Abstract
Various forms of mercury possess different rates of absorption, metabolism and excretion, and consequently, toxicity. Methylmercury (MeHg) is a highly neurotoxic organic mercurial. Human exposure is mostly due to ingestion of contaminated fish. Ethylmercury (EtHg), another organic mercury compound, has received significant toxicological attention due to its presence in thimerosal-containing vaccines. This study was designed to compare the toxicities induced by MeHg and EtHg, as well as by their complexes with cysteine (MeHg-S-Cys and EtHg-S-Cys) in the C6 rat glioma cell line. MeHg and EtHg caused significant (p < 0.0001) decreases in cellular viability when cells were treated during 30 min with each mercurial following by a washing period of 24 h (EC50 values of 4.83 and 5.05 μM, respectively). Significant cytotoxicity (p < 0.0001) was also observed when cells were treated under the same conditions with MeHg-S-Cys and EtHg-S-Cys, but the respective EC50 values were significantly increased (11.2 and 9.37 μM). L-Methionine, a substrate for the L-type neutral amino acid carrier transport (LAT) system, significantly protected against the toxicities induced by both complexes (MeHg-S-Cys and EtHg-S-Cys). However, no protective effects of L-methionine were observed against MeHg and EtHg toxicities. Corroborating these findings, L-methionine significantly decreased mercurial uptake when cells were exposed to MeHg-S-Cys (p = 0.028) and EtHg-S-Cys (p = 0.023), but not to MeHg and EtHg. These results indicate that the uptake of MeHg-S-Cys and EtHg-S-Cys into C6 cells is mediated, at least in part, through the LAT system, but MeHg and EtHg enter C6 cells by mechanisms other than LAT system.
Keywords: Methylmercury, Ethylmercury, Toxicity, L-Type neutral amino acid carrier transport
1. Introduction
Mercury is a highly toxic heavy metal present in the environment due to both natural processes and anthropogenic activities. It can be found mainly in three different forms: elemental mercury (Hg0), inorganic mercury salts (Hg2+) and organic mercury (Clarkson et al., 2003). Methylmercury (MeHg), an organic mercury compound, originates from methylation of inorganic mercury by sulfate-reducing bacteria in aquatic environment. Once in the aquatic food chain, MeHg bio-accumulates and magnifies, reaching high levels in predatory fish, thus representing a toxicological concern for humans subsiding on fish for their dietary intake (Hintelmann, 2010).
MeHg is the most toxic form of mercury in the environment (Clarkson and Magos, 2006) and despite its vast distribution among several tissues after absorption, the central nervous system (CNS) represents the main target of its toxicity, especially when exposures occur during early stages of neurodevelopment (Farina et al., 2011a). Although the molecular mechanisms by which MeHg enters the brain and exerts its toxicity have not been clearly elucidated (Simmons-Willis et al., 2002), several studies point to the high affinity of MeHg for thiols (-SH) and selenols (-Se) as the basis of their toxicity (Farina et al., 2009; Stringari et al., 2008; Rush et al., 2012a,b). With respect to MeHg’s transport from blood to the brain, it has been generally assumed that it occurs due to simple diffusion (Simmons-Willis et al., 2002). However, several studies have postulated that its transport from the blood to the CNS across the blood–brain barrier (BBB) occurs, at least in part, through the L-type neutral amino acid carrier transport (LAT) system (Aschner et al., 1990; Kajiwara et al., 1996; Clarkson et al., 2007; Yin et al., 2008) and that MeHg is transported as a MeHg-cysteine (MeHg-S-Cys) complex. Indeed, Hg uptake into brain after injection of MeHg-S-Cys complex was higher when compared with MeHg alone (Kerper et al., 1992). Interestingly, this uptake was partially inhibited by methionine, a substrate for the LAT system and the uptake of methionine was also inhibited by the MeHg-S-Cys complex, but not by MeHg alone (Kerper et al., 1992). Consistent with these observations, over-expression of LAT-1 (an important LAT subtype) in different cell lines increased Hg uptake, when MeHg-S-Cys was administrated, whereas knockdown of LAT-1 reduced the uptake of MeHg-S-Cys and attenuated its cytotoxicity (Simmons-Willis et al., 2002; Yin et al., 2008). Moreover, in vivo studies showed that the administration of MeHg-S-Cys complex caused a significant increase in Hg accumulation in brain (cortex and cerebellum) and liver compared with MeHg-treated mice (Roos et al., 2010). These findings corroborate the hypothesis that MeHg is transported as a complex with Cys (MeHg-S-Cys), by a mechanism of molecular mimicry with the amino acid L-methionine, one of the endogenous substrates of LAT-1 (Ballatori, 2002; Bridges and Zalups, 2010).
Another organic mercurial that has received significant toxicological attention is ethylmercury (EtHg), which shares with MeHg some specific chemical and toxic properties (Mutkus et al., 2005). In the early 1930s, ethylmercury thiosalicylate, known as thimerosal, was introduced as a preservative in many medicinal preparations and vaccines (Pless and Risher, 2000). Experimental studies indicate that animal exposure to thimerosal-Hg (which spontaneously generates EtHg and thiosalicylate in aqueous medium) can lead to accumulation of inorganic Hg in brain (for a review, see Dórea, 2011). Although it is known that thimerosal causes significant neurotoxicity in experimental (in vitro and in vivo) models, in vivo data indicate its shorter half-life compared with MeHg (Burbacher et al., 2005), which explains its lower neurotoxic potency. Accordingly, some studies on the potential neurotoxic effects of thimerosal in humans have failed to report adverse neurodevelopmental outcomes (Aschner and Ceccatelli, 2010; Dórea, 2010).
As previously described, MeHg-induced toxicity is mediated by its interaction with thiol groups (Aschner and Syversen, 2005; Franco et al., 2009) and the interaction of MeHg with the sulfhydryl amino acid cysteine is important for its entrance into the CNS (as a MeHg-S-Cys complex), via specific amino acid transporters, such as LAT-1 (Mokrzan et al., 1995; Heggland et al., 2009; Farina et al., 2011b). On the other hand, although it is well known that EtHg may share some chemical and toxic properties with MeHg (Mutkus et al., 2005) and that it interacts with thiols, there are no studies investigating its potential interaction with cysteine and the formation of an EtHg-S-Cys type complex, as well as its potential toxicity and transport via the LAT system. Thus, the aim of the present study was to compare the toxicities induced by MeHg and EtHg, as well as the products of their complexation with cysteine (MeHg-S-Cys and EtHg-S-Cys) in the C6 rat glioma cell line. To investigate the possible role of the LAT system in these processes, L-Met (a well known LAT-1 substrate) was used to elucidate potential mechanisms of toxicity, transport and protection.
2. Materials and methods
2.1. Chemicals
Reduced glutathione, methylmercuric (II) chloride, ethylmercuric chloride and L-methionine were obtained from Sigma (St. Louis, MO, USA). Rabbit polyclonal IgG anti-LAT-1 (sc-134994), monoclonal anti-β-actin primary antibody and protein A/G horseradish peroxidase-conjugated secondary antibody were from Santa Cruz (Santa Cruz, CA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Gibco Life Technologies Inc.). All other chemicals were of the highest commercially available grade.
2.2. Cell culture and treatments
C6 rat glioma cells (CCL-107) were obtained from the American Type Culture Collection (http://www.atcc.org) and cultured as a monolayer in polystyrene dishes and maintained in DMEM supplemented with 2 mM glutamine, 100 units/mL, penicillin, 100 μg/mL streptomycin and 10% fetal bovine serum (FBS) at 37 °C in a humidified atmosphere of 5% CO2. Cells were subcultured at ~80% confluence and used between the 10th and 16th passage.
Twenty-four hours after plating (confluence ~80%), cells were washed with Hank’s balanced salt solution (HBSS; 135 mM NaCl, 20 mM HEPES, 4 mM KCl, 1 mM Na2HPO4, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose) and incubated for 30 min with MeHg, EtHg or their Cys complexes (MeHg-S-Cys or EtHg-S-Cys) at concentrations ranging from 100 nM to 30 μM. MeHg-S-Cys and EtHg-S-Cys were synthesized by direct reaction of the mercurials with L-cysteine in water for 1 h. To ensure the absence of free mercurials in the complex solutions, the molar ratio of cysteine to MeHg and EtHg was 1.15 and the total complexation was confirmed by measuring free thiols with the Ellman’s reagent (Ellman, 1959). Treatments with the compounds (MeHg, EtHg or their complexes with Cys) were performed in a sulfhydryl molecule-free medium during 30 min. The short-time treatment with the mercurials (30 min) was chosen based on the relative fast kinetics of mercurials’ entrance into the cells. In some experiments, L-methionine (a substrate for the L-type neutral amino acid carrier transport system) was added to the incubation medium (HBSS) 15 min before the mercurial’s addition at a final concentration 1000 fold greater than the Hg-compounds; this amino acid remained in this medium during the mercurial’s treatment (30 min), totaling 45 min. After the short-time (30 or 45 min) treatments with the mercurials and/or L-methionine, the HBSS was replaced by fresh DMEM. Cells were placed on the 5% CO2 atmosphere (at 37 °C), where they were maintained for different periods before the evaluation of cell viability (24 h), glutathione levels (4 h) and Hg levels (30 min). Consequently, after a constant period (30 min) of exposure to each mercurial, the evaluations of Hg levels (item 2.3), glutathione levels (item 2.4) and cell viability (item 2.5) were purposely performed at different time-points (30 min, 4 h and 24 h, respectively) in an attempt to better understand the sequence of relevant events (mercurial’s entrance into cells, glutathione oxidation and disruption of cellular viability).
2.3. Hg levels in cells
Thirty minutes after mercurial’s treatment, mercury (Hg) measurements were carried out with a quadrupole inductively coupled plasma mass spectrometer (ICP-MS ELAN DRCII, PerkinElmer, SCIEX, Norwalk, CT, USA) coupled with a Perkin Elmer model L-200 LC pump, six-port injector (Rheodyne 9725) with a reverse-phase column (C18, 5 μm, 150 mm × 4 mm, Brownlee Columns PerkinElmer (USA)) and a pre-column (guard column) RP18 (7 μm, 15 mm × 3.2 mm, Brownlee Columns PerkinElmer (USA)). The methodology allows the detection of different species of mercurials, such as MeHg, EtHg and inorganic mercury. Instrument settings and operative conditions have been previously reported (Rodrigues et al., 2010).
2.4. Glutathione levels
Four hours after the 30 min mercurial’s treatment, GSH levels were measured as non-protein thiols based on the protocol developed by Ellman (1959). In short, the cells suspensions were precipitated in cooled trichloroacetic acid 10% and centrifuged at 5000 × g for 10 min, and the supernatant was incubated with 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) in a 1 M phosphate buffer, pH 7.0. Absorbance was measured at 412 nm. A standard curve of reduced GSH was used to calculate control cell GSH levels; the results of the other groups were expressed as percentage of control.
2.5. Cell viability assay
Twenty-four hours after the 30 min mercurial’s treatment, cell viability was evaluated based on the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT assay) (Vendrell et al., 2007) and on the leakage of lactate dehydrogenase into the cultured medium (Farina et al., 2009). Results of the MTT assays were expressed as percentage of control values (non-treated cells) after subtracting the blank values. All experiments were performed in triplicate.
2.6. Western blot analyses
Cells were rinsed with PBS and collected in ice-cold lysis buffer (50 mM Tris–HCl (pH 7.5), 1% Triton X-100, 100 mM NaCl, 5 mM EDTA (pH 8.0), 40 mM β-glycerolphosphate, 50 mM NaF, 200 μM orthovanadate, 5% glycerol and protease inhibitors), sonicated for 5 min and centrifuged at 14,000 × g for 45 min at 4 °C. As controls, tissue samples were obtained from mouse (heart) and rat (hippocampus). The tissues were homogenized (1:10, w/v) in ice-cold lysis buffer. All the homogenates (tissues and cells) were centrifuged at 14,000 × g, at 4 °C for 45 min, equivalent amounts of proteins were mixed in buffer (Tris 200 mM, glycerol 10%, SDS 2%, β-mercaptoethanol 2.75 mM and bromophenol blue 0.04%), boiled for 5 min and cooled immediately on ice. Samples (40 μg of protein) were subjected to SDS polyacrylamide gel electrophoresis on 10% polyacrylamide gels. Gels were run at 30 mA for about 120 min, with the electrophoresis tank placed in an iced water bath. Separated proteins were electroblotted onto nitrocellulose membranes at a constant current intensity of 400 mA for 90 min. The membranes were blocked for 1 h at room temperature in blocking buffer containing 5% nonfat dry milk. Blots were incubated overnight at 4 °C with primary polyclonal antibody against LAT-1 (1:500) in TBS–Tween–BSA buffer (20 mM Tris base, 140 mM NaCl, 0.05% Tween-20, 5% BSA). After washing, the blots were incubated for 1 h at room temperature with protein A/G-horseradish peroxidase conjugate (1:5000) in TBS–Tween buffer. Then, membranes were washed and developed with Immun-Star HRP Chemiluminescent reagents (Bio-Rad, Hercules, CA), and chemiluminescence was viewed with the Versadoc Imaging system (Bio-Rad). β-Actin was used as loading control. As negative and positive controls for LAT-1 expression, we used heart and hippocampus, respectively (Kanai et al., 1998; Boado et al., 1999). The mouse heart and rat hippocampus were derived from animals related to other ongoing studies from our laboratory, which were in accordance with the Guiding Principles of the Animal Care and Wellness Committee of the Universidade Federal de Santa Catarina (CEUA/UFSC PP00424 and PP00326).
2.7. Statistical analysis
The results were analyzed with the STATISTICA software system (version 8.0; StatSoft, Inc., 2008) and graphed with GraphPad Prism for Windows (version 5.0; GraphPad Software, San Diego, CA). For the concentration–response studies, significant differences were evaluated by one-way analysis of variance (ANOVA). Two-way ANOVA was performed to evaluate significant L-Met by mercurials’ interactions. After significant ANOVAs, Tukey’s multiple comparison test was performed and the differences were considered significant when p < 0.05. Results are expressed as mean ± SEM, except for the EC50 values and Hg levels, which are expressed as mean and 95% confidence intervals and standard deviation (SD), respectively. Pearson correlations were performed to evaluate the relationships between MTT reduction and LDH leakage.
3. Results
3.1. Concentration–response studies
Fig. 1 shows the toxicity of the studied mercury compounds in C6 cells, when mercurials were incubated during 30 min in a sulfhydryl-free incubating medium. After 24 h, significant decreases in cellular viability were observed after MeHg and EtHg exposures [half maximal effective concentration (EC50) values of 4.83 μM (4.34–5.33; 95% confidence intervals) and 5.05 μM (3.87–6.23; 95% confidence intervals), respectively] (Fig. 1A and B). Significant cell toxicities were also observed when cells were treated under the same conditions with the products of mercurial complexation with cysteine (MeHg-S-Cys and EtHg-S-Cys); however, the respective EC50 values were significantly (p < 0.001) increased [11.2 μM (9.81–12.59; 95% confidence intervals) and 9.37 μM (8.81–9.93; 95% confidence intervals)] (Fig. 1A and B). The EC50 values of MeHg and EtHg were not statistically different (Table 1), but MeHg and EtHg were significantly (p < 0.001) more toxic than their respective complexes (MeHg-S-Cys and EtHg-S-Cys).
Fig. 1.
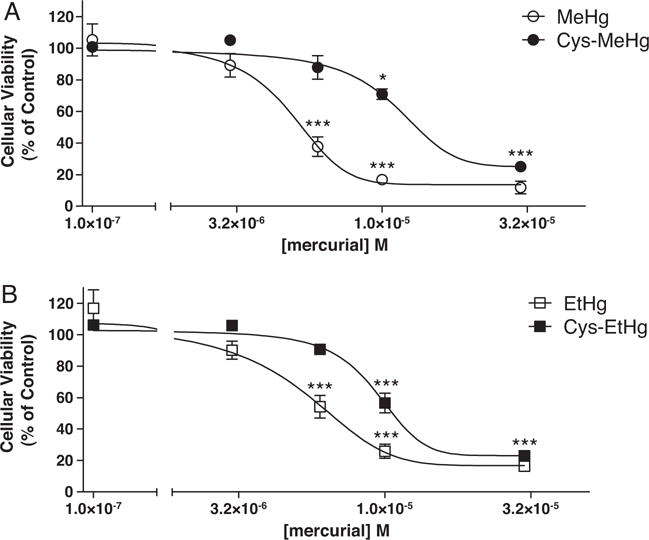
Dose–response curve of mercurial-induced cell toxicity. Rat glioma C6 cells (~80% confluence) were treated with (A) MeHg or MeHg-Cys, (B) EtHg or EtHg-Cys in HBSS. After 30 min of mercurial’s treatment, HBSS was replaced by fresh DMEM and the cells were incubated for additional 24 h at 37 °C in a humidified atmosphere of 5% CO2. Results of the MTT assays were expressed as percentage of control values (non treated cells). *p < 0.05 and ***p < 0.001 when compared to controls by one-way ANOVA followed by the Tukey’s HSD test. Data are expressed as mean ± SEM (N = 3 independent experiments).
Table 1.
EC50 values of mercurial-induced cell death.
| EC50 μM (95%CI)
|
||
|---|---|---|
| MeHg | EtHg | |
| Mercurial alone | 4.83 (4.34–5.33) | 5.05 (3.87–6.23) |
| Mercurial-Cys complex | 11.20 (9.81–12.59)* | 9.37 (8.81–9.93)#,+ |
EC50 values were determined by non-linear regression (sigmoidal concentration–response curves) from Fig. 1 data. EC50 values are expressed as mean and 95% confidence intervals (CI).
p< 0.001 when compared to MeHg.
p < 0.001 when compared to EtHg.
p < 0.01 when compared to MeHg-S-Cys.
In order to evaluate if an inhibition of cell division could potentially account for the significant decreased MTT metabolism observed in mercurial-treated cells (Fig. 1), we performed additional studies using an alternative test to evaluate cell viability, namely, lactate dehydrogenase (LDH) leakage (Farina et al., 2009). LDH is a soluble cytosolic enzyme present in most eukaryotic cells; it is released into the culture medium upon cell death due to damage or rupture of plasma membrane. Thus, the increase of LDH activity in culture medium is proportional to the number of lysed cells, an event that is not affected by decreased cell division. Cells were treated with different mercurial concentrations (0–10 μM) similarly to Fig. 1 conditions and, 24 h after treatments, LDH activity was measured in the culture medium while MTT reduction was measured in the platted cells. Correlation analyses showed significant negative correlations between LDH activity (measured in the culture medium) and MTT reduction (Supplemental File 1), which indicates that the decreased MTT metabolism observed in mercurial-treated cells is not related to inhibitory effects toward cell division.
Supplementary material related to this article found, in the online version, at http://dx.doi.org/10.1016/j.neuro.2013.05.015.
3.2. Effect of L-Met on the toxicity of Hg compounds
The second set of experiments was designed to investigate the effects of L-Met (a well known substrate of the LAT-1 transporter) pre-treatment on the toxicity induced by MeHg and EtHg, as well as their respective complexes with Cys. The concentrations of the studied mercurials were near the EC50 values calculated by nonlinear regression (sigmoidal concentration–response curves) from Fig. 1 data. L-Met (12 mM), added 15 min prior to the mercurial’s addition, significantly protected C6 cells from MeHg-S-Cys (12 μM) induced cell death, but no significant protective effects were observed in the presence of this amino acid when the cells were treated with 4.5 μM MeHg alone (Fig. 2A). In addition, L-Met (10 mM), also added 15 min prior to the mercurial’s addition, significantly protected C6 cells from EtHg-S-Cys (10 μM) induced cell death, but no significant protective effects of L-Met were observed when the cells were treated with 6 μM EtHg alone (Fig. 2B).
Fig. 2.
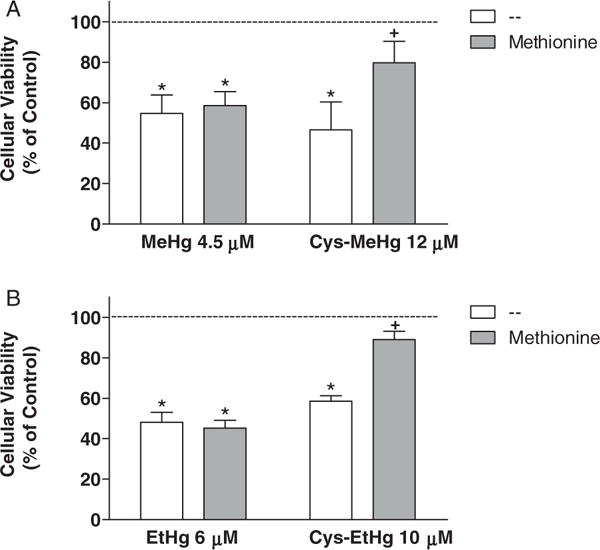
Effect of L-methionine on mercurial-induced cell toxicity. Rat C6 glioma cells were pre-treated with L-Met (at final concentrations 1000 fold greater than those of the respective Hg-compound) for 15 min before treatment with (A) MeHg (4.5 μM) or MeHg-Cys (12 μM), (B) EtHg (6 μM) or EtHg-Cys (10 μM) in HBSS. Thus, the concentrations of L-Met were 4.5 mM, 12 mM, 6 mM or 10 mM in wells treated with MeHg, MeHg-Cys, EtHg or EtHg-Cys, respectively. After the exposure to mercurials (30 min), HBSS was replaced by fresh DMEM and the cells were incubated for additional 24 h. Results of the MTT assays were expressed as percentage of control values (dashed line). The asterisks (*) mean significant differences (p < 0.001) when compared to control and the symbol + means significant difference (p < 0.001) when compared to cells treated only with the respective mercurial (without Cys) by two-way ANOVA followed by the Bonferroni test. Data are expressed as mean ± SEM (N = 6–9 independent experiments).
3.3. Effect of L-Met on intracellular Hg levels
Corroborating the findings on the protective effects of L-Met against MeHg-S-Cys- and EtHg-S-Cys-induced cytotoxicity (Fig. 2), this amino acid (added 15 min prior to the mercurial’s addition) caused a significant decrease in total Hg accumulation in cells incubated with MeHg-S-Cys and EtHg-S-Cys (Table 2). However, no significant effects of L-Met were observed when cells were incubated with MeHg and EtHg (Table 2).
Table 2.
Effects of L-methionine (L-Met) on total mercury concentration in C6 cells treated with MeHg, EtHg and their cysteine-complexes (MeHg-Cys and EtHg-Cys).
| Mercurial | L-Met | Total mercury concentration (ng/μL)
|
|||
|---|---|---|---|---|---|
| MeHg | MeHg-Cys | EtHg | EtHg-Cys | ||
| + | − | 7.28 (0.274)* | 5.12 (1.101)* | 5.35 (0.274)* | 3.72 (0.357)* |
| + | + | 7.87 (1.101)* | 2.08 (0.606)*,# | 5.96 (0.523)* | 2.71 (0.165)*,# |
| − | − | 0.0098 (0.0027) | |||
| − | + | 0.0118 (0.0035) | |||
Rat C6 glioma cells were pre-treated with 7 mM L-Met for 15 min before treatment with MeHg, EtHg, MeHg-Cys or EtHg-Cys (7 μM) in HBSS. After the exposure to mercurials (30 min), cells were washed twice with HBSS and, after 30 min, harvested in 200 μL Milli-Q water for mercury quantification. Data are expressed as ng Hg/μL of sample and represented as mean ± SD (N = 2 independent experiments).
Significantly different (p < 0.05) from control cells (no mercurial treatment) by two-way ANOVA followed by the Tukey’s HSD test.
Significantly different (p < 0.05) from cells treated only with the respective mercurial (no L-Met treatment) by two-way ANOVA followed by the Tukey’s HSD test.
In cells treated with MeHg, a significant MeHg effect [F(1,4) = 347.77; p = 0.00005] was observed on intracellular Hg levels, although L-Met showed no significant effects [F(1,4) = 0.53; p = 0.506]. Moreover, no significant MeHg by L-Met interaction [F(1,4) = 0.52; p = 0.508] was detected (Table 2). Notably, in cells treated with MeHg-S-Cys, significant MeHg-S-Cys [F(1,4) = 63.89; p = 0.0013] and L-Met [F(1,4) = 11.37; p = 0.0280] effects were observed. In addition, a significant MeHg-S-Cys by L-Met interaction [F(1,4) = 11.39; p = 0.0279] was detected, corroborating the significant decrease in Hg levels in cells treated with MeHg-S-Cys plus L-Met compared with cells treated with MeHg-S-Cys alone (Table 2).
In cells treated with EtHg, a significant EtHg effect [F(1,4) = 714.43; p = 0.00001] was observed on Hg levels, but L-Met showed no significant effects [F(1,4) = 2.09; p = 0.22]. No significant EtHg by L-Met interaction [F(1,4) = 2.07; p = 0.22] was observed (Table 2). Conversely, in cells treated with EtHg-S-Cys, significant EtHg-S-Cys [F(1,4) = 518.07; p = 0.00002] and L-Met [F(1,4) = 12.63; p = 0.023] effects were observed. A significant EtHg-S-Cys by L-Met interaction [F(1,4) = 12.73; p = 0.023] was detected, corroborating the significant reduction in Hg levels in cells treated with EtHg-S-Cys plus L-Met compared with cells treated with EtHg-S-Cys alone (Table 2).
In addition to the total Hg levels, speciation analyses were also performed. No MeHg was detected in cells treated with EtHg and EtHg-S-Cys. Moreover, no EtHg was detected in cells treated with MeHg and MeHg-S-Cys. Approximately 0.1% of the total mercury was in the inorganic form in cells treated with MeHg or MeHg-S-Cys (data not shown). In contrast, approximately 1% (10 fold higher) of the total mercury was in the inorganic form in cells treated with EtHg or EtHg-S-Cys (data not shown), in agreement with the higher dealkylation rate of EtHg compared with MeHg (Burbacher et al., 2005).
3.4. Glutathione levels
GSH is the most abundant intracellular non-protein thiol present in mammalian cells. In this study, we evaluated GSH levels in C6 cells after exposure to MeHg, EtHg and their complexes, MeHg-S-Cys and EtHg-S-Cys (Fig. 3). Moreover, we also evaluated the effect of L-Met pre-treatment at a final concentration 1000 fold greater than the Hg-compounds. The results clearly indicate that all the studied mercurials significantly reduced intracellular GSH levels at 4 h after the 30 min mercurial exposure. No differences in GSH levels were observed between cells treated with MeHg and EtHg (at concentrations approximating their respective EC50 values in the toxicity assay). In addition, no differences were found between cells treated with MeHg-S-Cys and EtHg-S-Cys (at concentrations approximating their respective EC50 values in the toxicity assay). The pre-treatment with L-Met was able to protect against GSH reduction only when the cells were incubated with the complexes (MeHg-S-Cys and EtHg-S-Cys).
Fig. 3.
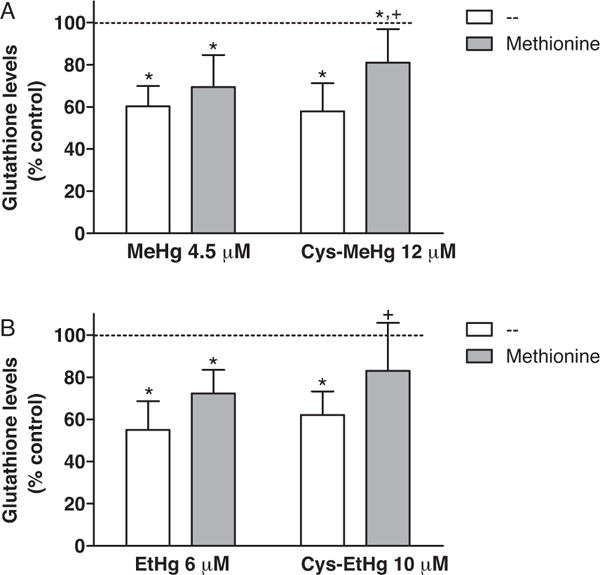
Glutathione levels after mercurial exposure. Rat C6 glioma cells were treated with MeHg, MeHg-Cys (A), EtHg, or EtHg-Cys (B) in HBSS (at concentrations approximating their respective EC50 value in the toxicity assay – Fig. 1). L-Methionine was added to the incubation medium (HBSS) 15 min before the mercurial’s addition at a final concentration 1000 fold greater than the Hg-compounds. After 30 min, HBSS was replaced by fresh DMEM and the cells were incubated for additional 4 h at 37 °C in a humidified atmosphere of 5% CO2. GSH concentration in control C6 cells (non treated) was 69.0 ± 2.2 nmol of GSH/mg of protein. GSH levels are expressed as percentage of control values (100%, dashed line). The asterisks (*) mean significant differences (p < 0.01) when compared to control and the symbol + means significant difference (p < 0.05) when compared to cells treated only with the respective mercurial (without Cys) by two-way ANOVA followed by the Bonferroni test. Data are expressed as mean ± SEM (N = 4 independent experiments).
3.5. Western blot analysis of LAT-1
We performed western blot analyses in order to investigate whether our cellular model (C6 rat glioma cells) expresses LAT-1. Fig. 4 clearly indicates the presence of LAT-1 in C6 cells and rodent hippocampus (positive control), although LAT-1 levels were not detected in rodent heart tissue (negative control), corroborating prior findings (Kanai et al., 1998; Boado et al., 1999).
Fig. 4.
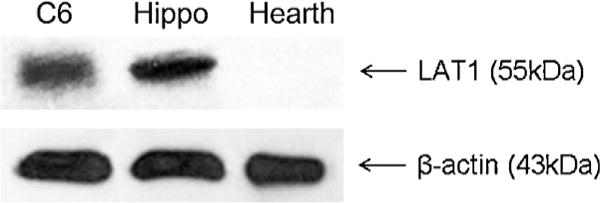
Western blot analysis of LAT-1 from different sources. Homogenates from rat glioma C6 cells, rat hippocampus (hippo; positive control) or mouse hearth (negative control) were submitted to SDS polyacrylamide gel electrophoresis, electroblotted and incubated overnight with primary polyclonal antibody against LAT-1 (1:500). β-Actin bands are also shown (protein load control).
4. Discussion
In this study, we observed that MeHg-S-Cys and EtHg-S-Cys, but not MeHg and EtHg, are substrates for the LAT transporter system in the C6 glioma cell line. Thus, this transporter plays a significant role in modulating the entry of cysteinyl-complexes of MeHg and EtHg (MeHg-S-Cys and EtHg-S-Cys) into glial cells. This event is of physiological significance, given that in biological systems mercurials are primarily bound to thiols. Conversely, MeHg and EtHg entry into C6 glioma cells is modulated by mechanism(s) distinct of the LAT system (not affected by L-Met), potentially involving simple diffusion (Simmons-Willis et al., 2002) or interaction with membrane sulfhydryl proteins and subsequent exchange reactions with intracellular thiols (Rabenstein et al., 1982; Farina et al., 2011a). Interestingly, intracellular Hg concentrations were higher in cells treated with MeHg or EtHg compared with those treated with equimolar concentrations of their respective complexes, MeHg-S-Cys or EtHg-S-Cys (Table 2). Taking into account that (i) cells were exposed to mercurials in a sulfhydryl-free medium, and that (ii) MeHg and EtHg entry into cells did not require the LAT system (was not affected by L-Met), these results indicate that transport mechanisms other that the LAT system significantly contribute to the entry of both MeHg and EtHg into cells. Moreover, because a 1000 fold higher concentration of L-Met was ineffective in fully preventing mercurial entry into cells treated with MeHg-S-Cys and EtHg-S-Cys, it is likely that these cysteinyl-complexes utilize different protein transporters or, alternatively, release the free mercurials into the extracellular milieu, allowing for their entry into cells via other mechanisms, such as simple diffusion or exchange thiol reactions.
Significant decreases in cellular viability were observed after MeHg and EtHg exposures. Notably, significant cell toxicities were also observed when cells were treated with MeHg-S-Cys and EtHg-S-Cys, although their respective EC50 values were increased (Fig. 1A and B). Based on the cellular viability test and the intracellular Hg levels, it is reasonable to suppose that both MeHg and EtHg exhibit analogous toxicities in rat glioma C6 cells under in vitro conditions. The inherently lower toxicities of MeHg-S-Cys or EtHg-S-Cys compared with those of MeHg and EtHg likely reflect the reduced entrance of the mercurials into cells in the cysteinyl-complex form. In fact, when present in the intracellular millieu, MeHg-S-Cys likely displays analogous toxicity to MeHg given the fast exchange of MeHg between sulfhydryl molecules (Rabenstein et al., 1982). This idea is reinforced by the findings that all the four studied mercurials (MeHg, EtHg, MeHg-S-Cys and EtHg-S-Cys) caused significant reductions in intracellular GSH levels when incubated at concentrations approximating their respective EC50 (Fig. 3).
L-Met (a LAT system substrate) significantly protected C6 cells from MeHg-S-Cys induced cell death, but no significant protective effects were observed in the presence of this amino acid when the cells were treated with MeHg alone (Fig. 2A). In addition, L-Met significantly protected C6 cells from EtHg-S-Cys induced cell death, but no significant protective effects of L-Met were observed when the cells were treated with EtHg alone (Fig. 2B). As L-Met is a substrate for LAT-1, these results are in agreement with the concept that the MeHg-S-Cys complex enters cells, at least in part, through this transporter. It has been previously shown that MeHg-Cys administration promotes significant Hg uptake in vivo (Aschner and Clarkson, 1989; Hirayama, 1980) and this effect was partially abolished by substrates of the LAT transporters (Aschner and Clarkson, 1989; Kerper et al., 1992). The same inhibition by LAT substrates in Hg uptake after MeHg-S-Cys administration was also observed in rat primary astrocyte cultures (Aschner et al., 1990) and cultured brain capillary endothelial cells (Mokrzan et al., 1995). This transport has been explained by MeHg-S-Cys mimicking the amino acid methionine, due to their similar structures (Aschner, 1989). However computational studies suggested that these two structures (methionine and MeHg-S-Cys complex) are in fact quite different. It has been proposed that the transport of MeHg-S-Cys was not related to its mimicry of methionine, but rather that the transporter LAT recognizes a specific region of this complex (Hoffmeyer et al., 2006).
Although we observed that MeHg and MeHg-Cys significantly decreased the ability to reduce MTT in C6 cells, similarly to the observations made by Roos et al. (2011) in liver slices, cultured C6 cells and liver slices behave differently when exposed to these mercurials. In fact, Roos et al. (2011) observed a higher Hg uptake in liver slices exposed to MeHg-Cys compared with those exposed to MeHg, suggesting a major contribution of the LAT system to the mercurial’s (MeHg-Cys) entry into liver slices. Accordingly, a higher rate of reactive oxygen species generation was observed in MeHg-Cys-exposed liver slices compared with those exposed to MeHg alone (Roos et al., 2011). Although we observed that L-Met treatment decreased Hg uptake in C6 cells exposed to MeHg-Cys (but not in cells exposed to MeHg), a higher Hg uptake was observed in C6 cells treated with MeHg compared with those treated with MeHg-Cys. These results indicate that mechanisms other than the LAT system are significantly important for MeHg entry into C6 cells.
The GSH antioxidant system is the major defense against oxidative damage in the CNS and a direct relationship between the GSH redox system and MeHg neurotoxicity has been proposed (Ballatori and Clarkson, 1982; Shanker et al., 2005; Franco et al., 2006; Amonpatumrat et al., 2008; Stringari et al., 2008; Ni et al., 2011). Herein, the four studied mercurials significantly reduced intracellular GSH levels (when added at concentrations approximating their respective EC50). No differences in GSH levels were observed between cells treated with MeHg or EtHg. In addition, no differences were found between cells treated with MeHg-S-Cys or EtHg-S-Cys. This indicates that not only MeHg and EtHg, but also their complexes with cysteine, are able to deplete endogenous GSH. This observation strongly suggests the occurrence of mercury’s exchange from the sulfur atom of cysteine to thiols (or thiolate) inherent to biomolecules (see Eq. (1)), corroborating proton nuclear magnetic resonance studies on mercurial binding to thiol molecules (Rabenstein et al., 1982). Such exchanges are likely responsible for the cell toxicities observed after MeHg-S-Cys and EtHg-S-Cys exposures.
| (1) |
Eq. (1) note: Mercury (as a methylmercury-cysteine complex, CH3-Hg-S-Cys) exchanges from the sulfur atom of cysteine to a thiol biomolecule (R-S−), generating “free” cysteine (Cys-S−) and the “oxidized” thiol biomolecule (CH3-Hg-S-R). The interaction of Hg with the thiol group (or thiolate anion) from a biomolecule might affect its physiological function, leading to toxic consequences. A similar reaction could occur with EtHg-S-Cys complex (CH3-CH2-Hg-S-Cys) instead of MeHg-S-Cys complex. Analogous to thiol biomolecules, selenoproteins (R-SeH or R-Se−) could also represent molecular targets, generating products of Hg-Se interaction (CH3-Hg-Se-R or CH3-CH2-Hg-Se-R).
LAT-1 functions as a bi-directional transporter of amino acids (Verrey, 2003). It is ubiquitously distributed in various cell types and tissues, and is highly abundant in brain, placenta and tumors (del Amo et al., 2008). Previous studies on the expression of LAT-1 (Kanai et al., 1998; Boado et al., 1999; Segawa et al., 1999; Yanagida et al., 2001) pointed to the heart and brain as tissues that express low (not detected) and high levels of LAT-1, respectively. Our western blot analyses (Fig. 4) indicates the presence of LAT-1 in C6 glioma cells (used herein as a cellular model to investigate mercurials’ transport) and in the rodent hippocampus, as well as its absence from heart tissue, corroborating prior findings by means of northern blot analyses (Kanai et al., 1998; Boado et al., 1999). The presence of LAT-1 in the rat C6 glioma cell line corroborates our data on the protective effects of L-Met against MeHg-S-Cys-induced toxicity. Based on the protective effects of L-Met against EtHg-S-Cys-induced toxicity, the positive detection of LAT-1 in C6 cells also reinforces the hypothesis that EtHg-S-Cys is also a substrate for this transporter system.
In conclusion, in the present study we investigated and compared the toxicity of different Hg compounds (MeHg, EtHg, MeHg-S-Cys and EtHg-S-Cys) in C6 glioma cells. Mercurial complexes with Cys appeared to be less toxic to C6 cells than MeHg or EtHg alone. The lower toxicities in the presence of MeHg-S-Cys or EtHg-S-Cys (compared with MeHg and EtHg) appear to be related to reduced mercurial entrance into the cells. In addition, treatment with L-Met was cytoprotective against MeHg-S-Cys and EtHg-S-Cys cytotoxicity, but not against MeHg and EtHg (data corroborated by Hg and GSH levels). Our data indicate that L-Met prevents MeHg-S-Cys- and EtHg-S-Cys-induced cytotoxicity secondary to attenuated mercurial entry into cells via the LAT transporter system. Thus, the results indicate that the uptake of MeHg-S-Cys and EtHg-S-Cys into C6 cells is mediated, at least in part, via the LAT system. Although the relationship between toxicity and MeHg-cysteine complexation is a relatively well studied topic (Yin et al., 2008; Roos et al., 2010, 2011), data on the relationship between toxicity and EtHg-cysteine complexation is scarce. In this context, the results obtained with EtHg-Cys in C6 cells represent a significant novelty concerning the toxicity elicited by this organic mercurial. When extrapolated to in vivo conditions, our results suggest that endogenous thiols likely influence the entry of EtHg into cells expressing LAT. With respect to MeHg and EtHg, even though both mercurials showed similar toxicities in C6 cells, the results cannot be directly extrapolated to in vivo conditions. In fact, the toxic potential of MeHg under in vivo conditions is higher than that of EtHg (Burbacher et al., 2005), which might be a consequence of differential metabolism pathways and excretion rates, and consequently its half-life.
Supplementary Material
Acknowledgments
Luciana Teixeira Zimmermann is a recipient of a post-doctoral fellowship from CAPES-PRODOC (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES – 2826/2010). The study was supported in part by funds from (i) IBN-Net/CNPq (MF, RBL and JBTR), (ii) Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (MF, JBTR, FBJr, RBL and JGD), (iii) Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (MF, JBTR, FBJr, RBL and JGD), (iv) INCT-CNPq-Excitotoxicity and Neuroprotection (MF and JBTR), and (v) US PHS NIH grants R01 ES07331 and ES000267 (MA).
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- Amonpatumrat S, Sakurai H, Wiriyasermkul P, Khunweeraphong N, Nagamori S, Tanaka H, et al. L-Glutamate enhances methylmercury toxicity by synergistically increasing oxidative stress. J Pharmacol Sci. 2008;108:280–9. doi: 10.1254/jphs.08118fp. [DOI] [PubMed] [Google Scholar]
- Aschner M, Ceccatelli S. Are neuropathological conditions relevant to ethylmercury exposure? Neurotox Res. 2010;18:59–68. doi: 10.1007/s12640-009-9113-2. [DOI] [PubMed] [Google Scholar]
- Aschner M, Clarkson TW. Methyl mercury uptake across bovine brain capillary endothelial cells in vitro: the role of amino acids. Pharmacol Toxicol. 1989;64:293–7. doi: 10.1111/j.1600-0773.1989.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Eberle NB, Goderie S, Kimelberg HK. Methylmercury uptake in rat primary astrocyte cultures: the role of the neutral amino acid transport system. Brain Res. 1990;521:221–8. doi: 10.1016/0006-8993(90)91546-s. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T. Methylmercury: recent advances in the understanding of its neurotoxicity. Ther Drug Monit. 2005;27:278–83. doi: 10.1097/01.ftd.0000160275.85450.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M. Brain, kidney and liver 203Hg-methyl mercury uptake in the rat: relationship to the neutral amino acid carrier. Pharmacol Toxicol. 1989;65:17–20. doi: 10.1111/j.1600-0773.1989.tb01119.x. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Developmental changes in the biliary excretion of methylmercury and glutathione. Science. 1982;216:61–3. doi: 10.1126/science.7063871. [DOI] [PubMed] [Google Scholar]
- Ballatori N. Transport of toxic metals by molecular mimicry. Environ Health Perspect. 2002;110(Suppl 5):689–94. doi: 10.1289/ehp.02110s5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood–brain barrier. Proc Natl Acad Sci USA. 1999;96:12079–84. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev. 2010;13:385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ Health Perspect. 2005;113:1015–21. doi: 10.1289/ehp.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury – current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–7. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–62. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Vyas JB, Ballatori N. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50:757–64. doi: 10.1002/ajim.20476. [DOI] [PubMed] [Google Scholar]
- del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci. 2008;35:161–74. doi: 10.1016/j.ejps.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Dórea JG. Integrating experimental (in vitro and in vivo) neurotoxicity studies of low-dose thimerosal relevant to vaccines. Neurochem Res. 2011;36:927–38. doi: 10.1007/s11064-011-0427-0. [DOI] [PubMed] [Google Scholar]
- Dórea JG. Making sense of epidemiological studies of young children exposed to thimerosal in vaccines. Clin Chim Acta. 2010;411:1580–6. doi: 10.1016/j.cca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JBT. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol. 2011a;256:405–17. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Campos F, Vendrell I, Berenguer J, Barzi M, Pons S, et al. Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicol Sci. 2009;112:416–26. doi: 10.1093/toxsci/kfp219. [DOI] [PubMed] [Google Scholar]
- Farina M, Rocha JBT, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci. 2011b;89:555–63. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, et al. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med. 2009;47:449–57. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Franco JL, Teixeira A, Meotti FC, Ribas CM, Stringari J, Garcia Pomblum SC, et al. Cerebellar thiol status and motor deficit after lactational exposure to methylmercury. Environ Res. 2006;102:22–8. doi: 10.1016/j.envres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Heggland I, Kaur P, Syversen T. Uptake and efflux of methylmercury in vitro: comparison of transport mechanisms in C6, B35 and RBE4 cells. Toxicol In Vitro. 2009;23:1020–7. doi: 10.1016/j.tiv.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Hintelmann H. Organomercurials. Their formation and pathways in the environment. Met Ions Life Sci. 2010;7:365–401. doi: 10.1039/BK9781847551771-00365. [DOI] [PubMed] [Google Scholar]
- Hirayama K. Effect of amino acids on brain uptake of methyl mercury. Toxicol Appl Pharmacol. 1980;55:318–23. doi: 10.1016/0041-008x(80)90093-9. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer RE, Singh SP, Doonan CJ, Ross ARS, Hughes RJ, Pickering IJ, et al. Molecular mimicry in mercury toxicology. Chem Res Toxicol. 2006;19:753–9. doi: 10.1021/tx0503449. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y, Yasutake A, Adachi T, Hirayama K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol. 1996;70:310–4. doi: 10.1007/s002040050279. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto Ki, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–32. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Kerper LE, Ballatori N, Clarkson TW. Methylmercury transport across the blood–brain barrier by an amino acid carrier. Am J Physiol. 1992;262:R761–5. doi: 10.1152/ajpregu.1992.262.5.R761. [DOI] [PubMed] [Google Scholar]
- Mokrzan EM, Kerper LE, Ballatori N, Clarkson TW. Methylmercury-thiol uptake into cultured brain capillary endothelial cells on amino acid system L. J Pharmacol Exp Ther. 1995;272:1277–84. [PubMed] [Google Scholar]
- Mutkus L, Aschner JL, Syversen T, Shanker G, Sonnewald U, Aschner M. In vitro uptake of glutamate in GLAST- and GLT-1-transfected mutant CHO-K1 cells is inhibited by the ethylmercury-containing preservative thimerosal. Biol Trace Elem Res. 2005;105:71–86. doi: 10.1385/BTER:105:1-3:071. [DOI] [PubMed] [Google Scholar]
- Ni M, Li X, Yin Z, Sidoryk-Węgrzynowicz M, Jiang H, Farina M, et al. Comparative study on the response of rat primary astrocytes and microglia to methylmercury toxicity. Glia. 2011;59:810–20. doi: 10.1002/glia.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pless R, Risher JF. Mercury, infant neurodevelopment, and vaccination. J Pediatr. 2000;136:571–3. doi: 10.1067/mpd.2000.106797. [DOI] [PubMed] [Google Scholar]
- Rabenstein DL, Isab AA, Reid RS. A proton nuclear magnetic resonance study of the binding of methylmercury in human erythrocytes. Biochim Biophys Acta Mol Cell Res. 1982;720:53–64. doi: 10.1016/0167-4889(82)90038-6. [DOI] [PubMed] [Google Scholar]
- Rodrigues JL, de Souza SS, de Oliveira Souza VC, Barbosa F., Jr Methylmercury and inorganic mercury determination in blood by using liquid chromatography with inductively coupled plasma mass spectrometry and a fast sample preparation procedure. Talanta. 2010;80:1158–63. doi: 10.1016/j.talanta.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Roos DH, Puntel RL, Lugokenski TH, Ineu RP, Bohrer D, Burger ME, et al. Complex methylmercury-cysteine alters mercury accumulation in different tissues of mice. Basic Clin Pharmacol Toxicol. 2010;107:789–92. doi: 10.1111/j.1742-7843.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- Roos DH, Puntel RL, Farina M, Aschner M, Bohrer D, Rocha JB, et al. Modulation of methylmercury uptake by methionine: prevention of mitochondrial dysfunction in rat liver slices by a mimicry mechanism. Toxicol Appl Pharmacol. 2011;252(1):28–35. doi: 10.1016/j.taap.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush T, Liu X, Lobner D. Synergistic toxicity of the environmental neurotoxins methylmercury and β-N-methylamino-L-alanine. Neuroreport. 2012a;23:216–9. doi: 10.1097/WNR.0b013e32834fe6d6. [DOI] [PubMed] [Google Scholar]
- Rush T, Liu X, Nowakowski AB, Petering DH, Lobner D. Glutathione-mediated neuroprotection against methylmercury neurotoxicity in cortical culture is dependent on MRP1. Neurotoxicology. 2012b;33:476–81. doi: 10.1016/j.neuro.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–51. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- Shanker G, Syversen T, Aschner JL, Aschner M. Modulatory effect of glutathione status and antioxidants on methylmercury-induced free radical formation in primary cultures of cerebral astrocytes. Brain Res Mol Brain Res. 2005;137:11–22. doi: 10.1016/j.molbrainres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Simmons-Willis TA, Koh AS, Clarkson TW, Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem J. 2002;367:239–46. doi: 10.1042/BJ20020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringari J, Nunes AKC, Franco JL, Bohrer D, Garcia SC, Dafre AL, et al. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol. 2008;227:147–54. doi: 10.1016/j.taap.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell I, Carrascal M, Vilaró M-T, Abián J, Rodríguez-Farré E, Suñol C. Cell viability and proteomic analysis in cultured neurons exposed to methylmercury. Hum Exp Toxicol. 2007;26:263–72. doi: 10.1177/0960327106070455. [DOI] [PubMed] [Google Scholar]
- Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–33. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Syversen T, Rocha JBT, Farina M, Aschner M. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J Neurochem. 2008;107:1083–90. doi: 10.1111/j.1471-4159.2008.05683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


