Abstract
Objective
To assess if early changes in enhancing tumor volume (eTV) and relative cerebral blood volume (rCBV) one month after convection-enhanced delivery (CED) of topotecan in patients with recurrent malignant glioma correlated with six-month disease progression status.
Materials and Methods
Sixteen patients were enrolled in a Phase Ib trial of convection-enhanced delivery of topotecan for recurrent malignant glioma. Each patient was evaluated with serial follow-up MR imaging at baseline and at 4 to 8 week intervals. Changes at one month compared to baseline in eTV and rCBV were evaluated as potential predictors of six-month progression status, classified as either progressive disease (PD) or nonprogressive disease (NPD). Relationships between percent changes in eTV and rCBV at one month with the probability of progressive disease at six-months were estimated using logistic regression analysis. Receiver operating characteristic (ROC) curves for varying percent change thresholds in eTV and rCBV were evaluated by using six-month progressive disease as the reference.
Results
There was a significant difference in the percent change in rCBV at one month in patients with PD as compared to those with NPD at six-months (+12% vs. -29%, p=0.02). Logistic regression analysis demonstrated on average that a 10% increase in rCBV at one month after CED of topotecan was associated with 1.7 times the odds of developing progressive disease at six-months (95% confidence interval (CI): 1.0, 2.9 p=0.05). ROC analysis for determining progressive disease at six-months showed a greater area under the curve with rCBV (0.867; 95% CI: 0.66, 1.00) than with change in enhancing tumor volume (0.767; 95% CI: 0.51, 1.00).
Conclusion
In this selected population of patients with recurrent malignant glioma treated with convection-enhanced delivery of topotecan, early changes in rCBV at four weeks after therapy may help predict progression status at 6 months.
Keywords: Convection-Enhanced Delivery, Perfusion MRI, Topotecan
Introduction
The dismal prognosis in patients with recurrent malignant glioma has driven interest in the development of a wide array of new therapies, including direct interstitial delivery techniques such as convection-enhanced delivery (CED). CED is a local delivery technique that bypasses the blood-brain barrier by delivering drugs through positive pressure bulk flow into the brain via stereotactically placed catheters. This method is designed to help overcome two major obstacles in malignant glioma therapy: 1) via bypassing the restrictive nature of the blood-brain barrier, which limits the efficacy of many promising chemotherapeutics and 2) by virtue of regional drug delivery, CED may be better suited to address the high rate of local recurrence in malignant glioma as compared to conventional intravenous chemotherapy.
One of the challenges in developing effective therapy for malignant glioma is the absence of non-invasive imaging biomarkers that can accurately determine antitumor effect early during the course of therapy. Contrast-enhanced MRI is the current key element in clinical assessment of treatment response prior to and after combined radiotherapy and temozolomide chemotherapy (RT/TMZ) based on the original Macdonald criteria1 and its recent update by the Response Assessment in Neuro-Oncology Working Group (RANO)2. Early treatment response may be transient and may not necessarily correlate with a long-term favorable outcome. This has proven problematic in conventional and experimental treatment response assessment, as early treatment related imaging changes manifesting as transient increases in contrast enhancement that spontaneously resolve can frequently be indistinguishable from disease progression, commonly referred to as pseudoprogression3. Patients with pseudoprogression have a spurious increase in contrast-enhancing tumor volume weeks to months after treatment for glioma that improves spontaneously, without any changes in their treatment regimen. There is some speculation as to the responsible mechanisms, though pseudoprogression likely reflects an exaggerated response to effective therapy4. Accurate prediction of which tumors that exhibit an increase in contrast-enhancing volume early after treatment represent progressive disease or pseudoprogression has not been definitively established using non-invasive imaging techniques. Definitive diagnosis is dependent upon histology, which must be obtained through invasive procedures. It is of significant clinical interest to identify a non-invasive method of determining which tumors that appear to be growing early after conventional or experimental therapy were truly progressing, and which were exhibiting pseudoprogression, and would eventually respond.
Optimal parameters for non-invasive evaluation of early treatment response to standard or novel therapies such as CED are currently not well defined. Recent advances in multi-parametric magnetic resonance imaging may provide quantitative information that can aid in monitoring therapeutic response and potentially predict clinical outcome early in the course of therapy. Contrast-enhanced perfusion MRI may offer increased specificity regarding the tumor microenvironment before, during and after therapy; this information is not readily available or apparent on conventional contrast-enhanced anatomic MRI.
Perfusion MRI based techniques have demonstrated utility in the grading of gliomas5-8 and have been shown to be a prognostic marker in predicting survival after therapy9-11. Higher-grade tumors tend to have elevated rCBV, which highlights the significant role of vascular proliferation in the biology of high-grade gliomas, given the associations between tumor grade, microvessel density and rCBV12. MR perfusion imaging can play a role in monitoring therapeutic response to either standard11 or experimental therapies in malignant glioma. For example, changes in the volume transfer coefficient as determined by dynamic contrast-enhanced MRI have been shown to correlate with survival even after a single dose of cediranib13, an experimental vascular endothelial growth factor inhibitor.
The purpose of this study was to examine changes in rCBV and enhancing tumor volume parameters in patients with recurrent malignant glioma treated with convection-enhanced delivery of topotecan. Our hypothesis was that changes in rCBV at one month after therapy would correlate with treatment response or failure as determined by six-month progression status and thus potentially circumvent some of the clinical challenges presented by the pseudoprogression phenomenon. In this paper, we used six-month progression status as the clinical endpoint of efficacy, which has been endorsed by the North American Brain Tumor Coalition and used in several other studies in evaluating therapies for newly diagnosed or recurrent malignant glioma patients14-15, in addition to having been shown to be a strong predictor of survival14.
Materials and Methods
Patient Population
Sixteen patients with recurrent or progressive supratentorial malignant glioma (World Health Organization grade III or IV) who underwent CED of topotecan at Columbia University Medical Center as part of a prospective Phase Ib open label, non-randomized trial were retrospectively selected for this Health Insurance Portability and Accountability Act-compliant Institutional Review Board-approved study. All patients in this study had prior therapy with external beam radiation, 15 of 16 patients had prior surgical resection (one patient had stereotactic biopsy and did not have primary surgical resection due to tumor involvement of eloquent regions), and a stereotactically accessible enhancing tumor volume on MRI less than 65cm3. The 16 patients (11 male, 5 female) had a median age of 50 years (range 22-71); 10 patients had glioblastoma multiforme (GBM), 2 had anaplastic astrocytoma, 2 had anaplastic oligodendroglioma, and 2 had anaplastic ependymoma. During their course of prior therapy, 12 patients were administered temozolide, one patient received BCNU only, one patient received BCNU, thiotepa and etoposide, two patients received imatinib mesylate and hydroxyurea and one patient received six courses of bevacizumab and irinotecan. (Please see Table 1 for demographic details). None of the patients received any treatment in the four weeks prior to topotecan CED. The treatment protocol used in this trial and the patients in this cohort have been previously described in detail in a prospective phase 1b study16. At the time of surgery, each patient had tissue biopsy to document tumor recurrence prior to placement of CED catheters. Briefly, one or two tunneled infusion catheters (2.5 mm outer diameter, CSF-peritoneal catheter; Integra; Plainsboro, NJ) were stereotactically placed directly into tumor or adjacent brain at sites chosen to maximize coverage of the tumor and adjacent infiltrated brain tissue based on a spherical distribution. The externalized catheters were connected to a Medfusion 2010 syringe pump (Medex, Inc; Carlsbad, CA). After patients received a 40 ml infusion of topotecan (GlaxoSmithKline; Philadelphia, PA), catheters were removed at the bedside. Patients returned for clinical follow-up evaluation and a contrast-enhanced MRI every four weeks for sixteen weeks, then every eight weeks thereafter, unless there were clinical or radiographic indications for more frequent monitoring.
Table 1.
Demographic details of the study population.
| Patient | Age/Sex | Pathology1 | 6month Progression Status2 | Previous Therapy3 | Tumor Site4 | Time of Dx --> Tx (months) | Pre/Post KPS |
|---|---|---|---|---|---|---|---|
| 1 | 54/M | AE | PD5 | S/RT/Ch | L P | 28 | 70/70 |
| 2 | 64/M | GBM | NPD | RT/Ch | L F/P | 5 | 70/60 |
| 3 | 35/F | AE | PD | S/RT/Ch | L F/P | 100 | 90/90 |
| 4 | 50/M | AO | PD | S/RT | L F | 52 | 80/80 |
| 5 | 67/M | AA | PD | S/RT | L P | 22 | 90/80 |
| 6 | 47/F | GBM | NPD | S/RT/Ch | L T | 59 | 90/90 |
| 7 | 46/M | GBM | NPD | S/RT/Ch | R F | 4 | 90/100 |
| 8 | 59/F | GBM | PD | S/RT/Ch | L P | 5 | 80/90 |
| 9 | 48/M | GBM | PD | S/RT/Ch | R P/O | 10 | 100/100 |
| 10 | 46/M | AO | PD | S/RT | L F | 47 | 100/100 |
| 11 | 50/M | GBM | PD | S/RT/Ch | L P | 26 | 100/90 |
| 12 | 66/M | GBM | PD5 | S/RT/Ch | R F | 11 | 80/80 |
| 13 | 71/F | AA | NPD | S/RT/Ch | R F | 10.5 | 60/50 |
| 14 | 28/M | GBM | NPD | S/RT/Ch | L F | 5.5 | 80/80 |
| 15 | 22/F | GBM | NPD | S/RT/Ch | L P | 7 | 80/80 |
| 16 | 66/M | GBM | PD | S/RT/Ch | R P | 18 | 80/80 |
AE – Anaplastic Ependymoma; GBM – Glioblastoma Multiforme; AO – Anaplastic Oligodendroglioma; AA – Anaplastic Astrocytoma
PD-Progressive disease; NPD-Nonprogressive disease
S: surgery, RT: radiation therapy, Ch: chemotherapy
P: Parietal; F: Frontal; O: Occipital
Patients 1 and 12 had decreasing tumor volume/nonprogressive disease at follow-up, though died of causes unrelated to CED therapy; for the purposes of imaging analysis for this study, these two patients were classified as having progressive disease. Patient 1 had nonprogressive disease at 8 weeks, though died at 8 weeks from comorbid complications unrelated to CED therapy. This patient had a history of preexisting seizures and was hospitalized with fever and seizures approximately 7 weeks post treatment, and suffered a fatal cardiac arrest five days later. This was not considered to be due to the study treatment, as the cause of death was presumed to be a pulmonary embolus, though this remains unproven, since an autopsy was declined by the family. Patient 12 had 4 months of progression-free survival, though died at 4 months after CED therapy from an unrelated bowel perforation.
Progressive disease was defined as a greater than 25% increase in enhancing tumor volume at six months (confirmed at seven months) as compared to baseline before CED therapy. Patients were classified as either developing progressive disease (PD) or nonprogressive disease (NPD). Among 16 patients at six months, six had stable disease or NPD. Ten of 16 patients had progressive disease at six months; two of these patients had decreasing tumor volume at follow-up but died of causes unrelated to CED therapy and for the purposes of imaging analysis for this study, were considered as having PD. The criteria used for progressive disease among this patient cohort for this study used a six-month time point compared to baseline, which differ from our previously published results,16 which considered progressive disease as >25% increase in contrast enhancing volume at one month or later after therapy until surgical resection or death. The criteria used in defining PD in this study takes into account the updated RANO response criteria developed between the time of the two studies. The use of different time points in defining progressive disease and the inclusion of two patients with decreasing tumor volumes who died of causes unrelated to CED though were classified as PD, account for differences in the number of patients classified as PD between our prior published results and the current study. Pseudoprogression was defined as an initial assessment of disease progression using standard (RANO) criteria 1-3 months after CED, and subsequent MRI's demonstrating stable or decreasing enhancing tumor volume for at least two months without changes in corticosteroid dose. Changes in FLAIR volume as described in RANO criteria were not utilized for the purposes of this study due to potential confounding effects on volume measurements from the direct interstitial nature of convection enhanced drug delivery. Among all 16 patients, eight had an increase in tumor volume relative to baseline at one month; four of these eight patients (representing 25% of all patients) had transient elevation in tumor volume followed by a subsequent decrease over time on follow-up imaging, and were considered to have pseudoprogression, though not histologically confirmed. To account for differences in MRI scheduling, the six-month interval to determine progression included a date range from 160 to 200 days.
Imaging Methods
All MR imaging data was obtained at Columbia University Medical Center on 1.5T clinical scanners (Signa HD, GE Healthcare, Milwaukee, Wisconsin, and Philips Achieva, Philips Healthcare, Netherlands) with an 8-channel head coil. Pretreatment MRIs were obtained at an average of four days (range 0-18) prior to topotecan infusion and at four and eight-week intervals following the infusion. MR imaging examinations were obtained according to a standardized protocol: sagittal noncontrast T1-weighted, axial fast spin-echo T2-weighted, axial fluid-attenuated inversion recovery (FLAIR), as well as postcontrast enhanced axial, coronal and sagittal T1-weighted images.
Dynamic susceptibility contrast enhanced (DSC) MR perfusion images were acquired at each imaging session with a gradient-recalled T2* weighted echo planar imaging sequence during the first pass of a standard dose (0.1 to 0.3 mmol/kg) bolus of gadopentate dimeglumine (Magnevist, Bayer Schering Pharma, Berlin, Germany), gadodiamide (GE Healthcare, Princeton, NJ) or gadobenate dimeglumine (Multihance, Bracco Diagnostic, Princeton, NJ). The scanning parameters included TR/TE 1800/27.6ms, 5 mm slice thickness, and a 256 × 256 matrix. Sixty image acquisitions were acquired at 1-second intervals, with the first 10 acquisitions acquired prior to contrast injection to establish a precontrast baseline. At the end of the 10th acquisition, an MR imaging-compatible power injector was used for the intravenous administration of contrast material through an antecubital vein at a rate of 3ml/sec followed by a saline flush of 20cc. No preload dose was administered.
MR Image Processing
Volume of interest analysis of contrast-enhancing tumor was calculated on contrast-enhanced T1-weighted images using manual segmentation (R.L.D), with exclusion of regions of necrosis and surgical defects. Enhancement area (mm2) was multiplied by image slice thickness (mm) to generate the volumes per slice, which were summed to generate total enhancement volume in cm3 (cm3 = mm3/1000).
Raw perfusion data were transferred to a commercially available image processing workstation (Advantage Workstation, GE Healthcare, Waukesha, WI, USA) and post-processed using FuncTool Software (GE Healthcare, Waukesha, WI, USA). Since CBV must be expressed relative to an internal reference, the ratio of the tumor with that of the normal contralateral white matter was used to determine the relative values referred to as rCBV. Since maximum rCBV within the tumor has been reported to be the most reliable value for inter- and intraobserver reproducibility17 in evaluation of tumor vascularity, the maximum rCBV was used as the benchmark parameter of tumor neovascularity. Solid tumor components with or without contrast enhancement on conventional MR images and rCBV maps (approximated by using the negative enhancement integral) were processed, co-registered and reviewed in consensus by two board certified radiologists with Certificates of Added Qualification in Neuroradiology (K.S. 5 years experience, R.L.D 30 years experience) blinded to patient progression status. The beginning and end of the first-pass bolus was evaluated on time-signal intensity curves to only allow the area under the curve of the first-pass bolus to be used for rCBV calculations. This was performed to decrease the confounding effects of contrast agent re-circulation effects and contrast leakage17. Four to eight regions of interest measuring 30mm2 within solid tumor were placed on regions showing the highest intratumoral rCBV on color-coded maps, with exclusion of regions of susceptibility, necrosis or partial volume effects; maximum rCBV values were recorded and used as the benchmark parameter of tumor neovascularity. The conventional MR images and dynamic images sets from the arterial and venous phases were examined to avoid volume-averaging of normal vessels, which can alter rCBV values. A 30mm2 ROI was placed in the contralateral normal appearing white matter to calculate the rCBV. This method has been reported to be the most reliable and reproducible way to obtain ROI-based perfusion metrics17,18.
Statistical Analysis
Statistical Package for Social Sciences (SPSS, Chicago, ILL) software was used for statistical analysis. Percentage change [calculated as % change = 100 * ((value at time 1) – (value at time 0)) / (value at time 0)] in rCBV and eTV at one month after topotecan CED was determined for the two clinical outcome groups (progressive disease, PD, and nonprogressive disease, ND). Logistic regression analysis was performed to assess the relationship between six-month progression status and percent changes at one month compared to baseline in eTV and rCBV. Receiver operator characteristic analysis (ROC) was performed by calculating sensitivity-specificity pairs to determine the optimal cutoff for each parameter by using six-month progression status as the reference criterion. Area under the curve and 95% confidence intervals were calculated for each ROC curve. An additional cutoff in the percent change in rCBV with maximum efficiency was calculated, determined as the proportion of true classifications among all classifications [E=(TP + TN)/(TP + TN + FN + FP), where E=efficiency, TP=true positive result, TN= true negative result, FN= false negative result, FP= false positive result. Sensitivity, specificity, negative and positive predictive values were calculated for the threshold percent change cutoff value with maximum efficiency. Statistical significance was evaluated by a p-value less than 0.05.
Results
Cerebral Blood Volume
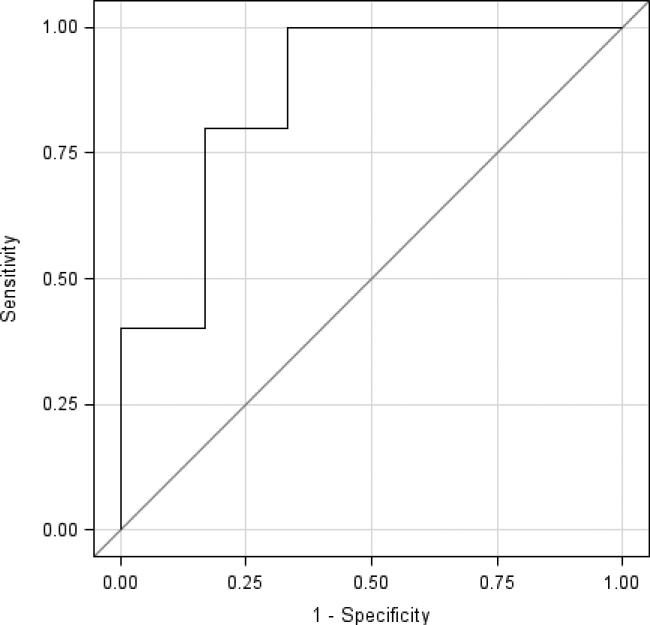
The mean percent change in the tumor rCBV at one month compared to baseline was significantly different between patients with PD and patients with NPD at 6 months (+11.9% vs. -28.9%, p=.02, Wilcoxon rank sum test) (see Table 2). The accuracy of rCBV in differentiating patients with PD compared to those with NPD at 6 months, as determined by the total area under the curve in ROC analysis, was classified as very good (0.8667; standard error 0.107) (See Figure 1). ROC analysis demonstrated that the optimal maximum cutoff for percent change in rCBV at one month from baseline for predicting nonprogressive disease at 6 months was -1.9% (sensitivity, 80%; specificity 83%; positive predictive value, 89%; negative predictive value, 83%, efficiency, 81.2%). The threshold percent change in rCBV associated with the highest efficiency in predicting nonprogressive disease at 6 months was -25.5% (sensitivity, 100%; specificity 67%; positive predictive value, 83%; negative predictive value, 100%, efficiency, 87.5%). Logistic regression analysis determined that on average, a 10% increase in rCBV one month after CED was associated with 1.7 times the odds of developing progressive disease at six months (95% CI: 1.0, 2.9; p=0.05) (See table 3). (Please see figures 3 and 4 for representative images of patients with progressive and nonprogressive disease).
Table 2.
Mean percent change in the tumor rCBV at one month compared to baseline.
| Mean percent change in rCBV at one month | Lower Quartile (25%) | Upper Quartile (75%) | |
|---|---|---|---|
| Nonprogressive Disease (n=6) | −28.9 | −61.0 | −4.40 |
| Progressive Disease (n=10) | 11.93 | −1.94 | 35.03 |
Mean percent change in tumor relative cerebral blood volume (rCBV) at one month after CED compared to baseline in patients with nonprogressive disease compared to those with progressive disease at six months (p=0.02)
Figure 1.
ROC curve for percent change in rCBV in differentiating patients with progressive disease or nonprogressive disease at six months (area under the curve: 0.8667).
Table 3.
A 10% increase in rCBV one month after CED was associated with 1.7 times the odds of developing progressive disease at six months.
| Odds Ratio | 95% Confidence Intervals | P value | |
|---|---|---|---|
| 10% increase in eTV | 1.2 | 0.92, 1.51 | 0.19 |
| 10% increase in rCBV | 1.7 | 1.0, 2.9 | 0.05 |
Prognostic values for a 10% increase from baseline in eTV and rCBV at one month correlated with the odds of developing six month progressive disease.
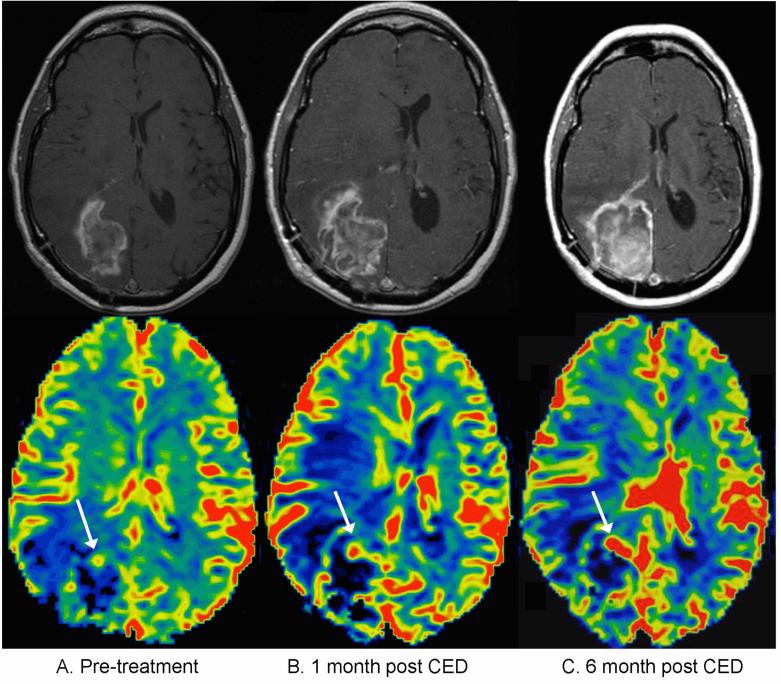
Figure 3.
Patient with true progressive disease, meaning an increase in contrast-enhancing tumor volume was observed within the first five months after treatment, and the patient had progressive disease at six months. A) Pretreatment contrast enhanced T1 weighted sequence (top) and corresponding cerebral blood volume map (bottom). The rCBV map color scale is weighted with red representing the highest values and black the lowest values. The white arrow indicates the portion of enhancing tumor with the highest rCBV. B) one-month post-treatment: an increase in eTV is noted with a corresponding increase in rCBV C) six months post treatment, showing progressively increasing eTV compatible with progressive disease as well as increasing rCBV.
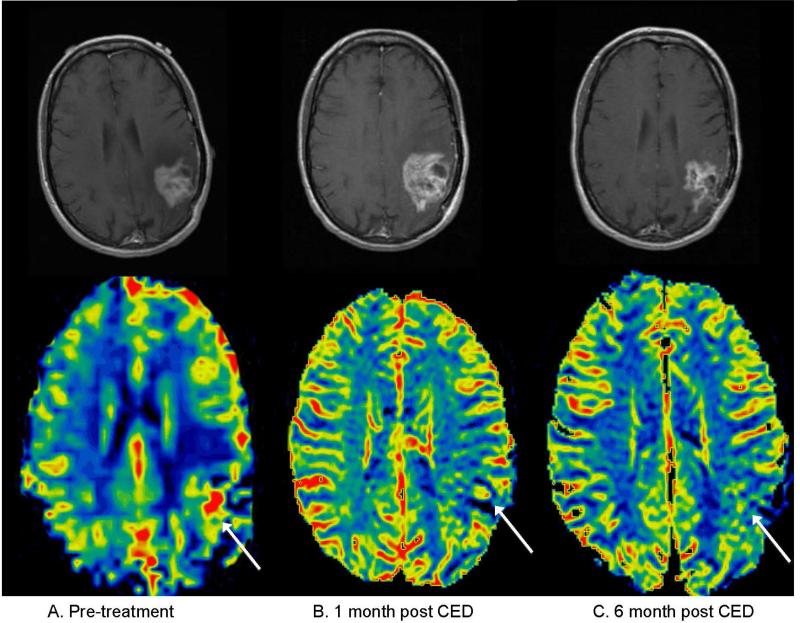
Figure 4.
Patient with pseudoprogression, meaning a transient increase in contrast-enhancing tumor volume was observed during the first five months after treatment, but the patient had non-progressive disease at six months. A) Pretreatment contrast enhanced T1 weighted sequence (top) and corresponding cerebral blood volume map (bottom), with white arrow highlighting region of maximum rCBV. B) One month post-treatment: there is an increase in eTV though with a decrease in rCBV compared to baseline. C) Six months post treatment, showing interval decrease in eTV and continued decline in rCBV.
Tumor Volumes
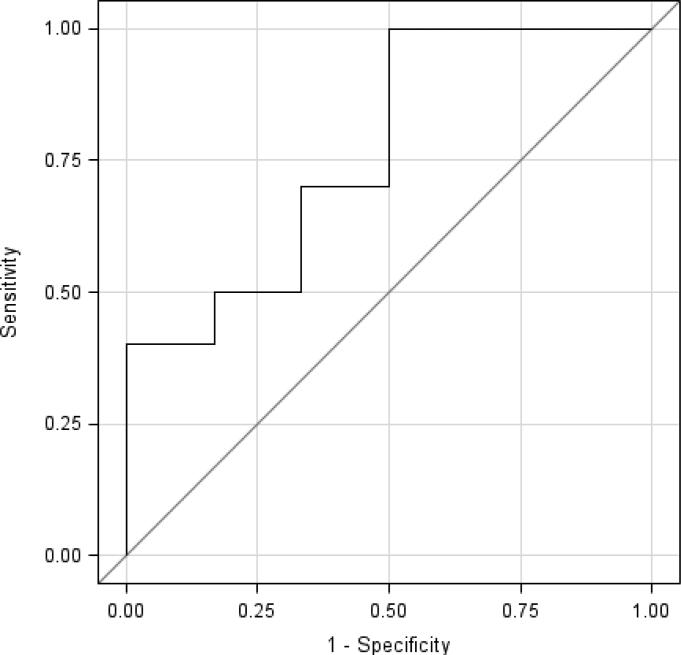
The mean percent change in enhancing tumor volume between patients with PD and patients with NPD at six months failed to reach significance (+119.5% vs. -7.48%, p=0.09, Wilcoxon rank sum test) (see Table 4). Logistic regression analysis determined that on average, for a 10% increase in enhancing tumor volume at one month after CED of topotecan, there was a trend towards 1.2 times the odds of developing progressive disease at six months (95% CI: 0.92, 1.51; p=0.19). ROC analysis showed that monitoring percent changes in enhancing tumor volumes was only fairly efficient for differentiating between the patients with PD and NPD at six months (area under the curve=0.7667, standard error=0.132). (See figure 2).
Table 4.
The mean percent change in enhancing tumor volume between patients with PD and patients with NPD at six months.
| Mean percent change in eTV at one month | Lower Quartile (25%) | Upper Quartile (75%) | |
|---|---|---|---|
| Nonprogressive Disease (n=6) | −7.48 | −65.9 | 52.2 |
| Progressive Disease (n=10) | 119.5 | −1.11 | 272.3 |
Mean percent change in enhancing tumor volume (eTV) at one month after CED compared to baseline in patients with nonprogressive disease compared to those with progressive disease at six months (p=0.09)
Figure 2.
ROC curve for percent change in eTV in differentiating patients with progressive disease or nonprogressive disease at six months (area under the curve: 0.7667).
Discussion
In this small pilot study of patients with recurrent malignant glioma treated with four days of continuous convection-enhanced delivery of topotecan, we found that the percent change in rCBV at one month after therapy was predictive of six-month progression status, while the percent change in enhancing tumor volume at one month did not reach significance.
In evaluating experimental therapies for glioma, accurate differentiation of patients with early progressive disease from those with nonprogressive disease is important in clinical management and to avoid a potential non-indicated change in therapy. Both progressive and nonprogressive disease can appear similar on conventional MRI and both can fulfill the MacDonald and RANO response criteria that are predominately based on changes in 2D enhancing tumor volume. In this small study, we found that changes in eTV were only fairly efficient in discriminating patients with PD from those with NPD. In response to this clinical and diagnostic dilemma in evaluating response to both conventional and experimental therapies, various advanced imaging paradigms are being utilized in order to differentiate PD from NPD including PET, MR spectroscopy, diffusion weighted imaging, and perfusion MRI, with perfusion based techniques currently showing the most promise, clinical utility and interest19.
The utility of perfusion-sensitive approaches in preoperative grading of gliomas has been demonstrated in a number of studies7,12,20-24, as well as in predicting histologic grade12. In addition to potentially aiding in differentiating radiation-induced necrosis from residual and/or recurrent tumor25, rCBV has been shown useful in predicting response to therapy8,11,26. In a study of patients with newly diagnosed glioblastoma treated with RT/TMZ, Mangla et al11 found that the percentage change in rCBV at one month after RT/TMZ was more predictive of 1-year survival than change in tumor size. They found a 5% increase in rCBV one month after therapy was 90% sensitive and 69% specific for predicting less than 1-year survival. We found somewhat similar results though in a more heterogenous study population of patients with recurrent grade III and IV gliomas treated with CED of topotecan. In our smaller study, an approximate 2% or greater decrease in rCBV at one month was 80% sensitive and 83% specific for predicting nonprogressive disease at six months. When using the threshold percent change in rCBV associated with the highest efficiency based on ROC curves, a decrease of less than 25.5% or an increase in rCBV at one month was a 100% sensitive and 67% specific in predicting progressive disease at six months. Our findings, in addition to the results from Mangla et. al., suggest that monitoring early temporal changes in rCBV may be helpful in evaluating treatment response in patients undergoing standard of care as well as experimental therapies and require more rigorous validation in larger randomized trials. Though evaluating overall survival is beyond the scope of a phase Ib trial and may not be an appropriate clinical end-point in light of the dismal prognosis in patients with recurrent malignant glioma, we found no correlation with rCBV or six-month progression status with overall survival. This may in part be contributed by the small sample size and heterogeneity of our study population.
Determining early treatment response after initiating standard of care (RT/TMZ) or novel therapies such as CED of topotecan for malignant glioma is often confounded by early transient increases in contrast enhancement, also known as pseudoprogression. Pseudoprogression is increasingly recognized as a common clinical problem with conventional therapy in evaluating treatment response. Additionally, patients with unrecognized pseudoprogression who are inadvertently enrolled in clinical trials for new therapies may lead to artificially improved outcomes and false positive response to experimental therapies. Although the exact physiologic basis for pseudoprogression is unclear, it may reflect a combination of a transient blood-brain barrier alteration response to newly-initiated therapy, and/or related to post-therapeutic cellular hypoxia with expression of hyopoxia-regulated molecules from the tumor and adjacent brain which cause increased vessel permeability and transient vasodilation3,27,28. This presumably manifests as an apparent increase in contrast enhancement most often in the first 2-6 months of therapy and is frequently indistinguishable from disease progression, followed by a subsequent variable decrease in enhancement3,27. The 25% incidence of pseudoprogression in patients treated with topotecan CED in this study is similar to the 15-50% rates of pseudoprogression reported in malignant glioma patients treated with RT/TMZ3, 29-32. Rather than reflect treatment failure, pseudoprogression in fact has been suggested to represent an exaggerated response to effective therapy29, in addition to being associated with slightly better survival rates compared to patients with no increase in tumor size at 1 month after RT/TMZ31. In this small pilot study we observed similar findings, with all four patients who had early pseudoprogression experiencing a lack of disease progression at six months. Furthermore, no pseudoprogression was observed in any patient who had progressive disease at six months. Temporal changes in rCBV in three of the four cases of pseudoprogression demonstrated a relative decrease in rCBV during a period of tumor volume increase, which may aid in confirming pseudoprogression.
To our knowledge, this is the first study serially demonstrating that pseudoprogression can occur in patients with recurrent malignant glioma treated via direct convection enhanced delivery. This is similar to what has been widely described with conventional RT/TMZ therapy. A study by Parney et al33 in 2005 described imaging changes in 14 patients with recurrent malignant glioma treated with convection enhanced delivery of interleukin 13 conjugated to a truncated pseudomonas exotoxin. The authors describe the challenges in determining therapeutic efficacy and difficulty in determining disease progression from treatment related changes when evaluating new or progressive contrast enhancement at follow-up. In hindsight, a significant portion of the study population was likely experiencing pseudoprogression, which accounted for the investigator's challenges in determining progression status based on conventional Macdonald criteria. They acknowledged that if traditional response criteria were used, new contrast enhancement in patients with nonprogressive disease retrospectively attributed to treatment related effects would inappropriately be considered progressive disease. This study in conjunction with our study highlight the importance of differentiating treatment related effects from progressive disease in future CED clinical trials evaluating new therapies.
Methylguanine methyltransferase (MGMT) promoter methylation status has been implicated in the incidence of pseudoprogression after RT/TMZ; pseudoprogression has been reported in 91% of GBM tumor samples with a methylated MGMT promoter, compared to a 41% incidence of pseudoprogression in GBM samples with an unmethylated MGMT promoter29. It is unclear what role MGMT promoter methylation status plays in pseudoprogression secondary to topotecan CED; MGMT status was not determined in this study but offers an opportunity for future investigation.
Our study has several limitations, including its retrospective nature and the small number of patients (who were part of a phase I trial of convection enhanced delivery of topotecan). Additionally, the study population was heterogenous with respect to multiple demographic factors including age and malignant glioma subtype, though statistical significance was found in the percent change in rCBV between patients with PD and NPD despite multiple confounding factors. In addition, our results are specific to the imaging parameters and image processing methods used in this study. An inherent current problem in perfusion MRI is the lack of standardization of technique and the numerous methods available for perfusion data analysis, which creates difficulty in comparing results obtained on different equipment and institutions. Since all patients in this study had recurrent malignant glioma and had received prior treatments consisting of different combinations of surgery, radiation and chemotherapy, the effects of susceptibility artifacts from prior post-therapeutic blood products on dynamic susceptibility contrast MR perfusion sequences can result in false rCBV values. Heavily T1-weighted dynamic contrast-enhanced (DCE) sequences are free of these susceptibility artifacts from old blood products; the retrospective nature of this study precluded the possibility to acquire DCE data and to additionally evaluate vascular permeability and document contrast agent distribution kinetics, though this clearly warrants future investigation. An additional limitation of the study was only one method of leakage correction, negative enhancement integration was utilized. Paulson et al34 reported variability in tumor rCBV depending on the choice of mathematic correction algorithms. Due to the retrospective nature of this study, no preload dosing leakage correction was performed, though inconsistency in preload methods for leakage correction have been reported in a preclinical study35. The use of a blood pool agent such as ferumoxytol which was not available at the time of this study, would likely offer more accurate estimates of rCBV, as it is minimally extravasated even with a disrupted blood-brain barrier when compared to gadolinium based agents, and does not require leakage correction36. An additional non-imaging related factor which may have altered rCBV values was the remote use of bevacizumab, an anti-angiogenic agent, in one patient out of sixteen prior to CED therapy. Anti-angiogenic therapies by their inherent mechanism of action, are known to alter rCBV values. Potential anti-angiogenic effects of Topotecan in humans as it relates to rcBV are unknown at this time, though an opportunity for future study.
Several MR perfusion techniques have been evaluated with respect to evaluating tumor progression. Seeger et al37 compared MRS and three perfusion techniques: arterial spin labeling, DCE, and DSC perfusion imaging in distinguishing recurrent high-grade gliomas from stable disease, and found DSC had the single best diagnostic performance. Voxel based techniques likely offer a more sensitive evaluation of perfusion data and estimates of histologic tumor fractions, though have some limitations. Histogram analysis is a method that accounts for heterogeneity within regions of interest, though with a loss of spatial specificity. Baek et al38 found that the percent change in parameters related to the CBV histographic pattern (skewness and kurtosis) could aid in differentiation of early progression from pseudoprogression in patients with newly diagnosed glioblastoma. A limitation of our study and many other prior studies is the binary classification of rCBV contrast enhancing lesions as tumor or treatment related effects, when in fact the majority of treated lesions are likely a mixture of both39. To better account for intratumoral heterogeity, in a study of 25 patients with recurrent GBM, Hu et al39 compared intervoxel rCBV variability using several different perfusion based metrics and histologic heterogeneity obtained from stereotactic biopsy samples in patients undergoing surgical re-resection for suspected tumor recurrence. When comparing rCBV mean, mode, maximum, width, and a newly developed thresholding metric referred to as perfusion MRI fractional tumor burden (pMRI-FTB), they found that pMRI-FTB provided the best estimate of relative histologic tumor fraction versus post treatment effects (pseudoprogression and/or radiation necrosis), and that the perfusion derived fractional tumor burden correlated with overall survival.
A more advanced and promising voxel based imaging analysis technique is parametric response mapping40-42 (PRM), in which serial parametric maps are co-registered voxel by voxel before and after therapy, which when applied to perfusion data, can quantify early hemodynamic changes after therapy. The current limitations of this technique are the technical demands of coregistration of image voxels over time, which makes routine clinical use currently challenging. PRMrCBV is a measure of the change between serial rCBV maps for each voxel in the region of interest. In a prospective study of patients with high grade glioma treated with RT/TMZ, Tsien et al41 applied PRM analysis to rCBV and found it to predict tumor response to standard therapy and differentiate disease progression from pseudoprogression. The same group subsequently in a similar patient cohort compared PRM with the percent change of whole tumor rCBV statistics (mean, median and percentiles) and with physiologic histogram segmentation (low, medium, or high rCBV) prior to, and one and three weeks after initiation of RT/TMZ. They found that the voxel based PRM approach was the only parameter that was predictive of 1-year survival42.
Larger studies are needed to validate our finding that early changes in rCBV in the first month of topotecan therapy via CED correlate with 6-month progression status. Additional validation of glioma subtype response to topotecan CED is needed. In conclusion, the results of this study suggest that in patients with recurrent malignant glioma treated with convection-enhanced delivery of topotecan, changes in rCBV at one month after therapy may help predict response to therapy and 6-month progression status.
Highlights.
Patients with malignant glioma received convection-enhanced delivery of topotecan
Temporal changes in rCBV and eTV were serially evaluated at one month intervals
Changes in rCBV one month after therapy predicted six month progression status
Monitoring changes in eTV was less efficient in predicting progression status
Pseudoprogression can occur in patients with malignant glioma treated with CED
Acknowledgements
This study was supported by NIH grant 5RO1CA89395.
Abbreviations list
- CED
Convection-enhanced delivery
- DSC
Dynamic susceptibility contrast
- eTV
Enhancing tumor volume
- NPD
Nonprogressive disease
- PD
Progressive disease
- PRM
Parametric response mapping
- rCBV
Relative cerebral blood volume
- RANO
Response Assessment in Neuro-Oncology Working Group
- RT/TMZ
Radiation Therapy/Temozolomide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest, financial or otherwise, to report, and each has contributed significantly to this manuscript. Informed consent was obtained from each patient in this study, and the protocols employed were approved by the Institutional Review Board of Columbia University Medical Center.
References
- 1.Macdonald DR, Cascino TL, Schold SC, Caincross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;1028(11):1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 3.Brandsma D, Stalphers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain MC. Pseudoprogression in glioblastoma. (letter and reply) J Clin Oncol. 2008;26:4359–4360. doi: 10.1200/JCO.2008.18.4440. [DOI] [PubMed] [Google Scholar]
- 5.Aronen HJ, Perkiö J. Dynamic susceptibility contrast MRI of gliomas. Neuroimaging Clin N Am. 2002;12(4):501–523. doi: 10.1016/s1052-5149(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 6.Knopp EA, Cha S, Johnson G, Mazumdar A, Golfinos JG, Zagzag D, Miller DC, Kelly PJ, Kricheff II. Glial neoplasms: dynamic contrast-enhanced T2*- weighted MR imaging. Radiology. 1999;211(3):791–798. doi: 10.1148/radiology.211.3.r99jn46791. [DOI] [PubMed] [Google Scholar]
- 7.Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24(10):1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 8.Law M, Oh S, Babb JS, Wang E, Inglese M, Zagzag D, Knopp EA, Johnson G. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging—prediction of patient clinical response. Radiology. 2006;238(2):658–667. doi: 10.1148/radiol.2382042180. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Tsien CI, Nagesh V, Junck L, Ten Haken R, Ross BD, Chenevert TL, Lawrence TS. Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected] Int J Radiat Oncol Biol Phys. 2006;64(3):876–885. doi: 10.1016/j.ijrobp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, Miller DC, Golfinos JG, Zagzag D, Johnson G. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast enhanced perfusion MR imaging. Radiology. 2008;247(2):490–498. doi: 10.1148/radiol.2472070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangla R, Singh G, Ziegelitz D, Milano MT, Korones DN, Zhong J, Ekholm SE. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010;256(2):575–584. doi: 10.1148/radiol.10091440. [DOI] [PubMed] [Google Scholar]
- 12.Sugahara T, Korogi Y, Kochi M, Ikushima I, Hirai T, Okuda T, Sigematsu Y, Liang L, Ge Y, Ushio Y, Takahashi M. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. Am J Roentgenol. 1998;171(6):1479–1486. doi: 10.2214/ajr.171.6.9843274. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, Jennings D, Wen PY, Lahdenranta J, Ancukiewicz M, di Tomaso E, Duda DG, Jain RK. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polley MC, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M. Six-month progression-free survival as an alternative primary efficacy endpoint to overall survival in newly-diagnosed glioblastoma patients receiving temozomolide. Neurooncology. 2010;12:274–282. doi: 10.1093/neuonc/nop034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freidman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 16.Bruce JN, Fine RL, Canoll P, Yun J, Kennedy BC, Rosenfeld SS, Sands SA, Surapaneni K, Lai R, Yanes CL, Bagiella E, DeLaPaz RL. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery. 2011;69(6):1272–9. doi: 10.1227/NEU.0b013e3182233e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetzel SG, Cha S, Johnson G, Lee P, Law M, Kasow DL, Pierce SD, Xue X. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology. 2002;224:797–803. doi: 10.1148/radiol.2243011014. [DOI] [PubMed] [Google Scholar]
- 18.Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223(1):11–29. doi: 10.1148/radiol.2231010594. [DOI] [PubMed] [Google Scholar]
- 19.Wen PY, Norden AD, Drappatz J, Quant E. Response assessment challenges in clinical trials of gliomas. Curr Oncol Rep. 2010;12(1):68–75. doi: 10.1007/s11912-009-0078-3. [DOI] [PubMed] [Google Scholar]
- 20.Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weiskoff RM, Harsh GR, Cosgrove GR, Halpern EF, Hochberg FH. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994;191(1):41–45. doi: 10.1148/radiology.191.1.8134596. [DOI] [PubMed] [Google Scholar]
- 21.Donahue KM, Krouwer HG, Rand SD, Pathak AP, Marszalkowski CS, Censky SC, Prost RW. Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med. 2000;43(6):845–853. doi: 10.1002/1522-2594(200006)43:6<845::aid-mrm10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Lev MH, Ozsunar Y, Henson JW, Rasheed AA, Barest GD, Harsh GR, Fitzek MM, Chiocca EA, Rabinov JD, Csavoy AN, Rosen BR, Hochberg FH, Schaefer PW, Gonzalez RG. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR Am J Neuroradiol. 2004;25(2):214–221. [PMC free article] [PubMed] [Google Scholar]
- 23.Sugahara T, Korogi Y, Kochi M, Ushio Y, Takahashi M. Perfusion-sensitive MR imaging of gliomas: comparison between gradient-echo and spin-echo echo-planar imaging techniques. AJNR Am J Neuroradiol. 2001;22(7):1306–1315. [PMC free article] [PubMed] [Google Scholar]
- 24.Yang D, Korogi Y, Sugahara T, Kitajima M, Shigematsu Y, Liang L, Ushio Y, Takahashi M. Cerebral gliomas: prospective comparison of multivoxel 2D chemical-shift imaging proton MR spectroscopy, echoplanar perfusion and diffusion-weighted MRI. Neuroradiology. 2002;44(8):656–666. doi: 10.1007/s00234-002-0816-9. [DOI] [PubMed] [Google Scholar]
- 25.Jain R, Narang J, Sundgren PM, Hearshen D, Saksena S, Rock JP, Gutierrez J, Mikkelsen T. Treatment induced necrosis versus recurrent/progressing brain tumor: going beyond the boundaries of conventional morphologic imaging. J Neurooncol. 2010;100(1):17–29. doi: 10.1007/s11060-010-0139-3. [DOI] [PubMed] [Google Scholar]
- 26.Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, Liang L, Ushio Y, Takahashi M. Post-therapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol. 2000;21(5):90–909. [PMC free article] [PubMed] [Google Scholar]
- 27.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 28.Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol. 2009;92(3):317–335. doi: 10.1007/s11060-009-9827-2. [DOI] [PubMed] [Google Scholar]
- 29.Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 30.Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 31.Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, van Es CA, van Bent MJ. Incidence of early pseudoprogression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 32.Gerstner ER, McNamara MB, Norden AD, Lafrankie D, Wen PY. Effect of adding temozolomide to radiation therapy on the incidence of pseudoprogression. J Neurooncol. 2009;94:97–101. doi: 10.1007/s11060-009-9809-4. [DOI] [PubMed] [Google Scholar]
- 33.Parney IF, Kunwar S, McDermott M, Berger M, Prados M, Cha S, Croteau D, Puri RK, Chang SM. Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13-PE38QQR for recurrent malignant glioma. J Neurosurg. 2005;102(2):267–275. doi: 10.3171/jns.2005.102.2.0267. [DOI] [PubMed] [Google Scholar]
- 34.Paulson ES, Schmainda KM. Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology. 2008;249(2):601. doi: 10.1148/radiol.2492071659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gahramanov S, Muldoon LL, Li X, Neuwelt EA. Improved perfusion MR imaging assessment of intracerebral tumor blood volume and antiangiogenic therapy efficacy in a rat model with ferumoxytol. Radiology. 2011;261(3):796. doi: 10.1148/radiol.11103503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gahramanov S, Muldoon LL, Varallyay CG, Li X, Kraemer DF, Fu R, Hamilton BE, Rooney WD, Neuwelt EA. Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology. 2013;266(3):842–852. doi: 10.1148/radiol.12111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seeger A, Braun C, Skardelly M, Paulsen F, Schittenhelm J, Ernemann U, Bisdas S. Comparison of Three Different MR Perfusion Techniques and MR Spectroscopy for Multiparametric Assessment in Distinguishing Recurrent High-Grade Gliomas from Stable Disease. Acad Radiol. 2013;20(12):1557–1565. doi: 10.1016/j.acra.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Baek HJ, Kim HS, Kim N, Choi YJ, Kim YJ. Percent change of perfusion skewness and kurtosis: a potential imaging biomarker for early treatment response in patients with newly diagnosed glioblastomas. Radiology. 2012;264(3):834–843. doi: 10.1148/radiol.12112120. [DOI] [PubMed] [Google Scholar]
- 39.Hu LS, Eschbacher JM, Heiserman JE, Dueck AC, Shapiro WR, Liu S, Karis JP, Smith KA, Coons SW, Nakaji P, Spetzler RF, Feuerstein BG, Debbins J, Baxter LC. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro-oncology. 2012;14(7):919–930. doi: 10.1093/neuonc/nos112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galbán CJ, Chenevert TL, Meyer CR, Tsien C, Lawrence TS, Hamstra DA, Junck L, Sundgren PC, Johnson TD, Ross DJ, Rehemtulla A, Ross BD. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat Med. 2009;15:572–576. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsien C, Galban CJ, Chenevert TL, Johnson TD, Hamstra DA, Sundgren PC, Junck L, Meyer CR, Rehemtulla A, Lawrence T, Ross BD. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J Clin Oncol. 2010;28(13):2293–9. doi: 10.1200/JCO.2009.25.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemasson B, Chenevert TL, Lawrence TS, Tsien C, Sundgren PC, Meyer CR, Junck L, Boes J, Galban S, Johnson TD, Rehemtulla A, Ross BD, Galban CJ. Impact of Perfusion Map Analysis on Early Survival Prediction Accuracy in Glioma Patients. Transl Oncol. 2013;6(6):766–774. doi: 10.1593/tlo.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]