Abstract
Modern methods of industrial poultry and egg production systems involve stressful practices that stimulate Escherichia coli (E. coli) activity causing endotoxic shock. This investigation was conducted to evaluate the expression of pro-inflammatory cytokines and cell death program genes and DNA damage induced by E. coli in the brain and liver tissues of laying hens. A total of two hundred and ten H&N brown layer hens with 20 week age, were used in this research. First, preliminary experiments were designed (60 hens in total) to establish the optimal exposure dose of E. coli and to determine the nearest time of notable response to be used in the remainder studies of this research. At 35-wk of age, 150 hens were randomly assigned into 2 groups with 3 replicates of 25 birds each; the first group was injected in the brachial wing vein with 107 E. coli colony/hen, while the second group was injected with saline and served as a control. The body temperature and plasma corticosterone concentration were measured 3 hr after injection. Specimens of liver and brain were obtained from each group and the gene expression of p38 mitogen-activated protein kinase, interlukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), Bax, and caspase-3 genes were measured by quantitative real-time PCR. DNA damage in the brain and liver tissues were also measured by comet assay. Hens treated with E. coli showed significant (P<0.05) increase of body temperature and plasma corticosterone (42.6°C and 14.5 ng/ml, respectively) compared to the control group (41.1°C and 5.5 ng/ml, respectively). Additional remarkable over-inflammation gene expression of p38, IL-1β and TNF-α.genes were also detected in the brain (2.2-fold, 2.0-fold and 3.3-fold, respectively) and the liver (2.1-fold, 1.9-fold and 3.0-fold, respectively) tissues of the infected chickens. It is also important to note that hens injected with E. coli showed an increase in DNA damage in the brain and liver cells (P<0.05). These results were synchronized with activating cell death program since our data showed significant high expression of Bax gene by 2.8- and 2.7-fold and caspase-3 gene by 2.5- and 2.7-fold in the brain and liver tissues of infected chickens, respectively (P<0.05). In conclusion, the current study indicates that E. coli injection induces inflammatory physiological response and triggers cell death program in the brain and liver. Our results provide more understanding to endotoxic shock by E. coli in chickens at cellular level. Further studies are required to confirm if such responses are destructive or protective to set the means through which a chicken mounts a successful defense against avian pathogenic E. coli.
Introduction
It is estimated that in order to feed the world population in 2050, there has to be a 70% increase in food production over today’s levels. The livestock sector, particularly poultry production, forms an essential component to meet these demands in both developed and undeveloped regions. Despite the improvement in poultry production systems and the parallel enhancement in the trade volume of their products over the past years, Avian Pathogenic Escherichia Coli (APEC) is the leading cause of morbidity and mortality in poultry and exerts significant economic and welfare costs and continues to pose a formidable challenge to poultry industry. For example, the commercial egg laying industry in US is comprised of over 303 million laying hens in April 2016, of which about 263 million table eggs are produced per day [1]. Because of massive egg production, virtually laying hens are exposed to a wide range of potential stressors including cages, housing-specific challenges, disease agents, poor bone strength, balanced rations, foot health, and pests and parasite load [2]. Such stress leads to activate E. coli in the digestive system of chickens which in turn induces endotoxin stress [3]. Clinically, APEC is a strain of E. coli that has the ability to enter the host through ingestion or inhalation, after which it translocate across mucosal layers, then colonize in other tissues via the bloodstream [4]. Poultry infections with APEC are frequently associated with sudden death, salpingitis, peritonitis, pericarditis, perihepatitis and airsacculitis. APEC infections also contributed to reduced, quality and hatching of eggs [5,6]. Unfortunately, prophylactic use of antibiotics to control APEC in poultry is still restricted owing to the risk of residues entering the food chain and it’s potential to evolve multi-drug resistant strains [7,8]. Vaccination against APEC can be problematic since, because of differences between disease causing serogroups, many vaccines do not protect well against a heterologous challenge [6]. Furthermore, the strong similarities in genome sequences of APEC strains and human extra-intestinal pathogenic E. coli indicates that they may also pose a threat to human health and other animals [9].
Escherichia coli (E. coli) is a Gram-negative, rod-shaped, facultative anaerobic bacterium that is commonly found in the lower intestine in endotherms organisms. E. coli O157:H7 is the most frequently isolated serotype of enterohemorrhagic E. coli (EHEC) from diseased persons in the United States, Japan, and the United Kingdom [10]. The Centers for Disease Control and Prevention (CDC) has estimated that E. Coli O157:H7 infections lead to 73,000 sicknesses, 2,200 hospitalizations, and 60 deaths annually in the United States [11]. The annual cost of sickness due to E. coli O157:H7 infections evaluated by 405 million dollars, including lost productivity, medical care, and premature deaths [12]. This severe effect could be due to that E. coli O157:H7 is a highly acid-resistant food-borne pathogen that survives in the bovine and human gastrointestinal tracts [13]. Acid resistance is associated with a lowering of the infectious dose of enteric pathogens [14]. The low infectious dose is one of the best known characteristics of E. coli O157:H7, making this serotypes highly infectious [10].
Gene expression patterns have been examined and described in chickens that differ in its resistance to bacterial infection [15,16], but lack of research has been conducted on the gene expression response to APEC [17,18]. These available studies have been focused on some pathways correlated with pro-inflammatory cytokines, cell death program and immune-competent cells released by/in spleen and peripheral blood leukocytes in broiler and layer chickens. Further, few literatures reported the immune response to lipopolysaccharide on the expression of pro-inflammatory cytokines and leukocytes in the ovary and oviduct of laying and molting hens [19]. However, the knowledge of molecular mechanisms response and pathways of E. coli related diseases in tissues and organs of commercial layers is relatively scarce. Gene expression of host tissues to infection is commonly utilized to assess and enhance understanding of its response to infection. Information from host tissues and gene expression facilitates the deduction of critical pathways that are important in immune response development.
One of the most important components involved in a wide variety of biological processes is the Mitogen-Activated Protein Kinases (MAPK), serine/threonine-specific protein kinases, that specifically convert extracellular stimuli into a wide range of cellular responses [20]. P38 MAPK are a class of mitogen-activated protein kinases that are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and are involved in cell differentiation, apoptosis and autophagy [21–23]. Disturbances of homeostasis by E. coli infection activate specific cells like leukocytes, fibroblasts and endothelial cells to release cytokines [24]. Cytokines have been classified into a number of groups based on their activity and the cells they are produced by or act upon, such as interleukins (IL), interferons (IFN), tumor necrosis factors (TNF), transforming growth factors (TGF), migratory inhibitory factors (MIF) and the smaller chemokines. Proinflammatory cytokines, such as interleukin1β (IL-1β), play a role in mediating inflammation during disease or injury [25]. IL-1β activity was increased in macrophage supernatants from birds suffering from poult enteritis and mortality syndrome [26]. It has been shown that expression of IL-1β mRNA was increased 80-fold in the gut of protozoa-infected chickens [27]. The IL-1β cytokines act on specific receptors of different target cells leading to a systemic reaction characterized by fever, leukocytosis, and increase in secretion of adrenocorticotrophic hormone (ACTH) and plasma concentration of corticosterone (CORT) [28]. Tumor Necrosis Factor alpha (TNF-α or cachetin) is a potent proinflammatory cytokine and is expressed by activated macrophages, lymphocytes, natural killer cells, and epithelial cells [29]. It is also implicated in fever [25] and induces diverse cellular responses that can vary from apoptosis to the expression of genes involved in both early inflammatory and acquired immune responses [30,31]. The release of TNF from chicken macrophages was detected after infection with Marek’s disease virus [32] or protozoa [33]. Injection of chickens with such TNF-like factors increases weight loss, which is partially reversible by treatment with antihuman-TNF antisera [34].
Apoptosis, or programmed cell death, is a multi-pathway biological process that regularly contributes to many physiological and pathological phenomena in multicellular organisms. At the cellular and molecular levels, apoptosis is identified by morphological and biochemical changes such as cell shrinkage, formation of apoptotic bodies, caspase activation, chromatin condensation, and DNA fragmentation [35]. At present, apoptosis regulation is often associated with caspase-3, Bcl-2 and Bax [36]. The Bax gene was the first identified pro-apoptotic member of the Bcl-2 protein family and it promotes apoptosis by binding to and antagonizing the Bcl-2 protein [37]. Many molecular processes of apoptosis are mainly mediated by particular cysteine proteases named caspase, which cleave hundreds of substrates to bring about the typical apoptotic morphology [38]. It has been reported that when caspases, an important mediator of apoptosis [39–41], were inactivated or blocked, the apoptic process was immediately suppressed [42]. In a study by Sun et al. [43] to identify genes and pathways that are expressed in bursa of Fabricius of infected chickens with APEC, a strong correlation has been observed between caspase-3 gene expression and the lesion scores of liver in response to APEC pathology. Therefore, most of treatments to inhibit Escherichia coli-induced apoptosis in chickens rely upon the inhibition of Bax translocation into mitochondria of infected tissues and prevention of caspase cascade activation [44].
The objective of this research is to gain greater understanding of chicken host response to endotoxin shock induced by the E. coli infection. mRNA expression of pro-inflammatory cytokines and cell death program genes were examined and analyzed using real-time PCR analysis in both the brain and liver of infected chickens. In addition, the gene toxicity potential of E. coli was also evaluated by comet assay to determine the induced DNA damage in brain and liver tissues of infected chickens.
Materials and Methods
Animals and ethical statement
The current study was conducted in the Poultry Services Center at Faculty of Agriculture, Cairo University. A total number of two hundred and ten of 20-wk-old laying chickens (H&N brown layer hens) were randomly housed in cages, 3 hens per cage, in opened poultry house with photoperiod regimen of 14L: 10D and changed gradually to be 16L: 8D at the experiment time (35 wk of age). A basal diet was formulated according to the recommendations of the National Research Council (NRC, 1994). Water and feed were provided ad libitum during the study.
Birds were monitored closely to detect any signs of stress (breathing difficulty, watery discharge of the peak, decreased appetite, ruffled feathers, or droopy looking). The experimental birds were observed every one hour for the first 6 hours, then every 2 hours for the following 6 hours, and every 3 hours for the following 12 hours of the rest of the first day of treatment. For the next 3 days, observation was done every 6 hours. Accordingly, when one or more of these signs appeared, body temperature is measured to determine the action taken. If the body temperature reached 43.5°C or higher, cervical dislocation was used to end the life of these birds. This process was accomplished to minimize suffering of infected birds and to allow humane endpoints. All experimental protocols were approved by Cairo University Ethics Committee for the Care and Use of Experimental Animals in Education and Scientific Research (CU-IACUC).
Experimental design
Sixty hens were assigned to carry out preliminary sets of experiments to establish the optimal exposure dose of E. coli and to determine the nearest time of notable response to be used in the remainder studies of this research. Several doses were used in a range of 105−109 colonies/hen, and the body temperature was used as a stress indicator after 2 hours. The highest body temperature, without mortality, was obtained at a concentration of 107 colonies/hen. Hens were injected with this dose and the body temperature was measured after 0, 1, 3, 6, 12, and 24 hours. Increasing in body temperature was detectable as early as 1 hour and was maximal 3 hours after injection. Contingent on the preliminary studies, 107 colonies/hen was used intravenously in a single shut, then tissues and blood samples were collected 3 hours after injection.
At 35 wk of age, a total of 150 hens were randomly assigned into 2 groups with 3 replicates of 25 birds each. Hens of the first group were injected intravenously in the brachial wing vein with 107 colonies/hen Escherichia coli O157:H7 in 0.5 ml of sterile saline. The Escherichia coli O157:H7 was obtained from the US State Department of Health (Washington D.C.-USA) through Cairo Microbiological Resources Center (Cairo-Egypt). The second group of hens was injected only with 0.5 ml sterile saline and served as a control group. Three hours after injection, the body temperature of 5 hens from each replicate was recorded for both groups; and 10 blood samples were collected from each replicate per group to measure corticosterone concentration in plasma. After that, 5 hens from each group were decapitated and specimens of liver and brain were subjected for RNA extraction protocol. The mRNA expression of protein kinase p38 gene, pro-inflammatory cytokines genes IL-1β and TNF-α, and cell death program genes Bax and caspase-3 were analyzed in each of the liver and brain using real time poly chain reaction (RT-PCR) technique. In addition, the DNA damage induced by E. coli injection in liver and brain cells obtained from 5 hens per group was assessed by a comet assay.
Stress indicators
Body temperature and plasma corticosterone concentration were used, in both groups, as indicators for stress induced by E. coli injection. Body rectal temperature was recorded using thermocouple rectal thermometer with a 3-cm insertion probe. Blood samples were withdrawn from the brachial wing vein in heparinized tubes and centrifuged at 2000 x g for 10 min at 4°C. The plasma was separated and stored at -20°C until analyzed. Plasma corticosterone concentration was measured by ELISA reader (BIOTEKELX808) using chicken corticosterone ELISA kits (MyBioSource, San Diego, CA-USA, cat# MBS701668). The intra- and inter-assay coefficient of variations was <8% and <10%, respectively. The analytical sensitivity of the assay was less than 0.0625 ng/ml and the dynamic range of the assay was 0.5–20 ng/ml.
Total RNA extraction and reverse transcription reaction
Total RNA was extracted from brain and liver tissues using RNeasy Midi Kits (Qiagen, USA) according to manufacturer's instruction. Total RNA was treated with 1 U of RQ1 RNase-free DNase (Invitrogen, Germany) to digest DNA residues, re-suspended in DEPC-treated water. Purity of total RNA was assessed spectrophotometrically at 260/280 nm. The integrity of extracted RNA was determined by using 1.5% agarose gel electrophoresis. Then total RNA was reverse-transcribed into cDNA by using RevertAidTM First Strand cDNA Synthesis Kit (MBI Fermentas, Germany) according to the manufacturer's directions. cDNA was stored at -20°C for relative quantitative real-time PCR.
Quantitative real-time PCR
PCR reactions were set up in 25 μL reaction mixtures containing 12.5 μL 1× SYBR® Premix Ex Taq™ (TaKaRa, Biotech. Co. Ltd., Germany) 0.5 μL 0.2 μM sense primers, 0.5 μL 0.2 μM antisense primer, 6.5 μL distilled water, and 5 μL of cDNA template. The reaction program was allocated to 3 steps of thermal cycling parameters. The first step was set to 95.0°C for 3 min. The second step consisted of 40 cycles in which each cycle divided to 3 steps: (a) at 95.0°C for 15 sec, (b) at 55.0°C for 30 sec, and (c) at 72.0°C for 30 sec. The last step consisted of 71 cycles which started at 60.0°C and then increased by 0.5°C every 10 sec up to 95.0°C. At the end of each qRT-PCR, a melting curve analysis was performed at 95.0°C to check the quality of the used primers. Each experiment included a distilled water control.
The qRT-PCR of p38, IL-1β, TNF-α, Bax and caspase-3 genes were normalized to the main expression of ß-actin and transformed using the comparative cycle threshold (CT) method to quantify expression levels as previously described by Ellestad et al. [45]. Sequence-specific primers (Table 1) for the real-time PCR were designed using the Primer blast web interface (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi).
Table 1. Details of primers used for real-time PCR quantitative analysis.
| Gene symbol | GenBank accession number | Primer sequences |
|---|---|---|
| P38 | CR339030 | F:TTGGTTCCACAACTCCAGCACAG |
| R:CCGCATCCAGCACCAGCATGT | ||
| IL-1β | NM_204524 | F: GGGCATCAAGGGCTACAA |
| R: TGTCCAGGCGGTAGAAGAT | ||
| TNF-α | BAC55966 | F: C ACAGAATGTAAGCCCTGTCC |
| R:T GGAGTTCTGCGATCCTGCATT | ||
| Bax | NM_007527 | F:CAGGGTTTCATCCAGGATCGAGCA |
| R: TCAGCTTCTTGGTGGACGCATC | ||
| Caspase-3 | GU230786.1 | F:TTCAGGCACGGATGCAGATG |
| R:TTCCTGGCGTGTTCCTTCAG | ||
| ß-actin | NM205518 | F:TGCGTGACATCAAGGAGAAG |
| R:TGCCAGGGTACATTGTGGTA |
DNA damage by comet assay
Brain and liver tissues from chicken were homogenized and isolated by centrifugation (280 g, 15min) in a density gradient of Gradisol L (Aqua Medica, Lodz, Poland). The concentration of the cells was adjusted to (1–3) x 105 cells/ ml by adding RPMI 1640 without glutamine to the single cell suspension. A freshly prepared suspension of cells in 0.75% low melting point agarose (Sigma) dissolved in phosphate buffer saline (PBS; sigma) was cast onto microscope slides pre-coated with 0.5% normal melting agarose and maintained at 37°C. After gelling on a cold metal plate for 1 minute, the cells were then lysed for 1h at 4°C in a buffer consisting of 2.5M NaCl, 100 mMEDTA, 1% Triton X-100, 10mM Tris, and pH10. After the lysis, DNA was allowed to unwind for 40 min in electrophoretic solution consisting of 300mM NaOH, 1mM EDTA, pH>13. Electrophoresis was conducted at 4°C for 30 min at electric field strength 0.73 V/cm (30mA). The slides were then neutralized with 0.4M Tris, pH 7.5, stained with 2ug/ml ethidium bromide (Sigma) and covered with cover slips. The slides were examined at 200 x magnification fluorescence microscope (Nikon Tokyo, Japan) equipped with UV filter block consisting an excitation filter (359nm) and barrier filter (461nm), and connected to a COHU 4910 video camera (Cohu, Inc., San Diego, CA, USA) and a personal computer–based image analysis system (Lucia-Comet v.4.51). Hundred images were randomly selected from each sample (5 hens per group) and the comet tail DNA was measured in brain and liver cells [46]. DNA damages were scored in 4 classes: Class 0 with no tail, Class 1 with tail length < diameter of nucleus, Class 2 with tail length between 1-2X of the diameter of nucleus, and Class 3 with tail length > 2X of the diameter of nucleus [47]. Visual scores of comet tails using the classified classes is presented in (S1 Fig).
Statistical analysis
All data were represented as mean ± standard deviation of the mean. A Student’s t-test was performed using SPSS 16 (SPSS Inc., Chicago, USA) to calculate the differences between control and E. coli-treatment groups. A P-value of less than 0.05 was considered significant.
Results
Stress indicators
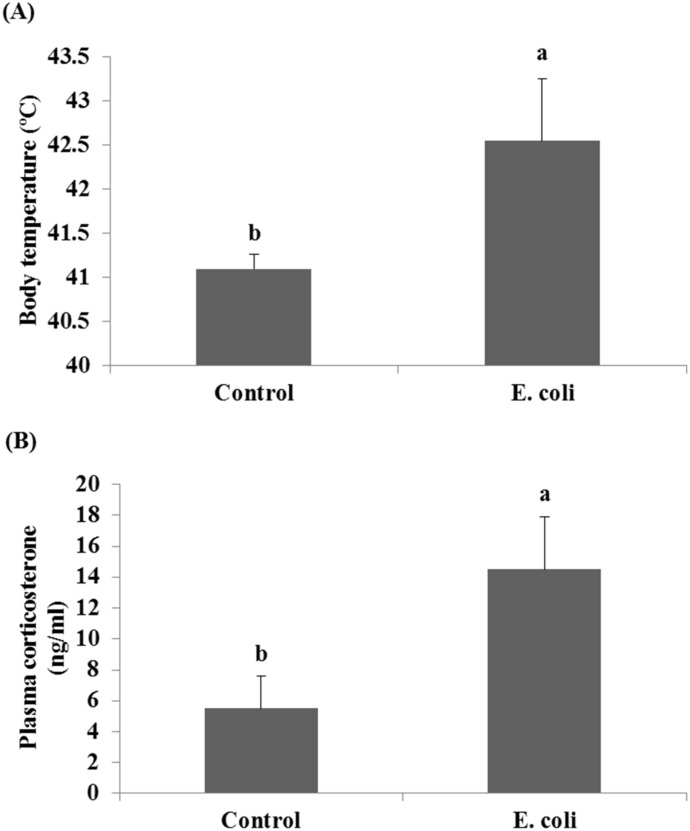
The effect of E. coli on the body temperature and the plasma corticosterone concentration as stress indicators of infected chickens has been shown in Fig 1. As shown in Fig 1A, a high significant fever was recorded for infected chickens compared to the control (42.6°C in infected birds vs. 41.1°C in control, P<0.05). These infected chickens with E. coli also expressed a significant (P<0.05) higher concentration in theplasma corticosterone (14.5 ng/ml) than that expressed by the control group (5.5 ng/ml), Fig 1B.
Fig 1. Effect of E. coli on the body temperature (A) and the plasm corticosterone concentration (B) of chickens.
Bars with different letters (a, b) are significantly different at P<0.05.
Quantitative real-time PCR
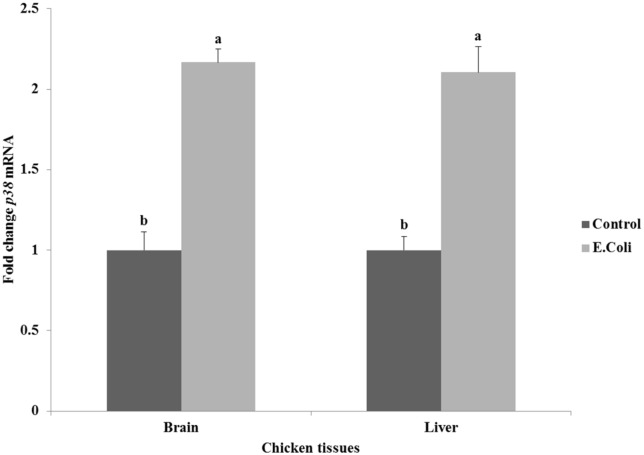
The effect of E. coli on the expression of protein kinase p38 gene in the brain and liver tissues of chickens is summarized in Fig 2. Infection with E. coli induced significant high expression of p38 gene by 2.1–2.2 fold (P<0.05) when compared to the control group in both tissues of brain and liver.
Fig 2. Effect of E. coli on the relative expression of protein kinase p38 gene in the brain and liver tissues of chickens.
a,b Mean values within tissue with unlike superscript letters are significantly different (P<0.05).
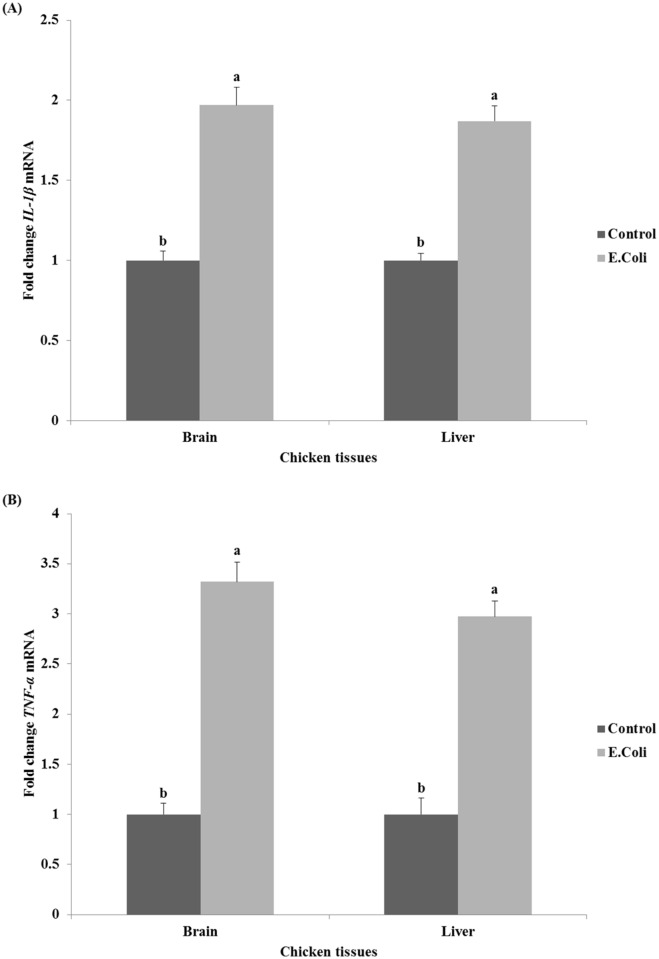
The relative expression of examined proinflammatory cytokines genes IL-1β and TNF-α in the brain and liver tissues after E. coli infection are illustrated in Fig 3. The current data showed that the relative expression of IL-1β and TNF-α genes followed the same pattern in brain and liver tissues. IL-1β gene expression increased significantly (P<0.05) by 2.0 and 1.9 fold in both the brain and liver tissues of infected chickens compared to control chickens, respectively (Fig 3A). Similarly, the expression of TNF-α gene increased significantly (P<0.05) by 3.3 and 3.0 fold in the brain and liver tissues of infected chickens compared to control chickens, respectively (Fig 3B).
Fig 3. Effect of E. coli on the relative expression of pro-inflammatory cytokines genes IL-1β (A) and TNF-α (B) in the brain and liver tissues of chickens.
a,b Mean values within tissue with unlike superscript letters are significantly different (P<0.05).
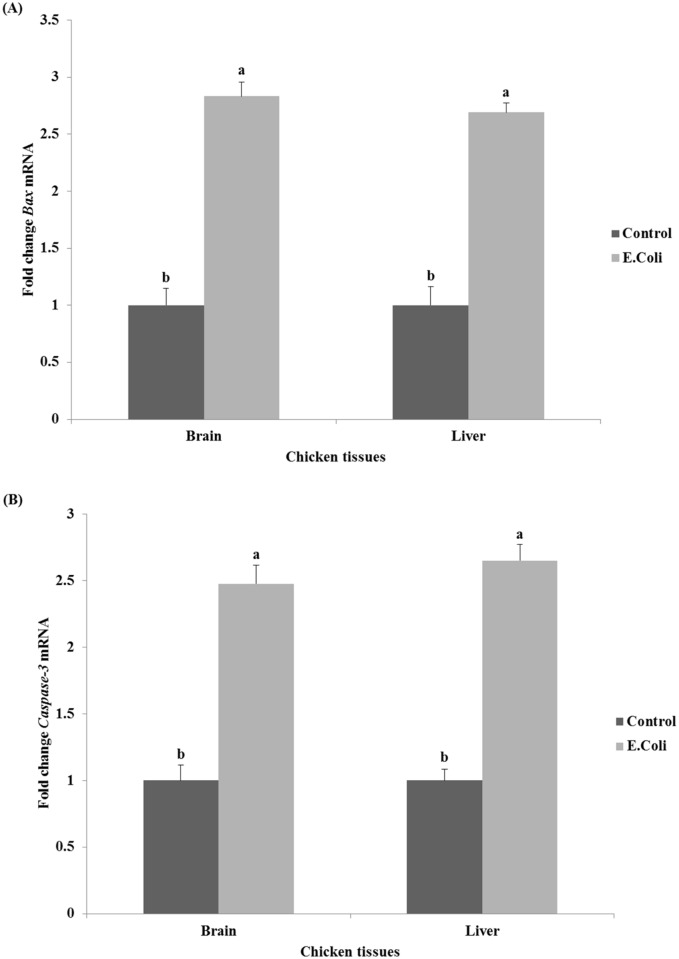
The relative expression of examined cell death program genes Bax and caspase-3 in the brain and liver tissues after E. coli infection are shown in Fig 4. The infected chickens with E. coli showed a significant (P<0.05) high expression of Bax gene in the brain and liver tissues in comparison with their controls (2.7–2.8-fold, Fig 4A). Furthermore, a significant increase in caspase-3 gene expression was found in the infected chickens (2.5-fold and 2.7-fold in the brain and liver tissues, respectively, P<0.05) when compared to control chickens (Fig 4B).
Fig 4. Effect of E. coli on the relative expression of cell death program genes Bax (A) and caspase-3 (B) in brain and liver tissues of chickens.
a,b Mean values within tissue with unlike superscript letters are significantly different (P<0.05).
DNA Damage detected by Comet assay
Table 2 demonstrates the results of the DNA damages by comet assay in liver and brain tissues of chickens after infection with E. coli. The chickens treated with E. coli showed a significant high levels of DNA damage compared with the control chickens (P<0.05). It is also important to note that, the rates of the DNA damage observed in liver tissues (11.2%) were higher than those observed in brain tissues (8.6%) as shown in Table 2. Furthermore, the rate in the infected chicken was generally higher compare to the DNA damage in untreated control chickens that scored in low rates ranged between 4.2 to 4.6 in brain and liver tissues, respectively.
Table 2. Visual score of DNA damage in brain and liver tissues of chicken treated with E. coli using comet assay.
| Treatment | No. of animals | No. of cells | Class of comet** | DNA damaged cells (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Analyzed* | Total comets | 0 | 1 | 2 | 3 | |||
| Brain | ||||||||
| Control | 5 | 500 | 21 | 479 | 16 | 5 | 0 | 4.2b |
| E. coli | 5 | 500 | 43 | 457 | 11 | 14 | 18 | 8.6a |
| Liver | ||||||||
| Control | 5 | 500 | 23 | 477 | 19 | 4 | 0 | 4.6b |
| E. coli | 5 | 500 | 56 | 444 | 10 | 22 | 24 | 11.2a |
*No of cells analyzed were 100 per an animal.
**Class 0 = no tail; Class 1 = tail length < diameter of nucleus; Class 2 = tail length between 1X and 2X the diameter of nucleus; and Class 3 = tail length > 2X the diameter of nucleus.
a,b Mean values within tissue with unlike superscript letters are significantly different (P<0.05).
Discussion
Avian pathogenic Escherichia coli (APEC) still exert severe economic loss in poultry industry and remains under focus of researchers and workers in this field. In laying hens, endotoxic shocks and egg production losses caused by E. coli are still particularly difficult to control yet the nature and consequences of host immune responses to infection are poorly understood, especially in extra-intestinal locations. This research describes the first events of E. coli interactions with laying chickens host, focusing on the gene expression analysis and the DNA damage which clearly occurred in the brain and liver tissues. To study these events, we carried out preliminary experiments on a group of chickens to determine the best dosage of E. coli to use in this study and to choose the nearest time of notable response in which chicken expressed high body temperature as a first indicator of stress by infection. We found that injection treatment with 107 colonies of E. coli per hen is enough to infect the chickens and the first stress indicators were appeared on infected chickens only 3 hours after the treatment.
The results revealed that the body temperature (Fig 1A) and the plasma corticosterone concentration (Fig 1B) were markedly increased in infected chickens when compared with control chickens. Old studies [48–50] reported a febrile response in broiler chickens after administration of lipopolysaccharide (LPS) from E. coli. We recorded that stress status as high body temperature in infected chickens begins following the 3 hr of E. coli injection. The stress status was associated with a significant increase in plasma corticosterone concentration. The corticosterone is the end product of the neuroendocrine molecules secreted from hypothalamic-pituitary-adrenal axis as an integrated response to stress [51]. These hormones can therefore modulate the activities of immune cells; in particular the production of proinflammatory cytokines and chemokines, which themselves can in turn modulate the activity of the hypothalamus and thus alter hormone production [52]. Besides the role of corticosterone in enhancing the formation of antibodies and the humoral-mediated immune response [53,54], it also mobilizes and produces glucose to meet the increased energy requirement during stress [55].
As shown in Fig 2, the infection with E. coli induced significant (P<0.05) high relative expression of p38 gene when compared with control in both tissues of brain (2.2-fold) and liver (2.1-fold). Our results are consistent with previous studies by Dziarski et al. [56] who reported that LPS produced by bacteria strongly activates all kinases, and Cao et al. [23] who reported also an increase in MAPK1 expression as a result of bacterial infection in ducks. Mitogen-activated protein kinases are involved in directing cellular responses to a diverse array of stimuli such as mitogens, osmotic stress, heat shock, and pro-inflammatory cytokines [20], and the E. coli infection in our study. They regulate gene expression, mitosis, proliferation, differentiation, cell survival, and apoptosis [22].
The significant increase in p38 gene expression in brain and liver of infected chickens was accompanied with a significant increase in the pro-inflammatory cytokines genes expression studied in our experiment. This elevated expression of pro-inflammatory cytokines may be the prerequisite to prevent the development of infection [19]. The current data showed that the relative expression of IL-1β in both the brain and liver tissues were significantly increased (P<0.05) approximately by 2-fold in infected chickens when compared to control chickens (Fig 3A). These results are in line with previous studies [26,27,57,58] where the IL-1β activity and mRNA expression has been shown to increase after viral and bacterial infections in the chicken. Moreover, Shaughnessy et al. [59] reported an increase in the expressions of IL-1β in avian intestinal tissues in response to Salmonella and Campylobacter bacteria. The study of Nii et al. [19] demonstrated that the IL-1βexpression was significantly up-regulated in both uterus and vagina of White Leghorn laying hens at 3 hr after LPS injection. Munyaka et al. [60] saw a similar response in the spleen and cecal tonsils of laying hens. At the same time, the relative expression of TNF-α gene significantly (P<0.05) increased by approximately 3.0–3.3-fold in the brain and liver of infected chickens when compared with their controls (Fig 3B). TNF-α (also known as cachetin) is a primary regulator of both the immune response and inflammation [61]. As previously observed by Tallant et al. [62] and Guma et al. [63], the increase in TNF-α gene expression as a result of E. coli infection may itself be a reason for the increase in p38 gene expression in the same group (Fig 2); and these two genes are essential to trigger the inflammation in infected chickens.
In the present study, we found that the relative expression of Bax gene was significantly increased (P<0.05) by 2.8-fold in both the brain and liver tissues of infected chickens when compared with untreated control chickens (Fig 4A). Some research and studies were found about Bax expression in chickens infected by E. coli, as it reported by Gao et al. [44] who found that Clostridium butyricum possesses the ability to prevent Escherichia coli-induced apoptosis in chicken embryo intestinal cells; and Sandford et al. [17] who reported changes in apoptosis-related genes in the challenged-susceptible birds. These reports supported our results concerning the main role of Bax in apoptosis after E. coli infection. We also observed a significant increase by 2-5-2.7-fold in caspase-3 gene expression in the tissues of infected chickens (P<0.05; Fig 4B). Similar results were obtained by Bastiani et al. [64] who found a strong activity of caspase-3 in a murine macrophages cell line 2 hr after incubation with an APEC strain. Recently, Sun et al. [43] revealed that novel pathways for apoptosis in bursa of Fabricius of susceptible chicken in response to APEC infection leads to the activation of caspase-3 and ends at the release of pro-apoptotic protein Bax.
On the other hand, the rates of DNA damage observed by comet assay in liver and brain tissues (11.2% and 8.6%, respectively) were higher in chickens treated with E. coli than those observed in untreated control chickens (ranged between 4.2 to 4.6 in brain and liver tissues, respectively; Table 2). Our results are in agreement with previous findings [65] which demonstrated that infection of eukaryotic cells with E. coli strains induced host-cell DNA double-strand breaks and activation of the DNA damage signaling cascade. The apoptotic morphology (plasma membrane blebbing, cytoplasm vacuolization, chromatin condensation and DNA degradation) is essentially the result of the proteolytic action of caspases family upon specific cellular substrates [66,67]. Such action of caspases could be also seen in our study hence the caspase-3 expression and DNA damage were significantly (P<0.05) high in infected chickens (Fig 4B and Table 2). Moreover, Bax expression was significantly increased in the same group of infected chickens (Fig 4A). It is thought that Bax is still required to activate effector caspases, particularly caspase-3/-9 activation, to accomplish DNA damage and apoptosis [68]. Other scientists explored that the Bax gene in primary neuronal cultures derived from mouse cortex was highly expressed after the DNA damage by overexpression of other mediator genes such as Peg3/Pw1and P53 (but not caspase pathways), resulting in neuronal cell death [69]. Such hypothesis was previously evidenced in neuronal cells of the ovary, placenta, testis and brain in mouse and human [70–72], and could explain the high expression of Bax gene accompanied with DNA damage incidence in the brain of infected chicken with E. coli in our study. TNF-α may also play a role in the DNA damage observed in our study. Rahman and McFadden [29] explained that TNF ligand homotrimer binds to the extracellular receptors to initiate some intracellular signaling pathways; including caspase family activation, which leads to cell death [73,74].
Referring back to the fever which occurred in the infected chickens in this study (Fig 1A), we think that this fever has been attributed to the endogenous pyrogenic action induced by the high IL-1β expressed in these chickens [25]. It may be also due to the high expression of TNF-α in infected chickens which has been implicated as a key mediator of fever in several animal models [25]. Pro-inflammatory cytokine genes including IL-1β and TNF-α were also studied in many organs of chicken embryos infected by mycoplasma disease [75]. They found that these genes were significantly up-regulated in the liver and spleen of infected embryos by macrophages and cells of the reticuloendothelial system; therefore, we also see from our results that E. coli infection in laying chickens may cause proliferation of similar repertoire of immune cells in the reticuloendothelial system of liver and brain tissues. Similar events were previously concluded in a work by Horn et al. [76] that endothelial and epithelial cells, heterophils, and macrophages, which were localized in lung tissues of infected chickens with avian E. coli, were involved in the defense and died at the infection sites. If this also happens in our experimental model, we can understand the activation of cell death program in liver and brain tissues after chickens’ infection with E. coli, wherein the Bax and caspase-3 genes were highly expressed (Fig 4). The proliferated macrophages in liver and brain tissues of infected chickens may participate in the uptake and digestion of E. coli bacteria as one of the known macrophages’ functions [77,78]. It was strongly suggested that upon uptake and digestion of E. coli bacteria, the caspase-9- and caspase-3-dependent branch of the apoptotic pathway was activated in a murine macrophages cell line [79]. Finally, the proteolytic action of caspases family on specific cell substrates [66,67] led to the apoptotic morphology aspects including DNA damages we detected in the infected chickens at the present study (Table 2).
In conclusion, the current study provides more understanding to APEC infection in chickens at cellular and molecular levels. The proinflammatory cytokine genes IL-1β and TNF-α are highly expressed accompanied with an increase in MAPK-p38 gene expression in the brain and liver tissues of infected chickens. Moreover, high expression of Bax and caspase-3 genes has been occurred in infected chickens associated with programmed cell death and DNA damage in brain and liver tissues. Further studies are required to clarify if such responses are destructive or protective, and such information may set the means through which a chicken is able to mount a successful defense against APEC.
Supporting Information
Class scores (0–3): Class (0) = no tail, Class (1) = tail length < diameter of nucleus, Class (2) = tail length between 1X and 2X the diameter of nucleus, and Class (3) = tail length > 2X the diameter of nucleus. (Original magnification: 200x; Scale bars: 50 μm).
(TIF)
Acknowledgments
The authors thank Prof. Dr. Magdi Mashaly (Professor of Poultry Immunology, Cairo University) and Prof. Dr. Abdel-Rahman Atta (Professor of Poultry Physiology, Cairo University) for the technical support during this study.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by Project of Rapid Climate Change in Poultry Cellular and Molecular Physiology (RCC-PCMP), funded from General Scientific Research Department at Cairo University (GSRD-CU). AOA was the principal investigator of the project and the fund was awarded to him during the management of the project. The funders have approved the study design, data collection and analysis, decision to publish, and preparation of the manuscript.
References
- 1.USDA National Agricultural Statistics Service. Poultry—Production and Value 2014 Summary. 2015.
- 2.Lay D Jr, Fulton R, Hester P, Karcher D, Kjaer J, Mench J, et al. Hen welfare in different housing systems. Poult Sci. 2011;90: 278–294. 10.3382/ps.2010-00962 [DOI] [PubMed] [Google Scholar]
- 3.Star L, Decuypere E, Parmentier HK, Kemp B. Effect of single or combined climatic and hygienic stress in four layer lines: 2. Endocrine and oxidative stress responses. Poult Sci. 2008;87: 1031–8. 10.3382/ps.2007-00143 [DOI] [PubMed] [Google Scholar]
- 4.Dziva F, Stevens MP. Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008;37: 355–366. 10.1080/03079450802216652 [DOI] [PubMed] [Google Scholar]
- 5.Russell SM. The effect of airsacculitis on bird weights, uniformity, fecal contamination, processing errors, and populations of Campylobacter spp. and Escherichia coli. Poult Sci. 2003;82: 1326–1331. Available: http://ps.fass.org/cgi/content/abstract/82/8/1326 [DOI] [PubMed] [Google Scholar]
- 6.Nolan L, Barnes JH, Vaillancourt JP, Abdul-Aziz T, Logue C. Colibacillosis In: Swayne D, Glisson J, McDougald L, Nolan L, Suarez D, Nair V, editors. Disease of poultry. Iowa State University Press, USA; 2013. pp. 751–805. [Google Scholar]
- 7.Arp LH, Jensen AE. Piliation, Hemagglutination, Motility, and Generation Time of Escherichia coli That Are Virulent or Avirulent for Turkeys. Avian Dis. 1980;24: 153–161. Available: http://www.jstor.org/stable/1589774. [Google Scholar]
- 8.Peighambari SM, Hunter DB, Shewen PE, Gyles CL. Safety, immunogenicity, and efficacy of two Escherichia coli cya crp mutants as vaccines for broilers. Avian Dis. 2002;46: 287–297. [DOI] [PubMed] [Google Scholar]
- 9.Johnson TJ, Kariyawasam S, Wannemuehler Y, Mangiamele P, Johnson SJ, Doetkott C, et al. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J Bacteriol. 2007;189: 3228–3236. 10.1128/JB.01726-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim JY, Yoon J, Hovde CJ. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J Microbiol Biotechnol. 2010;20: 5–14. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3645889&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 11.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5: 607–25. 10.3201/eid0505.990502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frenzen PD, Drake A, Angulo FJ, Group EIPFW. Economic cost of illness due to Escherichia coli O157 infections in the United States. J Food Prot. 2005;68: 2502–2720. Available: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=16355834 [DOI] [PubMed] [Google Scholar]
- 13.Price SB, Wright JC, DeGraves FJ, Castanie-Cornet M-P, Foster JW. Acid resistance systems required for survival of Escherichia coli O157:H7 in the bovine gastrointestinal tract and in apple cider are different. Appl Environ Microbiol. 2004;70: 4792–9. 10.1128/AEM.70.8.4792-4799.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlech WF, Chase DP, Badley A. A model of food-borne Listeria monocytogenes infection in the Sprague-Dawley rat using gastric inoculation: development and effect of gastric acidity on infective dose. Int J Food Microbiol. 1993;18: 15–24. 10.1016/0168-1605(93)90003-Y [DOI] [PubMed] [Google Scholar]
- 15.Ask B, van der Waaij EH, Stegeman JA, van Arendonk JAM. Genetic variation among broiler genotypes in susceptibility to colibacillosis. Poult Sci. 2006;85: 415–21. Available: http://www.ncbi.nlm.nih.gov/pubmed/16553269 [DOI] [PubMed] [Google Scholar]
- 16.Cavero D, Schmutz M, Philipp HC, Preisinger R. Breeding to reduce susceptibility to Escherichia coli in layers. Poult Sci. 2009;88: 2063–2068. 10.3382/ps.2009-00168 [DOI] [PubMed] [Google Scholar]
- 17.Sandford EE, Orr M, Shelby M, Li X, Zhou H, Johnson TJ, et al. Leukocyte transcriptome from chickens infected with avian pathogenic Escherichia coli identifies pathways associated with resistance. Results Immunol. Elsevier; 2012;2: 44–53. 10.1016/j.rinim.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeyen JRR, Kaiser P, Stevens MP, Dziva F. Analysis of immune responses induced by avian pathogenic Escherichia coli infection in turkeys and their association with resistance to homologous re-challenge. Vet Res. Veterinary Research; 2014;45: 19 10.1186/1297-9716-45-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nii T, Sonoda Y, Isobe N, Yoshimura Y. Effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokines and influx of leukocytes in the hen ovary. Poult Sci. 2011;90: 2332–2341. 10.3382/ps.2011-01596 [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Rodriguez S, Callejas-Rubio J-L, Ortego-Centeno N, Zumaquero E, Ríos-Fernandez R, Arias-Santiago S, et al. Altered AKT1 and MAPK1 gene expression on peripheral blood mononuclear cells and correlation with T-helper-transcription factors in systemic lupus erythematosus patients. Mediators Inflamm. 2012;2012: 495934 10.1155/2012/495934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calati R, Crisafulli C, Balestri M, Serretti A, Spina E, Calabrò M, et al. Evaluation of the role of MAPK1 and CREB1 polymorphisms on treatment resistance, response and remission in mood disorder patients. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44: 271–8. 10.1016/j.pnpbp.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 22.Upadhya D, Ogata M, Reneker LW. MAPK1 is required for establishing the pattern of cell proliferation and for cell survival during lens development. Development. 2013;140: 1573–82. 10.1242/dev.081042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao S, Han X, Ding C, Wang S, Tian M, Wang X, et al. Molecular cloning of the duck mitogen-activated protein kinase 1 (MAPK1) gene and the development of a quantitative real-time PCR assay to detect its expression. Poult Sci. 2014;93: 2158–2167. 10.3382/ps.2013-03796 [DOI] [PubMed] [Google Scholar]
- 24.Heinrich PC, Castell J V, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265: 621–36. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1133681&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wigley P, Kaiser P. Avian cytokines in health and disease. Rev Bras Ciência Avícola. 2003;5: 1–14. 10.1590/S1516-635X2003000100001 [DOI] [Google Scholar]
- 26.Heggen CL, Qureshi MA, Edens FW, Barnes HJ. Alterations in macrophage-produced cytokines and nitrite associated with poult enteritis and mortality syndrome. Avian Dis. 2000;44: 59–65. Available: http://www.ncbi.nlm.nih.gov/pubmed/10737645 [PubMed] [Google Scholar]
- 27.Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect Immun. 2001;69: 2527–34. 10.1128/IAI.69.4.2527-2534.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Mitarai Y, Yoshioka M, Koizumi N, Shibahara T, Nakajima Y. Serum levels of interleukin-6, alpha1-acid glycoprotein, and corticosterone in two-week-old chickens inoculated with Escherichia coli lipopolysaccharide. Poult Sci. 1998;77: 908–11. Available: http://www.ncbi.nlm.nih.gov/pubmed/9628544 [DOI] [PubMed] [Google Scholar]
- 29.Rahman MM, McFadden G. Modulation of tumor necrosis factor by microbial pathogens. PLoS Pathog. 2006;2: 0066–0077. 10.1371/journal.ppat.0020004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3: 745–56. 10.1038/nri1184 [DOI] [PubMed] [Google Scholar]
- 31.Mocellin S, Rossi CR, Pilati P, Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16: 35–53. 10.1016/j.cytogfr.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 32.Qureshi MA, Miller L, Lillehoj HS, Ficken MD. Establishment and characterization of a chicken mononuclear cell line. Vet Immunol Immunopathol. 1990;26: 237–50. Available: http://www.ncbi.nlm.nih.gov/pubmed/2176014 [DOI] [PubMed] [Google Scholar]
- 33.Byrnes S, Eaton R, Kogut M. In vitro interleukin-1 and tumor necrosis factor-alpha production by macrophages from chickens infected with either Eimeria maxima or Eimeria tenella. Int J Parasitol. 1993;23: 639–45. Available: http://www.ncbi.nlm.nih.gov/pubmed/8225766 [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Lillehoj HS, Ruff MD. In vivo role of tumor necrosis-like factor in Eimeria tenella infection. Avian Dis. 1995;39: 859–66. Available: http://www.ncbi.nlm.nih.gov/pubmed/8719221 [PubMed] [Google Scholar]
- 35.Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92: 57–70. Available: http://www.ncbi.nlm.nih.gov/pubmed/11750036 [DOI] [PubMed] [Google Scholar]
- 36.Shao Q, Jiang W, Jin Y. MiR-124 effect in neurons apoptosis in newborn rat with thyroid hypofunction. 2015;8: 14465–14471. [PMC free article] [PubMed] [Google Scholar]
- 37.Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. Elsevier; 1993;74: 609–619. 10.1016/0092-8674(93)90509-O [DOI] [PubMed] [Google Scholar]
- 38.Fischer U, Jänicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10: 76–100. 10.1038/sj.cdd.4401160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf BB, Schuler M, Echeverri F, Green DR. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J Biol Chem. 1999;274: 30651–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/10521451 [DOI] [PubMed] [Google Scholar]
- 40.Agas D, Marchetti L, Menghi G, Materazzi S, Materazzi G, Capacchietti M, et al. Anti-apoptotic Bcl-2 enhancing requires FGF-2/FGF receptor 1 binding in mouse osteoblasts. J Cell Physiol. 2008;214: 145–52. 10.1002/jcp.21170 [DOI] [PubMed] [Google Scholar]
- 41.Ling Y, Lu N, Gao Y, Chen Y, Wang S, Yang Y, et al. Endostar induces apoptotic effects in HUVECs through activation of caspase-3 and decrease of Bcl-2. Anticancer Res. 2009;29: 411–7. Available: http://www.ncbi.nlm.nih.gov/pubmed/19331180 [PubMed] [Google Scholar]
- 42.Oliver L, LeCabellec M-T, Pradal G, Meflah K, Kroemer G, Vallette FM. Constitutive presence of cytochrome c in the cytosol of a chemoresistant leukemic cell line. Apoptosis. 2005;10: 277–87. 10.1007/s10495-005-0802-x [DOI] [PubMed] [Google Scholar]
- 43.Sun H, Liu P, Nolan LK, Lamont SJ. Novel pathways revealed in bursa of fabricius transcriptome in response to extraintestinal pathogenic Escherichia coli (ExPEC) infection. PLoS One. 2015;10: 1–17. 10.1371/journal.pone.0142570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Q, Qi L, Wu T, Wang J. Ability of Clostridium butyricum to inhibit Escherichia coli-induced apoptosis in chicken embryo intestinal cells. Vet Microbiol. Elsevier B.V.; 2012;160: 395–402. 10.1016/j.vetmic.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 45.Ellestad LE, Malkiewicz SA, Guthrie HD, Welch GR, Porter TE. Expression and regulation of glucocorticoid-induced leucine zipper in the developing anterior pituitary gland. J Mol Endocrinol. 2009;42: 171–183. 10.1677/JME-08-0066 [DOI] [PubMed] [Google Scholar]
- 46.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281: 1312–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/9721091 [DOI] [PubMed] [Google Scholar]
- 47.Blasiak J, Arabski M, Krupa R, Wozniak K, Zadrozny M, Kasznicki J, et al. DNA damage and repair in type 2 diabetes mellitus. Mutat Res. 2004;554: 297–304. 10.1016/j.mrfmmm.2004.05.011 [DOI] [PubMed] [Google Scholar]
- 48.Jones CA, Edens FW, Denbow DM. Rectal temperature and blood chemical responses of young chickens given E. coli endotoxin. Poult Sci. 1981;60: 2189–94. Available: http://www.ncbi.nlm.nih.gov/pubmed/7036123 [DOI] [PubMed] [Google Scholar]
- 49.Fraifeld V, Blaicher-Kulick R, Degen AA, Kaplanski J. Is hypothalamic prostaglandin E2 involved in avian fever? Life Sci. 1995;56: 1343–6. Available: http://www.ncbi.nlm.nih.gov/pubmed/8614256 [DOI] [PubMed] [Google Scholar]
- 50.Baert K, Duchateau L, De Boever S, Cherlet M, De Backer P. Antipyretic effect of oral sodium salicylate after an intravenous E. coli LPS injection in broiler chickens. Br Poult Sci. 2005;46: 137–43. 10.1080/0071660500065151 [DOI] [PubMed] [Google Scholar]
- 51.Shini S, Huff GR, Shini a, Kaiser P. Understanding stress-induced immunosuppression: exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult Sci. 2010;89: 841–851. 10.3382/ps.2009-00483 [DOI] [PubMed] [Google Scholar]
- 52.Besedovsky HO, del Rey A. Regulating inflammation by glucocorticoids. Nat Immunol. 2006;7: 537 10.1038/ni0606-537 [DOI] [PubMed] [Google Scholar]
- 53.Braun W, Ishizuka M, Winchurch R, Webb D. On the role of cyclic AMP in immune responses. Ann N Y Acad Sci. 1971;185: 417–22. Available: http://www.ncbi.nlm.nih.gov/pubmed/4330508 [DOI] [PubMed] [Google Scholar]
- 54.Siegel HS. Immunological responses as indicators of stress. Worlds Poult Sci J. Cambridge University Press; 1985;41: 36–44. 10.1079/WPS19850003 [DOI] [Google Scholar]
- 55.Assenmacher I. THE PERIPHERAL ENDOCRINE GLANDS. Avian Biology. Elsevier; 1973. pp. 183–286. 10.1016/B978-0-12-249403-1.50011-6 [DOI] [Google Scholar]
- 56.Dziarski R, Jin YP, Gupta D. Differential activation of extracellular signal-regulated kinase (ERK) 1, ERK2, p38, and c-Jun NH2-terminal kinase mitogen-activated protein kinases by bacterial peptidoglycan. J Infect Dis. 1996;174: 777–85. Available: http://www.ncbi.nlm.nih.gov/pubmed/8843216 [DOI] [PubMed] [Google Scholar]
- 57.Leshchinsky T V, Klasing KC. Divergence of the inflammatory response in two types of chickens. Dev Comp Immunol. 2001;25: 629–38. Available: http://www.ncbi.nlm.nih.gov/pubmed/11472784 [DOI] [PubMed] [Google Scholar]
- 58.De Boever S, Beyaert R, Vandemaele F, Baert K, Duchateau L, Goddeeris B, et al. The influence of age and repeated lipopolysaccharide administration on body temperature and the concentration of interleukin-6 and IgM antibodies against lipopolysaccharide in broiler chickens. Avian Pathol. 2008;37: 39–44. 10.1080/03079450701784875 [DOI] [PubMed] [Google Scholar]
- 59.Shaughnessy RG, Meade KG, Cahalane S, Allan B, Reiman C, Callanan JJ, et al. Innate immune gene expression differentiates the early avian intestinal response between Salmonella and Campylobacter. Vet Immunol Immunopathol. 2009;132: 191–8. 10.1016/j.vetimm.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 60.Munyaka PM, Tactacan G, Jing M, O K, House JD, St Paul M, et al. Response of older laying hens to an Escherichia coli lipopolysaccharide challenge when fed diets with or without supplemental folic acid. Poult Sci. 2013;92: 105–13. 10.3382/ps.2012-02579 [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Czura CJ, Tracey KJ. Tumor necrosis factor In: Thomson A, Lotze M, editors. The Cytokine Handbook. Elsevier; 2003. pp. 837–860. 10.1016/B978-012689663-3/50039-9 [DOI] [Google Scholar]
- 62.Tallant T, Deb A, Kar N, Lupica J, de Veer MJ, DiDonato JA. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappa B and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 2004;4: 33 10.1186/1471-2180-4-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, et al. Constitutive intestinal NF-κB does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med. 2011;208: 1889–900. 10.1084/jem.20110242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bastiani M, Vidotto MC, Horn F. An avian pathogenic Escherichia coli isolate induces caspase 3/7 activation in J774 macrophages. FEMS Microbiol Lett. 2005;253: 133–140. 10.1016/j.femsle.2005.09.024 [DOI] [PubMed] [Google Scholar]
- 65.Nougayrède J-P, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. 2006;313: 848–51. 10.1126/science.1127059 [DOI] [PubMed] [Google Scholar]
- 66.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391: 43–50. 10.1038/34112 [DOI] [PubMed] [Google Scholar]
- 67.Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y, Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature. 1999;401: 168–73. 10.1038/43678 [DOI] [PubMed] [Google Scholar]
- 68.Kepp O, Rajalingam K, Kimmig S, Rudel T. Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. Embo J. 2007;26: 825–34. 10.1038/sj.emboj.7601533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson MD, Wu X, Aithmitti N, Morrison RS. Peg3/Pw1 is a mediator between p53 and Bax in DNA damage-induced neuronal death. J Biol Chem. 2002;277: 23000–23007. 10.1074/jbc.M201907200 [DOI] [PubMed] [Google Scholar]
- 70.Kuroiwa Y, Kaneko-Ishino T, Kagitani F, Kohda T, Li LL, Tada M, et al. Peg3 imprinted gene on proximal chromosome 7 encodes for a zinc finger protein. Nat Genet. 1996;12: 186–90. 10.1038/ng0296-186 [DOI] [PubMed] [Google Scholar]
- 71.Kim J, Ashworth L, Branscomb E, Stubbs L. The human homolog of a mouse-imprinted gene, Peg3, maps to a zinc finger gene-rich region of human chromosome 19q13.4. Genome Res. 1997;7: 532–40. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=310658&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li LL, Szeto IY, Cattanach BM, Ishino F, Surani MA. Organization and parent-of-origin-specific methylation of imprinted Peg3 gene on mouse proximal chromosome 7. Genomics. 2000;63: 333–40. 10.1006/geno.1999.6103 [DOI] [PubMed] [Google Scholar]
- 73.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114: 181–90. Available: http://www.ncbi.nlm.nih.gov/pubmed/12887920 [DOI] [PubMed] [Google Scholar]
- 74.Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21: 461–5. 10.1016/j.immuni.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 75.Bolha L, Bencina D, Cizelj I, Oven I, Slavec B, Rojs OZ, et al. Effect of Mycoplasma synoviae and lentogenic Newcastle disease virus coinfection on cytokine and chemokine gene expression in chicken embryos. Poult Sci. 2013;92: 3134–43. 10.3382/ps.2013-03332 [DOI] [PubMed] [Google Scholar]
- 76.Horn F, Corrêa AMR, Barbieri NL, Glodde S, Weyrauch KD, Kaspers B, et al. Infections with avian pathogenic and fecal escherichia coli strains display similar lung histopathology and macrophage apoptosis. PLoS One. 2012;7 10.1371/journal.pone.0041031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156: 3986–92. Available: http://www.ncbi.nlm.nih.gov/pubmed/8621940 [PubMed] [Google Scholar]
- 78.Vaux DL, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci U S A. 1996;93: 2239–44. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=39779&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Häcker H, Fürmann C, Wagner H, Häcker G. Caspase-9/-3 activation and apoptosis are induced in mouse macrophages upon ingestion and digestion of Escherichia coli bacteria. J Immunol. 2002;169: 3172–9. 10.4049/jimmunol.169.6.3172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Class scores (0–3): Class (0) = no tail, Class (1) = tail length < diameter of nucleus, Class (2) = tail length between 1X and 2X the diameter of nucleus, and Class (3) = tail length > 2X the diameter of nucleus. (Original magnification: 200x; Scale bars: 50 μm).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.