Abstract
Background
Biopsy diagnosis is the gold standard for differentiating benign and malignant prostatic enlargements. This study was aimed at supplementing biopsy diagnosis with immunophenotypic characters of prostatic lesions.
Methods
Twenty five cases each of nodular hyperplasia and adenocarcinoma prostate were compared for their morphologic appearances and immunophenotyping, by studying antibodies to prostate specific antigen (PSA), transglutaminase, chromogranin and high molecular weight keratin, proliferating cell nuclear antigen, cell death (apoptosis) and neovascularisation (CD 34).
Results
Markers of differentiation (PSA and transglutaminase) aided recognition of higher-grade tumours. PSA negativity avoided metaplasia being overcalled as carcinoma. Loss of basal cells around malignant prostatic acini as determined by high molecular weight keratin (HMWK), was useful in foci of atypical small acinar proliferation and in prostatic intraepithelial neoplasia. Assessment of proliferation indices identified subsets of tumours, within conventional morphologic Gleason's grades, with a higher growth fraction. Cell death determination and study of tumour vessels did not offer any improvement on morphology.
Conclusion
Immunophenotypic assessment helps in refining morphologic diagnosis of prostatic lesions. Differentiation and proliferation markers objectively assess tumour characteristics with their biologic growth potential and are recommended for diagnostic use. They also help in assessement of response to therapy.
Key Words: Immunophenotyping, Prostate, Benign, Malignant
Introduction
Nodular hyperplasia of prostate (NHP) is a common disorder of men increasing from 20% at 40 years, to 90% by the eighth decade of life. Prostatic carcinoma is the second highest cause of cancer related deaths amongst men in America [1]. In India, it constitutes about 4% of all male cancers [2]. Though 90% of such lesions may not manifest clinically in the lifetime of the host [3], occult carcinoma may present with extensive dissemination to bones and other organs. Digital rectal examination (DRE), transrectal ultrasound (TRUS) and serum prostate specific antigen (PSA) constitute complementary techniques for early detection [4, 5]. However, biopsy remains the gold standard for final diagnosis [6]. There is a growing recognition that prostatic carcinoma is predated by an ‘in situ’ ‘prostatic intraepithelial neoplasia’ (PIN) by atleast 10 years [7].
The diagnosis of the spectrum of preneoplastic and neoplastic lesions is being rendered more objective by the use of immunohistochemistry for phenotyping and prognosticating biologic behaviour. These include markers of differentiation like prostate specific antigen (PSA) and chromogranin, functional alterations (transglutaminase), loss of basal cell layer around glands (high molecular weight cytokeratin HMWCK), proliferative potential (cell-cycle associated proteins like proliferating cell nuclear antigen (PCNA) [8, 9], cell death (apoptosis)) and tumour microvasculature (CD 34) [10]. This study was undertaken to assess the pathologic features and determinants of prostate cancer in Indian patients that would provide an insight into its appearance and behaviour.
Material and Methods
Fifty cases of prostatic enlargement, presenting to the Army Hospital R&R, formed the study group. Clinical symptoms and signs, DRE, TRUS and PSA levels were recorded. Histological diagnosis separated two groups into 25 cases each of nodular hyperplasia prostate (including other benign lesions) and adenocarcinoma prostate.
The quantitation of core biopsies, transuretheral resection of prostate (TURP) chips and radical prostatectomy specimens was done followed by routine formalin-fixation, paraffin-embedding and staining with haematoxylin and eosin (H&E). Periodic acid Schiff and mucicarmine were used in selected cases (for glycogen and mucin). The immunohistochemical staining [11] was carried out by the labeled streptavidin-biotin method using monoclonal antibodies and kits (DAKO Corporation, USA). Antibodies to PSA, transglutaminase, chromogranin, HMWCK (34 ? E 12), PCNA, and CD 34 Class I were used. Positive staining for PSA, transglutaminase and chromogranin was observed as cytoplasmic staining of prostatic epithelial cells. HMWCK stained the basal cells, PCNA was seen as nuclear brown staining and CD34 stained endothelial cells. The detection and quantification of apoptosis was based on labelling of DNA strand breaks (TUNEL technology).
On histopathological findings morphologic patterns of hyperplasia, metaplasia, PIN and adenocarcinoma, Gleason's grade, spread and TNM staging were recorded. The following immunophenotypic features were used:
-
(a)
PSA: A semi-quantitative score from 0-4+.
-
(b)
HMWCK: for detection of basal cells around prostatic acini.
-
(c)
Transglutaminase for decreased expression.
-
(d)
Chromogranin for neuroendocrine differentiation.
-
(d)
CD 34 to stain endothelial cells for MVD.
-
(e)
The statistical analysis was done by Mann Whitney or Wilcoxon Two sample test/T-test for normal variance/ Kruskal Wallis one way analysis of variance as and wherever applicable.
Results
The mean age of the patients with NHP was 65.6 years (range 48-89 years) and of prostatic adenocarcinoma 73.8 years (range 54-93 years) (statistically significant). Carcinoma was diagnosed on trucut biopsies at a mean age of 68.9 years. Patients with PIN had a mean age of 70.4 years (range 56-77 years). Symptoms were referable to outflow obstruction of urine viz. frequency of micturition, hesitancy, dysuria, thin stream of urine, terminal dribbling and urinary retention. Two patients with prostatic adenocarcinoma had bony metastases. Mean duration of symptoms in NHP was 13.2 months (range 1-22 months) and in prostatic carcinoma 11.3 months (4-21 months). The difference was not significant. A suspicious DRE was recorded in five (20%) NHP and 18 (72%) cases of carcinoma. TRUS findings were suspicious in 1/9 patients of NHP and 10/12 patients of cancer. The serum PSA levels in NHP ranged from 0.85 to 42.4ng/ml (mean value 7.8 ± 0.8) and in carcinoma prostate from 3.2 to 180 ng/ml (mean 44.5± 45.3). The difference was statistically significant (Table 1).
Table 1.
Serum PSA levels in NHP and Carcinoma prostate
| PSA levels ng/ml | NHP n (%) | Carcinoma prostate (%) |
|---|---|---|
| <4 | 8 (32) | 2 (8.7) |
| 4-10 | 14 (56) | 10 (43.5) |
| >10 | 3 (12) | 11 (47.8) |
| Total | 25 (100) | 23 (100) |
Nineteen trucut biopsies, 30 transurethral resection of prostate specimens (TURP) and one radical prostatectomy were evaluated and a diagnosis of NHP was made in 25 cases(Fig. 1A, Fig. 1B). They also showed metaplasia (transitional, squamous or mucinous) in six, basal cell hyperplasia in two, prostatitis in six, atrophy in 10 and atypical adenomatous hyperplasia (AAH) in one case. In 14 benign cases where serum PSA was marginally elevated, seven showed significant inflammation while in others no associated cause for rise could be detected. Prostatic Intraepithelial Neoplasia (PIN) (Fig 1C) was seen in four cases associated with prostatic adenocarcinoma (TURP 3, trucut 1), (3 high grade and 1 low grade). One low grade PIN (4%) was associated with NHP (TURP).
Fig. 1A.
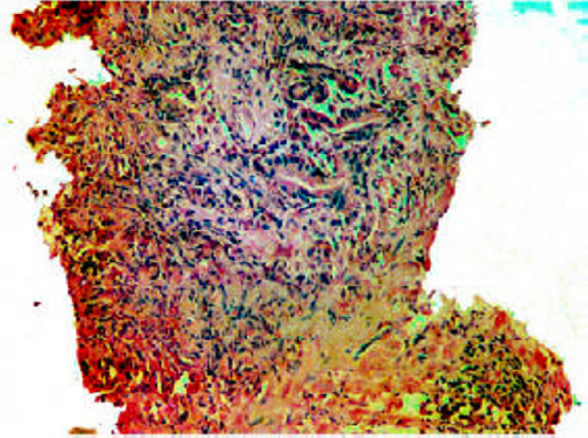
Trucut biopsy showing suspicious haphazard glands surrounded by severe inflammation (H&E × 10).
Fig. 1B.
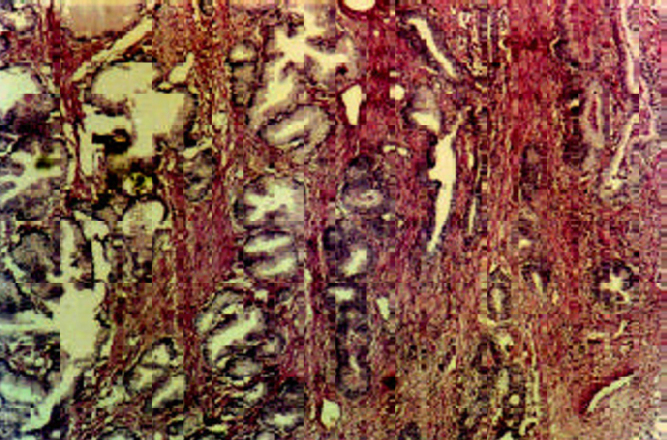
A focus of atypical adenomatous hyperplasia (arrow-heads) in nodular hyperplasia (H&E ×10).
Fig. 1C.
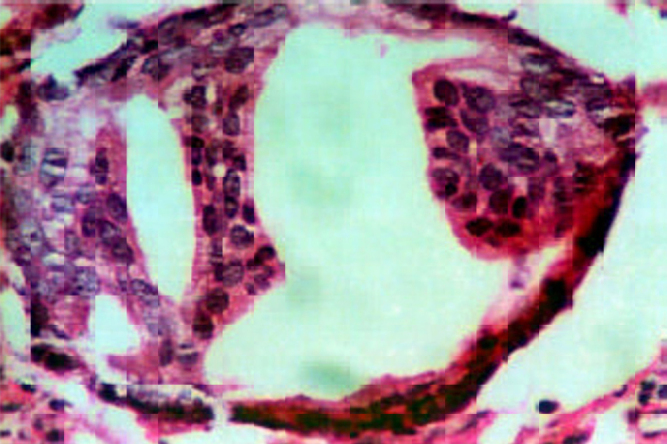
High grade prostatic intraepithelial neoplasia (PIN) with prominent nucleoli. (H&E × 20).
Prostatic adenocarcinoma (Fig 1D), was seen in 11 trucut biopsies, 13 TURP specimens (incidental) and one planned radical prostatectomy. In TURP the mean percentage of chips involved was 16.6% (range 9-20) (Stage T1b N0 M0). Histological pattern in 24 (96%) was acinar while one (4%) was a signet ring cell adenocarcinoma. Focal mucinous differentiation was seen in two (8%) cases (<25% of the tumour area). Perineural invasion was noted in eight (32%) and vascular invasion in three (12%) cases. Invasion around parasympathetic ganglia was seen in the radical prostatectomy. One (4%) was low Gleason's grade (score 2-4), 17 (68%) intermediate grade (score 5-7) and seven (28%) were high-grade tumours (score 8-10). The commonest pattern was Gleason's score 3. The PSA range of tumours of different grades is shown in Table 2. Thirteen (52%) were in stage I (T1b N0 M0), three (12%) cases in stage I (T1c N0 M0), eight (32%) cases in stage II (T2 N0 M0) and one (4%) case in stage III (T3 N0 M0).
Fig. 1D.
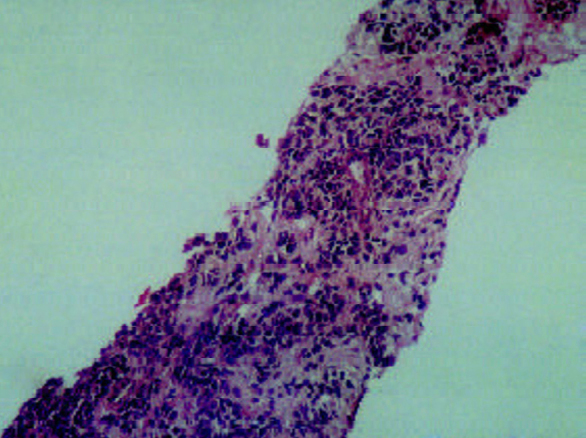
Poorly differentiated adenocarcinoma prostate (Gleason's score 4+5) in a trucut biopsy (H&E × 10)
Table 2.
Relationship of Gleason's grade with serum PSA levels.
| Grade of tumour | Serum PSA range ng/ml (n=number of cases) | Mean value | p value* |
|---|---|---|---|
| Low grade | 3.98 (1) | 3.98 | |
| Intermediate grade | 3.2-110.10 (17) | 34.26 ± 37.5 | 0.02 * |
| High grade | 20.83-180.2 (7) | 75.29 ± 52.8 |
Mann-Whitney or Wilcoxon two sample test (p value <0.05 was considered significant)
Immunophenotypic Characterisation
Tissue PSA staining was intense (4+) in benign lesions (except atrophic glands). With increasing grade of carcinoma serum PSA levels rose (Fig. 2A) while PSA staining was patchy and weak. The staining pattern of PSA and intensity of PSA expression in different grades of carcinoma is depicted in Tables 3, 4. Metaplastic epithelia (transitional/squamous) did not show staining. Chromogranin staining was not detected in any of our cases (controls being positive). Transglutaminase staining patterns paralleled the findings of PSA staining. With increasing grade of tumour i.e. lack of differentiation transglutaminase activity became weaker, becoming absent in solid areas and poorly differentiated areas.
Fig. 2A.
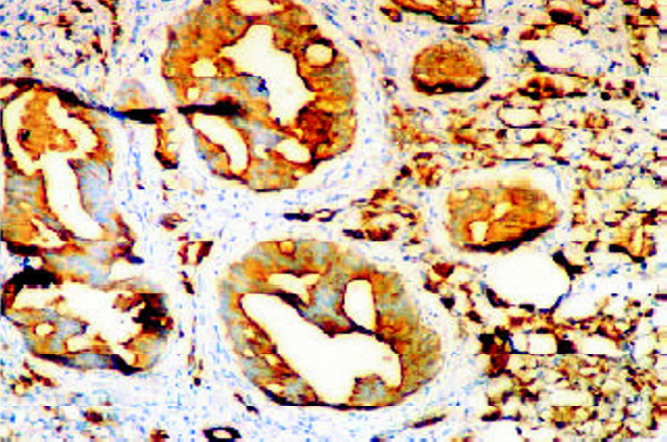
Staining for prostate specific antigen (PSA) in foci of PIN in a background of invasive carcinoma: intensity 3+ to 4+ (IHC with DAB × 10).
Table 3.
Intensity of PSA staining in benign prostatic lesions and PIN
| Intensity of staining | NHP n (%) | Atypical adenomatous hyperplasia n (%) | PIN Low grade n (%) | PIN High grade n (%) |
|---|---|---|---|---|
| 4 + | 22 (88) | − | 2 (100) | 1 (33.3) |
| 3 + | 3 (12) | 1 (100) | − | 2 (66.7) |
| 2 + | − | − | − | − |
| 1 + | − | − | − | − |
| 0 + | − | − | − | − |
| Total | 25 (100) | 1 (100) | 2 (100) | 3 (100) |
Table 4.
Prostatic adenocarcinoma: PSA expression and its relation to Gleason's grade
| Intensity of staining | Total cases n (%) | Low grade n (%) | Intermediate grade n (%) | High grade n (%) |
|---|---|---|---|---|
| 4+ | 6 (240) | 1 (100) | 5 (29.4) | − |
| 3+ | 9 (36) | − | 8(47.1) | 1 (14.3) |
| 2+ | 6 (24) | − | 4(23.5) | 2 (28.6) |
| 1+ | 3 (12) | − | − | 3 (42.8) |
| 0+ | 1 (4) | − | − | 1 (14.3) |
| Total | 25 (100) | 1 (100) | 17 (100) | 7 (100) |
Benign acini had a continuous layer of basal cells surrounding the prostatic glandular epithelium. In atrophic glands, though difficult to visualize on H&E, the basal cells could be visualized by HMWCK (Fig. 2B). Basal cell hyperplasia noted on H&E was better demonstrated with IHC. In low grade PIN the basal cell layer was continuous. In high grade PIN there were focal disruptions in 2/3 cases while it was continuous but with reduced intensity in one. Basal cells were absent in carcinoma.
Fig. 2B.
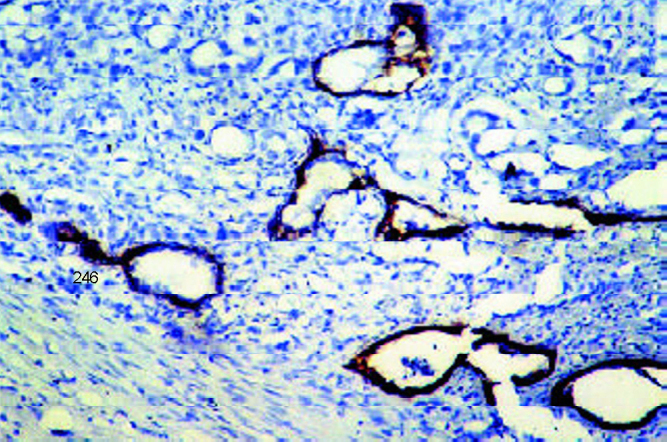
Complete loss of basal cells in malignant acini (in contrast entrapped benign acini have an intact basal cell layer (identified by HMWCK on IHC with DAB × 10).
Staining for PCNA was heterogeneous, however only the strongly stained nuclei were included in the count (Fig. 2C). The LI was low in benign lesions with a gradual increase from PIN to ascending grades of adenocarcinoma (Table 5A, Table 5B). The difference of mean LI of NHP vs PIN and PIN vs carcinoma was statistically significant. The difference in LI between low grade vs intermediate and high grade carcinoma was significant.
Fig. 2C.
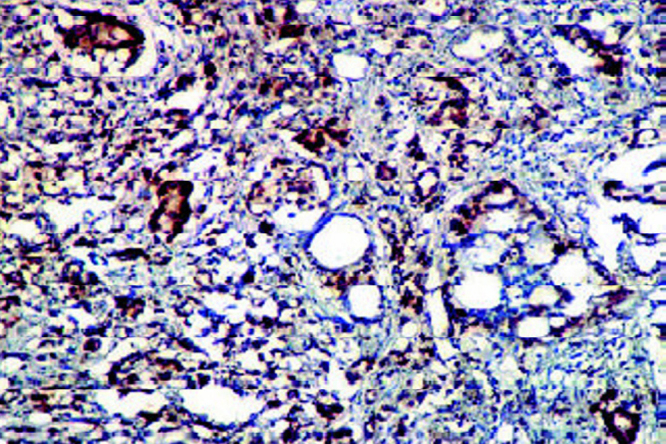
Proliferating cell nuclear antigen (PCNA) expression in a prostatic adenocarcinoma showing a high PCNA LI (IHC with DAB × 10).
Table 5A.
PCNA LI in Prostatic lesions
| Type of lesion | Number (n) | LI range (%) | Mean LI (%) | p value* |
|---|---|---|---|---|
| NHP | 25 | 0.6-1.1 | 0.76 ± 0.1} | 0.02 |
| PIN | 5 | 0.6-4.9 | 3.09 ± 1.7} | |
| Low grade Ca | 1 | 5.3 | 5.3 | |
| Intermediate grade Ca | 17 | 3.8-28.7 | 12.59 ± 5.5} | 0.03 |
| High grade Ca | 7 | 7.9-34.9 | 22.02 ± 8.3} |
– p<0.05 considered significant
Table 5B.
Prostatic lesions: PCNA LI by IHC
| Lesion | Mean PCNA LI (%) | p value* |
|---|---|---|
| NHP | 0.76 ± 0.1} | < 0.001 |
| PIN | 3.09 ± 1.7} | |
| Carcinoma prostate | 14.94 ± 7.7} |
– p<0.001 is significant
CD 34 staining showed randomly distributed thin walled vessels in NHP, more concentrated in areas of inflammation (Fig. 2D). In both trucut and TURP biopsies microvessel density (MVD) count was erratic (because of crushing in the former and small separate chips in the latter). However morphological observations showed randomly distributed vessels in low-grade tumours and accentuation in numbers at the advancing edge of high-grade tumours. Apoptotic cells were occasional in prostatic hyperplasias and metaplasias. In tumours, apoptotic rate varied from 2.5-12.2/1000 cells (low grade tumours) to 4-15.5/1000 cells (high grade tumours). Tumours with solid areas (high grade) showed less apoptosis compared to better-differentiated areas.
Fig. 2D.
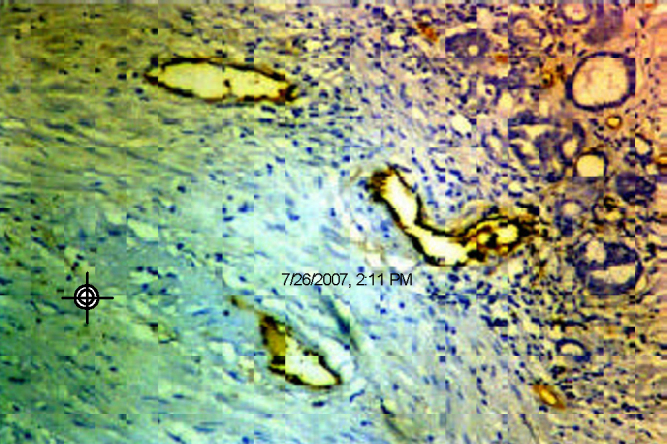
CD 34 stain marks the endothelial cells of vessels concentrated at the advancing edge of a carcinoma (IHC with DAB × 10).
Discussion
Carcinoma prostate, common among men in the West, is uncommon in our country ranking seventh and eighth among male cancers in Bangalore, Mumbai and Delhi respectively [1, 2]. The course of most prostate malignancies is often unpredictable. Heredity appears to be the most constant risk factor apart from hormonal influence [12]. The age specific prevalence of NHP and adenocarcinoma is remarkably similar with a gradual increase over 40 years [1]. The mean age of presentation of our patients was sixth and seventh decade of life.
TRUS, DRE and PSA levels form the basis for early detection of prostate carcinoma. In our experience the sensitivity and specificity of TRUS was better than DRE [13]. The mean serum PSA concentration in healthy men has been reported to be < 4ng/ml. Up to 43% patients of NHP have levels of 4-10 ng/ml and 12% >10 ng/ml [14, 15]. In our study serum PSA levels of NHP had a mean of 7.8 ± 8 ng/ml with 14 cases showing borderline and three high PSA due to inflammation, infarcts or instrumentation. PSA may not be the sole reliable criterion for diagnosis of malignancy as only about 75% cancers have an abnormal PSA, and 20% of prostate cancers with aggressive features have levels < 4 ng/mL (American Urological Association) [16]. In our series 2/23 cases had normal PSA levels. The likelihood of cancer above 50 years of age, goes up from 20 to 30% for levels between 2.5-4.0 ng/mL and above 4.0 ng/mL respectively [17]. Among benign lesions, atypical adenomatous hyperplasia (AAH) that varies in incidence from 19.6% (TURP specimens) to 24% (autopsy series in 20-40 year old men) is a cause for misdiagnosis [18, 19] and we had only one (4%) such case. Basal cell hyperplasia seen in 2/25 (8%) of our cases, has a potential for being misdiagnosed as carcinoma.
Bostwick et al [20], proposed the term prostatic intraepithelial neoplasia (PIN), for an intraluminal proliferation of secretory cells of the prostatic duct-acinar system with atypical cytology, and recommended its categorization into low and high grade. Many studies have shown the association of high grade PIN with prostate cancer [7, 20]. We found PIN associated with 16% of prostate cancers (majority high grade) and only 4% of NHP (low grade). Fenely et al [21], observed PIN in 11% cases in hospital practice, 20% in prostate cancer screening and 25% in urology practice. Our study supports the notion that low grade PIN occurs with NHP and can be ignored, whereas high grade PIN signifies coexistence with, or subsequent development of carcinoma. Our study was dominated by acinar patterns of prostatic adenocarcinoma. Perineural invasion was a common finding involving eight (32%) patients while vascular invasion was seen in only three (12%). Both connote a poor prognosis.
Gleason's grading system, based on the degree of architectural differentiation, is used for behaviour prediction [22]. In our series there was one (4%) low grade, 17 (68%) intermediate and seven (28%) high grade tumours. The commonest pattern had a score of 3. Current clinical and pathological staging (TNM) of early prostatic adenocarcinoma separates patients into 2 groups: those with palpable and non-palpable tumours [23] based on DRE which has low sensitivity, specificity and poor predictive value [4]. Thirteen (52%) cases in our study were detected on TURP and three (12%) cases had only PSA elevation without palpable prostate. Mai et al [24], showed incidental carcinoma in 8% cases. He also noted that there was no T1c cases in the non-PSA era but detected 28 such cases when serum PSA estimation became available.
Immunophenotyping revealed strong PSA staining for benign lesions as reported by others [8]. In atrophic glands and foci of inflammation, negativity may raise the possibility of carcinoma but morphological correlation solves the issue. PSA staining intensity was directly proportional to tumour differentiation. In intermediate grade tumours a subset stained poorly and is likely to be biologically aggressive. Others have reported similar findings [8, 25]. Transglutaminase expression paralleled PSA. In NHP, though serum PSA levels were variable, majority had good PSA staining (88%). In two cases of prostate carcinoma where PSA levels were < 4ng/ml and 12 cases where it was 4-10 ng/ml, staining in tissues was 4+ to 3+. Thus tissue expression of PSA did not always correlate directly with serum levels. The discrepancy may be due to differences between the amount of PSA produced and secreted and/or the sensitivity of detection of various isoforms [25]. HMWCK, highlighting basal cells was useful in separating AAH from carcinoma (being consistently absent in the latter) [26]. It is less useful in PIN. We improved HMWCK staining by combining antigen retrieval by trypsin treatment and microwave heating.
Proliferative activity (PCNA LI) was statistically higher in PIN and adenocarcinoma as compared to benign lesions and in higher grade tumours. Proliferation indices have correlated well with grades of tumour [27], though some have found lower LI. Apoptotic rate was consistently low in benign lesions and only marginally higher in PIN. In carcinomas it showed variability. This may be a reflection of poor cell viability rather than a protective intracellular signal to control the tumour burden. However, inhibitors of apoptosis have a direct bearing on drug resistance by tumour cells [28]. MVD counts were plagued by erratic staining and reliability of counting in TURP chips. The close relation of vessels to foci of PIN (with normal PSA) may be a neovascularisation promoting survival of this premalignant stage. There is a suggestion that high grade PIN that will progress to malignancy may have higher MVD [10].
Our morpho-immunophenotypic evaluation of prostatic lesions underscores the sound basis of good histological evaluation by routine H&E stains. Basal cell identification is useful in morphologically atypical cases. High grade PIN should alert the clinician to coexistence of carcinoma or need for close follow-up because PIN cannot be suspected by clinical examination, TRUS or PSA levels. Prognostication can be further enhanced by corroboration with markers of differentiation and assessment of proliferation indices. The application of tumour angiogenesis needs further study to establish guidelines for significance of counts. Future directions of prostate biology research are aimed at exploring a stem cell origin, biological markers for diagnosis and prognosis besides novel therapeutic approaches targeting angiogenesis, immunosurveillance and stromal-epithelial interactions [29, 30].
Conflicts of Interest
None identified
References
- 1.Boring CC, Squires TS, Tong T. Cancer Statistics CA. Cancer J Clin. 1994;44:7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Registry Programme. Consolidated Report Population Based Cancer Registries (PBCRs 1990–1996). Indian Council of Medical Research, New Delhi, Aug 2001.
- 3.Smith PH. The case for no initial treatment of localized prostate cancer. Urol Clin North Am. 1990;17:827–834. [PubMed] [Google Scholar]
- 4.Friedman GO, Hiatt RA, Quesenberry CP. Case control study of screening for prostate cancer by digital rectal examinations. Lancet. 1991;337:1526–1529. doi: 10.1016/0140-6736(91)93207-p. [DOI] [PubMed] [Google Scholar]
- 5.Fong YK, Milani S, Spaller S, Djavan B. Prostate-specific antigen testing in the new millennium. BJU Int. 2005;95:1167–1168. doi: 10.1111/j.1464-410X.2005.05532.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M, Epstein JI. The reporting of prostate cancer on needle biopsy: prognostic and therapeutic implications and the utility of diagnostic markers. Pathology. 2003;35:472–479. doi: 10.1080/00313020310001619163. [DOI] [PubMed] [Google Scholar]
- 7.Izawa JI, Lega I, Downey D, Chin JL, Luke PP. Do all patients with high-grade prostatic intraepithelial neoplasia on initial prostatic biopsy eventually progress to clinical prostate cancer? BJU Int. 2005;96:320–323. doi: 10.1111/j.1464-410X.2005.05623.x. [DOI] [PubMed] [Google Scholar]
- 8.Ellis DW, Leffers S, Davies JS. Multiple immunoperoxidase markers in benign hyperplasia and adenocarcinoma of the prostate. Am J Clin Pathol. 1984;81:279–284. doi: 10.1093/ajcp/81.3.279. [DOI] [PubMed] [Google Scholar]
- 9.Nemoto R, Kawamura H, Miyakawa I. Immunohistochemical detection of proliferating cell nuclear antigen (PCNA) / cyclin in human prostate adenocarcinoma. J Urol. 1993;149:165–169. doi: 10.1016/s0022-5347(17)36031-7. [DOI] [PubMed] [Google Scholar]
- 10.Sinha A, Quast BJ, Reddy PK, Lall V, Wilson MJ, Qian J, Bostwick DG. Microvessel density as a molecular marker for identifying high-grade prostatic intraepithelial neoplasia precursors to prostate cancer. Exp Mol Pathol. 2004;77:153–159. doi: 10.1016/j.yexmp.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Hsu SM, Raine L, Fanger H. The use of avidin antibody and avidin - hiotin peroxidase complex in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 12.Carter BS, Bova GS, Beaty TH. Hereditary prostate cancer: Epidemiologic and clinical features. J Urol. 1993;150:797. doi: 10.1016/s0022-5347(17)35617-3. [DOI] [PubMed] [Google Scholar]
- 13.Garzotto M, Hudson RG, Peters L. Predictive modelling for the presence of prostate carcinoma using clinical, laboratory, and ultrasound parameters in patients with prostate specific antigen levels < or = 10 ng/mL. Cancer. 2003;98:1417–1422. doi: 10.1002/cncr.11668. [DOI] [PubMed] [Google Scholar]
- 14.Oesterling JE, Chan DW, Epstein JI. Prostate specific antigen in the preoperative and postoperative evaluation of localized prostatic cancer treated with prostatectomy. J Urol. 1988;139:766–782. doi: 10.1016/s0022-5347(17)42630-9. [DOI] [PubMed] [Google Scholar]
- 15.Armitage TG, Cooper EH, Newling DW. The value of the measurement of serum prostate specific antigen in patients with benign prostatic hyperplasia and untreated prostate cancer. Br J Uro. 1988;62:584–589. doi: 10.1111/j.1464-410x.1988.tb04431.x. [DOI] [PubMed] [Google Scholar]
- 16.American Urological Association Prostate-specific antigen (PSA) best practice policy. Oncology. 2000;14:267–286. [PubMed] [Google Scholar]
- 17.Smith DS, Carvalhal GF, Mager DE. Use of lower prostate specific antigen cutoffs for prostate cancer screening in black and white men. J Urol. 1998;160:1734–1738. [PubMed] [Google Scholar]
- 18.Rekhi B, Jaswal TS, Arora B. Premalignant lesions of prostate and their association with nodular hyperplasia and carcinoma prostate. Indian J Cancer. 2004;41:60–65. [PubMed] [Google Scholar]
- 19.Brawn PN, Speights VO, Contin JU, Bayardo RJ, Kuhl DL. Atypical hyperplasia in prostates of 20–40 year old men. J Clin Pathol. 1989;42:383–386. doi: 10.1136/jcp.42.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bostwick DG, Brawer MK. Prostatic intraepithelial neoplasia and early invasion in prostate cancer. Cancer. 1987;59:788–794. doi: 10.1002/1097-0142(19870215)59:4<788::aid-cncr2820590421>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Feneley MR, Green JSA, Young MPA. Prevalence of prostatic intraepithelial neoplasia (PIN) in biopsies from hospital practice and pilot screening: clinical implications. Prostate Cancer Prostatic Dis. 1997;1:79–83. doi: 10.1038/sj.pcan.4500210. [DOI] [PubMed] [Google Scholar]
- 22.Gleason DF, Mellinger GT, and the Veterans Administration Cooperative Urological Research Group Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 23.Schroder FH, Harmanek P, Denis L. The TNM classification of prostate carcinoma. Prostate. 1992;4:129–138. doi: 10.1002/pros.2990210521. [DOI] [PubMed] [Google Scholar]
- 24.Mai KT, Isotalo PA, Green J. Incidental prostatic adenocarcinomas and putative premalignant lesions in TURP specimens collected before and after the introduction of prostate specific antigen screening. Arch Pathol Lab Med. 2000;124:1454–1456. doi: 10.5858/2000-124-1454-IPAAPP. [DOI] [PubMed] [Google Scholar]
- 25.Wier EG, Partin AW, Epstein JI. Correlation of serum prostate specific antigen and quantitative immunohistochemistry. J Urol. 2000;163:1739–1742. [PubMed] [Google Scholar]
- 26.O'Malley FP, Grignon OJ, Shum DT. Usefulness of immunoperoxidase staining with high molecular weight keratin in the differential diagnosis of small acinar lesions of the prostate. Virchows Archiv [A] Pathol Anat Histopathol. 1990;417:191–196. doi: 10.1007/BF01600133. [DOI] [PubMed] [Google Scholar]
- 27.Capello F, Rappa F, David S, Anzalone R, Zummo G. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res. 2003;23:1325–1331. [PubMed] [Google Scholar]
- 28.Nomura T, Yamasaki M, Nomura Y, Mimata H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep. 2005;14:993–997. [PubMed] [Google Scholar]
- 29.Schalken JA, van Leenders G. Cellular and molecular biology of the prostate: stem cell biology. Urology. 2003;62:11–20. doi: 10.1016/s0090-4295(03)00758-1. [DOI] [PubMed] [Google Scholar]
- 30.Watson RW, Schalken JA. Future opportunities for the diagnosis and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2004;7(1 Suppl):8S–13S. doi: 10.1038/sj.pcan.4500742. [DOI] [PubMed] [Google Scholar]


