Abstract
The role of primary motor cortex (M1) in the control of hand movements is still unclear. Functional magnetic resonance imaging (fMRI) studies of unimanual performance reported a relationship between level of precision of a motor task and additional ipsilateral M1 (iM1) activation. In the present study, we determined whether the demand on accuracy of a movement influences the magnitude of the inhibitory effect between primary motor cortices (IHI). We used transcranial magnetic stimulation (TMS) to measure active IHI (aIHI) of the iM1 on the contralateral M1 (cM1) in the premovement period of a left-hand motor task. Ten healthy participants manipulated a joystick to point to targets of two different sizes. For aIHI, the conditioning stimulus (CS) was applied to iM1, and the test stimulus (TS) to cM1, with an interstimulus interval of 10 ms. The amount of the inhibitory effect of the CS on the motor-evoked potential (MEP) of the subsequent TS was expressed as percentage of the mean MEP amplitude evoked by the single TS. Across different time points of aIHI measurements in the premovement period, there was a significant effect for target size on aIHI. Preparing to point to small targets was associated with weaker aIHI compared with pointing to large targets. The present findings suggest that, during the premovement period, aIHI from iM1 on cM1 is modulated by the demand on accuracy of the motor task. This is consistent with task fMRI findings showing bilateral M1 activation during high-precision movements but only unilateral M1 activity during low-precision movements.
Keywords: primary motor cortex, transcranial magnetic stimulation, interhemispheric inhibition, motor control
the role of the primary motor cortex (M1) ipsilateral to the performing hand (iM1) in the control of voluntary hand movements is still unclear. In functional magnetic resonance imaging (fMRI) studies of unilateral hand motor performance, strictly contralateral M1 (cM1) activation is demonstrated by some investigators (Butefisch et al. 2005; Catalan et al. 1998), while additional activation of iM1 is observed by others (Hummel et al. 2003; Lotze et al. 2006; Seidler et al. 2004; Winstein et al. 1997). More recent reports of a relationship between the level of precision or complexity of a motor task and this additional iM1 activation (Buetefisch et al. 2014a; Hummel et al. 2003; Seidler et al. 2004; Verstynen et al. 2005) may explain some of the inconsistencies in the earlier reports, as they suggest that iM1 activity changes as a function of difficulty of a motor task (Buetefisch et al. 2014a). While M1 has ipsilateral corticospinal projection, nonhuman and human primate data indicate that it is less likely that iM1 exerts its control through this pathway (Dancause et al. 2015).
In addition to the ipsilateral network, the motor areas of the two hemispheres are interconnected to each other, mainly through the corpus callosum. Although nonhuman primate data demonstrate that the majority of M1 callosal connections are with supplementary motor areas and are most prominent for the trunk and face representations, interhemispheric modulations of M1 neurons in the forelimb area can occur in the rostral portion of M1 (Dancause et al. 2015). This is consistent with the findings in humans showing that interhemispheric inhibition (IHI) between the two M1s can be demonstrated by means of transcranial magnetic stimulation (TMS). In this protocol, a conditioning stimulus (CS) is applied to one M1, which inhibits the size of the motor-evoked potential (MEP) produced by a subsequent test stimulus (TS) applied to the opposite M1 (Ferbert et al. 1992; Hanajima et al. 2001). It has been suggested that the CS activates excitatory transcallosal fibers, which project to local inhibitory GABAergic neurons in cM1 (Chen 2004; Daskalakis et al. 2002; Somogyi et al. 1998). The magnitude of the inhibitory effect between motor cortices changes as subjects prepare to move (Duque et al. 2007; Murase et al. 2004; Talelli et al. 2008a). Specifically, this so-called “active IHI” (aIHI) from iM1 on cM1 (in reference to the performing hand) is reduced when measured immediately before the onset of a movement (Duque et al. 2007; Murase et al. 2004). This indicates that changes in aIHI between M1s are involved in the control of unimanual skilled hand movements. This notion is further supported by the finding of abnormally reduced time-dependent modulation of aIHI in patients with motor stroke-related impaired hand performance (Murase et al. 2004). While there are reports of a relationship between the level of precision or complexity of a motor task and additional iM1 activation in fMRI studies (Buetefisch et al. 2014a; Hummel et al. 2003; Seidler et al. 2004; Verstynen et al. 2005), the relationship between changes in aIHI during the preparation of a movement (in the premovement period) and the demand on complexity or accuracy of a motor task has not been studied systematically in humans or animals.
The main goal of the present study was to investigate whether the demand on accuracy of a motor task had an effect on aIHI from iM1 on cM1 (in reference to the performing hand) during preparation of the motor task. We used a pointing task that allowed parametric variation of the level of difficulty by changing the size of the target (small and large, see Fig. 2) (Buetefisch et al. 2014a). We hypothesized that aIHI from iM1 on cM1 is reduced when subjects are pointing to a small target compared with pointing to the large target. This hypothesis was formulated on the aforementioned findings of an association between reduced aIHI from iM1 on cM1 and superior performance in a simple finger abduction task (Murase et al. 2004) and the task fMRI findings of bilateral M1 activation in more demanding hand motor task (Buetefisch et al. 2014a). As neurophysiology depends on age (Talelli et al. 2008a, 2008b; Ward and Frackowiak 2003), and the results of the present study may have important implications for studies on motor recovery after stroke, a middle-aged population (≥55 yr), more prone to stroke, was studied.
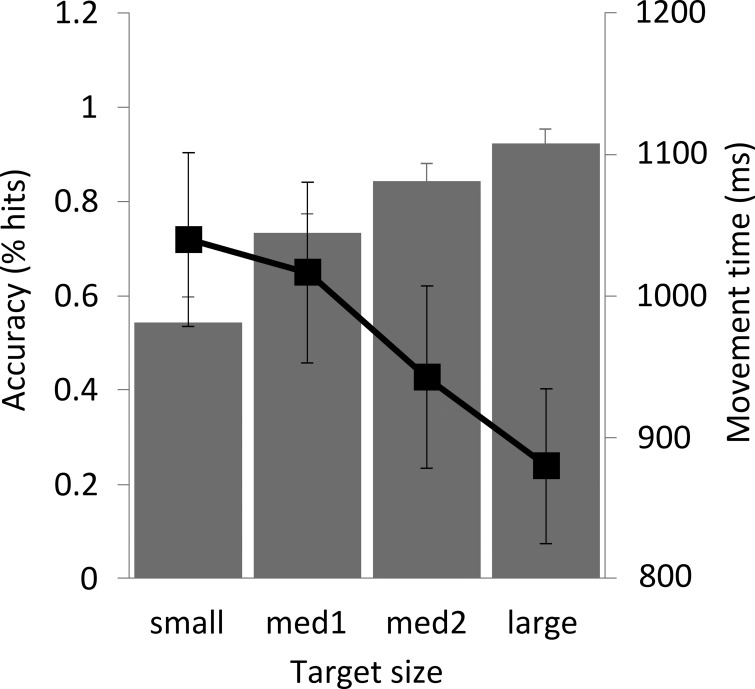
Fig. 2.
Subjects' performance on the motor task (n = 10). Pointing task accuracy is expressed as the mean percentage of hits for each target size. For the movement time, the mean time (±SE) from the go-signal to the button push is given. Note that pointing to the small target resulted in less accuracy and longer movement times compared with the large target. med1 and med2, Medium 1 and 2, respectively.
METHODS AND MATERIALS
The experiments were approved by the Institutional Review Board at Emory University, and conducted according to the Declaration of Helsinki. All subjects gave written, informed consent. Subjects were blinded to the stated hypothesis of the experiments. Investigators that performed the neurophysiological data analysis were blinded to the subject's performance.
Subjects
Ten healthy right-handed participants (mean age ± SE = 62.10 ± 1.86 yr, range 56–73 yr, 7 women, Table 1) fulfilled the following inclusion criteria and participated in the present study: age ≥55 yr, no neurological or psychiatric disorder, normal neurological examination, no intake of drugs with known effects on the central nervous system, no contraindication for TMS or MRI, normal brain as evaluated by MRI, normal cognitive function as determined by the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, see Table 1) (Randolph et al. 1998), ability to elicit a MEP > 0.2 mV at maximum stimulator output (MSO) < 80% (Daskalakis et al. 2002; Sanger et al. 2001) as determined in an inclusion experiment (see below for details), ability to perform the motor task at a minimum level of accuracy of 50% hits for the largest target (see below) as determined in a training session, and ability to give informed consent. Handedness was determined by the Edinburgh inventory for handedness (Oldfield 1971).
Table 1.
Subject demographics and neuropsychological and TMS measures
| CS to iM1 (%MSO) |
TS to cM1 (%MSO) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant No. | Age, yr | Sex | RBANS | rMT | rIHI intensity | aIHI intensity | rMT | rIHI intensity | aIHI intensity |
| 1 | 63 | F | 90 | 57 | 70 | 75 | 49 | 55 | 65 |
| 2 | 70 | M | 99 | 38 | 65 | 60 | 46 | 65 | 65 |
| 3 | 56 | F | 109 | 59 | 80 | 70 | 62 | 70 | 70 |
| 4 | 57 | M | 123 | 59 | 67 | 71 | 69 | 85 | 65 |
| 5 | 59 | F | 109 | 46 | 65 | 50 | 45 | 70 | 65 |
| 6 | 73 | F | 82 | 50 | 70 | 55 | 41 | 65 | 50 |
| 7 | 61 | F | 114 | 46 | 60 | 70 | 42 | 57 | 57 |
| 8 | 66 | M | 90 | 58 | 65 | 70 | 47 | 65 | 65 |
| 9 | 56 | F | 112 | 74 | 90 | 90 | 75 | 90 | 85 |
| 10 | 60 | F | 114 | 53 | 75 | 75 | 41 | 75 | 75 |
| Mean ± SE | 62.1 ± 1.86 | 104.2 ± 4.18 | 54.0 ± 3.12 | 70.7 ± 2.80 | 68.6 ± 3.58 | 51.70 ± 3.91 | 69.7 ± 3.52 | 66.2 ± 2.98 | |
Values are means ± SE; n = 10 subjects. The resting motor threshold (rMT) and applied intensities for test stimulus (TS) and conditioning stimulus (CS) used in the measurements of rIHI and aIHI are expressed in percentage of maximum stimulator output (%MSO). F, female; M, male; iM1, ipsilateral M1; cM1, contralateral M1; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status (Randolph et al. 1998).
Experimental Overview
After participants consented to the study and passed the initial screening for inclusion [medical assessment, TMS and MRI safety questionnaires, RBANS (see below for details) and handedness], they were tested in the inclusion experiment to determine their response to TMS and in the training session to determine their ability to perform the motor task. Structural MRIs were acquired for participants successfully meeting the TMS and motor task thresholds. Subjects who met all aspects of the inclusion criteria (see above) were then tested in the three main experiments. The experiments were conducted in a randomized order on separate occasions with at least 24 h between each experiment. In these three experiments, measures related to motor performance, corticospinal excitability, resting IHI (rIHI), and aIHI were obtained.
Neuropsychological Testing
The RBANS (Randolph et al. 1998) was administered to all subjects to establish normality of cognitive function (see inclusion criteria). The test is composed of 5 subtests and a total score, each with a mean of 100 and a standard deviation of 15. We determined that any score below 2 standard deviations from the mean on the total score would suggest abnormal cognitive function and would exclude a subject.
MRI of the Brain
Each subject completed a high-resolution 1-mm isotropic whole-head MPRAGE on a 3 Tesla Siemens Trio whole-body scanner (Siemens Medical Solutions, Malvern, PA) using a quadrature transmit-receive head coil. The scan was acquired in the sagittal plane with 176 slices (dimensions = 256 × 256 × 176, repetition time = 2,300 ms, echo time = 3.02 ms and flip angle = 8°). The MRI of the brain was reviewed by a board certified neurologist to establish anatomic normality of the brain. The MRI of the brain was then reconstructed in Brainsight (Brainsight, Rogue Research, Montreal, Canada) and served as each subject's reference for the coil position across experiments and within each experiment.
Experiment 1: Motor Performance
The motor task was designed as a pointing task that allowed parametric variation of the level of difficulty (easy and difficult) through changes in target size (Buetefisch et al. 2014a). According to the speed-accuracy tradeoff described in Fitts's law, decreasing the target size increases the level of difficulty and results in longer movement times (Fitts 1954). When subjects are forced to complete the task in a short, predefined time, accuracy is expected to decrease for smaller target sizes. To separate aspects of motor planning from aspects of the execution of the movement, we modeled our experiment after the instructed delay and reaction time paradigm used in neurophysiological studies of nonhuman primate M1 and premotor cortex (Kalaska 2009). A cue was given at a fixed time interval (500 ms) prior to the target presentation (Fig. 1). At the time of the cue, target size was known by the subjects, but information about the location of the target was delayed until the target presentation, which acted as a go signal (Fig. 1). The subjects sat comfortably in a dental chair ∼165 cm in front of a computer screen (29 × 51 cm). They were asked to manipulate a joystick between the thumb and the middle finger of their left hand to move a small joystick position cursor into target squares of different sizes and locations (shown for one target size and position in Fig. 1). The subjects' index finger rested on a push button on the top of the joystick. The joystick was attached with Velcro to a bed table which rested on the participant's lap and was positioned in such a way that the subject was able to rest the wrist on the base of the joystick comfortably at a 30–45° angle in reference to the ulnar aspect of the forearm. The manipulation of the joystick in response to the visual stimuli required wrist extension/flexion movements of ±5°, in addition to the finger movements. The arm was supported by pads so the participant did not have to use any muscles to actively support the arm. The nonoperating arm rested on this table. Stimulus presentation was controlled by Presentation software (www.neurobs.com).
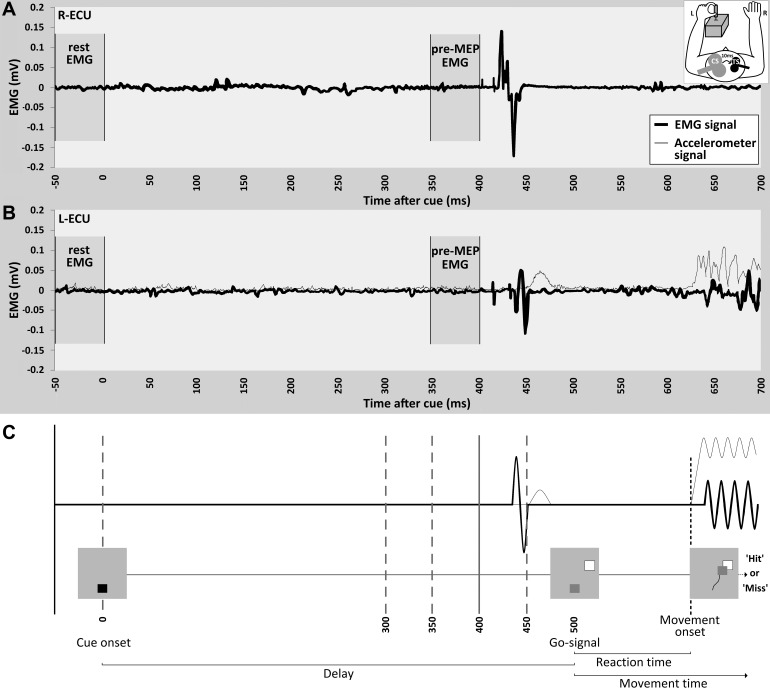
Fig. 1.
aIHI measurements. The EMG (black trace) and acceleration (gray trace) data for a single representative trial of an aIHI measurement are displayed for the right ECU (A) and left ECU (B). The acceleration signal derived from the accelerometer mounted on the joystick is superimposed on the EMG data of the left ECU (B). In this recording, the CS was given at 400 ms, and the TS at 410 ms. Note that the MEP evoked by the CS (A) precedes the MEP evoked by the TS (B) by 10 ms. C: schematic overview of the experimental setup. Different time points of aIHI measurements are indicated by vertical lines (0, 300, 350, 400, 450 ms). A visual cue was presented at the onset of the trial (0 ms). Following a delay of 500 ms, the target was presented which served as the “go-signal.” Participants were given a maximum of 2 s to move the cursor square to the target square. Visual feedback about the performance was given to the subject by displaying a “hit” or “miss” on the screen. The time interval preceding the cue was defined as rest-EMG (−50 to 0 ms), the time period of 50 ms prior to the CS was defined as pre-MEP EMG. Reaction time for this sample trial is 120 ms.
All trials began with the display of a visual cue, a small red square located in the middle of the screen (Fig. 1). After 500 ms, one of four differently sized target squares appeared in one of four possible positions, and the cue was replaced by the small green cursor square that indicated the joystick position (one target size and position pairing is shown in Fig. 1). Because the joystick returned passively to a neutral position after each movement and the neutral position corresponded to the middle of the screen, the cursor square was located in the middle of the screen at the beginning of each trial. The target squares were of small (5.3 × 5.3 mm), medium 1 (9.3 × 9.3 mm), medium 2 (13.2 × 13.2 mm), or large (17.2 × 17.2 mm) size and were presented at four possible locations (300°, 330°, 30° and 60°). Target presentations were blocked by size (7 targets per size block), but target location was random. Subjects were instructed to move the cursor square as quickly as possible to the presented target square. In this way, the appearance of the target square served as a go-signal (500 ms after the cue). Participants were given a maximum of 2 s to move the cursor to the target before the target disappeared. Participants were asked to push a button on top of the joystick once they arrived at the target. The button response was used for an estimation of participants' movement times. At the end of each trial, participants received feedback about their performance, with the word “hit” indicating a successful trial and “miss” indicating an unsuccessful trial. Between trials, a blank screen was presented for a variable amount of time to prevent anticipation of the visual cue and the beginning of the next trial (maximum intertrial time 3.5 s). A 12-s rest period (indicated by a “rest” display) occurred after every 28 trials. Subjects completed three blocks of trials at each target size (for a total of 21 trials for each target size and 84 total trials). Correct movements were defined as movements completed in the allocated time of 2 s that placed the cursor in the target square (defined as overlap of the target square and cursor square of an area of ≥25% of the cursor area, including the center of the cursor). For each trial, the movement time and the information about a “hit” or “miss” was extracted and stored by the Presentation software (www.neurobs.com) for offline analysis.
Prior to the main experiment, participants were trained on this motor task according to a standardized program to familiarize the subject with the motor task and to ensure that the stated inclusion criteria could be met. Subjects performed a maximum of three training runs of three blocks each (21 trials for every target size/run; 252 trials total). Baseline performance was determined at the end of the first training run. In subsequent runs, training continued until a minimal performance level of 50% hits for the largest target was achieved. For subjects who were already performing at this level or better at baseline, training continued until performance improved to >50% hits on the next smallest target. As it was possible to achieve these goals in less than three training runs, not all participants had the same amount of training.
Experiment 2: Corticospinal Excitability and Resting IHI Measurements
Subjects were comfortably seated in a dental chair surrounded by a frame that carried a coil holder to assist with the application of TMS to the brain (Brainsight, Rogue Research, Montreal, Canada). Electromyographic (EMG) activity (bandpass 1 Hz to 1 kHz) was recorded from the extensor carpi ulnaris muscle (ECU) with surface electrodes (11-mm diameter) in a belly-tendon montage. LabVIEW (National Instruments) was used for data acquisition. The raw EMG was sampled and digitized at a frequency of 5 kHz and stored for offline analysis. TMS was applied using Magstim 2002 stimulators connected via a Bistim module (Magstim). For determination of resting motor threshold (RMT) a 7-cm wing diameter figure-of-eight coil was used. An additional 5-cm wing diameter figure-of-eight coil was used for rIHI and aIHI measurements. In these experiments, the TS was applied through the small 5-cm wing diameter figure-of-eight coil, while the CS was applied through the larger 7-cm wing diameter figure-of-eight coil (Fig. 1). The use of the smaller coil permits the positioning of two coils on the participant's head for reliable stimulation of the two motor cortices at the optimal stimulation sites (hot spots). In addition, it allows for more focal stimulation and, therefore, higher spatial resolution of the observed effects. In the hot spot for the ECU, the MT was determined to the nearest 1% of MSO. The RMT was defined as the minimum stimulus intensity needed to evoke MEPs of >50 μV in 50% of the trials (Rossini et al. 1994). The coil was placed tangentially to the scalp in a 45° angle away from the midline (Werhahn et al. 1994).
For measurement of corticospinal excitability, stimulus response curves (SRC) were obtained for both M1s (Capaday et al. 1999; Chen et al. 1998a; Ridding and Rothwell 1997). MEPs were elicited by single TMS pulses applied to the ECU hot spot of either M1 at increasing stimulation intensities. The lowest intensity was set below the subject's RMT rounded down to the nearest 5% of MSO (Butefisch et al. 2008). Subsequent stimuli were given at increments of 5% up to 80% of MSO. At each stimulus intensity, 10 stimuli were given at an ISI of 6 s. Responses were set to zero for intensities below measurable MEP up to a level of 35% MSO.
For rIHI measurements, the subjects were at rest. In all subjects, rIHI was determined by the magnitude of the inhibitory effect of a CS applied to the ECU hot spot of the left M1 (corresponding to the iM1 in reference to the performing hand) on the MEP amplitude evoked by a subsequent TS applied to the ECU hot spot of the right M1 (corresponding to the cM1 in reference to the performing hand, Fig. 1). CS and TS were applied at an ISI of 10 ms. Paired stimuli were interleaved with 10 single TS. The intensity of CS and TS was adjusted to evoke MEP amplitudes of ∼1 mV (Table 1) (Boroojerdi et al. 1996; Chen 2004; Ferbert et al. 1992).
Experiment 3: Active IHI Measurements
aIHI from iM1 on cM1 (in reference to the performing hand) was measured in the premovement period of a motor task performed with the subject's left hand (Fig. 1). Subjects performed the same pointing task as described in experiment 1. CS and TS were applied as described for rIHI measurements. The premovement period was defined as time interval between the visual cue and the go signal (Fig. 1C). To ensure a sufficient number of trials at each combination of measurement time and target size, only the smallest and largest target sizes were tested, as we expected that the difference in the demand on accuracy was greatest for these two target sizes. Subjects executed 60 pointing movements with each target size (120 trials total).
Specifically, the aIHI measurements were collected at the appearance of the cue (0 ms), and at 300, 350, 400 or 450 ms after the cue (Fig. 1). These time points were selected to provide measures of aIHI before movement preparation (0 ms) and during movement preparation (300–450 ms). Pilot data suggested that movement onset occurred at ∼600 ms after the cue presentation (Fig. 1C). Onset of the movement was determined by increases in acceleration of the joystick as measured by an accelerometer mounted on the joystick (Fig. 1C) (Duque et al. 2007; Murase et al. 2004). The measurement of acceleration for determining the movement onset was necessary as the silent period that is observed after application of the TMS may mask the actual movement-related increase in EMG activity. Six paired and six single TS were given in a randomized order for each combination of time point and target size.
Data Analysis
EMG analysis.
MEP data were analyzed offline using LabVIEW software (National Instruments). For the analysis of rIHI, recordings were visually inspected at scale of −0.1 to +0.1 mV and excluded from further analysis when pre-MEP EMG activity was increased compared with resting state (Buetefisch et al. 2011, 2014b). For the purpose of comparison of the aIHI and rIHI measurements, the EMG background of all trials included in the analysis of rIHI was quantified. EMG pre-MEP was quantified as described for aIHI.
Because muscle activity prior to an MEP influences its amplitude (Devanne et al. 1997; Hess et al. 1986, 1987; Rosler et al. 2002), we quantified the background EMG to rule out the possibility that increases in EMG activity that are seen close to the onset of the movement (Chen et al. 1998b) accounted for the time or task-dependent changes seen in aIHI. Specifically, we defined pre-MEP EMG activity as the mean EMG activity in a 50-ms time window preceding the TMS pulse (pre-MEP EMG, Fig. 1B) and resting EMG as the mean EMG activity in a 50-ms time window preceding the cue (rest EMG, Fig. 1B). For each trial, we quantified the pre-MEP EMG and rest EMG. The comparison between pre-MEP EMG and rest EMG allowed objective identification of the trials with increased EMG background activation. Pre-MEP EMG was determined to be acceptable if it did not exceed the mean rest EMG + 3 SD. For rIHI and aIHI measurements, MEP amplitudes were expressed as a percentage of the mean test MEP amplitude evoked by TS.
Motor performance analysis.
As suprathreshold TMS may induce muscle twitches and can compromise the subjects' performance during the aIHI experiments, only data from experiment 1 were used for further analysis of motor performance. Accuracy was defined by the number of hits divided by the total number of trials per target size (% hits). Movement time (ms) was defined as the time from the target presentation (go signal) until the button push (Fig. 1C).
The accelerometer data were visualized and analyzed offline using LabVIEW software (National Instruments). In each trial, two increases in the acceleration signal were identifiable. The first increase displayed a tight temporal relationship to the MEP and was related to the TMS-evoked muscle twitch. This was followed by a second steep increase that was very distinct from baseline and was related to the onset of the voluntary movement. Movement onset was determined visually at the point when acceleration increased over the baseline (Fig. 1B). Reaction time was defined as the time between the target presentation and the second increase in acceleration (Fig. 1).
Statistical Methods
Data were analyzed using JMP Visualization Software (version 10, Cary, NC). The effect of target size on subjects' motor performance (experiment 1) was analyzed in two separate repeated-measures ANOVAs with SIZE (small, medium 1, medium 2, large) as an independent repeated measure and accuracy or movement time as the dependent measure.
For analysis of SRCs (experiment 2), the MEP amplitudes were plotted as a function of stimulus intensity and described using a 3-parameter sigmoid function (Boltzmann equation):
where S represents the stimulation intensity of the TMS expressed in %MSO, MEPMAX represents the maximum MEP amplitude, S50 represents the stimulation intensity (in %MSO) needed to evoke 50% of the maximum MEP amplitude, and K represents the slope parameter. This Boltzmann equation has been used to fit the averaged data points with the Levenberg-Marquardt least mean squares algorithm (Capaday 1997; Capaday et al. 1999; Devanne et al. 1997; Jensen et al. 2005). The three parameters were statistically compared between hemispheres by comparing the fit of a difference of curves to the fit of a single curve.
To test the hypothesis that aIHI from iM1 on cM1 is reduced when subjects are pointing to the small target compared with pointing to the large target, the following statistical analysis was done. In an initial step, a repeated-measures ANOVA was used to test whether the inhibitory effect of the CS on the TS-evoked MEP amplitude was significantly different from 100% (which indicates the absence of measurable aIHI) when measured over all time points. Time points were then investigated separately using Bonferroni-corrected pairwise comparisons. For aIHI measures, a repeated-measures ANCOVA with TIMING (0, 300, 350, 400, 450 ms) and SIZE (small vs. large) as within-subject variables was used to test the effect of TIMING and SIZE on the effect of the CS on TS-evoked MEP amplitudes. Control measurements (see below) that displayed significant differences between conditions were taken as covariates to determine whether they influenced the main results. Furthermore, the effect of timing of the TMS pulses on reaction times during the aIHI experiment was analyzed with a repeated-measures ANOVA using the within-subject variable TIMING (0, 300, 350, 400, 450 ms). To investigate whether the aIHI change over time follows a linear or nonlinear pattern, the repeated-measures ANOVA was followed by a polynomial contrast analysis. Pearson's correlation coefficients were used to investigate correlations between aIHI and motor performance or rIHI.
Additional statistical analysis with four repeated-measures ANOVAs were done to determine whether some of the observed effects on aIHI could be explained by differences in CS-evoked MEP amplitude, TS-evoked MEP amplitude or background EMG. First, the CS-evoked MEP amplitudes were compared with SIZE (small vs. large) and TIMING (0, 300, 350, 400, 450 ms) as within-subject variables. Second, the TS-evoked MEP amplitudes were compared with SIZE and TIMING as within-subject variable. Third, pre-MEP EMG in the muscle corresponding to iM1 stimulation (CS) was compared with rest EMG, i.e., EMG-ACTIVITY (rest EMG vs. pre-MEP EMG), TIMING and SIZE as within-subject variables. Fourth, pre-MEP EMG in the muscle corresponding to cM1 stimulation (TS) was compared with rest EMG, i.e., EMG-ACTIVITY, TIMING and SIZE as within-subject variables. Paired t-tests were used to compare differences in the pre-MEP EMG, CS-evoked MEP amplitude and TS-evoked MEP amplitude in the rIHI and aIHI experiments.
All statistical tests were compared with a two-sided P value of 0.05 for significance. Mean values are shown ± SE.
RESULTS
All participants completed all experiments. Subject characteristics are summarized in Table 1. There were no adverse reactions.
Experiment 1: Motor Performance
A repeated-measures ANOVA with SIZE (small, medium 1, medium 2, large) as the independent variable and motor task accuracy as the dependent variable showed that accuracy differed significantly between target sizes (F = 40.43, P < 0.001, Fig. 2). The main effect of size was not significant in a second repeated-measures ANOVA with movement time as the dependent variable (Fig. 2), but post hoc analysis with a paired t-test revealed a statistically significant difference between the movement times for the smallest and largest targets (t = 3.036, P = 0.029). These results confirm that the employed task indeed had different demands on precision and fulfilled the criteria of Fitts' law.
Experiment 2: Corticospinal Excitability (SRC) and rIHI
The stimulation parameters and MT are summarized in Table 1. The SRC for both M1s were described by the Boltzmann equation (iM1: r2 = 0.997; cM1: r2 = 0.981). A significant difference on parameter S50 was found (t = 3.34, P = 0.004) between iM1 (S50 = 59.18 ± 0.58) and cM1 (S50 = 53.34 ± 1.28). The MEPMAX also differed significantly (t = 3.98, P = 0.001) between iM1 (MEPMAX = 0.70 ± 0.02) and cM1 (MEPMAX = 0.93 ± 0.05). There was no difference between motor cortices for the K parameter (iM1: K = 0.16 ± 0.01; cM1: 0.16 ± 0.03). For rIHI, CS applied to iM1 had a statistically significant inhibitory effect on the MEP amplitude evoked by a subsequent TS applied to cM1 (t = −5.82, P < 0.001; Table 2).
Table 2.
rIHI and aIHI
| Target Size | Timing, ms | Mean ± SE | ANOVA | Post Hoc t-Tests |
|---|---|---|---|---|
| aIHI compared with baseline | ||||
| Small | 0 | 80.0 ± 8.1 | F = 2.38, P = 0.090 | t = −2.61, P = 0.428 |
| 300 | 86.9 ± 8.0 | t = −1.47, P = 1.00 | ||
| 350 | 82.4 ± 8.3 | t = −2.11, P = 0.964 | ||
| 400 | 87.3 ± 4.2 | t = −3.01, P = 0.219 | ||
| 450 | 96.1 ± 8.6 | t = −0.84, P = 1.00 | ||
| Large | 0 | 74.6 ± 10.0 | F = 3.92, P = 0.016 | t = −2.61, P = 0.423 |
| 300 | 68.9 ± 6.6 | t = −4.60, P = 0.019 | ||
| 350 | 85.7 ± 7.6 | t = −1.85, P = 1.00 | ||
| 400 | 87.2 ± 7.2 | t = −1.81, P = 1.00 | ||
| 450 | 83.4 ± 7.9 | t = −2.28, P = 0.735 | ||
| rIHI compared with baseline | ||||
| Paired Samples t-Test | ||||
| 56.1 ± 8.4 | t = −5.82, P < 0.001 | |||
Values are means ± SE. The amplitude of the conditioned MEP is expressed as %mean test MEP for rIHI and aIHI. The conditioned MEP amplitude measured at the different time points for both target sizes are given for aIHI. A repeated-measures ANOVA was used to test whether aIHI over all time points was significantly different from 100%. Time points were also investigated separately using Bonferroni-correct pairwise comparisons. Values in bold are statistically significant.
Experiment 3: Active IHI
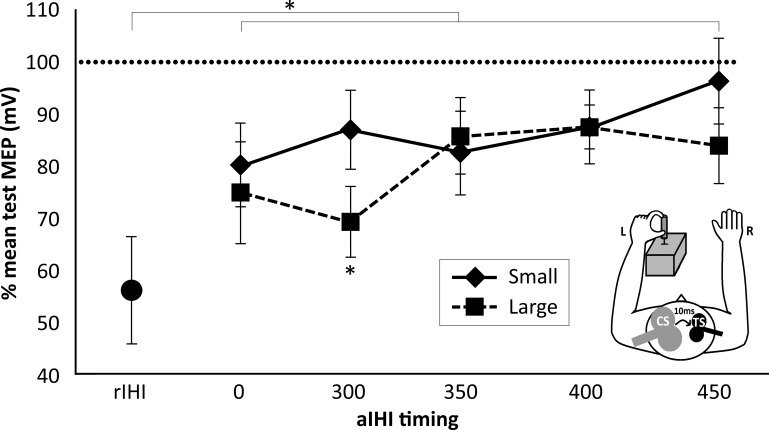
A repeated-measures ANOVA was used to test whether aIHI over all time points was significantly different from 100% (which indicates the absence of measurable aIHI). There was a significant inhibitory effect of CS on MEP evoked by TS when subjects pointed to the large target (F = 3.92, P = 0.016). Post hoc analysis with Bonferroni-corrected pairwise comparisons showed a significant inhibitory effect of CS on MEP evoked by TS at 300 ms after the cue for large targets (Table 2). No significant inhibitory effect was seen when subjects pointed to the small target.
As indicated in Fig. 3, the inhibitory effect of the CS on the MEP evoked by the TS differed, depending on the target size (SIZE) and time of measurement during the premovement period (TIMING). A repeated-measures analysis of covariance with TIMING (0, 300, 350, 400, 450 ms) and SIZE (small vs. large) as within-subject variables showed a significant effect of SIZE (F = 6.109, P = 0.039), whereas the main effect of TIMING and the interaction of SIZE × TIMING were not significant (Table 3, Fig. 3). As the TS-evoked MEP amplitude differed significantly between the two targets (see below), this difference was included as covariate in this analysis. There was no significant interaction with these results (Table 3), indicating that the observed difference in aIHI between the two targets cannot be explained by differences in the TS-evoked MEP amplitude.
Fig. 3.
Demand on motor skill and aIHI. The effect of the CS on the MEP amplitude evoked by subsequent TS pulse is expressed as percentage of mean test MEP at each time point and each target size for rIHI and aIHI. Values are means ± SE. *Significant differences.
Table 3.
Main analyses of aIHI
| Factor | ANCOVA |
|---|---|
| aIHI comparison between small and large targets: main analysis | |
| SIZE | F = 6.11, P = 0.039 |
| TIME | F = 1.42, P = 0.267 |
| SIZE × TIME | F = 1.43, P = 0.262 |
| DIF_TS (covariate) | F = 1.43, P = 0.267 |
| SIZE × DIF_TS (covariate) | F = 4.09, P = 0.078 |
| TIME × DIF_TS (covariate) | F = 0.85, P = 0.468 |
| SIZE × TIME × DIF_TS (covariate) | F = 1.43, P = 0.262 |
| Timing, ms | Post Hoc t-Tests |
|---|---|
| aIHI comparison between small and large targets: post hoc analysis (small vs. large targets) | |
| 0 | t = 0.99, P = 1.00 |
| 300 | t = 3.29, P = 0.045 |
| 350 | t = −0.58, P = 1.00 |
| 400 | t = 0.06, P = 1.00 |
| 450 | t = 1.67, P = 0.650 |
Main results comparing the effect of the variables SIZE, TIME, and the interaction between SIZE and TIME on aIHI in an analysis of covariance (ANCOVA), with differences in TS-evoked MEP amplitudes as covariate (DIF_TS). The statistically significant effect of SIZE was further investigated using Bonferroni-corrected pairwise comparisons. Values in bold are statistically significant.
To analyze the changes over time with greater statistical power, a polynomial within-subjects contrast analysis was performed which revealed a significant cubic effect for SIZE × TIMING (F = 10.29, P = 0.012). Bonferroni-corrected post hoc t-tests showed that the inhibitory effect of CS on the conditioned MEP evoked by the TS was significantly smaller at 300 ms when subjects pointed to the small target compared with the large target (t = 3.29, P = 0.045; Table 3). Differences at all other time points did not reach statistical significance (Fig. 3, Table 3).
A repeated-measures ANOVA was used to determine whether the timing of the TMS pulse during the premovement period (TIMING) or size of the target (SIZE) had an effect on the CS and TS-evoked MEP amplitudes and background EMG activity. Time- or size-dependent changes in these measures could confound the measurements of task-dependent changes in aIHI. The results are summarized in Table 4. For TS-evoked MEP amplitudes (cM1 stimulation), a significant effect of SIZE was found (F = 15.54, P = 0.003). TS-evoked MEP amplitudes were slightly larger when subjects pointed to the small target (1.21 ± 0.15 mV) than when they pointed to the large targets (1.13 ± 0.15 mV). Although the difference in TS-evoked amplitudes was relatively small, we added it as a covariate into the main analysis of the aIHI measures (see section Experiment 3: Active IHI). This covariate did not significantly interact with the main or interaction effects. This indicates that the observed differences between small and large aIHI cannot be explained by differences in TS size. There was no other statistically significant effect on TS-evoked MEP amplitudes or any of the other measures (Table 4). It is, therefore, unlikely that differences in background EMG, CS amplitude or TS amplitude explain the reported effects of TIME and SIZE on aIHI.
Table 4.
EMG and MEP amplitudes evoked by CS and TS
| Factor | EMG Background |
CS Amplitude | TS Amplitude | |
|---|---|---|---|---|
| Left M1 (CS) | Right M1 (TS) | |||
| TIMING | F = 1.54, P = 0.304 | F = 0.76, P = 0.455 | F = 2.63, P = 0.105 | F = 0.184, P = 0.859 |
| SIZE | F < 0.01, P = 0.956 | F = 0.31, P = 0.868 | F = 0.57, P = 0.470 | F = 15.54, P = 0.003 |
| TIMING × SIZE | F = 1.19, P = 0.405 | F = 1.72, P = 0.238 | F = 2.61, P = 0.105 | F = 1.80, P = 0.188 |
| EMG-ACTIVITY | F = 0.18, P = 0.682 | F = 0.61, P = 0.472 | ||
| EMG-ACTIVITY × SIZE | F = 2.88, P = 0.124 | F = 1.07, P = 0.349 | ||
| EMG-ACTIVITY × TIMING | F = 1.78, P = 0.251 | F = 0.57, P = 0.491 | ||
| EMG-ACTIVITY × SIZE × TIMING | F = 0.46, P = 0.767 | F = 0.56, P = 0.494 | ||
| rIHI vs. aIHI averaged over time | t = 0.99, P = 0.347 | t = 4.75, P = 0.001 | t = 2.47, P = 0.036 | t = 2.89, P = 0.018 |
| rIHI vs. aIHI at time point 0 ms | t = 0.69, P = 0.505 | t = 4.32, P = 0.002 | t = 1.96, P = 0.082 | t = 3.05, P = 0.014 |
A repeated-measures ANOVA was used to determine whether the timing of the TMS during the premovement period (TIMING) or the target size (SIZE) had an effect on the CS and TS-evoked MEP amplitudes and background EMG activity. Values in bold are statistically significant.
Relationship between rIHI and aIHI
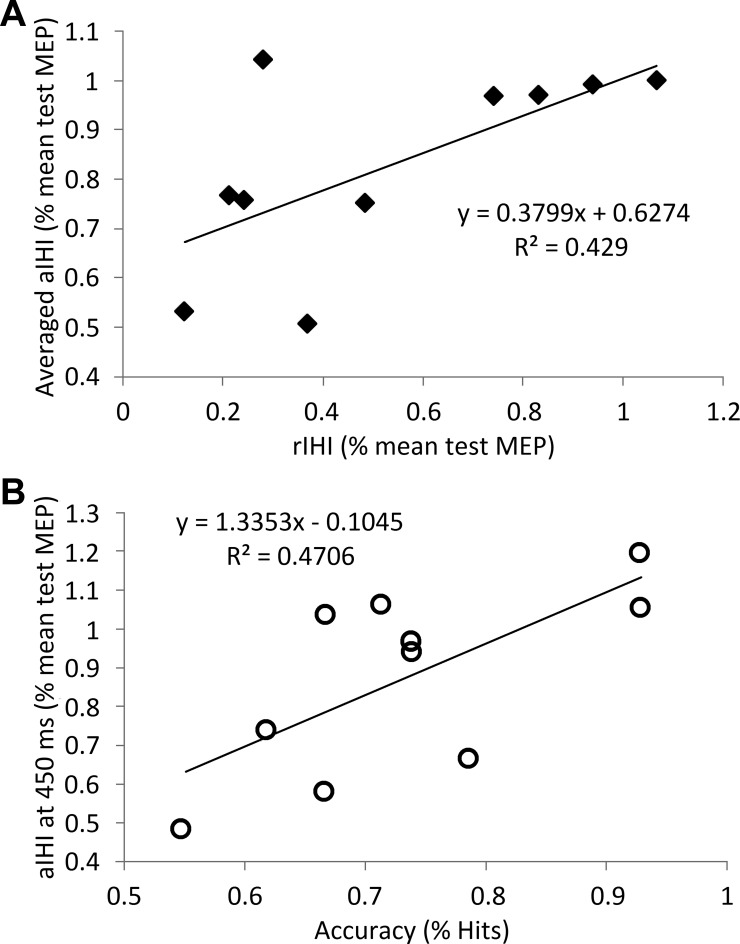
To explore the effect of movement preparation on aIHI, the inhibitory effect of the CS on the MEP amplitude evoked by the subsequent TS was compared during rest (rIHI) and movement preparation (aIHI). In an initial analysis, the rIHI measures were compared with aIHI measures averaged over all time points. In a second analysis, rIHI measures were compared with aIHI measures obtained at cue presentation (0 ms). There was a significant difference between rIHI (Fig. 3) and aIHI averaged over time points and target sizes (F = 19.99, P = 0.001). Bonferroni-corrected post hoc t-tests showed that the inhibitory effect of the CS was greater in rIHI compared with both small and large target aIHI (averaged over time points; small targets: t = 5.35, P = 0.001; large targets: t = 3.95, P = 0.010; Fig. 3). However, there was a positive correlation between rIHI and aIHI (averaged over size and time points, r2 = 0.429, P = 0.040, Fig. 4A). rIHI was positively correlated with aIHI at 0 ms, but this did not reach significance (r2 = 0.429, P = 0.106).
Fig. 4.
Relationship between measures of aIHI, rIHI and accuracy of motor performance. A: Pearson's correlation between rIHI and aIHI averaged over time points and target sizes. B: Pearson's correlation between accuracy of motor performance and aIHI at 450 ms averaged over target sizes. The amplitude of the conditioned MEP is expressed as percentage of mean test MEP for rIHI and aIHI.
To determine whether differences in the magnitude of rIHI and aIHI were due to differences in EMG background activity and/or MEP amplitudes evoked by the CS or TS, EMG activity and MEP amplitudes of rIHI and aIHI were compared with paired t-tests. As summarized in Table 4, EMG background activity in the ECU that corresponded to the cM1 (TS site) was significantly greater in the aIHI measurements averaged over all time points [aIHI (averaged): 0.018 ± 0.011, t = 4.75, P = 0.001] and at cue presentation (0 ms: 0.018 ± 0.012, t = 4.32, P = 0.002) than in rIHI (rIHI: 0.002 ± 0.001). In contrast, there was no difference in the EMG activity in the ECU corresponding to the iM1 (CS site).
TS-evoked MEP amplitudes were greater in the aIHI measurements averaged over all time points [aIHI (averaged): 1.17 ± 0.15; t = 2.89, P = 0.018] and at cue presentation [aIHI (0 ms): 1.19 ± 0.17; t = 3.05, P = 0.014] compared with the rIHI (0.77 ± 0.13) measurements. While CS-evoked MEP amplitudes did significantly differ between aIHI averaged over all time points and rIHI [rIHI: 0.62 ± 0.15, aIHI (averaged): 0.93 ± 0.16; t = 2.47, P = 0.036], comparison between rIHI and aIHI at cue presentation (0 ms) did not reveal any difference (Table 4). The difference between rIHI and aIHI (0 ms) TS was not significantly correlated with the difference between background EMG for rIHI and aIHI (0 ms; r2 = 0.203, P = 0.191).
Active IHI in Relation to Motor Task Performance
To explore the functional relevance of the changes in the magnitude of aIHI on the accuracy of motor performance (%target hits), the aIHI measurements obtained at different time points during the premovement period were plotted against %target hits (Fig. 4B). There was a significant negative relationship between weaker aIHI (indicated by a smaller inhibitory effect of the CS on the MEP amplitude evoked by the subsequent TS) and increased accuracy in the later premovement period (450 ms) (r2 = 0.471, P = 0.029). This relationship did not differ between target sizes (P = 0.953). There was no statistically significant relationship between aIHI at other time points and motor performance.
Effect of TMS (aIHI Measurements) on Reaction Time
We explored the effect of the timing of the TMS on reaction time using a repeated-measures ANOVA with time of TMS (TIME) as independent measure and reaction time as dependent measure. Reaction time was significantly influenced by the timing of the TMS (F = 4.40, P = 0.030). Average reaction time was 128.22 ± 43.43 ms when TMS was applied at 0 ms, but 201.81 ± 36.80 ms when TMS was applied at 450 ms. In contrast, there was no significant effect of target size on reaction time.
DISCUSSION
The new finding of the present study is that the demand on accuracy of a unilateral hand motor task modulates premovement aIHI from iM1 on cM1. As this has not been reported before, there is no other human or animal data available for direct comparison. We chose an experimental paradigm that mimics the seminal work on motor control studies in the nonhuman primates where neuronal recordings were obtained from dorsal premotor cortex and M1 during a pointing task (for a review, see Kalaska 2009). In nonhuman primate experiments, neuronal responses in dorsal premotor cortex and M1 during an instructed-delay period demonstrate activity during the entire delay period and are presumed to reflect early planning stages (Cisek et al. 2003; Crammond and Kalaska 2000). The objective of the present study was to determine the effect of the demand of accuracy on aIHI during movement planning. Because of the employed block design, the target size was known to the subject at the time of the cue at 0 ms. The cue provided additional information regarding the time to begin the movement. The lack of effect of target size on aIHI at 0 ms would suggest that, for M1, target size-related movement preparation was not initiated prior to the cue. The significant difference of aIHI between the large and small target at 300 ms after the cue would indicate that target size information was processed in M1 around this time period. A precise estimate of when target size information begins to be processed will require measurements at additional time points between 0 and 300 ms in future experiments.
The findings are in line with previous task-dependent fMRI results showing greater activity in iM1 when unilateral hand movements become more demanding in terms of accuracy or complexity (Buetefisch et al. 2014a; Hummel et al. 2003; Seidler et al. 2004; Verstynen et al. 2005). They are also in line with the evidence derived from reaction time experiments indicating time-dependent modulation of aIHI from iM1 on cM1 in the premovement period of a unilateral hand tasks (Duque et al. 2005b; Murase et al. 2004; Tazoe and Perez 2013). Although the direct comparison of the results is limited by the lack of an instructed delay period in the latter studies, which could have resulted in some differences in extent of planning after the go-signal (Crammond and Kalaska 2000), an increase of aIHI from iM1 on cM1 in the early premovement period was demonstrated. This was followed by a reduction of aIHI immediately prior to the onset of the movement (Duque et al. 2005b; Murase et al. 2004). In extending these earlier findings, we report now that this time-dependent modulation of aIHI was dependent on the demand on accuracy, as indicated by the significant cubic interaction between time and size. Specifically, for the large target condition, we observed a tendency for an initial increase of aIHI (Table 2, Fig. 3, aIHI was significant at 300 ms but not at any other time points) that was followed by a weakening aIHI closer to the movement onset (Table 2, Fig. 3), which is similar to the previous reports (Duque et al. 2005a; Murase et al. 2004). The new finding in the present study is that this time-dependent modulation of aIHI was different in a task with high demand on accuracy (small-target condition) where aIHI was weak (no significant aIHI at any time points) and time did not have an effect on aIHI (Table 2, Fig. 3). Taken together, these results would support the concept that changes in interhemispheric interactions between iM1 and cM1 are involved in the control of unimanual skilled finger motions in humans.
The mechanisms mediating the inhibitory effect of the CS on the MEP amplitude evoked by the subsequent TS are thought to involve transcallosal glutamatergic projections connecting with pyramidal tract neurons through GABAergic interneurons (Reis et al. 2008). Therefore, task-depending changes in aIHI could result from changes in the excitability of these neuronal networks.
As iM1 stimulation-evoked MEP amplitude did not show an effect of target size or the timing of the TMS, it is unlikely that the demonstrated changes in aIHI are related to changes in excitability at the spinal or subcortical level. Furthermore, while there are effects of size on cM1 stimulation, evoked MEP amplitudes were observed when TS-evoked MEP amplitude differences were included as a covariate into the main analysis of the aIHI measurements; there was no significant interaction with the main and interaction effects. It is, therefore, unlikely that this difference confounded the aIHI results. Moreover, the difference in TS amplitude was relatively small (0.08 mV), and it has been shown that IHI is not significantly affected when comparing TS amplitudes of 0.2 mV and 1 mV, under constant CS size (Chen 2004; Daskalakis et al. 2002). This indicates that the observed effects of demand on aIHI in a motor task cannot be explained by differences in TS-evoked MEP amplitudes.
Motor Performance
In the present study, a motor task was used that allowed the parametric increase in demand on accuracy by asking subjects to point to targets of either small- or large-target sizes (Buetefisch et al. 2011, 2014a). Consistent with Fitt's law, movement times for the smallest target were statistically significantly longer than movement times for the largest target, although the difference did not reach statistical significance when tested across all target sizes (Fig. 2, P = 0.06). The smaller impact of target size on the movement time in the present experiment could be explained by fact that the visual cue provided some information about the ensuing movement, which allowed the subject to initiate some early planning of the movement with respect to the target size (Crammond and Kalaska 2000). In addition, subjects also operated under a time constraint of having to complete the task in less than 2 s and may have opted for more misses (lower accuracy) relative to longer movement times. Because subjects' performance on the small and large target size were significantly different, the employed motor task is a valid method to test for the effects of demand on accuracy on a motor task on the inhibitory effect of iM1 on cM1 by means of TMS.
Different from other studies (Duque et al. 2007; Murase et al. 2004), reaction times were measured trial by trial during aIHI measurements. Consistent with previous reports, the timing of the TMS had a significant effect on reaction time, with shortened reaction times following early TMS applications, and delayed reaction times when TMS was applied closer to the movement onset (Stoeckel et al. 2009; Terao et al. 1997). There was no effect of target size on RT. This lack of effect of target size on RT could be explained by the TMS-related interference with RT, as TMS induced muscle twitches in the targeted muscles and may have interfered with motor execution differently, depending on aIHI timings. As such, the RT measurements should be regarded as estimates. Furthermore, as discussed above, the visual cue provided some information about the ensuing movement, which allowed the subject to initiate some early planning of the movement with respect to the target size (Crammond and Kalaska 2000).
Effect of Time and Demand on Accuracy on Premovement Evoked MEP Amplitudes (Single TMS Pulse)
We did not observe a significant effect of time on the TS (applied to cM1) or CS (applied to iM1) evoked MEP amplitudes (see below). In previous studies of M1 excitability changes during the premovement time, MEP amplitudes increased as a function of time (Chen et al. 1998b; Rossini et al. 1988). However, as these studies have reported the greatest increases of MEP amplitudes immediately prior to the onset of movements (Chen et al. 1998b), differences in the observed findings could be related to the differences in the time window. In the present study, TMS was only applied in the early planning phase prior to the go-signal.
In the present study, we observed a significant effect of target size on TS-evoked MEP amplitude (cM1 stimulation), where MEP amplitudes were slightly larger for the small-target compared with the large-target condition. This was not seen for iM1 stimulation. Similar to our results, cM1 evoked MEP amplitudes increased as a function of expectancy for the need to execute a movement following a delay in a reaction time task (van Elswijk et al. 2007). In single-cell recordings in monkey M1, task-related activity was observed during a instructed delay period prior to the go-signal (Cisek et al. 2003). Our findings could indicate that, during movement preparation, excitability in cM1 changes, depending on kinematic details of the ensuing movement that are related to the size of the target. However, as changes in MEP amplitude reflect changes in excitability along the entire corticospinal projections, we cannot determine whether these changes occurred just at the level of M1 and/or at the subcortical or spinal level.
Effect of Time and Demand on Accuracy on Premovement EMG Activity of ECU Muscles
As indicated by the lack of effect of time on EMG background activity, the present results of time-dependent changes in aIHI cannot be explained by the changes in EMG background activity. Differences in the results reported by other investigators (Duque et al. 2007; Murase et al. 2004) could be explained by the differences in the approach. In the present study, any trials with task preparation-related increases in EMG were discarded from further analysis. As demonstrated by the lack of correlation between changes in EMG activity and changes in TS-evoked MEP amplitude, EMG activity had no effect on the obtained aIHI measures. Comparison to other studies is limited, as EMG was not quantified in previous studies; the possibility of increased background EMG activity-related increases in TS amplitude cannot be ruled out (Duque et al. 2007; Murase et al. 2004).
Relationship between rIHI and aIHI
In the present study, aIHI from iM1 onto cM1 at target presentation was significantly weaker compared with rIHI. The different level of background EMG pre-MEP for the rIHI and aIHI measures is a confounding factor, and, therefore, the comparison between rIHI and aIHI measures is problematic. It should be noted, however, that, although the background EMG differs between rIHI and aIHI, the background EMG for aIHI is still very small (<0.02 μV). Estimates of MEP amplitudes as a function of EMG activity indicate a rate of 11.3 μV per 1 μV at intensity levels around the S50 value (Capaday et al. 1999). It is, therefore, unlikely that the substantial difference in rIHI and aIHI is merely explained by the difference in background EMG. This notion is supported by the differential effect of target size on EMG background (Table 4). We would argue that differences in rIHI and aIHI may reflect processes related to the planning of the movement. This notion is supported by target size-dependent increases in corticospinal excitability. As target presentation occurred in blocks, the size of the target was known to the subject prior to its appearance. Subjects could, therefore, anticipate some aspects of the movement, and preparation may have started prior to the actual presentation of the cue. As there was no effect of size when aIHI was measured at the time of cue presentation (0 ms) but an effect of size 300 ms after cue presentation, the notion of a more general anticipatory effect would be supported. This is in line with previous reports of weaker aIHI during the preparation of self-paced and ballistic movements compared with IHI at rest (Tazoe and Perez 2013).
Active IHI in Relation to Motor Task Performance
Superior performance on the motor task was positively correlated with weaker aIHI in the late premovement period (450 ms after the cue), but not with aIHI measurements at any other time points (Fig. 4B). Importantly, motor task performance was not correlated with rIHI. A lack of weakening in the aIHI toward the onset of movement and abnormal motor performance was found in stroke patients compared with healthy age-matched controls (Murase et al. 2004). Similar to the present results, there was no correlation between performance and rIHI.
Our results of modulation of aIHI, depending on the level of demand of a motor task, is compatible with findings in human imaging studies where iM1 is activated, depending on the demand of complexity or accuracy of the motor task (Buetefisch et al. 2011, 2014a; Konner 2002; Seidler et al. 2004; Verstynen et al. 2005; Verstynen and Ivry 2011). Our and other investigators' findings of an inverse correlation between weak aIHI from iM1 on cM1 in the late premovement period and superior performance (Duque et al. 2007; Murase et al. 2004; Tazoe and Perez 2013) would support the view that task-dependent differences in iM1 activity are mediated to some extent via the excitatory connections of the corpus callosum between the two motor cortices. This view would be supported by reported clumsiness of fine finger movements, along with difficulty of bimanual interactions (Meyer et al. 1995; Seitz et al. 2004) in patients with a lesioned or absent corpus callosum. In these patients, the inhibitory effect of CS on the MEP evoked by the subsequent TS cannot be elicited (Seitz et al. 2004), and the ipsilateral silent period is lacking or delayed (Meyer et al. 1995), further supporting the cortical site of the present finding. Furthermore, in an fMRI study, a relationship between rIHI and task-related iM1 activity was found (Talelli et al. 2008b). Specifically, peak forces for a hand grip were positively correlated with increases in the iM1 blood oxygenation level-dependent response when IHI between motor cortices was weak. This positive correlation changed to a negative correlation when IHI was strong.
In summary, the findings of the present study extend the evidence for task-dependent modulation of IHI and fMRI/TMS evidence of increased iM1 activity in motor tasks with higher demand on complexity or accuracy. Correlation between weaker aIHI from iM1 on cM1 and superior performance would support the notion that these transcallosal connections, among other cortico-cortical and thalamo-cortical projections, are important in motor preparation and the execution of unimanual hand movements. In future studies, a direct relationship between IHI, iM1 activity and task performance has to be established. Furthermore, as age has an impact on neuronal networks supporting motor performance, further studies have to substantiate whether the present findings generalize to a younger population.
GRANTS
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Human Development of the National Institutes of Health under Grants R01-NS-060830, R56-NS-070879, partially R21-HD-067906, and R01-NS-090677.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.W., M.W.H., G.H., and C.M.B. conception and design of research; M.W., G.M.K., F.R., S.R.B., and C.M.B. performed experiments; M.W., G.M.K., M.W.H., G.H., and C.M.B. analyzed data; M.W., S.R.B., and C.M.B. interpreted results of experiments; M.W. and C.M.B. prepared figures; M.W. and C.M.B. drafted manuscript; M.W., M.W.H., and C.M.B. edited and revised manuscript; M.W., G.M.K., F.R., S.R.B., M.W.H., G.H., and C.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our subjects for participation in the study, and Dr. Sebastian Buetefisch, Daphne Vincent and Kate Pirog Revill for technical support.
Present address of M. Wischnewski: Donders Centre for Cognition, Radboud University, Kapittelweg 29, 6525 EN Nijmegen, the Netherlands.
REFERENCES
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci 144: 160–170, 1996. [DOI] [PubMed] [Google Scholar]
- Buetefisch CM, Hines B, Shuster L, Pergami P, Mathes A. Motor demand-dependent improvement in accuracy following low-frequency transcranial magnetic stimulation of left motor cortex. J Neurophysiol 106: 1614–1621, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetefisch CM, Revill KP, Shuster L, Hines B, Parsons M. Motor demand dependent activation of ipsilateral motor cortex. J Neurophysiol 112: 999–1009, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetefisch CM, Revill KP, Shuster L, Hines B, Parsons M. Motor demand-dependent activation of ipsilateral motor cortex. J Neurophysiol 112: 999–1009, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butefisch CM, Kleiser R, Korber B, Muller K, Wittsack HJ, Homberg V, Seitz RJ. Recruitment of contralesional motor cortex in stroke patients with recovery of hand function. Neurology 64: 1067–1069, 2005. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 22: 4–21, 2008. [DOI] [PubMed] [Google Scholar]
- Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. J Neurosci Methods 74: 201–218, 1997. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol 81: 129–139, 1999. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain 121: 253–264, 1998. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 154: 1–10, 2004. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 80: 2870–2881, 1998a. [DOI] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol 44: 317–325, 1998b. [DOI] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol 89: 922–942, 2003. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on premovement activity. J Neurophysiol 84: 986–1005, 2000. [DOI] [PubMed] [Google Scholar]
- Dancause N, Touvykine B, Mansoori BK. Inhibition of the contralesional hemisphere after stroke: reviewing a few of the building blocks with a focus on animal models. Prog Brain Res 218: 361–387, 2015. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol 543: 317–326, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114: 329–338, 1997. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage 28: 940–946, 2005a. [DOI] [PubMed] [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex 15: 588–593, 2005b. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci 19: 204–213, 2007. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 453: 525–546, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47: 381–391, 1954. [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I. Interhemispheric facilitation of the hand motor area in humans. J Physiol 531: 849–859, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci Lett 71: 235–240, 1986. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol 388: 397–419, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Kirsammer R, Gerloff C. Ipsilateral cortical activation during finger sequences of increasing complexity: representation of movement difficulty or memory load? Clin Neurophysiol 114: 605–613, 2003. [DOI] [PubMed] [Google Scholar]
- Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol 99: 1558–1568, 2005. [DOI] [PubMed] [Google Scholar]
- Kalaska JF. From intention to action: motor cortex and the control of reaching movements. Adv Exp Med Biol 629: 139–178, 2009. [DOI] [PubMed] [Google Scholar]
- Konner M. The Tangled Wing: Biological Constraints on the Human Spirit. New York: Times Books, 2002. [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci 26: 6096–6102, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118: 429–440, 1995. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55: 400–409, 2004. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20: 310–319, 1998. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 586: 325–351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol 105: 340–344, 1997. [DOI] [PubMed] [Google Scholar]
- Rosler KM, Petrow E, Mathis J, Aranyi Z, Hess CW, Magistris MR. Effect of discharge desynchronization on the size of motor evoked potentials: an analysis. Clin Neurophysiol 113: 1680–1687, 2002. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Zarola F, Stalberg E, Caramia M. Premovement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res 458: 20–30, 1988. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol 530: 307–317, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. Neuroimage 22: 1775–1783, 2004. [DOI] [PubMed] [Google Scholar]
- Seitz R, Kleiser R, Butefisch C, Jorgens S, Neuhaus O, Hartung HP, Wittsack HJ, Sturm V, Hermann M. Bimanual recoupling by visual cueing in callosal disconnection. Neurocase 10: 316–325, 2004. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Rev 26: 113–135, 1998. [DOI] [PubMed] [Google Scholar]
- Stoeckel MC, Seitz RJ, Buetefisch CM. Congenitally altered motor experience alters somatotopic organization of human primary motor cortex. Proc Natl Acad Sci U S A 106: 2395–2400, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Ewas A, Waddingham W, Rothwell JC, Ward NS. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage 40: 1772–1781, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res 186: 59–66, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe T, Perez MA. Speed-dependent contribution of callosal pathways to ipsilateral movements. J Neurosci 33: 16178–16188, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Suzuki M, Sakai K, Hanajima R, Gemba-Shimizu K, Kanazawa I. Shortening of simple reaction time by peripheral electrical and submotor-threshold magnetic cortical stimulation. Exp Brain Res 115: 541–545, 1997. [DOI] [PubMed] [Google Scholar]
- van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci 19: 121–131, 2007. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol 93: 1209–1222, 2005. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Ivry RB. Network dynamics mediating ipsilateral motor cortex activity during unimanual actions. J Cogn Neurosci 23: 2468–2480, 2011. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain 126: 873–888, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol 93: 138–146, 1994. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS. Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J Neurophysiol 77: 1581–1594, 1997. [DOI] [PubMed] [Google Scholar]