Abstract
Cerebellar Purkinje cells (PCs) are primarily affected in many spinocerebellar ataxias (SCA). In this study we investigated functional activity of PCs in transgenic mouse model of SCA2, a polyglutamine neurodegenerative hereditary disorder. In our studies we used extracellular single-unit recording method to compare spontaneous activity of PCs in age-matched wild-type mice and SCA2-58Q transgenic mice. We discovered that the fraction of PCs with bursting and an irregular pattern of spontaneous activity dramatically increases in aged SCA2-58Q mice compared with wild-type littermates. Small-conductance calcium-activated potassium (SK) channels play an important role in determining firing rate of PCs. Indeed, we demonstrated that intraperitoneal (IP) injection of SK channel inhibitor NS8593 induces an irregular pattern of PC activity in wild-type mice. Furthermore, we demonstrated that IP injection of SK channel-positive modulator chlorzoxazone (CHZ) decreases spontaneous firing rate of cerebellar PCs. Finally, we have shown that IP injections with CHZ normalize firing activity of cerebellar PCs from aging SCA2-58Q mice. We propose that alterations in PC firing patterns is one of potential causes of ataxic symptoms in SCA2 and in other SCAs and that positive modulators of SK channels can be used to normalize activity of PCs and alleviate ataxic phenotype in patients with SCA.
Keywords: SCA2, Purkinje cells, electrophysiology, SK channels, NS8593, chlorzoxazone, transgenic mice, ataxia
NEW & NOTEWORTHY
Aging cerebellar Purkinje cells (PCs) in spinocerebellar ataxia 2 (SCA2) mice display irregular and bursting firing patterns in vivo. Injections with a positive modulator of SK-type calcium-activated potassium channels (chlorzoxazone) can normalize the firing pattern of SCA2 PCs in vivo. These results suggest that positive modulators of SK channels can be used to normalize activity of PCs and potentially alleviate ataxic phenotype in patients with spinocerebellar ataxia.
cerebellum represents a part of the brain that plays a fundamental role in the regulation of muscular activity and motor control. Cerebellar pathways play an important role in the coordination of movements. Cerebellar Purkinje cells (PCs) are considered to be the main dynamic element in the cerebellum because their axons represent the unique output coming from the cerebellar cortex to the cerebellar nuclei and other deep structures of the brain. PCs spontaneously fire action potentials at a constant frequency (De Zeeuw et al. 2011; Llinas and Sugimori 1980a, 1980b; Nam and Hockberger 1997; Raman and Bean 1997, 1999; Smith and Otis 2003; Womack and Khodakhah 2002). This tonic pacemaking activity of PCs is assumed to be crucial for the correct encoding of cortical cerebellar information (De Zeeuw et al. 2011; Hoebeek et al. 2005; Kasumu et al. 2012a).
In studies with cerebellar slices from ataxia mouse models it has been previously reported that the neuronal activity of aged PCs in various models of ataxia is abnormal compared with that of PCs from age-matched wild-type mice (Alvina and Khodakhah 2010b; Dell'Orco et al. 2015; Hansen et al. 2013; Kasumu et al. 2012a, 2012b; Mark et al. 2015; Shakkottai et al. 2011; Walter et al. 2006). These results suggested an idea that early ataxic symptoms can be caused by neuronal dysfunction, i.e., disturbances in firing patterns of PCs, and not by PCs death. PCs pacemaker activity is regulated by small-conductance calcium-activated potassium (SK) channel activity (Womack and Khodakhah 2003). These findings led to the hypothesis that modulators of SK channels can be used to regulate PC firing rates, leading to potential benefit in ataxia. Indeed, it has been demonstrated that two subtypes of nonselective SK channel positive modulators, chlorzoxazone (CHZ) and 1-ethyl-2-benzimidazolinone (1-EBIO), normalize PC firing and exert beneficial effects in a mouse model of episodic ataxia type 2 (EA2) (Alvina and Khodakhah 2010a, 2010b; Walter et al. 2006). Exposure to SKA-31, a riluzole analog optimized for positive modulation of SK channels, provided benefit in a mouse model of spinocerebellar ataxia type 3 (SCA3) by correcting abnormal PC firing and improving motor function in SCA3 mice (Shakkottai et al. 2011). Our laboratory demonstrated that a novel specific, positive modulator of SK2/3 channels (NS13001) stabilized PC firing rates and exerted beneficial effects in mouse model of spinocerebellar ataxia type 2 (SCA2) (Kasumu et al. 2012a). Riluzole yielded promising results in a recent phase II study in a mixed population of ataxia patients (Ristori et al. 2010) and in a more recent study with a larger cohort of patients with hereditary cerebellar ataxia (Romano et al. 2015). These clinical effects have been suggested to be related to the ability of riluzole to facilitate the activity of SK channels.
Previous studies with ataxic mouse models were mainly performed in vitro using acutely isolated cerebellar slices (Alvina and Khodakhah 2010b; Dell'Orco et al. 2015; Hansen et al. 2013; Kasumu et al. 2012a, 2012b; Shakkottai et al. 2011; Walter et al. 2006). However, functional properties of PCs may be affected by isolation of the slices and differ from their properties in vivo. Several in vivo recording studies have been performed with ataxic mice containing mutations in voltage-gated calcium channels (Gao et al. 2012; Hoebeek et al. 2005) and in mice expressing two different versions of CACNA1A calcium channel carboxy-terminal tail (Mark et al. 2015). Electrophysiological studies with calcium channel mutant mice led to the suggestion that dysregulated PC Ca2+ signaling may lead to abnormal PC firing patterns and ataxic phenotypes (De Zeeuw et al. 2011). This idea is consistent with the mechanism that was proposed to be responsible for ataxia in polyglutamine-expansion disorders (Hansen et al. 2013; Kasumu et al. 2012a, 2012b; Shakkottai et al. 2011). However, in vivo recordings of PC activity have not been previously performed in mouse models of polyglutamine-expansion disorders. To address this concern, in the present study we compared the functional properties of PCs from the intact cerebella of wild-type and SCA2-58Q transgenic mice using in vivo recording techniques. In addition, we evaluated effects of negative and positive modulators of SK channels on activity of wild-type and SCA2-58Q PCs in vivo.
MATERIALS AND METHODS
Compounds.
The SK channel inhibitor N-[(1R)-1,2,3,4-tetrahydro-1-naphthalenyl]-1H-benzimidazol-2-amine (NS8593) and SK channel activator 5-chloro-2-hydroxybenzoxazole (CHZ) were used. The compounds were supplied by Ataxion Therapeutics Biopharmaceutical Company. All compounds were diluted in 10% (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD) in saline to achieve better solubility. Fresh drug solutions were prepared immediately before the experiment from frozen aliquots of stock solutions. A fresh stock solution of HP-β-CD was produced each week, kept at +4°C, and used for 5 days without losing solvent properties.
Mice breeding and genotyping.
Experiments were conducted with transgenic SCA2-58Q mice and their wild-type (WT) littermates. SCA2-58Q transgenic mice (Pcp2-atxn2[Q58]5B9, B6D2F1 strain, a C57BL/6J×DBA/2J hybrid; Huynh et al. 2000) were kindly provided by Dr. Stefan Pulst (University of Utah, Salt Lake City, UT) and were crossed to the FVB (FVBN/NJ) background. The transgenic mice were crossed with WT mice on the FVB (FVBN/NJ) background for at least six generations in our laboratory as previously described (Kasumu et al. 2012a, 2012b). The genome of SCA2-58Q mice contains the insert of human mutant ataxin-2 protein, which has 58 CAG repeats. This transgene is driven by PC-specific L7/pcp2 promoter (Huynh et al. 2000). The mice were bred the following way: male hemizygous SCA2-58Q (FVB) mice were crossed with female WT (FVBN/NJ) mice to generate mixed litters. The genotyping was done via PCR for ATXN2 transgene as previously described (Kasumu et al. 2012a, 2012b). The volume of one PCR sample was 25 μl. The PCR mix per one sample contained 2.5 μl of 10× buffer for Taq polymerase, 0.5 μl of 10 mM dNTP, 1.5 μl of 25mM MgCl2, 0.125 μl of 20 μM primers (forward and reverse), 0.25 μl of Taq polymerase, 2 μl of DNA, and 18 μl of distilled water. The sequence of the forward primer was 5′-GCGAACACAAAGAGAAGGACCTGGA-3′, the sequence of the reverse primer was 5′-GCCCTTGCTTCCCGTTTTAA-3′, and the resulting PCR product was 232 bp in length. The animals were kept in groups of two to six in vivarium. The temperature was held at 22–24°C and included 12 daylight hours. The mice had access to standard food and water ad libitum. All procedures were approved according to the principles of the European Convention (Strasbourg 1986) and the World Medical Association Helsinki Declaration (1996) about humane treatment of animals.
Extracellular single-unit recordings in vivo.
The method for extracellular recording of PCs activity in vivo was adapted from a published report (Gao et al. 2012). To summarize, the mice were anesthetized with urethane with an initial concentration of 1,200 mg/kg, and then after 40 min this concentration was increased to 1,800 mg/kg. After anesthetic effects were achieved, the mice were immobilized using stereotaxic apparatus (RWD Life Science, San Diego, CA). A feedback-controlled heating pad (Harvard Apparatus, Holliston, MA) maintained the body temperature of the mice at 37°C. Next, the scalp under the cerebellum area was taken away, and a small burr hole was bored into the skull under the lambdoid suture. Extracellular recordings of PCs activity were performed from cerebellar lobules IV/V using borosilicate glass pipettes (1.5-mm outer diameter, 0.86-mm inner diameter; Sutter Instruments, Novato, CA) filled with 2.5 M NaCl and with a resistance of 3–10 MΩ. The pipettes were advanced into the cerebellum using a one-axis oil hydraulic micromanipulator (Narishige, Tokyo, Japan), and electrical activity was continuously recorded. The PC firing signal was identified by means of the complex spike occurrence. Complex spikes are caused by climbing fiber activation that involves the generation of calcium-mediated action potentials in the dendrites, whereas simple spikes are activated synaptically by the parallel fibers, otherwise known as the axons of the granule cells (Raman and Bean 1999). The PC activity was recorded from 1 to 5 h after the last injection of anesthetic. To classify PC activity, the recording of corresponding electrophysiological pattern was performed for at least 5 min. Electrical recordings were amplified using an AC/DC Differential Amplifier (A-M Systems, Carlsborg, WA), filtered (100-Hz high-pass and 10-kHz low-pass filters), digitized via analog-to-digital converter NI PCI-6221 (National Instruments, Austin, TX), and stored for off-line computer analysis. For data acquisition, the program Bioactivity Recorder (version 5.9) was used. Further analysis was performed using Clampfit (version 10.3.1.5) and Origin software.
Statistical analysis.
To analyze the statistical significant differences between groups, one-way ANOVA and Bonferroni posttest were used. To analyze the electrophysiological properties of PCs, average values of simple spike and complex spike firing frequency were detected. The data obtained are presented as relative firing frequencies [means ± SE, i.e., (Fi/F0) ± SE, where F0 is the value of the simple or complex spike's firing frequency before compound injection, Fi is the value of the simple or complex spike's firing frequency after compound injection, and SE is the standard error of mean].
For bistability analysis, the shape of the simple spike's interspike interval (ISI) distributions (CV, skewness, and kurtosis) were calculated as follows. CV (coefficient of variation) was calculated according to Eq. 1:
| (1) |
where SD is the standard deviation and ISI is the mean value of interspike intervals.
Skewness of the ISI distribution was calculated from Eq. 2:
| (2) |
where n is the total number of ISIs, xi is the ith realization of the ISI value, x̄ is the mean value of ISI, and s is the standard deviation.
Kurtosis of the ISI distribution was calculated according to Eq. 3:
| (3) |
where the values are the same as in Eq. 2 above.
To analyze the irregularity of PCs, we propose to introduce the parameter χ, which is calculated according to Eq. 4:
| (4) |
where CVb is the coefficient of variation of the ISI before injection and CVa is the coefficient of variation of ISI after the compound injection. Our results suggested that distribution of ISI intervals in many experiments is not Gaussian and cannot be described by any known distribution function. Therefore, mean value and variance of ISI intervals were calculated directly from the data.
The expected mean value of ISI is calculated according to Eq. 5:
| (5) |
The variance of ISI is calculated from Eq. 6:
| (6) |
Standard deviation is calculated from Eq. 7:
| (7) |
The CV is calculated from Eq. 8:
| (8) |
RESULTS
Negative pharmacological modulation of SK channels increases irregularity in firing patterns of PCs in vivo.
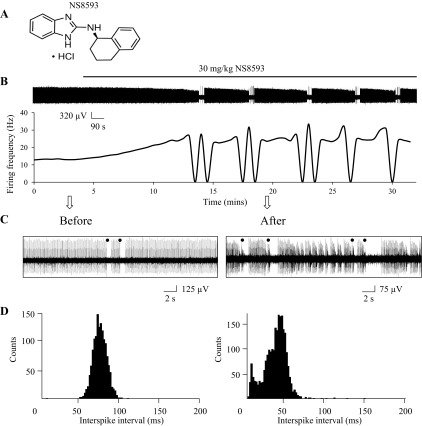
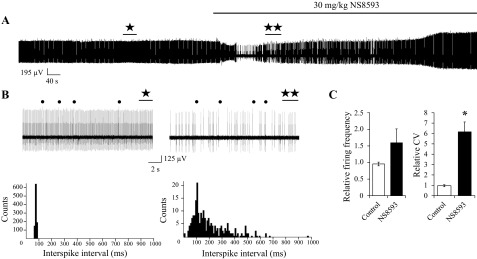
Recordings with cerebellar slices have demonstrated that small-conductance calcium-activated potassium channels (SK channels) play a key role in the control of pacemaker activity in the cerebellar PCs (Womack and Khodakhah, 2003). To confirm that SK modulators can affect PC firing activity in vivo, we performed a series of extracellular single-unit recordings of PC activity with anesthetized WT mice at the age of 2 mo. In earlier experiments we demonstrated that direct application of SK-positive modulators CyPPA and NS309 to open cerebellum resulted in time-dependent reduction in firing frequency of PCs in WT mice (Egorova et al. 2014). In the present study we set out to test functional effects of systemic administration of SK channel modulators. It was previously reported that intravenous administration of SK channel inhibitor NS8593 (Fig. 1A) leads to induction of bursting electrophysiological activity of dopaminergic neurons in substantia nigra (Herrik et al. 2010). In our experiments, intraperitoneal (IP) injections of 30 mg/kg NS8593 resulted in conversion of tonic PC firing pattern to bursting activity in 50% of experiments (7 of 13; Fig. 1, B and C). To study this conversion quantitatively, we performed the analysis of running average firing frequency (Fig. 1B, bottom). Silent periods can be clearly observed in the recording trace (Fig. 1B, top) and in the plot of running average firing frequency (Fig. 1B, bottom). In addition, we calculated the ISI distributions before and after injection with NS8593 (Fig. 1D). We observed two peaks on the ISI distribution histogram following injection with NS8593 (Fig. 1D, right). In the remaining experiments (6 of 13), injection of NS8593 resulted in an irregular firing pattern (Fig. 2, A and B). To analyze these changes quantitatively, we calculated distribution of the interspike intervals (ISI) before and after NS8593 injection in these experiments (Fig. 2B, bottom). As a control, we performed experiments with a group of mice injected with vehicle solution (n = 4). We determined that NS8593 administration increased the simple spikes firing rate by 63 ± 36% (n = 6) compared with the control group, but the difference was not statistically significant (Fig. 2C). The PC firing rate became significantly less regular following injection with NS8593, with a 523 ± 83% (n = 6, P < 0.05) increase in the coefficient of ISI variation (CV ISI) compared with that in the control group of mice (Fig. 2C). The complex spike firing frequency was not significantly affected, with an increase of only 3 ± 5% (data not shown). Generation of complex spikes in PCs was reported to be controlled by large-conductance calcium-activated potassium (BK) channels (Chen et al. 2010), which are not affected by NS8593 (Strobaek et al. 2006). From these experiments we concluded that inhibition of SK channels by systemic administration of NS8593 impairs regular firing of cerebellar PCs and causes bursting firing behavior in vivo.
Fig. 1.
Negative pharmacological modulator of SK channels induces bursting activity of PCs in vivo. A: chemical structure of SK channel negative modulator NS8593. B: continuous 30-min recording of PC activity. The time of 30 mg/kg NS8593 IP injection is indicated by a horizontal bar above the recording. A plot of the running average of firing frequency is shown below the recording. C: 20-s fragments of PC activity recordings before injection of NS8593 and 17 min after injection are shown on the expanded timescale. Complex spikes are marked by filled circles. D: ISI distributions were calculated from recordings 120 s in duration before (left) and after (right) injection with NS8593.
Fig. 2.
Negative pharmacological modulator of SK channels induces irregular firing pattern of PCs in vivo. A: continuous 12-min recording of PC activity. The time of 30 mg/kg NS8593 IP injection is indicated by a horizontal bar. B: 15-s recordings of PC activity before (⋆) and after (★⋆) injection of NS8593 are shown on the expanded timescale (top). Corresponding regions are labeled on continuous trace in A. Complex spikes are marked by filled circles. ISI distributions (bottom) are calculated from recordings 60 s in duration before (left) and after (right) injection of NS8593. C: effects of NS8593 IP injection on firing frequency (left) and variability (right) of PC firing. The firing frequency and ISI variability for each cell were normalized to the firing frequency and ISI variability (CV) of the same cell recorded before injection of NS8593. A similar analysis was performed for a control group of mice injected with vehicle alone. The data were averaged and are presented as means ± SE (n = 6 for NS8593 group, n = 4 for control group). *P < 0.05.
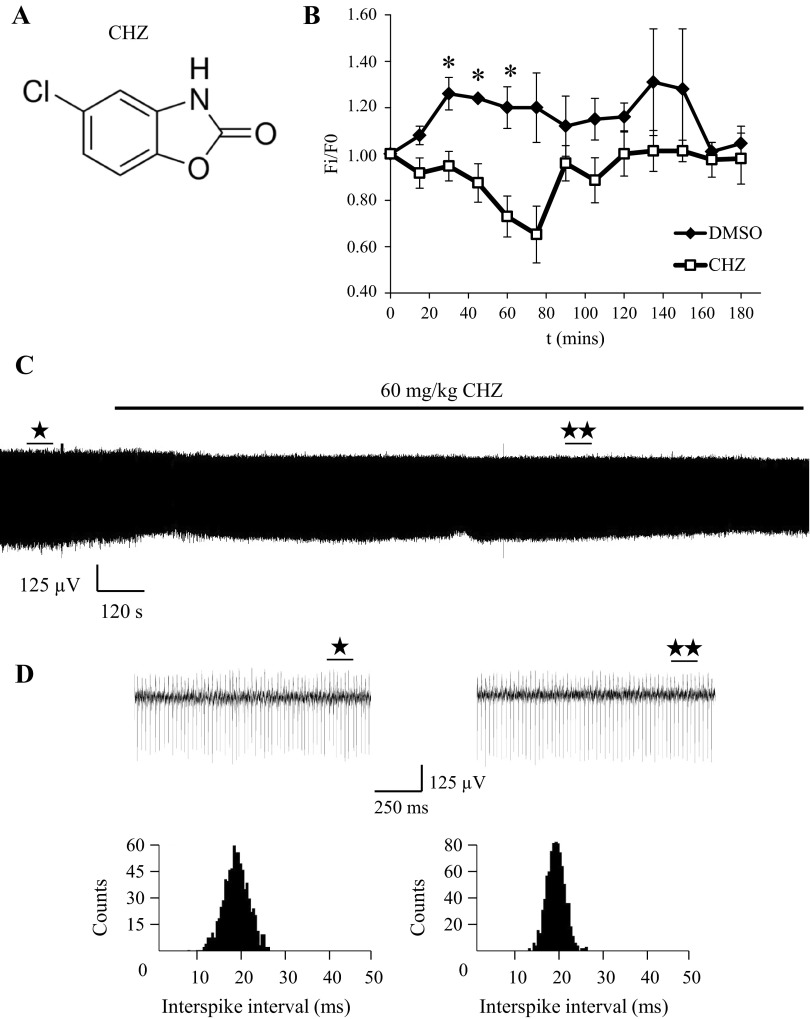
SK channel activator CHZ decreases firing frequency of cerebellar PCs in vivo.
In the next series of experiments we used a similar approach to evaluate functional effects of the positive SK channel modulator CHZ in WT mice at the age of 2 mo (Fig. 3A). CHZ was reported to enhance the activity of intermediate-conductance (IK) and SK channel subtypes (Cao et al. 2001). One hour following 60 mg/kg IP injection of CHZ in C57BL/6 mice, the total brain concentration of this compound is 28.5 μM with a free compound concentration of 3.6 μM (data provided by Ataxion). In our experiments the IP administration of 60 mg/kg CHZ (n = 6) led to a reversible reduction of PC firing frequency within 30–60 min after injection compared with vehicle-injected mice (n = 4; Fig. 3B). A typical example of a PC activity recording is shown in Fig. 3C. The fragments of recordings collected 5 min before and 40 min after CHZ injection are shown on the expanded timescale in Fig. 3D. Analysis of corresponding ISI distributions (Fig. 3D) demonstrated that the CV ISI was reduced on average by 17 ± 7% (n = 6) following injection of CHZ and by 4 ± 9% (n = 4) following injection of the vehicle solution. Changes in CV ISI were not statistically significant between the CHZ-injected and the vehicle-injected groups. Sixty minutes after injection, the firing frequency of complex spikes was decreased on average by 11 ± 6% (n = 6) following CHZ injection and by 5 ± 11% (n = 4) following control injection (data not shown). The changes in complex spike firing rate were not statistically significant. From these experiments we concluded that IP injection with CHZ causes a reduction in simple spike firing frequency and has no significant effect on variability of firing for tonically firing PCs in vivo.
Fig. 3.
Positive modulator of SK channels decreases spontaneous firing rate of PCs in vivo. A: chemical structure of CHZ, a positive modulator of SK channels. B: effects of CHZ on spontaneous firing frequency of PCs. For each cell the firing frequency is normalized to the firing frequency at the time point of IP drug injection (F0). The normalized and averaged firing frequency (Fi/F0) is plotted for different time points (means ± SE) for mice injected with 60 mg/kg CHZ (n = 6, □) and for mice injected with vehicle solution (n = 4, ⧫). *P < 0.05. C: continuous 45-min recording of PC activity. The time of 60 mg/kg CHZ IP injection is indicated by a horizontal bar above the trace. D: expanded traces of PC activity obtained before (⋆) and after (★⋆) injection of CHZ (top). Corresponding regions are labeled on the continuous trace in C. ISI distributions (bottom) are calculated from recordings 15 s in duration before and after CHZ injection.
Extracellular recording reveal alterations in spontaneous activity of PCs in aging SCA2 mice.
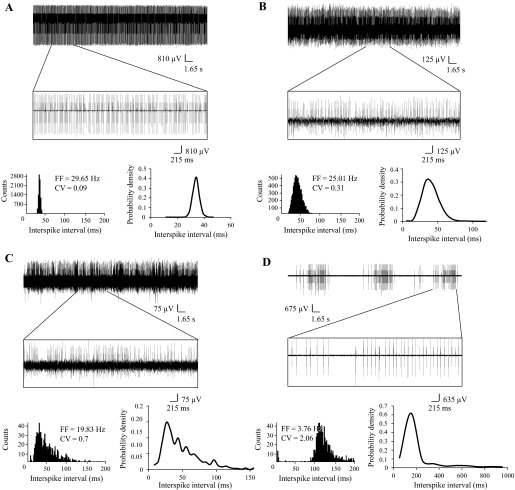
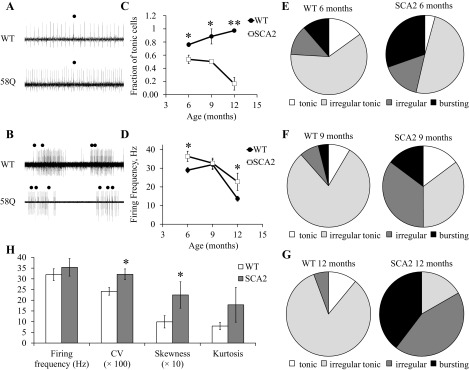
In previous experiments with cerebellar slices we demonstrated that aging PCs from SCA2-58Q mice on average fire less frequently and less regularly than age-matched PCs from WT mice (Kasumu et al. 2012a, 2012b). To validate these findings, we performed a series of in vivo recordings of PC activity in WT and SCA2-58Q mice at different ages. In these experiments we analyzed spontaneous activity of 170 PCs from WT and SCA2-58Q mice. In each experiment, PC activity was classified as tonic (Fig. 4A), irregularly tonic (Fig. 4B), irregular (Fig. 4C), and bursting (Fig. 4D). This classification was based on analysis of a 30-s recording period 5–10 min after initiation of the recording. The PC activity was classified as tonic (Fig. 4A) if the probability density function of ISI had a Gaussian shape (Fig. 4A, bottom left) and CV ISI was less than 0.15. The firing pattern of PC was characterized as irregularly tonic (Fig. 4B) if the probability density function of ISI had a Poisson shape (Fig. 4B, bottom left) and CV ISI was in the range between 0.15 and 0.5. The PC firing activity was classified as irregular (Fig. 4C) if patterns of activity with different firing frequencies were clearly observed (Fig. 4C, top), the probability density of ISI had a shape that could not be related to any known statistical distribution (Fig. 4C, bottom left), and CV ISI was greater than 0.5. The PCs were classified as bursting (Fig. 4D) if firing patterns consisted of clearly separated bursts of activity and silent periods (Fig. 4D, top), resulting in an ISI distribution that had two peaks (Fig. 4D, bottom right). A cell was also characterized as bursting if more than 5% of the ISIs fell outside 3 SD from the mean of all ISIs in the same cell (Kasumu et al. 2012a, 2012b).
Fig. 4.
Spontaneous in vivo firing patterns of SCA2-58Q PCs. A: example of PC with tonic activity pattern. Top, continuous 40-s recording of PC activity with enlarged 5-s fragment; bottom left, ISI distribution; bottom right, probability density distribution of ISI obtained from a 30-s fragment of the same recording. Average firing frequency (FF) for this cell is 29.65 Hz, and normalized CV is 0.09. B: example of PC with irregular tonic activity pattern. Top, continuous 40-s recording of PC activity with enlarged 5-s fragment; bottom left, ISI distribution; bottom right, probability density distribution of ISI obtained from a 30-s fragment of the same recording. Average FF for this cell is 25 Hz, and normalized CV is 0.31. C: example of PC with irregular activity pattern. Top, continuous 40-s recording of PC activity with enlarged 5-s fragment; bottom left, ISI distribution; bottom right, probability density distribution of ISI obtained from a 30-s fragment of the same recording. Average FF for this cell is 19.83 Hz, and normalized CV is 0.7. D: example of PC with bursting activity pattern. Top, continuous 40-s recording of PC activity with enlarged 5-s fragment; bottom left, ISI distribution; bottom right, probability density distribution of ISI obtained from a 30-s fragment of the same recording. Average FF for this cell is 3.76 Hz, and normalized CV is 2.06.
In our experiments we performed recordings of PCs activity from 6-, 9-, and 12 mo-old SCA2-58Q mice and from their age-matched WT littermates. Both tonic (Fig. 5A) and bursting (Fig. 5B) cells were observed in WT and SCA2-58Q mice. For each mouse an average proportion of tonic, irregular tonic, irregular, and bursting PCs was determined. To simplify the comparison, for each experimental group we calculated the fraction of PCs with tonic activity (which included tonic and irregularly tonic cells) and bursting activity (which included irregular and bursting cells). In 6-mo-old WT mice (m = 3 mice), 76 ± 3% of PCs (n = 26 cells) displayed tonic activity. The fraction of tonic cells in SCA2-58Q mice of the same age was 54 ± 6% (n = 34 cells, m = 4 mice), significantly (P < 0.05) lower (Fig. 5C). At 9 mo of age, 89 ± 12% of cells (n = 19 cells, m = 2 mice) displayed tonic firing behavior in WT mice and only 50 ± 3% (n = 28 cells, m = 3 mice, P < 0.05) in SCA2-58Q mice (Fig. 5C). At 12 mo of age, 97 ± 3% of PCs (n = 23 cells, m = 3 mice) had tonic activity in WT mice and only 17 ± 10% (n = 15 cells, m = 4 mice, P < 0.01) in SCA2-58Q mice (Fig. 5C). We also analyzed the average simple spike firing frequency for tonically firing WT and SCA2-58Q PCs for each age and genotype. We found that the average PC firing frequency for 6-mo-old WT mice was 29 ± 1 Hz (n = 22 cells), and for age-matched SCA2-58Q mice it was 36 ± 3 Hz (n = 24 cells, P < 0.05; Fig. 5D). At 9 mo of age, both WT and SCA2-58Q tonic PCs were firing with a similar average frequency of 32 Hz (Fig. 5D). At 12 mo of age, tonic WT cells were firing on average at 14 ± 1 Hz (n = 22 cells), and tonic SCA2-58Q cells were firing on average at 23 ± 5 Hz (n = 6 cells, P < 0.05; Fig. 5D).
Fig. 5.
In vivo electrophysiological phenotype of SCA2-58Q cerebellar PCs. A: examples of irregular tonic PC activity in 6-mo-old WT and SCA2-58Q mice. Recording traces 2 s in duration are shown. Complex spikes are marked by filled circles. B: examples of bursting PC activity in 6-mo-old WT and SCA2-58Q mice. Recording traces 15 s in duration are shown. Complex spikes are marked by filled circles. C: the combined fraction of tonic and irregular tonic cells is shown for WT and SCA2-58Q mice as a function of age. An average proportion of tonic and irregular tonic PCs was calculated for each mouse, averaged across different mice from each age group and genotype, and plotted. Data are means ± SE (n = 26, 19, and 23 cells for WT mice, m = 3, 2, and 3 mice, respectively; n = 34, 28, and 15 cells for SCA2-58Q mice, m = 4, 3, and 4 mice, respectively). Statistical comparison was performed between the genotypes for the same age group. *P < 0.05; **P < 0.01. D: the average firing frequency of tonic and irregular tonic cells is shown for WT and SCA2-58Q mice as a fraction of age. For each age group and genotype, an average firing frequency of tonic and irregular tonic PCs from all mice was determined. Data are means ± SE (n = 22, 15, and 22 cells for WT mice; n = 24, 14, and 6 cells for SCA2-58Q mice). Statistical comparison was performed between the genotypes for the same age group. *P < 0.05. E–G: distribution of observed patterns of PC activity for WT and SCA2 mice at 6 mo of age (E; m = 3 and 4 mice), 9 mo of age (F; m = 2 and 3 mice), and 12 mo of age (G; m = 3 and 4 mice). An average proportion of tonic, irregular tonic, irregular, and bursting PCs was calculated for each mouse, averaged across different mice from the same age group and genotype, and plotted on a pie chart. H: bistability analysis in PCs with irregular tonic activity from 9-mo-old WT and SCA2 mice (m = 3 and 2 mice, respectively). Average firing frequency and the shape of the simple spike ISI distributions (CV, skewness, and kurtosis) were calculated for WT (n = 14 cells) and SCA2 (n = 8 cells) PCs. *P < 0.05.
One potential explanation for changes in PC firing patterns is a change in bistability of the resting membrane potential (Loewenstein et al. 2005; Schonewille et al. 2006). To evaluate the levels of bistability in WT and SCA2 mice, we compared the features of irregular PC tonic activity in 9-mo-old WT and SCA2 mice (Fig. 5H). In the analysis of simple spike ISI distributions we discovered that the CV and the skewness of simple spike distributions were significantly higher in SCA2 PCs than in WT PCs, but the mean firing frequency was not significantly different (Fig. 5H). The kurtosis value was also elevated in SCA2 PCs, but the difference compared with that in WT PCs did not reach statistical significance (Fig. 5H). These results suggest that the level of PC bistability is higher in 9-mo-old anesthetized SCA2 mice than in age-matched anesthetized WT mice. This difference in bistability may contribute to differences in PC firing patterns observed in WT and SCA2 mice (Fig. 5).
We further analyzed the firing frequency of complex spikes for WT and SCA2-58Q PCs. We discovered that the average PC complex spike firing frequency for 6-mo-old WT mice was 387 ± 158 mHz (n = 22 cells), and for age-matched SCA2-58Q mice it was 355 ± 152 mHz (n = 24 cells; no significant difference, n.s.). At 9 mo of age, complex spike firing frequency for WT PCs was 275 ± 144 mHz (n = 19 cells), and complex spike firing frequency for SCA2-58Q PCs was on average 159 ± 64 mHz (n = 28 cells; n.s.). At 12 mo of age, tonic WT cells were firing complex spikes on average at 181 ± 118 mHz (n = 22 cells), and tonic SCA2-58Q cells were firing complex spikes on average at 154 ± 113 mHz (n = 6 cells; n.s.; data not shown). Therefore, we did not detect any significant difference in complex spike firing frequency between WT and SCA2-58Q PCs at any age.
We also compared the frequency of different activity patterns for each group of mice. For this comparison the frequency of each pattern of activity was averaged across different mice from each age group and genotype (Fig. 5, E–G). We discovered that in 6-mo-old WT mice (m = 3 mice) 15 ± 15% of PCs were tonic, 61 ± 12% were irregular tonic, 13 ± 9% were irregular, and 11 ± 7% were bursting (Fig. 5E). For SCA2-58Q mice of the same age (m = 4 mice), 4 ± 4% of PCs were tonic, 49 ± 8% were irregularly tonic, 16 ± 4% were irregular, and 30 ± 6% were bursting (Fig. 5E). For 9-mo-old WT mice (m = 2 mice), 9 ± 9% of PCs were tonic, 80 ± 3% were irregular tonic, 8 ± 8% were irregular, and 4 ± 4% were bursting (Fig. 5F). For SCA2-58Q mice of the same age (m = 3 mice), 15 ± 3% of PCs were tonic, 35 ± 5% were irregular tonic, 35 ± 5% were irregular, and 15 ± 8% were bursting (Fig. 5F). Finally, for WT mice from the oldest age group (12 mo old, m = 3 mice), 11 ± 7% of PCs were tonic, 83 ± 8% were irregularly tonic, and 6 ± 6% were irregular (Fig. 5G). No bursting cells were detected in this age group. For SCA2 mice at the same age (m = 4 mice), no PCs firing tonically were detected, 17 ± 10% of PCs were irregular tonic, 44 ± 21% were irregular, and 40 ± 15% were bursting (Fig. 5G). From this analysis we concluded that there is a dramatic shift from tonic to bursting firing pattern in aging SCA2-58Q mice but not in aging WT mice (Fig. 5, E–G). In the previous study we performed quantification of PC cell pathology in 12-mo-old WT and SCA2-58Q mice by using a dark cell degeneration (DCD) paradigm. In that analysis we discovered that 72% of WT PCs were scored as normal and only 12% of SCA2 PCs were defined as normal (Kasumu et al. 2012a). Thus there is a good correlation between fraction of tonically firing PC cells and “normal” PC cells as defined by DCD when 12-mo-old WT and SCA2-58Q mice are compared.
Obtained in vivo recordings suggest that the number of bursting and irregular cells increases and the number of tonic cells decreases dramatically in aging SCA2-58Q mice (Fig. 5, C and E–G). This conclusion is consistent with our previous studies with cerebellar slices from WT and SCA2-58Q mice (Kasumu et al. 2012a, 2012b). In studies with slices we also previously observed that tonic PCs in SCA2-58Q mice fire less frequently than in WT mice (Kasumu et al. 2012a, 2012b). Similar reduction in firing frequency of tonic cells was reported on the basis of cerebellar slice recordings from SCA2-127Q mouse PCs (Hansen et al., 013) and from the fast-activating/deactivating voltage-gated potassium channel Kv3.3 mutant mouse PCs (model of SCA13; Hurlock et al. 2008). Interestingly, we did not observe this phenomenon in our in vivo recordings (Fig. 5D). Instead, we discovered that the average firing frequency of tonic cells reduces with age in both WT and SCA2-58Q mice, but the firing frequency in SCA2-58Q mice is the same as or higher than in age-matched WT mice (Fig. 5D). From these results we concluded that an increase in the number of bursting cells and a reduction in the number of tonic cells is most likely the reason for impaired behavioral performance and ataxic phenotype in aging SCA2-58Q mice (Huynh et al. 2000; Kasumu et al. 2012a, 2012b; Liu et al. 2009).
CHZ normalizes firing activity of PCs from aging SCA2-58Q mice.
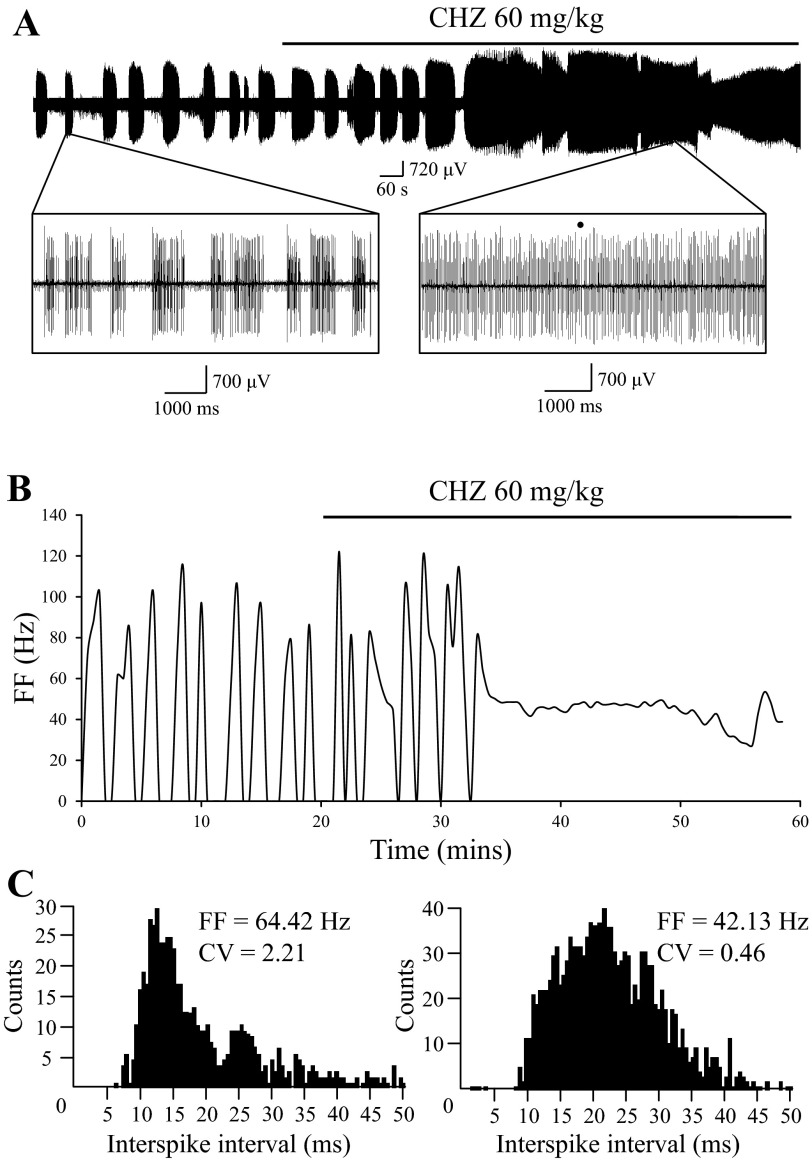
We previously reported that positive SK modulators can normalize bursting firing of SCA2-58Q PCs in cerebellar slice recordings and rescue the behavioral phenotype of aging SCA2-58Q mice (Kasumu et al. 2012a). To investigate if positive modulators of SK channels have similar effects on activity of SCA2 PCs in vivo, we performed extracellular recording of PCs activity in 6-mo-old SCA2-58Q mice before and after IP injections of 60 mg/kg CHZ. Mice 6 mo of age were chosen because they bear anesthesia and surgery better than older mice. For these experiments we selected SCA2-58Q PCs with obvious bursting activity (Fig. 6A). IP injection of 60 mg/kg CHZ resulted in conversion from bursting to a tonic firing pattern in the same cell (Fig. 6A) within 12 min after injection (Fig. 6B). To analyze these results, we calculated a running average of firing frequency, which clearly shows conversion in the firing pattern (Fig. 6B). Next, we analyzed the ISI distribution before and after CHZ injection, obtained from 30-s fragments of the recording (Fig. 6C). The ISI distribution before CHZ injection shows two peaks, whereas the ISI distribution after CHZ injection trends to the normal distribution. We also compared ISI distribution from the recordings 15 min before and 15 min after CHZ injection (Fig. 6A) to calculate the value of parameter χ. We used these distributions to determine corresponding CVb = 1.69 and CVa = 0.51 using Eqs. 5–8 (see materials and methods). With these values, we used Eq. 4 to calculate parameter χ = 0.70 (see materials and methods). The calculated value of parameter χ is close to 1.0, indicating that irregularity in firing of SCA2-58Q PCs was decreased dramatically following IP injection with 60 mg/kg CHZ. Similar results were obtained in another two independent experiments, with resulting χ values of 0.92 and 0.96. Thus we concluded that injection with CHZ results in a reliable increase in regularity of SCA2-58Q PC firing pattern.
Fig. 6.
Systemic administration of CHZ converts bursting PCs to tonic firing mode in SCA2-58Q mice. A: continuous 60-min recording of PC activity in 6-mo-old SCA2-58Q mice. The time of 60 mg/kg CHZ injection is indicated by a horizontal bar above the recording. Fragments of the recording before (8.5 s in duration) and after injection (7 s in duration) are shown on the expanded timescale as indicated. Complex spikes are marked by a filled circle. B: the running average of firing frequency is plotted against time for the experiment shown in A. The time of 60 mg/kg CHZ injection is indicated by a horizontal bar. C: ISI distribution before (left) and after (right) CHZ injection, obtained from 30-s fragments of the recording shown in A. Average firing frequency (FF) for the analyzed fragment before CHZ injection inside the bursts is 64.42 Hz, and the CV of ISI in the analyzed fragment is 2.21. Average FF for the analyzed fragment after CHZ injection is 42.13 Hz, and the CV of ISI is 0.46.
DISCUSSION
Electrophysiological properties of PCs in WT and ataxic mice.
The cerebellum plays an essential role in learning and control of coordinated movements. The precision and speed of these movements requires exact timing of cerebellar output. The inhibitory projections from the PC to the deep cerebellar nuclei (DCN) constitute the sole output of the cerebellar cortex (Ito 2002). PC electrical activity is tightly coordinated at millisecond resolution (de Solages et al. 2008; De Zeeuw et al. 2011; Heck et al. 2007; Person and Raman 2012). PCs spontaneously fire action potentials at a constant frequency in the range 17–150 Hz (Llinas and Sugimori 1980a; Llinas and Sugimori 1980b; Nam and Hockberger 1997; Raman and Bean 1997, 1999; Smith and Otis 2003; Womack and Khodakhah 2002). It is generally believed that the tonic pacemaking activity of PCs is crucial for the correct encoding of cortical cerebellar information to DCN and other motor coordination areas. It has been reported that with increasing age PCs start to fire at a trimodal pattern including the bursting and silent periods (Womack and Khodakhah 2002), which may be related to partial loss of motor control as a result of aging. Cerebellar PCs are affected in many ataxias (Carlson et al. 2009; Matilla-Duenas et al. 2010; Orr and Zoghbi 2007), and massive PC death is observed at the end stage of disease for many ataxic patients. However, it is becoming evident that early symptoms of ataxia may result not from PC death but from PC dysfunction and loss of firing precision. Consistent with this hypothesis, disruptions of regular PC pacemaking activity have been uncovered in studies with mouse models of EA2 (Alvina and Khodakhah 2010a; Walter et al. 2006), SCA3 (Shakkottai et al. 2011), SCA2 (Hansen et al. 2013; Kasumu et al. 2012a), SCA1 (Dell'Orco et al. 2015), and SCA6 (Mark et al. 2015) and in tottering mice with a mutation in P/Q-type voltage-gated calcium channels (Hoebeek et al. 2005).
However, most previous studies with ataxic mouse models were performed in vitro using acutely isolated cerebellar slices (Alvina and Khodakhah 2010b; Dell'Orco et al. 2015; Hansen et al. 2013; Kasumu et al. 2012a, 2012b; Shakkottai et al. 2011; Walter et al. 2006). Functional properties of PCs may be affected by isolation of the slices and differ from their properties in vivo. Extracellular single-unit in vivo recordings were performed with CACNA1A mutant mice encoding the pore-forming subunit of P/Q-type voltage-gated calcium channels (Hoebeek et al. 2005). These experiments revealed that this mutation provokes irregular simple spike activity of PCs without any change in other activity features. The authors concluded that the obtained data support the idea that regularity of firing influences sensorimotor processing (Hoebeek et al. 2005). Another group performed extracellular in vivo recordings of PCs in mice infected with adeno-associated viruses encoding two different human carboxy terminus (CT) splice variants of P/Q-type channel protein (CT-short without polyQs and CT-long with 27 polyQs as expressed in SCA6 patients). It was demonstrated that PCs from the mice expressing CT-longQ27 fire simple spikes less regularly than control cells and cells infected with CT-short (Mark et al. 2015). It has been proposed that many cerebellar ataxia mutations result in changes in spatiotemporal pattern of spiking activities and silent intervals (De Zeeuw et al. 2011). Most of these mutations represent mutations in various Ca2+ signaling proteins and Ca2+ sensors (De Zeeuw et al. 2011), consistent with an important role of intraneuronal Ca2+ homeostasis for PC cell maintenance and survival (Egorova et al. 2015).
In the present study we performed in vivo examination of electrophysiological properties of single PCs from transgenic mice encoding human polyglutamine-expanded protein ataxin-2. Previous electrophysiological analysis of polyQ-expansion mouse mutants was limited to cerebellar slice recordings (Hansen et al. 2013; Kasumu et al. 2012a, 2012b; Shakkottai et al. 2011). The genome of the mice used in our studies contains the insert of human mutant ataxin-2 protein with 58 CAG repeats. The most common mutant CAG repeats in SCA2 patients contain 39–40 repeats (Pulst et al. 2005), and we concluded that using the SCA2-58Q model would enable us to study physiological changes representative of human disease. Recently generated SCA2-82Q (Ingram et al. 2012) and SCA2-127Q (Hansen et al. 2013) mouse models may offer information about physiological changes resulting from large CAG expansions in the Atxn2 gene, as observed in some human SCA2 patients (Babovic-Vuksanovic et al. 1998; Mao et al. 2002).
In the present study we compared the functional properties of PCs from the intact cerebella of WT and SCA2-58Q transgenic mice at different ages using an in vivo recording technique. We performed and analyzed recordings from 170 PCs from WT and SCA2-58Q mice. In this analysis we discovered that PCs display variable firing patterns in vivo. To quantify these results, we classified observed firing patterns into four groups: tonic (Fig. 4A), irregularly tonic (Fig. 4B), irregular (Fig. 4C), and bursting (Fig. 4D). This classification was performed on the basis of analysis of ISI distributions from a 30-s recording period in each experiment. Consistent with the literature (Llinas and Sugimori 1980a, 1980b; Nam and Hockberger 1997; Raman and Bean 1997, 1999; Smith and Otis 2003; Womack and Khodakhah 2002), 80–90% of PCs in WT mice were firing in tonic or irregularly tonic patterns at all ages tested (Fig. 5, C and E–G). In contrast, we observed significant and age-dependent loss of firing precision of PCs in SCA2-58Q mice (Fig. 5, E–G). By 12 mo of age, only 20% of PCs in SCA2-58Q mice were firing in a tonic or irregularly tonic pattern, and 80% of PCs were firing in an irregular or bursting pattern (Fig. 5, C and E–G). These changes are consistent with and even more dramatic than our previous findings in cerebellar slices from aging SCA2-58Q mice (Kasumu et al. 2012a, 2012b).
In addition to studies of firing patterns, we performed the analysis of bistability of PCs irregular tonic activity in 9-mo-old WT and SCA2 mice. Obtained results revealed that in 9-mo-old SCA2 mice the level of bistability is higher than in WT mice of the same age. Although in 9-mo-old WT and SCA2 mice simple spike firing frequency is equal, the shape of simple spike ISI distributions (CV, skewness, and kurtosis) indicates a higher degree of instability in SCA2 mice compared with WT mice (Fig. 5H). Bistability in PCs may play a key role in the processing and storage of sensorimotor information in the cerebellar cortex (Loewenstein et al. 2005). Increased bistability in SCA2 mice may contribute to a less regular PC firing pattern in these mice (Schonewille et al. 2006).
We further discovered that the rate of spontaneous simple spike firing of tonic cerebellar PCs is reduced with age in these mice. An average rate of PC firing in WT mice was equal to 29 Hz at 6 mo of age and 14 Hz at 12 mo of age (Fig. 5D). Interestingly, we discovered that the tonically firing SCA2-58Q PCs fire at the same rate or faster than age-matched WT PCs (Fig. 5D). This is in contrast to the reduction in firing frequency of tonic PCs that we and others observed in experiments with cerebellar slices from SCA2 mouse models (Hansen et al. 2013; Kasumu et al. 2012a, 2012b). From these results we conclude that an increase in the number of bursting cells and a reduction in the number of tonic cells is the most likely reason for impaired behavioral performance and ataxic phenotype in aging SCA2-58Q mice (Huynh et al. 2000; Kasumu et al. 2012a, 2012b; Liu et al. 2009). This is consistent with the hypothesis that we proposed previously based on cerebellar slice recordings with SCA2-58Q mice (Kasumu et al. 2012a, 2012b). The reason for loss of precision firing by SCA2-58Q PCs may be due to toxic effects of mutant Atxn2-58Q and dysregulation of intracellular Ca2+ signaling mediated by inositol (1,4,5)-trisphosphate receptors (InsP3R1; Bezprozvanny 2011; Egorova et al. 2015; Kasumu and Bezprozvanny 2012). In the future it will be important to extend the same approach to analysis of in vivo PCs firing patterns in other polyQ-expansion ataxia models such as SCA1 and SCA3.
In addition to analysis of simple spikes, we also examined complex spike firing frequency of PCs in WT and SCA2-58Q aged mice. PCs generate complex spikes when activated by the olivocerebellar tract, and disturbances in olivocerebellar tract were reported previously for SCA1-82Q mice (Barnes et al. 2011). In our experiments we found that the rate of complex spike firing was reduced with age in both WT and SCA2-58Q mice. The average rate of complex spike firing was lower in 9- and 12-mo-old SCA2 mice than in WT mice, but the difference did not reach a statistically significant level because of large variability in the recorded values. The sagittal zones of cerebellar cortex, which include PCs layer, can be distinguished by the alternate presence and absence of expression of the glycolytic enzyme aldolase C or zebrin (De Zeeuw and Ten Brinke 2015; Zhou et al. 2014). Previous studies revealed that simple spike firing frequency is significantly lower in zebrin-positive PCs compared with zebrin-negative PCs (Zhou et al. 2014). The zebrin-positive PCs are mostly located in the caudal part of the cerebellum, whereas zebrin-negative PCs are mostly observed in the rostral part (Zhou et al. 2014). Electrophysiological recordings in our experiments were performed from cerebellar lobules IV/V in the rostral part of the cerebellum. We did not discriminate between zebrin-positive and zebrin-negative PCs in our experiments, but most likely our recordings were performed primarily in zebrin-negative PCs with some inclusion of zebrin-positive PCs. Simple spike regularity is not different between zebrin-positive and zebrin-negative PCs (Zhou et al. 2014), and therefore our main conclusions regarding reduced regularity in electrophysiological activity of aged SCA2-58Q PCs are not affected by pooling data together from zebrin-positive and zebrin-negative PCs. In further experiments we plan to investigate whether SCA2 PCs degenerate earlier in zebrin-negative zones than in zebrin-positive zones as has been proposed for ataxia models (De Zeeuw and Ten Brinke 2015).
Our recordings were performed in anesthetized mice. Previous studies revealed that there are significant differences in PC firing patterns in anesthetized and awake mice (Arancillo et al. 2015; Schonewille et al. 2006). It was discovered that simple spike and complex spike firing frequencies are higher in awake, behaving mice than in anesthetized mice (Arancillo et al. 2015). It was also discovered that PC cells in awake mice operate mostly in upstate, in contrast to anesthetized mice (Schonewille et al. 2006). However, the degree of regularity in PC firing pattern in anesthetized adult mice was not statistically different from that in awake mice (Arancillo et al. 2015). Moreover, the anesthesia regime did not mask complex spikes, although simple spike and complex spike firing frequencies were different (Arancillo et al. 2015). Importantly, in our studies both WT and SCA2 mice were anesthetized, and therefore observed differences cannot be explained by potential artifacts of anesthesia. In the future we plan to evaluate in vivo electrophysiological properties of PCs in awake, behaving WT and SCA2-58Q mice to avoid potential artifacts of anesthesia and to identify the activity patterns of PCs during different motor tasks.
SK channels as potential therapeutic target for ataxias.
Somatic SK channels are known to play a significant role in the maintenance of the cerebellar PCs pacemaker activity (Womack and Khodakhah 2003). In the present study we demonstrated that IP administration of the negative SK channel modulator NS8593 induced the conversion from tonic activity to irregular and bursting patterns in cerebellar PCs (Figs. 1 and 2). NS8593 is a reversible inhibitor of SK1, SK2, and SK3 channel subtypes, and its action is based on the decreasing Ca2+ sensitivity of these channels (Strobaek et al. 2006). This compound is selective and does not affect intermediate (IK)- and large-conductance (BK) calcium-activated potassium channels (Strobaek et al. 2006). Our results further support a critical role of SK channels in control of cerebellar PC firing regularity in vivo.
Positive activators of SK channels have been proposed to slow down and stabilize firing of PCs in ataxia mouse models. Perfusion with CHZ or 1-EBIO restored the precision of PC pacemaking in experiments with cerebellar slices from the mouse model of EA2 (the tottering mouse; Alvina and Khodakhah 2010a, 2010b; Walter et al. 2006). It was recently demonstrated that systemic application of CHZ promotes more regular simple spike firing in vivo by reducing the hyperexcitability of PCs in CACNA1AS218L transgenic mice (Gao et al. 2012). In our previous studies we demonstrated that NS309 and CyPPA, positive modulators of SK channels, normalized firing of PCs in cerebellar slices from the SCA2-58Q mouse model (Kasumu et al. 2012a). CHZ and 1-EBIO exerted beneficial effects in behavioral experiments with the mouse model of EA2 (Alvina and Khodakhah 2010a, 2010b; Walter et al. 2006). Exposure of SCA3 mice to SKA-31, a riluzole analog optimized for positive modulation of SK channels, provided benefit in a mouse model of SCA3 (Shakkottai et al., 2011). In our previous studies we demonstrated behavioral improvement and neuroprotective effects in SCA2-58Q mice treated with CyPPA or a novel specific positive modulator of SK2/3 channels, NS13001 (Kasumu et al. 2012a). In the present study we demonstrated that IP injection with CHZ resulted in a reversible reduction in firing rate of PCs in vivo (Fig. 3) and was able to convert bursting cells to tonic firing in SCA2-58Q mice (Fig. 6). These results provide further mechanistic support for the hypothesis that positive modulators of SK channels may help to stabilize abnormal firing of ataxic PCs and hold promise as potential therapeutic agents for ataxia. The methods of electrophysiological analysis utilized in the present study may be useful for evaluation of efficacy of such compounds in mouse models of ataxia.
GRANTS
I. Bezprozvanny is a holder of the Carl J. and Hortense M. Thomsen Chair in Alzheimer's Disease Research. P. A. Egorova is the holder of Presidential Stipend 3635.2016.4.
This work was supported by Ataxion, Inc., Ministry of Education and Science of the Russian Federation State Grant 17.1360.2014/K (to I. Bezprozvanny), Russian Scientific Fund Grant 14-25-00024 (to I. Bezprozvanny), UMNIK Grant 1726Γy1/2014 (to P. A. Egorova), and National Institute of Neurological Disorders and Stroke (NINDS) Grant R01NS056224 (to I. Bezprozvanny). The financial support was divided in the following way: experiments depicted in Figs. 1 and 2 were supported by the Russian Federation state grant, experiments depicted in Figs. 3 and 4 were supported by the Russian Scientific Fund, experiments depicted in Figs. 5 were supported by the Presidential Stipend and NINDS, and experiments depicted in Fig. 6 were supported by UMNIK and NINDS.
DISCLOSURES
I. Bezprozvanny is a member of the Scientific Advisory Board of Ataxion Inc.
AUTHOR CONTRIBUTIONS
P.A.E., O.L.V., and I.B. conception and design of research; P.A.E. and O.Z. performed experiments; P.A.E. and O.Z. analyzed data; P.A.E., O.Z., and I.B. interpreted results of experiments; P.A.E. and O.Z. prepared figures; P.A.E. and I.B. drafted manuscript; P.A.E., O.L.V., and I.B. edited and revised manuscript; P.A.E. and O.L.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to members of the Laboratory of Molecular Neurodegeneration, (Peter the Great St. Petersburg Polytechnic University) for advice and suggestions and to Polina Plotnikova for administrative assistance. We are thankful to Kelly Foster and Marty Jefson (Ataxion, Inc.) for comments on the manuscript.
REFERENCES
- Alvina K, Khodakhah K. KCa channels as therapeutic targets in episodic ataxia type-2. J Neurosci 30: 7249–7257, 2010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvina K, Khodakhah K. The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci 30: 7258–7268, 2010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancillo M, White JJ, Lin T, Stay TL, Sillitoe RV. In vivo analysis of Purkinje cell firing properties during postnatal mouse development. J Neurophysiol 113: 578–591, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babovic-Vuksanovic D, Snow K, Patterson MC, Michels VV. Spinocerebellar ataxia type 2 (SCA 2) in an infant with extreme CAG repeat expansion. Am J Med Genet 79: 383–387, 1998. [PubMed] [Google Scholar]
- Barnes JA, Ebner BA, Duvick LA, Gao W, Chen G, Orr HT, Ebner TJ. Abnormalities in the climbing fiber-Purkinje cell circuitry contribute to neuronal dysfunction in ATXN1[82Q] mice. J Neurosci 31: 12778–12789, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. Role of inositol 1,4,5-trisphosphate receptors in pathogenesis of Huntington's disease and spinocerebellar ataxias. Neurochem Res 36: 1186–1197, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Dreixler JC, Roizen JD, Roberts MT, Houamed KM. Modulation of recombinant small-conductance Ca2+-activated K+ channels by the muscle relaxant chlorzoxazone and structurally related compounds. J Pharmacol Exp Ther 296: 683–689, 2001. [PubMed] [Google Scholar]
- Carlson KM, Andresen JM, Orr HT. Emerging pathogenic pathways in the spinocerebellar ataxias. Curr Opin Genet Dev 19: 247–253, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kovalchuk Y, Adelsberger H, Henning HA, Sausbier M, Wietzorrek G, Ruth P, Yarom Y, Konnerth A. Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels. Proc Natl Acad Sci USA 107: 12323–12328, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Orco JM, Wasserman AH, Chopra R, Ingram MA, Hu YS, Singh V, Wulff H, Opal P, Orr HT, Shakkottai VG. Neuronal atrophy early in degenerative ataxia is a compensatory mechanism to regulate membrane excitability. J Neurosci 35: 11292–11307, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Solages C, Szapiro G, Brunel N, Hakim V, Isope P, Buisseret P, Rousseau C, Barbour B, Lena C. High-frequency organization and synchrony of activity in the Purkinje cell layer of the cerebellum. Neuron 58: 775–788, 2008. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoebeek FE, Bosman LW, Schonewille M, Witter L, Koekkoek SK. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci 12: 327–344, 2011. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Ten Brinke MM. Motor learning and the cerebellum. Cold Spring Harb Perspect Biol 7: a021683, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova P, Popugaeva E, Bezprozvanny I. Disturbed calcium signaling in spinocerebellar ataxias and Alzheimer's disease. Semin Cell Dev Biol 40: 127–133, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova PA, Karelina TV, Vlasova OL, Antonov SM, Bezprozvanny IB. [The effect of SK channel modulators on the simple spike firing frequency in discharge of cerebellar Purkinje cells in laboratory mice]. Zh Evol Biokhim Fiziol 50: 101–108, 2014. [PubMed] [Google Scholar]
- Gao Z, Todorov B, Barrett CF, van Dorp S, Ferrari MD, van den Maagdenberg AM, De Zeeuw CI, Hoebeek FE. Cerebellar ataxia by enhanced CaV2.1 currents is alleviated by Ca2+-dependent K+-channel activators in Cacna1a(S218L) mutant mice. J Neurosci 32: 15533–15546, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ST, Meera P, Otis TS, Pulst SM. Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2. Hum Mol Genet 22: 271–283, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DH, Thach WT, Keating JG. On-beam synchrony in the cerebellum as the mechanism for the timing and coordination of movement. Proc Natl Acad Sci USA 104: 7658–7663, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrik KF, Christophersen P, Shepard PD. Pharmacological modulation of the gating properties of small conductance Ca2+-activated K+ channels alters the firing pattern of dopamine neurons in vivo. J Neurophysiol 104: 1726–1735, 2010. [DOI] [PubMed] [Google Scholar]
- Hoebeek FE, Stahl JS, van Alphen AM, Schonewille M, Luo C, Rutteman M, van den Maagdenberg AM, Molenaar R, Goossens HH, Frens MA, De Zeeuw CI. Increased noise level of Purkinje cell activities minimizes impact of their modulation during sensorimotor control. Neuron 45: 953–965, 2005. [DOI] [PubMed] [Google Scholar]
- Hurlock EC, McMahon A, Joho RH. Purkinje-cell-restricted restoration of Kv3.3 function restores complex spikes and rescues motor coordination in Kcnc3 mutants. J Neurosci 28: 4640–4648, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh DP, Figueroa K, Hoang N, Pulst SM. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet 26: 44–50, 2000. [DOI] [PubMed] [Google Scholar]
- Ingram MA, Orr HT, Clark HB. Genetically engineered mouse models of the trinucleotide-repeat spinocerebellar ataxias. Brain Res Bull 88: 33–42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning. Ann NY Acad Sci 978: 273–288, 2002. [DOI] [PubMed] [Google Scholar]
- Kasumu A, Bezprozvanny I. Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias. Cerebellum 11: 630–639, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasumu AW, Hougaard C, Rode F, Jacobsen TA, Sabatier JM, Eriksen BL, Strobaek D, Liang X, Egorova P, Vorontsova D, Christophersen P, Ronn LC, Bezprozvanny I. Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem Biol 19: 1340–1353, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasumu AW, Liang X, Egorova P, Vorontsova D, Bezprozvanny I. Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar Purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci 32: 12786–12796, 2012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, Pulst SM, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci 29: 9148–9162, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol 305: 197–213, 1980a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol 305: 171–195, 1980b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, Hausser M. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat Neurosci 8: 202–211, 2005. [DOI] [PubMed] [Google Scholar]
- Mao R, Aylsworth AS, Potter N, Wilson WG, Breningstall G, Wick MJ, Babovic-Vuksanovic D, Nance M, Patterson MC, Gomez CM, Snow K. Childhood-onset ataxia: testing for large CAG-repeats in SCA2 and SCA7. Am J Med Genet 110: 338–345, 2002. [DOI] [PubMed] [Google Scholar]
- Mark MD, Krause M, Boele HJ, Kruse W, Pollok S, Kuner T, Dalkara D, Koekkoek S, De Zeeuw CI, Herlitze S. Spinocerebellar ataxia type 6 protein aggregates cause deficits in motor learning and cerebellar plasticity. J Neurosci 35: 8882–8895, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla-Dueñas A, Sánchez I, Corral-Juan M, Dávalos A, Alvarez R, Latorre P. Cellular and molecular pathways triggering neurodegeneration in the spinocerebellar ataxias. Cerebellum 9: 148–166, 2010. [DOI] [PubMed] [Google Scholar]
- Nam SC, Hockberger PE. Analysis of spontaneous electrical activity in cerebellar Purkinje cells acutely isolated from postnatal rats. J Neurobiol 33: 18–32, 1997. [DOI] [PubMed] [Google Scholar]
- Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci 30: 575–621, 2007. [DOI] [PubMed] [Google Scholar]
- Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature 481:502–505, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulst SM, Santos N, Wang D, Yang H, Huynh D, Velazquez L, Figueroa KP. Spinocerebellar ataxia type 2: polyQ repeat variation in the CACNA1A calcium channel modifies age of onset. Brain 128: 2297–2303, 2005. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 17: 4517–4526, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci 19: 1663–1674, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristori G, Romano S, Visconti A, Cannoni S, Spadaro M, Frontali M, Pontieri FE, Vanacore N, Salvetti M. Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology 74: 839–845, 2010. [DOI] [PubMed] [Google Scholar]
- Romano S, Coarelli G, Marcotulli C, Leonardi L, Piccolo F, Spadaro M, Frontali M, Ferraldeschi M, Vulpiani MC, Ponzelli F, Salvetti M, Orzi F, Petrucci A, Vanacore N, Casali C, Ristori G. Riluzole in patients with hereditary cerebellar ataxia: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 14: 985–991, 2015. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Khosrovani S, Winkelman BH, Hoebeek FE, De Jeu MT, Larsen IM, Van der Burg J, Schmolesky MT, Frens MA, De Zeeuw CI. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat Neurosci 9: 459–461; author reply 461, 2006. [DOI] [PubMed] [Google Scholar]
- Shakkottai VG, do Carmo Costa M, Dell'Orco JM, Sankaranarayanan A, Wulff H, Paulson HL. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J Neurosci 31: 13002–13014, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SL, Otis TS. Persistent changes in spontaneous firing of Purkinje neurons triggered by the nitric oxide signaling cascade. J Neurosci 23: 367–372, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobaek D, Hougaard C, Johansen TH, Sorensen US, Nielsen EO, Nielsen KS, Taylor RD, Pedarzani P, Christophersen P. Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons. Mol Pharmacol 70: 1771–1782, 2006. [DOI] [PubMed] [Google Scholar]
- Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci 9: 389–397, 2006. [DOI] [PubMed] [Google Scholar]
- Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci 22: 10603–10612, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons. J Neurosci 23: 2600–2607, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LW, Ruigrok TJ, Hoebeek FE, De Zeeuw CI, Schonewille M. Cerebellar modules operate at different frequencies. Elife 3: e02536, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]