Abstract
Previously, we found that orally administered acetic acid decreased lipogenesis in the liver and suppressed lipid accumulation in adipose tissue of Otsuka Long-Evans Tokushima Fatty rats, which exhibit hyperglycemic obesity with hyperinsulinemia and insulin resistance. Administered acetic acid led to increased phosphorylation of AMP-activated protein kinase (AMPK) in both liver and skeletal muscle cells, and increased transcripts of myoglobin and glucose transporter 4 (GLUT4) genes in skeletal muscle of the rats. It was suggested that acetic acid improved the lipid metabolism in skeletal muscles. In this study, we examined the activation of AMPK and the stimulation of GLUT4 and myoglobin expression by acetic acid in skeletal muscle cells to clarify the physiological function of acetic acid in skeletal muscle cells. Acetic acid added to culture medium was taken up rapidly by L6 cells, and AMPK was phosphorylated upon treatment with acetic acid. We observed increased gene and protein expression of GLUT4 and myoglobin. Uptake of glucose and fatty acids by L6 cells were increased, while triglyceride accumulation was lower in treated cells compared to untreated cells. Furthermore, treated cells also showed increased gene and protein expression of myocyte enhancer factor 2A (MEF2A), which is a well-known transcription factor involved in the expression of myoglobin and GLUT4 genes. These results indicate that acetic acid enhances glucose uptake and fatty acid metabolism through the activation of AMPK, and increases expression of GLUT4 and myoglobin.
Introduction
Obesity and type 2 diabetes are increasing throughout the world. The International Diabetes Federation Diabetes Atlas reported that there were 382 million people living with diabetes in the world in 2013. This number is expected to rise to 592 million by 2035 [1].
Obesity leads to excess lipid accumulation in adipose tissue, skeletal muscle, and liver. Lipid accumulation in muscle causes a decline of insulin sensitivity [2–5].
Previously, we found that orally administered acetic acid decreased lipogenesis in the liver and suppressed lipid accumulation in adipose tissue of Otsuka Long-Evans Tokushima Fatty rats, which exhibit hyperglycemic obesity with hyperinsulinemia and insulin resistance [6]. Acetic acid is a metabolite formed via β-oxidation of fatty acid in the liver mitochondria under starved conditions and is utilized in extrahepatic tissues as a biological fuel [7]. Under fed conditions, orally administered acetic acid is rapidly absorbed from the digestive organs and released into the blood stream [6]. Acetic acid absorbed into tissues is converted to acetyl-CoA with a formation of AMP via the activity of acetyl-CoA synthetase, leading to the activation of AMP-activated protein kinase (AMPK) [6]. Acetic acid contributed to protection against the accumulation of abdominal fat and of lipid in the liver.
AMPK, a heterotrimeric protein kinase, has been found to play a key role in regulation of whole-body energy balance by phosphorylating key metabolic enzymes in both biosynthetic and oxidative pathways [8–14]. AMPK is activated by a high AMP/ATP ratio in the cytosol, which occurs under heat shock, hypoxia, starvation, or physical exercise. AMPK activation results both from phosphorylation of Thr172 on AMPK’s α-subunits via upstream AMPK kinase and by way of allosteric activation of phosphorylated AMPK by 5’-AMP [8–15]. Administered acetic acid increased AMP concentration, resulting in an increase of the AMP: ATP ratio, and led to the activation of AMPK in skeletal muscle [15]. This phenomenon is very similar to that induced by endurance exercise training. Furthermore, treatment with acetic acid increased the gene expression of myoglobin and GLUT4 in skeletal muscle of rats. GLUT4 is a well-known gene that is induced by activation of AMPK in skeletal muscle [16]. Expression of the myoglobin gene is modulated by environmental stimuli including chronic hypoxia or endurance exercise training [17–20]. Mutational analysis of the myoglobin promoter confirmed that A/T-rich myocyte enhancer factor 2 (MEF2)-binding motifs were important in its gene regulation [21]. The human GLUT4 promoter is also regulated by the cooperative function of MEF2A [22], which is a transcription factor that plays a key role in skeletal muscle differentiation [23–25]. In differentiated myotubes, MEF2 is localized to the nucleus, indicating the importance of this transcription factor in specific skeletal muscle gene expression [26].
To investigate the function of acetic acid on AMPK activation and expression of genes such as myoglobin and GLUT4 that are involved with energy metabolism of skeletal muscle, we used L6 myotube cells and examined the effect of acetic acid. When added to the culture medium, acetic acid was rapidly taken up by L6 cells, and the phosphorylation of AMPK was stimulated. Transcripts and protein levels of myoglobin and GLUT4 were increased upon treatment with acetic acid. Furthermore, MEF2A levels in the nuclear fraction were increased. The uptake of glucose and fatty acid by cells were increased, while triglyceride accumulation was decreased upon treatment with acetic acid. These results indicate that treatment with acetic acid increases the expression of myoglobin and GLUT4 via the activation of AMPK and MEF2A, thus enhancing fatty acid metabolism and glucose uptake.
Materials and Methods
Materials
Rat L6 myoblasts (JCRB9081) were purchased from JCRB cell bank (Osaka, Japan). Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS) and 0.02% EDTA were from MP Biomedical (CA, USA); penicillin, streptomycin, and 0.25% trypsin, from Invitrogen (CA, USA). Antibodies against AMPKα, phosphorylated AMPKα, ACC, phosphorylated ACC, and GLUT4 were purchased from Cell Signaling (MA, USA), antibodies against myoglobin, MEF2A, PGC-1α and Sp1 were from Santa Cruz Biotechnology (CA, USA), and α-tubulin antibody was from Wako Pure Chemical Industries Ltd. (Osaka, Japan). AMPK agonist 5-amino-4-imidazolecarboxamide-1-beta-D-ribofuranoside (AICAR) and AMPK inhibitor adenine 9-β-D-arabinofuranoside (araA) were purchased from Sigma-ALDRICH (MO, USA). AMPK inhibitor Compound C was purchased from Merck (DA, Germany)
L6 cell culture
L6 myoblasts were grown in DMEM containing 10% (v/v) FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 at 37°C. For myotube differentiation, the medium was changed to DMEM containing 2% (v/v) horse serum when myoblasts were 80% confluent. Myotubes were harvested 8–11 days after differentiation, and experimental procedures were initiated.
Amount of acetic acid incorporated in cells
Differentiated L6 myotube cells were treated with 0.5 mM (1 μmol/2ml) acetic acid for 0–30 min, and then the each conditioned medium that treated with acetic acid for each time period was collected and measured the concentration of acetic acid. The concentration of acetic acid remaining in the media was measured using the acetic acid UV-method kit (R-Biopharm AG, Darmstadt, Germany) according to the manufacturer’s instructions. The rates of acetic acid uptake were calculated by using the amount of acetic acid remaining in the medium, which averaged 473.1 μM (ca. 0.95 μmol/2ml), 394.0 μM (ca. 0.79 μmol/2ml), 390.0 μM (ca. 0.78 μmol/2ml) in 10sec, 2min, 30min of the treatment of acetic acid, respectively.
Amount of glucose uptake into cells
Differentiated L6 myotube cells were treated with 0.5 mM acetic acid and 100 nM insulin for 24 and 48 hrs in the medium that the glucose concentration was 25 mM (50μmol/2ml). Each conditioned medium that treated with acetic acid or insulin for 24 and 48 hrs was collected and measured the concentration of glucose. The concentration of glucose remaining in the media was measured using a commercial assay kit (Glucose CII-Test Wako; Wako Pure Chemical Industries Ltd., Osaka, Japan). The amount of glucose uptake was calculated by using the amount of glucose remaining in the medium, which averaged 39 μmol and 32 μmol in 24 hr and 48 hr of the treatment of acetic acid, respectively, and 34 μmol and 20 μmol in 24 hr and 48 hr of the treatment of insulin, respectively. The amount of glucose remaining in the control medium averaged 44 μmol and 40 μmol in 24 hr and 48 hr, respectively.
Amounts of fatty acid uptake and triglyceride accumulation
Differentiated L6 myotube cells were incubated with the medium containing 0.6 μmol palmitic acid (300 μmol/L) for 24 and 48 hrs in the presence or absence of 0.5 mM acetic acid or 0.5 mM AICAR. After the incubation, mediums and cells were collected separately and the concentration of NEFA in the mediums and the concentration of TG in the cells were determined by using commercial assay kits (NEFA C-Test Wako and Triglyceride E-Test Wako, respectively; Wako Pure Chemical Industries Ltd., Osaka, Japan). The amount of fatty acid uptake was calculated by using the concentration of fatty acid remaining in the medium, which averaged 0.263 μmol and 0.075 μmol in 24 hr and 48 hr of the treatment of acetic acid, respectively, and 0.183 μmol and 0.149 μmol in 24 hr and 48 hr of the treatment of AICAR, respectively. The amount of fatty acid remaining in the control medium averaged 0.316 μmol and 0.159 μmol in 24 hr and 48 hr, respectively.
Nucleotide assay
Differentiated L6 myotube cells were treated with 0.5 mM acetic acid for 0–30 min and added to ice-cold 0.5 M perchloric acid, neutralized, and centrifuged. Concentrations of AMP, ADP, and ATP in the extracts of myotube cells were determined by reverse-phase HPLC analysis (SPD-10A, respectively; Shimazu, Kyoto, Japan) with an ODS column (HPLC PACKED COLUMN C18 CAPCELLPAK, respectively; Shimadzu Corporation, Kyoto, Japan). The mobile phase consisted of 100 mM phosphate buffer, pH 6.3 and 0.89% methanol. Quantification was performed at λ=259 nm. All chromatographic assays were carried out at room temperature with a flow of 1.0 ml/min. Adenosine nucleotides (ATP, ADP, and AMP) were identified and quantified based on the corresponding standard compounds.
Nuclear extraction
Myotubes were grown in 10-cm dishes, treated with acetic acid or AICAR as described above, and then washed immediately with ice-cold PBS. Hypotonic buffer (10 mM HEPES pH 7.9, 1.5 mM MgCl2, 0.1 mM EDTA, 0.1% NP-40, 1 mM DTT, 1 mM PMSF, and protease inhibitors (Nacalai Tesque, Kyoto, Japan)) were added and the cells were collected. After homogenization, the extract was centrifuged for 5 min at 3,000 rpm at 4°C, and the supernatant (cytoplasmic fraction) was collected. The nuclear pellet was resuspended in hypertonic buffer (20 mM HEPES pH 7.9, 1.5 mM MgCl2, 400 mM NaCl, 0.1 mM EDTA, 0.1% NP-40, 10% glycerol, 1 mM DTT, 1 mM PMSF, and protease inhibitors) by pipetting. The suspension was incubated and shaken for 1 hr on ice. Then the supernatant (nuclear fraction) was collected by centrifugation at 15,000 rpm for 5 min. Protein concentration of the nuclear extract was determined by Bradford assay.
AMPK activity
To measure AMPK activity, AMPK was immunoprecipitated from cell extracts with specific antibodies against the α2-subunits bound to protein G agarose beads. The kinase activity of the immunoprecipitates was measured using SAMS peptide [HMRSAMSGLHLVKRR] and the Kinase-Glo Luminescent Kinase Assay kit (Promega, Madison, USA) according to the manufacturer’s instructions.
Western blotting
L6 myotube cells were washed with ice-cold PBS and lysed with RIPA buffer (1x TBS pH 7.4, 0.5% deoxycholic acid, 0.1% SDS, 1% NP-40, 1 mM PMSF, 1 mM Na3VO4, 10 mM NaF, and protease inhibitors). Following centrifugation, supernatants were used for western blotting. Protein content of supernatants was determined by Bradford assay and an aliquot (15–30 μg of protein) of each extract from L6 cells was used for western blot to determine the contents of total AMPKα, phosphorylated Thr-172 AMPKα, total ACC, phosphorylated ACC, myoglobin, GLUT4, MEF2A, PGC-1α and α-tubulin. Samples were applied to 10–15% SDS-PAGE, and then proteins on the gel were transferred onto a polyvinylidene difluoride membrane (Merck, DA, Germany). The membranes were first incubated with primary antibodies overnight at 4°C, washed three times with TBST, and they were incubated with HRP-conjugated secondary antibodies for 60 min. For highly sensitive system, after the membranes were incubated with primary antibodies, they were incubated with biotin-conjugated secondary antibodies for 15–30min, and they were incubated with HRP-conjugated streptavidin for 15min. After washing three times with TBST, the chemiluminescent reaction was performed for 5 min with ImmunoStar LD (Wako Pure Chemical Industries Ltd., Osaka, Japan), according to the protocol supplied by the manufacturer. Chemiluminescent signals were visualized and quantified with ImageQuant LAS-4000 and Multi Gauge V3.2 analyzing software (Fujifilm, Tokyo, Japan).
Quantitative RT-PCR analysis
Differentiated myotubes were incubated with 0.5 mM acetic acid and/or other reagents for the indicated time, and then the cells were washed three times with RNase free PBS and harvested for RNA extraction. Total RNA was isolated from L6 cells by using Sepasol RNA I super G (Nacalai Tesque, Kyoto, Japan), and RNase inhibitor (TOYOBO, Osaka, Japan) was added according to the manufacturer’s instructions. Total RNA concentration was measured and cDNA was prepared with PrimeScript RT Reagent Kit with gDNA Eraser (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. Real-time quantitative PCR analyses were performed using the StepOnePlus detection system (Applied Biosystems, CA, USA) with KAPA SYBR FAST qPCR Kits (Kapa Biosystems, Wilmington, MA) for quantification of specific mRNA content. Data were normalized to β-actin mRNA and expressed relative to untreated control cells. The oligonucleotide primers were as follows: rat β-actin (actb) forward: 5’-GGAGATTACTGCCCTGGCTCCTA-3’, reverse: 5’-GACTCATGTACTCCTGCTTGCTG-3’, rat GLUT4 (Slc2a4), forward: 5’-GGGCGATTTCTCCCACATAC-3’, reverse: 5’-CTCATGGGCCTAGCCAATG-3’, rat MEF2A (mef2a), forward: 5’-ATGAGAGGAACCGACAGGTG-3’, reverse: 5’-TATCCGAGTTCGTCCTGCTT-3’, rat myoglobin (Mb), forward: 5’- CTAACAGCCGGCCTACACTC-3’, reverse: 5’-CGTGCTTCTTCAGGTCCTCT-3’, PGC-1α (ppargc1a) forward: 5’-GACCCCAGAGTCACCAAATGA-3’, reverse: 5’-GGCCTGCAGTTCCAGAGAGT-3’.
Immunofluorescence
L6 cells were fixed with 4% formaldehyde solution, incubated with 0.1% Triton X-100/PBS for 3 min, and blocked with 3% BSA/PBS for 10 min. Samples were incubated at room temperature for 1 hr with primary anti-MEF2A antibody (Santa Cruz Biotechnology) and anti-skeletal myosin antibody (SIGMA-ALDRICH, MO, USA). Then samples were washed three times with PBS and incubated with secondary antibody conjugated to Alexa Fluor at room temperature for 1 hr. Samples were counterstained with Hoechst 33258 (Polysciences, Inc., PA, USA) for 5 min and imaged using a confocal microscope (OLYMPUS FLUOVIEW FV1000, respectively; OLYMPUS, Tokyo, Japan).
Statistical analysis
For analysis of AMPK activity, specific activities of AMPK were compared using unpaired Student’s t-test. For the remaining analysis, one-way ANOVA followed by the Tukey-Kramer post hoc test for multiple comparisons was performed. P values < 0.05 were considered to represent statistical significance (*p<0.05, **p<0.01 compared to control). Groups without the same letter represent significantly different (p<0.05).
Results
Absorption of acetic acid by differentiated L6 myotube cells
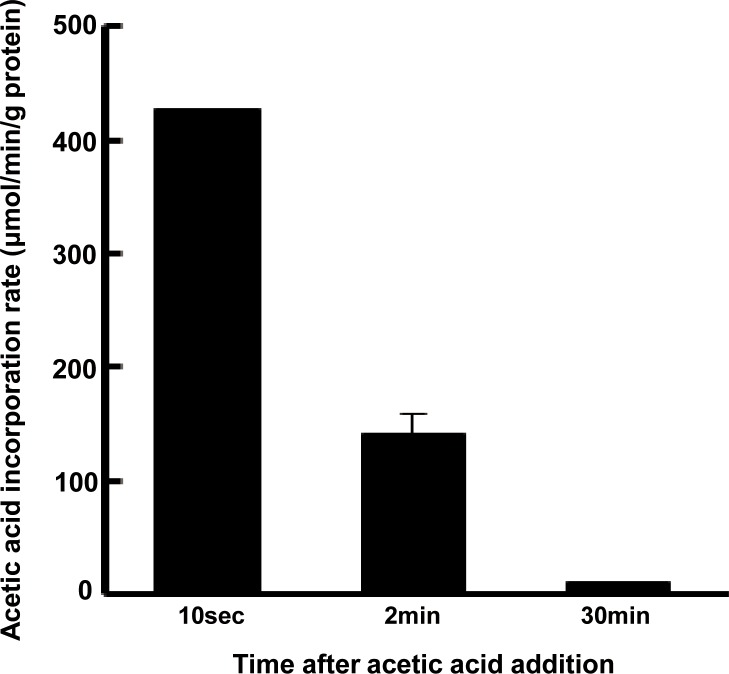
To assess the ability of L6 myotubes to absorb acetic acid, 0.5 mM acetic acid was added to cultured cells and the amount of acetic acid taken up by the cells was measured. Acetic acid was immediately taken up within 2 min of incubation (Fig 1).
Fig 1. Uptake of acetic acid by L6 myotube cells.
After treatment with acetic acid (0.5 mM), acetic acid content in the medium was determined at each indicated time point and the amount of uptake by the cells was calculated. Each value is shown as the mean ± SE (n = 3–6).
Acetic acid raises AMP concentration in differentiated L6 myotube cells
Following the absorption of acetic acid by cells, acetic acid is converted to acetyl-CoA concomitantly with the formation of AMP, a well-known activator of AMPK, via the catalytic activity of acetyl-CoA synthetase in the cytosol. AMP concentration in cells incubated with 0.5 mM acetic acid was consistently higher than that in non-treated steady state control cells (0 min), and significantly increased in 2 min compared to the steady state concentration (Table 1). The AMP:ATP ratio during incubation with acetic acid was significantly increased in 2 min of the addition of acetic acid.
Table 1. Stimulation of the AMP/ATP ratio in L6 myotube cells by treatment with acetic acid.
| (μmol / g of protein) | |||||
|---|---|---|---|---|---|
| Time | ATP | ADP | AMP | Total | AMP/ATP |
| 0 min | 20.95 ± 3.65 | 5.53 ± 0.71 | 0.86 ± 0.07 | 27.35 ± 4.45 | 0.041 ± 0.007 |
| 0.5 min | 18.52 ± 2.83 | 6.48 ± 0.16 | 2.06 ± 0.51 | 27.06 ± 2.61 | 0.111 ± 0.051 |
| 2 min | 19.73 ± 1.91 | 6.81 ± 0.49 | 4.43 ± 0.47* | 30.96 ± 1.17 | 0.224 ± 0.061* |
| 30 min | 18.95 ± 1.01 | 8.66 ± 0.31* | 3.38 ± 1.43 | 30.99 ± 0.57 | 0.179 ± 0.018 |
Adenine nucleotides (μmol/g of protein) in L6 cells treated with 0.5 mM acetic acid for the indicated times. Each value is shown as the mean ± SE (n = 3–4). Results were analyzed with one-way ANOVA followed by the Tukey-Kramer post hoc test for multiple comparisons. Statistical differences are shown as *p<0.05 compared to the 0 min time point.
Acetic acid induces phosphorylation of AMPK and ACC in differentiated L6 cells
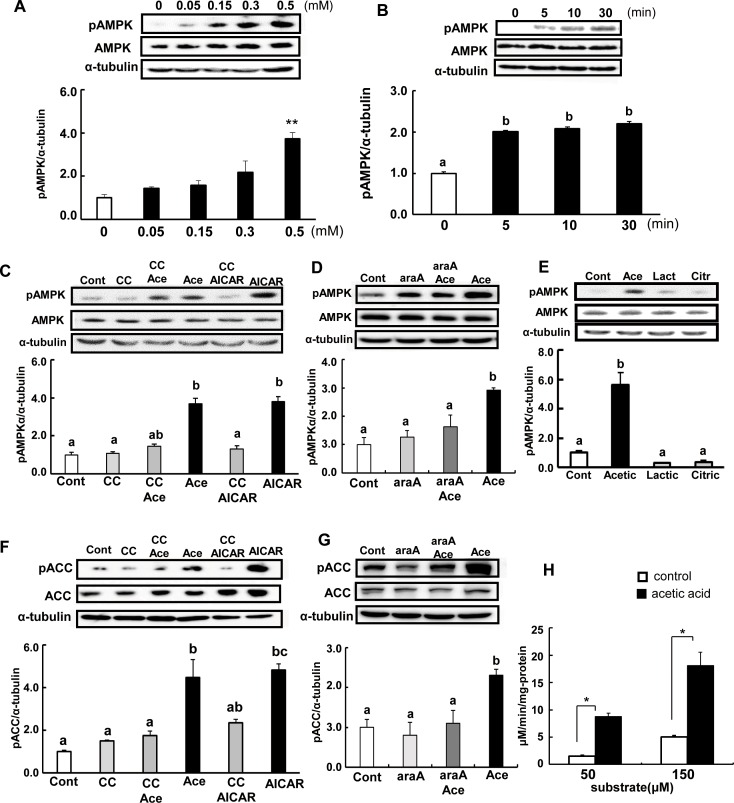
To determine whether the treatment with acetic acid can induce phosphorylation of AMPK at Thr172 in L6 cells, the change of phosphorylated AMPK levels was analyzed. Phosphorylation of AMPK was significantly increased in acetic acid-treated cells compared to in non-treated control cells. Ten minutes after the addition of the indicated amount of acetic acid to the culture medium, phosphorylated AMPK increased in a dose-dependent manner (Fig 2A).
Fig 2. Acetic acid induces phosphorylation of AMPK and ACC in L6 myotube cells.
L6 myotube cells were treated with 0, 0.05, 0.15, 0.3, and 0.5 mM acetic acid for 10 min (A). Cells were incubated with 0.5 mM acetic acid for the indicated time (B), 0.5 mM AICAR for 12 hr, and 10 μM compound C for 30 min (C), 2 mM araA for 20 min (D), and 0.5 mM lactic acid or 0.5 mM citric acid for 10 min (E), and analyzed for the phosphorylation of AMPK. Cells were treated with 0.5 mM acetic acid for 10 min and 0.5 mM AICAR for 12 hr, and 10 μM compound C for 30 min (F) and 2 mM araA for 20min (G), and analyzed for phosphorylation of ACC and ACCβ. After L6 myotube cells were treated with 0.5 mM acetic acid for 2 min, cell lysates were prepared. Then, AMPK was immunoprecipitated from cell extracts and AMPK activity was measured using the synthetic SAMS peptide as a substrate (50 μM and 150 μM) (H). Each bar represents the mean ±SE (n = 3–4). Results were analyzed with one-way ANOVA followed by the Tukey-Kramer post hoc test for multiple comparisons (A-G), or analyzed by an unpaired Student’s t-test (H). Statistical differences are shown as *p<0.05, **p<0.01 compared to non-treated (A). Groups without the same letter are significantly different (p<0.05) (B-G).
A time-course study revealed that incubation with 0.5 mM acetic acid increased the phosphorylation of AMPK after the addition of acetic acid (Fig 2B). Treatment with AICAR, an AMPK activator, also increased the phosphorylation of AMPK by about 2.2-fold at 12 hr after the addition. Pre-treatment of cells with compound C and araA, potent AMPK inhibitors, suppressed the acetic acid induced phosphorylation of AMPK (Fig 2C and 2D). Phosphorylation of AMPK was also analyzed after treatment with other acid compounds such as 0.5 mM lactic acid and 0.5 mM citric acid. Both compounds did not enhance the phosphorylation of AMPK (Fig 2E).
One downstream target of AMPK is phosphorylation of acetyl-CoA carboxylase (ACCβ) at Ser79. Phosphorylated ACC was significantly increased at 10 min after treatment with 0.5 mM acetic acid (Fig 2F), and the addition of compound C and araA together with acetic acid led to suppression of this phosphorylation (Fig 2F and 2G). This increase in phosphorylation was also seen in the treatment with AICAR, and inhibited by addition of compound C.
Moreover, we also conducted an evaluation of AMPK activity in L6 myotube cells using the SAMS peptide as a substrate of AMPK. AMPK activity was significantly increased in cells treated with acetic acid compared to non-treated control cells (Fig 2H).
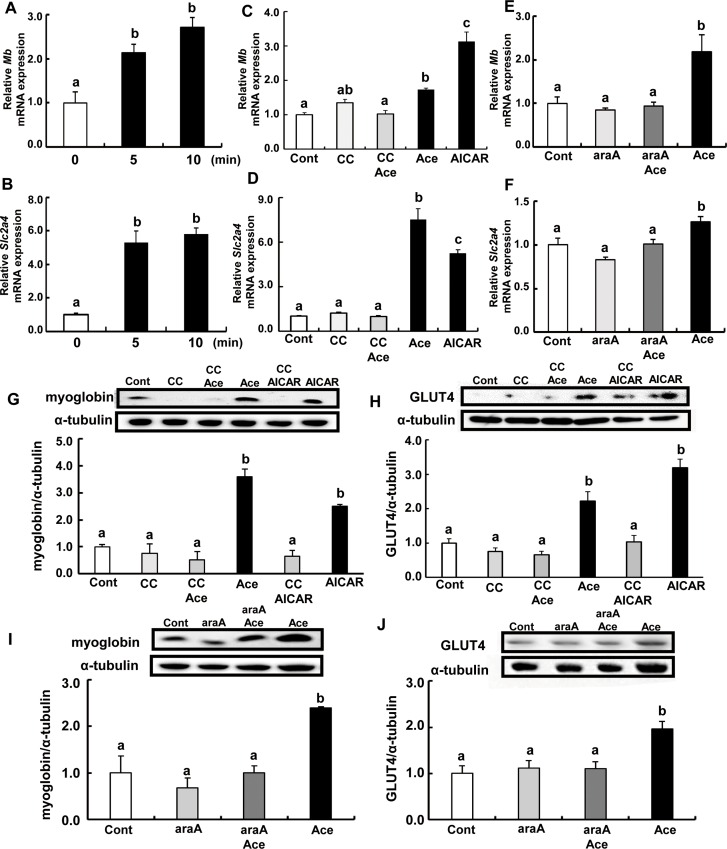
Acetic acid treatment increases mRNA and protein expression of myoglobin and GLUT4 in L6 myotube cells
Transcripts of myoglobin and GLUT4 were increased in cells treated with acetic acid. Time-course studies revealed that mRNA levels of myoglobin gene, Mb and GLUT4 gene, Slc2a4 were significantly higher than those of non-treated steady state control cells (Fig 3A and 3B). Furthermore, pre-treatment with compound C or araA and acetic acid completely suppressed the increase in expression of Mb and Slc2a4 genes (Fig 3C–3F). Expression of these proteins were also significantly increased and this stimulation was completely suppressed in the presence of compound C and araA (Fig 3G–3J).
Fig 3. Effects of acetic acid on the expression of myoglobin and GLUT4 in L6 myotube cells.
Total RNA was extracted from untreated L6 myotube cells or those treated with 0.5 mM acetic acid for the indicated time period (A; Mb, B; Slc2a4) or for 5 min after the addition of acetic acid (C; Mb, D; Slc2a4) or 0.5 mM AICAR for 12 hr, and 10 μM compound C for 30 min or 2mM araA for 20min. Real-time PCR analysis was carried out for determination of Mb (A, C, E) and Slc2a4 (B, D, F) mRNA levels in L6 myotube cells. Myoglobin or GLUT4 proteins were analyzed by western blotting on the treatment of 0.5 mM acetic acid for 10 min, 0.5 mM AICAR for 12 hours, and 10 μM compound C for 30 min (G, H) or 2 mM araA for 20 min (I, J). Each bar represents the mean ±SE (n = 3–6). Results were analyzed with one-way ANOVA followed by the Tukey-Kramer post hoc test for multiple comparisons. Groups without the same letter are significantly different (p<0.05).
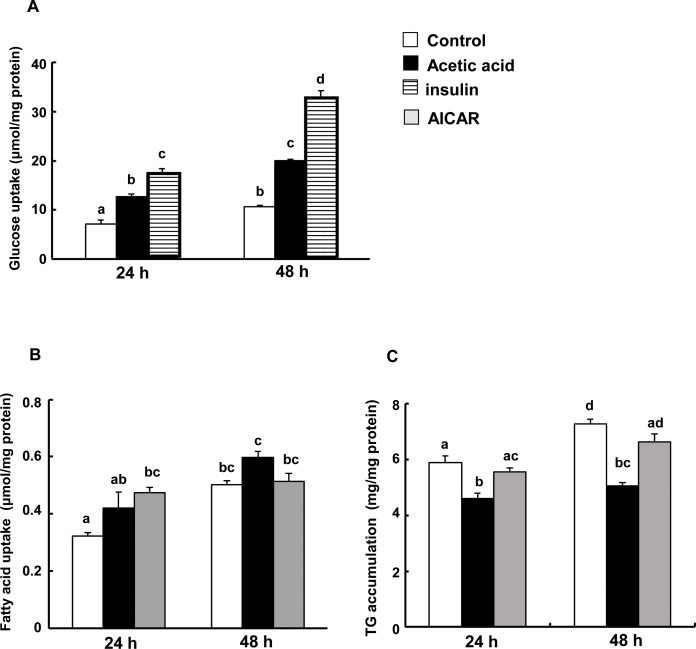
Acetic acid increases glucose and fatty acid uptake, and suppresses triglyceride accumulation in L6 myotube cells
To investigate whether the activation of AMPK by acetic acid stimulates uptake of glucose, glucose clearance was examined in cells treated with acetic acid. After insulin treatment, glucose uptake was approximately 3 times higher than that of non-treated controls. Similarly, acetic acid as well as AICAR treatment significantly increased glucose uptake (Fig 4A). Treatment with acetic acid tended to increase the utilization of fatty acid, but reduced their accumulation as triglyceride in the cells compared to non-treated control or AICAR treatment (Fig 4B and 4C).
Fig 4. Effects of acetic acid treatment on glucose, fatty acid uptake and triglyceride accumulation in L6 myotube cells.
(A)Glucose uptake by L6 cells. Differentiated L6 myotube cells were treated with 0.5 mM acetic acid and 100 nM insulin for 24 and 48 hrs in the medium (50μmol/2ml). Each conditioned medium was collected and measured the concentration of glucose. Amount of glucose uptake was calculated by using the amount of glucose remaining in the medium. (B) Fatty acid uptake by L6 cells. (C) TG accumulation in L6 cells. Differentiated L6 myotube cells were incubated with the medium containing 0.6 μmol palmitic acid (300 μmol/L) for 24 and 48 hrs in the presence or absence of 0.5 mM acetic acid or 0.5 mM AICAR. After the incubation, mediums and cells were collected separately and the concentration of NEFA in the mediums and the concentration of TG in the cells were determined. Each bar represents the mean ±SE (n = 3–4). Results were analyzed with one-way ANOVA followed by the Tukey-Kramer post hoc test for multiple comparisons. Groups without the same letter are significantly different (p<0.05).
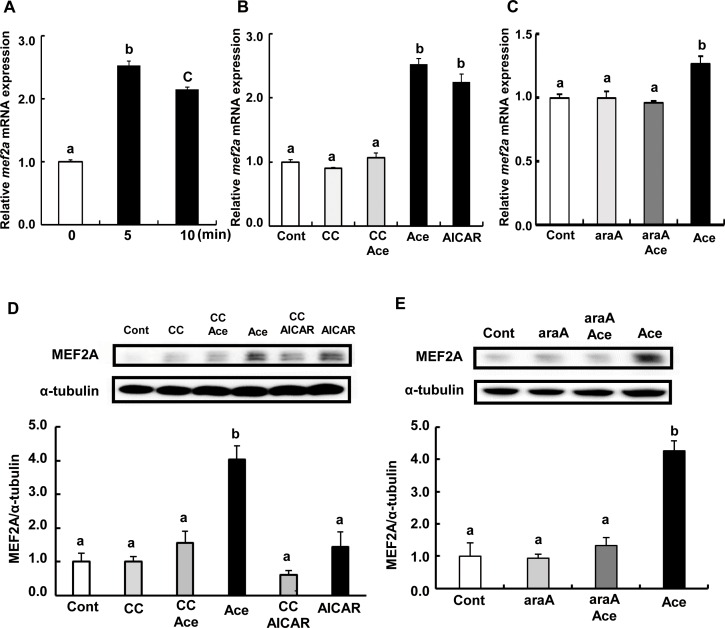
Acetic acid treatment increases mRNA and protein expression of MEF2A in L6 myotube cells
Myocyte enhancer factor 2A (MEF2A) plays an important role in the regulation of gene expression of myoglobin and GLUT4 in skeletal muscle [26]. MEF2 proteins are transcription factors involved in the differentiation of skeletal muscle, and possess diverse cellular functions in skeletal muscles or neurons. We focused on MEF2A and hypothesized that the increase in mb and Slc2a4 gene expression by acetic acid treatment might be associated with the function of MEF2A, as well as with the activation of AMPK.
Transcript levels of mef2a were significantly higher in cells treated with acetic acid than those of non-treated control (Fig 5A). Pre-treatment with compound C or araA completely suppressed the induction of mef2a mRNA by acetic acid (Fig 5B and 5C). Protein expression of MEF2A was also increased upon acetic acid treatment (Fig 5D and 5E), which was abolished by compound C and araA.
Fig 5. Effect of acetic acid on the expression of MEF2A in L6 myotube cells.
Total RNA was extracted from untreated L6 myotube cells or cells treated with 0.5 mM acetic acid for the indicated time period (A). L6 cells were treated with 0.5 mM acetic acid for 5 min, 0.5 mM AICAR for 12 hours, and pre-treated with 10 μM compound C for 30 min (B), and pre-treated with 2 mM araA for 20 min (C). Real-time PCR analysis was carried out for the determination of mef2A mRNA level in L6 myotube cells. MEF2A protein was analyzed by western blotting in 10 min treatment of 0.5 mM acetic acid, 0.5 mM AICAR for 12 hours, and 10 μM compound C for 30 min (D) or 2 mM araA for 20 min (E). Each bar represents the mean ±SE (n = 3–6). Results were analyzed with one-way ANOVA followed by the Tukey-Kramer post hoc test for multiple comparisons. Groups without the same letter are significantly different (p<0.05).
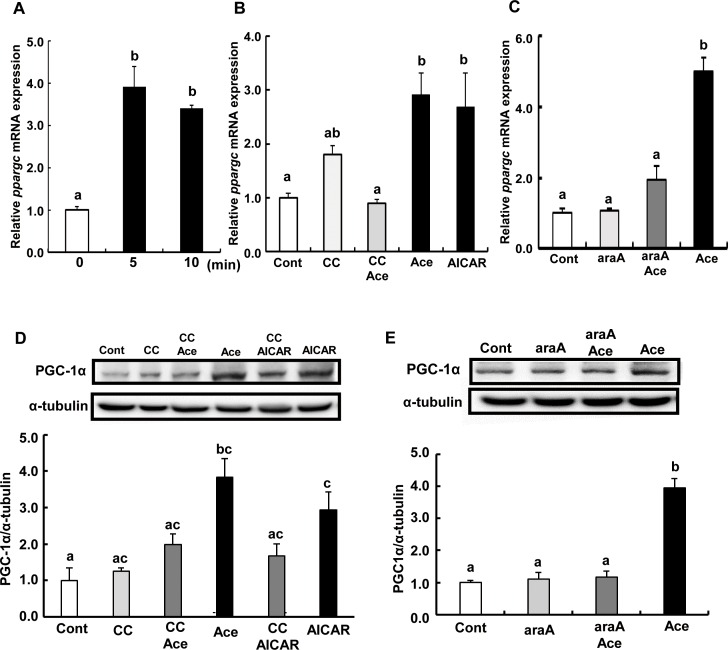
Acetic acid treatment increases mRNA and protein expression of PGC-1α in L6 myotube cells
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a transcriptional coactivator that mediates many biological processes related to energy metabolism. PGC-1α and MEF2 form a positive feedback loop [27]. Activation of AMPK has been implicated to be involved in the upregulation of ppargc1a expression [28].
Transcript levels of ppargc1a were significantly higher in cells treated with acetic acid than in non-treated control (Fig 6A). Pre-treatment with compound C or araA, and acetic acid completely suppressed the induction of ppargc1a mRNA (Fig 6B and 6C). Protein expression of PGC-1α was also increased upon treatment with acetic acid (Fig 6D and 6E).
Fig 6. Effect of acetic acid on the expression of PGC-1α in L6 myotube cells.
Total RNA was extracted from untreated L6 myotube cells or cells treated with 0.5 mM acetic acid for the indicated time period (A). L6 cells were treated with 0.5 mM acetic acid for 5 min, 0.5 mM AICAR for 12 hours, and pre-treated with 10 μM compound C for 30 min (B) or 2 mM araA for 20 min (C), and total RNA were isolated. Real-time PCR analysis was carried out for the determination of ppargc1a mRNA level in L6 myotube cells. PGC-1α protein level was analyzed by western blotting in 5 min treatment with 0.5 mM acetic acid, 0.5 mM AICAR for 12 hours, and 10 μM compound C for 30 min (D) or 2 mM araA for 20 min (E). Each bar represents the mean ±SE (n = 3–6). Results were analyzed with one-way ANOVA followed by the Tukey-Kramer post hoc test for multiple comparisons. Groups without the same letter are significantly different (p<0.05).
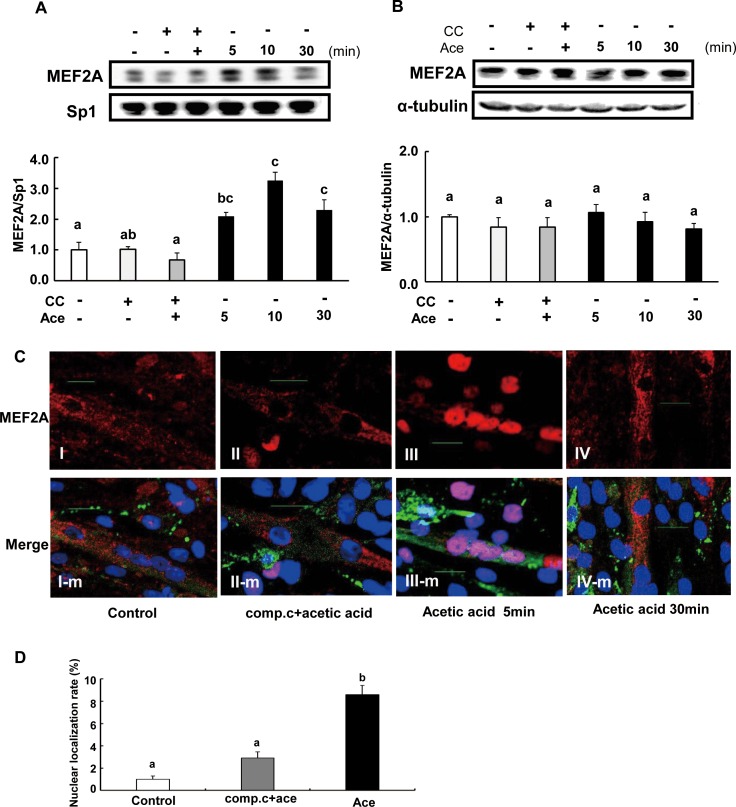
Acetic acid induces nuclear localization of MEF2A
The nuclear MEF2A protein level was measured by western blotting to see the nuclear localization of MEF2A. Nuclear MEF2A was significantly increased at 5 min (2.1-fold), 10 min (3.3-fold) and 30 min (2.3-fold) after treatment with acetic acid compared to non-treated control (Fig 7A). In contrast, there was no change in the cytosolic MEF2A levels (Fig 7B). However, nuclear localization of MEF2A was significantly reduced in the presence of compound C (Fig 7A). Furthermore, we analyzed L6 myotube cells by confocal immunofluorescence. Fig 7C is the typical image showing that MEF2A was localized in nucleus in L6 cells treated with acetic acid, while it was localized in the cytosol in the presence of compound C and in non-treated cells (Fig 7C). And the nuclear localization rate of MEF2A was significantly increased by the treatment of acetic acid (Fig 7D).
Fig 7. Effect of acetic acid treatment on nuclear MEF2A expression in L6 myotube cells.
L6 myotube cells were treated with 0.5 mM acetic acid, and nuclear fraction (A) and cytosolic fraction (B) were separated. MEF2A level was examined by western blotting as described in materials and methods. L6 myotube cells were cultured on glass cover slips coated with poly-L-lysine and treated with 0.5 mM acetic acid in the presence or absence of 10 μM compound C (C). Then cells were fixed and nuclear DNA was stained by Hoechst 33258 (blue). Cells were immunostained for MEF2A (red) and myosin (green). Scale bar = 20 μm. The nucleus immunostained with anti-MEF2A antibody were counted (8 mm2 area, n = 3) and the rate of nuclear localization of MEF2A was calculated (D). Each bar represents the mean ±SE (n = 3–4). Results were analyzed with one-way ANOVA followed by the Tukey-Kramer post hoc test for multiple comparisons. Groups without the same letter are significantly different (p<0.05).
Discussion
Skeletal muscle is one of the most important insulin-responsive organs in the body [29, 30], and an accumulation of locally derived fat metabolites in skeletal muscle is an important factor contributing to insulin resistance [29]. It has been demonstrated that AMPK plays a key role in regulating fat oxidation and glucose metabolism via upregulation of mitochondrial proteins, GLUT4, and several metabolic enzymes in mice, rats, or human skeletal muscles [8–14]. Present data indicate that acetic acid functions as an activator of AMPK and might be able to suppress lipogenesis via enhancing lipid metabolism. When acetic acid is taken up into tissues, it is converted to acetyl-CoA with concomitant formation of AMP by the catalytic activity of acetyl-CoA synthetase [31–36]. Acetic acid is easily absorbed by L6 myotube cells, and increases the AMP/ATP ratio in the cells. An increase in AMP/ATP ratio stimulates the phosphorylation of AMPK. Phosphorylated AMPK increased in a dose-dependent manner with the concentration of acetic acid (Fig 2A), which was in the range of physiological blood concentration of acetic acid. Pre-treatment of cells with compound C inhibited phosphorylation of AMPK by acetic acid (Fig 2C). Moreover, other acids such as lactic acid or citric acid were unable to increase the phosphorylation of AMPK (Fig 2E). Activated AMPK leads to an inactivation of ACC via phosphorylation, a decrease in malonyl-CoA content, a subsequent increase in fatty acid oxidation, and a block in fatty acid synthesis. Phosphorylation of ACC significantly increased at 10 min of acetic acid treatment (Fig 2F) and it was inhibited by pre-treatment with compound C and araA (Fig 2F and 2G). In our previous study, chronic intake of acetic acid induced gene expression of myoglobin and GLUT4 in skeletal muscle of rats, and the rats showed a higher rate of oxygen consumption and a smaller size of lipid droplets in white adipose tissue [15]. In this study, transcripts and protein levels of myoglobin and GLUT4 were increased in acetic acid-treated L6 myotube cells. Pre-treatment of acetic acid treated cells with compound C and araA completely suppressed the increase of myoglobin and GLUT4 gene and protein expression (Fig 3C, 3E and 3D–3J), suggesting that acetic acid induces myoglobin and GLUT4 transcription through the AMPK-mediated signaling pathway in L6 myotube cells. Myoglobin is expressed in cardiac and skeletal myocytes, and it plays a role in the diffusion of oxygen and to maintain mitochondrial respiration [37]. The myoglobin gene is expressed depending on myofiber subtypes, and the expression is regulated by environmental stimuli such as chronic hypoxia and endurance exercise training [17–20]. GLUT4 is one of the glucose transport proteins and is responsible for insulin-mediated glucose uptake in muscle and adipose tissue. It was shown that glucose homeostasis depends on the level of GLUT4 expression [38–40].
Transcription of myoglobin and GLUT4 genes is regulated by myocyte enhancer factor 2 (MEF2), which is a transcription factor involved in skeletal muscle differentiation [23–25, 41–44]. The MEF2 family is comprised of four members, MEF2A, B, C and D [26, 45], and belongs to the MCM1-agamous-deficiens-serum response factor (MADS) supergene family of DNA binding proteins [45–47]. MEF2B is a unique member of the MEF2 gene family [48] and its expression is restricted to myogenic lineages during early embryo development [49]. MEF2D is expressed in proliferating myoblasts prior to the onset of differentiation [26, 50]. MEF2C is expressed late in the differentiation program, and MEF2A protein appears in cells entering the differentiation pathway and the expression is regulated at multiple levels during development and differentiation [26]. The predominant MEF2 DNA-binding complex in muscle cells is composed of MEF2A homodimers [26]. There is a MEF2 binding site in GLUT4 promoters that contains the binding site of a MEF2A-MEF2D heterodimer [51]. Reduced MEF2A expression accounts for the reduction in DNA binding activity and directly correlates with the decrease in GLUT4 gene expression [51]. MEF2A is a substrate for p38 mitogen-activated protein (MAP) kinase (MAPK), and threonines 312 and 319 within the transcription activation domain of MEF2A are phosphorylated by p38 [52]. Phosphorylated MEF2A enhances MEF2-dependent gene expression. MAPK-independent pathways such as the AMPK-associated pathway have also been implicated in the regulation of MEF2 [53]. Treatment of human skeletal muscle cells with AICAR, a pharmacological activator of AMPK, stimulated MEF2 DNA binding activity [53]. AICAR-mediated MEF2 DNA binding was independent of p38 MAPK activation, and it was completely inhibited by an AMPK inhibitor, compound C [53]. AICAR-injected rats showed increased nuclear MEF2 DNA binding activity, and chronic AICAR treatment dramatically increased the expression of glucose transport protein GLUT4 in muscle [54]. In this study, we observed that MEF2A nuclear localization was increased upon treatment with acetic acid and it was inhibited by pre-treatment with compound C (Fig 7). Furthermore, transcripts of MEF2A and PGC-1α genes were significantly increased in cells treated with acetic acid (Fig 5A–5C and 6A–6C). PGC-1α plays a key role in the regulation of mitochondrial biogenesis and oxidative metabolism and its activity has been reported to be regulated by AMPK [55]. MEF2A and PGC-1α contain a MEF2A binding site in their promoter sequences [27, 56] and their gene expression was coordinated with one another [27]. Those findings implicate that acetic acid induced expression of both MEF2A and PGC-1α as well as of myoglobin and GLUT4 genes might be caused by an increase in nuclear localization of MEF2A via the activation of AMPK.
De Angelis et al. showed that transforming growth factor β (TGF-β) inhibited myogenesis and prevented the activation of the transcriptional complex related to MEF2A through localization of MEF2A in the cytoplasm [57]. Furthermore, activated AMPK inhibited TGF-β, Smad3 gene expression, and TGF-β-induced myofibroblast differentiation [58–60]. In this study, MEF2A, which contains a nuclear localization sequence at its C-terminus [26, 61], was localized in the nucleus shortly after treatment with acetic acid and exported to the cytoplasm 30 min after treatment. Investigation of the transport mechanism of MEF2A upon treatment with acetic acid is currently underway.
mRNA and protein expression levels of MEF2A were significantly increased upon treatment with acetic acid in L6 myotube cells (Fig 5). In addition, pre-treatment of acetic acid-treated cells with compound C completely suppressed the increase in MEF2A mRNA and protein expression levels (Fig 5B and 5D). A ChIP assay revealed that the MEF2A promoter contains a MEF2A binding site and that MEF2A would associate with the site to control the MEF2A transcription [56]. These results implicate that increased expression of MEF2A by acetic acid might be caused by increased nuclear localized MEF2A via the activation of AMPK.
Activation of AMPK is linked to lipid catabolism and improvement of insulin sensitivity [14]. Numerous AMPK activators have been described including adiponectine or berberine. Adiponectine, which is an adipocytokine preventing metabolic syndrome or atherosclerosis, activates AMPK in skeletal muscle and improves insulin sensitivity [28, 62]. Berberine, a food component, has an effect on AMPK activation, adipose tissues, and macrophages [63, 64]. In this study, acetic acid activated AMPK, induced gene and protein expression of myoglobin and GLUT4, stimulated glucose incorporation, and suppressed lipid accumulation in L6 cells. Thus, acetic acid has the potential to prevent metabolic disorders through the activation of AMPK.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 15K07437 for Grant-in-Aid for Scientific Research (C) and 26850087 for Grant-in-Aid for Young Scientists (B). The founders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the following sources of funding: JSPS KAKENHI 15K07437 for Grant-in-Aid for Scientific Research (C) and JSPS KAKENHI 26850087 for Grant-in-Aid for Young Scientists (B).
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014. February; 103 [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, et al. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991. September; 88(3):960–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996. June 15; 97:2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnan C, Gilbert M and Kahn BB. Chronic free fatty acid infusion in rats results in insulin resistance but no alteration in insulin-responsive glucose transporter levels in skeletal muscle. Lipids. 1996. November; 31(11):114–1149 [DOI] [PubMed] [Google Scholar]
- 5.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, et al. Effect of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999. January; 103(2):253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashita H, Fujisawa K, Ito E, Idei S, Kawaguchi N, Kimoto M, et al. Improvement of obesity and glucose tolerance by acetate in type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem. 2007. May 71(5):1236–1243 [DOI] [PubMed] [Google Scholar]
- 7.Yamashita H, Kaneyuki T, Tagawa K. Production of acetate in the liver and its utilization in peripheral tissues. Biochim Biophys Acta, 2001. May 31; 1532(1–2):79–87 [DOI] [PubMed] [Google Scholar]
- 8.Moore F, Weekes J, Hardie DG. Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. Eur J Biochem. 1999. August 1; 199(3):691–697 [DOI] [PubMed] [Google Scholar]
- 9.Winder WW and Harie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996. February; 270(2 Pt 1):E299–E304 [DOI] [PubMed] [Google Scholar]
- 10.Winder WW and Harie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol. 1999. July; 277(1 Pt 1):E1–E10 [DOI] [PubMed] [Google Scholar]
- 11.Hardie DG. The AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003. December; 144(12):5179–5183 [DOI] [PubMed] [Google Scholar]
- 12.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002. January 17; 415(6869):339–343. [DOI] [PubMed] [Google Scholar]
- 13.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003. July 3; 546(1):113–120. [DOI] [PubMed] [Google Scholar]
- 14.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011. April; 93(4):891S–896S. 10.3945/ajcn.110.001925 [DOI] [PubMed] [Google Scholar]
- 15.Yamashita H, Maruta H, Jozuka M, Kimura R, Iwabuchi H, Yamato M, et al. Effects of acetate on lipid metabolism in muscles and adipose tissues of type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem. 2009. March 23; 73(3):570–576 [DOI] [PubMed] [Google Scholar]
- 16.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003. December; 144(12):5179–5183. [DOI] [PubMed] [Google Scholar]
- 17.Kanatous SB, DiMichele LV, Cowan DF, Davis RW. High aerobic capacities in the skeletal muscles of pinnipeds: adaptations to diving hypoxia. J Appl Physiol (1985). 1999. April; 86(4):1247–1256. [DOI] [PubMed] [Google Scholar]
- 18.Neufer PD, Ordway GA, Williams RS. Transient regulation of c-fos, αB-crystallin, and hsp70 in muscle during recovery from contractile activity. Am J Physiol. 1998. February; 274(2 Pt 1):C341–C346. [DOI] [PubMed] [Google Scholar]
- 19.Underwood LE, Williams RS. Pretranslational regulation of myoglobin gene expression. Am J Physiol. 1987. April; 252(4 Pt 1):C450–C453. [DOI] [PubMed] [Google Scholar]
- 20.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976; 38:273–291. [DOI] [PubMed] [Google Scholar]
- 21.Gossett LA, Kelvin DJ, Sternberg EA, Olson EN. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989. November; 9(11):5022–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murgia M, Jensen TE, Cusinato M, Garcia M, Richter EA, Schiaffino S. Multiple signalling pathways redundantly control glucose transporter GLUT4 gene transcription in skeletal muscle. J Physiol. 2009. September 1;587(Pt 17):4319–4327 10.1113/jphysiol.2009.174888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black BL, Molkentin JD, Olson EN. Multiple roles for the MyoD basic region in transmission of transcriptional activation signals and interaction with MEF2. Mol Cell Biol. 1988. January; 18(1):69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaushal S, Schneider JW, Nadal-Ginard B, Mahdavi V. Activation of the myogenic lineage by MEF2A, a factor that induces and cooperates with MyoD. Science. 1994. November 18; 266(5188):1236–1240 [DOI] [PubMed] [Google Scholar]
- 25.Molkentin JD, Black BL, Martin JF, Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995. December 29; 83(7):1125–1136 [DOI] [PubMed] [Google Scholar]
- 26.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998; 14:167–196 [DOI] [PubMed] [Google Scholar]
- 27.Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci U S A. 2003. June 10; 100(12):7111–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwabu M1, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, et al. Adiponectin and AdipoR1 regulate PGC-1a and mitochondria by Ca2+ and AMPK/SIRT1. Nature. 2010. April 29; 464(7293):1313–1319. 10.1038/nature08991 [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001. June 19; 98(13):7522–7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron AD, Brechtel G, Wallace P, Edellman, SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988. December; 255(6 Pt 1):E769–E774 [DOI] [PubMed] [Google Scholar]
- 31.Ballard FJ. Supply and utilization of acetate in mammals. Am J Clin Nutr. 1972. August; 25(8):773–779. [DOI] [PubMed] [Google Scholar]
- 32.Knowles SE, Jarrett IG, Filsell OH, Ballard FJ. Production and utilization of acetate in mammals. Biochem J. 1974. August; 142(2):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. Plasma acetate turnover and oxidation. J Clin Invest. 1979. September; 64(3):708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bremer J., and Osmundsen H., Fatty acid oxidation and its regulation In “Fatty Acid Metabolism and Its Regulation,” ed. Numa S., Elsevier Science Publishers, Amsterdam, pp. 113–154 (1984). [Google Scholar]
- 35.Pouteau E, Piloquet H, Maugeais P, Champ M, Dumon H, et al. Kinetic aspect of acetate metabolism in healthy humans using [1-13C] acetate. Am J Physiol. 1996. July; 271(1 Pt 1):E58–E64. [DOI] [PubMed] [Google Scholar]
- 36.Mittendorfer B, Sidossis LS, Walser E, Chinkes DL, Wolfe RR. Regional acetate kinetics and oxidation in human volunteers. Am J Physiol. 1998. June; 274(6 Pt 1):E978–E983. [DOI] [PubMed] [Google Scholar]
- 37.Wittenberg BA, Wittenberg JB. Transport of oxgen in muscle. Annu Rev Physiol. 1989; 51:857–878. [DOI] [PubMed] [Google Scholar]
- 38.Tsao TS, Burcelin R, Katz EB, Huang L, Charron MJ. Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. Diabetes. 1996. January; 45(1):28–36. [DOI] [PubMed] [Google Scholar]
- 39.Treadway JL, Hargrove DM, Nardone NA, McPherson RK, Russo JF, et al. Enhanced peripheral glucose utilization in transgenic mice expressing the human GLUT4 gene. J Biol Chem. 1994. November 25; 269(47):29956–29961. [PubMed] [Google Scholar]
- 40.Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, et al. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993. October 25; 268(30):22243–22246. [PubMed] [Google Scholar]
- 41.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002. August 15; 418(6899):797–801. [DOI] [PubMed] [Google Scholar]
- 42.Thai MV, Guruswamy S, Cao KT, Pessin JE, Olson AL. Myocyte enhancer factor 2 (MEF2)-binding site is required for GLUT4 gene expression in transgenic mice. Regulation of MEF2 DNA binding activity in insulin-deficient diabetes. J Biol Chem. 1998. June 5; 273(23):14285–14292 [DOI] [PubMed] [Google Scholar]
- 43.Holmes BF, Sparling DP, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT4 enhancer factor and myocyte enhancer factor 2 by AMP-activated protein kinase. Am J Physiol Endocrinol Metab. 2005. December; 289(6):E1071–E1076. [DOI] [PubMed] [Google Scholar]
- 44.Liu ML, Olson AL, Edgington NP, Moye-Rowley WS, Pessin JE. Myocyte enhancer factor 2 (MEF2) binding site is essential for C2C12 myotube-specific expression of the rat GLUT4/muscle-adipose facilitative glucose transporter gene. J Biol Chem. 1994. November; 269(45):28514–2821 [PubMed] [Google Scholar]
- 45.Olson EN, Perry M, Schulz RA. Regulation of muscle differentiation by the MEF2 family of MADS box transcription factors. Dev Biol. 1995. November; 172(1):2–14 [DOI] [PubMed] [Google Scholar]
- 46.Pollock R, Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991. December; 5(12A):2327–2341 [DOI] [PubMed] [Google Scholar]
- 47.Yu YT, Breitbart RE, Smoot LB, Lee Y, Mahdavi V, Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992. September; 6(9):1783–1798 [DOI] [PubMed] [Google Scholar]
- 48.Morisaki T, Sermsuvitayawong K, Byun SH, Matsuda Y, Hidaka K, Morisaki H, et al. Mouse Mef2b gene: unique member of MEF2 gene family. J Biochem. 1997. November; 122(5):939–946 [DOI] [PubMed] [Google Scholar]
- 49.Molkentin JD, Firulli AB, Black BL, Martin JF, Hustad CM, Copeland N, et al. MEF2B is a potent transactivator expressed in early myogenic lineages. Mol Cell Biol. 1996. July; 16(7):3814–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han TH, Prywes R., Regulatory role of MEF2D in serum induction of the c-jun promoter. Mol Cell Biol. 1995. June; 15(6): 2907–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mora S, Pessin JE. The MEF2A isoform is required for striated muscle-specific expression of the insulin-responsive GLUT4 glucose transporter. J Biol Chem. 2000. May 26; 275(21):16323–16328 [DOI] [PubMed] [Google Scholar]
- 52.Zhao M, New L, Kravchenko VV, Kato Y, Gram H, et al. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999. January; 19(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Khalili L, Chibalin AV, Yu M, Sjödin B, Nylén C, et al. MEF2 activation in differentiated primary human skeletal muscle cultures requires coordinated involvement of parallel pathways. Am J Physiol Cell Physiol. 2004. June; 286(6):C1410–C1416. [DOI] [PubMed] [Google Scholar]
- 54.Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, et al. Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol (1985). 2001. September; 91(3):1073–1083. [DOI] [PubMed] [Google Scholar]
- 55.Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS One. 2008; 3(10):e3614 10.1371/journal.pone.0003614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramachandran B, Yu G, Gulick T. Nuclear respiratory factor 1 controls myocyte enhancer factor 2A transcription to provide a mechanism for coordinate expression of respiratory chain subunits. J Biol Chem. 2008. May 2; 283(18):11935–11946. 10.1074/jbc.M707389200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Angelis L, Borghi S, Melchionna R, Berghella L, Baccarani-Contri M, Parise F, et al. Inhibition of myogenesis by transforming growth factor beta is density-dependent and related to the translocation of transcription factor MEF2 to the cytoplasm. Proc Natl Acad Sci U S A. 1998. October 13; 95(21):12358–12363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra R, Cool BL, Laderoute KR, Foretz M, Viollet B, Simonson MS., AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem. 2008. April 18; 283(16):10461–10469 10.1074/jbc.M800902200 [DOI] [PubMed] [Google Scholar]
- 59.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012. March 13; 3:735 10.1038/ncomms1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma K. Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int Suppl (2011). 2014. November; 4 (1):113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.61Yu YT. Distinct domains of myocyte enhancer binding factor-2A determining nuclear localization and cell type-specific transcriptional activity. J Biol Chem. 1996. October 4; 271(40):24675–24683 [PubMed] [Google Scholar]
- 62.Zhou L1, Deepa SS, Etzler JC, Ryu J, Mao X, et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem. 2009. August 14; 284(33):22426–22435. 10.1074/jbc.M109.028357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006. August; 55(8):2256–2264. [DOI] [PubMed] [Google Scholar]
- 64.Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009. April; 296(4):E955–E964. 10.1152/ajpendo.90599.2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.