Abstract
Background
Disease burden does not account fully for functional status, morbidity, or mortality. Older adults with extensive disease but relative vigor (adapters) may have protective factors which mitigate the consequences of disease, while greater frailty relative to disease may indicate lack of adaptation. Our goal was to ascertain if adapters compress morbidity. If this was observed, it may suggest adapters harbor protective factors, which could subsequently be identified and translated to promote healthy aging.
Design, Setting, Participants
Cardiovascular Health Study, a prospective, community-based cohort study of adults ≥65 years old in four US cities
Measurements
Participants were categorized into 3 groups by extent of disease, assessed non-invasively, and vigor/frailty: expected agers (N=3528, extent of disease similar to vigor/frailty – reference group), adapters (N=882, higher disease with relative vigor), and premature frail (N=855, lower disease with relative frailty) and compared on years of able life (YAL), years of self-reported healthy life (YHL), and mortality using multivariable regression and survival analysis.
Results
After adjustment, adapted agers had 0.97 (95% CI 0.60, 1.33) more YAL and 0.54 (0.19, 0.90) more YHL than expected agers, and the premature frail had −0.99 (−1.36, −0.62) less YAL and −0.53 (−0.89, −0.17) less YHL compared to expected agers. Compared to expected agers, adapters had 0.9 years more and prematurely frail had 1.5 years less total life (P<0.001). Adapters and prematurely frail spent 55% and 37% of their remaining life able and healthy, compared to 47% of expected agers (P<0.001).
Conclusions
Despite similar levels of disease burden, older adults who were relatively vigorous appeared to compress morbidity and live longer. Older adults with higher frailty lengthened morbidity and had greater mortality. Adaptive factors may compress morbidity and decrease mortality.
Keywords: Adaptation, function, morbidity, frailty, longevity
INTRODUCTION
As individuals age or develop disease in different organs, they also tend to develop frailty. Individuals whose level of vigor/frailty tracks with their extent of disease may be termed “expected agers.” In contrast, some individuals with marked organ system impairment do not exhibit frailty – the consequence of organ dysfunction is blunted, physiologically compensated, or clinically unapparent. These individuals may be called “adapted agers.” Still others with lower disease burden but higher than expected frailty may be characterized as “prematurely frail.” We theorize that older adults with organ system impairment but retained vigor (adapted agers) may have intrinsic protective factors that mitigate the consequences of organ system impairment, showing adaptation to accumulated, intrinsic damage. If these adaptive factors could be identified, they may serve as novel points of intervention to increase active life expectancy even in the setting of high disease burden.
Identifying modifiable factors that can reduce the duration of morbidity as a fraction of remaining life, i.e., compress morbidity, is a prime goal of clinical medicine and aging research.1,2 We hypothesized that, compared to expected agers, adapted agers compress morbidity and have reduced mortality rates. If adapters demonstrate this pattern, it may support further study of their unique factors that promote morbidity compression.
METHODS
Study Population
The Cardiovascular Health Study (CHS) is an ongoing community-based study of cardiovascular risk in 5888 men and women over the age of 65 years, from four regions of the United States, now with support for the study of longevity and healthy aging outcomes.3,4 The cohort was enrolled in 1989–1990 and was supplemented with added minority recruitment in 1992–1993. Participants and eligible household members were identified from Medicare eligibility lists. To be eligible, participants were ≥65 years old, did not have cancer under active treatment, could not be wheelchair- or bed-bound in the home, did not need a proxy, and did not plan to move out of the area within 3 years. We used data from the 1992–1993 examination (N=5265) to include all of the minority participants and to align years of follow-up for both cohorts. The CHS is approved by the institutional review boards of all participating institutions.
Measuring Extent of Disease
Clinical disease included available measures of cardiovascular disease (CVD), cancer, chronic obstructive pulmonary disease (COPD), dementia, diabetes, arthritis, osteoporosis, depression, kidney disease, and diseases of the eye. The CHS confirmed the presence of cardiovascular disease at study entry, and adjudicated incident CVD events during follow-up as previously reported.5 Ascertainment of non-CVD illness relied on self-report, medications data, an ancillary study of dementia, and/or hospitalization data. We specifically did not use available subclinical measures of disease such as cognitive function tests or glucose in order to allow for exploration of the impact of subclinical disease on the associations of aging groups with outcomes.
Coronary heart disease (CHD) was defined as angina, myocardial infarction, bypass surgery, or angioplasty. Other cardiovascular diseases included claudication, congestive heart failure (CHF), and stroke. COPD included chronic bronchitis, emphysema, or asthma, self-reported as current and confirmed by a doctor at baseline, or hospitalized between baseline and the 1992–93 visit (original cohort only) with an ICD-9 code of 491, 492, or 493 in any position. Participants under active treatment for cancer were excluded from study entry. Ascertainment of cancer other than non-melanoma skin cancer was based on self-report or hospitalization for cancer between baseline and year 5 for the original cohort. Diabetes, clinical depression and Parkinson’s disease were based on medication use, ascertained by a medication inventory at each clinic visit.5 Kidney disease, arthritis, osteoporosis, and diseases of the eye (glaucoma or retinal problems) were ascertained by self-report. Dementia was defined only on participants who were in the CHS Memory Ancillary study, N=3660.6 Participants who had an MRI from 1991–1994 were included in the Memory study and evaluated for prevalent dementia at the time of the MRI and for incident dementia through 1999. In this analysis, participants were classified with dementia if they were adjudicated to have prevalent dementia at the time of the MRI and the MRI date was before or within 1 year after the date of the 1992–93 visit. Because participants needed to be able to answer all study questionnaires, including cognitive tests, without a proxy as a criterion for study entry, participants for whom dementia was not evaluated were classified as non-demented, with the recognized limitation that some misclassification occurred. Finally, we included self-reported health (excellent, very good, good, fair, or poor) and number of medications as a way of capturing severity of illness or other diseases. Missing self-report and medication values were filled in as much as possible from available data in prior years and otherwise set to 0 if missing.
Measuring Vigor/Frailty: The Scale of Aging Vigor in Epidemiology (SAVE)
The SAVE is a rescaled version of the widely employed CHS frailty scale developed by Fried et al, using the same components as the original scale (weakness, slowness, unintentional weight loss, exhaustion, low energy).7,8 Because of its ceiling effect, the original 5-point CHS frailty scale is not able to identify vigorous older adults with little frailty, which is critical for identifying the adapted aging group.8 To remove the ceiling effect and achieve greater differentiation of vigor, we considered tertiles of each component. The best tertile received a score of 0, the middle tertile a score of 1, and the worst tertile a score of 2. Adding the five component scores created the new vigor/frailty scale from 0 (most vigorous) to 10 (frailest) (Supplementary Text Online).
Weight change in the past year was measured using self-report of weight loss. Physical activity was based on the Modified Minnesota Leisure Time Activities questionnaire and involved self-report of performing any of 18 activities in the prior week, along with the frequency and duration of these activities. Kilocalories of energy expended in a week on leisure time activity were calculated. Motor performance was assessed by gait speed for crossing a 15 ft (4.5 m) length at usual pace by a trained examiner according to a standardized protocol. Strength was assessed using isometric hand grip strength dynamometry. Two items from the Center for Epidemiologic Studies Short Depression Scale (CES-D) were used to characterize exhaustion. These were (1) “I felt that everything I do is an effort” and (2) “I cannot get going.”
Years of Able Life, Years of Healthy Life, and Mortality
Outcomes representing physical function and overall health were based on participant self-report at semi-annual contacts over a maximum of 20 years. Our questions were, “Do you have any difficulty with [activity]?” annually until 1999, and semi-annually from 2005–06 to the present time. Between 2000 and 2005, the question was asked semi-annually and was phrased, “Has there been a change in your ability to [activity]?” with follow-up questions about the nature of the change, e.g., more or less difficulty. Years of able life (YAL) was calculated as the number of years the participant did not report any difficulty with activities of daily living (ADL).9 Years of healthy life (YHL) was calculated as the number of years the participant reported good or better health on a scale of Excellent/Very Good/Good/Fair/Poor.10 Years of healthy and able life (YHAL) was calculated as the number of years the participant was in good health and had no ADL difficulty. Methods for imputing missing values have been previously reported.11 Deaths were ascertained through participant surveillance that has occurred every six months from study inception. Confirmation of deaths was conducted through reviews of obituaries, medical records, death certificates, the Center for Medicare Studies health care utilization database, and the National Death Index. Contacts and proxies were also interviewed for participants unavailable for follow-up. Ascertainment of mortality in CHS is 100% and in this analysis includes 20 years of follow-up. Years of Life (YOL) was also calculated as the number of visit years in which the participant was alive in order to use the same granularity of data for YOL as for YAL, YHL, and YHAL. The proportion of able, healthy, and healthy and able years was calculated as the number of able, healthy, and healthy and able years divided by YOL.
Potential Confounders
Age, sex, race, education, income, marital status, smoking status, and alcohol consumption were determined by self-report.4 Weight and height were measured with standard protocols and used to calculate body mass index (BMI) in kg/m2. Similarly, waist circumference and blood pressure were measured using standard protocols.4 Depressive symptoms were defined as a score >7 on a modified 10-item Center for Epidemiologic Studies Short Depression Scale (CES-D) test after removing the two CES-D questions included in the SAVE scale.12,13 Cognitive function was measured with the Digit Symbol Substitution Test (DSST).14 Social network size was measured using the Lubben Social Network Score.15
Fasting blood samples were collected at the 1992–93 exam using standardized protocols and quality assurance.4,16 Glucose, insulin, albumin, cholesterol, white blood cell count, hemoglobin, and hematocrit were measured using standard clinical methods. C-reactive protein (CRP) was assessed with a high-sensitivity enzyme-linked immunosorbent assay (interassay CV 5.5%). Coagulation factor values were reported as a percentage of normal plasma pool, and standardization was performed by assaying reference plasma from the World Health Organization. The mean monthly CV for the factor VII assay was 5.3%. Plasma fibrinogen was measured using a semiautomated modified clot-rate method (mean monthly CV 3.1%). Adiponectin was measured with an enzyme-linked immunosorbent assay; intra- and inter-assay CVs were 2.5–4.7% and 5.8–6.9%, respectively.
Pulmonary function tests were conducted in 1989–90 for the original CHS cohort and 1993–94 for both cohorts, and the average of the two measurements was used for original cohort participants. Spirometry was conducted according to the standards of the American Thoracic Society.4 Carotid ultrasound was obtained in the left and right internal and common carotid arteries to assess near and far wall thicknesses and Doppler flow. The mean of the maximum wall thickness of the internal carotid artery was used to represent the extent of vascular disease.17 Fasting glucose was assessed as described previously.18 Cystatin-C, a serum marker of glomerular filtration rate, was assessed using a BNII nephelometer that used a particle-enhanced immunonephelometric assay.19 Brain MRI was obtained according to a standard scanning protocol and read using a semiquantitative atlas-based scale at a central MRI Reading Center.11
Statistical Analysis
Linear regression was used to estimate the SAVE score from the clinical diseases, age, age2, age3, race and sex. Residuals from the regression were used to define 3 aging groups based on values less than, within, or above one standard deviation (1.85) of the residuals. Participants whose observed SAVE index was >1.85 points higher than their regression estimated index were considered prematurely frail, and participants whose observed SAVE index was lower than their expected by more than 1.85 points were classified as adapted agers, and those whose SAVE index was within 1 standard deviation of expected constituted the expected agers.
Associations of the resulting groups with participant characteristics were determined by Chi-Square tests for categorical variables and by the Kruskal-Wallis test for continuous measures. Linear regression was used to estimate YOL, YHL, YAL, YHAL, and their proportions for the premature frail and adapted aging groups compared to the expected aging group. Covariates were entered in several stages, beginning with adjustment for demographic variables. At each stage, covariates were retained if statistically significant or if the estimated regression coefficient for the group variable changed by 10% or more. Successive stages of adjustment included lifestyle factors of smoking, alcohol consumption, and body size; social network score; biomarkers; subclinical disease measures of internal carotid wall thickness, fasting glucose and the Digit Symbol Substitution Test (DSST) score, followed by subclinical disease measures available on a reduced sample, namely FVC, Cystatin-C, and white matter grade from MRI. Interactions of group with C-reactive protein and DSST were specified a priori and were tested by forming cross-product terms to test if inflammation or cognitive processing specifically might be involved in adaptation. Cox regression was used to determine the hazard ratio of mortality associated with the premature frail and adapted aging groups compared to the expected aging group. The proportional hazards assumption was tested with Schoenfeld residuals. Participants were followed until the minimum of time to death or 20 years. Missing covariate values were carried forward from a prior visit, if possible, or imputed at baseline as previously described.20 White matter grade, FVC, and Cystatin-C were not imputed due to substantial numbers missing and exclusion criteria for the MRI, the fact that FVC was not measured at baseline for the second cohort and required survival to be ascertained, and Cystatin-C was measured from stored bloods later in the study and specimen availability was impacted by disease status. Differences in proportion of deaths due to a specific cause were assessed with the Chi-Square test. A two-sided alpha of p<0.05 was used to determine statistical significance. All analyses were done with STATA, version 12.
RESULTS
Clinical disease and demographics explained 30% of the variability in the SAVE index, with self-reported health and demographics accounting for 25% of the total variability. Table 1 shows the prevalence of the diseases in the cohort and the regression coefficients from the model of the SAVE index. Eye disease, COPD, CHD, and male sex were the only factors that were not statistically significant in the full model.
Table 1.
Clinical Disease prevalence and association with the SAVE scale.
| Clinical disease | Prevalence | Beta (95% CI) | p-value |
|---|---|---|---|
| Self-reported health | <0.001 | ||
| Excellent | 336 (6.4) | −0.97 (−1.19, −0.75) | |
| Very good | 1564 (29.7) | −0.53 (−0.66, −0.41) | |
| Good | 2166 (41.1) | 1.00 (reference) | |
| Fair | 1028 (19.5) | 0.90 (0.76, 1.05) | |
| Poor | 171 (3.3) | 1.78 (1.48, 2.08) | |
| Parkinson’s | 43 (0.8) | 0.90 (0.33, 1.46) | 0.002 |
| Dementia | 196 (3.7) | 0.59 (0.31, 0.86) | <0.001 |
| Stroke | 416 (7.9) | 0.51 (0.29, 0.73) | <0.001 |
| Claudication | 168 (3.2) | 0.47 (0.18, 0.76) | 0.002 |
| Diabetes | 495 (9.4) | 0.39 (0.21, 0.57) | <0.001 |
| Osteoporosis | 601 (11.4) | 0.37 (0.21, 0.54) | <0.001 |
| Kidney disease | 123 (2.3) | 0.36 (0.03, 0.70) | 0.035 |
| Depression | 389 (7.4) | 0.36 (0.16, 0.56) | <0.001 |
| CHF | 344 (6.5) | 0.34 (0.12, 0.56) | 0.002 |
| Arthritis | 2632 (50.0) | 0.32 (0.21, 0.42) | <0.001 |
| Cancer | 326 (6.2) | 0.18 (−0.35, 0.39) | 0.102 |
| # of medications, mean (sd) |
2.59 (2.3) | 0.11 (0.08, 0.13) | <0.001 |
| Eye disease | 516 (9.8) | 0.04 (−0.13, 0.21) | 0.641 |
| COPD | 762 (14.5) | 0.02 (−0.13, 0.16) | 0.823 |
| CHD | 1173 (22.3) | −0.10 (−0.24, 0.03) | 0.131 |
| Other covariates | |||
| Age, yrs mean(sd) | 75.1 (5.5) | 0.12 (0.10, 0.13) | <.001 |
| Age2 | 0.0046 (0.0025, 0.0067) | <.001 | |
| Age3 | −0.0003 (−0.00043, −0.00016) | <.001 | |
| Male sex | 2166 (41.1) | 0.01 (−0.10, 0.12) | 0.847 |
| Black race | 885 (16.8) | 0.32 (0.18, 0.47) | <.001 |
Entries in Table are N (%) unless otherwise noted.
There were 3528 expected agers (67.0%), 882 adapters (16.8%), and 855 premature frail (16.2%). The mean (SD) age of the cohort was 75.1 (5.5) years and 58.9% were women (Table 2). Due to the inclusion of demographics in the regression model, there were no differences across aging groups by age, sex, or race. Compared to expected agers, adapted agers were less likely to be current smokers and to report depressive symptoms, and had less subclinical disease (Table 2). Premature frail adults had less education and income, were more likely to be current smokers and alcohol abstainers, had higher BMI and CRP, were more likely to report depressive symptoms, and had more subclinical disease.
Table 2.
Association of risk factors with adaption groups
| Risk factor | Prematurely frail N=855 |
Expected agers N=3528 |
Adapted agers N=882 |
Total N=5265 |
p-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, yrs | 75.3 (5.5) | 75.0 (5.5) | 75.3 (5.5) | 75.1 (5.5) | .29 |
| Female | 502 (58.7) | 2080 (59.0) | 517 (58.6) | 3099 (58.9) | .98 |
| Black race | 150 (17.5) | 587 (16.6) | 148 (16.8) | 885 (16.8) | .82 |
| Education, yrs | 12.0 (3.3) | 12.4 (3.2) | 12.6 (3.1) | 12.3 (3.2) | <.001 |
| Income group | <.001 | ||||
| <12K | 249 (29.1) | 906 (25.7) | 205 (23.2) | 1360 (25.8) | |
| 12K–24,999 | 317 (37.1) | 1228 (34.8) | 270 (30.6) | 1815 (34.5) | |
| 25K–49,999 | 200 (23.4) | 918 (26.0) | 270 (30.6) | 1388 (26.4) | |
| >=50K | 89 (10.4) | 476 (13.5) | 137 (15.5) | 702 (13.3) | |
| Married | 532 (62.2) | 2227 (63.1) | 574 (65.1) | 3333 (63.3) | .43 |
| Lifestyle | |||||
| Smoking | <.001 | ||||
| Never | 363 (42.5) | 1632 (46.3) | 410 (46.4) | 2405 (45.7) | |
| Former | 373 (43.6) | 1543 (43.7) | 422 (47.9) | 2338 (44.4) | |
| Current | 119 (13.9) | 353 (10.1) | 50 (5.7) | 522 (9.9) | |
| Pack-years | 20.1 (28.8) | 17.2 (25.5) | 15.5 (24.0) | 17.4 (25.9) | .007 |
| Years quit (former smokers only) | 20.0 (13.1) | 20.5 (13.0) | 20.6 (13.3) | 20.4 (13.0) | .79 |
| Drink alcohol | 348 (40.7) | 1559 (44.2) | 413 (46.8) | 2320 (44.1) | .036 |
| Waist, cm | 99.1 (14.5) | 97.7 (13.2) | 94.3 (12.1) | 97.4 (13.4) | <.001 |
| BMI, kg/m2 | 27.2 (5.4) | 27.0 (4.8) | 26.0 (4.2) | 26.9 (4.8) | <.001 |
| Social network score | 31.8 (8.3) | 33.0 (7.7) | 34.1 (7.6) | 33.0 (7.8) | <.001 |
| Blood pressure | |||||
| Systolic BP, mmHg | 136 (22) | 137 (22) | 137 (22) | 137 (22) | .33 |
| Anti-hypertension meds | 431 (50.4) | 1783 (50.5) | 474 (53.7) | 2688 (51.0) | .35 |
| Biomarkers | |||||
| CRP, mg/L | 6.6 (11.3) | 5.0 (8.8) | 4.4 (8.4) | 5.2 (9.2) | <.001 |
| Hemoglobin, g/dl | 13.6 (1.4) | 13.7 (1.4) | 13.7 (1.3) | 13.7 (1.4) | .40 |
| Hematocrit, % | 40.7 (4.1) | 40.8 (3.8) | 40.8 (3.9) | 40.8 (3.9) | .81 |
| White blood count | 6.62 (2.17) | 6.37 (2.45) | 6.27 (4.66) | 6.41 (3.10) | <.001 |
| Fibrinogen, mg/dl | 342 (76) | 329 (69) | 322 (65) | 330 (70) | <.001 |
| Factor VII, % | 113 (27) | 113 (28) | 112 (28) | 112 (28) | .74 |
| Adiponectin1, mg/L | 14.2 (8.2) | 13.9 (8.0) | 13.8 (7.4) | 14.0 (7.9) | .42 |
| Total cholesterol, mg/dl | 207 (39) | 209 (39) | 210 (38) | 209 (39) | .32 |
| HDL cholesterol, mg/dl | 52.4 (14.4) | 53.3 (14.4) | 54.4 (15.2) | 53.3 (14.6) | .015 |
| LDL cholesterol, mg/dl | 126 (35) | 128 (34) | 128 (33) | 128 (34) | .49 |
| Triglycerides, mg/dl | 147 (88) | 144 (87) | 140 (89) | 144 (87) | .29 |
| Albumin, g/dl | 3.95 (0.30) | 3.97 (0.27) | 3.99 (0.27) | 3.97 (0.27) | .03 |
| Fetuin-A1, g/L | 0.47 (0.10) | 0.48 (0.10) | 0.48 (0.09) | 0.48 (0.10) | .23 |
| Subclinical Disease | |||||
| Internal carotid wall thickness, mm | 1.45 (0.56) | 1.46 (0.58) | 1.40 (0.54) | 1.45 (0.57) | .03 |
| Common carotid wall thickness, mm |
1.09 (0.22) | 1.08 (0.23) | 1.06 (0.22) | 1.08 (0.23) | .005 |
| Glucose, mg/dl | 112 (39) | 109 (34) | 107 (31) | 109 (35) | .008 |
| Cystatin C2, mg/l | 1.18 (0.44) | 1.12 (0.33) | 1.08 (0.27) | 1.12 (0.34) | <.001 |
| FVC3, L | 2.67 (0.82) | 2.90 (0.87) | 2.99 (0.84) | 2.88 (0.86) | <.001 |
| White matter grade4 | 2.55 (1.74) | 2.22 (1.43) | 2.16 (1.29) | 2.26 (1.46) | .003 |
| DSST | 33.7 (14.7) | 37.7 (14.5) | 39.5 (13.7) | 37.3 (14.5) | <.001 |
| CESD5 Depression>=7 | 296 (34.6) | 797 (22.6) | 115 (13.0) | 1208 (22.9) | <.001 |
Available on N=4715
Available on N=4734
Available on N=5037
Available on subset only N=3579
Subscore without the 2 questions in the SAVE scale.
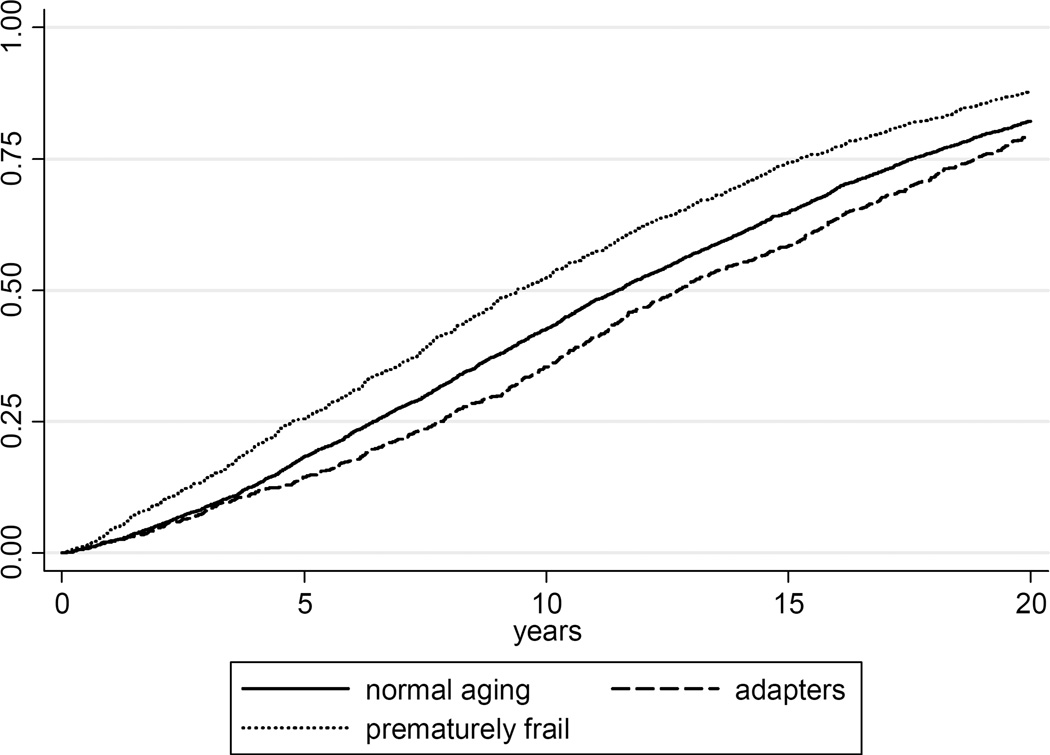
Table 3 depicts the crude mortality rate, YOL, YAL, YHL, YHAL, and proportion of remaining life able or healthy classified by the aging groups. Despite controlling for age, sex, and clinical disease prior to defining the groups, there were significant differences in all measures across the groups. Prematurely frail participants had, on average, 1.5 fewer years of life and 2 full years fewer of able life than the expected agers. Adapted agers had about 1 year more of life than the expected agers, and 1.5 years more of able life, enjoying on average ¾ of their remaining years free of ADL disability, compared with 65% in the expected aging group and 55% in the prematurely frail group. Differences in cumulative death rate were notably consistent across the entire length of follow up: adapted agers had the lowest death rate and the prematurely frail had the highest death rate over 20 years (Figure 1).
Table 3.
Years of Life, Healthy Life and Mortality Rates by aging groups
| Prematurely frail | Expected aging | Adaptive aging | p-value | |
|---|---|---|---|---|
| Person -years | 8,703 | 41,183 | 11,089 | |
| # Deaths (%) | 752 (88.0) | 2,898 (82.1) | 699 (79.2) | <.001 |
| Mortality rate per 1,000 person-yrs (95% CI) |
86.4 (80.4, 92.8) |
70.4 (67.9, 73.0) |
63.0 (58.5, 67.9) |
<.0011 |
| Age at death (N=4349) | 84.7 (6.5) | 85.7 (6.2) | 86.8 (6.4) | <.001 |
| Years of Life (YOL) | 10.0 (6.2) | 11.5 (6.2) | 12.4 (6.1) | <.001 |
| Years of Healthy Life (YHL) |
5.9 (5.2) | 7.5 (5.8) | 8.7 (5.9) | <.001 |
| Years of Able Life (YAL) | 6.1 (5.5) | 8.1 (6.0) | 9.6 (6.0) | <.001 |
| Years of Healthy and Able life (YHAL) |
4.2 (4.6) | 6.0 (5.5) | 7.4 (5.6) | <.001 |
| Proportion able (YAL/YOL) |
0.55 (0.31) | 0.65 (0.29) | 0.74 (0.24) | <.001 |
| Proportion healthy (YHL/YOL) |
0.55 (0.31) | 0.61 (0.30) | 0.66 (0.28) | <.001 |
| Proportion healthy and able (YHAL/YOL) |
0.37 (0.30) | 0.47 (0.31) | 0.55 (0.29) | <.001 |
Log-rank test of survivor function
Figure 1.
Kaplan Meier cumulative death plot by aging group over 20 years of follow up.
Table 4 compares years of able, healthy, and healthy and able life for each aging group compared with the expected agers, after adjusting for potential confounders. After adjustment for demographics, lifestyle factors, social support, blood pressure, and biomarkers, the adapted agers had approximately 1 year more and the prematurely frail 1 year less of able, healthy, and both healthy and able life than the expected agers. Further adjustment for subclinical disease attenuated the associations, most notably for the prematurely frail group. There were no significant interactions of aging group with CRP or DSST score.
Table 4.
Regression coefficients (95% confidence interval) representing number of years of able (YAL), years of healthy life (YHL), or years of healthy and able life (YHAL) in each aging group compared to the expected agers, sequentially adjusted for other factors
| YAL | YHL | YHAL | ||||
|---|---|---|---|---|---|---|
| Model | Prematurely frail N=855 |
Adapted agers N=882 |
Prematurely frail N=855 |
Adapted agers N=882 |
Prematurely frail N=855 |
Adapted agers N=882 |
| 1: Demographics1 | −1.70 (−2.10, −1.31) | 1.47 (1.08, 1.86) | −1.35 (−1.74, −0.96) | 1.09 (0.71, 1.48) | −1.58 (−1.95, −1.22) | 1.31 (0.95, 1.67) |
| 2: 1 + Lifestyle2 | −1.54 (−1.93, −1.16) | 1.29 (0.91, 1.67) | −1.18 (−1.56, −0.80) | 0.97 (0.59, 1.34) | −1.41 (−1.77, −1.06) | 1.13 (0.78, 1.49) |
| 3: 2 + Social support3 | −1.46 (−1.84, −1.07) | 1.23 (0.85, 1.61) | −1.10 (−1.48, −0.72) | 0.89 (0.52, 1.27) | −1.34 (−1.70, −0.99) | 1.07 (0.71, 1.42) |
| 4: 3 + Blood pressure4 | −1.49 (−1.87, −1.11) | 1.31 (0.94, 1.69) | −1.13 (−1.51, −0.76) | 0.99 (0.62, 1.36) | −1.37 (−1.72, −1.02) | 1.15 (0.80, 1.50) |
| 5: 4 + Biomarkers5 | −1.37 (−1.75, −0.99) | 1.26 (0.88, 1.63) | −0.99 (−1.37, −0.62) | 0.94 (0.57, 1.31) | −1.26 (−1.62, −0.91) | 1.11 (0.76, 1.45) |
| 6: 5 + Subclinical disease6 | −0.99 (−1.36, −0.62) | 0.97 (0.60, 1.33) | −0.53 (−0.89, −0.17) | 0.54 (0.19, 0.90) | −0.82 (−1.16, −0.48) | 0.73 (0.40, 1.06) |
| 7: 6 + Limited Subclinical disease7 |
−0.84 (−1.24, −0.44) | 0.84 (0.46, 1.22) | −0.28 (−0.67, 0.11) | 0.40 (0.23, 0.77) | −0.67 (−1.03, −0.30) | 0.63 (0.27, 0.98) |
| 8: 7 + White matter grade8 | −0.44 (−0.92, 0.04) | 0.76 (0.32, 1.19) | −0.01 (−0.48, 0.46) | 0.22 (−0.20, 0.65) | −0.37 (−0.82, 0.07) | 0.52 (0.12, 0.92) |
Age, gender, race, education, income, marital status
Smoking, pack years of smoking, alcohol, waist circumference, body mass index
Social network score
Systolic blood pressure, antihypertensive medication
(log) CRP, (log) white blood count, hemoglobin and total cholesterol
Internal carotid wall thickness, fasting glucose, and the Digit Symbol Substitution Test (DSST) score
Forced vital capacity, Cystatin-C
White matter grade
DISCUSSION
With the observation that some older adults with high disease burden still remain vigorous (adapted agers), we undertook this analysis to see if this group compressed morbidity at the end of life, potentially signaling that they harbor protective factors that may blunt the effects of disease and promote function. We favored this intuitive model given that there are many older individuals who have high levels of disease but who are seemingly vigorous. We found that, compared to expected agers, adapters lived longer and had less disability and better self-rated health, ultimately showing compressed morbidity with a greater proportion of life lived able and healthy. In contrast, prematurely frail older adults had higher mortality and spent more of their remaining life disabled, despite lower than expected clinical disease for their level of frailty. As conceptualized and measured here, adaptation may delay mortality and even more so morbidity, resulting in compression of morbidity in a desirable way.
The profile of adapted agers is intriguing. Our regression models define these individuals as having better than expected function for similar levels of age, gender, race, and, importantly, chronic disease burden. When explored further, they have better intrinsic and extrinsic markers of health. This includes lower CRP and fibrinogen, higher HDL, less subclinical disease, as well as lower pack-years of smoking, higher education and income, and higher social network score. These individuals had the longest life expectancy with preserved years of able life, resulting in compressed morbidity. These associations were robust after extensive adjustment for potentially explanatory confounders. It may be that adapted agers truly do have adaptive factors which compress morbidity and mask the effect of underlying disease which may ultimately present as a catastrophic illness, followed shortly by death. Adapted agers may die before they manifest phenotypes of frailty. Adapted agers do appear to be a unique group warranting more careful exploration to identify factors which may promote morbidity compression.
In contrast, the prematurely frail have higher mortality and spend more of their remaining life disabled and feeling less healthy. This group included more women, among whom there is a known greater burden of disability, chronic pain, and affective disorders, and had higher subclinical disease. There is perhaps a cycle of reporting poorer health and depressive symptoms as a result of comparing their outward disability to their less-disabled peers, which may also make them less likely to become able. The premature frail may illustrate opportunity – if their subclinical disease and/or disability can be mitigated, they may achieve long lives with good health, rather than living a shorter life with an expanded period of morbidity.
Our results underscore two important clinical points. First, even among individuals with similar levels of diagnosed clinical disease, there is wide variation in vigor and frailty as well as subclinical disease. Although diagnosed disease burden, usually represented as a comorbidity count, has been used for decades as a predictor of morbidity and mortality, influencing researchers, providers, and payers, it is an imperfect representation of physiologic fitness and does not explain the bulk of the variance in these outcomes. Second, subclinical disease may be more strongly associated with morbidity and mortality than clinically diagnosed disease.21 This is exemplified by the adaptors having much lower levels of subclinical disease across several organ systems compared to expected agers, and especially by the prematurely frail, who had the highest levels of subclinical disease. As mortality prediction models become increasingly utilized in clinical practice for treatment planning purposes,22 it should be noted that subclinical disease markers will likely be more powerful predictors than clinically diagnosed disease if they can be measured safely, accurately, reproducibly, affordably, and efficiently.21
We acknowledge several limitations in this analysis. We measured the SAVE scale only once and individuals’ vigor/frailty likely changed throughout time. The phraseology for the question on activity disability changed slightly during the follow up period, though the data is highly internally consistent across how the question was phrased, suggesting little misclassification occurred when the question was slightly altered. Some of the chronic diseases were ascertained by self-report and dementia ascertainment was only confirmed in a subset. The definition of aging groups based on a standard deviation cut-point in residuals from a regression is arbitrary, but results were similar when the interquartile range of residuals was used in sensitivity analyses. The cutpoints used to form the three aging groups were substantially wide enough to allow for some variation without gross misclassification. While mortality is a hard outcome, ADL disability can be fluid because individuals can become disabled and then have health improvements which ameliorate their disability, even into the last year of life.23 Our method of counting years free of ADL disability allowed for recovery. It is different from life-table methods in that years are summed on the individual level rather than the population level. Other methods are possible to examine the association between aging groups and health outcomes, such as modeling transitions between states, and may produce different results, though transition state modeling specifically requires more frequently updated data on the SAVE scale, which is not available in CHS. Our goal was to model total years of healthy and able life rather than transitions between states, and transitions are accounted for in the summing of the total years of reported healthy and able life. Despite these potential weaknesses, the results were consistent across multiple outcomes, suggesting that misclassification of outcome did not have a substantial impact on our findings. Finally, while CHS has a wealth of data, the study does not capture every possible illness nor are all illnesses assessed with the accuracy and consistency of adjudicated CVD, therefore residual confounding is possible. Nonetheless, we believe this would have a minimal effect given the degree of adjustment carried out for both intrinsic and extrinsic covariates.
In conclusion, we show that older adults with better than expected vigor have lower mortality and, disproportionately, even lower morbidity, leading to a compression of morbidity. In contrast, older adults with worse than expected frailty have higher mortality and substantially greater morbidity, and a higher proportion of life lived disabled before death. Adapted agers may be a unique group to plumb further to identify factors which promote longevity by blunting the effect of disease, while the prematurely frail may be most amenable to interventions which reduce morbidity to extend the healthy and able period at the end of life.
Supplementary Material
Acknowledgments
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Role of sponsor(s): The investigators retained full independence in the conduct of this research.
Footnotes
Disclosure of potential conflicts of interest: All authors have no potential conflicts of interest.
Authors’ contributions: conception and design (JLS, AMA, CHH, SMT, SBK, ABN); acquisition of data (AMA, CHH, JRK, RCK, SBK, ABN); analysis and interpretation of data (JLS, AMA, ABN); drafting of the manuscript (JLS, AMA, ABN); critical revision of the manuscript (JLS, AMA, CHH, SMT, DK, KJM, JRK, JHI, RCK, SBK, ABN); statistical analysis (AMA); obtaining funding (AMA, JRK, RCK, ABN); administrative, technical, or materials support (AMA, ABN); supervision (ABN).
Author access to data: All authors had access to data at all times.
REFERENCES
- 1.Fries JF, Bruce B, Chakravarty E. Compression of morbidity 1980–2011: A focused review of paradigms and progress. J Aging Res. 2011;2011 doi: 10.4061/2011/261702. ID,261702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fries JF. Measuring and monitoring success in compressing morbidity. Ann Intern Med. 2003;139:455–459. doi: 10.7326/0003-4819-139-5_part_2-200309021-00015. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort--the cardiovascular health study all stars study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 5.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 6.Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Sanders JL, Boudreau RM, Fried LP, et al. Measurement of organ structure and function enhances understanding of the physiological basis of frailty: The Cardiovascular Health Study. J Am Geriatr Soc. 2011;59:1581–1588. doi: 10.1111/j.1532-5415.2011.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diehr P, O'Meara ES, Fitzpatrick A, et al. Weight, mortality, years of healthy life, and active life expectancy in older adults. J Am Geriatr Soc. 2008;56:76–83. doi: 10.1111/j.1532-5415.2007.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehr P, Patrick DL, Spertus J, et al. Transforming self-rated health and the SF-36 scales to include death and improve interpretability. Med Care. 2001;39:670–680. doi: 10.1097/00005650-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study, Stroke. J Cerebr Circu. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 12.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Schulz R, Beach SR, Ives DG, et al. Association between depression and mortality in older adults: The Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 14.Wechsler D. Wechsler adult intelligence scale-revised. New York: Psychological Corporation; 1988. [Google Scholar]
- 15.Lubben JE. Assessing social networks among elderly populations. J Health Promo Maint. 1988;11:42–52. [Google Scholar]
- 16.Cushman M, Cornell ES, Howard PR, et al. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 17.O'Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group, Stroke. J Cereb Circu. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 18.Barzilay JI, Kronmal RA, Gottdiener JS, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: The Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2236–2241. doi: 10.1016/j.jacc.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 19.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol: JASN. 2005;1:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 20.Arnold AM, Kronmal RA. Multiple imputation of baseline data in the cardiovascular health study. Am J Epidem. 2003;157:74–84. doi: 10.1093/aje/kwf156. [DOI] [PubMed] [Google Scholar]
- 21.Newman AB, Boudreau RM, Naydeck BL, et al. A physiologic index of comorbidity: Relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. doi: 10.1093/gerona/63.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz M, Covinsky K, Widera EW, et al. Predicting 10-year mortality for older adults. JAMA. 2013;309:874–876. doi: 10.1001/jama.2013.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill TM, Gahbauer EA, Han L, et al. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.