescent microbeads demonstrates a significant reduction of BV2 cell population that takes up more than 3 beads per cell in the 24-h copper (0.5 µM) exposure followed by 0.5 µM fAβ stimulation for 1 h. Each graph represents mean ± SEM of 3 separate experiments in triplicates (n = 9). *P < 0.05 compared to the fAβ-stimulated group. B, The expression of YM-1 and Arg-1 in 0.5 µM fAβ alone or copper (0.5 µM, 24 h) exposure followed by 0.5 µM fAβ stimulation for 1 h. Each graph represents mean ± S.E.M. of 3 separate experiments in triplicates (n = 9). *P < 0.05 compared to the fAβ-stimulated group. C, Immunoblot analysis of phospho-SYK in BV2 cells. The band intensity of phospho-SYK or total SYK was first normalized by tubulin, then the ratio phospho-SYK/total SYK was calculated. Each graph represents mean ± S.E.M. of 2 separate experiments in triplicates (n = 6). *P < 0.05 or **P < 0.01 compared to the fAβ-stimulated group, #P < 0.05 compared to the control group.
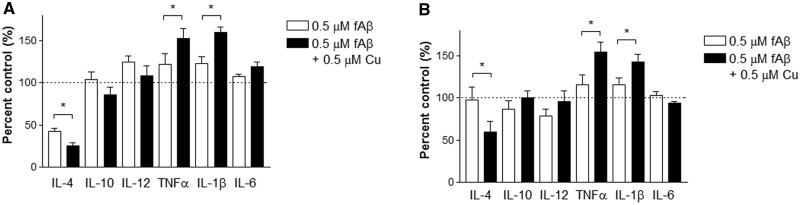
The perturbed inflammatory activation of BV2 cells following the copper exposure was also evident by the selected inflammatory cytokine profile in the conditioned media. Cytokine arrays (TNFα, IFNγ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, and KC) identified several cytokines were significantly altered in the presence of copper. These cytokines were secreted into conditioned media with wide ranges. The actual concentration of each cytokine found in the control-conditioned media from the 3-h study is following: TNFα (11.62 pg/ml), IFNγ (0.0703 pg/ml), IL-1β (0.142 pg/ml), IL-2 (0.581 pg/ml), IL-4 (0.643 pg/ml), IL-5 (1.33 pg/ml), IL-6 (13.37 pg/ml), IL-10 (0.947 pg/ml), IL-12 (13.38 pg/ml), and KC (0.259 pg/ml). The actual concentration of each cytokine found in the control-conditioned media from the 24-h study is following: TNFα (22.64 pg/ml), IFNγ (0.0255 pg/ml), IL-1β (0.09 pg/ml), IL-2 (0.436 pg/ml), IL-4 (0.171 pg/ml), IL-5 (1.20 pg/ml), IL-6 (13.85 pg/ml), IL-10 (0.81 pg/ml), IL-12 (14.37 pg/ml), and KC (0.304 pg/ml). Pro-inflammatory cytokines, IL-1β and TNFα, were significantly elevated in BV2 cells exposed to 0.5 µM copper 3-h or 24-h prior to fAβ exposure (0.5 µM), while anti-inflammatory cytokine, IL-4, was significantly downregulated (Figure 4A and B). Copper alone was not different from control levels in BV2 cells (data not shown). Together, these findings showed that the copper exposure triggered pro-inflammatory activation of BV2 cells.
FIG. 4.
Copper exposure activates pro-inflammatory responses in BV2 cells when stimulated with fAβ. Conditioned media were collected and quantitatively analyzed for selected pro- and anti-inflammatory cytokines released from BV2 cells after 1-h fAβ (0.5 µM) stimulation. A, BV2 cells pre-exposed to 0.5 µM copper for 3 h or B, BV2 cells pre-exposed to 0.5 µM copper for 24 h. Each graph represents mean ± S.E.M. of 3 separate experiments in triplicates (n = 9). *P < 0.05 compared to the fAβ-stimulated group without pre-exposure to copper (open bar).
Down-Regulation of Endothelial LRP1 Following the Secretion of ProInflammatory Cytokines
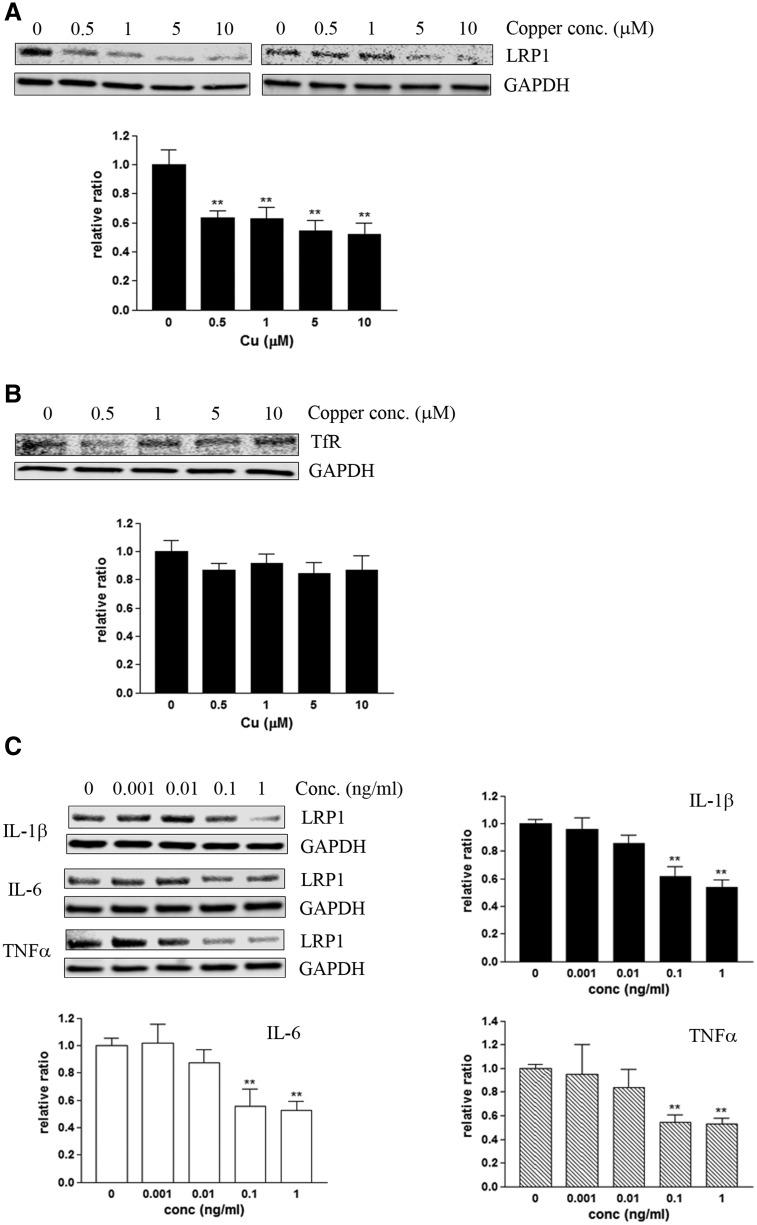
A recent in vivo study by Singh and colleagues unveiled that the trace amount of copper exposure down-regulates LRP1 in brain capillaries, resulting in impairment of LRP1-mediated Aβ clearance (Singh et al., 2013). We have confirmed that 24 h of copper exposure significantly decreased the steady-state levels of LRP1 in human primary microvascular endothelial cells (MVECs) in a concentration-dependent manner (Figure 5A). This effect of copper on LRP1 was evident at 0.5 µM or higher concentration of copper in the growth media for 24 h, which was higher concentration than that in previously reported conditions (Singh et al., 2013). This was probably in part because our growth media contained sera, which might decrease the bioavailability of free copper in the media. The reduction of LRP1 by copper appeared to be specific as transferrin receptor (TfR), another cell surface receptor abundantly expressed in the endothelial cells, was not affected in MVECs (Figure 5B).
FIG. 5.
Copper and pro-inflammatory cytokines down-regulate LRP1 in microvascular endothelial cells. Human primary microvascular endothelial cells (MVECs) were exposed to copper or human recombinant IL-1β, IL-6 or TNFα for 24 h, and the steady-state levels of LRP1 was quantitatively measured by immunoblot. A, Copper down-regulates LRP1 in MVECs in a concentration-dependent manner. B, Transferrin receptor (TfR) was not altered in MVEC exposed to copper. C, IL-1β, IL-6 or TNFα alone also significantly down-regulates LRP1 in MVECs in a concentration-dependent manner. Each graph represents mean ± S.E.M. of 3 separate experiments (n = 6–9). *P < 0.05 or **P < 0.01 compared to the control group.
Interestingly, we identified that not only copper by itself, but also pro-inflammatory cytokines significantly down-regulated LRP1 levels in these endothelial cells. When human recombinant pro-inflammatory cytokines, IL-1β, IL-6, and TNFα, were individually applied to MVECs at various concentrations (0.001–1 ng/ml) for 24 h, the steady-state levels of LRP1 were markedly down-regulated in a concentration-dependent manner (Figure 5C). TNFα appeared to be the most potent cytokine to down-regulate LRP1, followed by IL-1β and IL-6.
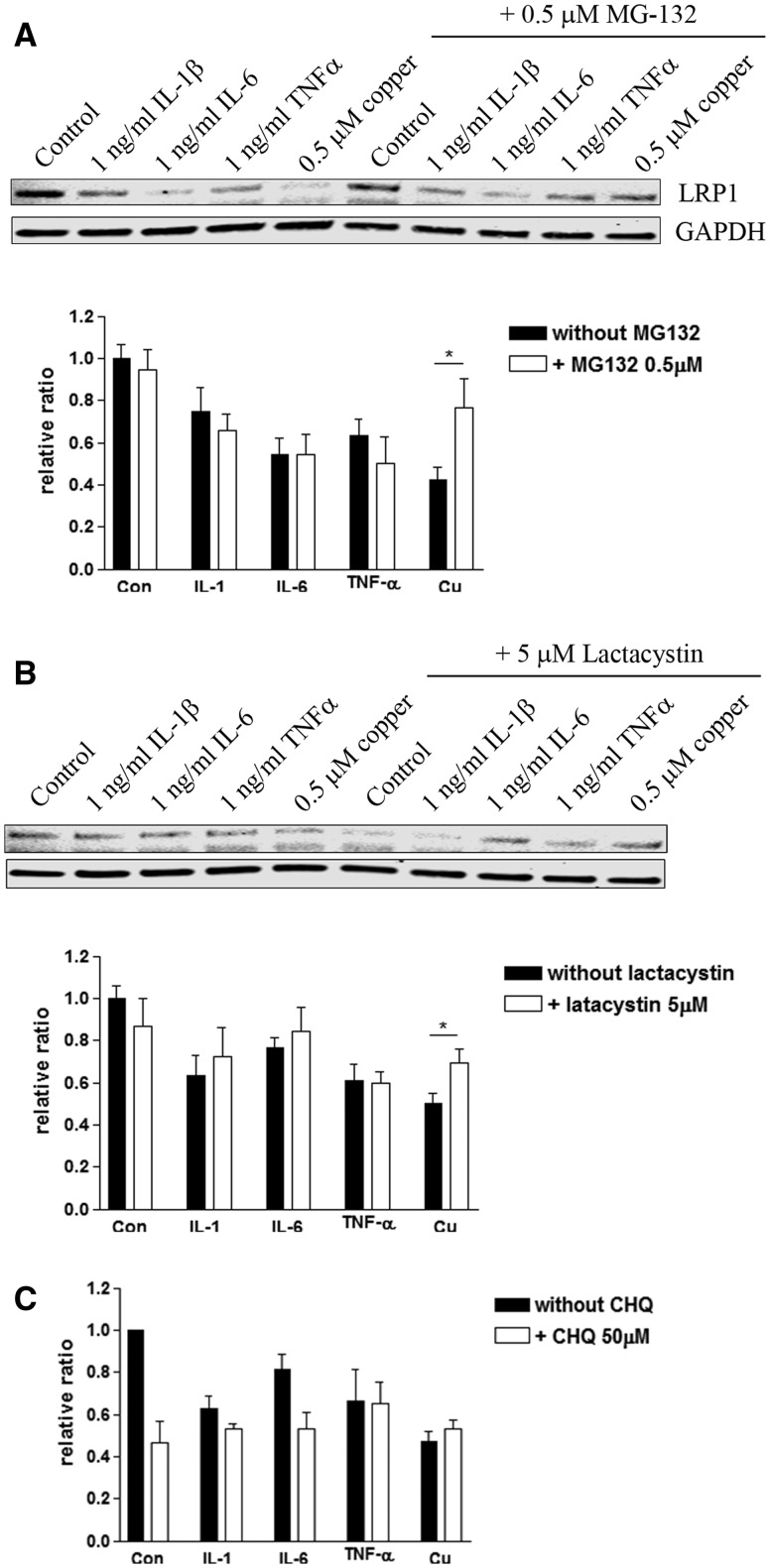
Recent evidence suggests that copper promotes proteasome-mediated degradation of LRP1 in the endothelial cells (Singh et al., 2013). To test whether not only copper- but also IL-1 β-, IL-6-, and TNFα-induced loss of LRP1 was mediated by the activation of proteasome degradation, we co-treated MVECs with proteasome inhibitors, MG-132 (0.5 µM, Supplementary Figure 1) or lactacystein (5 µM, Supplementary Figure 1), for 24 h with copper (0.5 µM), IL-1β (1 ng/ml), IL-6 (1 ng/ml), or TNFα (1 ng/ml). Interestingly, we observed a partial restoration of LRP1 when proteasome inhibitors were applied with copper in MVECs (Figures 6A and B). However, inhibition of proteasome did not restore cytokine-induced downregulation of LRP1 (Figures 6A and B). We also examined whether copper- or cytokine-induced loss of LRP1 in MVEC was mediated by lysosomal degradation. Co-treatment of lysosomal inhibitor, chloroquine (CHQ, 50 µM) did not rescue LRP1 (Figure 6C). CHQ treatment alone significantly down-regulated LRP1, suggesting long-term lysosome inhibition had adverse effects.
FIG. 6.
Cytokine-induced downregulation of LRP1 is not mediated by proteasome degradation. A, MVECs were exposed to copper, IL-1β, IL-6 or TNFα in the presence or absence of proteasome inhibitors, MG-132 (0.5 µM, A) or lactacystin (5 µM, B), for 24 h, and the steady-state levels of LRP1 were measured. The graph represents mean ± S.E.M. of 3 separate experiments (n = 8–11). *P < 0.05 compared to the group without proteasome inhibitor. C, MVECs were exposed to copper, IL-1β, IL-6 or TNFα in the presence or absence of a lysosomal inhibitor, CHQ, for 24 h, and the steady-state levels of LRP1 were measured. The graph represents mean ± S.E.M. of 3 separate experiments in duplicates (n = 6). No significance was observed.
Copper Exposure Exacerbates AD-like Neuropathology in 3xTg-AD Model
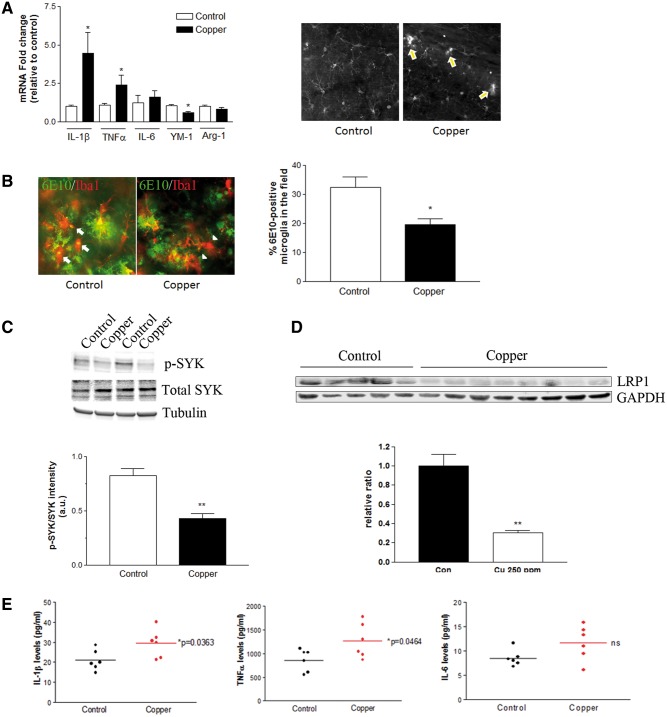
We have previously reported the impact of copper-containing drinking water on 3xTg-AD mouse model of AD (Kitazawa et al., 2009). We analyzed inflammatory components and microglial activation on these copper-exposed mouse brains and found early signs of clusters of activated microglia and significant elevation of IL-1β and TNFα at as early as 3 months of copper exposure, preceding Aβ plaque pathology in the brain (Figure 7A). At 9 months exposure, a clear increase in Aβ plaque pathology was observed in 3xTg-AD mice receiving copper-containing drinking water (Kitazawa et al., 2009). Both astrocytes and microglia were highly activated around plaques, yet phagocytically active microglia appeared to be markedly reduced in the copper exposed mice as indicated by reduced number of activated microglia co-immunostained with 6E10 antibody (Figure 7B). Immunoblot analysis of brain homogenates also revealed a marked reduction of phospho-SYK, an indication of active SYK, in the copper-exposed group (P < 0.01, Figure 7C), confirming impairment of microglial phagocytic activation in copper-exposed mice. A significant down-regulation of LRP1 in the brain was not detected until 12 months of copper exposure to these mice (Figure 7D). Multiplex analysis of pro-inflammatory cytokines in these brain homogenates showed significant increases of IL-1β and TNFα, but not IL-6 (Figure 7E). In our in vitro studies, these pro-inflammatory cytokines, especially IL-1β and TNFα, had a robust impact on LRP1 expression in MVECs, suggesting that the loss of LRP1 and impairment of microglial phagocytic activation together acted as a vicious cycle to exacerbate a pathological buildup of Aβ in the brain of mice that were chronically exposed to copper-containing water. Collectively, our in vitro and in vivo findings strongly suggest that copper significantly dysregulates phagocytosis and neuroinflammation at early stages, leading to a loss of LRP1 in the endothelial cells, and an accelerated buildup of Aβ in the brain.
FIG. 7.
Aberrant activation of neuroinflammation in the brain of copper-exposed 3xTg-AD mice. Three months old 3xTg-AD mice were treated with 250 ppm copper-containing drinking water for 3–12 months. A, The mRNA expression of selected cytokines and phagocytosis markers in the brain after the 3 months of control water (n = 10, open bars) or copper exposure (n = 10, closed bars). *P < 0.05 compared to the control group. Representative immunofluorescent staining of Iba1-positive microglia in hippocampus of 3xTg-AD mice received control or copper-containing drinking water for 3 months shows early signs of microglial activation. Arrows indicate the clustering and activating microglia in the absence of Aβ plaques in the copper-exposed mice. B, Representative double immunofluorescent staining of Iba1-positive microglia (red) and 6E10-positive Aβ plaques (green) in hippocampus of 3xTg-AD mice received control or copper-containing drinking water for 9 months. Arrows indicate microglia colocalized with 6E10-positive Aβ, and arrowheads indicate microglia located nearby Aβ plaques but no obvious 6E10-positive Aβ within. The graph represents the percentage of Iba1-positive microglia that colocalize 6E10 positive Aβ. *P < 0.05 compared to the control group (n = 5–8). C, The activation of SYK was determined by immunoblot of phospho-SYK and total SYK in the brain homogenates from 9 months treatment (**P < 0.01 compared to control, n = 4). D, The down-regulation of brain LRP1 was detected in 3xTg-AD mice exposed to copper-containing drinking water for 12 months (**P < 0.01, control n = 5, copper-exposed n = 8). E, Brain cytokine levels were measured by ELISA following 12 months copper exposure (n = 6).
DISCUSSION
Our study has demonstrated that the exposure to copper primes macrophage/microglia cell to inhibit its phagocytic phenotype and activate pro-inflammatory cytokine release upon Aβ stimulation, presumably through inactivation of SYK, a master regulator of inflammatory and immune responses in the cell (Tohyama and Yamamura, 2009). Increased secretion of pro-inflammatory cytokines, IL-1β, IL-6, and TNFα contribute significantly to the downregulation of endothelial LRP1 in vitro. Interestingly, the molecular mechanism of cytokine-mediated loss of LRP1 in MVECs may be distinct from that of copper-mediated loss of LRP1 as it was not rescued by proteasome inhibitors. Further studies are needed to clarify its mechanisms.
Impairment of phagocytosis and loss of endothelial LRP1 may facilitate pathological buildup of Aβ (Kiyota et al., 2012; Storck et al., 2016). Results from our study, together with previously reported findings, have unveiled multifactorial mechanisms of copper on neuroinflammation and Aβ deposition in the brain, and mechanistically supported the exposure to copper increases the risk of developing AD throughout the life. In humans, aging, the most prominent risk factor for AD, is known to decrease the brain LRP1 and LRP1-mediated transcytotic clearance of Aβ (Shibata et al., 2000; Silverberg et al., 2010). Decreased levels of LRP1 are also found in AD brains and are inversely proportional to the Aβ burden (Deane et al., 2008; Kang et al., 2000; Jeynes and Provias, 2008). Environmental copper exposure and inflammation are now known trigger to reduce endothelial LRP1 and increase the risk for AD. We have confirmed the mechanism of copper-mediated loss of LRP1 as proteasome-dependent, as previously reported (Singh et al., 2013). Interestingly, cytokine-mediated loss of LRP1 is proteasome- or lysosome-independent. Further studies will be required to unveil the underlying mechanism of cytokine-mediated down-regulation of LRP1. In animal models, environmental enrichment is shown to increase the expression of LRP1 and reduced brain Aβ levels (Herring et al., 2008). Taken together, environmental impact on brain LRP1 may significantly influence the onset of AD throughout the life.
Inflammation has recently gained much attentions in the field of AD. While epidemiological studies have long supported the use of anti-inflammatory medications to lower the risk for AD (Szekely and Zandi, 2010), its cellular and molecular mechanisms still remain to be fully elucidated. Studies from animal models have mixed results, suggesting complex, multifactorial inflammatory cascades in the brain. For example, acute activation of brain inflammation attenuates Aβ pathology (DiCarlo et al., 2001; Herber et al., 2007), while chronic disturbance of inflammation exacerbates AD-like neuropathology in animal models (Kitazawa et al., 2005; Sheng et al., 2003). Selective overexpression or inhibition of particular cytokine, such as IL-1β, TNFα, IL-6, IFNγ, or IL-10, differentially affect the phenotypic behavior of microglia in complicated manners and modulate AD-like neuropathology in mice (Chakrabarty et al., 2010; Chakrabarty et al., 2015; Ghosh et al., 2013; Heneka et al., 2013; Kitazawa et al., 2011; Kiyota et al., 2012). We cannot exclude a possibility that the strain and age of mice, transgenes, and other factors may influence the phenotypes, behaviors and responses to these cytokines.
The involvement of heavy metals in the increased risk for AD has long been discussed. We have previously exposed 3xTg-AD mouse model to copper-containing drinking water for a period of 3–12 months (Kitazawa et al., 2009). Although the dose of copper used in this experiment was much higher than that is allowed through drinking water in humans by EPA, we found the levels of APP and BACE were significantly increased in the brain, suggesting that upregulation of APP processing and increased production of Aβ caused exacerbation of AD-like pathology in mice received copper-containing water. Even in more environmentally-relevant doses, chronic exposure to copper through drinking water exhibits robust phenotypic and pathological results (Singh et al., 2013; Sparks et al., 2006; Sparks and Schreurs, 2003). Interestingly, however, chronic exposure to high levels of copper in drinking water in APP23 mice attenuated the generation and toxicity of reactive oxygen species (ROS) and Aβ plaques in the brain by stabilizing copper/zinc superoxide dismutase (Cu/Zn SOD) (Bayer et al., 2003). While no convincing explanation or mechanism to support all findings in copper and AD is currently available, it is not surprising that copper exhibits such beneficial effects as it is an essential heavy metal. However, dyshomeostasis of brain copper levels likely contributes to the pathogenesis of AD as clearance and redistribution of copper by metal chelators, such as clioquinol or trientine, effectively reduced the Aβ plaque formation in mouse models of AD (Cherny et al., 2001; Wang et al., 2013). Collectively, these in vivo studies clearly indicate the impact of chronic copper toxicity on Aβ production and clearance, hence increasing the risk for developing AD.
The pathogenic impact of environmental risk factors including chronic copper exposure on developing AD still remains controversial. Growing evidence supports low-level, life-long copper exposure from drinking water may have significant adverse effect and increase the onset of sporadic AD in subset of the patients. It is also important to investigate whether any particular risk genes and environmental factors synergistically facilitate Aβ deposition and neurodegeneration. While definitive causes of and effective treatment strategies for AD remain to be discovered, restoration of brain LRP1 or inflammatory homeostasis through life may potentially reduce the risk of developing AD.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENT
This study is supported by the NIH/NIEHS R01 ES024331 (M.K.).
References
- Bayer T. A., Schafer S., Simons A., Kemmling A., Kamer T., Tepest R., Eckert A., Schussel K., Eikenberg O., Sturchler-Pierrat C., et al. (2003). Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc. Natl. Acad. Sci. USA 100, 14187–14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G. J., Kanzer S. H., Zimmerman E. A., Celmins D. F., Heckman S. M., Dick R. (2010). Copper and ceruloplasmin abnormalities in Alzheimer's disease. Am. J. Alzheimer's Dis. Other Dement 25, 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhead J. L., Gray L. W., Lutsenko S. (2011). Systems biology approach to Wilson's disease. Biometals 24, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P., Jansen-West K., Beccard A., Ceballos-Diaz C., Levites Y., Verbeeck C., Zubair A. C., Dickson D., Golde T. E., Das P. (2010). Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. Faseb J. 24, 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P., Li A., Ceballos-Diaz C., Eddy J. A., Funk C. C., Moore B., DiNunno N., Rosario A. M., Cruz P. E., Verbeeck C., et al. (2015). IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron 85, 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny R. A., Atwood C. S., Xilinas M. E., Gray D. N., Jones W. D., McLean C. A., Barnham K. J., Volitakis I., Fraser F. W., Kim Y., et al. (2001). Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30, 665–676. [DOI] [PubMed] [Google Scholar]
- Deane R., Sagare A., Zlokovic B. V. (2008). The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer's disease. Curr. Pharm. Des. 14, 1601–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo G., Wilcock D., Henderson D., Gordon M., Morgan D. (2001). Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol. Aging 22, 1007–1012. [DOI] [PubMed] [Google Scholar]
- Donahue J. E., Flaherty S. L., Johanson C. E., Duncan J. A., 3rd, Silverberg G. D., Miller M. C., Tavares R., Yang W., Wu Q., Sabo E., et al. (2006). RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 112, 405–415. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Wu M. D., Shaftel S. S., Kyrkanides S., LaFerla F. M., Olschowka J. A., O'Banion M. K. (2013). Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J. Neurosci. 33, 5053–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Chang P., Silverstein S. C. (1993). Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J. Exp. Med. 177, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough M. A., Camakaris J., Bush A. I. (2013). Metal dyshomeostasis and oxidative stress in Alzheimer's disease. Neurochem. Int. 62, 540–555. [DOI] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T. C., et al. (2013). NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner F. L., Ransohoff R. M., Becher B. (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16, 358–372. [DOI] [PubMed] [Google Scholar]
- Herber D. L., Mercer M., Roth L. M., Symmonds K., Maloney J., Wilson N., Freeman M. J., Morgan D., Gordon M. N. (2007). Microglial activation is required for Abeta clearance after intracranial injection of lipopolysaccharide in APP transgenic mice. J. Neuroimmune Pharmacol. 2, 222–231. [DOI] [PubMed] [Google Scholar]
- Herring A., Yasin H., Ambree O., Sachser N., Paulus W., Keyvani K. (2008). Environmental enrichment counteracts Alzheimer's neurovascular dysfunction in TgCRND8 mice. Brain Pathol. (Zurich, Switzerland) 18, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Ueno T., Ohtsuki S., Terasaki T. (2010). Lack of brain-to-blood efflux transport activity of low-density lipoprotein receptor-related protein-1 (LRP-1) for amyloid-beta peptide(1-40) in mouse: involvement of an LRP-1-independent pathway. J. Neurochem. 113, 1356–1363. [DOI] [PubMed] [Google Scholar]
- Jeynes B., Provias J. (2008). Evidence for altered LRP/RAGE expression in Alzheimer lesion pathogenesis. Curr. Alzheimer Res. 5, 432–437. [DOI] [PubMed] [Google Scholar]
- Kaler S. G. (2011). ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. 7, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. E., Pietrzik C. U., Baum L., Chevallier N., Merriam D. E., Kounnas M. Z., Wagner S. L., Troncoso J. C., Kawas C. H., Katzman R., and., et al. (2000). Modulation of amyloid beta-protein clearance and Alzheimer's disease susceptibility by the LDL receptor-related protein pathway. J. Clin. Invest. 106, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M., Cheng D., Laferla F. M. (2009). Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J. Neurochem. 108, 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M., Cheng D., Tsukamoto M. R., Koike M. A., Wes P. D., Vasilevko V., Cribbs D. H., LaFerla F. M. (2011). Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer's disease model. J. Immunol. 187, 6539–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M., Oddo S., Yamasaki T. R., Green K. N., LaFerla F. M. (2005). Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J. Neurosci. 25, 8843–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T., Ingraham K. L., Swan R. J., Jacobsen M. T., Andrews S. J., Ikezu T. (2012). AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 19, 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J., Landreth G. E. (2005). Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 25, 8240–8249. 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht J., Landreth G. (2004). Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J. Neurosci. 24, 9838–9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta C. H., Brothers H. M., Wilcock D. M. (2015). Neuroinflammation in Alzheimer's disease; A source of heterogeneity and target for personalized therapy. Neuroscience 302, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt M., Lee H. G., Perry G., Smith M. A., Zhu X. (2004). Sources and mechanisms of cytoplasmic oxidative damage in Alzheimer's disease. Acta Neurobiol. Exp. (Wars) 64, 81–87. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G. (2007). NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol. Aging 28, 639–647. [DOI] [PubMed] [Google Scholar]
- Medeiros R., Kitazawa M., Passos G. F., Baglietto-Vargas D., Cheng D., Cribbs D. H., LaFerla F. M. (2013). Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am. J. Pathol. 182, 1780–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. C., Evans D. A., Tangney C. C., Bienias J. L., Schneider J. A., Wilson R. S., Scherr P. A. (2006). Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch. Neurol. 63, 1085–1088. [DOI] [PubMed] [Google Scholar]
- Multhaup G., Ruppert T., Schlicksupp A., Hesse L., Bill E., Pipkorn R., Masters C. L., Beyreuther K. (1998). Copper-binding amyloid precursor protein undergoes a site-specific fragmentation in the reduction of hydrogen peroxide. Biochemistry 37, 7224–7230. [DOI] [PubMed] [Google Scholar]
- Multhaup G., Scheuermann S., Schlicksupp A., Simons A., Strauss M., Kemmling A., Oehler C., Cappai R., Pipkorn R., Bayer T. A. (2002). Possible mechanisms of APP-mediated oxidative stress in Alzheimer's disease. Free Radic. Biol. Med. 33, 45–51. [DOI] [PubMed] [Google Scholar]
- Nazer B., Hong S., Selkoe D. J. (2008). LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol. Dis. 30, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X. D., Zhu Y. G., Lin N., Zhang J., Ye Q. Y., Huang H. P., Chen X. C. (2011). Microglial phagocytosis induced by fibrillar beta-amyloid is attenuated by oligomeric beta-amyloid: implications for Alzheimer's disease. Mol. Neurodegen. 6, 45. 10.1186/1750-1326-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Lee D. W., Park K. S. (2013). Elevated serum copper and ceruloplasmin levels in Alzheimer's disease. Asia Pac. Psychiatry. [DOI] [PubMed] [Google Scholar]
- Sheng J. G., Bora S. H., Xu G., Borchelt D. R., Price D. L., Koliatsos V. E. (2003). Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol. Dis. 14, 133–145. [DOI] [PubMed] [Google Scholar]
- Shibata M., Yamada S., Kumar S. R., Calero M., Bading J., Frangione B., Holtzman D. M., Miller C. A., Strickland D. K., Ghiso J., and., et al. (2000). Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 106, 1489–1499. 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg G. D., Messier A. A., Miller M. C., Machan J. T., Majmudar S. S., Stopa E. G., Donahue J. E., Johanson C. E. (2010). Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. J. Neuropathol. Exp. Neurol. 69, 1034–1043. 10.1097/NEN.0b013e3181f46e25. [DOI] [PubMed] [Google Scholar]
- Singh I., Sagare A. P., Coma M., Perlmutter D., Gelein R., Bell R. D., Deane R. J., Zhong E., Parisi M., Ciszewski J., et al. (2013). Low levels of copper disrupt brain amyloid-beta homeostasis by altering its production and clearance. Proc. Natl. Acad. Sci. USA 110, 14771–14776. 10.1073/pnas.1302212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks D. L., Friedland R., Petanceska S., Schreurs B. G., Shi J., Perry G., Smith M. A., Sharma A., Derosa S., Ziolkowski C., and., et al. (2006). Trace copper levels in the drinking water, but not zinc or aluminum influence CNS Alzheimer-like pathology. J. Nutr. Health Aging 10, 247–254. [PMC free article] [PubMed] [Google Scholar]
- Sparks D. L., Schreurs B. G. (2003). Trace amounts of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 100, 11065–11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squitti R., Barbati G., Rossi L., Ventriglia M., Dal Forno G., Cesaretti S., Moffa F., Caridi I., Cassetta E., Pasqualetti P., et al. (2006). Excess of nonceruloplasmin serum copper in AD correlates with MMSE, CSF [beta]-amyloid, and h-tau. Neurology 67, 76–82. [DOI] [PubMed] [Google Scholar]
- Squitti R., Ghidoni R., Siotto M., Ventriglia M., Benussi L., Paterlini A., Magri M., Binetti G., Cassetta E., Caprara D., et al. (2014). Value of serum nonceruloplasmin copper for prediction of mild cognitive impairment conversion to Alzheimer disease. Ann. Neurol. 75, 574–580. [DOI] [PubMed] [Google Scholar]
- Storck S. E., Meister S., Nahrath J., Meissner J. N., Schubert N., Di Spiezio A., Baches S., Vandenbroucke R. E., Bouter Y., Prikulis I., et al. (2016). Endothelial LRP1 transports amyloid-beta1-42 across the blood-brain barrier. J. Clin. Invest. 126, 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely C. A., Zandi P. P. (2010). Non-steroidal anti-inflammatory drugs and Alzheimer's disease: the epidemiological evidence. CNS Neurol. Disord. Drug Targets 9, 132–139. [DOI] [PubMed] [Google Scholar]
- Tohyama Y., Yamamura H. (2009). Protein tyrosine kinase, syk: a key player in phagocytic cells. J. Biochem. 145, 267–273. [DOI] [PubMed] [Google Scholar]
- Ventriglia M., Bucossi S., Panetta V., Squitti R. (2012). Copper in Alzheimer's disease: a meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimers Dis. 30, 981–984. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Xie J. W., Xu Y., Wang T., Cai J. H., Wang X., Zhao B. L., An L., Wang Z. Y. (2013). Trientine reduces BACE1 activity and mitigates amyloidosis via the AGE/RAGE/NF-kappaB pathway in a transgenic mouse model of Alzheimer's disease. Antioxid. Redox. Signal. 19, 2024–2039. 10.1089/ars.2012.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wang M., Wang B., Li M., Chen H., Yu X., Yang K., Chai Z., Zhao Y., Feng W. (2012). Immunogold labeling and X-ray fluorescence microscopy reveal enrichment ratios of Cu and Zn, metabolism of APP and amyloid-beta plaque formation in a mouse model of Alzheimer's disease. Metallomics 4, 1113–1118. [DOI] [PubMed] [Google Scholar]
- Xu G., Green C. C., Fromholt S. E., Borchelt D. R. (2012). Reduction of low-density lipoprotein receptor-related protein (LRP1) in hippocampal neurons does not proportionately reduce, or otherwise alter, amyloid deposition in APPswe/PS1dE9 transgenic mice. Alzheimers Res. Ther. 4, 12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Luo X., Xu H., Ma Q., Yuan J., Li X., Chang R. C., Qu Z., Huang X., Zhuang Z., et al. (2015). Identification of the key molecules involved in chronic copper exposure-aggravated memory impairment in transgenic mice of Alzheimer's disease using proteomic analysis. J. Alzheimers Dis. 44, 455–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.