Abstract
Objective
There were no reports on predicting long-term efficacy of fecal microbiota transplantation (FMT) for ulcerative colitis (UC). This study aimed to detect short-term changes of cytokines and C-reactive protein (CRP) in patients with UC undergoing FMT, and to evaluate the predictive value of CRP and cytokines for the long-term efficacy of FMT.
Methods
Nineteen patients with moderate to severe UC (Mayo score ≥ 6) were treated with single fresh FMT through mid-gut. Serum samples were collected before and three days post-FMT. Clinical responses were evaluated by a minimum follow-up of three months. Patients with clinical improvement and remission at the assessment point of three-month were included as response group, while patients without clinical improvement or remission were included as non-response group. Serum concentrations of cytokines (IL-1β, IL-2, IL-4, IL-6, IL-10, IL-11, IL-17A, IFN-γ, TNF, TNFR-1, TNFR-2, MCP-1, G-CSF, GM-CSF) and CRP were assayed to predict the clinical response of FMT.
Results
In total, 10.5% (2/19) of patients achieved clinical remission and 47.4% (9/19) achieved clinical improvement (Response group, including clinical remission and clinical improvement), 42.1% (8/19) failed to benefit from FMT (Non-response group). In both Response group and Non-response group, the level of CRP at three days after FMT didn’t show significant decrease compared with that before FMT (p>0.05). However, in Response group, CRP level at three months after FMT decreased significantly than that before FMT (p<0.05). Compared with healthy controls (n = 9), patients with UC showed a higher baseline level of serum IL-6, TNFR-2 and G-CSF, and a lower level of IL-2 and IL-4 (p<0.05). In both Response group and Non-response group, none of the eleven detectable cytokines showed a significant difference between the value at three days after FMT and that before FMT (p>0.05).
Conclusions
Patients with moderate to severe UC presented a complex disorder of cytokines. However, the efficacy of FMT for UC might not be predicted by the short-term surveillance of cytokines and CRP.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by remitting and relapsing inflammation and epithelial injury[1,2]. Dysbiosis of the intestinal microbiota has been implicated in the etiology of this disease[3]. Fecal microbiota transplantation (FMT), a concept for the reconstruction of the intestinal homeostasis, originated in China in the fourth century[4]. Previous reports have shown the potential therapeutic role of FMT for the treatment of inflammatory bowel disease (IBD)[5–13]. In our previous study[14], the clinical response was better at one month after FMT, which might indicate slow or delayed response to FMT. Angelberger et al[15] also reported one patient with UC achieved positive clinical response at 12 weeks after FMT. Since FMT might bring long-term efficacy for the patients, we intended to find an immediate blood marker or markers for indicating the long-term outcome of FMT. C-reactive protein (CRP) was conventionally used to reflect the activity of IBD; however, both recent studies[15] and our group[14] observed that the temporary increase of CRP occurred after FMT in some cases, regardless whether they benefited from FMT or not. Cytokines have been proven to play a crucial role in controlling intestinal inflammation and associated clinical symptoms[16]. Disorders of cytokines on the circulating levels have been reported in patients with IBD[17–20]. The normal production of cytokines, including interleukin (IL)-1β[21], IL-2[22], IL-6[23], tumor necrosis factor (TNF)[16,24], interferon (IFN)-γ[25], IL-17[26], have been shown to be different from the normal controls. IBD is also related to gut dysbiosis, which plays a key role in regulating the host immune system[27–29]. However, it remains unknown whether the restoring of gut microbiota would affect the production of cytokines in IBD.
To determine whether there are markers which can be used to predict the long-term outcome of FMT, the changes of 14 cytokines in patients with UC at three days after FMT were evaluated to clarify the correlation between short-term changing of cytokines and long-term clinical efficacy of FMT.
Materials and Methods
Recruitment of patients
A prospective study as a part of clinical trial (NCT 01790061) was carried out by the Medical Center for Digestive Diseases at the Second Affiliated Hospital of Nanjing Medical University, Nanjing, China, from June 2013 to March 2015. This study was reviewed and approved by the Second Affiliated Hospital of Nanjing Medical University Institutional Review Board. All eligible subjects provided written informed consent prior to participation in the study. The diagnosis of UC consists of typical clinical, endoscopic, and histological criteria. All included patients aged 18–70 years old, had moderate to severe extensive UC (total Mayo score≥6)[30]. All participants were followed up more than three months. Exclusion criteria were: subjects who had an acute or chronic infectious disease; any clinically significant disorder e.g. cancer; subjects who were on any medication with a known effect on immunological factors, such as corticosteroids or immunomodulators; cases with incomplete samples or data.
Efficacy assessment of FMT
The efficacy of FMT was evaluated by patients’ clinical symptoms involving abdominal pain, stool frequency, rectal bleeding and laboratory tests at different observation time points including three days, one week, one month and three months after FMT. To avoid frequent endoscopic examinations, the scores of endoscopic examination at one week and one month were supposed to be equal to that before FMT. Clinical remission is defined as total Mayo score ≤ 2 points, with no individual subscore ≥1. Clinical improvement is defined as a decrease from baseline in the total Mayo score of at least 3 points or at least 30%, along with a reduction in the rectal bleeding subscore (RBS) of at least 1 point or an absolute RBS of ≤ 1. All patients who achieved clinical remission were also included in the analysis of clinical improvement. For analysis, at the surveillance point of three-month, patients with clinical improvement and remission were included as response group, while patients without clinical improvement or remission were included as non-response group.
Procedure of FMT
Firstly, patients were given metoclopramide 10 mg by intramuscular injection and esomeprazole magnesium 40 mg intravenously one hour before FMT. Fresh fecal material from selected donors was purified through our laboratory process based on a newly developed automatic purification system (GenFMTer, FMT Medical, Nanjing, China) as previously reported[14] since April 2014. Then the purified microbiota suspension was transplanted into the mid-gut of patients through an endoscopic infusion tube inserted into the gastroscope channel under anesthesia. Importantly, complete suction of stomach fluid must be performed before the endoscope was inserted into duodenum for infusion of microbiota suspension. Taken together, the entire procedure from the microbiota preparation to infusion should be completed within one hour.
Determination of the cytokine levels
To evaluate the temporary changing of serum cytokines, blood samples were collected from UC patients (n = 19) on admission day and three days post-FMT. Because patients’ response to FMT can generally be confirmed by clinical parameters within three days after FMT according to our clinical experience, then the timepoint of three days was selected for assessing short-term response of cytokines. In addition, blood samples were collected from 9 healthy volunteers as control group. The control group was matched with the UC group on baseline characteristics, including age, gender, ethnicity, Body Mass Index (BMI). All subjects underwent laboratory evaluation including blood test (complete blood count), CRP, erythrocyte sedimentation rate (ESR) and biochemical tests.
The 14 cytokines known to or hypothesized to be involved in inflammatory processes and that were investigated were interleukin IL-1β, IL-2, IL-4, IL-6, IL-10, IL-11, IL-17A, IFN-γ, TNF, TNF receptor type 1 (TNFR-1), TNFR-2, monocyte chemoattractant protein (MCP)-1, granulocyte-colonystimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF). Fasting blood tests were sampled at seven or eight in the morning before FMT and on day 3 after FMT. Blood samples were first centrifuged at 2500 rpm for 20 min and the serum samples were then stored at −80°C until analysis. The circulating levels of fourteen cytokines were analyzed using BD Cytometric Bead Array Flex Set System kits (BD Biosciences, USA) according to the manufacturer’s instructions.
Statistical analysis
Data were performed using SPSS (Chicago, IL, USA) or GraphPad (La Jolla, CA, USA). When the normality of the distribution of variables was acceptable, the independent sample t-test and paired student’s t test were used. When the normality of the distribution of variables was not acceptable, the Mann–Whitney U test and Wilcoxon signed-rank test were used to analyze differences between groups. Comparisons of categorical variables between groups were performed using a Fisher’s exact test. A value of p < 0.05 (two-tailed) was considered to indicate significance.
Results
Patient characteristics
A total of 19 patients (Table 1) with moderate-severe UC (Mayo score ≥ 6) were enrolled. In patients, the mean age was 39.2 year-old (range 19–60 years), and the mean disease duration was 8.0 years (range 1–21 years), 57.9% (11/19) had severe (Mayo score ≥ 11) disease and 42.1% (8/19) had moderate UC. In the control group, the mean age was 38.1 year-old, and 44.4% (4/9) were male.
Table 1. The characteristics of the included patients.
| Items | Results | |
|---|---|---|
| Patient | Total number | 19 |
| Age (years), ± SD (range) | 39.2 ± 14.1 (19–60) | |
| Sex, male% (n) | 36.8 (7) | |
| Disease duration (years, ± SD) | 8.0 ± 5.8 | |
| Mayo scores ( ± SD) | 10.5 ± 1.7 | |
| Smoking, yes % (n) | 10.5 (2) | |
| With history of steroid, yes % (n) | 21.1 (4) | |
| With history of immunomodulator, yes %(n) | 21.1 (4) | |
| CRP before FMT (mg/l, ± SD) | 16.42 ± 10.53 | |
| ESR before FMT (mm, ± SD) | 28.53 ± 18.04 | |
| WBC before FMT (*109/mm3, ± SD) | 7.50± 2.33 | |
| Hb (g/l, ± SD) | 121.05 ± 21.34 | |
| ALB (g/l, ± SD) | 41.63 ± 10.69 | |
| IgG (g/l, ± SD) | 14.29 ± 3.09 | |
| IgA(g/l, ± SD) | 2.23 ± 0.75 | |
| IgM (g/l, ± SD) | 1.24 ± 0.39 |
Note: SD, (Standard Deviation); CRP, (C-reactive protein); ESR (erythrocyte sedimentation rate).
Response to FMT
UC-related abdominal pain, stool frequency, bloody purulent stool, ESR, CRP and other parameters were assessed for all patients after FMT. According to the criteria of Mayo score, 31.6% (6/19) of patients benefited from the FMT at our first surveillance point of three-day. While at the three-month observing point, 57.9% (11/19) of patients achieved clinical response (named as response group), and 42.1% (8/19) of patients failed to benefit from the FMT (named as non-response group). Among the response group, 10.5% (2/19) met the criteria of clinical remission, and 47.4% (9/19) achieved clinical improvement. In addition, four patients in response group, who failed to achieve clinical improvement at three days, were observed to successfully achieve clinical efficacy three months post-FMT. In non-response group, two patients presented transient clinical improvement at three days, but did not achieve clinical improvement at three months.
In addition, we tested the correlation between the potential impact factors (including patients’ age, disease duration, smoking history etc.) and patients’ clinical response at three months post-FMT. As shown in Table 2, there was no significant difference among all investigated factors between two groups.
Table 2. Patients’ characteristics and clinical response.
| Characteristics | Response(n = 11) | Non-Response(n = 8) | P |
|---|---|---|---|
| Age (years) | 42.91 ± 15.27 | 34.00 ± 11.22 | 0.181 |
| Sex (male/female) | 3/8 | 4/4 | 0.377 |
| Disease duration (years, m ± SD) | 7.27 ± 5.57 | 8.88 ± 6.33 | 0.566 |
| Age at onset (years, m ± SD) | 35.64 ± 12.30 | 25.13 ± 10.84 | 0.070 |
| Smoking (no/yes) | 10/1 | 7/1 | 0.678 |
| With history of steroid (no/yes) | 9/2 | 6/2 | 0.574 |
Note: SD, (Standard Deviation).
Safety of FMT
No severe adverse events were observed during and after FMT procedure, as well as during the three months’ follow-up. 36.8% of patients (7/19) had a transient increased diarrhea frequency within 24h after FMT, and mainly occurred within three hours after FMT. One patient occurred mild skin pruritus six hours after FMT, and two patients presented with borborygmus. All symptoms resolved without any medical intervention. Except the above events, there was no more adverse event during the follow-up of more than three months.
Changing levels of CRP and clinical response
The CRP levels at three days post-FMT didn’t show a significant difference compared with that before FMT in both response group and non-response group (p>0.05). Two patients who showed an increased level of CRP at three days still maintained clinical improvement at the three-month follow-up point. However, the patients who achieved clinical improvement and clinical remission at three months post-FMT had a significantly lower CRP level than that before FMT (6.64±3.93mg/l vs. 15.73±8.96 mg/l, p = 0.003) (Fig 1), while CRP in non-response group didn’t show a similar trend (p>0.05).
Fig 1. Surveillance of CRP in response group (n = 11).
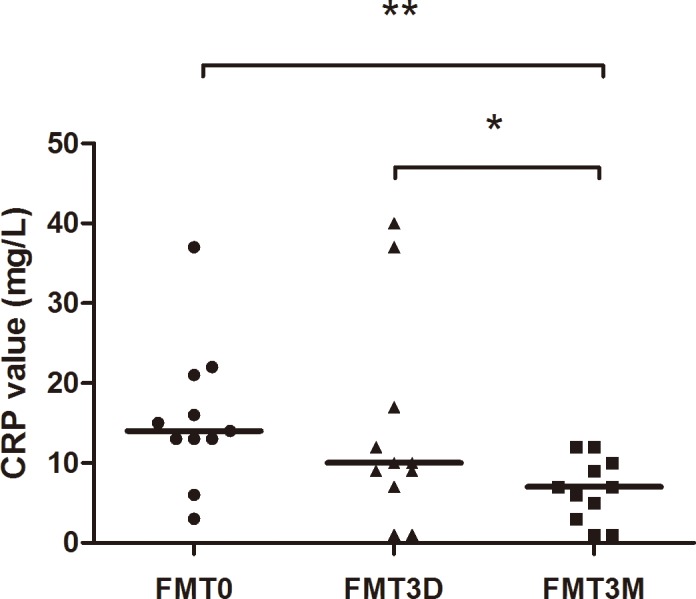
There is no statistical difference on CRP levels in pre-FMT and three days post-FMT. Compared with the value pre-FMT and three days post-FMT, the level of CRP decreased three months post-FMT, ** p<0.01 and * p<0.05, respectively. FMT0: 0 day pre-FMT, FMT3D: three days post-FMT, FMT3M: three months post-FMT.
Changes of cytokines after FMT
The baseline values of cytokines in UC patients before FMT were evaluated and then compared with healthy controls. As shown in Table 3, patients with UC presented a distinct cytokine profile compared with the healthy controls. Among the 14 cytokines, three cytokines (IL-1β, IL-11 and GM-CSF) were not detectable in both groups. The levels of serum IL-6, TNFR-2 and G-CSF in UC patients were significantly higher than that in the healthy persons (p<0.05), while IL-2 and IL-4 were significantly decreased (p<0.05). Six cytokines (IL-10, IL-17A, IFN-γ, TNF, TNFR-1 and MCP-1) did not show a significant difference between the UC patients and healthy persons.
Table 3. Concentrations of cytokines in UC and the healthy controls.
| cytokine | Controls (n = 9) | UC (n = 19) | P |
|---|---|---|---|
| IL-1β | nd | nd | - |
| IL-2 | 3.66 (1.91–5.80) | 1.25 (nd-4.48)* | 0.003 |
| IL-4 | 3.57 (2.76–5.18) | 2.12 (nd-2.87)* | 0.020 |
| IL-6 | 4.89 (3.415–5.56) | 9.185 (5.375–18.148)* | 0.002 |
| IL-10 | 3.55 (0.88–3.785) | 1.92 (nd-3.388) | 0.275 |
| IL-11 | nd | nd | - |
| IL-17A | 19.34 (14.62–24.16) | 20.41 (11.97–30.73) | 0.594 |
| IFN-γ | 4.05 (2.67–4.53) | 2.46 (nd-3.953) | 0.072 |
| TNF | 2.98 (nd-3.55) | 3.47 (nd-4.78) | 0.236 |
| TNFR-1 | 34.68 (9.925–73.865) | 44.22 (14.268–144.578) | 0.640 |
| TNFR-2 | 634.55 (365.71–1005.33) | 1685.52 (1017.775–2223.61)* | 0.004 |
| MCP-1 | 10.73 (4.805–17.170) | 19.635 (11.453–30.443) | 0.161 |
| G-CSF | nd | 0.675 (nd-2.628)* | 0.007 |
| GM-CSF | nd | nd | - |
Note: Values are expressed as the median (interquartile range) in pg/ml.
* p<0.05, vs. control group (Mann-Whitney test with Bonferroni correction for multiple comparisons).
nd: non-detectable, IBD: inflammatory bowel disease, IL: interleukin, IFN: inter feron, TNF: tumor necrosis factor, TNFR-1: tumor necrosis factor receptor-1, TNFR-2: tumor necrosis factor receptor-1, MCP: monocyte chemoattractant protein, G-CSF: granulocyte-colony stimulating factor, GM-CSF: granulocyte-macrophage colony-stimulating factor.
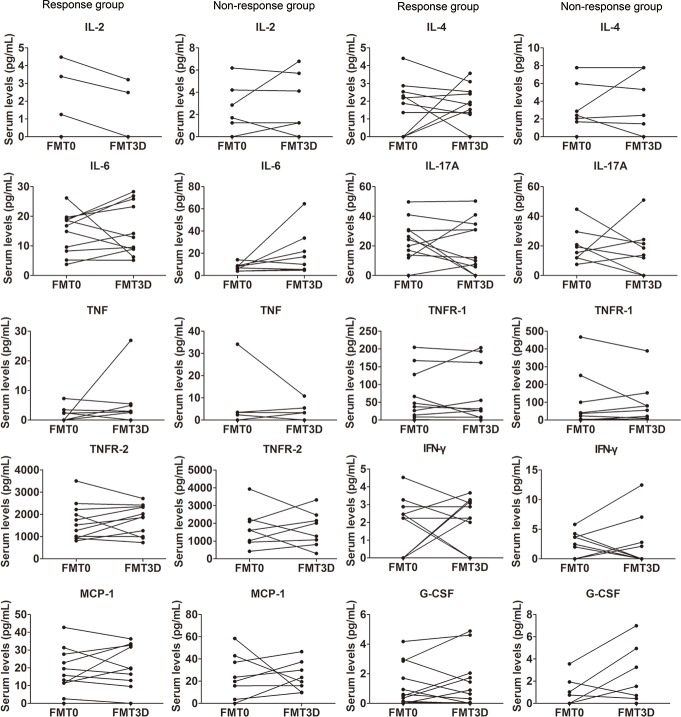
Concentrations of cytokines at three days after FMT were analyzed and compared with the original values before FMT in patients with UC. All of the eleven detectable cytokines didn’t show a significant difference between the values at three days after FMT and the values before FMT (p>0.05), regardless whether the patients benefited from FMT or not (Fig 2).
Fig 2. Changes of cytokines before and after FMT in patients with UC.
The serum concentrations of cytokines (IL-1β, IL-2, IL-4, IL-6, IL-10, IL-11, IL-17A, IFN-γ, TNF, TNFR-1, TNFR-2, MCP-1, G-CSF, GM-CSF) between pre-FMT group and three days post-FMT group were not significantly different (p>0.05).
Discussion
In the present study, 10.5% (2/19) of patients achieved clinical improvement and 47.4% (9/19) achieved clinical remission at three months after FMT. These results indicated that the therapeutic role of FMT for UC was consistent with Moayyedi’s study[6]. However, the efficacy of FMT for UC was still in debate. Rossen et al[13] and Kump et al[31] reported no benefit was achieved from FMT in patients with UC. The controversy in the efficacy of FMT might be explained by the difference in the methodology of FMT[32].
FMT had been reported to ameliorate the abdominal pain and reduce the frequency of diarrhea immediately in the patients with Crohn’s diseases in our previous study[14]. However, some studies[14,15] reported that a delayed positive clinical response to FMT was observed in IBD. In this study, we observed that four patients with UC, who failed to achieve clinical improvement at three days, successfully achieved clinical efficacy at three months post-FMT, ultimately. Two patients achieved transient clinical response at three days post-FMT, but relapsed soon. These results implied that both immediate and delayed clinical response should be highlighted during assessment of FMT efficacy.
Some quantifiable laboratory assessments including CRP, ESR, leukocytes, platelets and lymphocytes subset analysis were used to evaluate the disease activity of IBD in our previous study and some other studies[7,33,34]. These available serum lab assays may be helpful to answer the question whether the patient is getting better with the treatment of FMT. This is also the reason why these markers were included in this study.
CRP is a sensitive and reliable index of inflammatory process. The level of CRP increases in patients with trauma, inflammation and infection[35]. Determination of CRP is very important in diagnosis, treatment and monitoring of inflammatory conditions, because an elevated CRP level is always associated with pathological changes. In the patients who benefited from FMT, the level of CRP did not decrease three days after FMT compared with that before FMT, indicating that the three days’ value of CRP might not be able to predict the immediate clinical efficacy. However, in the response group, CRP decreased significantly at the assessment point of three months after FMT.
Among the cytokines investigated in the present study, IL-6, TNFR-2 and G-CSF were shown to be significantly upregulated in UC patients, while IL-2 and IL-4 were significantly downregulated, as compared with the controls. IL-6, in particular, is known to have a strong proinflammatory reaction[36], certain other cytokines, such as IL-4, may play a part in decreasing the inflammatory activity[37].
TNF is a proinflammatory cytokine, which has been reported to play a critical role in promoting chronic inflammation due to its pleiotropic functions[38–40]. Furthermore, anti-TNF monoclonal antibodies have been proved to be effective in the treatment of UC and CD[41]. TNF-α, as a potent cytokine promoting IBD, showed a trend of rise in serum of UC patients. However, it didn’t show a significant difference between UC patients and the controls in this study. The similar results were reported in pediatric patients with inflammatory bowel disease[42]. These results were completely unexpected, but may contribute to explain why certain IBD patient failed to benefit from anti-TNF therapy.
Levels of TNFR1 and TNFR2 were also tested in this study. TNF may exert various proinflammatory functions in colitis by binding to its receptors TNFR1 and TNFR2 followed by the intracellular activation of the transcription factor nuclear factor-κB (NF-κB). Recent clinical and experimental studies have shown that membrane-bound TNF, rather than soluble TNF, plays an important role in driving intestinal inflammation. Consistent with this, neutralization of membrane-bound TNF has been shown to induce T cell apoptosis and was effective in suppressing experimental colitis in mice, whereas activation of TNFR2 (which is induced by membrane-bound but not soluble TNF) on T cells was found to aggravate colitis activity[43,44]. It may account for the fact that serum concentrations of TNFR-2 were significantly elevated in IBD patients.
IL-2 is a proinflammatory factor mainly produced by Th1 cells, which could activate NK cells and macrophages and strengthen the sterilization ability of immunocyte to pathogens. It’s unexpected that the concentration of IL-2, which has been previously reported to be upregulated at the mucosal level, was significantly downregulated particularly considering that in IBD patients. In accordance with our results, recent studies have found that expression of IL-2 is downregulated at the level of the inflamed mucosa in murine models[16], indicating that there may be a negative correlation between the level of IL-2 and the severity of UC. More studies are needed to further verify our hypothesis.
Therefore, the results of the present study demonstrated that the characteristics of immune response in IBD patients might be more complicated than originally considered, and may be associated with certain aspect of immunodeficiency. It’s important to evaluate and figure out the precise balance between proinflammatory and anti-inflammatory cytokines during the occurrence and development of IBD.
In non-response group, all of the eleven detectable cytokines didn’t show a statistic difference at three days after FMT compared with the values before FMT. Surprisingly, there was also no significant integral difference on response group in pre-therapy and post-treatment. Even a number of these molecules, including IL-2, IL-4, IL-6, TNFR-2 and G-CSF, which have been previously reported to be statistically significant when compared with the normal controls, didn’t show a statistical difference before and after treatment in UC patients. These results indicated that clinical efficacy in patients with UC couldn’t be predicted by the cytokine changes at a short-term surveillance point of three days post-FMT. In another word, these results indicated that detection of cytokines is possibly not a strong evidence for the evaluation of curative effect in patients with UC.
There are some limitations in the present study. First, the small sample size may be responsible for some surprising results and a larger sample study is needed to conduct further analysis. Then CRP data should have been shown for both response and non-response groups. However, because of the consideration on the cost of tests and ethical reasons, we didn’t do the CRP tests since we confirmed some patients who had no response to FMT according to clinical assessments. In addition, the serum concentrations of cytokines three months after FMT were not tested in this study. Further studies are necessary to improve the understanding of IBD etiology and clarify whether there are differences in serum levels of cytokines between three days and three months after FMT.
In conclusion, FMT has showed its therapeutic role in UC. Patients with UC presented a complex disorder of cytokines. However, the efficacy of FMT for UC cannot be predicted by the short-term surveillance of cytokines and CRP.
Ethics Statement
This study was reviewed and approved by the Second Affiliated Hospital of Nanjing Medical University Institutional Review Board. All eligible subjects provided written informed consent prior to participation in the study.
Supporting Information
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by publicly donated funds, the Intestine Initiative Foundation, Clinical Science and Technology Foundation of Jiangsu Province (BL2014097) and the National Gastroenterology Research Project (2015BAI13B07).
References
- 1.Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc 2014; 89: 1553–63. 10.1016/j.mayocp.2014.07.002 . [DOI] [PubMed] [Google Scholar]
- 2.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011; 365: 1713–25. 10.1056/NEJMra1102942 . [DOI] [PubMed] [Google Scholar]
- 3.Babickova J, Gardlik R. Pathological and therapeutic interactions between bacteriophages, microbes and the host in inflammatory bowel disease. World J Gastroenterol 2015; 21: 11321–30. 10.3748/wjg.v21.i40.11321 ; PubMed Central PMCID: PMCPmc4616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol 2012; 107: 1755; author reply p. 1755–6. 10.1038/ajg.2012.251 . [DOI] [PubMed] [Google Scholar]
- 5.Sha S, Liang J, Chen M, Xu B, Liang C, Wei N, et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther 2014; 39: 1003–32. 10.1111/apt.12699 . [DOI] [PubMed] [Google Scholar]
- 6.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015; 149: 102–109.e6. 10.1053/j.gastro.2015.04.001 . [DOI] [PubMed] [Google Scholar]
- 7.Cui B, Li P, Xu L, Zhao Y, Wang H, Peng Z, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. Journal Transl Med 2015; 13: 298 10.1186/s12967-015-0646-2 ; PubMed Central PMCID: PMCPmc4567790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1989; 1: 164 . [DOI] [PubMed] [Google Scholar]
- 9.Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust 1989; 150: 604 . [DOI] [PubMed] [Google Scholar]
- 10.Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H Jr, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr 2013; 56: 597–601. 10.1097/MPG.0b013e318292fa0d . [DOI] [PubMed] [Google Scholar]
- 11.Gordon H, Harbord M. A patient with severe Crohn's colitis responds to Faecal Microbiota Transplantation. J Crohns Colitis 2014; 8: 256–7. 10.1016/j.crohns.2013.10.007 . [DOI] [PubMed] [Google Scholar]
- 12.Kao D, Hotte N, Gillevet P, Madsen K. Fecal microbiota transplantation inducing remission in Crohn's colitis and the associated changes in fecal microbial profile. J Clin Gastroenterol 2014; 48: 625–8. 10.1097/mcg.0000000000000131 . [DOI] [PubMed] [Google Scholar]
- 13.Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015; 149: 110–118.e4. 10.1053/j.gastro.2015.03.045 . [DOI] [PubMed] [Google Scholar]
- 14.Cui B, Feng Q, Wang H, Wang M, Peng Z, Li P, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol 2015; 30: 51–8. 10.1111/jgh.12727 . [DOI] [PubMed] [Google Scholar]
- 15.Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol 2013; 108: 1620–30. 10.1038/ajg.2013.257 . [DOI] [PubMed] [Google Scholar]
- 16.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 2002; 20: 495–549. 10.1146/annurev.immunol.20.100301.064816 . [DOI] [PubMed] [Google Scholar]
- 17.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014; 14: 329–42. 10.1038/nri3661 . [DOI] [PubMed] [Google Scholar]
- 18.Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis 2015; 21: 409–18. 10.1097/mib.0000000000000236 ; PubMed Central PMCID: PMCPmc4481731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol 2014; 20: 6–21. 10.3748/wjg.v20.i1.6 ; PubMed Central PMCID: PMCPmc3886033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang W, Su J, Zhang X, Cheng X, Zhou J, Shi R, et al. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm Res 2014; 63: 943–50. 10.1007/s00011-014-0768-7 . [DOI] [PubMed] [Google Scholar]
- 21.Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, et al. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med 2012; 209: 1595–609. 10.1084/jem.20111453 ; PubMed Central PMCID: PMCPmc3428945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breese E, Braegger CP, Corrigan CJ, Walker-Smith JA, MacDonald TT. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology 1993; 78: 127–31. ; PubMed Central PMCID: PMCPmc1421783. [PMC free article] [PubMed] [Google Scholar]
- 23.Kai Y, Takahashi I, Ishikawa H, Hiroi T, Mizushima T, Matsuda C, et al. Colitis in mice lacking the common cytokine receptor gamma chain is mediated by IL-6-producing CD4+ T cells. Gastroenterology 2005; 128: 922–34. . [DOI] [PubMed] [Google Scholar]
- 24.Atreya R, Zimmer M, Bartsch B, Waldner MJ, Atreya I, Neumann H, et al. Antibodies against tumor necrosis factor (TNF) induce T-cell apoptosis in patients with inflammatory bowel diseases via TNF receptor 2 and intestinal CD14(+) macrophages. Gastroenterology 2011; 141: 2026–38. 10.1053/j.gastro.2011.08.032 . [DOI] [PubMed] [Google Scholar]
- 25.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology 2009; 137: 1736–45. 10.1053/j.gastro.2009.07.049 . [DOI] [PubMed] [Google Scholar]
- 26.Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 2011; 208: 1127–33. 10.1084/jem.20101712 ; PubMed Central PMCID: PMCPmc3173242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caballero S, Pamer EG. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu Rev Immunol 2015; 33: 227–56. 10.1146/annurev-immunol-032713-120238 ; PubMed Central PMCID: PMCPmc4540477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Backhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe 2012; 12: 611–22. 10.1016/j.chom.2012.10.012 . [DOI] [PubMed] [Google Scholar]
- 29.Schuijt TJ, van der Poll T, de Vos WM, Wiersinga WJ. The intestinal microbiota and host immune interactions in the critically ill. Trends Microbiol 2013; 21: 221–9. 10.1016/j.tim.2013.02.001 . [DOI] [PubMed] [Google Scholar]
- 30.D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007; 132: 763–86. 10.1053/j.gastro.2006.12.038 . [DOI] [PubMed] [Google Scholar]
- 31.Kump PK, Grochenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis 2013; 19: 2155–65. 10.1097/MIB.0b013e31829ea325 . [DOI] [PubMed] [Google Scholar]
- 32.Cui B, Xu F, Zhang F. Methodology, Not Concept of Fecal Microbiota Transplantation, Affects Clinical Findings. Gastroenterology 2016; 150: 285–6. 10.1053/j.gastro.2015.05.065 . [DOI] [PubMed] [Google Scholar]
- 33.Chang S, Malter L, Hudesman D. Disease monitoring in inflammatory bowel disease. World J Gastroenterol 2015; 21: 11246–59. 10.3748/wjg.v21.i40.11246 ; PubMed Central PMCID: PMCPmc4616202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sands BE. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology 2015; 149: 1275–1285.e2. 10.1053/j.gastro.2015.07.003 . [DOI] [PubMed] [Google Scholar]
- 35.Du Clos TW. Function of C-reactive protein. Ann Med 2000; 32: 274–8. . [DOI] [PubMed] [Google Scholar]
- 36.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med 2000; 6: 583–8. 10.1038/75068 . [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Guo Z, Lv Z, Sun Y, Cao W, Zhang R, et al. The beneficial effect of Rheum tanguticum polysaccharide on protecting against diarrhea, colonic inflammation and ulceration in rats with TNBS-induced colitis: the role of macrophage mannose receptor in inflammation and immune response. Int Immunopharmacol 2008; 8: 1481–92. 10.1016/j.intimp.2008.04.013 . [DOI] [PubMed] [Google Scholar]
- 38.Xanthoulea S, Pasparakis M, Kousteni S, Brakebusch C, Wallach D, Bauer J, et al. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J Exp Med 2004; 200: 367–76. 10.1084/jem.20040435 ; PubMed Central PMCID: PMCPmc2211976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res 2005; 15: 24–7. 10.1038/sj.cr.7290259 . [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012; 119: 651–65. 10.1182/blood-2011-04-325225 ; PubMed Central PMCID: PMCPmc3265196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011; 106: 644–59, quiz 660. 10.1038/ajg.2011.73 . [DOI] [PubMed] [Google Scholar]
- 42.Kleiner G, Zanin V, Monasta L, Crovella S, Caruso L, Milani D, et al. Pediatric patients with inflammatory bowel disease exhibit increased serum levels of proinflammatory cytokines and chemokines, but decreased circulating levels of macrophage inhibitory protein-1beta, interleukin-2 and interleukin-17. Exp Ther Med 2015; 9: 2047–2052. 10.3892/etm.2015.2370 ; PubMed Central PMCID: PMCPmc4473502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holtmann MH, Douni E, Schutz M, Zeller G, Mudter J, Lehr HA, et al. Tumor necrosis factor-receptor 2 is up-regulated on lamina propria T cells in Crohn's disease and promotes experimental colitis in vivo. Eur J Immunol 2002; 32: 3142–51. . [DOI] [PubMed] [Google Scholar]
- 44.Perrier C, de Hertogh G, Cremer J, Vermeire S, Rutgeerts P, Van Assche G, et al. Neutralization of membrane TNF, but not soluble TNF, is crucial for the treatment of experimental colitis. Inflamm Bowel Dis 2013; 19: 246–53. 10.1002/ibd.23023 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.