Abstract
Pegylated interferon α-2a (Peg-IFN-α) represents a therapeutic alternative to the prolonged use of nucleos(t)ide analog (NA) in chronic hepatitis B (CHB) infection. The mechanisms leading to a positive clinical outcome remain unclear. As immune responses are critical for virus control, we investigated the effects of Peg-IFN-α on both innate and adaptive immunity, and related it to the clinical evolution. The phenotypic and functional features of the dendritic cells (DCs), natural killer (NK) cells and HBV-specific CD4/CD8 T cells were analyzed in HBeAg-negative CHB patients treated for 48-weeks with NA alone or together with Peg-IFN-α, before, during and up to 2-years after therapy. Peg-IFN-α induced an early activation of DCs, a potent expansion of the CD56bright NK subset, and enhanced the activation and functionality of the CD56dim NK subset. Peg-IFN-α triggered an increase in the frequencies of Th1- and Th17-oriented HBV-specific CD4/CD8 T cells. Peg-IFN-α reversed the unresponsiveness of patients to a specific stimulation. Most of the parameters returned to baseline after the stop of Peg-IFN-α therapy. Peg-IFN-α impacts both innate and adaptive immunity, overcoming dysfunctional immune responses in CHB patients. These modulations were not associated with seroconversion, which questioned the benefit of the add-on Peg-IFN-α treatment.
Introduction
Pegylated interferon α-2a (Peg-IFN-α) therapy represents a promising therapeutic alternative to the prolonged use of nucleos(t)ide analogs (NA) in chronic hepatitis B (CHB) infection [1–4]. Although Peg-IFN-α potentially leads to HBsAg seroconversion, its mechanisms of immunomodulation remain poorly known.
HBV modulates innate and adaptive immunity to escape clearance, generating weak and dysfunctional immune responses. Dysfunctions in dendritic cells (DCs), natural killer (NK) cells and T cells have been identified in patients with CHB infection. The virus may actively alter the function of plasmacytoid DCs (pDCs) [5], leading to a failure of the subsequent pDC-NK cross-talk in CHB patients [6]. Defects in the activation and antiviral functions of NK cells have also been described [7]. In addition, HBV-specific T-cell responses are often weak in patients who evolve toward chronic HBV infection [8], whereas multi-specific and vigorous HBV-specific T-cell responses directed toward epitopes located within the major HBV proteins [i.e. the nucleoscapsid (HBc), the surface antigen (HBs), the HBx antigen, and the polymerase (POL)] are required to successfully control HBV infection [9].
Peg-IFN-α represents a promising way to boost innate and adaptive immunity to overcome dysfunctional immune responses. IFN-α is a pleiotropic cytokine that displays strong antiviral and immunomodulatory properties [10]. It is produced in large amounts by pDCs during the early stages of viral infection. IFN-α can directly inhibit viral replication and enhance antiviral responses by acting on different immune effectors such as NK and T cells [10, 11]. NK cells play a pivotal role in antiviral immunity by controlling viral replication through direct cytotoxicity or by the production of immunoregulatory cytokines including IFN-γ and TNF-α that can modulate adaptive immune responses [12][13]. Virus-specific T cells are crucial in the later stages of viral infection. Following their activation by innate effectors, such as DCs and activated NK cells, virus-specific CD8+ T lymphocytes and CD4+ T-helper cells can control the infection through the secretion of pro-inflammatory cytokines and by differentiation into cytotoxic effectors that can lyse the infected cells [14].
The clinical benefit of Peg-IFN-α (as mono- or combination therapy) is superior to NA alone, whereas there is no difference in the virological response between treatment with Peg-IFN-α as monotherapy or in combination with NA [3, 4, 15, 16]. The precise impact of this therapy on the key antiviral effectors and the mechanism leading to a positive clinical outcome remain not fully understood. Only one study compared immunological changes triggered by Peg-IFNα alone, NA alone or the combination of both, but on limited immune parameters and at very early time points (within the first two weeks of therapy) [17]. Peg-IFN-α as a monotherapy activates DCs [18], expands and modulates the function of CD56bright NK cells [19, 20], and drives either an improvement or no changes in HBV-specific T-cell responses [21–23]. These studies were performed in separate cohorts of patients, thus preventing correlations between the immune parameters. As well, the kinetics of the immunologic changes was not detailed, which prevented the distinction of early and late effects. Finally, the studies did not feature long-term follow-up after the cessation of the treatment or comparison of the combined therapy with NA alone.
To overcome these limitations, the current sub-study investigated the impact of Peg-IFN-α on all major antiviral immune effectors including pDCs, mDCs, CD56brightCD16+/- and CD56dimCD16+/- NK-cell subsets, and T cells in the same cohort of HBeAg-negative CHB patients. We compared patients receiving a 48-week course of Peg-IFN-α in addition to NA with patients treated with NA alone. The immune parameters were studied at baseline, at different time points during the Peg-IFN-α therapy, and up to 2 years after the end of the treatment. We provide a dynamic longitudinal analysis of the features of both innate and adaptive responses, and related this to changes in the clinical parameters. This work contributes to a better understanding of the impact of Peg-IFN-α on immunity, revealing yet unexplored immune effects of the combination therapy. The observed alterations being not prognostically relevant, our work also questioned the benefit of the add-on Peg-IFN-α treatment over the NA or Peg-IFN-α monotherapies.
Materials and Methods
Patients
The study participants comprised 23 HBsAg-positive and HBeAg-negative CHB patients treated by analogs who had undetectable HBV-DNA for at least one year and who were enrolled in a multicenter, randomized, phase 3 study of Peg-IFN-α (ANRS HB06-PEGAN: NCT01172392). Fourteen patients remained on NA alone (control group) and nine received an additional 180μg of Peg-IFN-α (Pegasys; F Hoffmann-La Roche) once a week for 48 weeks (Peg-IFN-α group) (Table 1). Seven out of 14 patients in the control group and 7 out of 9 patients in the Peg-IFN-α group were HLA-A*02:01+ (Table 1). The study protocol was conducted according to the Declaration of Helsinki and French law for biomedical research, and approved by the Ethics Committee “Comite de Protection des Personnnes” (CPP) Sud-Méditerranée-I and the French Regulatory Authority “Agence Nationale de Securite du Medicament et des produits de sante” (ANSM). All participants gave the written informed consent. Heparinized blood samples were obtained at baseline and after 4 (Peg-IFN-α only), 12, 24, 48, 96 and 144 weeks of treatment. Peripheral blood mononuclear cells (PBMCs) were purified by Ficoll-Hypaque density gradient centrifugation (Eurobio) and the total lymphocyte concentration was determined. All the experiments were performed with freshly purified PBMCs.
Table 1. Clinical features of patients at baseline and during the course of the treatment.
| Baseline | HBsAg (log IU/ml) | Anti-HBs Ab status | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case # | Group | Age (year) | Sex | Analog | HBV DNA (IU/ml) | AST (IU/ml) | ALT (IU/ml) | Metavir activity | Metavir fibrosis | W0 | W12 | W24 | W48 | W96 | W144 | W0 | W48 | W96 | W144 |
| 1b | NA | 55 | M | tenofovir | <20 | 22 | 21 | 2 | 4 | 2,85 | 2,97 | 2,93 | 2,89 | 2,83 | ND | neg | neg | neg | neg |
| 2 | 54 | M | entecavir | <20 | 27 | 24 | 1 | 1 | 3,17 | 3,15 | 3,16 | 3,02 | 2,82 | ND | neg | neg | neg | neg | |
| 3 | 56 | M | tenofovir | <20 | 38 | 54 | ND | 4 | 1,72 | 1,72 | 1,72 | 1,65 | 1,60 | ND | neg | neg | neg | neg | |
| 4b | 34 | M | entecavir | <20 | 27 | 51 | 1 | 0 | 3,71 | 3,74 | 3,51 | 3,54 | 3,49 | ND | neg | ND | neg | ND | |
| 5b | 31 | M | tenofovir | <20 | 28 | 28 | 1 | 0 | 4,07 | 3,97 | 3,99 | 4,13 | 3,98 | ND | neg | neg | neg | ND | |
| 6 | 51 | M | tenofovir | <20 | 22 | 35 | ND | ND | 3,08 | 2,99 | 3,04 | 3,05 | 3,05 | 2,80 | neg | ND | neg | neg | |
| 7b | 39 | M | entecavir | <20 | 19 | 51 | 3 | 3 | 3,11 | 3,08 | 3,13 | 3,11 | 3,09 | 2,92 | neg | neg | neg | neg | |
| 8 | 54 | M | tenofovir | <20 | 23 | 29 | 2 | 2 | 3,41 | 3,32 | 3,34 | 3,16 | 3,08 | ND | neg | ND | neg | neg | |
| 9 | 35 | M | tenofovir | <20 | 35 | 38 | 2 | 4 | 3,39 | 3,28 | 3,36 | 3,19 | 3,12 | ND | neg | neg | neg | ND | |
| 10b | 48 | F | lamivudine | <20 | 19 | 15 | 3 | 1 | 2,60 | 2,55 | 2,38 | 2,56 | 2,51 | 2,24 | neg | neg | neg | neg | |
| 11 | 65 | M | tenofovir | <20 | 30 | 25 | 1 | 4 | 0,61 | 0,61 | 0,18 | 0,18 | -0,52 | ND | neg | neg | neg | pos | |
| 12 | 37 | M | entecavir | <20 | 43 | 35 | 2 | 1 | 4,32 | 4,30 | 4,21 | 4,14 | 4,42 | ND | neg | neg | neg | ND | |
| 13b | 62 | F | lamivudine | <20 | 23 | 19 | 1 | 2 | 3,36 | ND | 3,40 | 3,22 | 3,35 | ND | neg | neg | neg | neg | |
| 14b | 35 | M | tenofovir | <20 | 24 | 24 | 2 | 4 | 3,51 | 3,44 | 3,50 | 3,57 | 3,47 | 3,54 | neg | neg | neg | ND | |
| 15b | NA + Peg-IFNα | 41 | M | entecavir | <20 | 28 | 39 | 3 | 2 | 3,66 | 3,55 | 3,53 | 3,00 | 3,53 | 3,37 | neg | neg | neg | neg |
| 16b | 44 | M | entecavir | <20 | 23 | 41 | 1 | 2 | 3,35 | 3,21 | 3,24 | 3,10 | 3,36 | ND | neg | neg | neg | neg | |
| 17b | 63 | M | tenofovir | <20 | 31 | 42 | 1 | 1 | 2,93 | 2,97 | 2,83 | 2,59 | 2,65 | ND | neg | neg | neg | ND | |
| 18 | 57 | M | entecavir | <20 | 19 | 28 | 2 | 4 | 2,88 | 2,89 | 2,79 | 2,67 | 2,61 | 2,34 | neg | neg | neg | neg | |
| 19b | 49 | M | entecavir | <20 | 32 | 20 | ND | ND | 3,90 | 3,78 | 2,70 | 1,28 | 3,26 | 2,90 | neg | neg | neg | neg | |
| 20a,b | 49 | F | tenofovir | <20 | 17 | 13 | 1 | 2 | 2,38 | 2,24 | 2,36 | 2,20 | 1,61 | ND | neg | neg | neg | ND | |
| 21b | 67 | M | tenofovir | <20 | 40 | 56 | 2 | 2 | 3,78 | 3,64 | 3,24 | 1,83 | 3,08 | 3,06 | neg | neg | neg | neg | |
| 22 | 34 | M | entecavir | <20 | 24 | 22 | ND | ND | 4,36 | 4,27 | 4,27 | 4,34 | 4,35 | ND | neg | neg | neg | ND | |
| 23b | 61 | M | adefovir | <20 | 25 | 25 | 2 | 4 | 2,89 | 2,84 | 2,31 | 2,21 | 1,86 | ND | neg | neg | ND | ND | |
a: patient stopped treatment after W12
b: patients HLA-A*02:01+
NA: nucleos(t)ide analog
ND: not determined.
Phenotypic analysis
PBMCs were stained with fluorochrome-labeled anti-human antibodies or isotype-matched controls. T cells were defined with CD3/CD4/CD8/CD25 (BD), and Foxp3 (eBioscience) antibodies (Abs), and NK subsets were defined using CD3, CD16, and CD56 Abs (BD). The activation status was evaluated using the CD69 marker (BD). pDCs and mDCs were defined with BDCA2 (Miltenyi) and HLA-DR,/CD11c/Lin Abs (BD), and their activation status using CD40/CD80/CD86 Abs (BD). The stained cells were analyzed by flow cytometry using a FACSCantoII device equipped with Diva software (BD). Absolute numbers (cells/ml blood) were obtained by multiplying their percent by the total lymphocyte number. To ensure quality control during the study, we performed a systematic standardization of the fluorescence intensities using cytometer setup and tracking beads (BD).
NK function measurements
Cultures were performed in RPMI1640-Glutamax supplemented with 1% non-essential amino acids, 100μg/mL gentamycin, 10% fetal calf serum (Invitrogen) and 1mM sodium pyruvate (SigmaAldrich). PBMCs were cultured in the presence or absence of recombinant human interleukin (rhIL)12 and rhIL18 (R&D Systems) for 18h. To measure IFN-γ secretion, 1μl/ml brefeldinA (BD) was added for the last 4h. Cells were labeled with CD3, CD16, and CD56 Abs (BD) and stained for intracellular IFN-γ (BD). NK cytotoxic activity was evaluated by a CD107 degranulation assay. The cultures were washed and co-cultured with K562 cells (5:1 ratio) for 3h. Anti-human CD107a/b Abs (BD) were added at the start of the re-stimulation together with GolgiSTOP for the last 2h. The cells were labeled with CD3, CD16, and CD56 Abs before flow cytometry analysis.
Evaluation of HBV-specific T-cell responses
PBMCs were stimulated during 14 days without or with pools of 15-mer peptides overlapping by 10-residues covering the overall sequence of HBc, HBs, HBx, and POL antigens of HBV genotype D (pooled in 14 mixtures). The cultures were re-stimulated at day 7 with the same peptide pools, IL-2 (Proleukine) and 30Gy-irradiated allogeneic PBMCs. To determine the frequency of specific T cells, intracellular cytokine staining was performed at day 14 upon specific stimulation. Expanded T-cell lines were incubated for 5h with the corresponding peptide pools in the presence of brefeldinA for the last 4h. The cells were washed and stained with CD3/CD8 Abs (BD) followed by the intracellular staining of TNF-α, IFN-γ and IL-10. To determine the global cytokine profile, expanded T-cell lines were resuspended at 1x106cells/ml, and restimulated with the corresponding peptide pools before measurement of cytokine secretion in the supernatant 24h later by a cytometric bead array (CBA) assay (BD).
Measurements of specific CD8 T cells using tetramers
For HLA-A*02:01+ patients, specific T cells were measured directly from PBMCs by tetramer labeling using HBc18-27 (FLPSDFFPSV) and HBs335-343 (WLSLLVPFV) HLA-A*02:01-tetramers (Beckman Immunomics) followed by staining with CD3/CD8 Abs (BD).
Induction of HBV-specific CD8 T-cell responses using pDC-based stimulation
The ability of HLA-A*02:01+ patients to respond to a stimulation by peptide-loaded HLA-A*02:01+ pDCs was evaluated as previously described [24]. PBMCs were co-cultured for 14 days with HBc18-27 and HBs335-343 peptide-loaded pDCs (1:10 ratio) and re-stimulated weekly in the presence of 200UI/ml IL-2 (Proleukine, Chiron). Specific T-cell responses were measured by tetramer labeling as previously described.
Cytokine quantification
Plasma samples were collected before and at each time point of treatment, and the levels of IP-10 were quantified by a CBA assay (BD).
Statistical analyses
Statistical analysis was performed using the Mann-Whitney non-parametric U-test, Wilcoxon matched pairs test, and Spearman correlation using Prism software (GraphPad Software).
Results
Peg-IFN-α increases the basal activation levels of DC subsets
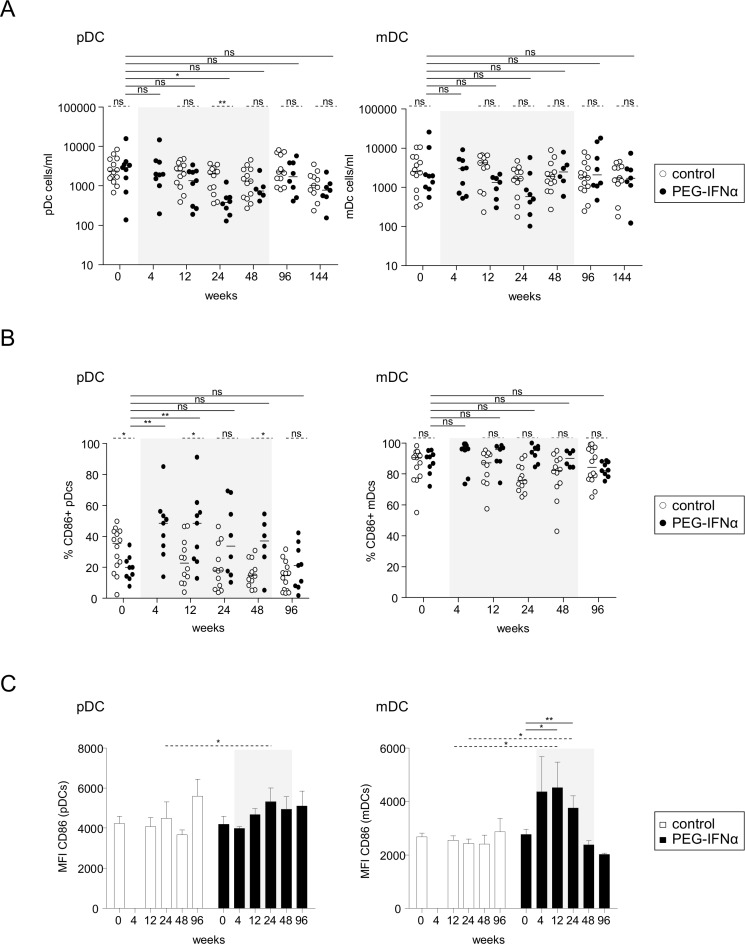
We first investigated the impact of Peg-IFN-α on the features of DC subsets. The frequency, absolute numbers, and activation status of pDCs (HLA-DR+BDCA2+) and mDCs (Lin-HLA-DR+CD11c+) were analyzed in both treatment arms before and at different times during treatment. We observed a continuous trend of a decrease in the frequencies and absolute numbers of DCs, achieving significance for pDCs at week (W) 24 (Fig 1A). Peg-IFN-α induced an up-regulation of CD86 expression on pDCs and mDCs (Fig 1B and 1C) without clearly affecting CD40 levels (S1 Fig). All the parameters returned to baseline after the cessation of the treatment. Despite the reduction of DC numbers over time, the activation status of pDCs and mDCs was modulated as soon as 4 weeks by Peg-IFN-α therapy.
Fig 1. Modulation of DC subsets by Peg-IFN-α.
Patients with CHB infection were treated with NA alone (open circles, n = 11–14) or together with Peg-IFN-α (black circles, n = 7–9). DC subsets were analyzed by flow cytometry before and at different time points of treatment. (A) Absolute numbers of pDCs and mDCs. (B) Basal percentages of CD86 expression on pDCs and mDCs. (C) Mean fluorescence intensity of CD86 on pDCs and mDCs. CD86 was not evaluable for W144. The gray area represents the period of Peg-IFN-α administration. Bars represent median. P-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). *p<0.05, **p<0.01, ***p<0.001.
Peg-IFN-α modulates NK-cell distribution and function
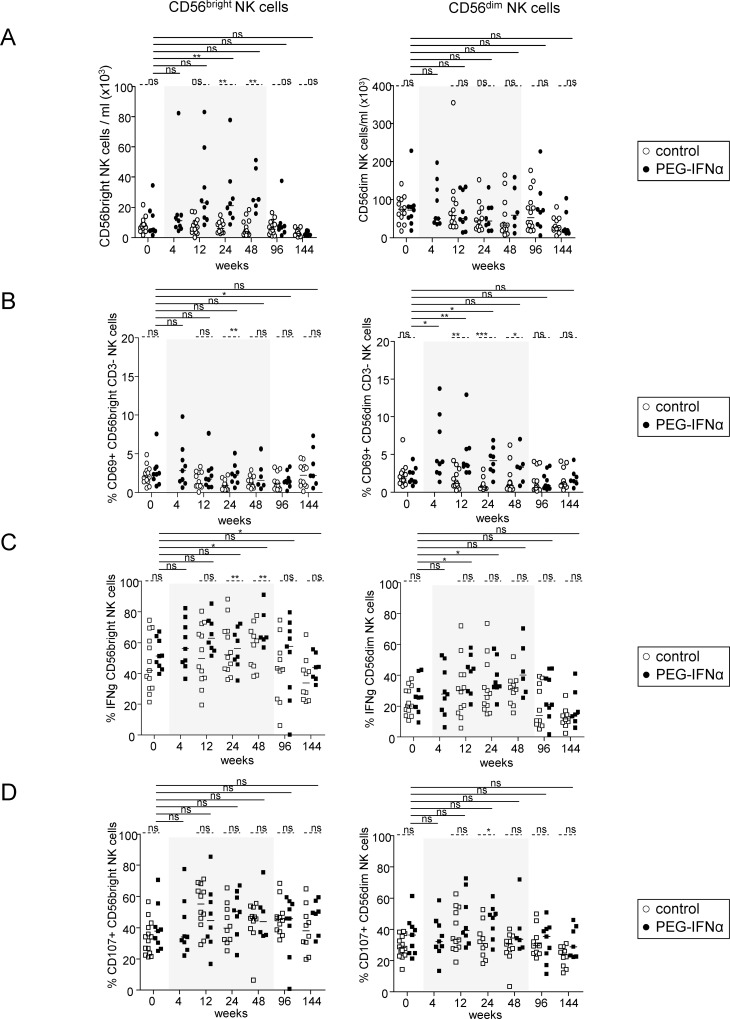
We next assessed the impact of Peg-IFN-α on NK-cell subsets. Peg-IFN-α drove an increase in total NK-cell frequency (S2A Fig), attributable to a continuous enhancement of the CD56bright NK-cell subset (Fig 2A), including both CD16+ and CD16- CD56bright NK cells (S2B Fig). NK cells were activated by Peg-IFN-α as soon as 4 weeks, as revealed by the increase in CD69 expression W4 post-treatment (S3A Fig). This modulation was seen only within the CD16+ and CD16- CD56dim NK cells (Fig 2B, S3B Fig). A significant increase of the ability of NK cells to secrete IFN-γ following IL12/IL18 stimulation in the Peg-IFN-α group was evident from W12 to W48 post-treatment compared to baseline and to the control group (Fig 2C, S4A and S4B Fig). In addition, the cytotoxic activity of NK cells assessed by CD107 surface expression upon IL12/IL18 stimulation and co-culture with the NK-cell target K562 was significantly improved in patients treated with Peg-IFN-α, peaking at W24 post-treatment (Fig 2D, S4C and S4D Fig). Thus, Peg-IFN-α drastically modulated NK-cell distribution and activation, and potentiated the functionality of the CD56bright and CD56dim NK-cell subsets. All the parameters returned to baseline after the cessation of treatment.
Fig 2. Modulation of NK-cell features by Peg-IFN-α.
Patients with CHB infection were treated with NA alone (open circles, n = 12–13) or together with Peg-IFN-α (black circles, n = 8–9). CD56+CD3- total NK cells and CD56bright/dim NK subsets were analyzed by flow cytometry before and during Peg-IFN-α treatment. (A) Absolute numbers of CD56bright and CD56dim NK cells. (B) Basal level of CD69 expression. (C) IFN-γ secretion was evaluated by intracellular staining upon IL12/IL18 stimulation. (D) The cytotoxic activity was evaluated upon IL12/IL18 stimulation after co-culture with K562 by measuring CD107 surface expression. Bars represent median. P-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). *p<0.05, **p<0.01, ***p<0.001.
Peg-IFN-α triggers Th1- and Th17-oriented HBV-specific CD4 and CD8 T-cell responses
We then investigated the immunomodulatory effects of Peg-IFN-α on the T-cell subsets. A gradual decrease in the absolute numbers of CD4/CD8 T cells was evident at W24 and W48 following Peg-IFN-α treatment accompanied by a slight modulation of their activation status (S5A and S5B Fig). Peg-IFN-α also induced a transient and modest modulation of the absolute numbers of regulatory T cells (Treg) (S5C Fig).
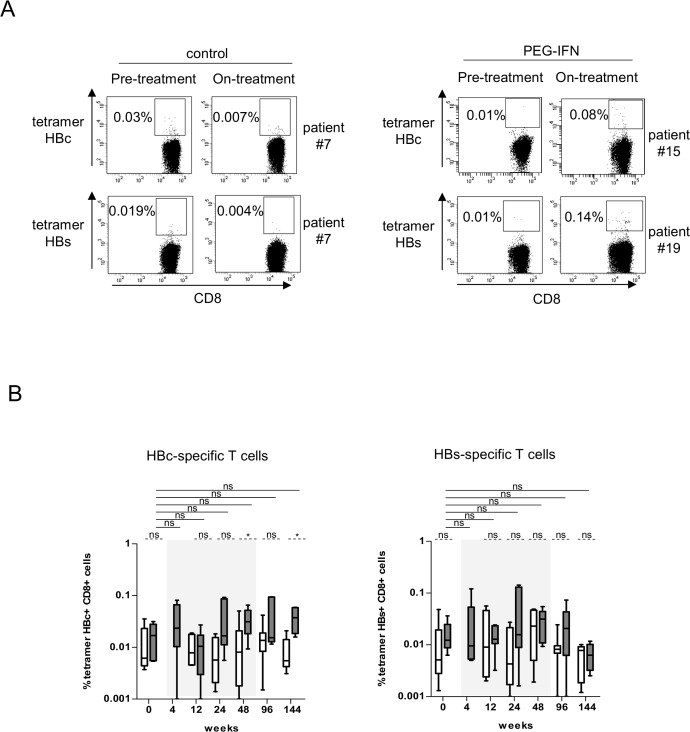
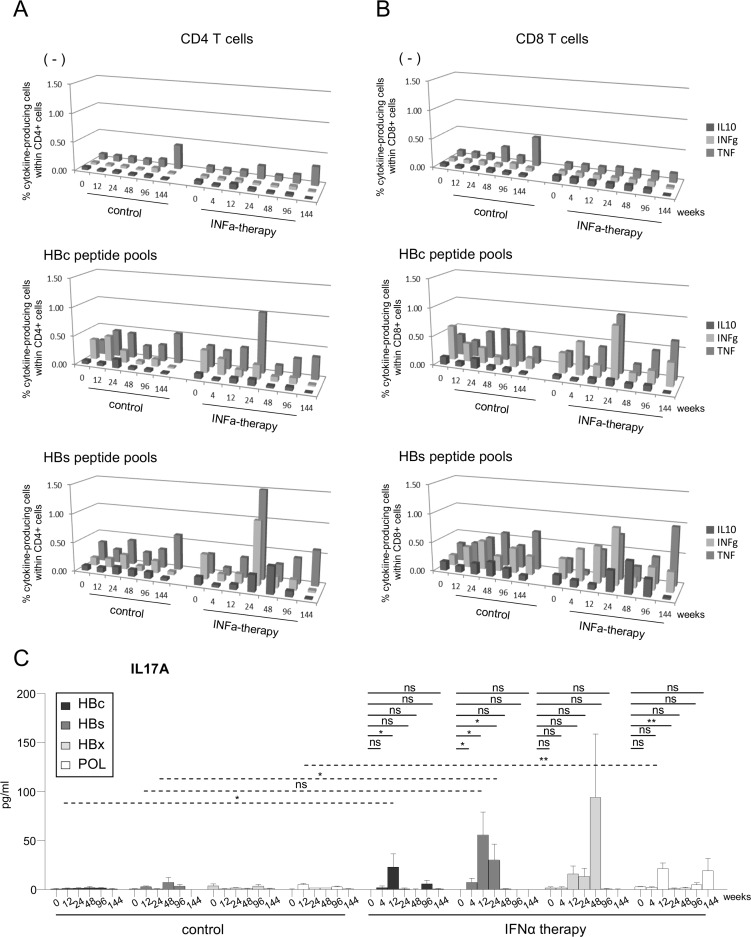
To assess whether Peg-IFN-α therapy could improve the virus-specific T-cell responses, we first evaluated the ex-vivo frequency of HBc18-27 and HBs335-343-specific CD8 T cells directly from the blood of HLA-A*02:01+ patients using multimers. Notably, the frequency of circulating HBV-specific CD8 T cells following Peg-IFN-α therapy increased in some patients (Fig 3). We further analyzed the frequency and Th orientation of HBV-specific CD4/CD8 T cells following stimulation with overlapping peptide pools derived from HBc, HBs, HBx, and POL antigens. Upon specific stimulation, we observed the improvement of TNF-α and/or IFN-γ producing HBc- and HBs-specific T cells among CD4 (Fig 4A) and CD8 (Fig 4B) T cells during the course of Peg-IFN-α therapy compared to the control group and to the control stimulation. No elicitation of HBx- and pol-specific immune responses were noticed (S6 Fig). To obtain a more extended view of the Th profile of the activated T cells, we also measured the global cytokine profile in the culture supernatants following the specific stimulation of PBMCs with HBV-derived peptide pools. Strikingly, we observed a substantial secretion of IL-17A in response to the HBV antigens in patients treated with Peg-IFN-α compared to NA alone (Fig 4C). Nine out of 9 patients from the Peg-IFN-α group displayed such a Th17 profile at least at one time point, in response to 1 to 3 HBV antigens. Thus, Peg-IFN-α triggered the immune system toward Th1-biased HBV-specific T-cell responses and Th17 profile.
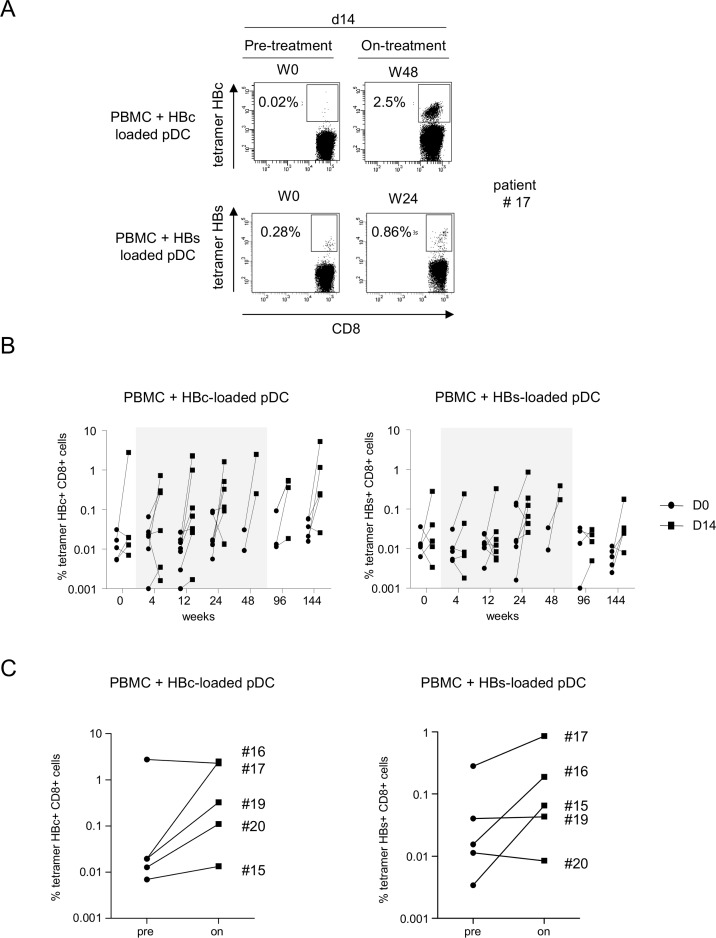
Fig 3. Direct HBV-specific CD8 T-cell response evolution during Peg-IFN-α therapy.
Direct HBV-specific CD8 T cells measurements following Peg-IFN-α treatment using tetramers. (A) Representative dot plots of the tetramer labeling of HBc18-27- and HBs335-343-specific T cells (gated on CD8 T cells) before and 24 weeks after treatment in patients treated with nucleos(t)ide analog alone (left panel) or together with Peg-IFN-α (right panel). (B) Evolution of the basal percentages of HBc18-27- (left panel) and HBs335-343- (right panel) specific CD8 T cells in patients with CHB infection treated with nucleos(t)ide analog alone (white bars, n = 7) or together with Peg-IFN-α (grey bars, n = 7). The gray area represents the period of Peg-IFN-α administration.
Fig 4. Frequencies and Th1/Th17 orientation of HBV-specific CD4/CD8 T cells during the course of Peg-IFN-α treatment.
(A-B) Frequencies of HBV-specific CD4/CD8 T cells were evaluated before and during Peg-IFN-α treatment upon the stimulation of PBMCs with peptide pools and intracellular TNF-α, IFN-γ and IL-10 cytokine staining from patients treated with NA alone (n = 5–8) or together with Peg-IFN-α (n = 3–5). (A) Frequencies of cytokine-producing HBc-/HBs-specific CD4 T-cell responses (mean values). (B) Frequencies of cytokine-producing HBc-/HBs-specific CD8 T-cell responses (mean values). (C) IL-17A production was analyzed in supernatants of PBMCs stimulated with peptide pools derived from the HBc, HBs, HBx and POL antigens before and during Peg-IFN-α treatment in patients with CHB infection treated with NA alone (n = 10) or together with Peg-IFN-α (n = 9). P-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). *p<0.05, **p<0.01, ***p<0.001.
Peg-IFN-α treatment reverses unresponsiveness to specific stimulation by HBV-derived peptide-loaded pDCs
We previously developed a powerful technology based on a human HLA-A*02:01+ pDC line to trigger functional HBV-specific T-cell responses ex-vivo from chronic HBV patients [24]. We identified responder and non-responder patients, allowing the defining of an immunologic responsiveness to this strategy. To investigate the effects of Peg-IFN-α on the ability of HLA-A*02:01+ patients to respond to this immunotherapeutic tool, PBMCs were stimulated once a week with the pDC line loaded with the HLA-A*02:01-restricted HBc- and HBs-derived peptides, and antigen-specific T cells evaluated using tetramers. Before the start of the treatment (W0), four of five patients tested were unable to respond to the pDC-based stimulation (Fig 5A and 5B) as no amplification of HBc- and HBs-specific T cells were observed upon 14 days of stimulation. Notably, during the course of Peg-IFN-α therapy, the proportion of responder patients increased to 4 of 5 patients (Fig 5B and 5C). Strikingly, this potentiality was maintained for more than 2 years after the cessation of Peg-IFN-α therapy (W144). Thus, Peg-IFN-α treatment potently reversed the unresponsiveness to specific stimulation by HBV-derived peptide-loaded pDCs.
Fig 5. Peg-IFN-α reverses the unresponsiveness of patients to the stimulation by HBV-derived peptide-loaded pDCs.
PBMCs were stimulated with pDCs loaded with HLA-A*02:01-restricted HBc18-27 and HBs335-343 peptides and the amplification of HBV-specific T cells was assessed using HLA-A*02:01-tetramers. (A) Representative dotplots of the tetramer labelling of HBc18-27- (upper panel) and HBs335-343- (lower panel) specific T cells (gated on CD8 T cells) at day (D)14 before and on Peg-IFN-α therapy. (B) Evolution of the percentages of HBc18-27- and HBs335-343-specific T cells at D0 and D14 of culture during Peg-IFN-α therapy. (C) Comparative levels of HBc18-27- and HBs335-343-specific CD8 T cells at D14 between baseline and on-treatment (n = 5). The gray area represents the period of Peg-IFN-α administration.
Correlations between immunologic and clinical parameters
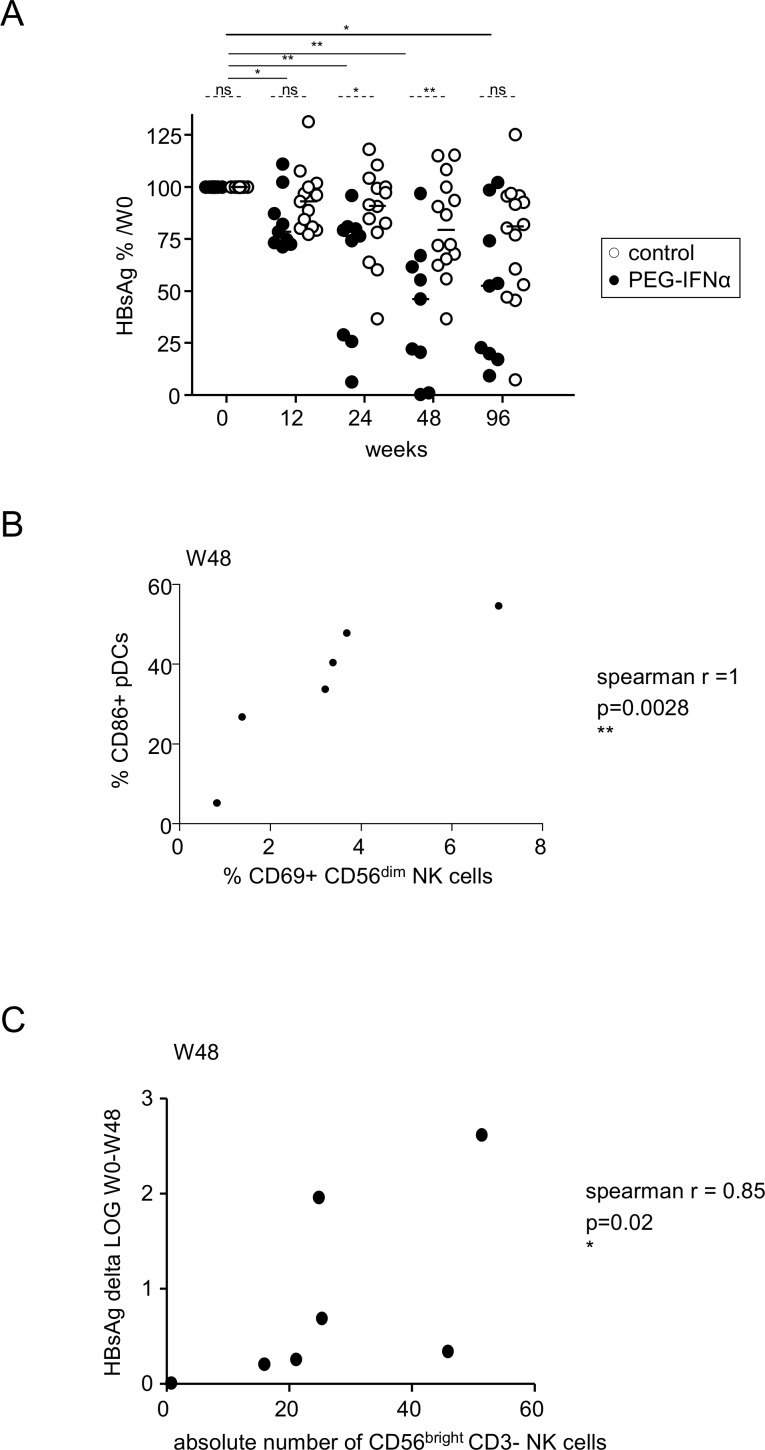
One of the major signatures of a clinical response to Peg-IFN-α therapy is a decrease in plasma HBsAg levels. In contrast to the control group, the HBsAg level significantly decreased throughout the treatment of patients with the additional Peg-IFN-α therapy compared to baseline (Fig 6A). No correlation between remodeling of the DC/NK/T-cell compartment and the ability to respond to Peg-IFN-α therapy (defined by HBsAg seroconversion at W96) was revealed. However, two patients (#19, #21) underwent a decrease of 2 log IU/ml in HBsAg titer during Peg-IFN-α therapy (Table 1). These patients had the highest levels of both CD86+ pDCs and CD69+CD56dim NK cells at W48. Interestingly, the proportion of CD86-expressing pDCs strongly correlated with the proportion of CD69-expressing CD56dim NK cells (Fig 6B). Moreover, we observed an inverse correlation between the absolute numbers of CD56bright NK cells and the decline in HBsAg (Fig 6C). In parallel to changes in innate and adaptive immunity, a significant increase in plasma interferon-inducible protein-10 (IP-10) levels was evident throughout the course of the Peg-IFN-α treatment, which may participate in the modulations observed (S7 Fig). Collectively, our data suggest a relationship between the immunologic changes observed and the evolution of viral parameters following Peg-IFN-α therapy.
Fig 6. Correlation between immunological parameters and the evolution of HBsAg in patients treated with Peg-IFN-α.
(A) Evolution of HBsAg during the course of Peg-IFN-α treatment (percentage of W0). P-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). Bars represent median. *p<0.05, **p<0.01, ***p<0.001 (B) The modulation of NK cells by Peg-IFN-α correlates with pDC activation. Correlation between the percentage of CD69+CD56dim NK cells and the percentage of CD86+ pDCs after 48 weeks of treatment (Spearman correlation). (C) The modulation of NK cells by Peg-IFN-α correlates with the decrease in HBsAg. Spearman correlation between the absolute number of CD56bright NK cells and the decline in HBsAg after 48 weeks of treatment.
Discussion
We provide a dynamic description of the innate and adaptive immunological changes during and up to 2 years after the course of an additional Peg-IFN-α therapy in CHB patients compared to patients receiving NA alone, whose clinical outcomes are known to be different. The findings increase the understanding of Peg-IFN-α immunomodulatory effects, and reveal yet unexplored immune effects of the combination therapy. However, as no seroconversion occurred in our cohort of patients, this work questions which function, direct antiviral or indirect immunomodulation, is more relevant in the efficacy of Peg-IFN-α in the treatment of CHB patients in the add-on settings.
Our data bring a larger view of the immunologic changes triggered by Peg-IFN-α therapy, also with a better subsetting definition and the full kinetics of the immune modulations, reinforcing the results of recent clinical trials [17–20, 23]. Firstly, Peg-IFN-α triggered a marked expansion of the CD56bright NK-cell subset accompanied by an activation and an enhanced functionality of the CD56dim NK-cell subset. CD56bright NK cells represent an intermediate stage of NK-cell differentiation, as a precursor of the CD56dim subset. Their unique immunoregulatory role [25] is illustrated by their preferential interactions with DCs, and their important role in early immune responses and in the shaping of subsequent adaptive responses [26]. Expansion of CD56bright NK cells has been described in several diseases, especially in HCV infection [27], in patients with multiple sclerosis treated with IFN-β [28], and interestingly in patients with active systemic lupus erythematosus [29], a disease mediated by elevated type-I IFN levels. Of note, we observed an inverse correlation between the absolute numbers of CD56bright NK cells and the decline in HBsAg titer. The distinct conclusions on NK functionality compared to other studies [19, 20] may be due to the different ways of data analysis, either as absolute numbers of functional NK cells [19] or as percentages of functional cells within the NK population [20], the time point (point of kinetics or the end of treatment) and the groups that were compared (responders versus non-responders, pre- and on-treatment points).
Moreover, Peg-IFN-α triggered as soon as 4 weeks an activation of pDCs and mDCs, which are critical in antiviral responses [30]. Indeed, type-I IFN is essential for DC maturation and activation [31], and to favor the cross-presentation of antigens by DCs [32], which can help promote subsequent cross-priming of antiviral T cells and facilitate viral clearance. Since DCs are essential to activate NKs and antiviral responses [33], modulation of DCs by Peg-IFN-α may affect the subsequent DC-NK cross-talk and potentiate NK-cell and T-cell functions. This hypothesis is supported by the strong correlation observed between the percentages of activated CD69+CD56dim NK cells and CD86+ pDCs. Thus, Peg-IFN-α may restore the pDC/NK cross-talk [6], subsequently enhancing antiviral NK-cell responses that could optimize the elimination of infected hepatocytes.
We also revealed key effects of Peg-IFN-α on adaptive immunity. We observed the elicitation of HBc- and HBs-specific CD4 and CD8 immune responses during Peg-IFN-α therapy. This is in contrast with prior observations that Peg-IFN-α did not improve peripheral HBV-specific T-cell responses [17, 22]. The discrepancy may be due to the time of analysis (very early versus late), and/or the use of frozen cells stimulated once with the peptide pools, whereas we performed two rounds of stimulation on freshly isolated PBMCs. Our data are consistent with the previously described restoration of HBV-specific T-cell responses following Peg-IFN-α therapy [23, 34, 35]. In addition, we have shown for the first time the elicitation of Th17 cells upon Peg-IFN-α treatment, preceding the induction of Th1-oriented immunity. Although IL17-producing T cells are described as pro-inflammatory cells and have been associated with liver damage [36], plasma IL17A levels and Th17 cell frequency are negatively correlated with viral load in patients with CHB infection [37]. Such a Th17 profile is revealed only upon specific stimulation with HBV-derived peptide pools and only in the Peg-IFN-α group, suggesting that Th17 cells are either HBV-specific T cells or that HBV-specific T cells subsequently activate other T cells to produce IL17. Interestingly, Th17 cells have been involved in the establishment of long-term immune memory and for promoting B-cell class switch [38]. Thus, they may regulate cellular and humoral antiviral immune responses, therefore favoring the elicitation of HBV-specific immune responses crucial for the immune control of HBV infection.
By potently modulating both the virus fitness and immunity, Peg-IFN-α appears to trigger a systemic immune activation and overcome the functional impairments to subsequently drive antiviral immunity. Despite evident immunologic changes, patients did not achieve HBsAg seroconversion in this cohort. In the overall ANRS HB06 PEGAN trial, HBsAg clearance at W48 has been achieved in only 8% of the patients treated by Peg-IFN-α (7/90) (Bourliere et al. J Hepatol 62, S2, S249, 2015). HBsAg clearance was associated with a low baseline HBs Ag titers and a history of HBeAg seroconversion prior to the inclusion into the trial, which suggests a potential spontaneous anti-viral immune activation. Immune modulations triggered by the therapy might even though improve clinical parameters. The two patients who underwent the highest decline in HBsAg during Peg-IFN-α therapy had the highest level of activated pDCs and NK cells at the end of treatment. The inverse correlation between the absolute numbers of CD56bright NKs and the down-regulation of HBsAg suggested a relationship between the immunologic and virological changes. Strikingly, we identified immunologic changes that persisted for up to 2 years after the cessation of the treatment in the absence of HBsAg seroconversion, as illustrated by the reversion of the unresponsiveness to the peptide-loaded pDCs.
Peg-IFN-α therapy profoundly affects both innate and adaptive immunity, but only transiently and without leading to HBsAg seroconversion. By identifying yet unexplored immune effects of the combination therapy, our study illustrates the pleiotropic action of IFN-α. However, as shown by the other groups, most of the immune changes returned to baseline after the cessation of Peg-IFN-α treatment. The immune alterations induced by Peg-IFN-α treatment failing in the majority of cases to induce seroconversion, they are probably less determinant than the direct antiviral effect of this cytokine. Our work questioned the benefit of the add-on Peg-IFN-α treatment over the NA or Peg-IFN-α monotherapies.
Supporting Information
Patients with CHB infection were treated with nucleos(t)ide analog alone (open circles, n = 11–14) or together with Peg-IFN-α (black circles, n = 7–9). Basal percentages of CD40 expression on DC subsets were analyzed by flow cytometry before and at different time points of treatment. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
Patients with CHB infection were treated with nucleos(t)ide analog alone (open circles, n = 12–13) or together with Peg-IFN-α (black circles, n = 8–9). CD56+CD3- total NK cells as well as NK subsets defined based on CD56bright / dim and CD16 expression were analyzed by flow cytometry before and at different time points of treatment. (A) Frequencies of total NK cells, CD56bright NK cells and CD56dim NK cells. (B) Absolute numbers of CD56brightCD16- and CD16+ NK cells and CD56dimCD16- and CD16+ NK cells. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
The basal level of CD69 expression was analyzed on the NK subsets from patients with CHB infection treated with nucleos(t)ide analog alone (open circles, n = 12–13) or together with Peg-IFN-α (black circles, n = 8–9) by flow cytometry before and at different time points of treatment. (A) CD69 expression on total NK cells. (B) CD69 expression on CD56brightCD16- NK cells and CD56brightCD16+ NK cells and CD56dimCD16- NK cells and CD56dimCD16+ NK cells. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p- values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
Patients with CHB infection were treated with nucleos(t)ide analog alone or together with Peg-IFN-α. (A,B) IFN-γ secretion was evaluated by intracellular staining in total NK cells without or with IL12/IL18 stimulation before and at different time points of treatment. (A) Representative dotplots from patients with CHB infection treated either with nucleos(t)ide analog alone (upper panel) or together with Peg-IFN-α (lower panel) (gated on total CD56+CD3- NK cells). (B) Comparative proportions of IFNγ+ NK cells upon IL12/IL18 stimulation in patients treated with NA alone (open squares, n = 12–14) or together with Peg-IFNα (black squares, n = 8–9) at different time points of treatment. (C,D) NK cytotoxic activity was evaluated in total NK cells without or with IL12/IL18 stimulation after co-culture with K562 by measuring CD107 surface expression before and at different time points of treatment. (C) Representative dotplots from patients with CHB infection treated either with nucleos(t)ide analog alone (upper panel) or together with Peg-IFN-α (lower panel) (gated on total CD56+CD3- NK cells). (D) Comparative proportions of CD107+ NK cells upon IL12/IL18 stimulation and co-culture with K562 in patients treated with NA alone (open squares, n = 12–14) or together with Peg-IFN-α (black squares, n = 8–9) at different time points of treatment. For clarity, only the IL12/IL18 condition is shown. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
Patients with CHB infection were treated with nucleos(t)ide analog alone (open circles, n = 11–14) or together with Peg-IFN-α (black circles, n = 8–9). T-cell subsets were analyzed by flow cytometry before and at different time points of treatment. (A) Evolution of CD4 and CD8 T-cell numbers following Peg-IFN-α treatment. Fold increase in absolute CD4 and CD8 T-cell numbers before and at different time points of treatment. P-values were calculated using the Wilcoxon test within the same group of patients toward W0 (*p<0.05) and the Mann-Whitney test between the two groups of patients at the indicated time point (#p<0.05). (B) Evolution of the basal level of CD69 expression on CD4 T cells (left panels) and CD8 T cells (right panels). (C) Evolution of the absolute regulatory T-cell (Treg) numbers during the course of treatment. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
The frequencies of HBV-specific CD4/CD8 T cells were evaluated before and during the course of the treatment upon stimulation of PBMC with overlapping peptide pools and intracellular TNFα, IFN-γ and IL-10 cytokine staining from patients treated with nucleos(t)ide analog alone (n = 12–13) or together with Peg-IFN-α (n = 8–9). (A) Frequencies of cytokine-producing HBx- and pol-specific CD4 T-cell responses. (B) Frequencies of cytokine-producing HBx- and pol-specific CD8 T-cell responses.
(TIFF)
Plasma levels of IP-10 from patients with CHB infection treated with nucleos(t)ide analogue together with Peg-IFN-α (n = 7–9). The gray area represents the period of Peg-IFN-α administration. The p- values were calculated using the Wilcoxon test.
(TIFF)
Acknowledgments
We are indebted to the patients who agreed to participate in this study. We thank the co investigator coordinator Dr Yves Benhamou, the methodologist Pr Fabrice Carrat, and all the investigators belonging to the ANRS HB06 PEGAN study group (leader: Dr Marc Bourlière, contact: mbourliere@hopital-saint-joseph.fr): Pr. Dominique Roulot, Unité fonctionnelle d'Hépatologie, Hôpital Avicenne, Bobigny; Pr. Dominique Valla, Pr. Patrick Marcellin, Service d'Hépatologie, Hôpital Beaujon, Clichy; Pr. Catherine Buffet, Dr. Pierre Attali, Service d’Hépato-gastro-entérologie, Hôpital Bicêtre, Le Kremlin Bicêtre; Pr. Stanislas Pol, Dr. Fontaine Hélène, Pôle d'Hépato-gastroentérologie, Hôpital Cochin, Paris; Dr. Ariane Mallat, Dr. Christophe Hezode, Service d'Hépato-gastroentérologie, Hôpital Henri Mondor, Creteil; Pr. Michel Beaugrand, Dr. Nathalie Ganne-Carrié, Service d'Hépato-gastroentérologie, Hôpital Jean Verdier, Bondy; Pr. Denis Castaing, Pr. Didier Samuel, Centre Hépato-Biliaire, Hôpital Paul Brousse, Villejuif; Pr. Thierry Poynard, Dr. Yves Benhamou, Pr. Vlad Ratziu, Service d'Hépato-gastroentérologie, Hôpital Pitié Salpêtrière, Paris; Pr. Olivier Chazouillères, Dr. Lawrence Serfaty, Service d'Hépato-gastroentérologie, Hôpital Saint Antoine, Paris; Pr. Daniel Sereni, Dr. Caroline Lascoux-Combe, Service de médecine interne, Hôpital Saint Louis, Paris; Pr. Jean-Michel Molina, Service des Maladies Infectieuses, Hôpital Saint Louis, Paris; Dr. Jean-Didier Grangé, Service d'Hépato-gastroentérologie, Hôpital Tenon, Paris; Dr. Magali Picon-Coste, Service de Gastroentérologie et Hépatologie, Centre Hospitalier du Pays d'Aix, Aix en Provence; Pr. Paul Calès, Service des Maladies du Foie et de l’Appareil Digestif, Hôpital de l'Hôtel Dieu, Angers; Pr. Victor de Ledinghen, Service d'Hépato-gastroentérologie, Hôpital de Haut Lévêque, Bordeaux; Dr. Thierry Sapey, Service de Médecine F, Centre Hospitalier de Châteauroux, Châteauroux; Pr. Gilles Bommelaer, Pr. Armand Abergel, Pôle Digestif Hépato Biliaire, Hôpital d'Estaing, Clermont-Ferrand; Dr. Hervé Hagège, Dr. Isabelle Rosa-Hézode, Service d'Hépato-gastroentérologie, Centre Hospitalier Intercommunal, Créteil; Pr. Jean-Pierre Zarski, Pr Vincent Leroy, Service d'Hépato-gastroentérologieHôpital Albert Michallon, Grenoble; Dr. Alain Blanchi, Dr. Christophe Pilette, Service d’Hépato-gastroentérologie, Centre Hospitalier du Mans, Le Mans; Pr. Antoine Cortot, Pr. Philippe Mathurin, Service des Maladies de l'Appareil Digestif, Hôpital Claude Huriez, Lille; Pr. Fabien Zoulim, Service d'Hépato-gastroentérologie, Hôpital de la Croix Rousse, Lyon; Pr. Danielle Botta-Fridlund, Service d’Hépato-Gastroentérologie, Hôpital de la Conception, Marseille; Pr. Marc Bourlière, Service d'Hépato-gastroentérologie, Fondation Hôpital Saint Joseph, Marseille; Pr. Dominique Larrey, Service d'Hépato-gastroentérologie, Hôpital Saint Eloi, Montpellier; Pr. Marc-André Bigard, Pr. Jean-Pierre Bronowicki, Service d'Hépato-gastroentérologie, Hôpital de Brabois, Vandoeuvre-lès-Nancy; Pr. Albert Tran, Service d'Hépatologie, Hôpital de l'Archet, Nice; Dr. Xavier Causse, Service d'Hépato-gastroentérologie, Hôpital de La Source, Orléans; Pr. Pierre Brissot, Pr. Dominique Guyader, Service d'Hépatologie, Hôpital Pontchaillou, Rennes; Pr. Eric Lerebours, Dr. Ghassan Riachi, Service d'Hépato-gastroentérologie, Hôpital Charles Nicolle, Rouen; Pr. Jean-Pierre Vinel, Pr. Laurent Alric, Fédération Digestive, Hôpital Purpan, Toulouse; Pr. Etienne Metman, Dr. Yannick Bacq, Service d'Hépato-gastroentérologie, Hôpital Trousseau, Tours; Pr Eric Nguyen-Khac, Service d’Hépato-gastroentérologie, Centre Hospitalier d’Amiens, Amiens.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the French Blood Service (EFS) and the study was sponsored and funded by ANRS(France REcherche Nord & sud Sida-hiv Hépatites).
References
- 1.Tujios SR, Lee WM. Update in the management of chronic hepatitis B. Current opinion in gastroenterology. 2013;29(3):250–6. Epub 2013/03/23. 10.1097/MOG.0b013e32835ff1e9 . [DOI] [PubMed] [Google Scholar]
- 2.EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. Journal of hepatology. 2012;57(1):167–85. Epub 2012/03/23. 10.1016/j.jhep.2012.02.010 . [DOI] [PubMed] [Google Scholar]
- 3.Lampertico P, Vigano M, Di Costanzo GG, Sagnelli E, Fasano M, Di Marco V, et al. Randomised study comparing 48 and 96 weeks peginterferon alpha-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut. 2013;62(2):290–8. Epub 2012/08/04. 10.1136/gutjnl-2011-301430 . [DOI] [PubMed] [Google Scholar]
- 4.Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. The New England journal of medicine. 2005;352(26):2682–95. Epub 2005/07/01. 10.1056/NEJMoa043470 . [DOI] [PubMed] [Google Scholar]
- 5.Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PloS one. 2011;6(1):e15324 Epub 2011/01/20. 10.1371/journal.pone.0015324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinet J, Dufeu-Duchesne T, Bruder Costa J, Larrat S, Marlu A, Leroy V, et al. Altered functions of plasmacytoid dendritic cells and reduced cytolytic activity of natural killer cells in patients with chronic HBV infection. Gastroenterology. 2012;143(6):1586–96 e8. Epub 2012/09/11. 10.1053/j.gastro.2012.08.046 . [DOI] [PubMed] [Google Scholar]
- 7.Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: facts and controversies. Eur J Clin Invest. 2010;40(9):851–63. Epub 2010/07/06. 10.1111/j.1365-2362.2010.02332.x . [DOI] [PubMed] [Google Scholar]
- 8.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature reviews Immunology. 2005;5(3):215–29. Epub 2005/03/02. 10.1038/nri1573 . [DOI] [PubMed] [Google Scholar]
- 9.Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, et al. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117(6):1386–96. Epub 1999/12/02. . [DOI] [PubMed] [Google Scholar]
- 10.Swiecki M, Colonna M. Type I interferons: diversity of sources, production pathways and effects on immune responses. Curr Opin Virol. 2011;1(6):463–75. Epub 2012/03/24. 10.1016/j.coviro.2011.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nature reviews Immunology. 2012;12(2):125–35. Epub 2012/01/10. 10.1038/nri3133 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanier LL. Evolutionary struggles between NK cells and viruses. Nature reviews Immunology. 2008;8(4):259–68. Epub 2008/03/15. 10.1038/nri2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(2):728–32. Epub 2010/12/29. 10.1073/pnas.1012356108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. Epub 2007/11/28. 10.1146/annurev.pathol.1.110304.100230 . [DOI] [PubMed] [Google Scholar]
- 15.Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. The New England journal of medicine. 2004;351(12):1206–17. Epub 2004/09/17. 10.1056/NEJMoa040431 . [DOI] [PubMed] [Google Scholar]
- 16.Rijckborst V, ter Borg MJ, Cakaloglu Y, Ferenci P, Tabak F, Akdogan M, et al. A randomized trial of peginterferon alpha-2a with or without ribavirin for HBeAg-negative chronic hepatitis B. Am J Gastroenterol. 2010;105(8):1762–9. Epub 2010/05/13. 10.1038/ajg.2010.186 . [DOI] [PubMed] [Google Scholar]
- 17.Tan AT, Hoang LT, Chin D, Rasmussen E, Lopatin U, Hart S, et al. Reduction of HBV replication prolongs the early immunological response to IFNalpha therapy. Journal of hepatology. 2014;60(1):54–61. Epub 2013/09/03. 10.1016/j.jhep.2013.08.020 . [DOI] [PubMed] [Google Scholar]
- 18.Boltjes A, Op den Brouw ML, Biesta PJ, Binda RS, van der Molen RG, Boonstra A, et al. Assessment of the effect of ribavirin on myeloid and plasmacytoid dendritic cells during interferon-based therapy of chronic hepatitis B patients. Mol Immunol. 2013;53(1–2):72–8. Epub 2012/07/21. 10.1016/j.molimm.2012.06.016 . [DOI] [PubMed] [Google Scholar]
- 19.Micco L, Peppa D, Loggi E, Schurich A, Jefferson L, Cursaro C, et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. Journal of hepatology. 2013;58(2):225–33. Epub 2012/10/11. 10.1016/j.jhep.2012.09.029 . [DOI] [PubMed] [Google Scholar]
- 20.Stelma F, de Niet A, Tempelmans Plat-Sinnige MJ, Jansen L, Takkenberg RB, Reesink HW, et al. Natural Killer Cell Characteristics in Patients With Chronic Hepatitis B Virus (HBV) Infection Are Associated With HBV Surface Antigen Clearance After Combination Treatment With Pegylated Interferon Alfa-2a and Adefovir. The Journal of infectious diseases. 2015. Epub 2015/03/21. 10.1093/infdis/jiv180 . [DOI] [PubMed] [Google Scholar]
- 21.Liu YZ, Hou FQ, Ding P, Ren YY, Li SH, Wang GQ. Pegylated interferon alpha enhances recovery of memory T cells in e antigen positive chronic hepatitis B patients. Virol J. 2012;9:274 Epub 2012/11/20. 10.1186/1743-422X-9-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penna A, Laccabue D, Libri I, Giuberti T, Schivazappa S, Alfieri A, et al. Peginterferon-alpha does not improve early peripheral blood HBV-specific T-cell responses in HBeAg-negative chronic hepatitis. Journal of hepatology. 2012;56(6):1239–46. Epub 2012/02/14. 10.1016/j.jhep.2011.12.032 . [DOI] [PubMed] [Google Scholar]
- 23.Thimme R, Dandri M. Dissecting the divergent effects of interferon-alpha on immune cells: time to rethink combination therapy in chronic hepatitis B? Journal of hepatology. 2013;58(2):205–9. Epub 2012/11/20. 10.1016/j.jhep.2012.11.007 . [DOI] [PubMed] [Google Scholar]
- 24.Martinet J, Leroy V, Dufeu-Duchesne T, Larrat S, Richard MJ, Zoulim F, et al. Plasmacytoid dendritic cells induce efficient stimulation of antiviral immunity in the context of chronic hepatitis B virus infection. Hepatology. 2012;56(5):1706–18. Epub 2012/06/19. 10.1002/hep.25879 . [DOI] [PubMed] [Google Scholar]
- 25.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–51. Epub 2001/05/09. . [DOI] [PubMed] [Google Scholar]
- 26.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–65. Epub 2009/03/13. 10.1111/j.1365-2567.2008.03027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy MJ, Ryan EJ, Hegarty JE, et al. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57(8):1121–8. Epub 2008/03/29. 10.1136/gut.2007.130963 . [DOI] [PubMed] [Google Scholar]
- 28.Saraste M, Irjala H, Airas L. Expansion of CD56Bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2007;28(3):121–6. Epub 2007/07/03. 10.1007/s10072-007-0803-3 . [DOI] [PubMed] [Google Scholar]
- 29.Schepis D, Gunnarsson I, Eloranta ML, Lampa J, Jacobson SH, Karre K, et al. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology. 2009;126(1):140–6. Epub 2008/06/20. 10.1111/j.1365-2567.2008.02887.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woltman AM, Boonstra A, Janssen HL. Dendritic cells in chronic viral hepatitis B and C: victims or guardian angels? Gut. 2010;59(1):115–25. Epub 2009/12/17. 10.1136/gut.2009.181040 . [DOI] [PubMed] [Google Scholar]
- 31.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nature reviews Immunology. 2008;8(8):594–606. Epub 2008/07/22. 10.1038/nri2358 . [DOI] [PubMed] [Google Scholar]
- 32.Schiavoni G, Mattei F, Gabriele L. Type I Interferons as Stimulators of DC-Mediated Cross-Priming: Impact on Anti-Tumor Response. Frontiers in immunology. 2013;4:483 Epub 2014/01/09. 10.3389/fimmu.2013.00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33(6):955–66. Epub 2010/12/07. 10.1016/j.immuni.2010.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehermann B, Lau D, Hoofnagle JH, Chisari FV. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. The Journal of clinical investigation. 1996;97(7):1655–65. Epub 1996/04/01. 10.1172/JCI118592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprinzl MF, Russo C, Kittner J, Allgayer S, Grambihler A, Bartsch B, et al. Hepatitis B virus-specific T-cell responses during IFN administration in a small cohort of chronic hepatitis B patients under nucleos(t)ide analogue treatment. Journal of viral hepatitis. 2014;21(9):633–41. Epub 2013/11/21. 10.1111/jvh.12189 . [DOI] [PubMed] [Google Scholar]
- 36.Zhang JY, Zhang Z, Lin F, Zou ZS, Xu RN, Jin L, et al. Interleukin-17-producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology. 2010;51(1):81–91. Epub 2009/10/21. 10.1002/hep.23273 . [DOI] [PubMed] [Google Scholar]
- 37.Xue-Song L, Cheng-Zhong L, Ying Z, Mo-Bin W. Changes of Treg and Th17 cells balance in the development of acute and chronic hepatitis B virus infection. BMC gastroenterology. 2012;12:43 Epub 2012/05/03. 10.1186/1471-230X-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121(13):2402–14. Epub 2013/01/18. 10.1182/blood-2012-09-378653 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patients with CHB infection were treated with nucleos(t)ide analog alone (open circles, n = 11–14) or together with Peg-IFN-α (black circles, n = 7–9). Basal percentages of CD40 expression on DC subsets were analyzed by flow cytometry before and at different time points of treatment. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
Patients with CHB infection were treated with nucleos(t)ide analog alone (open circles, n = 12–13) or together with Peg-IFN-α (black circles, n = 8–9). CD56+CD3- total NK cells as well as NK subsets defined based on CD56bright / dim and CD16 expression were analyzed by flow cytometry before and at different time points of treatment. (A) Frequencies of total NK cells, CD56bright NK cells and CD56dim NK cells. (B) Absolute numbers of CD56brightCD16- and CD16+ NK cells and CD56dimCD16- and CD16+ NK cells. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
The basal level of CD69 expression was analyzed on the NK subsets from patients with CHB infection treated with nucleos(t)ide analog alone (open circles, n = 12–13) or together with Peg-IFN-α (black circles, n = 8–9) by flow cytometry before and at different time points of treatment. (A) CD69 expression on total NK cells. (B) CD69 expression on CD56brightCD16- NK cells and CD56brightCD16+ NK cells and CD56dimCD16- NK cells and CD56dimCD16+ NK cells. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p- values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
Patients with CHB infection were treated with nucleos(t)ide analog alone or together with Peg-IFN-α. (A,B) IFN-γ secretion was evaluated by intracellular staining in total NK cells without or with IL12/IL18 stimulation before and at different time points of treatment. (A) Representative dotplots from patients with CHB infection treated either with nucleos(t)ide analog alone (upper panel) or together with Peg-IFN-α (lower panel) (gated on total CD56+CD3- NK cells). (B) Comparative proportions of IFNγ+ NK cells upon IL12/IL18 stimulation in patients treated with NA alone (open squares, n = 12–14) or together with Peg-IFNα (black squares, n = 8–9) at different time points of treatment. (C,D) NK cytotoxic activity was evaluated in total NK cells without or with IL12/IL18 stimulation after co-culture with K562 by measuring CD107 surface expression before and at different time points of treatment. (C) Representative dotplots from patients with CHB infection treated either with nucleos(t)ide analog alone (upper panel) or together with Peg-IFN-α (lower panel) (gated on total CD56+CD3- NK cells). (D) Comparative proportions of CD107+ NK cells upon IL12/IL18 stimulation and co-culture with K562 in patients treated with NA alone (open squares, n = 12–14) or together with Peg-IFN-α (black squares, n = 8–9) at different time points of treatment. For clarity, only the IL12/IL18 condition is shown. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
Patients with CHB infection were treated with nucleos(t)ide analog alone (open circles, n = 11–14) or together with Peg-IFN-α (black circles, n = 8–9). T-cell subsets were analyzed by flow cytometry before and at different time points of treatment. (A) Evolution of CD4 and CD8 T-cell numbers following Peg-IFN-α treatment. Fold increase in absolute CD4 and CD8 T-cell numbers before and at different time points of treatment. P-values were calculated using the Wilcoxon test within the same group of patients toward W0 (*p<0.05) and the Mann-Whitney test between the two groups of patients at the indicated time point (#p<0.05). (B) Evolution of the basal level of CD69 expression on CD4 T cells (left panels) and CD8 T cells (right panels). (C) Evolution of the absolute regulatory T-cell (Treg) numbers during the course of treatment. The gray area represents the period of Peg-IFN-α administration. Bars represent median. The p-values were calculated using the Wilcoxon test (straight lines) or the Mann-Whitney test (dashed lines). * p<0.05, ** p<0.01, *** p<0.001.
(TIFF)
The frequencies of HBV-specific CD4/CD8 T cells were evaluated before and during the course of the treatment upon stimulation of PBMC with overlapping peptide pools and intracellular TNFα, IFN-γ and IL-10 cytokine staining from patients treated with nucleos(t)ide analog alone (n = 12–13) or together with Peg-IFN-α (n = 8–9). (A) Frequencies of cytokine-producing HBx- and pol-specific CD4 T-cell responses. (B) Frequencies of cytokine-producing HBx- and pol-specific CD8 T-cell responses.
(TIFF)
Plasma levels of IP-10 from patients with CHB infection treated with nucleos(t)ide analogue together with Peg-IFN-α (n = 7–9). The gray area represents the period of Peg-IFN-α administration. The p- values were calculated using the Wilcoxon test.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.