Abstract
Development of the neuronal circuitry involves both Hebbian and homeostatic plasticity mechanisms that orchestrate activity-dependent refinement of the synaptic connectivity. AMPA receptor subunit GluA4 is expressed in hippocampal pyramidal neurons during early postnatal period and is critical for neonatal long-term potentiation; however, its role in homeostatic plasticity is unknown. Here we show that GluA4-dependent plasticity mechanisms allow immature synapses to promptly respond to alterations in network activity. In the neonatal CA3, the threshold for homeostatic plasticity is low, and a 15-h activity blockage with tetrodotoxin triggers homeostatic upregulation of glutamatergic transmission. On the other hand, attenuation of the correlated high-frequency bursting in the CA3-CA1 circuitry leads to weakening of AMPA transmission in CA1, thus reflecting a critical role for Hebbian synapse induction in the developing CA3-CA1. Both of these developmentally restricted forms of plasticity were absent in GluA4−/− mice. These data suggest that GluA4 enables efficient homeostatic upscaling and responsiveness to temporal activity patterns during the critical period of activity-dependent refinement of the circuitry.
Keywords: GluA4, hippocampus, homeostatic plasticity
maturation of neuronal circuits is guided by activity-dependent plasticity that refines and primes the network to perform proper physiological brain functions. Individual synapses are strengthened or weakened via Hebbian mechanisms, while homeostatic plasticity provides a vital counterbalancing mechanism to maintain stability (Hanse et al. 2009; Pozo and Goda 2010; Turrigiano and Nelson 2004; Zhang and Poo 2001). A critical mechanism mediating both Hebbian and homeostatic plasticity is the regulation of the postsynaptic dl-α-amino-3-hydroxy-5-methylisoxazole-propionic acid (AMPA) receptors (AMPARs). For example, in cortical neuron cultures, tetrodotoxin (TTX)-induced synaptic scaling has been shown to be dependent on synaptic accumulation of GluA1 and GluA2-containing AMPARs (e.g., Gainey et al. 2009; O'Brien et al. 1998; Thiagarajan et al. 2005). Less is known about the corresponding mechanisms in the intact developing networks displaying distinct mechanisms of synaptic transmission (e.g., Bolshakov and Siegelbaum 1995; Lauri et al. 2006; Xiao et al. 2004) and characteristic pattern of network activity (Karlsson et al. 2006; Lahtinen et al. 2002; Leinekugel et al. 2002; Palva et al. 2000).
In the developing hippocampus, the early activity comprises intermittent, highly synchronous bursts of action potentials interleaved by asynchronous neuronal firing. This immature-type activity pattern coincides temporally with the development of the CA3-CA1 synapses and is thought to provide the associative signals necessary for Hebbian plasticity (Ben-Ari 2001; Palva et al. 2000). The occurrence of the synchronous bursts of activity is highly sensitive to the balance between GABAergic and glutamatergic transmission within the immature CA3 network (e.g., Bolea et al. 1999; Lamsa et al. 2000; Lauri et al. 2005). Both glutamatergic and GABAergic synapses in area CA3 show a low-threshold for homeostatic plasticity (Colin-Le et al. 2004; Huupponen et al. 2007), suggested to maintain the critical level of excitability necessary for the generation of the stereotypical activity patterns. Furthermore, the ongoing high-frequency burst firing of the CA3 pyramidal neurons is vital for the emerging functional connectivity to the CA1 target neurons: the absence of the synchronous CA3 input results in downregulation of synaptic AMPARs in the CA1 pyramidal neurons and increases the proportion of silent synapses (Huupponen et al. 2013).
The GluA4 AMPAR subunit is transiently and prominently expressed in pyramidal cells at the time of intense synapse formation and reorganization (Monyer et al. 1991; Zhu et al. 2000). Previous data indicate that GluA4 expression is necessary and sufficient to alter the signaling requirements of long-term potentiation (LTP) (Luchkina et al. 2014; Yasuda et al. 2003). Thus, at immature GluA4 expressing synapses, PKA activation is sufficient for LTP induction, while, later on in development, LTP requires activation of CaMKII together with other kinases (Luchkina et al. 2014; Yasuda et al. 2003). These data suggest that GluA4 provides a simple mechanism for activity-dependent regulation of AMPA receptors during Hebbian LTP. However, the role of GluA4 in homeostatic plasticity is not known.
In this study, we have explored the role of GluA4 in the synaptic response to altered patterns of endogenous network activity in the immature hippocampus. We show that, in the absence of GluA4, AMPA transmission is less prone to changes in intrinsic activity. Together with previous findings (Esteban et al. 2003; Luchkina et al. 2014; Zhu et al. 2000), these data suggest that expression of GluA4 at immature synapses is responsible for their greater sensitivity to activity-dependent regulation.
MATERIALS AND METHODS
Experiments were performed on wild-type (WT) and GluA4-deficient (GluA4−/−) mice (4- to 7-day-old), kindly provided by Hannah Monyer (Fuchs et al. 2007). All experiments with animals were done in accordance with the University of Helsinki Animal Welfare Guidelines and approved by the local committee for laboratory animal usage.
Hippocampal slices (400 μm) were prepared using standard methods (Lauri et al. 2006). The slices were used 1–4 h after cutting (“acute slices”) or prepared for incubation. In these experiments, the slices were washed with 1-ml incubation solution containing the following (in mM): 105 NaCl, 3 KCl, 1 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 15 d-glucose, and 25 HEPES, placed on Millicell CM 0.4-μm membrane inserts (Millipore, Bedford, MA) with 1 ml of the above solution, with or without 1 μM TTX or 50 μM carbenoxolone (CBX), and transferred to an incubator (35°C, 5% CO2 in air). Neonatal slices remain viable for 15–22 h under these incubation conditions (Huupponen et al. 2007).
The organotypic hippocampal slices were prepared and maintained, following the Stoppini method (Stoppini et al. 1991) with slight modifications. Briefly, brains from postnatal day 6 (P6) or P7 mice (WT and GluA4−/−) were placed in ice-cold Gey's balanced salt solution (Sigma-Aldrich), supplemented with glucose (6.5 mg/ml) and mounted in liquid agarose (2%). The slices (400 μm) were cut using McIlwain tissue chopper, transferred into Gey's balanced salt solution that was aerated with carbogen (95% O2 and 5% CO2), and were left to recover for 1 h at room temperature. Slices were then placed on semipermeable membrane inserts (Millipore) and cultured in serum-free media containing 96% Neurobasal A medium (Gibco), 2% B27 supplement (Gibco), 1% glutamine (Gibco), and 1% chloramphenicol (Bioline). Media was changed every 2nd day. TTX (1 μM) was added to the culture medium 15–22 h (TTX17) or 45–50 h (TTX48) before electrophysiological recordings. Recordings were done at 7–8 days in vitro.
For electrophysiological recordings, the slices were placed in a submerged recording chamber and perfused with extracellular solution (artificial cerebrospinal fluid) containing the following (mM): 124 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 10–15 d-glucose, 2 CaCl2, bubbled with 5% CO2/95% O2, at 32°C. Whole cell voltage-clamp recordings were made with glass electrodes (3–5 MΩ). For the recordings of AMPAR-mediated miniature excitatory postsynaptic currents (mEPSCs), 100 μM picrotoxin and 1 μM TTX were added to artificial cerebrospinal fluid, and CA1 or CA3 pyramidal cells were voltage clamped at −70 mV with electrodes filled with a solution containing the following (in mM): 130 CsMeSO4, 10 HEPES, 0.5 EGTA, 4 Mg-ATP, 0.3 Na-GTP, 5 QX-314, 8 NaCl, 280–285 mOsm, pH 7.2. Spontaneous network activity was recorded from CA3 pyramidal neurons in acute slices with electrodes filled with a solution containing the following (in mM): 135 potassium-gluconate, 10 HEPES, 5 EGTA, 2 KCl, 2 CaOH2, 4 Mg-ATP, 0.3 Na-GTP, pH 7.2, and voltage clamped at −58 mV. Under these recording conditions, glutamatergic and GABAergic events are observed as inward and outward currents, respectively (Lauri et al. 2005).
All compounds were from Tocris Bioscience, except CBX, which was from Sigma-Aldrich. Data were collected using Axoscope 9.2 (Axon Instruments). Uncompensated series resistance was monitored, and cells were discarded if this parameter varied by >20%. Spontaneous events were analyzed using the template search algorithm in the MiniAnalysis 6.0.3 program (Synaptosoft Decatur). All of the detected events were verified visually, and events with amplitude less than three times the baseline root-mean-square noise level were rejected. For statistical analysis, Student's two-tailed t-test was used, and P < 0.05 was considered as statistically significant.
RESULTS
Neonatal mice lacking GluA4 have no apparent defects in basal glutamatergic transmission or network activity, but show impaired homeostatic scaling in area CA3.
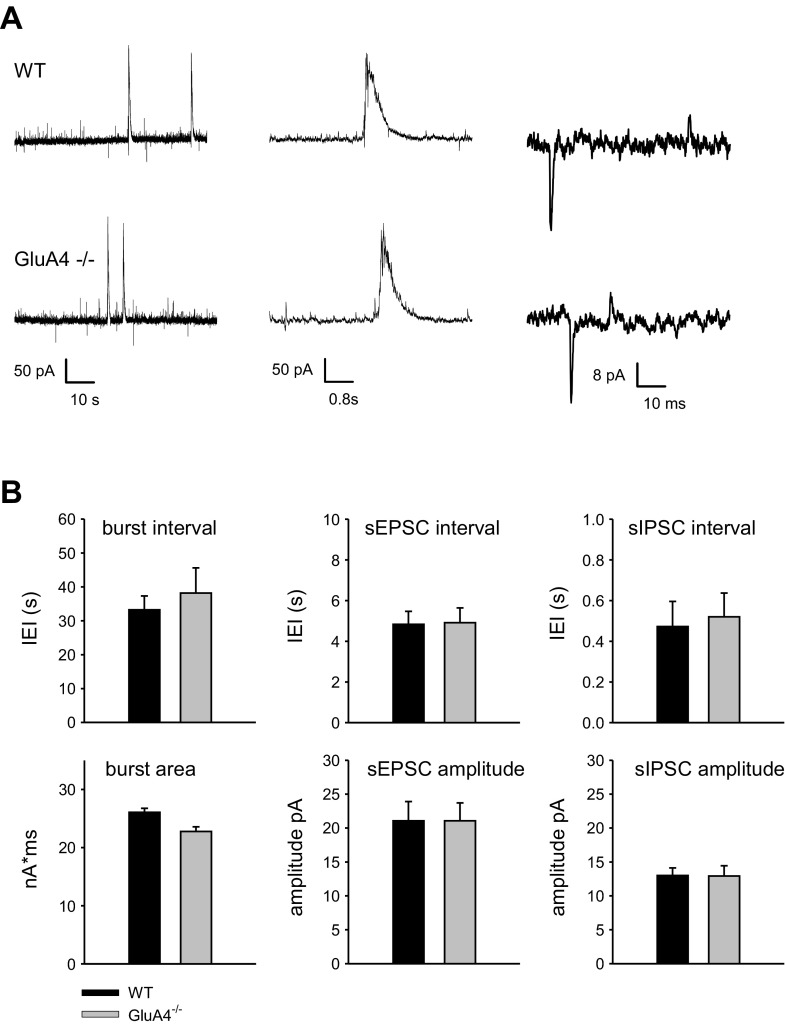
To study the role of GluA4 at developing synapses, acute slices were prepared from neonatal (P4–P6) WT and GluA4−/− mice (Fuchs et al. 2007). At this developmental stage, hippocampal network displays endogenous network activity that is characterized by intermittent bursts of synchronous activity, interspersed with isolated glutamatergic events (spontaneous excitatory postsynaptic currents) and more frequent GABAergic events (spontaneous inhibitory postsynaptic currents) (Ben-Ari et al. 1989; Lamsa et al. 2000). Whole cell recordings of spontaneous activity in CA3 pyramidal neurons indicated no differences in the frequency or area of the network bursts between WT and GluA4−/− mice strains [WT: interevent interval (IEI) 33.2 ± 4.1 s, area 26.1 ± 0.7 nA × ms, n = 6; GluA4−/−: IEI 38.2 ± 7.4 s, area 22.8 ± 0.8 nA × ms, n = 10]. Furthermore, no significant differences in the frequency or amplitude of the spontaneous excitatory postsynaptic currents (WT: IEI 4.8 ± 0.6 s, amplitude 20.2 ± 2.7 pA; GluA4−/−: IEI 4.9 ± 0.9 s, amplitude 20.1 ± 2.7 pA) and spontaneous inhibitory postsynaptic currents (WT: IEI 0.47 ± 0.1 s, amplitude 13.0 ± 1.1 pA; GluA4−/−: IEI 0.52 ± 0.1 s, amplitude 12.9 ± 1.5 pA) were detected between the genotypes (Fig. 1).
Fig. 1.
Spontaneous activity is not altered in GluA4−/− mice. A: example traces of spontaneous activity in CA3 pyramidal neurons from WT and GluA4−/− mice (P4–P6). The traces in the middle depict a typical network burst. Isolated glutamatergic and GABAergic synaptic events are observed as inward and outward currents, respectively. B: averaged data on the frequency and size of network bursts, spontaneous excitatory postsynaptic currents (sEPSCs), and spontaneous GABAergic postsynaptic currents (sIPSCs) in WT (n = 6) and GluA4−/− slices (n = 10). Values are mean ± SE.
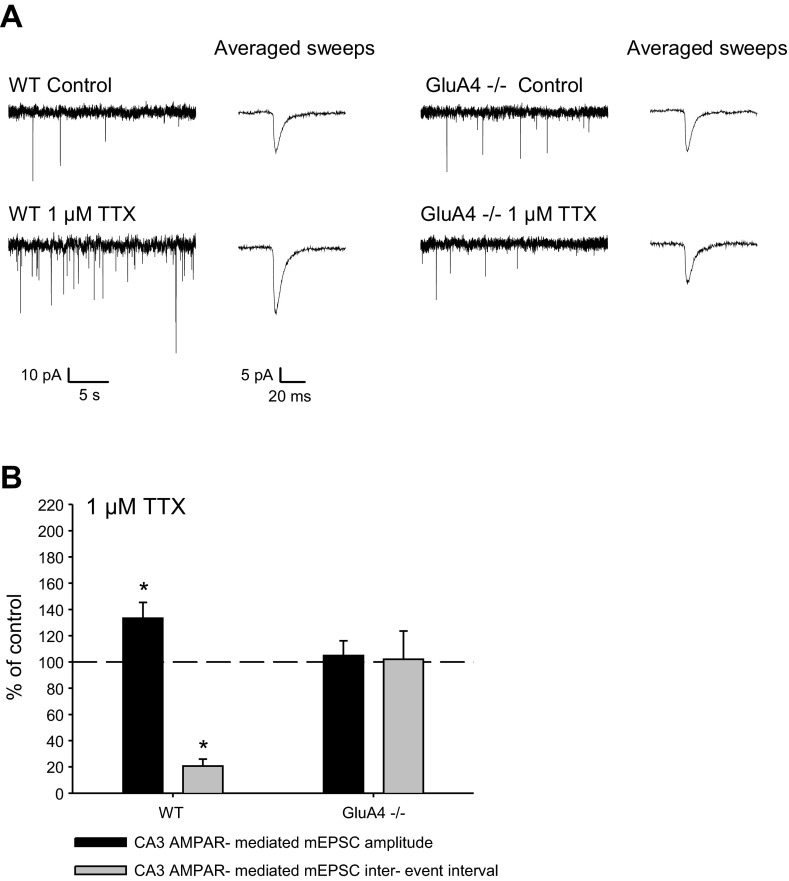
The lack of significant differences in the spontaneous activity levels enabled us to use these mice as a model to study the role of GluA4 in homeostatic regulation in area CA3. To this end, all action potential-dependent transmission was blocked by incubating acute slices from WT or GluA4−/− mice (P4–P6) for 15–22 h with TTX (1 μM), after which the efficacy of glutamatergic transmission was estimated by recording mEPSCs from CA3 pyramidal neurons. In control slices, no difference in mEPSC frequency or amplitude was detected between the genotypes (WT: IEI 3.4 ± 1.0 s, amplitude 22.4 ± 2.1 pA, n = 7; GluA4−/−: IEI 2.7 ± 1.4 s, amplitude 19.2 ± 1.3 pA, n = 8). As expected (Huupponen et al. 2007; Lauri et al. 2003; Turrigiano et al. 1998), TTX treatment resulted in significant upregulation of mEPSC frequency and amplitude in WT slices (IEI 20.6 ± 5.3% of control; amplitude 133.3 ± 12.0% of control, n = 5–7) (Fig. 2). Interestingly, the same treatment had no effect on AMPAR-mediated mEPSCs in GluA4−/− slices (IEI 102.1 ± 21.5% of control; amplitude 104.9 ± 11.3% of control, n = 6–8). These results suggest that GluA4 is critical for the quick (15–22 h) homeostatic upregulation of glutamatergic transmission in the neonatal CA3 pyramidal neurons.
Fig. 2.
The fast homeostatic response to activity deprivation is absent in neonatal GluA4−/− mice. Example traces (A) and pooled data (B) of mEPSCs from CA3 pyramidal cells from WT (control n = 7; TTX n = 5) and GluA4−/− (control n = 8; TTX n = 6) mice after 15–22 h activity block with 1 μM TTX are shown. The traces in expanded time scale depict average of 8 events from each recording. Values are mean ± SE. *P < 0.05.
Extended activity deprivation (48 h) induces homeostatic plasticity via GluA4-independent mechanisms.
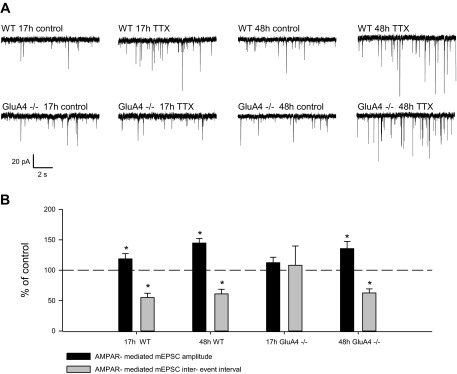
The threshold for the induction of homeostatic plasticity is increased in parallel with the maturation of the hippocampal neurons. While upregulation of glutamatergic transmission is readily observed after >7-h TTX treatment at P4–P6 CA3, longer time periods of inactivity are required for homeostatic response later on in development (Huupponen et al. 2007). To investigate the role of GluA4 in the homeostatic response to prolonged TTX treatment (48 h), we used organotypic slices from WT and GluA4−/− mice. In these experiments, TTX was added 15–22 h (TTX17) or 45–50 h (TTX48) before recording mEPSCs from CA3 pyramidal neurons at 7–8 days in vitro. In WT organotypic slices, the mEPSC frequency and amplitude were significantly higher following brief 17-h (IEI 55.1 ± 7.1% of control; amplitude 118.6 ± 8.6% of control, n = 9–11) and prolonged 48-h (IEI 61.1 ± 7.5% of control; amplitude 144.6 ± 7.7%, n = 6) TTX treatment compared with control slices. In contrast, in the GluA4−/− slices, the homeostatic upregulation of mEPSC frequency and amplitude was seen only after prolonged 48-h activity deprivation (TTX17: IEI 108.1 ± 31.8% of control; amplitude 112.4 ± 8.9% of control, n = 10–11; TTX48: IEI 62.6 ± 6.6% of control; amplitude 135.5 ± 11.8% of control, n = 8–11) (Fig. 3). Again, no significant differences in the mEPSCs were detected between genotypes in the control slices (WT: IEI 0.7 s ± 0.09 s, amplitude 21.7 ± 1.1 pA, n = 15; GluA4−/−: IEI 0.74 ± 0.1 s, amplitude 18.8 ± 0.9 pA, n = 19).
Fig. 3.
Prolonged activity deprivation induces homeostatic plasticity in a GluA4-independent manner. A: examples of mEPSC recordings from CA3 pyramidal neurons in control and TTX-treated (15–22 h or 45–50 h) organotypic hippocampal cultures from WT and GluA4−/− mice. Recordings were made at 7–8 days in vitro. B: pooled data on mEPSC frequency and amplitude in GluA4−/− and WT cultures after 15–22 h TTX (WT control n = 9, TTX n = 11; GluA4−/− control n = 11, TTX n = 10) or 45–50 h TTX (WT control n = 6, TTX n = 6; GluA4−/− control n = 8, TTX n = 11) treatment. Values are mean ± SE. *P < 0.05.
These data suggest that, in the developing CA3 hippocampal network, the low threshold for homeostatic regulation depends on expression of GluA4. Longer periods of inactivity (48) induce homeostatic upregulation of glutamatergic transmission via distinct GluA4-independent mechanisms.
GluA4 subunit is responsible for the lability of glutamatergic transmission at immature CA1 synapses.
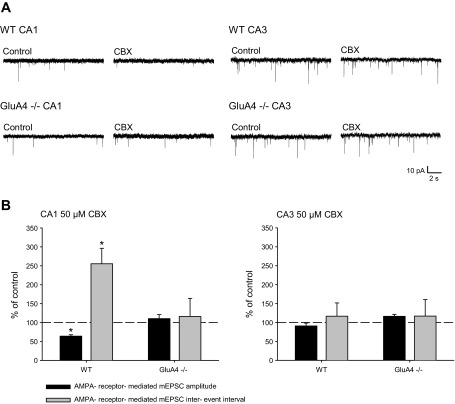
In immature CA1, glutamatergic transmission is sensitive to ongoing synchronous activity, provided by the endogenously occurring network bursts in CA3 pyramidal neurons (Huupponen et al. 2013). During the bursts, CA3 neurons typically fire a train of action potentials at high frequency (Palva et al. 2000). This temporal pattern can be disrupted by application of the gap-junction inhibitor CBX, which results in asynchronous firing of the pyramidal neurons (Huupponen et al. 2013). Long-term (15–20 h) desynchronization of the endogenous neonatal activity by CBX leads to downregulation of AMPAR-mediated transmission at CA1 pyramidal neurons (Huupponen et al. 2013), consistent with the majority of AMPA receptors being functionally labile at immature synapses and requiring continuous high-frequency activity for their maintenance (Hanse et al. 2009). To characterize the role of GluA4 in this process, we treated neonatal WT and GluA4−/− slices with CBX (15–20 h) and subsequently recorded mEPSC from CA3 and CA1 pyramidal neurons. In the WT slices, CBX treatment (15–20 h) led to downregulation of glutamatergic transmission in the CA1 pyramidal neurons, observed as lower frequency (IEI 255.5 ± 40.1% of control, n = 5–6) and amplitude (64.2 ± 3.8% of control) of mEPSCs compared with control slices. In contrast, CBX treatment had no effect on mEPSCs in slices from GluA4−/− mice (IEI 116.2 ± 47.8% of control, amplitude 110.6 ± 10.6% of control, n = 8) (Fig. 4). Consistent with our laboratory's previous data (Luchkina et al. 2014), there were no significant differences in the mEPSC frequency (WT: IEI 2.52 ± 0.97 s, n = 11; GluA4−/−: 2.53 ± 0.61 s, n = 12), amplitude (WT: 16.6 ± 0.90 pA, GluA4−/−: 16.0 ± 1.30 pA) or kinetics (decay90-37 WT 5.1 ± 0.6 ms, GluA4−/−: 5.0 ± 0.2 ms) between the genotypes in CA1 pyramidal neurons in acute control slices.
Fig. 4.
Synaptic response to network desynchronization in area CA1 depends on GluA4 expression. A: example traces of mEPSCs, recorded from CA1 or CA3 pyramidal cells in WT and GluA4−/− slices incubated (15–22 h) under control conditions or in the presence of 50 μM CBX. B: pooled data illustrating mean amplitude and frequency of mEPSCs under various experimental conditions (CA1: WT control n = 5, CBX n = 6; GluA4−/− control n = 8, CBX n = 8; CA3: WT control n = 7, CBX n = 6; GluA4−/− control n = 8, CBX n = 6). The traces in expanded time scale depict average of 8 events from each recording. Values are mean ± SE. *P < 0.05.
In area CA3, the chronic (15–20 h) CBX treatment had no significant effect on mEPSCs in either in WT or GluA4−/− slices (WT: IEI 116.7 ± 35.0% of control, amplitude 91.0 ± 7.6% of control, n = 6–7; GluA4−/−: IEI 117.1 ± 43.6% of control, amplitude 116.3 ± 5.3% of control, n = 6–8), similar to that previously observed in rat slices (Huupponen et al. 2007) (Fig. 4).
These results suggest that GluA4 expression is required to maintain the sensitivity of the developing CA3-CA1 synapses to patterned activity that is endogenously generated in the area CA3. In WT (GluA4 containing) synapses, AMPARs are labile and require ongoing synchronous activity for their maintenance, while GluA4 lacking AMPARs are more stable and able to resist the CBX-induced desynchronization.
DISCUSSION
During the first postnatal weeks, the GluA4 AMPAR subunit is transiently expressed in hippocampal pyramidal cells (Monyer at al. 1991; Zhu et al. 2000), but its physiological role in the maturation of synaptic circuitry is poorly understood. Here we show that expression of GluA4 in the developing hippocampus facilitates synaptic changes to altered network activity. In the area CA3, GluA4 enables low-threshold homeostatic regulation, thought to maintain excitability at a level that is suitable for generation of physiological immature-type activity patterns. In the CA1 target neurons, GluA4 facilitates tuning of the AMPA transmission to the temporally correlated activity patterns that are endogenously generated in the CA3. These developmentally restricted GluA4-dependent plasticity mechanisms may be critical for appropriate formation of the CA3-CA1 circuitry.
GluA4 enables low-threshold homeostatic upregulation in area CA3 of the immature hippocampus.
The mechanisms of homeostatic plasticity are dependent on the developmental stage of the neurons and on the activity manipulation that is used (Burrone et al. 2002; Echegoyen et al. 2007; Hartman et al. 2006; Hou et al. 2008; Huupponen et al. 2007; Ibata et al. 2008; Sutton et al. 2006; Wierenga et al. 2006). In the neonatal CA3 circuitry, the threshold for induction of homeostatic plasticity is low and is increased in parallel with the maturation of the circuitry. Hence, even though a 15- to 22-h activity deprivation causes homeostatic upregulation of mEPSCs at P4, it is not sufficient to induce plasticity at P8 (Huupponen et al. 2007). According to our results, expression of GluA4 could fully explain the low threshold for homeostatic plasticity at immature synapses. Electrophysiological characterization of GluA4−/− mice showed that loss of GluA4 had no significant effect on the frequency of synchronous network bursts or on the spontaneous glutamatergic transmission in the developing hippocampus. However, the neonatal homeostatic response to 15- to 22-h TTX treatment was absent. Prolonged activity deprivation was able to induce homeostatic upregulation also in the absence of GluA4, suggesting that additional GluA4-independent mechanisms, such as TNF-α-dependent homeostatic mechanisms requiring longer induction times (40–48 h) (Beattie et al. 2002; Steinmetz and Turrigiano 2010; Stellwagen and Malenka 2006) coexist.
While evident that the regulation of AMPARs is critical for synaptic scaling, the subunits reported to be responsible for these effects vary, depending on the preparation (e.g., Gainey et al. 2009; Garcia-Bereguiain et al. 2013; Thiagarajan et al. 2005). Recently, data from AMPAR knockout mice indicated that none of the subunits GluA1-3 is absolutely required for homeostatic upregulation (Altimimi and Stellwagen 2013). Similar findings concerning hippocampal LTP (Granger et al. 2013) have raised the hypothesis that the protein composition of the postsynaptic density, rather than the trafficking of individual subunits, is critical for postsynaptic plasticity.
The role of GluA4 subunit in the context of homeostatic regulation has not been studied before. Our present data indicate that, in the absence of GluA4, the properties of homeostatic plasticity are altered, while the homeostatic regulation per se can still be observed in response to prolonged activity block. Similarly, GluA4 is not indispensable for neonatal LTP, but alters its signaling properties (Luchkina et al. 2014; Yasuda et al. 2003). Thus these data suggest that the subunit composition of the AMPARs can contribute to the induction requirements of activity-dependent plasticity, and, furthermore, that GluA4 enables plasticity in response to stimuli that are not strong enough to trigger plasticity at GluA4-lacking synapses. Interestingly, AMPAR activity itself has been recently implicated in the induction of synaptic upscaling (Fong et al. 2015). An intriguing possibility is that the calcium influx via homomeric GluA4 receptors efficiently triggers homeostatic upregulation at immature synapses. During development, this mechanism would be lost in parallel with the loss of the calcium-permeable AMPARs (Kumar et al. 2002; Stubblefield and Benke 2010).
GluA4 facilitates tuning of the AMPA transmission to the temporally correlated activity patterns at immature CA1 synapses.
During the time the CA3-CA1 connectivity is developing, immature CA1 synapses are highly sensitive to the patterned activity generated in the CA3 network. Low-frequency activity in the absence of synchronous high-frequency input leads to destabilization of postsynaptic AMPARs and to an increase in the relative amount of silent synapses in the CA1 (Hanse et al. 2009; Huupponen et al. 2013; Xiao et al. 2004). This phenomenon is thought to guide refinement of the circuitry by eliminating inputs that are not participating in the endogenously generated population activity at the immature hippocampal network (Hanse et al. 2009). Experimentally, the lability of AMPA transmission can be observed as downregulation of mEPSC frequency and amplitude at neonatal CA1 (but not CA3) pyramidal neurons in response to chronic (15–22 h) desynchronization of the network activity by CBX (Huupponen et al. 2013). Interestingly, in contrast to the WTs, CBX treatment had no effect on glutamatergic transmission in GluA4−/− mice. These data, together with the previously observed decrease in the level of GluA4 after CBX treatment (Huupponen et al. 2013), suggest that ongoing synchronous activity is necessary for maintenance of transmission, specifically at GluA4 subunit-containing synapses. Furthermore, the substantial reduction in the mEPSC frequency and amplitude in response to CBX treatment in the WT animals suggests that GluA4-containing AMPAR compose a large subpopulation of the synaptic AMPARs at this developmental stage.
Basal glutamatergic transmission as well as network activity in the GluA4−/− were similar to the WT, suggesting that compensatory GluA4-independent mechanisms account for glutamatergic transmission and plasticity in these mice. In fact, the expression of GluA1 AMPAR subunit is increased in the GluA4−/− mice, and CaMKII-dependent “adult-type” LTP is observed already at the early developmental stages (Luchkina et al. 2014). We cannot fully exclude the possibility that the increased expression of GluA1 might influence the loss of plasticity in response to altered network activity in GluA4−/− mice. However, when expressed in the adult, GluA4 supports immature-type PKA-dependent LTP mechanism, despite the high endogenous expression of GluA1 (Luchkina et al. 2014), indicating that GluA4 expression is sufficient to account for altered mechanism of synaptic plasticity.
Possible mechanisms and physiological significance.
GluA4 is endogenously expressed at a developmental stage when the most of the glutamatergic synapses lack spines and are equipped with distinct scaffolding proteins compared with mature synapses (e.g., Elias et al. 2008; Fiala et al. 1998). While the molecular mechanisms underlying trafficking of GluA4 at immature synapses are not completely understood, the existing data indicate that relatively weak physiological stimuli are sufficient for its synaptic delivery (Zhu et al. 2000). GluA4 lacks the COOH-terminal PDZ ligand interaction motif, as well as the CaMKII phosphorylation site, which are critical in regulating the synaptic trafficking of GluA1 (Hayashi et al. 2000; Shi et al. 2001). The lack of such regulatory elements and structural constraints may ease trafficking of GluA4 in and out of the synapses and thereby allow the GluA4-expressing synapses to readily respond to altered network activity.
During early development, the refinement of the circuitry requires effective and quick mechanisms for activity-dependent modification of glutamatergic connectivity. At the same time, activity-driven changes, such as activation of silent synapses (Durand et al. 1996; Isaac et al. 1995) and alterations in short-term dynamics of transmission (Lauri et al. 2006), render the developing network vulnerable to the runaway excitation. GluA4 expression at the immature synapses appears to contribute to both of these phenomena. Thus GluA4 allows synaptic strength to be highly responsive to alterations in the level of synchrony within the network. On the other hand, the GluA4-dependent homeostatic regulation facilitates balancing of network excitability at the time of intense remodeling of synaptic contacts.
GRANTS
This work was supported by the Academy of Finland and the Sigrid Juselius Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.H., T.T., and S.E.L. designed research; T.A. made organotypic slices; J.H. conducted experiments and analyzed data; J.H. and S.E.L. wrote the paper.
REFERENCES
- Altimimi HF, Stellwagen D. Persistent synaptic scaling independent of AMPA receptor subunit composition. J Neurosci 33: 11763–11767, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNF alpha. Science 295: 2282–2285, 2002. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci 24: 353–360, 2001. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol 416: 303–325, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolea S, Avignone E, Berretta N, Sanchez-Andres JV, Cherubini E. Glutamate controls the induction of GABA-mediated giant depolarizing potentials through AMPA receptors in neonatal rat hippocampal slices. J Neurophysiol 81: 2095–2102, 1999. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Siegelbaum AS. Regulation of hippocampal transmitter release during development and long-term potentiation. Science 269: 1730–1734, 1995. [DOI] [PubMed] [Google Scholar]
- Burrone J, O'Byrne M, Murthy V. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature 420: 414–418, 2002. [DOI] [PubMed] [Google Scholar]
- Colin-Le, Brun I, Ferrand N, Caillard O, Tosetti P, Ben-Ari Y, Gaïarsa J-L. Spontaneous synaptic activity is required for the formation of functional GABAergic synapses in the developing rat hippocampus. J Physiol 559: 129–139, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature 381: 71–75, 1996. [DOI] [PubMed] [Google Scholar]
- Echegoyen J, Neu A, Graber KD, Soltesz I. Homeostatic plasticity studied using in vivo hippocampal activity-blockade: synaptic scaling, intrinsic plasticity and age-dependence. PloS One 2: e700, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias GM, Elias LAB, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci U S A 105: 20953–20958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban AJ, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136–143, 2003. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci 18: 8900–8911, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong M, Newman JP, Potter SM, Wenner P. Upward synaptic scaling is dependent on neurotransmission rather than spiking. Nat Commun 6: 6339, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FEN, Bannerman DM, Rozov A, Whittington AM, Traub RD, Rawlins JNP, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 53: 591–604, 2007. [DOI] [PubMed] [Google Scholar]
- Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci 29: 6479–6489, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bereguiain MA, Gonzalez-Islas C, Lindsly C, Butler E, Hill AW, Wenner P. In vivo synaptic scaling is mediated by GluA2-lacking AMPA receptors in the embryonic spinal cord. J Neurosci 33: 6791–6799, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493: 495–500, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanse E, Taira T, Lauri S, Groc L. Glutamate synapse in developing brain: an integrative perspective beyond the silent state. Trends Neurosci 32: 532–537, 2009. [DOI] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci 9: 642–649, 2006. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267, 2000. [DOI] [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci U S A 105: 775–780, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huupponen J, Molchanova SM, Lauri SE, Taira T. Ongoing intrinsic synchronous activity is required for the functional maturation of CA3-CA1 glutamatergic synapses. Cereb Cortex 23: 2754–2764, 2013. [DOI] [PubMed] [Google Scholar]
- Huupponen J, Molchanova SM, Taira T, Lauri SE. Susceptibility for homeostatic plasticity is down-regulated in parallel with maturation of the rat hippocampal synaptic circuitry. J Physiol 581: 505–514, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57: 819–826, 2008. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron 15: 427–434, 1995. [DOI] [PubMed] [Google Scholar]
- Karlsson K, Mohns E, di Prisco GV, Blumberg MS. On the co-occurrence of startles and hippocampal sharp waves in newborn rats. Hippocampus 965: 959–965, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci 22: 3005–3015, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahtinen H, Palva JM, Sumanen S, Voipio J, Kaila K, Taira T. Postnatal development of rat hippocampal gamma (20–80 Hz) rhythm in vivo. J Neurophysiol 88: 1469–1474, 2002. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Palva JM, Ruusuvuori E, Kaila K, Taira T. Synaptic GABAA activation inhibits AMPA-kainate receptor-mediated bursting in the newborn (P0-P2) rat hippocampus. J Neurophysiol 83: 359–366, 2000. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Lamsa K, Pavlov I, Riekki R, Johnson BE, Molnar E, Rauvala H, Taira T. Activity blockade increases the number of functional synapses in the hippocampus of newborn rats. Mol Cell Neurosci 22: 107–117, 2003. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Segerstråle M, Vesikansa A, Maingret F, Mulle C, Collingridge GL, Isaac JTR, Taira T. Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. J Neurosci 25: 4473–4484, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Vesikansa A, Segerstråle M, Collingridge GL, Isaac JTR, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron 50: 415–429, 2006. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsáki G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science 296: 2049–2052, 2002. [DOI] [PubMed] [Google Scholar]
- Luchkina NV, Huupponen J, Clarke VRJ, Coleman SK, Keinänen K, Taira T, Lauri SE. Developmental switch in the kinase dependency of long-term potentiation depends on expression of GluA4 subunit-containing AMPA receptors. Proc Natl Acad Sci U S A 111: 4321–4326, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Seeburg PH, Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron 6: 799–810, 1991. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron 21: 1067–1078, 1998. [DOI] [PubMed] [Google Scholar]
- Palva JM, Lamsa K, Lauri SE, Rauvala H, Kaila K, Taira T. Fast network oscillations in the newborn rat hippocampus in vitro. J Neurosci 20: 1170–1178, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66: 337–351, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331–343, 2001. [DOI] [PubMed] [Google Scholar]
- Steinmetz CC, Turrigiano GG. Tumor necrosis factor-α signaling maintains the ability of cortical synapses to express synaptic scaling. J Neurosci 30: 14685–14690, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature 440: 1054–1059, 2006. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37: 173–182, 1991. [DOI] [PubMed] [Google Scholar]
- Stubblefield EA, Benke TA. Distinct AMPA-type glutamatergic synapses in developing rat CA1 hippocampus. J Neurophysiol 104: 1899–1912, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125: 785–799, 2006. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47: 725–737, 2005. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391: 892–896, 1998. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Walsh MF, Turrigiano GG. Temporal regulation of the expression locus of homeostatic plasticity. J Neurophysiol 96: 2127–2133, 2006. [DOI] [PubMed] [Google Scholar]
- Xiao MY, Wasling P, Hanse E, Gustafsson B. Creation of AMPA-silent synapses in the neonatal hippocampus. Nat Neurosci 7: 236–243, 2004. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci 6: 15–16, 2003. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci 4, Suppl: 1207–1214, 2001. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-316 containing AMPA receptors by spontaneous activity. Nat Neurosci 3: 1098–1106, 2000. [DOI] [PubMed] [Google Scholar]