Abstract
Purpose
Metastatic lymph node ratio (MLNR) was reported to be an important prognostic factor in several tumors. However, depth of primary tumor invasion is also important in cervical cancer prognostic analysis. In this study, the objective was to determine if MLNR can be used to define a high-risk category of patients with squamous cell carcinoma of the cervix (SCC). And we combined MLNR and depth of invasion to investigate whether prognosis of SCC can be predicted better.
Patients and methods
We performed a retrospective review of patients with SCC who underwent radical hysterectomy and pelvic lymphadenectomy at QiLu Hospital of Shandong University from January 2007 to December 2009. Prognostic factors for disease-free survival (DFS) and overall survival (OS) were identified by univariate and multivariate analyses.
Results
One hundred and ninety-eight patients met the inclusion criteria and were included in the analysis. By cut-point survival analysis, MLNR cutoff was designed as 0.2. On multivariate analysis, an MLNR >0.2 was associated with a worse OS (hazard ratio [HR] =2.560, 95% CI 1.275–5.143, P=0.008) and DFS (HR =2.404, 95% CI 1.202–4.809, P=0.013). Depth of invasion cutoff was designed as invasion >1/2 cervix wall and was associated with a worse OS (HR =1.806, 95% CI 1.063–3.070, P=0.029) and DFS (HR =1.900, 95% CI 1.101–3.279, P=0.021). In addition, subgroup analysis revealed significant difference in OS and DFS rates between different MLNR categories within the same depth of invasion category (P<0.05), however, not between different depth of invasion categories within the same MLNR category (P>0.05).
Conclusion
MLNR may be used as the independent prognostic parameter in patients with SCC. Combined MLNR and depth of invasion can predict both OS and DFS better in SCC than one factor. Besides, MLNR appears to be a better prognostic value than depth of invasion for SCC.
Keywords: squamous cell carcinoma of the cervix, metastatic lymph node ratio, radical hysterectomy, prognosis
Introduction
Cervical cancer is the fourth leading cause of cancer deaths, as well as the third most common cancer, found in women worldwide. Although the treatment has been improved, the overall survival (OS) of cervical cancer remains poor.1,2 Cervical cancer comprises 80% of squamous cell carcinoma of the cervix (SCC).3 Many clinical and pathologic factors, such as positive lymph node metastases, size of primary tumor, depth of primary tumor invasion, lymphovascular space invasion, close or positive margins, and parametrial involvement, have been found at increasing risk for recurrence. Among these risk factors, the presence of lymph node metastases is an independent prognostic factor for OS. It has been reported that cervical cancer patients with metastatic lymph nodes, compared to node-negative patients, have worse prognosis after operation.4 In addition, cervical cancer staging clinically depends on the International Federation of Gynecology and Obstetrics (FIGO) staging system of uterine cervical cancer, and the status of lymph node is not included in it. However, because lymph node metastasis is an important risk factor for recurrence, it is essential for an accurate knowledge of lymph node status to describe prognosis in SCC.
The status of lymph node metastasis is an important prognostic factor in many solid tumors. The metastatic lymph node ratio (MLNR), the ratio of positive nodes to the total number of total retrieved nodes, which is reported to show metastatic lymph node status more clearly, has been used to predict prognosis of breast, colorectal, esophageal, and gastric cancers.5–9 Recently, there has been interest in using MLNR as a prognostic tool to assess the comprehensive nature of lymphadenectomy in gynecologic malignancies, including cervical and endometrial cancers. Nevertheless, the study of MLNR in prognostic evaluation for patients with SCC is few and disputable.
The objective of this study was to investigate the correlation between MLNR and prognosis of SCC. We also aimed to determine if the depth of primary tumor invasion combined with MLNR could predict the survival of patients with SCC preferably.
Patients and methods
Patients
In this study, a series of 198 patients with cervical squamous cell carcinoma who underwent radical hysterectomy and pelvic lymph adenectomy at QiLu Hospital of Shandong University, from January 2007 to December 2009, were included in the analysis. The study was approved by the Institutional Review Board of the Department of Radiation Oncology, QiLu Hospital of Shandong University. The patients provided written informed consent to be included in this study. Patients were excluded when they received preoperative chemotherapy or radiotherapy, as well as they could not be contacted during follow-ups. Clinicopathological, tumor-specific, and adjuvant therapy data were obtained from the patients’ medical recording system of QiLu Hospital. Descriptive statistics were used to summarize the demographic and clinical characteristics of the patients. Patients’ histological documentation was assessed by two independent gynecologic pathologists.
Statistical analysis
The MLNR was defined as the ratio of metastatic lymph nodes to total lymph nodes harvested. The largest log-rank test statistic was applied to detect the optimal cutoff point for the number of the lymph node ratio as predictors of survival.10 Then, the patients were retrospectively divided into two groups according to MLNR for analysis. The depth of primary tumor invasion was measured from the most superficial epithelial–stromal interface of the adjacent intraepithelial process to the lower limits of invasion.11 The follow-up was completed in October 2014. Survival time was calculated from the date of surgery to the event or the last follow-up. Survival analyses were performed by Kaplan–Meier curves with log-rank tests for significance. Statistical analyses included univariate analysis and multivariate analysis. Univariable Cox regression analyses were performed using disease recurrence or death as the outcomes with a significance level of P<0.05. Multivariate analysis was carried out with a Cox proportional hazards model to evaluate MLNR and other prognostic factors with respect to disease-free survival (DFS) and overall survival (OS). Hazard ratios (HRs) and 95% CIs were calculated. A value of P<0.05 was considered as statistically significant. All statistical analyses were conducted using the SPSS statistical software package (Version 20.0; IBM Corporation, Armonk, NY, USA).
Results
One hundred and ninety-eight patients (100%) with SCC were included in the analysis. Some of them were found to have positive lymph node metastases at the time of radical hysterectomy and pelvic lymphadenectomy. Clinicopathologic characteristics were shown in Table 1. Median total lymph nodes harvested were 16 (range: 2–31 nodes).
Table 1.
Clinicopathologic characteristics
| Characteristics | n (range, %) |
|---|---|
| Median age, years (range) | 44.0 (39.0–52.0) |
| Pathologic type | |
| Squamous | 198 (100%) |
| Histological grade | |
| Well | 45 (22.7%) |
| Moderate | 61 (30.8%) |
| Poor | 92 (46.5%) |
| Size of primary tumor (cm) | |
| ≤4 | 161 (81.3%) |
| >4 | 37 (18.7%) |
| Number of metastatic lymph nodes | |
| 0 | 147 (74.2%) |
| 1 | 14 (7.1%) |
| 2–5 | 25 (12.6%) |
| >5 | 12 (6.1%) |
| Metastatic lymph node ratio | |
| <0.2 | 171 (86.4%) |
| ≥0.2 | 27 (13.6%) |
| Depth of primary tumor invasion | |
| ≤1/2 | 105 (53%) |
| >1/2 | 93 (47%) |
| Stage (FIGO) | |
| IA2 | 29 (14.6%) |
| IB1 | 67 (33.8%) |
| IB2 | 24 (12.1%) |
| IIA | 51 (25.8%) |
| IIB | 27 (13.6%) |
| Treatment regimen | |
| Surgery only | 84 (42.4%) |
| Surgery plus postoperative R | 57 (28.8%) |
| Surgery plus postoperative C | 26 (13.1%) |
| Surgery plus postoperative CRT | 31 (15.7%) |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; R, radiotherapy; C, chemotherapy; CRT, chemoradiotherapy.
The median DFS of this cohort was 56.7 months, and 5-year DFS rate was 55.5%. The median OS of this cohort was 61.3 months, and 5-year OS rate was 56.6%. Kaplan–Meier survival analysis revealed a correlation between MLNR and OS and DFS times. Patients with MLNR >0.2 had significantly worse DFS (P<0.001) and OS (P<0.001) than those with MLNR <0.2. Kaplan–Meier curves of DFS and OS based on MLNR are shown in Figures 1 and 2. Survival analysis showed significant difference in OS (P<0.001) and DFS (P<0.001) among different depth of invasion categories in these entire data. Kaplan–Meier curves of DFS and OS based on depth of primary tumor invasion are shown in Figures 3 and 4.
Figure 1.
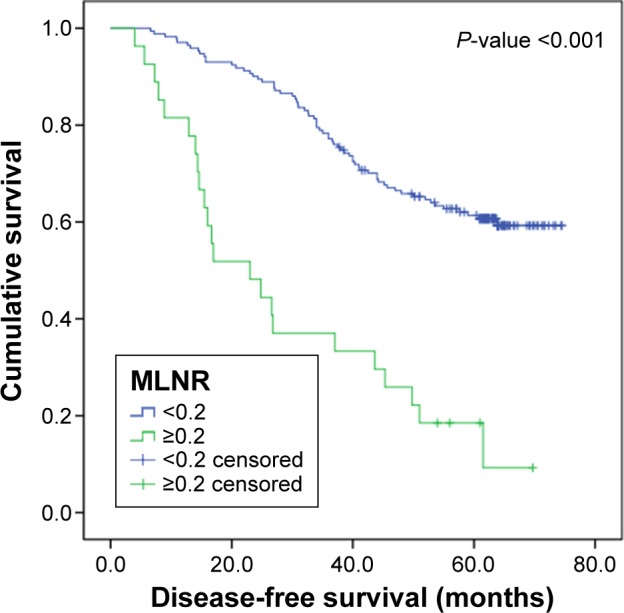
DFS curve for 198 patients with SCC according to the MLNR (MLNR <0.2 and MLNR ≥0.2).
Abbreviations: DFS, disease-free survival; SCC, squamous carcinoma of the cervix; MLNR, metastatic lymph node ratio.
Figure 2.
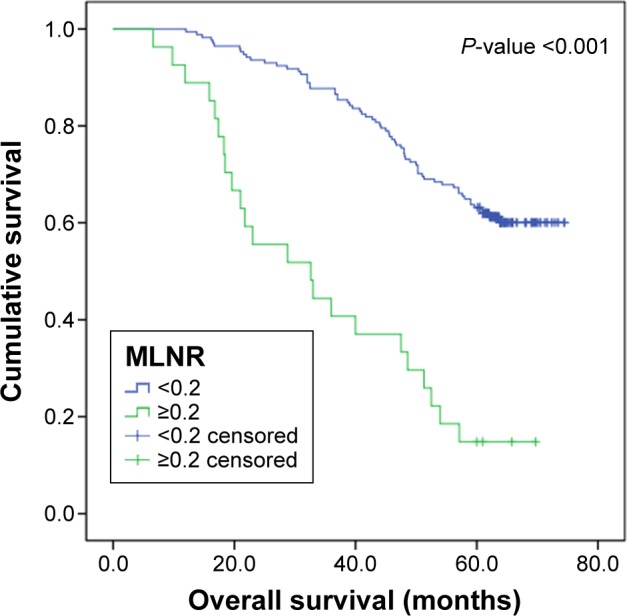
OS curve for 198 patients with SCC according to the MLNR (MLNR <0.2 and MLNR ≥0.2).
Abbreviations: OS, overall survival; SCC, squamous carcinoma of the cervix; MLNR, metastatic lymph node ratio.
Figure 3.
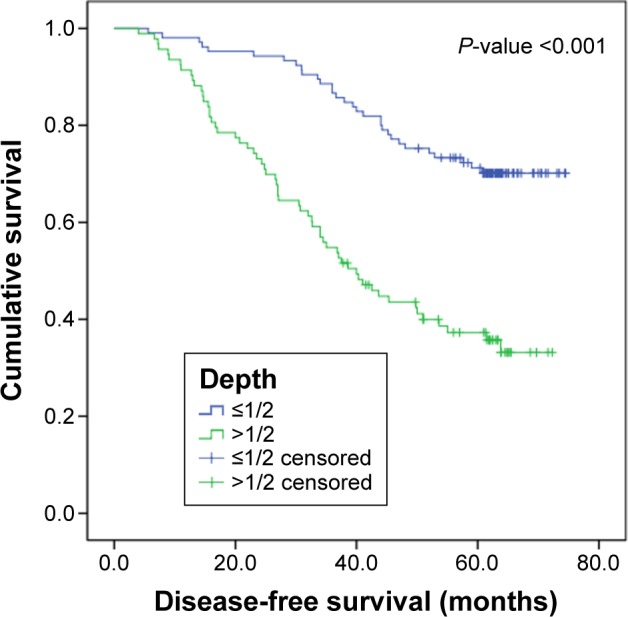
DFS curve for 198 patients with SCC according to the depth of primary tumor invasion (depth ≤1/2 and depth >1/2).
Note: Depth, depth of primary tumor invasion.
Abbreviations: DFS, disease-free survival; SCC, squamous carcinoma of the cervix.
Figure 4.
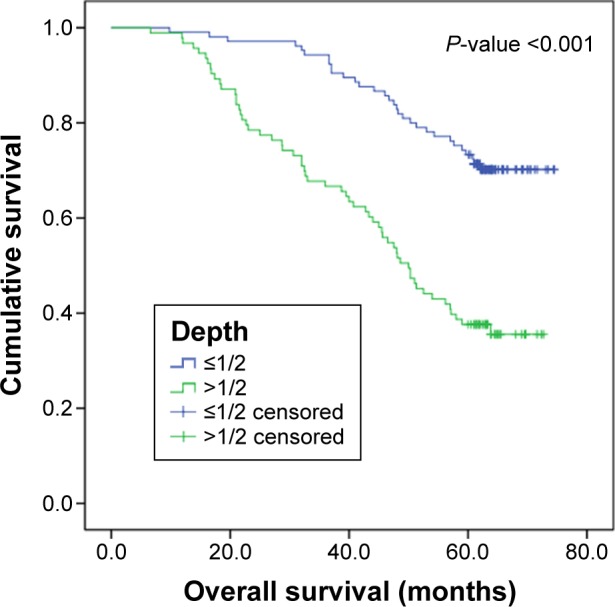
OS curve for 198 patients with SCC according to the depth of primary tumor invasion (depth ≤1/2 and depth >1/2).
Note: Depth, depth of primary tumor invasion.
Abbreviations: OS, overall survival; SCC, squamous carcinoma of the cervix.
Univariate survival analysis indicated that size of primary tumor, depth of primary tumor invasion, MLNR, and radiotherapy were potential prognostic factors correlated with OS and DFS (all P<0.05; Table 2). Multivariate analyses were performed using Cox proportional hazards regression. Multivariate analysis demonstrated that MLNR ≥0.2 was independent adverse prognostic factor for OS (HR =2.560, 95% CI 1.275–5.143, P=0.008) and DFS (HR =2.404, 95% CI 1.202–4.809, P=0.013). On multivariate analysis for OS, size of primary tumor >4 cm (HR =2.771, 95% CI 1.663–4.617, P<0.001), stage (HR 1.825, 95% CI 1.046–3.183, P=0.034), and depth of primary tumor invasion >1/2 (HR =1.806, 95% CI 1.063–3.070, P=0.029) were significantly associated with a worse OS. For DFS, size of primary tumor >4 cm (HR =2.854, 95% CI 1.714–4.753, P<0.001), stage (HR =1.832, 95% CI 1.052–3.188, P=0.032), and depth of primary tumor invasion >1/2 (HR =2.008, 95% CI 1.200–3.358, P=0.008) were independent prognostic factors on multivariate analysis. Besides, radiotherapy was also associated with improved OS (HR =0.463, 95% CI 0.292–0.735, P=0.001) and DFS (HR =0.485, 95% CI 0.306–0.768, P=0.002) (Table 3). The number of positive lymph nodes was not significantly associated with progression-free survival or OS on multivariate analysis.
Table 2.
Univariate analysis of factors associated with OS and DFS
| OS
|
DFS
|
|||||
|---|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | HR | 95% CI | |
| Histological grade | ||||||
| Well | 0.021 | 1.000 | Ref | 0.022 | 1.000 | Ref |
| Moderately | 0.069 | 1.868 | 0.953–3.661 | 0.079 | 1.829 | 0.933–3.585 |
| Poorly | 0.006 | 2.432 | 1.293–4.577 | 0.006 | 2.410 | 1.281–4.535 |
| Size of primary tumor | ||||||
| ≤4 cm | <0.001 | 1.000 | Ref | <0.001 | 1.000 | Ref |
| >4 cm | <0.001 | 3.622 | 2.319–5.659 | <0.001 | 3.782 | 2.416–5.919 |
| Depth of primary tumor invasion | ||||||
| ≤1/2 | <0.001 | 1.000 | Ref | <0.001 | 1.000 | Ref |
| >1/2 | <0.001 | 3.067 | 1.982–4.746 | <0.001 | 3.198 | 2.065–4.955 |
| Number of metastatic lymph nodes | ||||||
| 0 | <0.001 | 1.000 | Ref | <0.001 | 1.000 | Ref |
| 1 | 0.052 | 2.017 | 0.994–4.095 | 0.045 | 2.062 | 1.015–4.186 |
| 2–5 | 0.001 | 2.554 | 1.474–4.425 | 0.001 | 2.510 | 1.450–4.344 |
| >5 | <0.001 | 10.521 | 5.476–20.213 | <0.001 | 8.847 | 4.628–16.913 |
| Metastatic lymph node ratio | ||||||
| <0.2 | <0.001 | 1.000 | Ref | <0.001 | 1.000 | Ref |
| ≥0.2 | <0.001 | 4.313 | 2.668–6.974 | <0.001 | 4.223 | 2.614–6.820 |
| Stage (FIGO) | ||||||
| IA2 | 0.002 | 1.000 | Ref | 0.001 | 1.000 | Ref |
| IB1 | 0.187 | 1.872 | 0.746–4.471 | 0.194 | 1.810 | 0.740–4.430 |
| IB2 | 0.009 | 3.556 | 1.365–9.263 | 0.011 | 3.467 | 1.332–9.028 |
| IIA | 0.003 | 3.795 | 1.578–9.129 | 0.003 | 3.834 | 1.593–9.227 |
| IIB | 0.002 | 4.312 | 1.686–11.062 | 0.002 | 4.495 | 1.757–11.501 |
| Treatment regimen | ||||||
| Surgery only | 0.010 | 1.000 | Ref | 0.010 | 1.000 | Ref |
| Surgery plus postoperative R | 0.011 | 0.502 | 0.296–0.853 | 0.020 | 0.535 | 0.315–0.908 |
| Surgery plus postoperative C | 0.563 | 1.184 | 0.668–2.100 | 0.364 | 1.304 | 0.735–2.312 |
| Surgery plus postoperative CRT | 0.042 | 0.491 | 0.247–0.976 | 0.045 | 0.495 | 0.249–0.984 |
| Radiotherapy | ||||||
| No | 0.001 | 1.000 | Ref | 0.001 | 1.000 | Ref |
| Yes | 0.001 | 0.478 | 0.308–0.741 | 0.001 | 0.489 | 0.315–0.758 |
Abbreviations: OS, overall survival; DFS, disease-free survival; HR, hazard ratio; Ref, reference; FIGO, International Federation of Gynecology and Obstetrics; R, radiotherapy; C, chemotherapy; CRT, chemoradiotherapy.
Table 3.
Multivariate analysis of factors associated with OS and DFS
| OS
|
DFS
|
|||||
|---|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | HR | 95% CI | |
| Metastatic lymph node ratio | 0.008 | 2.560 | 1.275–5.143 | 0.013 | 2.404 | 1.202–4.809 |
| Positive nodes (n>0) | 0.799 | 1.089 | 0.565–2.097 | 0.995 | 1.019 | 0.527–1.970 |
| Depth of primary tumor invasion | 0.029 | 1.806 | 1.063–3.070 | 0.021 | 1.900 | 1.101–3.279 |
| Size of primary tumor | <0.001 | 2.771 | 1.663–4.617 | <0.001 | 2.854 | 1.714–4.753 |
| Stage (IIB) | 0.034 | 1.825 | 1.046–3.183 | 0.032 | 1.832 | 1.052–3.188 |
| Radiotherapy | 0.001 | 0.463 | 0.292–0.735 | 0.002 | 0.485 | 0.306–0.768 |
Abbreviations: OS, overall survival; DFS, disease-free survival; HR, hazard ratio.
Significant differences in OS and DFS rates were found between different MLNR categories within the same depth of invasion category (P<0.05; Table 4). However, different depth of invasion categories was not significantly associated with different OS or DFS within the same MLNR category, especially MLNR ≥0.2 (P>0.05; Table 5).
Table 4.
OS and DFS based on depth of primary tumor invasion category according to the MLNR category
| OS
|
DFS
|
|||||
|---|---|---|---|---|---|---|
| MLNR <0.2 (%) | MLNR ≥0.2 (%) | P-value | MLNR <0.2 (%) | MLNR ≥0.2 (%) | P-value | |
| Depth ≤1/2 | 59.6 | 35.7 | <0.001 | 57.0 | 20.4 | <0.001 |
| Depth >1/2 | 49.5 | 34.6 | 0.001 | 42.5 | 30.6 | 0.013 |
Note: Depth, depth of primary tumor invasion.
Abbreviations: OS, overall survival; DFS, disease-free survival; MLNR, metastatic lymph node ratio.
Table 5.
OS and DFS based on MLNR category according to the depth of primary tumor invasion category
| OS
|
DFS
|
|||||
|---|---|---|---|---|---|---|
| Depth ≤1/2 | Depth >1/2 | P-value | Depth ≤1/2 | Depth >1/2 | P-value | |
| MLNR <0.2 (%) | 59.6 | 49.5 | <0.001 | 57.0 | 42.5 | <0.001 |
| MLNR ≥0.2 (%) | 35.7 | 34.6 | 0.920 | 20.4 | 30.6 | 0.362 |
Note: Depth, depth of primary tumor invasion.
Abbreviations: OS, overall survival; DFS, disease-free survival; MLNR, metastatic lymph node ratio.
Discussion
In this study, we found that MLNR ≥0.2 was associated with a worse significant DFS and OS in patients with SCC than MLNR <0.2. This proved prognostic value of MLNR as an independent factor in patients with SCC who underwent radical hysterectomy and pelvic lymphadenectomy. Meanwhile, we found that other pathologic risk factors such as size of primary tumor >4 cm and depth of primary tumor invasion >1/2 were associated with a significantly worse DFS and OS. Consistent with historical data involving the use of adjuvant radiation in SCC, adjuvant radiotherapy in our cohort was associated with improved DFS and OS. Compared to the number of metastatic lymph nodes, MLNR showed better prognostic value in survival prediction for SCC.
Cervical cancer, according to histology, can be divided into squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma. SCC is mostly common in cervical cancer. In addition, cervical cancer remains a clinically staged disease, and lymph node status is not included in the FIGO staging system. However, several factors related to nodal status have been shown to affect the prognosis in SCC. These factors include the number of involved metastatic nodes, size of the metastatic deposits, and localization of the metastatic nodes in the pelvis.12–14 The presence of lymph node metastasis is an independent prognostic factor for prognostic value.4 Thus, more and more attention has been focused on accurate knowledge of lymph node status in SCC.
Then, MLNR, the ratio of positive nodes to the total number of total retrieved nodes, was defined to describe the lymph node status of patients more accurately and was used to estimate DFS and OS in SCC. In previous studies, the association between low MLNR and improvement in survival for patients has been proved in various malignancies, such as breast cancer, gastric cancer, and colorectal cancer.7,15–17 Meanwhile, MLNR was also proposed to be an independent prognostic factor in esophageal cancer, gallbladder cancer, and pancreatic cancer.18–20 There has been recent interest in using MLNR as a prognostic tool in gynecologic malignancies, for instance, endometrial cancers. Previous multicenter retrospective studies in endometrial cancer have found MLNR to be associated with worse OS.21 Furthermore, Chan et al22 found that increasing MLNR (≤10%, 10–50%, and ≥50%) was associated with survival decrease from 77.3% to 60.7% to 40.9% in endometrioid corpus cancer with lymph node metastasis and that MLNR was an important independent prognostic factor.
There have been a few previous retrospective studies evaluating the impact of MLNR in cervical cancer. Some studies examined whether involvement of a higher number of lymph nodes mate was associated with worse survival among patients with cervical cancer. A retrospective study of 95 patients who underwent radical hysterectomy and pelvic ± paraaortic lymphadenectomy for cervical cancer was conducted to assess predictors of survival. They found that progression-free survival and OS rates decreased with higher MLNR, and MLNR appears to be a useful tool to identify patients with worse prognosis in node-positive early-stage cervical cancer.23 In this study, our results showed a significant decrease in survival as the percentage of positive nodes increased. MLNR was pointed to predict survival more accurately than the number of lymph node metastases. Theoretically, the MLNR may obviate possible confounding effect related to the number of lymph nodes excised and the number of regional lymph nodes that varies in each individual and be more accurate to represent the status of pelvic lymph nodes.24 Nevertheless, the data of MLNR in prognostic evaluation for patients with cervical cancer are few and disputable, although Horn et al14 proved that the number of metastatic lymph node was an independent prognostic factor in cervical carcinomas. Similarly, Polterauer et al25 showed that MLNR was an independent prognostic parameter in patients with lymph node metastasis cervical cancer and superior to the number of metastatic lymph node in evaluation of OS.
Our study evaluated the prognostic value of MLNR in SCC. We divided the whole cases into two groups based on their MLNR for survival analysis. We found that patients with MLNR >0.2 had a worse OS and DFS than patients with MLNR <0.2. Our result was consistent with many previous studies, which clarified the significance of MLNR in OS and DFS of patients with cervical cancer. One of the selected criteria of our study was patients with cervical cancer not receiving any therapy before radical hysterectomy and pelvic ± paraaortic lymphadenectomy. We intended to investigate the long-term outcome of patients after radical hysterectomy, and preoperative therapy can affect the outcome after radical hysterectomy to a certain extent. Preoperative therapy helps in downstaging cervical cancer, thus enhancing the chance of curative resection and improving the prognosis of patients with cervical cancer.26,27 So we excluded patients who had preoperative therapy from our study to eliminate this effect. However, Chen et al28 retrospectively analyzed 588 patients with cervical cancer taking into account cisplatin-based chemotherapy before surgery. One hundred and seventy-two patients who received one to three cycles of cisplatin-based chemotherapy before surgery were included in this study. They found that the prognostic role of the ratio of metastatic lymph nodes was maintained regardless of patients receiving neoadjuvant chemoradiation or not.
Furthermore, as we all know it is difficult to demonstrate whether MLNR is better than the depth of the primary tumor invasion. We need to take the results in the exact context to evaluate. In this study, we have figured out that both MLNR and the depth of the primary invasion were associated with DFS and OS in SCC. But after comparison of survival rates between patient subsets in either of the depth of the primary tumor invasion classification, we found significant difference in OS and DFS rates between different MLNR categories within the same category of the depth of invasion but not between different depth of invasion categories within the same MLNR category. These results confirmed that MLNR showed better prognostic value than depth of the primary tumor invasion for SCC. Lymph node metastasis is closely related with the recurrence and survival of cervical squamous cell carcinoma. Therefore, closer relation was found between MLNR and survival rates.
In this study, MLNR ≥0.2 and depth of primary tumor >4 cm as well as size of primary tumor were associated with worse OS and DFS in SCC; adjuvant radiotherapy in our cohort was associated with improved DFS and OS. However, our study did not provide the evidence that the lower the MLNR gained, the better the prognosis achieved. It has been proved that postoperative complications occurred more frequently with increasing number of lymph nodes retrieved.29 Furthermore, Soliman et al emphasized that more extensive pelvic lymphadenectomies are associated with longer operating times, greater blood loss, and postoperative complications.30 That is to say, optimal MLNR can increase the likelihood of proper staging and improve patient outcome with minimal complications.
Our study is limited by its retrospective nature and its inherent bias. Moreover, the sample size was small since there were only 198 cases included. Large-sample clinical analysis is required for further study.
Conclusion
MLNR may be used as the independent prognostic parameter in patients with cervical squamous cell carcinoma who underwent radical hysterectomy and pelvic lymphadenectomy. In addition, combined MLNR and depth of primary tumor invasion can predict both OS and DFS in SCC better than one factor. Besides, MLNR appears to be a better prognostic value than depth of invasion for SCC. Further studies are needed to verify whether MLNR could be used to select the appropriate preventive measures for an individual with poor prognosis to improve outcomes.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Monk BJ, Tian C, Rose PG, Lanciano R. Which clinical/pathologic factors matter in the era of chemoradiation as treatment for locally advanced cervical carcinoma? Analysis of two Gynecologic Oncology Group (GOG) trials. Gynecol Oncol. 2007;105(2):427–433. doi: 10.1016/j.ygyno.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew A, George PS. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix-worldwide. Asian Pac J Cancer Prev. 2009;10(4):645–650. [PubMed] [Google Scholar]
- 4.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73(2):177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, Yang PJ, Zhong Y, Clancy TE, Lin MT, Wang J. Lymph node ratio-based staging system outperforms the seventh AJCC system for gastric cancer: validation analysis with National Taiwan University Hospital Cancer Registry. Am J Clin Oncol. 2014 Aug 7; doi: 10.1097/COC.0000000000000110. Epub. [DOI] [PubMed] [Google Scholar]
- 6.Shida A, Fujioka S, Kawamura M, et al. Prediction of lymph node metastasis in patients with submucosa-invading early gastric cancer. Anticancer Res. 2014;34(8):4471–4474. [PubMed] [Google Scholar]
- 7.Park J, Byun BH, Noh WC, et al. Lymph node to primary tumor SUV ratio by 18F-FDG PET/CT and the prediction of axillary lymph node metastases in breast cancer. Clin Nucl Med. 2014;39(4):e249–e253. doi: 10.1097/RLU.0b013e3182a75477. [DOI] [PubMed] [Google Scholar]
- 8.Jiang K, Zhu Y, Liu Y, et al. Lymph node ratio as an independent prognostic indicator in stage III colorectal cancer: especially for fewer than 12 lymph nodes examined. Tumour Biol. 2014;35(11):11685–11690. doi: 10.1007/s13277-014-2484-x. [DOI] [PubMed] [Google Scholar]
- 9.Chen SL, Steele SR, Eberhardt J, Zhu K, Bilchik A, Stojadinovic A. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg. 2011;253(1):82–87. doi: 10.1097/SLA.0b013e3181ffa780. [DOI] [PubMed] [Google Scholar]
- 10.Budczies J, Klauschen F, Sinn BV, et al. Cutoff finder: a comprehensive and straight forward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12):e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crum CP, Lee KR. Diagnostic Gynecologic and Obstetric Pathology. 1 ed. Philadelphia PA: Elsevier Saunders; 2006. [Google Scholar]
- 12.Stehman FB, Bundy BN, DiSaia PJ, Keys HM, Larson JE, Fowler WC. Carcinoma of the cervix treated with radiation therapy. I. A multi-variate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67(11):2776–2785. doi: 10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Aoki Y, Sasaki M, Watanabe M, et al. High risk group in node-positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecol Oncol. 2000;77(2):305–309. doi: 10.1006/gyno.2000.5788. [DOI] [PubMed] [Google Scholar]
- 14.Horn LC, Hentschel B, Galle D, Bilek K. Extracapsular extension of pelvic lymph node metastases is of prognostic value in carcinoma of the cervix uteri. Gynecol Oncol. 2008;108(1):63–67. doi: 10.1016/j.ygyno.2007.08.086. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzon L, Mercantini P, Ferri M, et al. Lymph-node ratio classification strongly correlates with cancer survivals of patients who underwent R0 resection for gastric cancer with more than 15 nodes harvested. Eur Surg Res. 2014;53(1–4):1–10. doi: 10.1159/000360937. [DOI] [PubMed] [Google Scholar]
- 16.Ke B, Song XN, Liu N, Zhang RP, Wang CL, Liang H. Prognostic value of the lymph node ratio in stage III gastric cancer patients undergoing radical resection. PLoS One. 2014;9(5):e96455. doi: 10.1371/journal.pone.0096455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YL, Wang CY, Wu CC, et al. Prognostic influences of lymph node ratio in major cancers of Taiwan: a longitudinal study from a single cancer center. J Cancer Res Clin Oncol. 2015;141(2):333–343. doi: 10.1007/s00432-014-1810-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu YP, Ma L, Wang SJ, et al. Prognostic value of lymph node metastases and lymph node ratio in esophageal squamous cell carcinoma. Eur J Surg Oncol. 2010;36(2):155–159. doi: 10.1016/j.ejso.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Birnbaum DJ, Viganò L, Russolillo N, Langella S, Ferrero A, Capussotti L. Lymph node metastases in patients undergoing surgery for a gallbladder cancer. Extension of the lymph node dissection and prognostic value of the lymph node ratio. Ann Surg Oncol. 2015;22(3):811–818. doi: 10.1245/s10434-014-4044-4. [DOI] [PubMed] [Google Scholar]
- 20.Smith BJ, Mezhir JJ. An interactive Bayesian model for prediction of lymph node ratio and survival in pancreatic cancer patients. J Am Med Inform Assoc. 2014;21(e2):e203–e211. doi: 10.1136/amiajnl-2013-002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polterauer S, Khalil S, Zivanovic O, et al. Prognostic value of lymph node ratio and clinicopathologic parameters in patients diagnosed with stage IIIC endometrial cancer. Obstet Gynecol. 2012;119(6):1210–1218. doi: 10.1097/AOG.0b013e318255060c. [DOI] [PubMed] [Google Scholar]
- 22.Chan JK, Kapp DS, Cheung MK, et al. The impact of the absolute number and ratio of positive lymph nodes on survival of endometrioid uterine cancer patients. Br J Cancer. 2007;97(5):605–611. doi: 10.1038/sj.bjc.6603898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming ND, Frumovitz M, Schmeler KM, et al. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol. 2015;136(1):48–53. doi: 10.1016/j.ygyno.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9(8):775–784. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 25.Polterauer S, Hefler L, Seebacher V, et al. The impact of lymph node density on survival of cervical cancer patients. Br J Cancer. 2010;103(5):613–616. doi: 10.1038/sj.bjc.6605801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo L, Liu X, Wang L, et al. Outcome of International Federation of Gynecology and Obstetrics Stage IIB Cervical Cancer From 2003 to 2012: An Evaluation of Treatments and Prognosis: A Retrospective Study. Int J Gynecol Cancer. 2015;25(5):910–918. doi: 10.1097/IGC.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Liang C, Zhang L, Huang S, Wu X. Clinical efficacy of modified preoperative neoadjuvant chemotherapy in the treatment of locally advanced (stage IB2 to IIB) cervical cancer: randomized study. Gynecol Oncol. 2008;110(3):308–315. doi: 10.1016/j.ygyno.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Zhang L, Tian J, Fu X, Ren X, Hao Q. Significance of the absolute number and ratio of metastatic lymph nodes in predicting postoperative survival for the International Federation of Gynecology and Obstetrics Stage IA2 to IIA Cervical Cancer. Int J Gynecol Cancer. 2013;23(1):157–163. doi: 10.1097/IGC.0b013e3182778bcf. [DOI] [PubMed] [Google Scholar]
- 29.Suprasert P, Charoenkwan K, Khunamornpong S. Pelvic node removal and disease-free survival in cervical cancer patients treated with radical hysterectomy and pelvic lymphadenectomy. Int J Gynaecol Obstet. 2012;116(1):43–46. doi: 10.1016/j.ijgo.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Soliman PT, Frumovitz M, Sun CC, et al. Radical hysterectomy: a comparison of surgical approaches after adoption of robotic surgery in gynecologic oncology. Gynecol Oncol. 2011;123(2):333–336. doi: 10.1016/j.ygyno.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


