Abstract
The retina adjusts its signaling gain over a wide range of light levels. A functional result of this is increased visual acuity at brighter luminance levels (light adaptation) due to shifts in the excitatory center-inhibitory surround receptive field parameters of ganglion cells that increases their sensitivity to smaller light stimuli. Recent work supports the idea that changes in ganglion cell spatial sensitivity with background luminance are due in part to inner retinal mechanisms, possibly including modulation of inhibition onto bipolar cells. To determine how the receptive fields of OFF cone bipolar cells may contribute to changes in ganglion cell resolution, the spatial extent and magnitude of inhibitory and excitatory inputs were measured from OFF bipolar cells under dark- and light-adapted conditions. There was no change in the OFF bipolar cell excitatory input with light adaptation; however, the spatial distributions of inhibitory inputs, including both glycinergic and GABAergic sources, became significantly narrower, smaller, and more transient. The magnitude and size of the OFF bipolar cell center-surround receptive fields as well as light-adapted changes in resting membrane potential were incorporated into a spatial model of OFF bipolar cell output to the downstream ganglion cells, which predicted an increase in signal output strength with light adaptation. We show a prominent role for inner retinal spatial signals in modulating the modeled strength of bipolar cell output to potentially play a role in ganglion cell visual sensitivity and acuity.
Keywords: bipolar cell, γ-aminobutyric acid, glycine, amacrine cell
the retina must signal under many light conditions to encode the environment accurately. This involves retinal adaptation to increasing luminance levels to avoid saturation and increase visual acuity. The retina signals multiple light levels by using photoreceptors with dim (rod) and bright (cone) sensitivities as well as adaptation of both the photoreceptors themselves (Tamura et al. 1991; Woodruff et al. 2008) and downstream networks (Dunn et al. 2006, 2007; Green et al. 1975; Green and Powers 1982; Naka et al. 1979; Page-McCaw et al. 2004; Shapley and Enroth-Cugell 1984). Photoreceptor light information is sent to bipolar cells that synapse onto ganglion cells, the output neurons of the retina, and inhibitory amacrine cells, which shape bipolar cell-ganglion cell signaling (Dong and Werblin 1998; Eggers and Lukasiewicz 2010; 2006b; Eggers et al. 2007; Sagdullaev et al. 2006) by releasing either glycine or GABA (Kolb 1997; Menger et al. 1998; Pourcho and Goebel 1985, 1983; Vaney 1990). Morphologically wide GABAergic amacrine cells (Pourcho and Goebel 1983) are thought to primarily carry wide lateral information for center-surround receptive fields (Dacey et al. 2000; Ichinose and Lukasiewicz 2005; Sinclair et al. 2004; Zhang and Wu 2009), whereas narrow glycinergic amacrine cells (Pourcho and Goebel 1985) provide inhibition between different pathways (Mazade and Eggers 2013; Molnar et al. 2009; Werblin 2010). However, glycinergic amacrine cells, such as the AII amacrine cell of the rod pathway can be highly coupled, depending on the light state, to extend the spread of their signaling (Bloomfield et al. 1997). The balance between the excitatory center and inhibitory surround allows ganglion cells fine spatial tuning to specific sizes of light, vital for visual resolution.
Ganglion cells increase their spatial sensitivity and visual acuity with increased ambient illumination (Barlow et al. 1957; Dedek et al. 2008; Farrow et al. 2013), and retinal network adaptation is thought to be crucial for this process (Dunn et al. 2006; Wu and Yang 1992). Additionally, previous work has shown that the increase in ganglion cell sensitivity to small lights was due to a decrease in excitatory center size (Merwine et al. 1995; Troy et al. 1999) or decreases in surround size and strength (Dedek et al. 2008; Farrow et al. 2013; Merwine et al. 1995; Troy et al. 1999; Troy and Enroth-Cugell 1993). Inner retinal processing likely plays an important role in ganglion cell spatial resolution increases with light (Dedek et al. 2008), because the connections between bipolar and ganglion cells are important for light adaptation (Dunn et al. 2007; Farrow et al. 2013; Oesch and Diamond 2011). Given other reports that show inhibition to bipolar cells is important for determining the spatial resolution and receptive field properties of ganglion cells (Buldyrev and Taylor 2013; Flores-Herr et al. 2001; Protti et al. 2014; Russell and Werblin 2010), modulation of bipolar cell inhibition by light is a potential mechanism for increasing visual acuity.
Modulation of inhibitory inputs to the pathway that responds to the offset of light (OFF) in particular may be important in retinal network adaptation, because inhibitory inputs switch between rod and cone sources (Chun et al. 1993; Deans et al. 2002; Mazade and Eggers 2013; McGuire et al. 1984; Strettoi et al. 1990, 1992; Trexler et al. 2001). This change from rod- to cone-mediated input and light adaptation of cones could change the distribution of inhibitory input to OFF (cone) bipolar cells and affect ganglion cell center-surround organization, but this is not known. In the present study we investigated how the spatial extent of inhibition to OFF bipolar cells changes with light adaptation and used our findings to model the strength of OFF bipolar cell output to downstream ganglion cells. We found that the spatial inhibition to OFF bipolar cells became narrower with significantly reduced magnitude and that OFF bipolar cells were more depolarized with light adaptation. A model using these parameters showed that these changes are important for determining stronger signal output to ganglion cells for small light stimuli.
METHODS
Mouse retinal slice preparation.
All animal protocols were approved by the University of Arizona Institutional Animal Care and Use Committee. As described previously (Eggers and Lukasiewicz 2006a; Eggers et al. 2013b; Mazade and Eggers 2013), male mice (C57BL/6J strain; Jackson Laboratories, Bar Harbor, ME) 35–60 days of age were euthanized using carbon dioxide and their eyes enucleated. The cornea and lens were removed, and the eyecup was incubated for 20 min in cold extracellular solution (see Solution and drugs) with 800 U/ml hyaluronidase to dissolve the remaining vitreous humor. The hyaluronidase solution was then replaced with ice-cold, oxygenated extracellular solution, and the retina was dissected out of the eyecup. After removal, the retina was trimmed into one large, flat rectangle by removing the periphery and leaving only the central retina surrounding the optic disc. A nitrocellulose membrane filter paper (0.45-μm pore size; Millipore Ireland) was placed on the retina section that was transferred to a hand chopper. An average of six 250-μm slices were cut, rotated 90°, and mounted onto glass coverslips using vacuum grease. Several methods were used to limit potential circuitry disruption from use of retinal slice preparations. Only cells located near the center of the slice and deep within the tissue, avoiding the surface, were targeted for recordings to maximize accurate spatial connectivity. Additionally, retinal slices were on average 2 mm wide, so wide-field amacrine cell processes in the horizontal plane of the slice are likely undisturbed. Spatial stimuli were presented perpendicular to the horizontal plane of the slice so that neurons that extended in this direction were preferentially activated. Retinal slices of these dimensions were used to counteract the difficulty of visualizing cells for patch clamping, similar to other studies that have used slices to investigate light-evoked inhibitory inputs to bipolar cells (Eggers and Lukasiewicz 2010; Eggers et al. 2007, 2013; Tanaka and Tachibana 2013; Vigh et al. 2011). The tissue was maintained in oxygenated extracellular solution at room temperature. All dissection procedures were performed under infrared illumination to preserve the light sensitivity of the preparations.
Solutions and drugs.
The extracellular recording solution used for dissection and to examine light-evoked currents contained (in mM) 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 2 CaCl2, 20 glucose, and 26 NaHCO3 and was bubbled with 95% O2-5% CO2. For voltage-clamp recordings, the intracellular solution contained (in mM) 120 CsOH, 120 gluconic acid, 1 MgCl2, 10 HEPES, 10 tetraethylammonium chloride (TEA-Cl), 10 phosphocreatine-Na2, 4 Mg-ATP, 0.5 Na-GTP, 10 EGTA, and 50 μM Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and was adjusted to pH 7.2 with CsOH. To isolate the inhibitory receptor inputs, we used SR-95531 (SR; 20 μM) to block GABAA receptors (GABAARs), (1,2,5,6-tetrahydropyridine-4yl)methyphosphinic acid (TPMPA; 50 μM) to block GABACRs, and strychnine (1 μM) to block glycine receptors. For current-clamp recordings, the intracellular solution contained (in mM) 120 KOH, 120 gluconic acid, 1 MgCl2, 10 HEPES, 10 EGTA, 10 TEA-Cl, 10 phosphocreatine-Na2, 4 Mg-ATP, 0.5 Na-GTP, and 50 μM Alexa Fluor 488 (Invitrogen) and was adjusted to pH 7.2 with CsOH. All drug solutions were washed on the slice for 5 min before recordings began using a gravity-driven superfusion system (Cell Microcontrols, Norfolk, VA) at a rate of ∼1–2 ml/min. Unless otherwise indicated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Whole cell recordings.
Whole cell patch-clamp recordings, sampled at 10 kHz, were made from bipolar cells in retinal slices. Light-evoked inhibitory postsynaptic currents (L-IPSCs) were recorded from retinal bipolar cells voltage-clamped to 0 mV, the reversal potential of nonselective cation channel currents. Bipolar cell recordings were stable, and no rundown of the light response was observed over the recording period. Light-evoked excitatory post synaptic currents (L-EPSCs) were recorded from bipolar cells voltage-clamped to −60 mV, the reversal potential for chloride channel-mediated currents. Resting membrane potentials were recorded passively in current-clamp (I-clamp) mode. Liquid junction potentials of 20 mV were corrected for at the beginning of each recording. Electrodes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) on a P97 Flaming/Brown puller (Sutter Instruments, Novato, CA) and had resistances of 5–7 MΩ. Mice were dark-adapted overnight, and all recording procedures were performed in the dark under infrared illumination to preserve the light sensitivity of the slices. Recordings were made in extracellular solution heated to 32°C, using thin stage and inline heaters (Cell Microcontrols, Norfolk, VA). Responses were filtered at 6 kHz with the four-pole Bessel filter on a Multi-clamp 700B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA) and digitized with a Digidata 1140 data acquisition system (Molecular Devices).
Morphological identification of cell subtypes.
Alexa Fluor 488 included in the recording pipette was used to label OFF bipolar cells. They were classified as OFF bipolar cells on the basis of their axonal morphologies and stratification within the inner plexiform layers and the position of their somas in the inner nuclear layer (Ghosh et al. 2004). The cells were imaged with a Nikon Digital Sight camera with Elements software using a Nikon Intensilight C-HGFIE fluorescent lamp (Nikon Instruments, Tokyo, Japan). Detailed analysis of axon terminal morphology and response properties was performed previously on all OFF bipolar cells (Mazade and Eggers 2013), and these same criteria were used for identification in the current study.
Light stimuli.
Bar stimuli (25 μm wide) were presented using a white organic light-emitting diode (OLED Microdisplay, EMA-100503 SXGA Monochrome White XL; eMagin, Bellevue, WA) projected through the camera port of the microscope, which elicited strong responses in both dark- and light-adapted conditions (Fig. 1B), comparable to previous work on bipolar cell inhibition (Eggers and Lukasiewicz 2010; Eggers et al. 2007; Mazade and Eggers 2013; Pang et al. 2012). Responses to OFF bipolar cells were repeatable over the course of the recording, with the magnitude highly dependent on the adapting state and particular manipulation (isolating specific inhibitory inputs). Recordings were from cells located within the regions of mixed green/UV cone opsin input (Applebury et al. 2000; Haverkamp et al. 2005), which ensured that all possible pathways were present; however, the white OLED stimulus used would not likely activate S-cone photoreceptors (UV) (Wang et al. 2011). For all experiments, a long light stimulus (1 s) was used to determine the type of inhibition and excitation to all recorded bipolar cells as well as to elicit the strongest responses despite the use of a small stimulus. OFF bipolar cells showed robust OFF L-EPSCs at the offset of this stimulus. The adapting background light was applied for 5 min to light-adapt the retinal slice and was sustained throughout all light-adapted recordings.
Fig. 1.
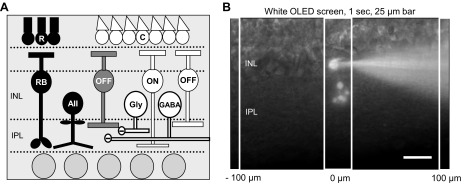
OFF bipolar cell inhibition. A: schematic of OFF bipolar cell inhibitory input pathways. Rod photoreceptors (R) activated by dim light release glutamate onto rod bipolar cells (RB), which release glutamate onto AII amacrine cells (AII). AII amacrine cells release glycine onto OFF cone bipolar cells (OFF; black pathway). Cone photoreceptors (C) are activated by brighter light and release glutamate onto OFF and ON cone (ON) bipolar cells. Activation of these bipolar cells in turn releases glutamate onto other wide-field GABAergic (GABA) and narrow-field glycinergic (Gly) amacrine cells, which also have inputs onto OFF bipolar cells (white pathways). INL, inner nuclear layer; IPL, inner plexiform layer. B: diagram of experimental protocol. Example cell morphology of an Alexa 488-filled OFF type 3 bipolar cell in a retinal slice preparation. To examine spatial inhibitory input to OFF bipolar cells, 25-μm bars of white light were presented to the retinal slice for 1 s, as shown, using a white OLED screen mounted on the microscope. Bars of light were presented every 100 μm from the recorded cell, extending in both directions, with 30 s between stimuli. Scale bar, 25 μm.
For dark-adapted experiments, the stimulus intensity was 7.83 × 104 photons·μm−2·s−1 (3.91 × 104 R*·rod−1·s−1) on a completely dark background (room luminance of 0.05 photons·μm−2·s−1) since the OLED screen did not use a backlight. For light-adapted experiments the light intensity of the background was 1,150 photons·μm−2·s−1 (575 R*·rod−1·s−1), with the same stimulus intensity as in dark-adapted experiments with a Weber contrast (CW) of 67. The light-adapted background intensity was shown to maximally activate rods (Wang and Kefalov 2009) and produced significant changes in inner retinal inhibition (Mazade and Eggers 2013). The stimulus and background intensities are similar to previous work on bipolar cell inhibition (Mazade and Eggers 2013) and incorporate meaningful changes in Weber contrast inherent in seeing an unchanged visual stimulus in the environment with only a shift in the ambient illumination. For experiments used for the spatial model, stimuli with similar step changes in intensity from the background luminance were used in both light conditions. A dim background of 158 photons·μm−2·s−1 (79 R*·rod−1·s−1) with a stimulus intensity of 7.65 × 104 photons·μm−2·s−1 (3.82 × 104 R*·rod−1·s−1) was used in the dark-adapted condition (CW = 483), with the same parameters used as previously described for the light-adapted condition. Light intensities were calculated (limited by OLED screen parameters) so that the Michelson contrasts (CM) in the dark-adapted (0.995) and light-adapted (0.971) conditions were similar, like the stimuli and strategy used in a previous study (Farrow et al. 2013). All stimuli were controlled with custom MATLAB (The MathWorks, Natick, MA) software by controlling the intensity, size, location, and duration of the bar stimuli through the OLED screen.
Data analysis and statistics.
L-IPSC and L-EPSC traces from a given response condition were averaged using Clampfit software (Molecular Devices), and the charge transfer (Q) and peak amplitude were measured in each condition. Because of the significant amount of spontaneous activity, it was difficult to measure a peak from OFF bipolar cell L-IPSCs. Therefore, to estimate the peak, the sampling rate of averaged traces was reduced (50-fold) and each point replaced with the average of those data points to limit variations caused by spontaneous activity. To determine changes in total current, Q was measured in Clampfit over the length of the response, typically 1–2 s, using the same time parameters in each condition for the same cell. The baseline Q value was added to the Q standard deviation and was subtracted from all raw Q measurements to negate any current due to baseline or spontaneous events. All example response traces show responses to the center bar stimulus directly over the recorded cell or 200 μm away from the cell. For the I-clamp experiments, baseline voltage was averaged over ∼200 s of stable baseline, to account for variation, in each condition to calculate the resting membrane potential.
For spatial distribution curves, light-evoked Q values were normalized to the maximal response in the dark-adapted condition to control for variability between bipolar cell L-IPSCs, caused by spontaneous activity incorporated into the light response, so that spatial extent could be accurately compared and visualized between light conditions. Raw peak amplitude values were used to more accurately reflect response magnitude changes. The normalized and raw data were plotted against the distance of the stimulus from the cell. To construct the spatial surround distribution graphs, only OFF bipolar cells in which the full range of stimulus distances were tested in both light conditions were used for averaging as well as statistics to compare changes in the surround. However, to compare between the dark- and light-adapted conditions at each stimulus distance, bar graphs were constructed using data from all bipolar cells, including data from cells in which a smaller range of spatial stimulus positions were tested. Additionally, responses at the same stimulus distance from both sides of the retinal slice were averaged to reduce potential variation throughout the slice. As a result, the data presented in the bar graphs provide a more accurate comparison of response magnitude at each distance from the cell between the two light conditions.
To measure timing differences between light conditions, the transient and sustained components of center L-IPSCs were measured. The transient L-IPSC component was measured as the first 20% Q of the response based on the 1-s light stimulus, similar to the method described by Nobles et al. (2012). Sustained L-IPSC components were measured by subtracting the transient Q from the total Q of the L-IPSC for each light condition. Proportions were calculated by dividing the transient and sustained values by the total Q.
Two-way analysis of variance (ANOVA) with the Student-Newman-Keuls post hoc test was used to compare spatial distributions before and after light adaptation as well as between response characteristics at each stimulus distance. Student's t-test (2-tailed, paired) was used to compare resting membrane potentials and timing parameters before and after light adaptation. Differences were considered significant when P < 0.05 and P < 0.01. All averaged data are reported as means ± SE.
Spatial inhibition model.
A model of input signal strength to a ganglion cell was constructed based on the spatial, magnitude, and resting potential changes reported in this study. Average OFF bipolar cell spatial distributions and average peak amplitude values of the center response were used from both dark- and light-adapted conditions. Average spatial inhibition and excitation curves were fitted with a Gaussian curve from which standard deviations were obtained for both light conditions. The standard deviations were then used as a base for constructing model OFF bipolar cell inhibitory or excitatory spatial receptive fields. These distributions were then normalized and multiplied by a scaled peak amplitude (Isc; Eq. 1), which was calculated using measured average peak amplitudes of the center responses (Imeasured) at holding potentials (Vhold) of either 0 mV (L-IPSC) or −60 mV (L-EPSC),
| (1) |
where Vrest is the resting membrane potential of dark- or light-adapted OFF bipolar cells, Vrev is the reversal potential for either cation or chloride channels, Vhold is the holding voltage for measuring excitatory or inhibitory currents, and G = Imeasured/(Vrev − Vhold). Excitatory and inhibitory spatial Gaussian distributions based on the scaled currents were constructed. A difference-of-Gaussians was calculated by subtracting the inhibitory from the excitatory Gaussian distributions. To account for the fact that glutamate release is necessary for bipolar cell signals to ganglion cells, bipolar cell scaled currents were used to calculate real membrane voltage values (Vreal; Eq. 2) by converting scaled currents (Isc) to the amount of voltage change they would cause, using an average input resistance (R) measured from OFF bipolar cells and adding the value to the resting membrane potential (Vrest) in each light condition:
| (2) |
Bipolar cell voltage values under dark and light adaptation were then used as a baseline for whether the bipolar cells would signal to downstream ganglion cells, incorporating release probability as described in results, and thus the bipolar cell signal output strength.
RESULTS
The spatial extent of total OFF bipolar cell inhibition is reduced after light adaptation.
OFF bipolar cells receive glycine-, GABAA-, and GABACR-mediated inhibition including glycinergic inputs from the rod pathway via the unique AII amacrine cell connection and glycinergic and GABAergic inputs from cone pathways (Eggers et al. 2007; Euler and Wässle 1998; Ivanova et al. 2006) (Fig. 1A) that change with light adaptation (Mazade and Eggers 2013). Previous work showed changes in the excitatory center size (Merwine et al. 1995; Troy et al. 1999) and inhibitory surround size and strength (Dedek et al. 2008; Farrow et al. 2013; Merwine et al. 1995; Troy et al. 1999; Troy and Enroth-Cugell 1993) of ganglion cells with light adaptation that may be a result of spatial changes at the bipolar cell level (Dedek et al. 2008). To test this, we mapped the spatial extent of OFF bipolar cell inhibition using a long light stimulus (1 s) that was flashed onto a retinal slice every 100 μm, with 30 s between stimuli (Fig. 1B).
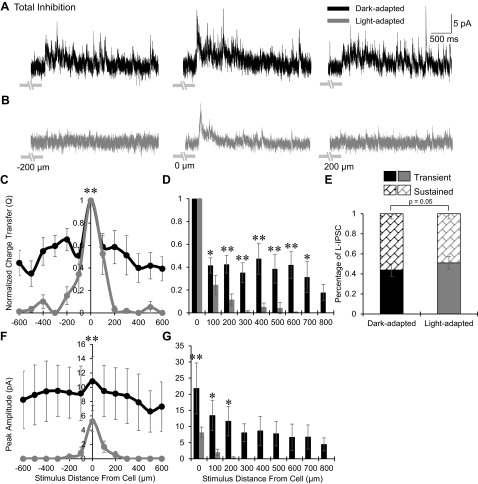
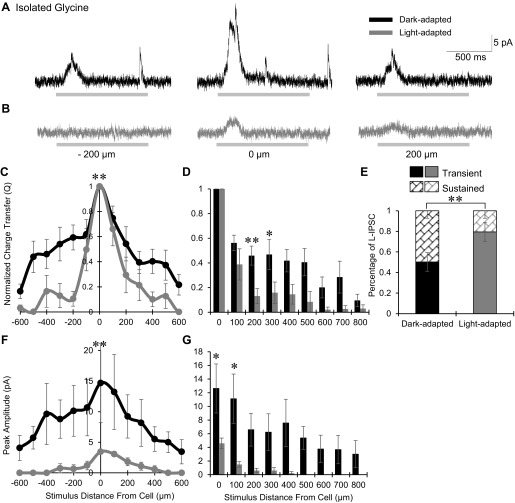
We predicted that the spatial distribution of inhibition to OFF bipolar cells would become wider with light adaptation due to the larger proportion of inputs from wide GABAergic amacrine cells (Mazade and Eggers 2013). Surprisingly, we found that the total inhibition became significantly narrower and smaller under light-adapted conditions (Fig. 2). OFF bipolar cell L-IPSCs in the dark were robust and sustained with inhibition activated at distances more than 200 μm away from the recorded cell (Fig. 2A). After light adaptation, most OFF bipolar cells did not receive any inhibition if stimuli were presented more than 200 μm away (Fig. 2B). The spatial distribution of Q values normalized to the center bar became significantly narrower with light adaptation (P < 0.01; Fig. 2C). To compare the normalized Q value between light conditions at each stimulus distance, data from experiments where a smaller range of spatial distances were tested were included and both sides of the spatial distribution curves were averaged to decrease response variation within the slice and obtain a more representative comparison (see methods). There was a significantly smaller proportion of inhibition in the light-adapted than in the dark-adapted case at many distances from the recorded cell (Fig. 2D). OFF bipolar cell center L-IPSCs also became significantly more transient, with a decrease in sustained response with light adaptation (P < 0.05; Fig. 2E). The L-IPSC peak amplitude spatial distribution also became significantly smaller and narrower in light-adapted conditions (P < 0.01; Fig. 2F) with no inhibition remaining from stimuli farther than 300 μm (Fig. 2G). The average peak amplitude for the center stimulus under dark adaptation was 21.8 ± 7.9 pA, which significantly decreased to 8.2 ± 1.6 pA after light adaptation (P < 0.01).
Fig. 2.
Light adaptation narrows the total spatial inhibitory input to OFF bipolar cells. A and B: example L-IPSCs recorded from an OFF type 3 bipolar cell in dark- and light-adapted conditions (CW = 67, CM = 0.971), black and gray traces, respectively, in response to a 1-s flash of a 25-μm bar of light, presented at −200, 0, and 200 μm away from the recorded cell. Light adaptation greatly reduced L-IPSCs 200 μm away from the OFF bipolar cell and decreased the center L-IPSC. Light stimulus is indicated by gray disconnected bars below L-IPSCs; OFF type 3 bipolar cells respond at the offset of light. C: spatial inhibition curves of Q, normalized to the center bar stimulus, in dark (n = 8)- and light (n = 5)-adapted conditions. The spatial inhibitory distribution became significantly narrower with light adaptation. D: L-IPSC Q, normalized to the center L-IPSC, compared between dark (0–300 μm, n = 17; 400–800 μm, n = 8)- and light (0–300 μm, n = 14; 400–800 μm, n = 5)-adapted conditions at each stimulus distance. The proportion of inhibition was significantly smaller after light adaptation starting at 100 μm away from the cell, after which there was little to no inhibition present. E: the average proportion of center L-IPSC response that is transient or sustained in dark- and light-adapted conditions (n = 14). L-IPSCs became significantly more transient with light adaptation. F: spatial inhibition curves of peak amplitude in the dark- and light-adapted conditions. The peak amplitude distribution was significantly narrower and smaller with light adaptation. G: L-IPSC peak amplitude compared between dark- and light-adapted conditions at each stimulus distance. The proportion of inhibition was significantly smaller under light adaptation at 0–200 μm away from the cell, after which there was no inhibitory input present. *P < 0.05; **P < 0.01.
Previous studies determined that although OFF bipolar cell subtypes receive input from distinct pathways (Mazade and Eggers 2013; Tsukamoto et al. 2001), all OFF bipolar cells had similar glycine/GABA inhibitory proportion changes with light adaptation (Mazade and Eggers 2013). Despite this, there may still be differences in spatial inhibitory input between the subtypes. To investigate this, we compared the spatial distribution of inhibition between OFF cone bipolar cell types 1, 2, and 4 (OFF1,2,4), previously shown to receive inhibition at the onset of light especially from the rod pathway, and OFF cone bipolar cell type 3 (OFF3), which receives inhibitory input only from cone activated pathways at the offset of light (Mazade and Eggers 2013). There was no significant difference between the OFF1,2,4 (n = 6) and OFF3 (n = 9) bipolar cell spatial inhibitory Q, normalized to the largest center L-IPSC (data not shown, P = 0.70), or normalized peak amplitude distribution of the L-IPSCs under dark adaptation (P = 0.37). Likewise, there was no difference between OFF1,2,4 (n = 5) and OFF3 (n = 9) bipolar cell normalized spatial Q (P = 0.13) or peak amplitude (P = 0.58) distributions in light-adapted conditions (cells where a smaller range of spatial positions were tested were also included in this comparison). However, as expected from previous work (Mazade and Eggers 2013), OFF1,2,4 bipolar cells had significantly greater L-IPSC peak amplitudes than OFF3 bipolar cells under dark adaptation (center OFF1,2,4 bipolar cell = 23.6 ± 8.7 pA, OFF3 bipolar cells = 10.1 ± 3.9 pA, P < 0.01) and light (center OFF1,2,4 bipolar cell = 12.8 ± 2.6 pA, OFF3 bipolar cells = 5.7 ± 1.1 pA, P = 0.058). Larger OFF1,2,4 L-IPSC peak amplitudes under dark adaptation are likely due in part to the large glycinergic AII amacrine cell input from the rod pathway (Mazade and Eggers 2013); however, both OFF bipolar cell groups had similar percent magnitude changes with increased luminance (∼50% decrease). Since all OFF bipolar cell subtypes showed similar spatial distributions in dark- and light-adapted conditions, results from all types were pooled for the remainder of this study, because the narrowing of spatial input is likely a general property of OFF bipolar cell inhibition. Taken together, these results suggest that circuitry connections are in place to provide widespread inhibition to OFF bipolar cells under dark adaptation, but changes with light adaptation act to shorten the spatial extent of inhibitory signaling regardless of specific circuitry differences between subtypes.
Isolated GABAergic spatial inhibitory input to OFF bipolar cells narrows with light adaptation.
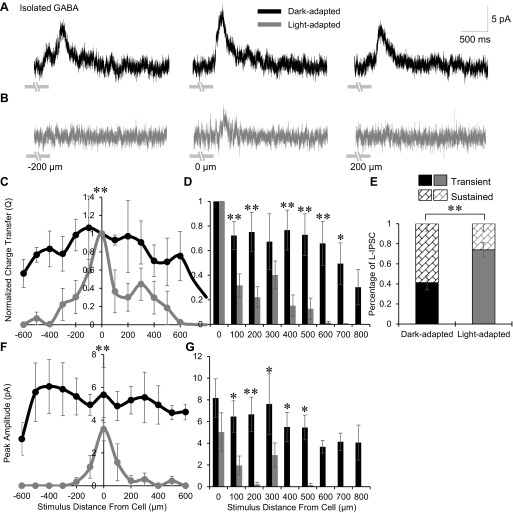
The narrowing and reduction of OFF bipolar cell spatial inhibition (Fig. 2) could be caused by changes in GABAergic inhibitory inputs, glycinergic inhibitory inputs, or both. To determine this, we first investigated how pharmacologically isolated GABAergic spatial input (in strychnine) to OFF bipolar cells changes with light adaptation. GABAergic amacrine cells are wide-field and have processes that can span hundreds of microns laterally across the retina potentially mediating very wide spatial signaling (Kolb 1997; Menger et al. 1998; Pourcho and Goebel 1985, 1983; Vaney 1990). As expected, dark-adapted isolated GABAergic input, consisting of both GABAAR- and GABACR-mediated inhibition, was significantly wider than total inhibition (from Fig. 2C, P < 0.01). Bar stimuli centered on the cell elicited large L-IPSCs that remained even when the stimulus was 200 μm away (Fig. 3A). However, after light adaptation, L-IPSCs became smaller in the center with decreasing responses farther from the cell (Fig. 3B). The spatial distribution of normalized Q values became significantly narrower after light adaptation (P < 0.01; Fig. 3C). At each stimulus distance from the cell, the proportion of GABAergic inhibition was smaller in light-adapted conditions than in dark-adapted with significant decreases beginning at 100 μm away (Fig. 3D). OFF bipolar cell center L-IPSCs also shifted to being significantly more transient, with a large decrease in sustained portion of the response with light adaptation (P < 0.01; Fig. 3E). The GABAergic peak amplitude distribution of L-IPSCs became smaller and narrower with light adaptation (P < 0.01; Fig. 3F). Although the average GABAergic peak amplitude at the center did not significantly decrease when light-adapted (dark, 8.1 ± 1.8 pA; light, 5.0 ± 1.8 pA; P = 0.22), the peak amplitudes for the majority of stimuli outside the center were significantly smaller (Fig. 3G). These results suggest that GABAergic inhibition contributes to the changes in the spatial extent, magnitude, and timing observed in the total L-IPSCs with light adaptation.
Fig. 3.
Light adaptation narrows the isolated GABAerigc spatial inhibitory input to OFF bipolar cells. A and B: example GABAergic L-IPSCs recorded from an OFF type 3 bipolar cell in dark- and light-adapted conditions (CW = 67, CM = 0.971), black and gray traces, respectively (1-s flash of a 25-μm bar of light, −200, 0, and 200 μm away from the recorded cell). Light adaptation greatly reduced L-IPSCs 200 μm away from the OFF bipolar cell and decreased the center L-IPSC. Light stimulus is indicated by gray disconnected bars under L-IPSCs, OFF type 3 bipolar cells respond at the offset of light. C: spatial inhibition curves of GABAergic Q, normalized to the center bar stimulus, in dark (n = 5)- and light (n = 4)-adapted conditions. The spatial inhibitory distribution became significantly narrower with light adaptation. D: GABAergic L-IPSC Q (normalized to the center L-IPSC) compared between dark (0–200 μm, n = 10; 300–800 μm, n = 4) and light (0–200 μm, n = 7; 300–800 μm, n = 4)-adapted conditions at each stimulus distance. The proportion of inhibition was significantly smaller at multiple locations with no inhibition present after 500 μm. E: the average proportion of center GABAergic L-IPSC response that is transient or sustained in dark- and light-adapted conditions (n = 7). L-IPSCs became significantly more transient with light adaptation. F: spatial inhibition curves of GABAergic peak amplitude in the dark- and light-adapted conditions. The peak amplitude distribution was significantly narrower and smaller with light adaptation. G: GABAergic L-IPSC peak amplitude compared between dark- and light- adapted conditions at each stimulus distance. The proportion of inhibition was significantly smaller at multiple locations with no inhibition present after 300 μm. *P < 0.05; **P < 0.01.
Isolated GABACR spatial inhibitory input to OFF bipolar cells is abolished with light adaptation.
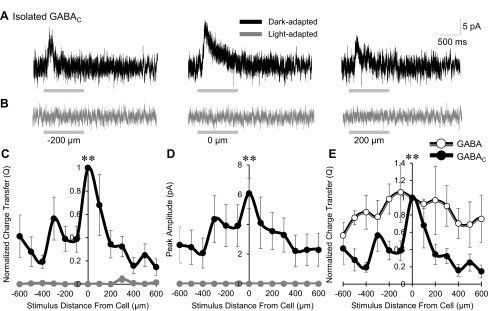
We found that light adaptation caused a significant narrowing of spatial GABAergic input to OFF bipolar cells. Under light-adapted conditions, this spatially reduced input presents an initial challenge; how could wide-field cells whose processes span hundreds of microns laterally across the retina, many of which use action potentials, change to only provide spatially close inhibition? However, previous work has shown that there are inhibitory connections between GABAergic cells that decrease inhibition onto downstream bipolar cells (Eggers et al. 2007). These amacrine cell-amacrine cell serial inhibitory connections, mediated by GABAARs, are activated when large light stimuli excite circuitry distant from the recorded bipolar cell (Eggers et al. 2007, 2013b). It is possible that under light-adapted conditions, stimuli distant from the target cell more strongly activate this serial inhibition, which would decrease the amount of inhibition that GABAergic amacrine cells are able to provide to OFF bipolar cells. To investigate this, we pharmacologically isolated GABACR-mediated inhibitory input (in SR and strychnine), thereby blocking GABAAR-mediated serial connections, and measured spatial input and peak amplitude of the L-IPSCs. Under dark-adapted conditions, OFF bipolar cells received robust GABACR-mediated inhibition at the center bar and smaller L-IPSCS 200 μm away from the recorded cell (Fig. 4A). With light adaptation, OFF bipolar cells received no GABACR-mediated inhibition at any stimulus distance (Fig. 4B). The normalized GABACR-mediated spatial inhibitory Q and peak amplitude under dark adaptation were moderately wide (Fig. 4, C and D), similar to total inhibition (from Fig. 2C, P = 0.08), with no inhibition remaining after light adaptation (P < 0.01). GABACR-mediated spatial input was significantly narrower than isolated GABAergic input (P < 0.01; Fig. 4E). The magnitudes of GABACR-mediated inhibition are likely partially inflated, as blocking inhibitory GABAAR-mediated inhibition on the amacrine cells themselves will increase their signal output. Given that GABACR-mediated inhibition was eliminated in the light-adapted retina, we could not test our hypothesis about serial connections. However, serial inhibitory input may still play a role in GABAergic narrowing.
Fig. 4.
Light adaptation abolishes GABACR input to OFF bipolar cells. A and B: example GABACR-mediated L-IPSCs recorded from an OFF type 4 bipolar cell in the dark- and light-adapted conditions (CW = 67, CM = 0.971), black and gray traces, respectively (1-s flash of a 25-μm bar of light, −200, 0, and 200 μm away from the recorded cell). Light adaptation abolished all GABACR-mediated input. Light stimulus is indicated by gray bars under L-IPSCs; OFF type 4 bipolar cells respond at the onset of light. C and D: spatial inhibition curves of GABACR-mediated Q, normalized to the center bar stimulus, and of peak amplitude in the dark- and light-adapted conditions. There was no GABACR-mediated inhibition after light adaptation (n = 6). E: dark-adapted spatial distribution curves of normalized GABACR-mediated (n = 6) and total GABAergic (n = 4) spatial input. GABACR-mediated spatial input was significantly narrower than total GABAergic input. **P < 0.01.
Isolated glycinergic spatial inhibitory input to OFF bipolar cells narrows with light adaptation.
Previous studies have shown that OFF bipolar cells receive proportionally larger glycinergic input under dark adaptation (Eggers and Lukasiewicz 2006b; Mazade and Eggers 2013). Since GABAergic spatial inhibition becomes narrower in light-adapted conditions, we wanted to determine whether glycinergic amacrine cells were also contributing to OFF bipolar cell spatial changes with light adaptation. Using the same stimulus parameters described above, pharmacologically isolated glycinergic spatial inhibitory input (in SR and TPMPA) was relatively wide under dark adaptation and was not significantly different than the total inhibitory spatial input (from Fig. 2C, P = 0.30). Center bar stimuli elicited robust L-IPSCs with smaller but still robust L-IPSCs at 200 μm away (Fig. 5A). However, light-adapted L-IPSCs were smaller in the center and absent in most cells at 200 μm away (Fig. 5B). The spatial distribution of normalized Q values became significantly more narrow in the light-adapted condition (P < 0.01; Fig. 5C), similar to total inhibition. There was a significantly smaller proportion of glycinergic inhibition in the light-adapted than in the dark-adapted condition, with little inhibition at large distances from the recorded cell (Fig. 5D). OFF bipolar cell center L-IPSCs became significantly more transient, with a large decrease in sustained portion of the response, with light adaptation (P < 0.01; Fig. 5E). The peak amplitude distribution of glycinergic input became significantly smaller and narrower in the light-adapted retina (P < 0.01; Fig. 5F). Both the average peak amplitude for the center bar (12.8 ± 3.8 pA in the dark, 4.6 ± 0.8 pA after light adaptation, P < 0.05) and at multiple stimulus distances decreased, with no light-adapted inhibition remaining from stimuli farther than 400 μm (Fig. 5G). These results suggest that narrowing of glycinergic spatial inhibition to OFF bipolar cells and reduction in peak amplitude at least in part contributes to the total light-adapted narrowing of spatial inhibition.
Fig. 5.
Light adaptation narrows the isolated glycinergic spatial inhibitory input to OFF bipolar cells. A and B: example glycinergic L-IPSCs recorded from an OFF type 1/2 bipolar cell in dark- and light-adapted conditions (CW = 67, CM = 0.971), black and gray traces, respectively, (1-s flash of a 25-μm bar of light, −200, 0, and 200 μm away from the recorded cell). Light adaptation significantly reduced L-IPSCs 200 μm away from the OFF bipolar cell and decreased the center L-IPSC. Light stimulus is indicated by gray bars under L-IPSCs; OFF type 1/2 bipolar cells respond at the onset of light. C: spatial inhibition curves of glycinergic Q, normalized to the center bar stimulus, in dark (n = 10)- and light (n = 4)-adapted conditions. The spatial inhibitory distribution became significantly narrower with light adaptation. D: glycinergic L-IPSC Q, normalized to the center L-IPSC, compared between dark (0–300 μm, n = 20; 400–800 μm, n = 10)- and light (0–300 μm, n = 9; 400–800 μm, n = 4)-adapted conditions at each stimulus distance. The proportion of inhibition was significantly smaller at multiple locations with little to no inhibition remaining after 500 μm away from the recorded cell. E: the average proportion of center glycinergic L-IPSC response that is transient or sustained in dark- and light-adapted conditions (n = 9). L-IPSCs became significantly more transient with light adaptation. F: spatial inhibition curves of glycinergic peak amplitude in the dark- and light-adapted conditions. The peak amplitude distribution was significantly narrower and smaller with light adaptation. G: glycinergic L-IPSC peak amplitude compared between dark- and light-adapted conditions at each stimulus distance. The proportion of inhibition was significantly smaller under light adaptation at multiple locations with no inhibition beginning at 500 μm away from the recorded cell. *P < 0.05; **P < 0.01.
GABAergic spatial input is wider than glycinergic spatial input in dark- but not light-adapted conditions.
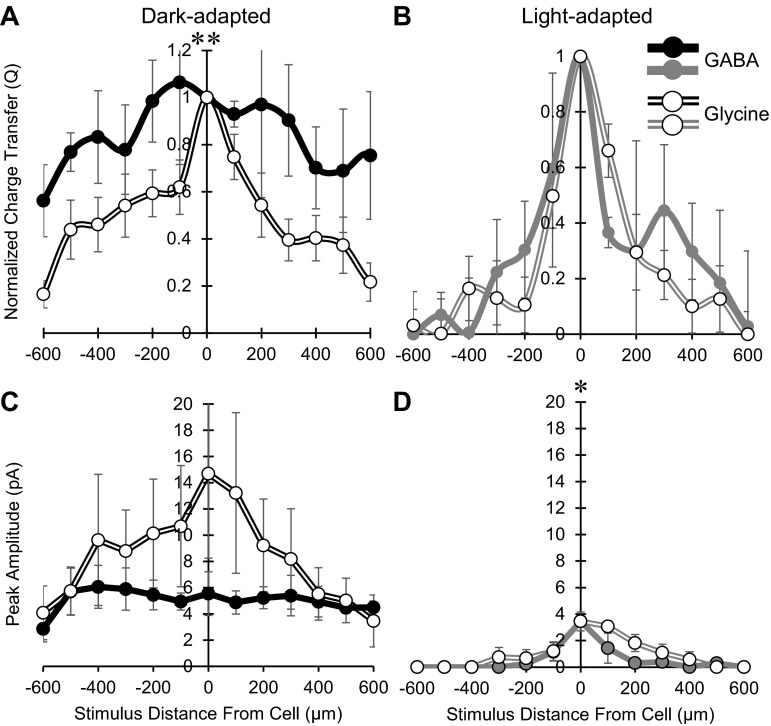
GABAergic spatial inputs to OFF bipolar cells were quite wide in dark-adapted conditions, and we found that glycinergic spatial input was also wide. This was surprising because the narrow morphology of glycinergic amacrine cells suggests that they would not send inhibitory signals over long lateral distances. Since these two neurotransmitters come from distinct sources, we compared the spatial inputs between glycinergic and GABAergic connections. In dark-adapted conditions, the spatial distribution of isolated glycinergic Q values (normalized to the center bar) was significantly narrower than that of isolated GABAergic responses (P < 0.01; Fig. 6A). With light adaptation, both isolated glycinergic and isolated GABAergic spatial inputs to OFF bipolar cells became significantly narrower and were not different from each other (P = 0.52; Fig. 6B). The isolated glycinergic peak amplitude distribution was on average larger than the isolated GABAergic response distribution under dark adaptation (P = 0.09), whereas the glycinergic peak amplitude distribution was slightly, but significantly larger than the GABAergic distribution after light adaptation (P < 0.05; Fig. 6, C and D). Isolated glycinergic responses had a significant decrease in magnitude at the center bar with light adaptation (∼64% decrease, P < 0.05; Fig. 5), whereas GABAergic input did not (Fig. 3), so the proportion of GABA to glycine input to OFF bipolar cells increased (shown in Fig. 6, C and D), similar to our previous findings (Mazade and Eggers 2013). This suggests the use of a full-field green stimulus in the previous study and the white bar stimulus in the current study are not differentially affecting the inhibitory inputs to OFF bipolar cells. This dark-adapted relationship was not unexpected, as wide-field GABAergic amacrine cells would mediate wider spatial signals than glycinergic amacrine cells. However, since glycinergic spatial input still extended hundreds of microns across the retina, there must be circuitry organization to lengthen the extent of their signaling. Despite the dark-adapted differences, both distinct inputs have similar spatial extents with light adaptation.
Fig. 6.
GABAergic spatial inhibition is wider but weaker that glycinergic input in dark- but not light-adapted conditions. A: spatial inhibition curves of Q, normalized to the center bar stimulus, in the dark-adapted retina of isolated GABAergic (n = 4, ●) and isolated glycinergic (n = 10, ○) bipolar cell L-IPSCs. GABAergic spatial input was significantly wider than glycinergic spatial input. B: spatial inhibition curves of Q, normalized to the center bar stimulus, in the light-adapted retina of isolated GABAergic (n = 4) and isolated glycinergic (n = 4) bipolar cell L-IPSCs. There was no difference between the two groups (P = 0.52). C: spatial inhibition curves of peak amplitude in the dark-adapted retina of isolated GABAergic and isolated glycinergic bipolar cell L-IPSCs. The glycinergic peak amplitude distribution was on average larger than the GABAergic peak response amplitudes, but not significant, likely due to large variation in peak magnitude (P = 0.09). D: spatial inhibition curves of peak amplitudes in the light-adapted retina of isolated GABAergic and isolated glycinergic bipolar cell L-IPSCs. The glycinergic distribution was slightly but significantly larger than the GABAergic distribution. *P < 0.05; **P < 0.01.
Light adaptation narrowed total spatial inhibition from dim light conditions.
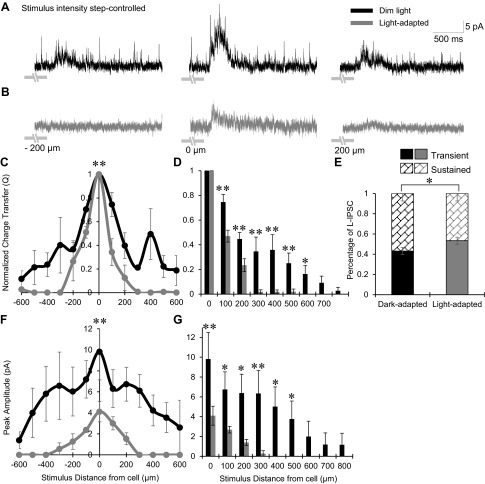
We have found that the total spatial inhibition, whether coming from either glycinergic or GABAergic sources, becomes significantly narrower and smaller in light-adapted conditions (Figs. 2–6). These large changes in inhibition at the bipolar cell likely affect their output onto downstream ganglion cells. We sought to model how the spatial and magnitude decreases in inhibitory input to OFF bipolar cells might affect the strength of their output to OFF ganglion cells. The experiments in Figs. 2–6 were conducted under true dark-adapted and in light-adapted conditions, in response to the same intensity bar stimulus (7.83 × 104 photons·μm−2·s−1) to get a large activation with small bar sizes. To construct a model of this synapse we first performed additional experiments using stimuli where the intensity step of the stimulus from the background was similar between dark- and light-adapted conditions, which may more accurately reflect the operation of the OFF bipolar cell pathway. To accomplish this, we mapped the total spatial inhibition to OFF bipolar cells under dark-adapted conditions by applying a dim background light (see methods), which was not bright enough to fully activate the rod pathway as in the light-adapted preparation, and matched the intensity step of the stimuli in dark- and light-adapted conditions so the Michelson contrast was controlled, similar to the method of Farrow et al. (2013). In dim light, bar stimuli produced similar L-IPSCs as in the previous experiments (Fig. 7A) and light adaptation reduced the response to stimuli 200 μm away from the recorded cell (Fig. 7B). The normalized spatial inhibitory Q distribution in dim light was significantly different (narrower) than the dark-adapted spatial distribution shown in Fig. 2 (P < 0.01), likely because of the slight adaptation to the dim background light, but also became significantly more narrow after light adaptation (n = 4, P < 0.01; Fig. 7, C and D). Center bar L-IPSCs became significantly more transient in the light-adapted condition (n = 4, P < 0.05; Fig. 7E), similar to results in Fig. 2. The peak amplitude distribution of L-IPSCs also became narrower with light adaptation (Fig. 7, F and G). The center bar L-IPSC peak amplitude was not different between the dim light and complete darkness experiments in dark- or light-adapted states (dark, P = 0.68; light, P = 0.56).
Fig. 7.
Light adaptation narrowed the total spatial inhibitory input to OFF bipolar cells under conditions with similar stimulus intensity steps. A and B: example L-IPSCs recorded from an OFF type 3 bipolar cell in dim and light intensity step-controlled conditions, black and gray traces, respectively (1-s flash of a 25-μm bar of light, −200, 0, and 200 μm away from the recorded cell). The dark-adapted (dim light) CM = 0.995 (CW = 483) and the light-adapted CM = 0.971 (CW = 67), where the step in stimulus intensity from the background was similar. Light adaptation greatly reduced L-IPSCs 200 μm away from the OFF bipolar cell and decreased the center L-IPSC. Light stimulus is indicated by gray disconnected bars under L-IPSCs; OFF type 3 bipolar cells respond at the offset of light. C: spatial inhibition curves of Q, normalized to the center bar stimulus, in dim- and light-adapted conditions (n = 4). The spatial inhibitory distribution became significantly narrower with light adaptation. D: L-IPSC Q, normalized to the center L-IPSC, compared between dim- and light-adapted conditions at each stimulus distance. The proportion of inhibition was significantly smaller under light adaptation from 100 to 600 μm away from the cell with little inhibition remaining after 200 μm. E: the average proportion of center L-IPSC response that is transient or sustained in dim and light intensity step-controlled conditions (n = 4). L-IPSCs became significantly more transient with light adaptation. F: spatial inhibition curves of peak amplitude in both light conditions. The peak amplitude distribution was significantly narrower and smaller with light adaptation. G: L-IPSC peak amplitude compared between dim- and light-adapted conditions at each stimulus distance. The proportion of inhibition was significantly smaller under light adaptation at 0–500 μm away from the cell with no inhibition present after 300 μm. *P < 0.05; **P < 0.01.
Light adaptation changes OFF bipolar cell Vrest and L-IPSCs without changing L-EPSCs.
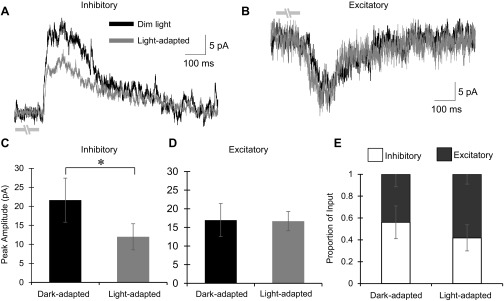
To determine if the changes in the spatial extent of inhibitory signaling to bipolar cells contribute to increased sensitivity of ganglion cells to small stimuli, we used the values and spatial changes from the experiments in Fig. 7 to create a model for how modulation of bipolar cell inhibition changes the strength of bipolar cell signal output. To create a more realistic model, we measured several different OFF bipolar cell response parameters under dark- and light-adapted conditions. First, we measured the amounts of inhibition and excitation in dim and light-adapted conditions, with the stimulus intensity step similar between the two, within the same cell. This is important in determining the output from OFF bipolar cells to OFF ganglion cells, which depends on the balance of these inputs. L-IPSCs and L-EPSCs were recorded from OFF bipolar cells in response to a 25-μm bar of light flashed over the center of the recorded cell, similar to the central bar of spatial mapping experiments. As expected from the spatial experiments, peak amplitude of the L-IPSCs was significantly decreased from 21.6 ± 5.8 pA under dim light conditions to 12.0 ± 3.4 pA in light-adapted conditions (n = 12, P < 0.05; Fig. 8, A and C). In contrast, peak amplitudes of the L-EPSCs were not significantly different between dim (16.95 ± 4.4 pA) and light-adapted (17.0 ± 2.6 pA, n = 8, P = 0.92) conditions (Fig. 8, B and D). With a large decrease in inhibition and no change in excitation, the balance of excitatory and inhibitory input to OFF bipolar cells shifted toward excitation (P = 0.07; Fig. 8E). Additionally, we measured the spatial extent of excitatory input to OFF bipolar cells under dim and light-adapted conditions (data not shown). This was performed by using a 25-μm bar of light and stimulating every 50 μm away from the cell for 150 μm in each direction, with 30 s between each stimulus. There was no difference between dim and light-adapted states (dark, n = 7; light, n = 3; spatial Q, P = 0.38; spatial peak amplitude, P = 0.30), and cells typically only had robust L-EPSCs in response to the 25-μm bar when it was directly over the cell with much smaller responses directly near the cell. All OFF bipolar cell subtypes were pooled for these experiments, as they were when measuring inhibitory inputs, since previous reports have shown that the different subtypes of OFF bipolar cells contact relatively similar numbers of cone photoreceptors (Wässle et al. 2009). Since this would give the subtypes similar spatial coverage, it is unlikely that the receptive field centers between subtypes would be dramatically different within the stimulus size parameters we used in this study.
Fig. 8.
Light adaptation decreases inhibition to OFF bipolar cells in response to a small light stimulus without changing excitatory input. A: example L-IPSC from an OFF type 3 bipolar cell (1-s flash of a 25-μm bar of light directly over the recorded cell). The L-IPSC decreased with light adaptation (dim, CW = 483 and CM = 0.995; light, CW = 67 and CM = 0.971). Light stimulus is indicated by gray disconnected bars under L-IPSCs; OFF type 3 bipolar cells respond at the offset of light. B: example L-EPSC from the same cell in A. There was no change in the excitatory response with light adaptation. C: peak amplitude of the L-IPSCs in dim- and light-adapted intensity step-controlled conditions (n = 12). There was a significant decrease in the magnitude of the response with light adaptation. D: peak amplitude of the L-EPSCs in the dim- and light-adapted intensity step-controlled conditions (n = 8). There was no change in the magnitude of the response. E: the proportion of excitatory and inhibitory input to OFF bipolar cells in dim- and light-adapted conditions. In dim light, OFF bipolar cells receive a large amount of inhibitory input (56.0 ± 15.0%). However, after light adaptation, OFF bipolar cells receive a larger proportion of excitatory input (58.2 ± 9.1%). *P < 0.05.
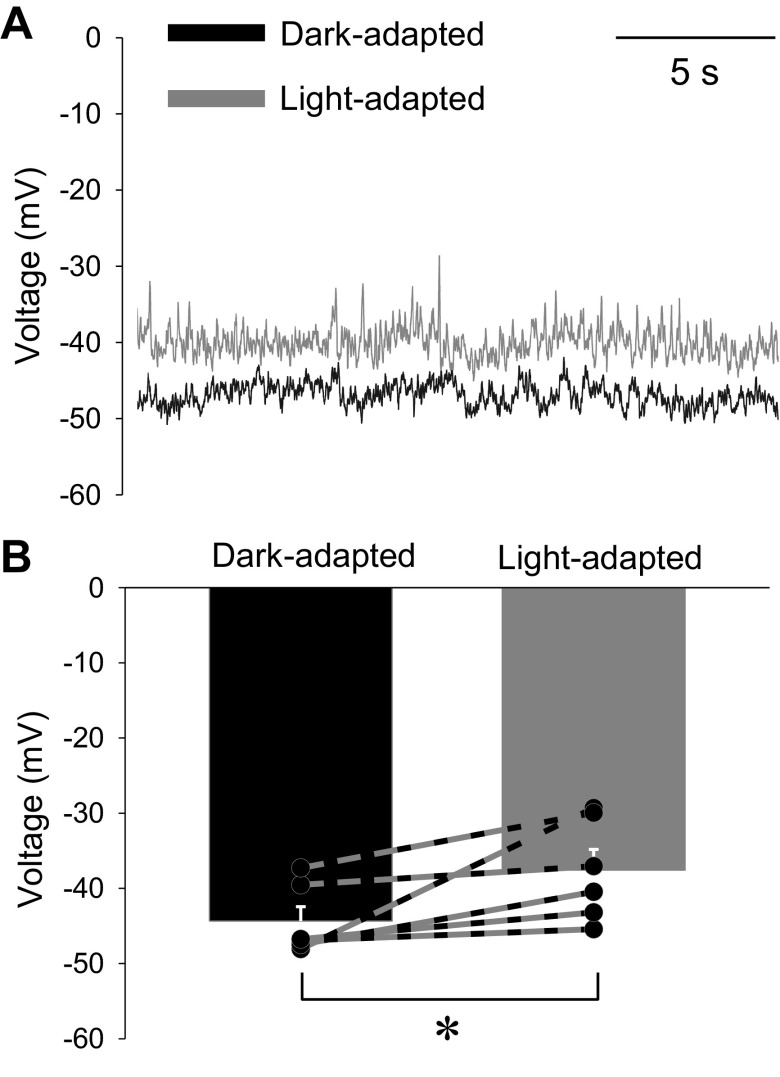
Next, we measured Vrest using current-clamp recordings of OFF bipolar cells in dark- and light-adapted conditions. Under dark adaptation, OFF bipolar cells have a relatively depolarized resting potential likely due to the large amount of constant glutamate release from the highly active cone photoreceptors. The dark-adapted OFF bipolar cell Vrest was on average −44.4 ± 1.9 mV, similar to that reported in a previous study (Arman and Sampath 2012) (Fig. 9, A and B). With light adaptation, there is less release from photoreceptors (Choi et al. 2005), suggesting that the OFF bipolar cells should become hyperpolarized relative to dark-adapted conditions. However, we found that in light-adapted conditions, the OFF bipolar cell Vrest became even more depolarized, averaging −37.6 ± 2.8 mV (total range from −30 to −45 mV; Fig. 9, A and B), which was significantly more depolarized than in dark-adapted conditions (P < 0.05). The more depolarized Vrest is likely due to a decrease in inhibitory inputs to the OFF bipolar cells as a result of light adaptation (Mazade and Eggers 2013), despite the reduction in glutamate release. Determining the resting state of OFF bipolar cells in the dark- and light-adapted conditions allows for the incorporation of OFF bipolar cell activation in the construction of the spatial model.
Fig. 9.
OFF bipolar cells become more depolarized under light-adapted conditions. A: example current-clamp recordings from OFF bipolar cells under dark- and the light-adapted conditions from an OFF type 1/2 bipolar cell. B: the mean and individual resting membrane potentials of OFF bipolar cells in the dark- and light-adapted conditions. The resting membrane voltage is significantly more depolarized after light adaptation, from −44.4 ± 1.9 to −37.6 ± 2.8 mV (n = 6). *P < 0.05.
Light adaptation increases the modeled signal output strength of OFF bipolar cells.
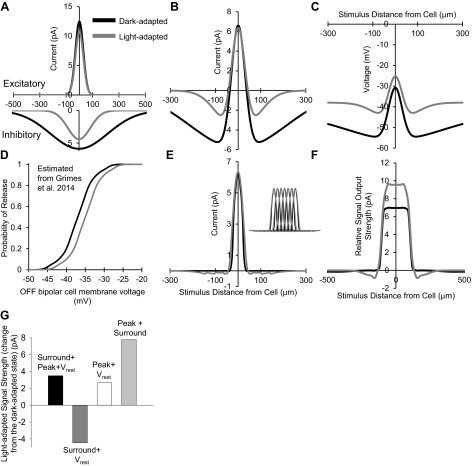
The experimental findings of this study show that under dark-adapted conditions, OFF bipolar cells had wide and strong spatial inhibition and thus large receptive field surrounds. Under light-adapted conditions however, there was no change in the excitatory center size, but the narrowing and reduction of spatial inhibition implied a smaller and weaker surround (Table 1). Additionally, the resting membrane potential became significantly more depolarized so the cells are in a state of higher activation. To predict how these changes would alter the output of OFF bipolar cells onto downstream ganglion cells, we used the data from Figs. 7–9 to create a model. The excitatory center and inhibitory surround standard deviations of Gaussian distributions fit to the data (Fig. 7) were used to construct prototypical Gaussian receptive field curves in dark- and light-adapted conditions (Table 1). These dark- and light-adapted curves were scaled to the conductance-scaled peak amplitude of the L-IPSCs and L-EPSCs (see methods, Eq. 1) in dark- and light-adapted conditions. There is little difference between the bipolar excitatory center distributions, whereas the inhibitory surround is much wider and larger in the dark-adapted condition than in the light-adapted state, reflecting the experimental data (Fig. 10A).
Table 1.
Spatial model parameters
| Model Parameter | Dark-Adapted (Dim Light) | Light-Adapted |
|---|---|---|
| Inhibitory fitted Gaussian distribution σ, μm | 213 | 83 |
| Excitatory fitted Gaussian distribution σ, μm | 30 | 30 |
| Average measured inhibitory peak amplitude (all cells), pA | 21.62 ± 5.79 | 11.99 ± 3.43 |
| Average measured excitatory peak amplitude (all cells), pA | 16.95 ± 4.44 | 16.67 ± 2.59 |
| Conductance-scaled inhibitory peak amplitude, pA | 5.87 | 4.20 |
| Conductance-scaled excitatory peak amplitude, pA | 12.47 | 11.05 |
Data are means (±SE where applicable).
Fig. 10.
Light-adapted narrowing and reduction of spatial inhibition increases the modeled strength of OFF bipolar cell output. A: OFF bipolar cell excitatory center (top) and inhibitory surround (bottom) Gaussian distributions based on conductance-adjusted scaled peak amplitudes. There was no significant change in excitatory center spatial distributions with light adaptation; however, the light-adapted inhibitory spatial distribution became smaller and narrower. B: difference of excitatory and inhibitory Gaussian distributions. Inhibition had less impact on the OFF bipolar cell center-surround distribution in light-adapted conditions than in dark-adapted conditions. C: calculated simulated potentials from the excitatory postsynaptic potentials caused by a current from the center-surround distributions. Under light-adapted conditions, the combination of excitatory and inhibitory current inputs depolarizes the OFF bipolar cell more at a given stimulus distance than under dark adaptation. D: OFF bipolar cell release probability based on values obtained from Fig. S4Ain Grimes et al. (2014) for rod bipolar cells. Dark-adapted release values were estimated from the rod bipolar cell release curve at a membrane voltage of ∼44 mV, and light-adapted release values were estimated from the rod bipolar cell release curve at a membrane voltage of ∼39 mV. E: threshold- and release-corrected center-surround distributions in the dark- and light-adapted conditions. Inset: 7 threshold/release-corrected bipolar cell center-surround current distributions constructed with the center peak of each cell 30 μm apart, summed to obtain total bipolar cell output. F: model of the total current output of 7 summed threshold/release-corrected OFF bipolar cell receptive fields (correlated to the input the OFF ganglion cell would receive). This bipolar cell signal strength represents the probability that the collective OFF bipolar cell group comprising the center receptive field of the OFF ganglion cell would cause release leading to a voltage change in the ganglion cell. Light adaptation increases the strength of the input to the OFF ganglion cell excitatory center to small stimuli. G: light-adapted signal output strength under several model manipulations plotted as the change from the dark-adapted state. Dark-adapted signal strength increased with light adaptation (black bar) when the surround, peak, and Vrest changes were included. When only bipolar cell peak L-IPSC magnitude changes were excluded, the signal strength decreased from dark-adapted conditions (dark gray bar). When only bipolar cell surround size changes were excluded, the signal strength increased from dark-adapted conditions, but less so than in normal conditions (white bar). Finally, when only Vrest changes were excluded, the signal strength increased past normal light-adapted changes (light gray bar). Both the surround size and peak changes are important for increasing the light-adapted signal strength, whereas the change in Vrest limits the extent of the increase.
The differences of the excitatory and inhibitory Gaussian distributions were then used to produce center-surround receptive field curves (Fig. 10B). Given that neurotransmitter release is required for a bipolar cell to signal to a downstream ganglion cell, linear transfer of bipolar cell currents to ganglion cells cannot be assumed. To estimate what signals would be sent to a downstream cell, we calculated the voltage change that each current level would cause at each point in the bipolar cell center-surround distribution using our average measured input resistance of 1.9 ± 0.2 GΩ for all OFF bipolar cells in control, dark-adapted conditions (see methods, Eq. 2). Although input resistance may decrease slightly in the light-adapted retina due to an increase in voltage-gated currents, we estimate this effect to be small, based on a previous paper that showed a depolarization to ∼38 mV (our measured light-adapted Vrest) from a −50-mV Vhold (close to our measured dark-adapted Vrest) in OFF bipolar cells caused a 20-pA Ca2+ current (Fig. 4D in Cui et al. 2012), which would lead to a calculated input resistance of 1.9 GΩ (Eq. 2). Since there was no significant change from dark-adapted conditions in this case, 1.9 GΩ was used for voltage calculations in each light state. The calculated voltage change was added to the OFF bipolar cell Vrest in the dark- and light-adapted conditions to obtain the simulated peak voltage a stimulus at each distance would elicit (Fig. 10C). For example, the peak current at the center stimulus in Fig. 10B would depolarize the cell to −30.8 mV under dark adaptation and to −25.3 mV under light adaptation.
The simulated real voltages were then used to determine whether the OFF bipolar cell at the given voltage would initiate glutamate release onto the OFF ganglion cell, using the activation voltage of bipolar cell Ca2+ channels (CaV) of −50 mV (Singer and Diamond 2006). Any voltage below −50 mV was assumed to cause no glutamate release, so a minimum threshold was applied to the currents in Fig. 10B so that any current that would cause a hyperpolarization of more than −50 mV was equal to 0. Since CaV channel activation by voltage occurs in a nonlinear manner, we used the release probability measured at the rod bipolar cell ribbon synapse (Fig. S4A in Grimes et al. 2014). This release data was the only data available from mouse bipolar cells, so the values were used with the assumption that release probability is similar between different bipolar cells as they all use ribbon synapses. Assuming no CaV channel inactivation, rod bipolar cell probability of release values were taken from the release curves that most closely matched the voltage of our OFF bipolar cells in each light state. The probability of release values at each OFF bipolar cell membrane voltage were estimated and reconstructed from Grimes et al. 2014 (Fig. 10D) and multiplied by the current values in our OFF bipolar cells. After these nonlinear components were added, the new threshold-corrected center-surround bipolar cell current distributions were constructed (Fig. 10E). Seven bipolar cells (OFF bipolar cell dendritic diameter ∼30 μm) were chosen as the number of bipolar cells that cover a large percentage of the excitatory center (dendrites, ∼300 μm) of an OFFα ganglion cell in a single two-dimensional plane in the mouse (Ghosh et al. 2004; Thyagarajan et al. 2010). Seven threshold-corrected bipolar cell center-surround current distributions were constructed with the center peak of each cell being 30 μm apart, the width of the bipolar cell dendrites (Fig. 10E, inset). These center-surround receptive fields were summed to create the total amount of relative bipolar cell current that would cause glutamate release onto the center of the downstream ganglion cell (Fig. 10F). These spatial curves represent the signal strength, or the probability that the ganglion cell will receive stronger output from the bipolar cell at its excitatory center, of all bipolar cell outputs to the ganglion cell.
Given that the peak of this simulated ganglion cell response was higher in light-adapted conditions, the reduced spatial inhibition, with no change in excitation, allows for the bipolar cells to provide stronger output to spatially small and distinct light stimuli. To quantify this change, currents from stimuli extending 90 μm in either direction from the center (from Fig. 10F) were averaged in dark- and light-adapted conditions. These peak values were subtracted from the maximum magnitude of the surround to get a total increase in signal strength from the OFF bipolar cells. Dark-adapted relative signal strength was 6.97 pA, which increased to 10.46 pA with light adaptation (Fig. 10G, black bar). To determine if the reduction in L-IPSC peak amplitude, reduction in inhibitory spatial size, or the change in Vrest is important to increase signal strength, the model was tested by excluding each of the three parameters. When the model was manipulated so that the L-IPSC magnitude (peak amplitude) was unchanged from dark-adapted conditions (dark gray bar), the light-adapted signal strength decreased from dark-adapted conditions by 4.45 pA, suggesting that peak amplitude changes are playing a large role in the light-adapted increase in signal strength. Likewise, when the model was manipulated so that the surround size of inhibition was unchanged from dark-adapted conditions (white bar), the light-adapted signal strength increased by 2.65 pA from the dark-adapted condition but was smaller than the normal light-adapted signal strength by almost 1 pA. The surround size reduction is also important for determining the signal strength, but less so than the magnitude of the surround. Last, when the Vrest change with light adaptation was excluded from the model, the light-adapted signal strength increased by 7.76 pA from the dark-adapted strength. This increase was 4.23 pA higher than the normal light-adapted increase and suggests that the depolarization of the resting membrane potential is inhibitory and works to limit the strength of the bipolar cell signal. The interactions between the surround size and inhibitory magnitude are both players in determining the signal strength to downstream ganglion cells, whereas changes in the resting membrane potential likely add another layer of modulation to the system.
DISCUSSION
A key aspect of retinal adaptation is increasing visual acuity, or increasing the signal strength of small light stimuli, when there is abundant background illumination. This allows for a new signaling threshold for the comparison of distinct light stimuli to detect more subtle differences in contrast in the visual scene. In this study we show that spatial inhibitory input to the inner retina becomes narrower, smaller, and more transient with increasing background light and that these changes could affect bipolar cell output strength. Our results suggest that the increase in ganglion cell sensitivity to small light stimuli with light adaptation may be in part due to changes in inhibition in the inner retina.
Dark-adapted spatial inhibition relies on large inhibitory surrounds.
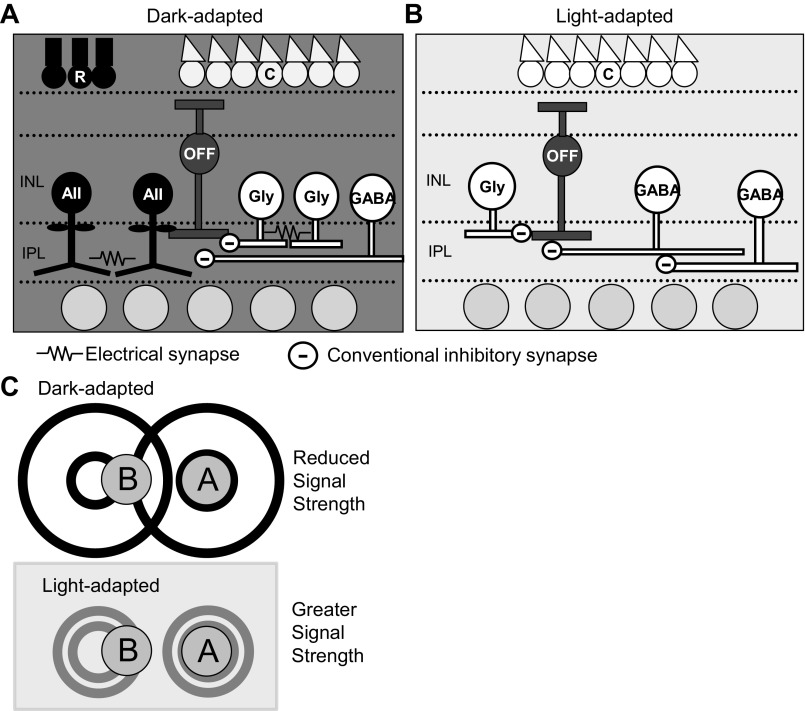
Our data show that OFF bipolar cell inhibition in dark-adapted conditions consists of relatively large, wide, and sustained responses to small light stimuli. This inhibitory input consists of both widely distributed glycinergic and GABAergic inhibition (Fig. 11A). The wide GABAergic inhibition under dark adaptation is expected because of the long processes of GABAergic amacrine cells, likely mediated by GABAARs since isolated GABACR input resulted in smaller responses than isolated GABAergic input at far distances from the cell (Fig. 4E). Many GABAergic amacrine cells mediate wide inhibitory surrounds (Dacey et al. 2000; Ichinose and Lukasiewicz 2005; Sinclair et al. 2004; Zhang and Wu 2009) and can be coupled and/or fire action potentials to further extend their spatial signaling (Cook and Werblin 1994; Heflin and Cook 2007; Volgyi et al. 2009). Isolated GABAergic input was wider than total input (Figs. 2 and 3), likely because glycinergic inputs from narrow glycinergic amacrine cells (Pourcho and Goebel 1985) dominate OFF bipolar cell L-IPSCs (Mazade and Eggers 2013). However, it is also possible that blocking potential glycinergic inputs to GABAergic amacrine cells could be widening the spatial extent of GABAergic inhibition, as has been shown previously when blocking GABAergic connections between amacrine cells (Eggers and Lukasiewicz 2010).
Fig. 11.
Spatial circuitry models in dark- and light-adapted conditions. A: in dark-adapted conditions, OFF bipolar cells receive wide spatial inhibition from wide-field GABAergic amacrine cells. Coupling between both AII and other glycinergic amacrine cells likely contribute to increasing the wide spatial spread of glycinergic signals to OFF bipolar cells. B: in light-adapted conditions, OFF bipolar cells receive spatially narrow glycinergic input, likely due to uncoupling of AII and other glycinergic amacrine cells. Light stimuli distant from the bipolar cell likely active serial inhibitory connections between GABAergic amacrine cells, which would shorten spatial GABAergic signals to OFF bipolar cells. C: functional schematic of changing bipolar cell center-surround sizes. In dark-adapted conditions, OFF bipolar cells receive wide and strong inhibition, so their inhibitory surrounds are large. If 2 small spots of light are presented to the retina, spot A stimulates excitatory output from the center of one OFF bipolar cell, whereas spot B stimulates surround inhibitory connections to that same cell. Overall output is reduced in this instance due to the addition of inhibitory input. In light-adapted conditions, OFF bipolar cells receive narrow and weaker inhibition, so their inhibitory surrounds are small. In these conditions, spot B does not stimulate the inhibitory surround, and there is no reduction in excitatory bipolar cell output from spot A. Thus the strength of the bipolar cell output in the light-adapted case is stronger.
Although dark-adapted spatial inputs from glycinergic amacrine cells were significantly narrower than inputs from GABAergic amacrine cells (Fig. 6), they were not as different as expected by amacrine cell morphology. Additionally, despite differences in amplitude and time course, the spatial distribution of glycinergic inhibition was not different from total inhibition, suggesting that glycinergic spatial input was not altered by blocking GABAARs mediating serial inhibition. Previous work has shown that the GABAAR antagonist SR-95531 can have antagonistic effects on glycine currents (Wang and Slaughter 2005). However, this is not likely affecting the overall changes we report with light adaptation because only the response to low, nonsynaptic, concentrations of glycine were affected, and we would expect there to be further decreases from control conditions when blocking GABAARs, which we did not observe.
Glycinergic inputs come from two distinct sources: rod pathway-mediated AII amacrine cell synaptic contacts and cone-activated amacrine cell inputs from both ON and OFF pathways (Mazade and Eggers 2013), all of which should be narrow-field amacrine cells (Pourcho and Goebel 1985). How then could dark-adapted glycinergic inhibition to OFF bipolar cells be activated from stimuli presented hundreds of micrometers away? One way this could be achieved is through electrical gap junction coupling between amacrine cells that could increase their excitatory spread. AII amacrine cells, which give input to most OFF bipolar cells (OFF1,2,4) (Mazade and Eggers 2013; Tsukamoto et al. 2001) are highly coupled in the very dim light conditions shown from labeling studies (Bloomfield et al. 1997; Xin and Bloomfield 1999a, 1997), which mediated signal spread through the network (Veruki et al. 2010). This glycinergic signal spread has been shown to extend far beyond the dimensions of the dendritic arbor further suggesting the use of gap junctions in narrow-field amacrine cell networks (Chen et al. 2011). It is possible that other types of glycinergic amacrine cells could also be coupled to extend non-AII glycinergic signaling to OFF3 bipolar cells, such as the A3 amacrine cell in the macaque (Klump et al. 2009). Labeling studies show numerous gap junctions located within the IPL in distinct bands, many of which likely connect amacrine cells (Marc et al. 1988). The wide spatial extent of glycinergic input suggests that the retina may attempt to match the GABAergic and glycinergic circuits so that all OFF bipolar cells receive uniform spatial signaling, creating defined and wide inhibitory receptive-field surrounds under dark adaptation.
Light adaptation shortens the extent of inhibitory spatial signaling.
Whereas spatial inhibitory input to OFF bipolar cells is relatively wide under dark adaptation, increasing the background luminance causes a dramatic shift (Fig. 2). This is also true when the background is increased to a dim but still dark-adapted level (Fig. 7). The dim background light caused an intermediate narrowing of the OFF bipolar cell spatial surround between true dark and light-adapted conditions, suggesting a gradual transition to reduced spatial inhibitory input as background intensity increases. Both the glycinergic and GABAergic inhibitory input to OFF bipolar cells became significantly narrower, smaller, and more transient after light adaptation (Figs. 3, 5, and 6). Previous reports have shown that AII amacrine cell gap junctions are uncoupled (Bloomfield et al. 1997; Xin and Bloomfield 1999a; 1997) and activation is decreased (Dacheux and Raviola 1986; Mazade and Eggers 2013) after light adaptation, which would narrow and reduce glycinergic inhibition. Uncoupling of other glycinergic amacrine cells may contribute as well (Fig. 11B). Additionally, horizontal cells in the outer retina, which also contribute to surround receptive fields of bipolar cells (Zhang and Wu 2009), become uncoupled with light adaptation (Xin and Bloomfield 1999b), although amacrine cells likely play a larger role in light adaptation of spatial signaling (Dedek et al. 2008). Uncoupling is a likely explanation, given that glycinergic inhibition in light-adapted slices is similar to what we would expect given the narrow morphology of glycinergic amacrine cells. Last, there may be serial inhibitory connections between glycinergic amacrine cells that become activated in light-adapted conditions and which also act to narrow OFF bipolar cell inhibition (Eggers and Lukasiewicz 2010; Eggers et al. 2013b).
The total GABAergic spatial inhibition also decreased in light-adapted conditions and consisted solely of GABAAR-mediated input, as suggested by the results of Fig. 4, where GABACR-mediated input was shown to be abolished. The modulation of light-adapted OFF bipolar surrounds by solely GABAARs can also explain the more transient time course of GABAergic input with light adaptation in Fig. 3E, since slower GABACR input reported in previous work (Eggers and Lukasiewicz 2011) is no longer prominent. Since GABACR-mediated currents are abolished, GABAAR-and GABACR-mediated input to OFF bipolar cells may come from different amacrine cells (Moore-Dotson et al. 2015), or light adaptation could preferentially decrease GABACR-mediated input. The narrowing of spatial inhibition was initially unexpected, because GABAergic amacrine cells have wide-field morphologies whose processes can span hundreds of micrometers laterally across the retina (Kolb 1997). However, previous reports have shown that there are GABAAR-mediated inhibitory serial connections between amacrine cells that narrow spatial inhibition to bipolar cells (Eggers and Lukasiewicz 2006a, 2010; Roska et al. 1998; Zhang et al. 1997) and may be more active after light adaptation (Eggers et al. 2013b) (Fig. 11B). Isolating serial inhibitory inputs to the OFF bipolar cells by bath-applying receptor antagonists may affect many pathways indirectly, so serial inhibition between amacrine cells is not likely the sole mechanism for the inhibitory spatial changes we measured.
GABAergic blockade has been shown to affect ganglion cell receptive field surrounds under dark adaptation (Flores-Herr et al. 2001), which, combined with our results, further suggests that changing GABAergic input to retinal neurons is likely a main mechanism for changing light-adapted retinal receptive fields. Additionally, both glycinergic and GABAergic L-IPSCs became significantly more transient, with a loss of the sustained component. This suggests that inhibition provides a sharper, more finely tuned input mediated by cone circuitry for faster modulation of stimuli in a changing environment. Overall, both glycinergic and GABAergic spatial input to OFF bipolar cells decreased with light adaptation, suggesting the retina works to match the spatial signaling of distinct pathways.
Narrower and smaller spatial inhibition to the inner retina increases signal output strength.
We found that several OFF bipolar cell signaling and cellular properties change with light adaptation, in addition to spatial changes. OFF bipolar cells are more depolarized at rest in dark-adapted conditions than rod bipolar cells (Vrest = −50.3 mV) and ON cone bipolar cells (Vrest = −66.5 mV) (Oesch and Diamond 2011; Saszik and Devries 2012). This is likely due to the high glutamate release from the cone photoreceptors and the ionotropic glutamate receptors on OFF bipolar cells, although the addition of TEA-Cl in our recording pipette or the use of perforated patch recordings, as used by Oesch and Diamond (2011), may also partially account for differences in Vrest between the bipolar cell subtypes. Although the glutamate release rate is reduced after light adaptation, the hyperpolarization of the AII amacrine network (Grimes et al. 2014) and subsequent reduction in glycine release onto OFF bipolar cells (Mazade and Eggers 2013) likely contribute to the decrease in inhibition we observed. Our model suggests that this depolarization may work to limit the extent of the increase in the bipolar cell signal strength that can be achieved with light adaptation, acting like an auditor to more finely control the changes in bipolar cell release.
These results are different from the recent finding that ON bipolar cells hyperpolarize with light (Grimes et al. 2014). The decrease in AII amacrine cell excitatory input and AII amacrine cell hyperpolarization with light (Grimes et al. 2014; Mazade and Eggers 2013; White et al. 2015) would have distinct effects on the ON and OFF pathways. ON bipolar cells hyperpolarize due to electrical coupling with AII amacrine cells (Grimes et al. 2014). In contrast, AII amacrine cell hyperpolarization would decrease inhibitory input to OFF bipolar cells (Mazade and Eggers 2013), which would lead to depolarization of the OFF bipolar cell resting membrane potential. Grimes et al. (2014) showed that this ON bipolar cell hyperpolarization increased rectification and visual acuity. Our model also implies an increase in visual acuity with light adaptation due to changes in the magnitude and spatial sensitivity of inhibition, but our results suggest that the depolarization of the OFF bipolar cell with light may be limiting this increase, similar to the effects of adaptation on the ON bipolar cell. It is likely that the retina utilizes two different strategies for increasing the strength of the visual signal of ON and OFF pathways to increase visual acuity.
Our data and model suggest that in dark-adapted conditions, OFF bipolar cells have large inhibitory surrounds so that if two small light stimuli are presented on the retina, one light (spot A) stimulates the center of the bipolar cell receptive field while the other light (spot B) is close enough to stimulate the wide surround of the same bipolar cell (Fig. 11C). As a result, strong inhibition would be stimulated, decreasing bipolar cell excitatory output. In light-adapted conditions, the inhibitory surrounds of the OFF bipolar cells are reduced so that if two spatially close light stimuli are presented, spot B no longer stimulates the inhibitory surround. Thus excitatory bipolar cell output would increase, and the downstream ganglion cell would see a stronger input. The reduction in the magnitude of the spatial surround of the bipolar cell is necessary to obtain the maximum output strength with light adaptation. Our results add support to growing evidence (Dedek et al. 2008; Protti et al. 2014) that inner retinal inhibition is important for modulating ganglion cell spatial sensitivity to determine visual acuity.
In summary, we show that the spatial inhibition to OFF bipolar cells changes from widely distributed in dark-adapted conditions to a narrowly distributed in light-adapted conditions due to the narrowing spatial extent of both glycinergic and GABAergic amacrine cell input. These data predict that the output of OFF bipolar cells onto downstream ganglion cells is stronger with light adaptation, thereby fine-tuning the spatial sensitivity of ganglion cells to smaller light stimuli. Bipolar cell inhibition may be one important mechanism for controlling the gain of ganglion cell center strength under different light conditions to help determine visual acuity and resolution.
GRANTS
This project was funded by National Institutes of Health (NIH) Grant EY018131 (to E. D. Eggers), University of Arizona NIH Graduate Training in Systems and Integrative Physiology Grant 5T32GM008400 (to R. E. Mazade), and the ARCS Foundation (to R. E. Mazade).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.E.M. and E.D.E. conception and design of research; R.E.M. performed experiments; R.E.M. analyzed data; R.E.M. and E.D.E. interpreted results of experiments; R.E.M. prepared figures; R.E.M. drafted manuscript; R.E.M. and E.D.E. edited and revised manuscript; R.E.M. and E.D.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Peter Lukasiewicz and Richard Levine for helpful comments on this manuscript, Dr. Johnnie Moore-Dotson and Mike Flood for helpful comments on the project, and Adam Bernstein for technical assistance.
Present address of R. E. Mazade: Dept. of Biological and Vision Sciences, Alonso Laboratory, State Univ. of New York College of Optometry, 33 West 42nd St., New York, NY 10036.
REFERENCES
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27: 513–523, 2000. [DOI] [PubMed] [Google Scholar]
- Arman AC, Sampath AP. Dark-adapted response threshold of OFF ganglion cells is not set by OFF bipolar cells in the mouse retina. J Neurophysiol 107: 2649–2659, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol 137: 338–354, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis Neurosci 14: 565–576, 1997. [DOI] [PubMed] [Google Scholar]
- Buldyrev I, Taylor WR. Inhibitory mechanisms that generate centre and surround properties in ON and OFF brisk-sustained ganglion cells in the rabbit retina. J Physiol 591: 303–325, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hsueh HA, Werblin FS. Amacrine-to-amacrine cell inhibition: spatiotemporal properties of GABA and glycine pathways. Vis Neurosci 28: 193–204, 2011. [DOI] [PubMed] [Google Scholar]
- Choi S, Borghuis BG, Rea R, Levitan ES, Sterling P, Kramer RH. Encoding light intensity by the cone photoreceptor synapse. Neuron 48: 555–562, 2005. [DOI] [PubMed] [Google Scholar]
- Chun MH, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol 332: 421–432, 1993. [DOI] [PubMed] [Google Scholar]
- Cook PB, Werblin FS. Spike initiation and propagation in wide field transient amacrine cells of the salamander retina. J Neurosci 14: 3852–3861, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Ivanova E, Qi L, Pan ZH. Expression of Cav3.2 T-type Ca2+ channels in a subpopulation of retinal type-3 cone bipolar cells. Neuroscience 224: 63–69, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey D, Packer OS, Diller L, Brainard D, Peterson B, Lee B. Center surround receptive field structure of cone bipolar cells in primate retina. Vision Res 40: 1801–1811, 2000. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci 6: 331–345, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron 36: 703–712, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Pandarinath C, Alam NM, Wellershaus K, Schubert T, Willecke K, Prusky GT, Weiler R, Nirenberg S. Ganglion cell adaptability: does the coupling of horizontal cells play a role? PLoS One 3: e1714, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CJ, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol 79: 2171–2180, 1998. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the Mammalian retina. J Neurosci 26: 3959–3970, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Lankheet MJ, Rieke F. Light adaptation in cone vision involves switching between receptor and post-receptor sites. Nature 449: 603–606, 2007. [DOI] [PubMed] [Google Scholar]
- Eggers ED, Klein JS, Moore-Dotson JM. Slow changes in Ca2+ cause prolonged release from GABAergic retinal amacrine cells. J Neurophysiol 110: 709–719, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol 103: 25–37, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci 28: 95–108, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Mazade RE, Klein JS. Inhibition to retinal rod bipolar cells is regulated by light levels. J Neurophysiol 110: 153–161, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582: 569–582, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wässle H. Different contributions of GABAA and GABAC receptors to rod and cone bipolar cells in a rat retinal slice preparation. J Neurophysiol 79: 1384–1395, 1998. [DOI] [PubMed] [Google Scholar]
- Farrow K, Teixeira M, Szikra T, Viney TJ, Balint K, Yonehara K, Roska B. Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron 78: 325–338, 2013. [DOI] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, Wässle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci 21: 4852–4863, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004. [DOI] [PubMed] [Google Scholar]
- Green DG, Dowling JE, Siegel IM, Ripps H. Retinal mechanisms of visual adaptation in the skate. J Gen Physiol 65: 483–502, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DG, Powers MK. Mechanisms of light adaptation in rat retina. Vision Res 22: 209–216, 1982. [DOI] [PubMed] [Google Scholar]
- Grimes WN, Schwartz GW, Rieke F. The synaptic and circuit mechanisms underlying a change in spatial encoding in the retina. Neuron 82: 460–473, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci 25: 5438–5445, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflin SJ, Cook PB. Narrow and wide field amacrine cells fire action potentials in response to depolarization and light stimulation. Vis Neurosci 24: 197–206, 2007. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. Inner and outer retinal pathways both contribute to surround inhibition of salamander ganglion cells. J Physiol 565: 517–535, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Muller U, Wässle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci 23: 350–364, 2006. [DOI] [PubMed] [Google Scholar]
- Klump KE, Zhang AJ, Wu SM, Marshak DW. Parvalbumin-immunoreactive amacrine cells of macaque retina. Vis Neurosci 26: 287–296, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. Amacrine cells of the mammalian retina: neurocircuitry and functional roles. Eye 11: 904–923, 1997. [DOI] [PubMed] [Google Scholar]
- Marc RE, Liu WL, Muller JF. Gap junctions in the inner plexiform layer of the goldfish retina. Vision Res 28: 9–24, 1988. [PubMed] [Google Scholar]
- Mazade RE, Eggers ED. Light adaptation alters the source of inhibition to the mouse retinal OFF pathway. J Neurophysiol 110: 2113–2128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci 4: 2920–2938, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger N, Pow DV, Wässle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol 401: 34–46, 1998. [DOI] [PubMed] [Google Scholar]
- Merwine DK, Amthor FR, Grzywacz NM. Interaction between center and surround in rabbit retinal ganglion cells. J Neurophysiol 73: 1547–1567, 1995. [DOI] [PubMed] [Google Scholar]
- Molnar A, Hsueh HA, Roska B, Werblin FS. Crossover inhibition in the retina: circuitry that compensates for nonlinear rectifying synaptic transmission. J Comput Neurosci 27: 569–590, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Dotson JM, Klein JS, Mazade RE, Eggers ED. Different types of retinal inhibition have distinct neurotransmitter release properties. J Neurophysiol 113: 2078–2090, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka KI, Chan RY, Yasui S. Adaptation in catfish retina. J Neurophysiol 42: 441–454, 1979. [DOI] [PubMed] [Google Scholar]
- Nobles RD, Zhang C, Muller U, Betz H, McCall MA. Selective glycine receptor alpha2 subunit control of crossover inhibition between the on and off retinal pathways. J Neurosci 32: 3321–3332, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch NW, Diamond JS. Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci 14: 1555–1561, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw PS, Chung SC, Muto A, Roeser T, Staub W, Finger-Baier KC, Korenbrot JI, Baier H. Retinal network adaptation to bright light requires tyrosinase. Nat Neurosci 7: 1329–1336, 2004. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Paul DL, Wu SM. Rod, M-cone and M/S-cone inputs to hyperpolarizing bipolar cells in the mouse retina. J Physiol 590: 845–854, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol 233: 473–480, 1985. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol 219: 25–35, 1983. [DOI] [PubMed] [Google Scholar]
- Protti DA, Di Marco S, Huang JY, Vonhoff CR, Nguyen V, Solomon SG. Inner retinal inhibition shapes the receptive field of retinal ganglion cells in primate. J Physiol 592: 49–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Nemeth E, Werblin FS. Response to change is facilitated by a three-neuron disinhibitory pathway in the tiger salamander retina. J Neurosci 18: 3451–3459, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TL, Werblin FS. Retinal synaptic pathways underlying the response of the rabbit local edge detector. J Neurophysiol 103: 2757–2769, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron 50: 923–935, 2006. [DOI] [PubMed] [Google Scholar]
- Saszik S, Devries SH. A mammalian retinal bipolar cell uses both graded changes in membrane voltage and all-or-nothing Na+ spikes to encode light. J Neurosci 32: 297–307, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM, Enroth-Cugell C. Visual adaptation and retinal gain controls. Prog Retin Res 3: 263–346, 1984. [Google Scholar]
- Sinclair JR, Jacobs AL, Nirenberg S. Selective ablation of a class of amacrine cells alters spatial processing in the retina. J Neurosci 24: 1459–1467, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol 95: 3191–3198, 2006. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol 295: 449–466, 1990. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol 325: 152–168, 1992. [DOI] [PubMed] [Google Scholar]
- Tamura T, Nakatani K, Yau KW. Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol 98: 95–130, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Tachibana M. Independent control of reciprocal and lateral inhibition at the axon terminal of retinal bipolar cells. J Physiol 591: 3833–3851, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan S, van Wyk M, Lehmann K, Lowel S, Feng G, Wässle H. Visual function in mice with photoreceptor degeneration and transgenic expression of channelrhodopsin 2 in ganglion cells. J Neurosci 30: 8745–8758, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Li W, Mills SL, Massey SC. Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional. J Comp Neurol 437: 408–422, 2001. [DOI] [PubMed] [Google Scholar]
- Troy JB, Bohnsack DL, Diller LC. Spatial properties of the cat X-cell receptive field as a function of mean light level. Vis Neurosci 16: 1089–1104, 1999. [DOI] [PubMed] [Google Scholar]
- Troy JB, Enroth-Cugell C. X and Y ganglion cells inform the cat's brain about contrast in the retinal image. Exp Brain Res 93: 383–390, 1993. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci 21: 8616–8623, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Prog Retin Res 9: 49–100, 1990. [Google Scholar]
- Veruki ML, Oltedal L, Hartveit E. Electrical coupling and passive membrane properties of AII amacrine cells. J Neurophysiol 103: 1456–1466, 2010. [DOI] [PubMed] [Google Scholar]
- Vigh J, Vickers E, von Gersdorff H. Light-evoked lateral GABAergic inhibition at single bipolar cell synaptic terminals is driven by distinct retinal microcircuits. J Neurosci 31: 15884–15893, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol 512: 664–687, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Kefalov VJ. An alternative pathway mediates the mouse and human cone visual cycle. Curr Biol 19: 1665–1669, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Slaughter MM. Effects of GABA receptor antagonists on retinal glycine receptors and on homomeric glycine receptor alpha subunits. J Neurophysiol 93: 3120–3126, 2005. [DOI] [PubMed] [Google Scholar]
- Wang YV, Weick M, Demb JB. Spectral and temporal sensitivity of cone-mediated responses in mouse retinal ganglion cells. J Neurosci 31: 7670–7681, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci 29: 106–117, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci 27: 1–8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ, Cheruvu SC, Sarris M, Liyanage SS, Lumbers E, Chui J, Wakefield D, McCluskey PJ. Expression of classical components of the renin-angiotensin system in the human eye. J Renin Angiotensin Aldosterone Syst 16: 59–66, 2015. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH, Fain GL. Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J Neurosci 28: 2064–2074, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Yang XL. Modulation of synaptic gain by light. Proc Natl Acad Sci USA 89: 11755–11758, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci 16: 653–665, 1999a. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Dark- and light-induced changes in coupling between horizontal cells in mammalian retina. J Comp Neurol 405: 75–87, 1999b. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Comp Neurol 383: 512–528, 1997. [DOI] [PubMed] [Google Scholar]
- Zhang AJ, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci 29: 789–797, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chang-Sub J, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci 14: 553–563, 1997. [DOI] [PubMed] [Google Scholar]