Abstract
Aims
To assess the incidence and risk of arterial and venous thromboembolic events (ATEs and VTEs) associated with antivascular endothelial growth factor (VEGF) agents, including VEGF receptor-tyrosine kinase inhibitors and VEGF monoclonal antibodies, in advanced non-small-cell lung cancer (NSCLC) patients.
Methods
We performed a broad search of PubMed for relevant trials. Prospective randomized trials evaluating therapy with or without anti-VEGF agents in patients with advanced NSCLC were included for analysis. Data on VTEs and ATEs were extracted. The overall incidence, Peto odds ratio (Peto OR), and 95% confidence intervals (CIs) were pooled according to the heterogeneity of included trials.
Results
A total of 13,436 patients from 23 trials were included for analysis. Our results showed that anti-VEGF agents significantly increased the risk of developing high-grade ATEs (Peto OR: 1.44, 95% CI: 1.00–2.07, P=0.048), but not for all-grade ATEs (Peto OR: 0.94, 95% CI: 0.56–1.59, P=0.82) compared with controls. Additionally, no increased risk of all-grade and high-grade VTEs (Peto OR: 0.94, 95% CI: 0.67–1.31, P=0.71 and Peto OR: 0.95, 95% CI: 0.73–1.22, P=0.67, respectively) was observed in advanced NSCLC patients receiving anti-VEGF agents.
Conclusion
The use of anti-VEGF agents in advanced NSCLC patients significantly increased the risk of high-grade ATEs, but not for VTEs. Clinicians should be aware of the risk of severe ATEs with administration of these drugs in advanced NSCLC patients.
Keywords: anti-VEGF agents, toxicity, arterial thromboembolic events, venous thromboembolic events, meta-analysis
Introduction
Angiogenesis, the formation of new blood vessels, is critical for tumor progression, invasion, and metastasis in many solid tumors.1–3 Basic research shows that this process is mainly driven by vascular endothelial growth factor (VEGF), and thus angiogenesis inhibitors targeting the VEGF signal pathway are a potential treatment options for solid tumors.4,5 In the past two decades, many novel anti-VEGF agents, including VEGF monoclonal antibodies and multitarget VEGF receptor (VEGFR)-tyrosine kinase inhibitors (TKIs)/monoclonal antibodies, have been proven to improve survival benefits in many solid tumors including non-small-cell lung cancer (NSCLC). Until now, three anti-VEGF agents, including bevacizumab, ramucirumab, and nintedanib, have been approved by the US Food and Drug Administration (FDA) for the treatment of advanced NSCLC,6–9 and it is anticipated that the use of these agents in cancer patients would be increased in the near future.
However, the VEGF signal pathway plays a critical role in physiological functions and homeostasis in the cardiovascular and renal systems. Previous research has found that VEGF is of great importance to regulate angiogenesis and the vascular tone.10 Other vascular proteins, such as tissue factor (TF) and endothelial nitric oxide synthase, have also been involved in controlling endothelial thrombogenicity and regulation of vascular tone. While TF and its distinct isoforms can induce the expression of VEGF, and interact with VEGF and its pro-/antiangiogenic isoforms could in turn leads to modifications of essential biological processes.11 Thus, inhibition of angiogenesis pathway could cause a variety of adverse effects.12 Indeed, a variety of toxicities associated with anti-VEGF signal pathway including hypertension,13–17 proteinuria or renal dysfunction,18–21 congestive heart failure,22–25 hemorrhage,26,27 and gastrointestinal perforation28–30 have been reported in previous studies. Although several meta-analyses have been conducted to assess the risk of arterial and venous thromboembolic events (VTEs and ATEs) associated with anti-VEGF agents, all these studies include different tumor types.31–38 It has been reported that some tumor-dependent intrinsic mechanisms have been related to VTEs or ATEs, and patient baseline characteristics differ between tumor types. Additionally, time to treatment failure and follow-up duration vary according to tumor types, and these factors are closely related to the likelihood of developing and detecting VTEs and ATEs. As a result, the risk of VTEs and ATEs associated with anti-VEGF agents in advanced NSCLC remains unknown. We thus conducted a meta-analysis of published trials to investigate the risk of ATEs and VTEs associated with the use of anti-VEGF agents in advanced NSCLC patients.
Methods
Data source
We performed this systematic review adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statements.39 We performed a board search of PubMed for relevant trials published between January 1, 1990 and November 31, 2015. The search included the following terms: “anti-VEGF agents”, “VEGFR-TKIs”, “bevacizumab”, “aflibercept”, “sorafenib”, “sunitinib”, “vandetanib”, “pazopanib”, “axitinib”, “motesanib”, “ramucirumab”, “cediranib”, “regorafenib”, “cabozantinib”, “brivanib”, “tivozanib”, “nintedanib”, “angiogenesis inhibitors”, “non-small-cell lung cancer”, and “randomized clinical trial”. Each publication was reviewed, and in case of duplicate publication only, the most complete, recent, and updated report of the clinical trial was included in the meta-analysis.
Inclusion criteria for our study were 1) patients having pathologically confirmed NSCLC, 2) trials comparing treatment with or without anti-VEGF agents, and 3) reporting data on VTEs or ATEs toxicity. We assessed the quality of reports of clinical trials by using the five-item Jadad scale including randomization, double-blinding, and withdrawals as previously described.40
Data extraction
Two independent investigators reviewed the titles and abstracts of potentially relevant studies. We retrieved the full text of relevant studies for further review by the same two reviewers. A third senior investigator resolved any discrepancies between reviewers. The same pair of reviewers extracted study details independently using a standardized pilot-tested form. A third investigator reviewed all data entries. We extracted the following information data: first author, study period, treatment regimens, sample size, number evaluable for toxicity, median age, median overall survival, and progression-free survival. We considered the following adverse outcomes as VTEs/ATEs: thrombosis/thrombus/embolism (excluded vascular access related-thrombosis if reported separately), arterial thrombosis, cerebral infarct, cerebral ischemia, cerebrovascular accident, myocardial infarction, and myocardial ischemia. We assessed and recorded adverse events according to the National Cancer Institute’s common toxicity criteria (Version 2 or 3).41
Statistical analysis
Statistical analysis for overall risk of VTEs/ATEs was performed using comprehensive meta-analysis software Version 2.0 (Biostat, Englewood, NJ, USA). We used the Peto method to calculate odds ratios (ORs) and 95% confidence intervals (CIs) because this method provided the best CI coverage and was more powerful and relatively less biased than the fixed or random effects analysis when dealing with low event rates.42 Between-study heterogeneity was estimated using the χ2-based Q-statistic. Heterogeneity was considered statistically significant when Pheterogeneity <0.05 or I2>50%.43 Meta-analysis was performed using a random effects model in response to the expected clinical heterogeneity among the trials.42 A two-sided P-value <0.05 was considered significant. The presence of publication bias was evaluated using the Begg and Egger tests.
Results
Search results
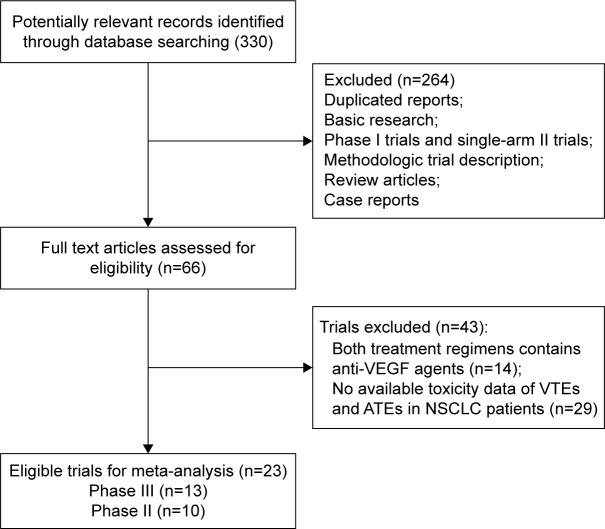
A total of 330 studies were identified from the database search, and 66 trials were retrieved for full-text evaluation. Forty-three trials were excluded for the reasons shown in Figure 1. Finally, a total of 13,436 patients from 23 randomized controlled trials were included for the meta-analysis.6,8,9,44–63 The baseline characteristics of each trial are summarized in Table 1. Fifteen trials were performed in first-line settings and eight second-line settings. According to the inclusion criteria of each trial, patients were required to have an adequate renal, hepatic, and hematologic function. We roughly assessed the quality of each included study according to the Jadad score, and 14 trials had Jadad score of 5, and nine trials had Jadad score of 3.
Figure 1.
Selection process for randomized controlled trials included in the meta-analysis.
Abbreviations: VEGF, vascular endothelial growth factor; VTEs, venous thromboembolic events; ATEs, arterial thromboembolic events; NSCLC, non-small-cell lung cancer.
Table 1.
Baseline characteristics of 23 included trials for analysis
| Authors | Phase | Total patients | Therapy line | Treatment arms | Median age (years) | Median PFS, months | Median OS, months | Number for analysis | Jadad score |
|---|---|---|---|---|---|---|---|---|---|
| Johnson et al63 | II | 99 | First-line | Bevacizumab 2.5 mg/kg/wk + PTX + CBP | NR | 4.3 | 11.6 | 32 | 3 |
| Bevacizumab 5 mg/kg/wk + PTX + CBP | NR | 7.4 | 17.7 | 34 | |||||
| PTX + CBP | NR | 4.2 | 14.9 | 32 | |||||
| Sandler et al8 | III | 878 | First-line | Bevacizumab 5 mg/kg/wk + PTX + CBP | NR | 6.2 | 12.3 | 427 | 3 |
| PTX + CBP | NR | 4.5 | 10.3 | 440 | |||||
| Heymach et al61 | II | 127 | Second-line | Vandetanib 100 mg + Doc | 61 | 4.4 | 13.1 | 42 | 5 |
| Vandetanib 300 mg + Doc | 60 | 4 | 7.9 | 44 | |||||
| Placebo + Doc | 58 | 2.8 | 13.4 | 41 | |||||
| Herbst et al62 | II | 120 | First-line | Bevacizumab 5 mg/kg/wk + chemotherapy | 63.5 | 4.8 | 12.6 | 40 | 5 |
| Bevacizumab 5 mg/kg/wk + erlotinib | 68 | 4.4 | 13.7 | 39 | |||||
| Placebo + chemotherapy | 65 | 3 | 8.6 | 41 | |||||
| Reck et al60 | III | 1,043 | First-line | Bevacizumab 5 mg/kg/wk + GEM + DDP | 59 | 6.7 | NR | 329 | 5 |
| Bevacizumab 2.5 mg/kg/wk + GEM + DDP | 57 | 6.5 | NR | 330 | |||||
| Placebo + GEM + DDP | 59 | 6.1 | NR | 327 | |||||
| Herbst et al57 | III | 636 | Second-line | Bevacizumab 5 mg/kg/wk + erlotinib | 64.8 | 3.4 | 9.3 | 319 | 3 |
| Erlotinib | 65 | 1.7 | 9.2 | 317 | |||||
| Scagliotti et al59 | III | 926 | First-line | Sorafenib 400 mg bid po + PTX + CBP | 62 | 4.6 | 10.7 | 436 | 5 |
| Placebo + PTX + CBP | 63 | 5.4 | 10.6 | 459 | |||||
| de Boer et al58 | III | 534 | Second-line | Vandetanib 100 mg + PEM | 60 | 4.1 | 10.5 | 260 | 5 |
| Placebo + PEM | 60 | 2.8 | 9.2 | 273 | |||||
| Natale et al56 | III | 1,240 | Second-line | Vandetanib 300 mg qd po | 61 | 2.6 | 6.8 | 623 | 3 |
| Erlotinib | 61 | 2 | 7.7 | 614 | |||||
| Lee et al55 | III | 924 | Second-line | Vandetanib 300 mg qd po | 60 | 1.9 | 8.5 | 619 | 5 |
| Placebo | 60 | 1.8 | 7.8 | 303 | |||||
| Niho et al54 | II | 180 | First-line | Bevacizumab 5 mg/kg/wk + PTX + CBP | 61 | 6.9 | 22.8 | 119 | 3 |
| PTX + CBP | 60 | 5.9 | 23.4 | 58 | |||||
| Paz-Ares et al53 | III | 772 | First-line | Sorafenib 400 mg bid po + GEM + DDP | 60 | 6 | 12.4 | 385 | 5 |
| Placebo + GEM + DDP | 58 | 5.5 | 12.5 | 387 | |||||
| Scagliotti et al51 | III | 1,090 | First-line | Motesanib 125 mg qd po + PTX + CBP | 60 | 5.6 | 13 | 533 | 5 |
| Placebo + PTX + CBP | 60 | 5.4 | 11 | 539 | |||||
| Ramlau et al52 | III | 913 | First-line | Aflibercept 6 mg/kg + Doc | 59.6 | 5.2 | 10.1 | 456 | 5 |
| Placebo + Doc | 59.6 | 4.1 | 10.4 | 457 | |||||
| Groen et al50 | II | 132 | Second-line | Sunitinib 37.5 mg qd po + erlotinib | 59 | 2.8 | 8.2 | 65 | 5 |
| Placebo + erlotinib | 61 | 2 | 7.6 | 67 | |||||
| Belani et al49 | II | 170 | First-line | Axitinib 5 mg bid po (continuous) + PEM + DDP | 62 | 8 | 17 | 55 | 3 |
| Axitinib 5 mg bid po (modified) + PEM + DDP | 62 | 7.9 | 14.7 | 58 | |||||
| PEM + DDP | 59 | 7.1 | 15.9 | 55 | |||||
| Garon et al9 | III | 1,253 | Second-line | Ramucirumab 10 mg/kg + Doc | 62 | 4.5 | 10.5 | 627 | 5 |
| Placebo + Doc | 61 | 3 | 9.1 | 618 | |||||
| Gridelli et al48 | II | 124 | First-line | Vandetanib 100 mg qd po + GEM | 75 | 6.1 | 8.7 | 61 | 5 |
| Placebo + GEM | 75.48 | 5.6 | 10.2 | 63 | |||||
| Laurie et al48 | III | 306 | First-line | Cediranib 20 mg qd po + PTX + CBP | 63 | 5.5 | 12.2 | 153 | 5 |
| Placebo + PTX + CBP | 62 | 5.5 | 12.1 | 153 | |||||
| Reck et al6 | III | 1,314 | Second-line | Nintedanib 200 mg bid po + Doc | 60 | 3.4 | 10.9 | 652 | 5 |
| Placebo + Doc | 60 | 2.7 | 7.9 | 655 | |||||
| Zinner et al44 | II | 361 | First-line | PTX + CBP + bevacizumab followed by maintenance bevacizumab | 65.4 | 5.49 | 11.7 | 179 | 3 |
| PEM + CBP followed by maintenance PEM | 65.8 | 4.44 | 10.5 | 182 | |||||
| Doebele et al45 | II | 140 | First-line | Ramucirumab 10 mg/kg + PEM + platinum | 67 | 7.2 | 13.9 | 69 | 3 |
| PEM + platinum | 69 | 5.6 | 10.4 | 71 | |||||
| Seto et al46 | II | 154 | First-line | Bevacizumab 5 mg/kg/wk + erlotinib | 67 | 16 | NR | 77 | 3 |
| Erlotinib | 67 | 9.7 | NR | 77 |
Abbreviations: PTX, paclitaxel; CBP, carboplatin; DDP, cisplatin; GEM, gemcitabine; Doc, docetaxel; PEM, pemetrexed; PFS, progression-free survival; OS, overall survival; qd, quaque die; bid, bis in die; po, per os; wk, weekly; NR, not reported.
Incidence of VTEs and ATEs
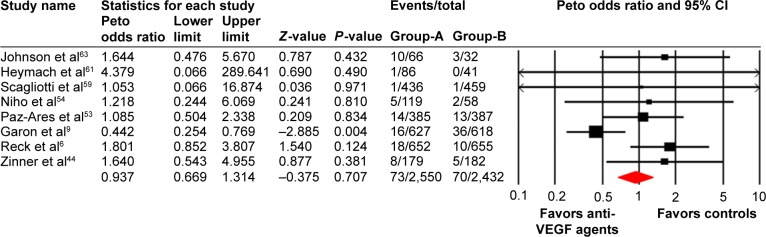
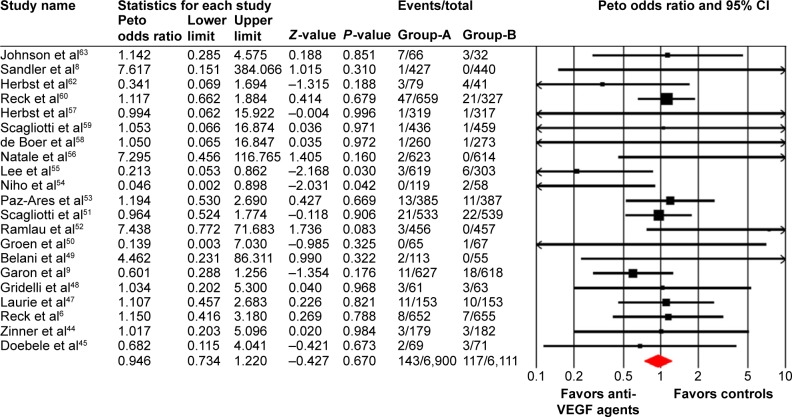
A total of 2,550 patients from eight treatment arms who received anti-VEGF agents were available for all-grade VTEs incidence analysis. Using a random effects model, the summary incidence of all-grade VTEs was 3.4% (95% CI: 2.0%–5.9%; Table 2). As for high-grade VTEs, a total of 6,900 patients from 21 treatment arms were included, and the pooled incidence was 1.8% (95% CI: 1.1%–2.9%; Table 2).
Table 2.
Incidence of venous thromboembolic events (VTEs) and arterial thromboembolic events (ATEs) in advanced non-small-cell lung cancer patients receiving antivascular endothelial growth factor agents
| Adverse events | Number of trials | Events | Total patients | I2 (%) | Incidence (95% confidence interval) |
|---|---|---|---|---|---|
| VTEs | |||||
| All-grade | 8 | 73 | 2,550 | 79.2 | 3.4 (2.0%–5.9%) |
| High-grade | 21 | 143 | 6,900 | 83.2 | 1.8 (1.1%–2.9%) |
| ATEs | |||||
| All-grade | 4 | 28 | 1,784 | 87.9 | 2.1 (0.7%–6.0%) |
| High-grade | 16 | 79 | 4,824 | 60.6 | 1.8 (1.2%–2.7%) |
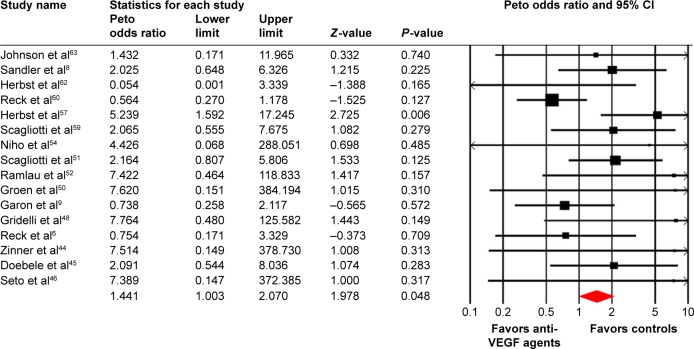
Four included trials reported the all-grade ATEs, and the pooled incidence was 2.1% (95% CI: 0.7%–6.0%; Table 2). As for high-grade ATEs, a total of 4,824 patients from 16 trials were included for analysis with a pooled incidence of 1.8% (95% CI: 1.2%–2.7%; Table 2) using a random effects model.
Peto odds ratio of VTEs and ATEs
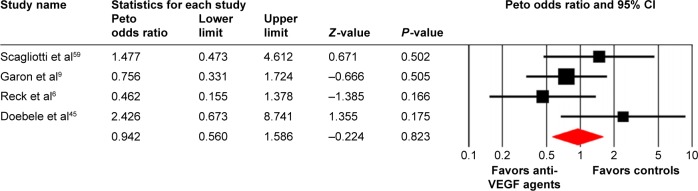
A meta-analysis of the Peto OR for all-grade and high-grade VTEs attributable to anti-VEGF agents compared with controls was performed. The pooled results showed that the use of anti-VEGF agents did not increase the risk of all-grade (Peto OR: 0.94, 95% CI: 0.67–1.31, P=0.71; Figure 2) and high-grade VTEs compared with controls (Peto OR: 0.95, 95% CI: 0.73–1.22, P=0.67; Figure 3) using a fixed effects model. We then investigated the risk of ATEs associated with anti-VEGF agents. Our results showed that the use of anti-VEGF agents significantly increased the risk of high-grade ATEs (Peto OR: 1.44, 95% CI: 1.00–2.07, P=0.048; Figure 4) using a fixed effects model (I2=32.1, P=0.11), whereas the use of anti-VEGF agents did not increase the risk of all-grade ATEs when compared to controls (Peto OR: 0.94, 95% CI: 0.56–1.59, P=0.82; Figure 5).
Figure 2.
Odds ratio of all-grade venous thromboembolic events associated with antivascular endothelial growth factor (VEGF) agents vs control.
Notes: Group-A is the number of patients included for analysis in VEGF group; Group-B is the number of patients included for analysis in controlled group.
Abbreviations: CI, confidence interval; I2, level of heterogeneity among included trials.
Figure 3.
Odds ratio of high-grade venous thromboembolic events associated with antivascular endothelial growth factor (VEGF) agents vs control.
Notes: Group-A is the number of patients included for analysis in VEGF group; Group-B is the number of patients included for analysis in controlled group.
Abbreviation: CI, confidence interval.
Figure 4.
Odds ratio of high-grade arterial thromboembolic events associated with antivascular endothelial growth factor (VEGF) agents vs control.
Notes: Group-A is the number of patients included for analysis in VEGF group; Group-B is the number of patients included for analysis in controlled group.
Abbreviation: CI, confidence interval.
Figure 5.
Odds ratio of all-grade arterial thromboembolic events associated with antivascular endothelial growth factor (VEGF) agents vs control.
Abbreviation: CI, confidence interval.
Publication bias
We used Begg’s funnel plot and Egger’s test to assess the publication bias of literatures. No evidence of obvious asymmetry was detected by Begg’s test for VTEs (all-grade: P=0.62 and high-grade: P=0.80, respectively) and ATEs (all-grade: P=0.18 and high-grade: P=0.65, respectively). Similarly, Egger’s test still did not suggest any evidence of publication bias for VTEs (all-grade: P=0.27 and high-grade: P=0.19, respectively) and ATEs (all-grade: P=0.08 and high-grade: P=0.11, respectively).
Discussion
Thromboembolic events are a major cause of morbidity and mortality in patients with advanced tumors.64,65 Although the presence of malignancies itself and its associated physiologic changes are risk factors for thromboembolism, several anticancer therapies, including cytotoxic agents, angiogenesis inhibitors, and hormonal therapies, might increase the risk of developing a thromboembolic event.66 During past decades, several anti-VEGF agents have been approved by the FDA for use in a variety of solid tumors due to its survival benefits; concerns have arisen regarding the risk of thromboembolic events with the use of these drugs. Previous meta-analyses consistently supported a significant increase in ATEs from both VEGFR-TKIs and bevacizumab across a range of advanced solid tumors. In one trial-level meta-analysis conducted by Choueiri et al35 found that sunitinib or sorafenib treatment significantly increased the risk of ATEs (relative risk [RR] =3.03, P=0.015). Similarly, another individual patient-level meta-analysis (hazard ratio [HR]: 2.0, P=0.031)37 and two trial-level meta-analyses (RR: 1.46, P=0.007 and RR: 1.44, P=0.013), respectively67,68 also demonstrated that the risk of ATEs with bevacizumab and chemotherapy was higher than that in chemotherapy alone. A recent meta-analysis conducted by Qi et al38 also found an increased risk of developing ATEs in cancer patients receiving VEGFR-TKIs (OR: 2.26, P=0.001). As far as we know, this was the largest study investigating the risk of VTEs and ATEs with anti-VEGF agents in advanced NSCLC patients with a total of 13,436 patients from 23 trials. In the present study, we found that the use of anti-VEGF agents significantly increased the risk of high-grade ATEs, but not for all-grade ATEs. As anti-VEGF agents are increasingly used in the treatment of advanced NSCLC patients, it is critically important for clinicians to be aware of the risk of ATEs associated with anti-VEGF agents and monitor and treat it appropriately.
Several studies have been conducted to investigate the risk of VTEs associated with anti-VEGF agents in cancer patients, but the results are controversial. In one meta-analysis evaluating the impact of bevacizumab, the HR was 0.89 (P=0.44) and in the second study conducted by Hurwitz et al,34 the OR was 1.13 (P=0.13). In a third trial-level meta-analysis (n=7,956), bevacizumab treatment significantly increases the risk of developing VTEs (RR: 1.33, P<0.001).36 For VEGFR-TKIs and VTEs risk, two similar meta-analyses also found that the use of VEGFR-TKIs did not significantly increase the risk of VTEs (RR: 0.91, P=0.64 and RR: 1.10, P=0.64).32,33 In the present study, we also found that anti-VEGF therapies did not significantly increase the risk of all-grade and high-grade VTEs when compared to controls. Based on our findings, NSCLC patients with a recent but controlled VTEs probably should not be denied an anti-VEGF agent.
The mechanism of causing thromboembolic events might be related to the anti-VEGF effect with anti-VEGF agents: the VEGF pathway regulates endothelial cell proliferation, survival, and helps maintain vascular integrity.69 Inhibition of this pathway might lead to vascular wall defects and exposes procoagulant phospholipids.70 Additionally, VEGF also increases the production of nitric oxide (NO) and prostacylin (PGI2, prostaglandin I2), and suppresses the pathways involved in the endothelial cell activation and apoptosis.71 Hence, perturbation of endothelial cell function by inhibiting VEGF pathway may promote thromboembolism. Moreover, VEGF inhibition may also increase the expression of proinflammatory cytokines, causing damage and in situ thrombus formation.72 Additionally, VEGF is known to affect the expression of TF, the primary initiator of blood coagulation. TF and its distinct isoforms (alternatively spliced [as]TF, and full-length [fl]TF) can induce the expression of and interact with VEGF and its pro-/antiangiogenic isoforms, which in turn leads to modifications of essential biological processes, such as thrombogenicity, angiogenesis, cell proliferation, tissue growth, and migration. 73 These processes as well as the interaction of VEGF with TF-associated pathways play an essential role in cancer as well as in other diseases such as cardiovascular disease.74 Goldin-Lang et al75 showed that the expression of asTF and flTF was increased in NSCLC patients. This was associated with increased risk of thrombotic events in these patients.75
Limitations
Several limitations in our analysis need to be acknowledged. First, our study is a meta-analysis of published data, and we lack individual patient information. Therefore, intervening variables at the patient level are unavailable in the analysis. Second, toxicity data in randomized controlled trials have been reported to be suboptimal and variable as toxicity is usually not the primary outcome measure. Third, different anti-VEGF agents are included for analysis in the meta-analysis, which increases the clinical heterogeneity of the meta-analysis. Finally, the study might have a potential publication bias even though we detected no publication bias using the Begg and Egger tests.
Conclusion
Treatment with anti-VEGF agents in advanced NSCLC patients is associated with a significantly increased risk of high-grade ATEs compared to control, but not for VTEs. Based on our findings, patients with recent but controlled VTEs should not be denied the anti-VEGF treatment, and clinicians should pay more attention to the risk of high-grade ATEs associated with these drugs and must provide rigorous continuous monitoring.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175(3):409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol. 1998;153(4):1249–1256. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 5.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 6.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 7.Naito T, Seto T, Takeda K, et al. Phase II clinical trial of S-1 plus oral leucovorin in previously treated patients with non-small-cell lung cancer. Lung Cancer. 2014;86(3):339–343. doi: 10.1016/j.lungcan.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 9.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 10.Eisenreich A. Regulation of vascular function on posttranscriptional level. Thrombosis. 2013;2013:948765. doi: 10.1155/2013/948765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121(Pt 20):3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29(6 Suppl 16):10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 13.Qi WX, Shen Z, Tang LN, Yao Y. Risk of hypertension in cancer patients treated with aflibercept: a systematic review and meta-analysis. Clin Drug Investig. 2014;34(4):231–240. doi: 10.1007/s40261-014-0174-5. [DOI] [PubMed] [Google Scholar]
- 14.Qi WX, Shen Z, Lin F, et al. Incidence and risk of hypertension with vandetanib in cancer patients: a systematic review and meta-analysis of clinical trials. Br J Clin Pharmacol. 2013;75(4):919–930. doi: 10.1111/j.1365-2125.2012.04417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi WX, Lin F, Sun YJ, et al. Incidence and risk of hypertension with pazopanib in patients with cancer: a meta-analysis. Cancer Chemother Pharmacol. 2013;71(2):431–439. doi: 10.1007/s00280-012-2025-5. [DOI] [PubMed] [Google Scholar]
- 16.Ranpura V, Pulipati B, Chu D, Zhu X, Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens. 2010;23(5):460–468. doi: 10.1038/ajh.2010.25. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Wang Z, Zhao Y. Incidence and risk of hypertension with ramucirumab in cancer patients: a meta-analysis of published studies. Clin Drug Investig. 2015;35(4):221–228. doi: 10.1007/s40261-015-0272-z. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48(1):9–17. doi: 10.1080/02841860802314720. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49(2):186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Wu S, Kim C, Baer L, Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21(8):1381–1389. doi: 10.1681/ASN.2010020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang ZF, Wang T, Liu LH, Guo HQ. Risks of proteinuria associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a systematic review and meta-analysis. PLoS One. 2014;9(3):e90135. doi: 10.1371/journal.pone.0090135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choueiri TK, Mayer EL, Je Y, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29(6):632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 23.Qi WX, Shen Z, Tang LN, Yao Y. Congestive heart failure risk in cancer patients treated with vascular endothelial growth factor tyrosine kinase inhibitors: a systematic review and meta-analysis of 36 clinical trials. Br J Clin Pharmacol. 2014;78(4):748–762. doi: 10.1111/bcp.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghatalia P, Morgan CJ, Je Y, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94(2):228–237. doi: 10.1016/j.critrevonc.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Qi WX, Fu S, Zhang Q, Guo XM. Bevacizumab increases the risk of severe congestive heart failure in cancer patients: an up-to-date meta-analysis with a focus on different subgroups. Clin Drug Investig. 2014;34(10):681–690. doi: 10.1007/s40261-014-0222-1. [DOI] [PubMed] [Google Scholar]
- 26.Qi WX, Tang LN, Sun YJ, et al. Incidence and risk of hemorrhagic events with vascular endothelial growth factor receptor tyrosine-kinase inhibitors: an up-to-date meta-analysis of 27 randomized controlled trials. Ann Oncol. 2013;24(12):2943–2952. doi: 10.1093/annonc/mdt292. [DOI] [PubMed] [Google Scholar]
- 27.Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79(1–2):27–38. doi: 10.1159/000314980. [DOI] [PubMed] [Google Scholar]
- 28.Qi WX, Sun YJ, Tang LN, Shen Z, Yao Y. Risk of gastrointestinal perforation in cancer patients treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2014;89(3):394–403. doi: 10.1016/j.critrevonc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Qi WX, Shen Z, Tang LN, Yao Y. Bevacizumab increases the risk of gastrointestinal perforation in cancer patients: a meta-analysis with a focus on different subgroups. Eur J Clin Pharmacol. 2014;70(8):893–906. doi: 10.1007/s00228-014-1687-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Zhang J, Zhang L, Liu P, Xie Y, Zhou Q. Risk of gastrointestinal perforation in cancer patients receiving ramucirumab: a meta-analysis of randomized controlled trials. J Chemother. 2015 Jun 23; doi: 10.1179/1973947815Y.0000000053. Epub. [DOI] [PubMed] [Google Scholar]
- 31.Zuo PY, Chen XL, Liu YW, Xiao CL, Liu CY. Increased risk of cerebrovascular events in patients with cancer treated with bevacizumab: a meta-analysis. PLoS One. 2014;9(7):e102484. doi: 10.1371/journal.pone.0102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonpavde G, Je Y, Schutz F, et al. Venous thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2013;87(1):80–89. doi: 10.1016/j.critrevonc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Qi WX, Min DL, Shen Z, et al. Risk of venous thromboembolic events associated with VEGFR-TKIs: a systematic review and meta-analysis. Int J Cancer. 2013;132(12):2967–2974. doi: 10.1002/ijc.27979. [DOI] [PubMed] [Google Scholar]
- 34.Hurwitz HI, Saltz LB, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol. 2011;29(13):1757–1764. doi: 10.1200/JCO.2010.32.3220. [DOI] [PubMed] [Google Scholar]
- 35.Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28(13):2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 36.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300(19):2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 37.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99(16):1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 38.Qi WX, Shen Z, Tang LN, Yao Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit Rev Oncol Hematol. 2014;92(2):71–82. doi: 10.1016/j.critrevonc.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 41.NCI, Cancer Therapy Evaluation Program CTC v 2.0 and Common Terminology Criteria for Adverse Events Criteria V3.0 (CTCAE) [Assessed January 27, 2013]. Available from: http://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm.
- 42.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351–1375. doi: 10.1002/sim.1761. [DOI] [PubMed] [Google Scholar]
- 43.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 44.Zinner RG, Obasaju CK, Spigel DR, et al. PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2015;10(1):134–142. doi: 10.1097/JTO.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doebele RC, Spigel D, Tehfe M, et al. Phase 2, randomized, open-label study of ramucirumab in combination with first-line pemetrexed and platinum chemotherapy in patients with nonsquamous, advanced/metastatic non-small cell lung cancer. Cancer. 2015;121(6):883–892. doi: 10.1002/cncr.29132. [DOI] [PubMed] [Google Scholar]
- 46.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 47.Laurie SA, Solomon BJ, Seymour L, et al. Randomised, double-blind trial of carboplatin and paclitaxel with daily oral cediranib or placebo in patients with advanced non-small cell lung cancer: NCIC Clinical Trials Group study BR29. Eur J Cancer. 2014;50(4):706–712. doi: 10.1016/j.ejca.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 48.Gridelli C, Novello S, Zilembo N, et al. Phase II randomized study of vandetanib plus gemcitabine or gemcitabine plus placebo as first-line treatment of advanced non-small-cell lung cancer in elderly patients. J Thorac Oncol. 2014;9(5):733–737. doi: 10.1097/JTO.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 49.Belani CP, Yamamoto N, Bondarenko IM, et al. Randomized phase II study of pemetrexed/cisplatin with or without axitinib for non-squamous non-small-cell lung cancer. BMC Cancer. 2014;14:290. doi: 10.1186/1471-2407-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groen HJ, Socinski MA, Grossi F, et al. A randomized, double-blind, phase II study of erlotinib with or without sunitinib for the second-line treatment of metastatic non-small-cell lung cancer (NSCLC) Ann Oncol. 2013;24(9):2382–2389. doi: 10.1093/annonc/mdt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scagliotti GV, Vynnychenko I, Park K, et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol. 2012;30(23):2829–2836. doi: 10.1200/JCO.2011.41.4987. [DOI] [PubMed] [Google Scholar]
- 52.Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol. 2012;30(29):3640–3647. doi: 10.1200/JCO.2012.42.6932. [DOI] [PubMed] [Google Scholar]
- 53.Paz-Ares LG, Biesma B, Heigener D, et al. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol. 2012;30(25):3084–3092. doi: 10.1200/JCO.2011.39.7646. [DOI] [PubMed] [Google Scholar]
- 54.Niho S, Kunitoh H, Nokihara H, et al. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer. 2012;76(3):362–367. doi: 10.1016/j.lungcan.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Lee JS, Hirsh V, Park K, et al. Vandetanib versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR) J Clin Oncol. 2012;30(10):1114–1121. doi: 10.1200/JCO.2011.36.1709. [DOI] [PubMed] [Google Scholar]
- 56.Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(8):1059–1066. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 57.Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377(9780):1846–1854. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Boer RH, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2011;29(8):1067–1074. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 59.Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(11):1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 60.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 61.Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo- controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol. 2007;25(27):4270–4277. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- 62.Herbst RS, O’Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25(30):4743–4750. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 63.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 64.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 65.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 66.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–5382. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 67.Schutz FA, Je Y, Azzi GR, Nguyen PL, Choueiri TK. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22(6):1404–1412. doi: 10.1093/annonc/mdq587. [DOI] [PubMed] [Google Scholar]
- 68.Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49(3):287–297. doi: 10.3109/02841860903524396. [DOI] [PubMed] [Google Scholar]
- 69.Stone JR, Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium. 2002;9(4):231–238. doi: 10.1080/10623320214733. [DOI] [PubMed] [Google Scholar]
- 70.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96(12):1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49(3):568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 72.Hesser BA, Liang XH, Camenisch G, et al. Down syndrome critical region protein 1 (DSCR1), a novel VEGF target gene that regulates expression of inflammatory markers on activated endothelial cells. Blood. 2004;104(1):149–158. doi: 10.1182/blood-2004-01-0273. [DOI] [PubMed] [Google Scholar]
- 73.Eisenreich A, Boltzen U, Malz R, Schultheiss HP, Rauch U. Overexpression of alternatively spliced tissue factor induces the pro-angiogenic properties of murine cardiomyocytic HL-1 cells. Circ J. 2011;75(5):1235–1242. doi: 10.1253/circj.cj-10-0783. [DOI] [PubMed] [Google Scholar]
- 74.Leppert U, Eisenreich A. The role of tissue factor isoforms in cancer biology. Int J Cancer. 2015;137(3):497–503. doi: 10.1002/ijc.28959. [DOI] [PubMed] [Google Scholar]
- 75.Goldin-Lang P, Tran QV, Fichtner I, et al. Tissue factor expression pattern in human non-small cell lung cancer tissues indicate increased blood thrombogenicity and tumor metastasis. Oncol Rep. 2008;20(1):123–128. [PubMed] [Google Scholar]