Abstract
Objectives
Understanding the epidemiology of pain in patients on hemodialysis (HD) is crucial for further improvement in managing pain. The aim of this study was to systematically review available evidence on the prevalence and severity of pain in adult end-stage renal disease patients on chronic intermittent HD.
Materials and methods
We carried out a systematic review of the literature and developed a comprehensive search strategy based on search terms on pain and HD. We searched the databases MEDLINE, Scopus, PsycINFO, and CINAHL from the earliest date of each database to July 24, 2014. Manuscripts in all languages were taken into consideration. Two authors performed each step independently, and all disagreements were resolved after discussion with the third author. The quality of studies was estimated using the STROBE checklist and Cochrane risk-of-bias tool.
Results
We included 52 studies with 6,917 participants. The prevalence of acute and chronic pain in HD patients was up to 82% and 92%, respectively. A considerable number of patients suffered from severe pain. Various locations and causes of pain were described, with most of the studies reporting pain in general, pain related to arteriovenous access, headache, and musculoskeletal pain.
Conclusion
The findings of this systematic review indicate high prevalence of pain in HD patients and considerable gaps and limitations in the available evidence. Pain in this population should be recognized as a considerable health concern, and the nephrology community should promote pain management in HD patients as a clinical and research priority to improve patients’ quality of life and pain-related disability.
Keywords: pain, hemodialysis, prevalence, intensity, epidemiology
Introduction
The prevalence of chronic kidney disease is increasing worldwide, and is expected to continue increasing.1 There are five stages of chronic kidney disease, with end-stage renal disease (ESRD) its final stage. With worsening of their kidney disease, patients develop many complications associated with a high risk of comorbidities and mortality.2–4 Therefore, health care professionals caring for ESRD patients should aim not only to extend patients’ life span but also improve their quality of life.5 ESRD patients of all ages also have poor quality of life.6–8 One of the most important qualitative parameters for evaluating patients’ quality of life is bodily pain.9 Therefore, it is important to understand and relieve bodily pain in this population, in order to improve their quality of life and quality of care.
ESRD is defined as loss of renal function requiring renal replacement therapy with any form of chronic dialysis or transplantation or occasionally conservative management in the elderly or those with significant comorbidities.10–12 Incidentally, acute kidney injury requiring dialysis is not considered ESRD unless renal function fails to recover.13
Pain is common in ESRD patients.14 Based on data from surveys, when asked, up to 50%–60% of dialysis patients admit to feeling pain, often very severe and not effectively managed, although many will not mention this to their doctors at clinic visits.14,15 Pain is the major cause of depression, disturbed sleep patterns, impaired dialysis adequacy (if unable to endure full sessions), and likeliness of withdrawal from dialysis.16
Therefore, the objectives of this systematic review were to provide an updated analysis of epidemiological studies on pain in patients on hemodialysis (HD), to use both systematic and narrative methods to provide an objective summary of the literature, to assess study quality, and to provide recommendations for practice and research. Understanding the epidemiology of pain in patients on HD is crucial for further improvements in managing pain.
Materials and methods
A systematic review of literature was carried out in accordance with the guidelines of the Center for Reviews and Dissemination17 and the MOOSE study.18 A priori protocol of the systematic review was designed and registered in the PROSPERO database (registration number CRD42015024894).
Inclusion/exclusion criteria
We included all studies that reported epidemiology of pain in HD patients. Case reports and interventional studies reporting the effectiveness of interventions for the treatment of pain, as well as studies concerning peritoneal dialysis patients, continuous dialysis procedures, any other non-HD renal replacement therapy (eg, renal transplantation), plasmapheresis, children as participants and psychological studies concerning HD pain, were not included.
Search strategy and record screening
The databases MEDLINE, Scopus, PsycINFO, and CINAHL were searched from the earliest date of each database to July 24, 2014, with the help of a library information specialist. The complex search strategy was initially designed for MEDLINE (Table 1), and was then thoroughly adapted for each database. There were no publication type limits. Studies in any language were considered. The search results were exported to the EndNote X7.4 program (Thomson Reuters, New York, NY, USA), and duplicates were removed. Titles and abstracts of records retrieved by bibliographic search were initially screened by two authors (TB and EB) independently. Disagreements were resolved by the third author (LP). Once agreement was reached, the full text of each potentially eligible study was retrieved and analyzed by two authors independently. References and citations of included studies were downloaded from the Web of Science and screened by two authors independently (TB and EB) to identify additional citations that may have been missed through electronic database-search methods.
Table 1.
MEDLINE search strategy
| 1) (h?emodialy$ or h?emo$filtrat$ or ultrafiltrat$).tw |
| 2) exp Renal Dialysis/or exp Hemofiltration/ or exp Hemodiafiltration/or exp Ultrafiltration |
| 3) 1 or 2 |
| 4) (pain$ or dolo?r or hurt$ or ache or aching or pang? or $algia or $dynia or discomfort or nocicept$ or analge$ or an?esthe$ or pain?kill$ or antihyperalg$ or algesi$ or anguish$ or suffer$).tw |
| 5) exp Pain/or exp Nociception/or exp Analgesia/ or exp Anesthesia/or exp Anesthetics/or exp Analgesics/or exp Stress, Psychological/ or exp Stress, Physiological/ |
| 6) 4 or 5 |
| 7) 3 and 6 |
Data extraction
A data-extraction form was designed specifically for the study, and piloted and applied to all patients treated with HD without separation of individual subgroups. The following data were extracted: type of study, manuscript language, country, number of patients, age, sex, and race/ethnicity of patients, time of HD, type of pain studied, time recall for pain assessment, prevalence of pain, causes of pain, pain-intensity measuring tool, and pain-intensity results.
Assessment of study quality
STROBE checklist was used19 for assessing the quality of observational studies, where each of the 22 points of the STROBE criteria was assigned equal weight, and a total score was calculated. The Cochrane risk-of-bias tool was used20 for randomized controlled trials.
Results
Search results
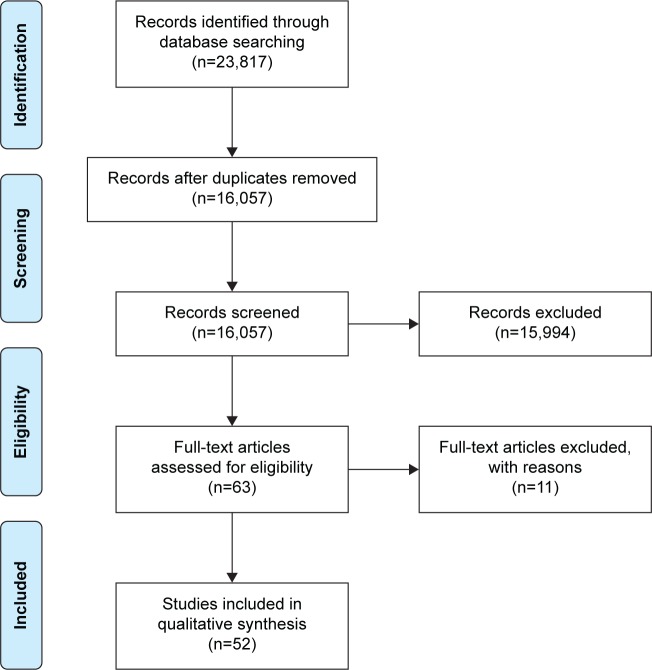
The database search yielded a total of 16,057 (MEDLINE 8,907, Scopus 6,639, CINAHL 425, PsycINFO 86) records. Based on the screening, the authors assessed that 63 full-text studies could contain relevant data. Analysis of full texts indicated that a total of 52 studies met our a priori inclusion criteria and were included in this review (Figure 1). Characteristics of included studies are presented in Table 2. The aim of the current review was to summarize epidemiological findings, and thus the summary of data using meta-analysis was not conducted. The findings were synthesized and described systematically.
Figure 1.
Study flow diagram.
Table 2.
Characteristics of included studies
| Study | Language | Country | n | Average age (years)a | Sex: % of males | Time on HDa | Race/ethnicity (%) | Painful condition studied |
|---|---|---|---|---|---|---|---|---|
| Aitken et al55 | English | Scotland | 449 | 60.5±0.72 | 56.6 | 5.19±2.3 years (range 0–27) (time from creation of AVF) | Not reported | AVF pain: acute pain on cannulation and chronic pain between dialysis sessions (most days for at least 4 weeks) |
| Alessandri et al78 | English | Italy | 24 | 58±4 (range 41–75) | 63 | HDH patients: 37±3 months HDH-free: 45±4 months |
Not reported | HDH (IHS 2004 criteria) |
| Al-Hilali et al33 | English | Kuwait | 40 | 48.6±14.9 | 38 | 34.5±2.7 months | Not reported | Intradialytic symptoms; baseline measure was used for this study |
| André et al21 | English | Brazil | SHD 36 DHD 5 |
SHD 36±15 DHD 41±12 |
SHD 64 DHD 100 |
SHD 57±36 months DHD 50±21 months |
B/W: SHD 19/17 DHD 2/3 |
Intradialysis symptoms during last 6 months, including headache |
| Antoniazzi et al39 | Portuguese | Brazil | 50 | 60% aged 20–49; 38% ≥50 | 56 | 42.4 months | Not reported | All patients had headaches strictly related to HD sessions (IHS 1988 criteria) |
| Antoniazzi et al48 | English | Brazil | 123 | 48.2 | 58 | 42.2 months | Not reported | All types of headache; HDH (IHS 1988 criteria) |
| Antoniazzi et al49 | English | Brazil | 132 | Not reported | Not reported | Not reported | Not reported | All types of headache; HDH (IHS 1988 criteria) |
| Bana et al72 | English | USA | 44 | 38±19 | 73 | Not reported | Not reported | All types of headache during HD |
| Barakzoy and Moss15 | English | USA | 143 | Not reported for all participants | 37 | Not reported | White: 83 Black: 17 |
Current pain at the time of survey |
| Barrett et al79 | English | Canada | 147 | 54±17 | 61 | 3.7±3.2 years | Not reported | Somatic symptoms in HD patients (time recall for pain unclear) |
| Binik et al61 | English | Canada | 80 (53 HD, 27 Tx) | 45.3 | 61 | Range 1.4–2.8 years | Not reported | All types of pain on and off dialysis (time frame for reporting pain not reported) |
| Bouattar et al36 | French | Morocco | 67 | 43.5±12.9 | 42 | 122±67 months (range 3–312) | Not reported | Chronic pain of all types (duration above 3 months) |
| Bourbonnais and Tousignant57 | English | Canada | 25 | 31–80 68% >61 |
60 | Range 3 months–30 years | Not reported | Pain either during HD or upon returning home |
| Braz and Duarte40 | Portuguese | Brazil | 162 | 47.3 | 58 | 44.1 months | Caucasian: 26 Non-Caucasian: 74 |
Musculoskeletal manifestations in HD patients (time recall for pain unclear) |
| Calls et al43 | Spanish | Spain | 27 | 66.7±13.6 | 48 | 66.4±61.3 months | Not reported | Intradialytic and chronic pain (definition of chronic pain not reported) |
| Caplin et al60 | English | UK | 508 | Median 64 | 54 | Median 37 months (range 18–64) | White: 45 Black: 29 South Asian: 20 Other: 2 |
Symptoms specifically experienced during the HD session |
| Caplin et al34 | English | UK | 519 | 63 (range 50–75) | 56 | Median 32 months (range 13–52.5) | White: 37 Black: 34 South Asian: 15 Chinese: 7 Other: 7 |
All symptoms specifically experienced during the HD session |
| Carreon et al80 | English | USA | 75 | 59±14 | 67 | 4.4±5.8 years | White: 64 | Bone/joint pain over the past 7 days |
| Chattopadhyay et al81 | English | UK | 15 | Median 45 (range 31–59) | 73 | 182 months (range 120–210) | Not reported | Shoulder-pain syndrome on long-term HD (time frame for reporting pain not reported) |
| Claxton et al62 | English | USA | 62 | 59 | 55 | 4±4 years | White: 26 African-American: 35 |
Physical symptoms over the prior week |
| Cristofolini et al50 | English | Brazil | 205 | 51.9±14.8 | 51 | Median (range) With LBP: 2.8 years (0–17) Without LBP: 1.5 years (0–29) |
Not reported | Chronic LBP (duration above 3 months) |
| Davison14 | English | Canada | 205 | 60±16.9 | 58 | 45.0±38.5 months | Caucasian: 78.5 Asian: 7.8 First Nations: 6.3 African: 1.5 Other: 5.9 |
Chronic pain of all types (duration above 3 months), pain in previous 24 hours, and current pain |
| Davison et al82 | English | Canada | 205 | 60±16.9 | 58 | 33.2±49.4 months Median 15.6 months |
Caucasian: 78.4 Asian: 7.8 First Nations: 6.4 African: 1.5 Other: 5.9 |
Chronic pain of all types (duration above 3 months) |
| Davison et al83 | English | Canada | 591 | 61.3±16.3 | 9.3 | 3.3±2.8 years | White: 72.6 Aboriginal: 11.3 Others: 16.1 |
Dialysis patients’ symptom burden, including pain (time frame for reporting pain not reported) |
| Jesus et al51 | English | Brazil | 177 | 46.22±14.3 | 59 | 32.9±22.8 months | Not reported | All types of headache; HDH (IHS 2004 criteria) |
| Armendáriz et al44 | Spanish | Spain | 35 | Not reported | Not reported | Not reported | Not reported | Evaluation of pain before, during, and after dialysis |
| Djurić et al41 | Serbian | Serbia | 143 | Range 20–86 | 65 | HDH patients: 57.2±60.4 months HDH-free: 49.2±44.1 months |
Not reported | All types of headache, HDH (IHS 2004 criteria) |
| El Harraqui et al37 | French | Morocco | 66 | 55.3±13.3 | 45 | 82±56 months (range 4–252) | Not reported | Pain’s epidemiology and characteristics in chronic HD, including AVF puncture pain |
| Elsurer et al84 | English | Turkey | 95 | 53.9±14.3 (range 20–82) | 52 | Median 48 months (range 15–300) | Not reported | Chronic bone pain (>3 months) |
| Er et al85 | English | Turkey | 95 | 51.5 (range 22–78) | Not reported | 5.21±3.89 years | Not reported | Pain in HD patients: instant (24 hours), acute (>3 days), and chronic (>3 months) |
| Fidan et al86 | English | Turkey | 50 | 56.04±16.77 | 48 | Median 24 months (range 2–276) | Not reported | All patients had MS symptoms at the time of the study |
| Fortina et al38 | Italian | Italy | 100 | 68±33 (range 33–88) | 73 | Not reported | Not reported | Chronic pain of all types (definition of chronic pain not reported) |
| Gamondi et al87 | English | Switzerland | 123 | Range 36–90 | 61 | 3.5 years (range 1–22) | Not reported | Pain during the past 4 weeks |
| Goksan et al88 | English | Turkey | 63 | 44±10 | 52 | 5±3.8 years | Not reported | All patients HDH (IHS 1988 and 2003 criteria) |
| Goksel et al59 | English | Turkey | 250 | 44.9±16.9 (range 15–75) | 42 | HDH patients: 47.7±42 months Control: 41.3±32 months |
Not reported | Prevalence of all types of headache; further studies only patients with headache experiences during HD sessions and in the 72 hours after a session (IHS 2004 criteria) |
| Golan et al52 | English | Israel | 100 | 64.5 | 45 | 40.4±42.0 months (range 3–204) | Arab: 31 Non-Arab: 69 |
Chronic pain (duration above 3 months) |
| Harris et al89 | English | USA | 128 | 57.3±13.8 (range 24–86) | 60.2 | 39.9±40.9 months | African-American: 91.4 White: 7 Asian or Pacific Islander: 1.6 |
Pain during HD treatment (excluding needle insertion) and at times between dialysis treatment (nondialysis days) during past month |
| Iacono et al90 | English | USA | 45 | 53 | Not reported | 4.2 years | Not reported | In-center HD chronic pain |
| Khan et al91 | English | Pakistan | 42 | Range 20–75 | 64 | Not reported | Not reported | Symptomatology in ESRD patients on HD |
| Kimmel et al92 | English | USA | 165 | 60.9 | 52 | 44 months | African-American: 33.3 White: 63 Asian: 1.2 Other: 2.5 |
Symptoms of patients on HD, including pain (time frame for reporting pain not reported) |
| Konishiike et al53 | English | Japan | 166 | 58.4 (range 35–78) | 57 | 9 years (range 0.3–22.7) | Not reported | Shoulder pain at the time of survey in long-term HD patients |
| Malaki et al54 | English | Iran | 26 | 35.7±21.9 | 23 | 34.8±18 months (range 9–75 months) | Not reported | Leg pain present at least for three to four times per week, persisting for several weeks |
| Mercadante et al32 | English | Italy | 95 | 63.4±14.4 | 41.8 | 5±4.5 years | Not reported | Chronic pain (IASP 1986 criteria) |
| Milinković et al58 | English | Serbia | 318 | 55.19±15.5 | 63 | HDH patients: 49.43±39.56 months HDH-free: 55.63±53.2 |
Not reported | All types of headache; HDH (IHS 2004 criteria) |
| Nikić et al42 | Serbian | Serbia | 126 | 58 (range 20–79) | 62 | HDH patients: 73.34±63.29 months HDH-free: 59.01±53.17 |
Not reported | All types of headache; HDH (IHS 2004 criteria) |
| Parfrey et al93 | English | Canada | 77 | 52±2 | 53 | 3.5±0.4 years | Not reported | Nonspecific symptoms currently experienced in dialysis patients, including headache |
| Rodriguez Calero et al46 | Spanish | Spain | 38 | 65±14.8 | 47 | 65.13±78.5 months | Not reported | Current intradialytic pain |
| Rodriguez Calero et al45 | Spanish | Spain | 32 | 66.7±13.6 | 53 | Pain patients: 61.5±60.3 months Pain-free: 47.8±43.8 months |
Not reported | Chronic pain of all types (definition of chronic pain not reported) |
| Rodriguez Calero et al47 | Spanish | Spain | 27 | 66.7±13.6 | 48 | Pain patients: 60.5±58.5 Pain-free: 45.7±44.2 months |
Not reported | Chronic and intradialytic pain (definition of chronic pain not reported) |
| Shayamsunder et al94 | English | USA | 156 | Not reported | Not reported | Not reported | Not reported | Pain in the HD population, including pain on needle insertion (no details about the type of cannulation or pain reporting period) |
| Vergne et al35 | French | France | 66 | 66.8±13.1 | 73 | 47 months, median 25 months | Not reported | AV-access puncture pain (rope-ladder technique) |
| Weisbord et al95 | English | USA | 179 | 61.9 | 62 | 4±4.1 years | Black: 48 White: 51 Asian: 1 |
Symptoms, including pain, in previous 7 days |
Note:
Average age and time on hemodialysis presented as mean or mean ± SD, unless indicated differently.
Abbreviations: AV, arteriovenous; AVF, arteriovenous fistula; B/W, black/white ratio; DHD, daily hemodialysis; ESRD, end-stage renal disease; HD, hemodialysis; HDH, HD headache; IASP, International Association for the Study of Pain; IHS, International Headache Society; LBP, low-back pain; MS, musculoskeletal; SD, standard deviation; SHD, standard HD; Tx, transplant.
Excluded studies
Eleven studies were excluded for various reasons: reporting headache and cramps as number and percentage of sessions with the clinical event without specifying number of affected patients;21 reporting prevalence of various handicaps of HD patients, but no information about pain prevalence;22 not presenting results separately for patients on different types of dialysis;23 seven were interventional studies with no data on baseline prevalence of pain;24–30 and one was a case report.31
Included studies
A total of 52 studies with 6,917 patients (range 15–591) were included. Studies were grouped according to the type of pain investigated, including prevalence of pain in general, prevalence by location, including pain related to arteriovenous (AV) access, headache, limb pain, chest pain, abdominal pain, and “other and procedural” pain, as well as such causes of pain as musculoskeletal pain, ischemic pain, and neuropathic pain.
A total of 49 studies were observational, including one letter to the editor, which contained original data that were extracted.32 Data about baseline pain prevalence were extracted from three interventional studies as well.33–35 All studies were published in peer-reviewed journals except one, which was freely available on an institution’s Web site.35
Included studies were published between 1972 and 2014, and 39 of them were published in English. Studies were published in French,35–37 Italian,38 Portuguese,39,40 Serbian,41,42 and Spanish.43–47 Studies were conducted mainly in Europe and North America (Table 2), while the remaining studies were conducted in South America,21,25,39,48–51 Asia,33,52–54 and Africa.24,37 Distribution of age, ratio of male to female, and time on HD were very heterogeneous between the studies (Table 2). Race/ethnicity of patients was reported by only 13 studies (Table 2).
Prevalence and severity of pain in general
Nineteen studies with 2,377 patients (range 27–591) that were included examined the general prevalence of pain, both chronic and acute, in the analyzed cohorts of HD patients (Table 3). The reported prevalence of chronic pain ranged from 33% to 82%, while the prevalence of acute pain (current pain, intradialytic pain, pain during the past 4 weeks) ranged from 21% to 92% (Table 3).
Table 3.
Characteristics of studies reporting general pain in HD patients
| Study | Painful condition studied | Prevalence of general pain (%) | Pain-intensity measurement tool | Pain-intensity results |
|---|---|---|---|---|
| Barakzoy and Moss15 | Current pain of all types at the time of survey | 54 | SF-MPQ (VAS) | Current pain Moderate or severe: 86% Initial pain Severe: 76% |
| Bouattar et al36 | Chronic pain of all types (duration above 3 months) | 50.7 | Descriptive verbal scale | Chronic pain Low: 3% Moderate: 41% Severe: 44 Very severe: 12% |
| Calls et al43 | Intradialytic and chronic pain of all types (definition of chronic pain not reported) | During the session: 92.5 Off-session: 77.7 |
VAS, MPQ, PMI | Intradialytic vs chronic pain VAS: 3.28±2.22 vs 2.67±2.13 PMI: 0.81±0.76 vs −0.12±0.94) MPQ: similar in both situations |
| Davison14 | Chronic pain of all types, (duration above 3 months), pain during past 24 hours, current pain | 50 | BPI, MPQ | Previous 24 hours Mild (0–4): 17.5 Moderate (5–6): 27.2 Severe (7–10): 55.3 (BPI 7.03±2.40) Current Mild: 44.7 Moderate: 28.2 Severe: 55.3 (BPI 4.99±2.96) MPQ PRI 22.2±13.8, PPI 2.6±1.6, NWC 8.7±5.2 |
| Davison et al82 | Chronic pain of all types (duration above 3 months) | 50.2 | BPI | Moderate or severe: 41.4% |
| Davison et al83 | Dialysis patients’ symptom burden, including pain (time frame for reporting pain not reported) | 72.4 | mESAS | Moderate or severe: 46.5% |
| Armendáriz et al44 Er et al85 | Evaluation of pain before, during, and after dialysis Instant pain (24 hours), acute (>3 days), and chronic (>3 months) |
71.4 63.1 A: 26 C: 33 |
VAS MMPQ |
VAS results not reported Not specified whether instant or chronic Mild: 28.3 Disturbing: 23.3 Severe: 31.7 Very severe: 10 Intolerable: 6.7 |
| Gamondi et al87 | Pain during the past 4 weeks | 66 | VAS and BPI | Previous 4 weeks Mild: 17% Moderate: 21% Intense: 61% At the time of interview No pain: 68% Mild: 16% Moderate: 8.5% Intense: 6% |
| Golan et al52 | Chronic pain (duration above 3 months) | 51 | BPI | Chronic pain Mild: 49% Moderate: 31.4% Severe: 19.6% |
| Harris et al89 | Pain during HD treatment (excluding needle insertion) and at times between dialysis treatment (nondialysis days) during past months | HD: 30.7 Off HD: 44.1 |
Modified MPQ | During dialysis: 2.1±3.5 Highest level of pain: 3.9% Between dialyses: 3.1±3.8 Highest level of pain: 7.9% |
| Kimmel et al92 | Symptoms of patients on HD, including pain (time frame for reporting pain not reported) | 21 | Not measured | NA |
| Mercadante et al32 | Chronic pain (IASP 1986 criteria) | 48 | NRS | 5.59±1.63 (95% CI 5.12–6.03) |
| Rodriguez Calero et al46 | Intradialysis pain of all types | 92 | VAS, MPQ, PMI | Intradialysis pain: 3.31±2.22 |
| Rodriguez Calero et al45 | Chronic pain of all types (definition of chronic pain not reported) | 82 | VAS, MPQ, PMI, BPI | Chronic pain: 2.41±2.13 Severe (VAS >7.5): no case reported |
| Rodriguez Calero et al47 | Chronic and intradialytic pain of all types (definition of chronic pain not reported) | ID: 92 C: 78 |
VAS, MPQ, PMI, BPI | ID: 3.28±2.22 C: 2.67±2.13 |
| Shayamsunder et al94 | Pain in ESRD patients treated with chronic HD | During HD: 30 On non-HD days: 44 |
Not measured | NA |
| Iacono et al90 | In-center HD chronic pain | 60 | 5-point Likert scale | Mean 3.3 |
| Fortina et al38 | Chronic pain of all types (definition of chronic pain not reported) | 37 | VAS | Not reported for general pain |
Abbreviations: A, acute; BPI, Brief Pain Inventory; C, chronic; CI, confidence interval; ESRD, end-stage renal disease; HD, hemodialysis; IASP, International Association for the Study of Pain; ID, intradialytic pain; mESAS, modified Edmonton Symptom Assessment System; MPQ, McGill Pain Questionnaire; MMPQ, McGill–Melzack Pain Questionnaire; NA, not applicable; NRS, numeric rating scale; NWC, number of words chosen; PMI, Pain Management Index; PPI, present pain intensity; PRI, Pain Rating Index; SF-MPQ, short-form McGill Pain Questionnaire; VAS, visual analog scale.
Rodriguez Calero et al showed very high prevalence of intradialytic pain, with only 8% of patients reporting no pain at all. Analgesics were prescribed to 18% of patients, and the Pain Management Index (PMI) showed clear undertreatment of pain, which was more accentuated among patients who reported more intense pain.46
Characteristics of pain were reported by only a few studies. Bouattar et al reported that patients described their chronic pain as continuous (21%), frequent (18%), intermittent (47%), and rare (15%).36 Severity of pain in general was reported with various pain scales in all studies except one. The pain-assessment scales used in studies reporting general pain were the short-form McGill Pain Questionnaire (MPQ), visual analog scale (VAS), PMI, Brief Pain Inventory, modified Edmonton Symptom Assessment System, and the McGill–Melzack Pain Questionnaire (Table 3). While the average reported pain intensity tended to be low, multiple studies indicated high prevalence of patients with moderate or severe pain (Table 3). Reported prevalence of severe/intensive pain ranged from 0%45 to 76%.15
Prevalence and severity of AV-access pain
In the included studies, pain related to AV access was described in various terms, relating not only to AV fistula (AVF), which is why the broader term “AV access” is used herein to describe pain reported in these studies.
Ten studies with a total of 1,028 patients (range 25–449) analyzed prevalence and/or severity of pain related to AV access (Table 4). If available, type of AV access was extracted (Table 4). Only two studies provided details about AV access. Aitken et al55 indicated that there was a trend toward more severe pain with rope-ladder cannulation (27.7%) compared to buttonhole cannulation (18.2%); however, this difference did not reach statistical significance (P=0.09). In Vergne et al, some patients had rope-ladder cannulation of AVF, but some also had a graft.35
Table 4.
Type of pain studied, prevalence and assessment tools for AV-access pain
| Study | Timing and type of AV-access pain studied | Type of cannulation | Prevalence of AV-access pain (%) | Pain-assessment tool for AV-access pain | Pain-intensity results |
|---|---|---|---|---|---|
| Aitken et al55 | Both acute pain (on cannulation) and chronic pain (between dialysis sessions, most days for at least 4 weeks) related to AVF | Rope-ladder, buttonhole | Any pain AP 25 CP 50 Severe (VAS >5) AP 24 CP 3.2 |
VAS, BPI, and MPQ | Pain on cannulation Median 3 (IQR 0.5–4.5) Severe pain 24.4% Between dialyses 0 (IQR 0–1) Severe pain 3.2% |
| Binik et al61 | Acute (current) pain related to fistula | Not reported | On HD 15 Dialysis pain reported by post-Tx 7.4 |
Structured interview and MPQ | On HD 3.6 |
| Bourbonnais and Tousignant57 | Acute needling pain from both insertion of needle and removal | Not reported | 12 | Not measured | NA |
| Calls et al43 | Both acute intradialytic and chronic pain related to vascular access Definition of chronic pain not reported |
Not reported | AP 21.9 CP 21.8 |
Not measured | NA |
| El Harraqui et al37 | Chronic pain associated with vascular access Chronic pain defined as pain of >3 months’ duration |
Not reported | 15.1 | Not measured | NA |
| Golan et al52 | Pain related to repeated dialysis access cannulation as a form of chronic pain (needle insertion in patients with a fistula or graft) | Not reported | 80.2 | Not measured | NA |
| Rodriguez Calero et al46 | Acute intradialytic assessment of pain related to vascular access | Not reported | 21 | Not measured | NA |
| Rodriguez Calero et al47 | Chronic pain related to vascular access | Not reported | 37 | Not measured | NA |
| Shayamsunder et al94 | Pain in ESRD patients treated with chronic HD, including acute intradialytic pain on needle insertion | Not reported | 78.4 | Not measured | NA |
| Vergne et al35 | Acute AVF (natural or PTFE graft) puncture pain | Rope-ladder | 57.5 | NRS | Low pain 79% Moderate 13% Severe 8% |
Abbreviations: AP, acute cannulation pain; AV, arteriovenous; AVF, arteriovenous fistula; BPI, brief pain inventory; CP, chronic cannulation pain; ESRD, end-stage renal disease; HD, hemodialysis; IQR, interquartile range; MPQ, McGill Pain Questionnaire; NA, not applicable; NRS, numeric rating scale; PTFE, polytetrafluoroethylene; Tx, transplant; VAS, visual analog scale.
The majority of studies were observational, with two interventional studies reporting baseline pain intensity.35,56 The prevalence of acute and chronic pain or both was studied, ranging from 12%57 to 80.2%52 (Table 4). Severity of pain was not always reported. Different pain-assessment scales were used, including the MPQ, VAS, Brief Pain Inventory, PMI, and numeric rating scale.
Prevalence and severity of headache
A total of 24 studies with 3,444 patients (range 24–519) analyzed prevalence and/or severity of headache in HD patients. Some studies looked for headache as part of the overall symptom burden in HD patients; a subset of studies analyzed different types of headache in HD patients, while some analyzed specifically HD headache (HDH) (Table 5). Two studies were interventional,33,34 and prevalence of headache before the intervention was reported for those studies. The other studies were observational.
Table 5.
Studies of headache prevalence
| Study | Timing and type of headache studied | Prevalence of headache (%) | Pain-assessment tool for headache | Pain-intensity results |
|---|---|---|---|---|
| Alessandri et al78 | HDH (IHS 2004 criteria) | HDH: 50 | Not described in methods | Most patients had mild or moderate severity of pain |
| Al-Hilali et al33 | Intradialytic symptoms, including headache; baseline measure was used for this study | H: 37.5 (before the intervention) | Not measured | NA |
| André et al21 | Intradialysis symptoms during last 6 months, including headache | H: 13 | Not measured | NA |
| Antoniazzi et al39 | All patients had headaches strictly related to HD sessions (IHS 1988 criteria) | H: 70.7 HDH: 68 (in the second half of HD: 86) |
Not measured | NA |
| Antoniazzi et al48 | All types of headache; HDH (IHS 1988 criteria) | H: 70.7 (during HD: 57.5) HDH: 28 |
Not measured | NA |
| Antoniazzi et al49 | All types of headache; HDH (IHS 1988 criteria) | HDH: 21.2 | Not measured | NA |
| Bana et al72 | All types of headache during HD | H: 70 | Descriptive measure of headache severity | An average of 6 headache units per dialysis |
| Barrett et al79 | Somatic symptoms in HD patients, including headache (time recall for pain unclear) | H: 45 | Perception of symptom severity | Mentioned in methods, but results not reported |
| Binik et al61 | All types of pain on and off dialysis, including headache | On HD: 62.3 Off HD: 43 Reported by post-Tx: 66.7 |
MPQ | On HD 6.2 Off HD 7 |
| Bouattar et al36 | Chronic pain of all types, including headache | H: 11.8 | Descriptive verbal scale | Low 3% Moderate 41% Severe 44% Very severe 12% |
| Calls et al43 | Intradialytic and chronic pain, including headache | IH: 31.5 CH: 6.2 |
Not measured | NA |
| Caplin et al60 | Symptoms specifically experienced during the HD session, including headache | IH: 53.6 | Not measured | NA |
| Caplin et al34 | All symptoms specifically experienced during the HD session | IH: 56 | Not measured | NA |
| Jesus et al51 | All types of headache; HDH (IHS 2004 criteria) | H: 76.1 HDH: 6.7 |
VAS | Moderate 63.6% VAS ≥8 (severe) 36.4% |
| Djurić et al41 | All types of headache; HDH (IHS 2004 criteria) | H: 19 HDH: 5.6 |
VAS | VAS <8 (severe) 87.5% |
| Gamondi et al87 | Pain duration, intensity, perception, and localization in patients reporting pain during the 4 weeks before the interview | H: 31 | Not measured | NA |
| Goksan et al88 | All patients HDH (IHS 1988 and 2003 criteria) | HDH: 48 | Descriptive scale for intensity of headache | Moderate 73% Severe 27% Very severe 87% |
| Goksel et al59 | Prevalence of all types of headache; further studies, only patients with headache experiences during HD sessions and in the 72 hours after a session; IHS 2004 criteria | HDH: 30 | VAS | Mean ± SD 6.06±2.4 |
| Golan et al52 | Chronic pain (lasting 3 months and more), including headache | CH: 54.9 | Not measured | NA |
| Milinković et al58 | All types of headache; HDH (IHS 2004 criteria) | HDH: 6.6 | VAS | Severe (VAS >8) 52% |
| Nikić et al42 | All types of headache; HDH (IHS 2004 criteria) | H: 32.5 HDH: 34 |
VAS | Mild 14.3% Moderate 50% Severe (VAS >8) 35.7% |
| Parfrey et al93 | Nonspecific symptoms experienced in dialysis patients, including headache | H: 41 (during or just after HD 56) | Not reported for HD patients separately | NA |
| Rodriguez Calero et al47 | Intradialytic and chronic pain, including headache | IH: 31.5 CH: 6.2 |
Not measured | NA |
| Weisbord et al95 | Physical and emotional symptoms in maintenance-HD patients, including headache | H: 19 | DSI | Mean 3.03 |
Abbreviations: CH, chronic headache; DSI, Dialysis Symptom Index; H, headache; HD, hemodialysis; HDH, HD headache; IH, intradialytic headache; IHS, International Headache Society; MPQ, McGill Pain Questionnaire; NA, not applicable; Tx, transplant; VAS, visual analog scale.
Reported prevalence rates of headache in HD patients varied considerably. For presentation of these results, it is important to emphasize that some studies reported prevalence of all headaches, while others reported specifically prevalence of HDH according to International Headache Society (IHS) diagnostic criteria. Some studies reported both. The reported prevalence of all kinds of headaches ranged from 11.8%36 to 76.1%.51 The reported prevalence of HDH, diagnosed according to the 1988 or 2004 IHS criteria, ranged from 6.6%58 to 68%39 (Table 5).
Severity of headache was assessed with various scales, including descriptive scales, the MPQ, and the VAS. Different severity of headache was observed in the included studies, indicating that headache pain can be very debilitating. Analyzing types of headache, Goksel et al reported average pain intensity on the VAS as 6.06±2.4.59 The prevalence of severe pain ranged from 36%51 to 88%41 (Table 5).
Prevalence of limb pain
Six studies with 422 patients (range 26–205) reported the prevalence of lower- and/or upper-limb pain without analyzing causes of that pain. Studied pain was chronic, acute, or both (Table 6). The reported prevalence of chronic lower-leg pain was very similar in the three studies examining this type of pain, while the prevalence of lower-leg pain lasting several weeks was 42% and current intradialytic pain reported by 34% of patients in the three studies that examined it. Chronic upper-limb pain prevalence was more heterogeneous (Table 6). A sixth study reported the prevalence of chronic peripheral neuropathy as 13% without specifying the affected body part.14 None of the studies reported pain severity of limb pain.
Table 6.
Prevalence of limb pain
| Study | Painful condition studied | Prevalence of limb pain (%) |
|---|---|---|
| Bouattar et al36 | Chronic pain of all types (duration above 3 months), including upper- and lower-limb pain | UL 74 LL 62 |
| Calls et al43 | Intradialytic and chronic pain (definition of chronic pain not reported), including upper- and lower-limb pain | LL: ID 34, C 63 UL: ID 11, C 19 |
| Davison14 | Chronic pain (duration above 3 months), including painful PN | PN 13 |
| Malaki et al54 | Leg pain present at least three to four times per week, persisting for several weeks | LL 42 |
| Rodriguez Calero et al46 | Current intradialytic pain | LL 34 |
| Rodriguez Calero et al45 | Chronic pain of all types (definition of chronic pain not reported), including lower-limb pain | LL 63 |
| Rodriguez Calero et al47 | Intradialytic and chronic pain (definition of chronic reported), including upper- and lower-limb pain pain not | UL: ID 10.5, C 18.7 LL: ID 34.2, C 62.5 |
Abbreviations: C, chronic; ID, intradialytic; LL, lower limb; PN, peripheral neuropathy; UL, upper limb.
Prevalence and severity of musculoskeletal pain
A total of 21 studies with 2,778 patients (range 15–519) reported the prevalence of musculoskeletal pain in HD patients (Table 7). The studies reported different types of pain, ranging from carpal tunnel syndrome to muscle cramps. Data on pain severity for musculoskeletal pain indicated that such pain can be considerable (Table 7).
Table 7.
Studies of MS-pain prevalence and intensity
| Study | Timing and type of MS pain studied | Prevalence of MS pain (%) | Pain-assessment tool for MS pain | Pain-intensity results |
|---|---|---|---|---|
| Al-Hilali et al33 | Intradialytic symptoms, including muscle cramps; baseline measure was used for this study | Cramps: 23 | Not measured | NA |
| André et al21 | Intradialysis symptoms during last 6 months, including cramps | Cramps: 18 (type of cramps not specified) | Not measured | NA |
| Barrett et al79 | Somatic symptoms in HD patients, including joint pain and cramps; time recall for pain not reported | Joint pain: 43 Cramps: 53 (type of cramps not specified) |
Not measured | NA |
| Binik et al61 | All types of pain on and off dialysis, including cramps and bone pain | On HD: cramps 81, bone 11 Off HD: cramps 62, bone 15, back 5.7 Post-Tx reporting dialysis pain: cramps 51.9, bone 3.7 |
MPQ | On HD: cramps 10.7, bone 11.3 Off HD: cramps 9.9, bone 8.4, back |
| Bouattar et al36 | Chronic pain of all types (duration above 3 months) | OA: 76.5 Posttraumatic: 2.9 |
Not measured | NA |
| Bourbonnais and Tousignant57 | Pain during either HD or upon return home | Cramps and muscle pain: 25 Joint and back pain: 1,456 |
Not measured | NA |
| Braz and Duarte40 | MS manifestations in HD patients (time recall for pain unclear) | Arthralgia: 16 Bone pain: 7 CTS: 1 Myalgia: 2 |
Not measured | NA |
| Calls et al43 | Intradialytic and chronic pain (definition of chronic pain not reported) | MS pain ID: 33 C: 77 CTS: ID: 3.7 C: 7.4 Backache ID: 28.9 C: 37.5 |
Not measured | NA |
| Caplin et al60 | Symptoms specifically experienced during the HD session | Cramps: 74 (type of cramps not specified) Backache: 51 |
Not measured | NA |
| Caplin et al34 | All symptoms specifically experienced during the HD session | Cramps: 74.3 (type of cramps not specified) | Not measured | NA |
| Carreon et al80 | Bone/joint pain over the past 7 days | Bone/joint pain: 37 | DSI (Likert scale 1–5) | Median: 3±1 Distribution 0–1 (no pain) 0 1–2 (mild) 29% 2–3 (moderate) 36% 3–4 (severe) 21% 4–5 (excruciating) 14% |
| Chattopadhyay et al81 | SP syndrome on long-term HD (time frame for reporting pain not reported) | SP: 67 CTS: 73 KP: 7 |
Not measured | NA |
| Claxton et al62 | Physical symptoms over the prior week | Bone/joint pain: 53 | DSI; Likert scale 0–4 | 2.8 for bone/joint pain |
| Cristofolini et al50 | Chronic LBP (duration above 3 months) | LBP: 36 | Not measured | NA |
| Davison14 | Chronic pain of all types (duration above 3 months) | MS: 63 CTS: 1.9 |
Not measured | NA |
| Elsurer et al84 | Chronic bone pain (duration above 3 months) | Bone pain: 52 | VAS | VAS (mean ± SD) 39.8±32.1 |
| Fidan et al86 | All patients had MS symptoms at the time of the study | Arthralgia: 60 Myalgia: 62 Cramps: 82 Bone pain: 48 |
VAS | VAS hand (mean ± SD) 2.58±2.51 VAS upper extremities (mean ± SD) 2.58±2.51 |
| Fortina et al38 | Chronic pain of all types (definition of chronic pain not reported) | OA: 24 | VAS (0–4) | Mean score by different scorers Patients 3 Physicians 1.9 Paramedics 1 |
| Gamondi et al87 | Pain during the past 4 weeks | MS: 64 Cramps: 25 |
Not measured | NA |
| Golan et al52 | Chronic pain (duration above 3 months) | BP: 26 OMP: 22 CJP: 12 |
Not measured | NA |
| Iacono et al90 | In-center HD chronic pain | CJP: 59 BP/NP: 55 Legs/feet: 55 Hands/arms: 37 |
Not measured | NA |
| Khan et al91 | Symptomatology in ESRD patients on HD | Bone pain: 52 Cramps: 40 |
Not measured | NA |
| Konishiike et al53 | Shoulder pain at the time of survey in long-term HD patients | SP: 36 | Not measured | NA |
| Rodriguez Calero et al47 | Intradialysis and chronic pain (definition of chronic pain not reported), including CTS, backache and MS pain | MS: ID 33, C 77 CTS: ID 3.7, C 7.4 Backache: ID 28.9, C 37.5 |
Not measured | NA |
Abbreviations: BP, back pain; C, chronic; CJP, chronic joint pain; CTS, carpal tunnel syndrome; DSI, Dialysis Symptom Index; ESRD, end-stage renal disease; HD, hemodialysis; ID, intradialytic; KP, knee pain; LBP, low-back pain; MPQ, McGill Pain Questionnaire; MS, musculoskeletal; NA, not applicable; NP, neck pain; OA, osteoarticular; OMP, other MS pain; SD, standard deviation; SP, shoulder pain; Tx, transplant; VAS, visual analog scale.
Prevalence of chest pain
Six studies with 747 patients (range 27–508) reported the prevalence of chest pain in HD patients.36,43,46,47,60,61 Two studies reported the prevalence of intradialytic pain as exactly 2.6%,43,46 while the third found chest pain during HD sessions to be 25%.60 Chronic chest pain was reported to be 5.9%36 and 9.3%.43 Binik et al reported chest pain both on and off dialysis in 13% of the sample.61 Only Binik et al reported pain severity using MPQ score as 6.4 on dialysis and 6.9 off dialysis.61
Prevalence and severity of abdominal pain
Six studies reported the prevalence of abdominal pain.36,43,46,47,52,61 Prevalence of intradialytic abdominal pain was reported as 16% in two studies,43,46 chronic abdominal pain as 18% in two studies36,52 and 9.3% in one study.43 Binik et al reported the prevalence of abdominal pain both on and off dialysis as 17%.61 Only one study reported the severity of abdominal pain as being 7.3 on MPQ score for on-HD abdominal pain.61
Prevalence of other pain
Several studies reported the prevalence of “other” pain, but this type of pain was rarely specified. Golan et al reported that 13% of patients had chronic pain from various other sources, such as phantom pain, steal syndrome, and nonspecific diffuse pain.52 Davison found prevalence of other combined chronic pain (including trauma, polycystic kidney disease, malignancy, and calciphylaxis) to be 18.4%.14 Claxton et al reported the prevalence of other pain over the prior week as 18%, but without specifying any details about the location or causes of that pain.62 Calls et al43 and Rodriguez Calero et al47 (using the same raw data) reported the prevalence of other (polycystic kidney disease, neoplasia) pain during HD sessions as 3.7% and chronic pain as 7.4%. Severity of pain listed as “other” was not reported in these studies.
Prevalence of procedural pain
Five studies reported the prevalence of poorly defined “procedural pain”.14,43,45–47 Calls et al reported that 26% of patients suffered from procedural pain, including cramps, headaches, and pain related to vascular access.43 Davison reported that 6.8% of patients experienced significant pain due to recurrent symptoms related to HD that included “cramping, headaches, and access-related pain, such as pain from needling fistulas and pain in the fistula hand”. This represented 14% of patients reporting a problem with pain.14 Rodriguez Calero et al reported that 25.9% patients identified the procedure itself as the cause of the pain,47 while another study showed a slightly higher prevalence (29%).46 Finally, according to their most recent results, 38% suffered from procedure-related pain.45 Therefore, while three studies did not explain what procedural pain was,45–47 the other two explained that procedural pain can have different locations and causes,14,43 which were reported as specific types of pain in other studies included in this review.
Prevalence of ischemic pain
Prevalence of ischemic pain as a cause of pain was reported in three studies.43,45,46 In a study on current dialytic pain, with a prevalence of 32% it was reported as the most prevalent cause of pain,46 while in another study on intradialytic ischemic pain its prevalence was even higher – 37%.43 Prevalence of ischemic pain as a cause of chronic pain was reported as 25%45 and 30%.43
Prevalence of neuropathic pain
According to the literature, while a high percentage of HD patients have shown electrophysiological evidence of nerve damage, only a small proportion have been reported as suffering from neuropathic pain.63–65 Generally, it occurs more frequently in males. Only three studies reported the prevalence of neuropathic pain.14,15,52 The older the study was, the higher the prevalence shown. Davison reported a prevalence of neuropathic pain as 12.6%.14 Three years later, Barakzoy and Moss reported it as 31%,15 while Golan et al yielded a figure of 41.2%.52
Quality of included studies
The STROBE checklist indicated that 49 observational studies were generally of moderate quality: scores ranged in sum from 6 to 18, with a median of 13 points. Three interventional studies assessed using the Cochrane risk-of-bias tool had high or unclear risk of bias in six of seven domains. Conflict of interest in the included studies was reported in only eleven of 52 studies: nine acknowledged support from public governmental grants/institutions or a private foundation; one indicated support from a small educational grant, but the source of the grant was not mentioned; while one study simply indicated that there was no conflict of interest.
Discussion
The results of this systematic review offer a comprehensive view of epidemiological studies on pain in HD patients, indicating that pain can be very prevalent and severe in HD patients. Although some studies did not examine pain as a single concept, but reported specifically pain affecting certain body parts, such as headache or musculoskeletal pain, a uniform conclusion of the included studies indicates that pain is very prevalent in HD patients.
We found that the prevalence of acute and chronic pain in HD patients can be up to 82% and 92%, respectively, which is consistent with previous research. A previously published systematic review of symptom prevalence in ESRD, which included 59 studies, reported a mean HD-pain prevalence of 47% (8%–82%).66 Identifying prevalence rates has pertinent implications for investigating the fundamental pathophysiology and developmental pathways of pain in HD. Beyond reporting prevalence of general pain, the prevalence of various types of pain in HD patients was also reviewed in this study. Most of the studies reported the prevalence of pain related to AV access, headache, and musculoskeletal pain.
Pain related to AV access is a particular type of pain that can be expected in all HD patients. Vascular access is required to permit HD. AVF is the most effective and efficient method of achieving vascular access.55 However, if HD is performed three times per week via AVF, this will repeatedly expose patients to the stress and pain of approximately 320 needle punctures/year. It is often necessary to make more than one attempt at cannulation to maintain an adequate blood flow.25 It is necessary to use large needles to achieve the required rate of flow for dialysis, which can often lead to bruising and pain, especially in patients with new fistulae.55
It has been suggested in the literature that AVF cannulation is an easy and painless procedure.28 However, it has been shown that repeated insertion of the AVF needles can cause considerable pain, both on and between dialysis sessions, with subsequent fear and anxiety.25 Patients consider pain during needle insertion the most common problem regarding dialysis vascular access.25,67 AVF-cannulation pain may adversely impact quality of life, and pain is cited as the primary reason for patients failing to tolerate dialysis via AVF.68 Even though severe pain leading to regular avoidance of dialysis or abandonment of AVF is rare, over 10% of patients have experienced pain severe enough to require early cessation of HD at least once.55
There have been a number of published systematic reviews69–71 on the impact of the different puncturing technique on the incidence of AVF-cannulation pain, all with equivocal results, showing various limitations, such as incomplete literature search69 or even overall poor quality and substantial heterogeneity among studies that precluded pooling of outcomes.70 Pain arising from AVF access was common and often multimodal in nature, frequently leading to avoidance or shortening of dialysis sessions and even abandonment of otherwise well-functioning AVF. Furthermore, pain is often a sign of underlying anatomical problems with AVF, and should always be investigated in the first instance.55
A considerable number of studies in this systematic review analyzed the prevalence and/or severity of headache. Bana et al first described headache during HD in 1972, and reported its prevalence as 70%.72 Before 1988, the taxonomy of headache was not uniform, and diagnostic criteria were rarely based on operational rules. In 1988, the IHS instituted a classification system that has become the standard for headache diagnosis and clinical research. The classification was endorsed by all the national headache societies represented in the IHS and also by the World Federation of Neurology.73 The 1988 IHS criteria for headache related to HD consider that the headaches must begin during HD and terminate within 24 hours. However, it has been noted that some headaches cannot be classified.48 The IHS revised the criteria for HDH in 2004, and described this condition as a headache that starts during an HD session and resolves within 72 hours after the session.74 Our systematic review has shown the prevalence of headache, and particularly HDH, to be very high and among the most common problems in the HD population.
With regard to musculoskeletal pain, Braz and Duarte indicated that they excluded patients with previously confirmed rheumatologic disease or who said they had any osteoarticular manifestation before the HD treatment (episode of arthritis of unknown etiology, bursopathy, and diffuse bone pain, among others, not properly investigated or undiagnosed) to prevent these as potential confounding factors.40 Such a statement was not present in other studies reporting general, limb, or musculoskeletal pain. Therefore, it is highly likely that high prevalence of limb and musculoskeletal pain in HD patients indicates comorbidities, and not such pain related to HD.
One of the strengths of this study is the inclusion of literature published in languages other than English and gray literature. In this way, we were able to locate multiple studies that were conducted outside Europe and North America. These studies indicated that pain is a considerable burden in developing countries as well.
Although this paper provides valuable data on the prevalence and severity of pain in HD patients, there were numerous limitations in the available evidence from primary studies. Data included in this systematic review indicate gaps that we still need to overcome in future literature on pain in HD patients. First, very few studies on the prevalence and severity of pain in HD patients were conducted in developing countries. Studies from those settings would be welcome for informing practice and research needs. Second, future studies need to pay particular attention to reporting, specifically for which period a patient is reporting pain. Several included studies did not report recall time for pain. Chronic pain was mostly defined as pain duration ≥3 months, but some studies did not define what they considered chronic pain, while some indicated that they measured chronic pain as pain lasting at least 4 weeks.55 Future studies should clearly indicate what they consider to be chronic pain and when exactly the pain was measured, ie, what the recall period expected of patients was (ie, current pain, pain in the last week, pain lasting ≥3 months).
Third, sample sizes need to be bigger. Half of the studies presented herein were small, with fewer than 100 patients included. The estimates of pain were sometimes based on median prevalence rates that may have been affected by the small sample size used, and should thus be interpreted with caution. Future studies should include a sufficient number of patients to gain a more representative sample. Fourth, validated pain-assessment tools should be used. The studies included in this review used various scales for pain assessment, which hinders comparability of pain intensity. While some studies used measures of pain intensity/severity to report average pain intensity, others reported the prevalence of different pain intensities in the analyzed sample. Future studies should all use the VAS for pain reporting, together with other pain-assessment scales. Additionally, studies should also report average pain, as well as percentage of patients experiencing different pain intensities. Furthermore, some of the included studies did not provide clear definitions of certain modalities of pain, such as cramps, making it difficult to judge whether these were indeed musculoskeletal cramps that are typical for HD patients.75,76 Finally, the quality of the included studies was low to moderate. The authors of future studies should consult checklists for conducting and reporting trials, in order to improve the quality of available evidence.
The role of systematic reviews is to provide reliable actionable evidence, and also to point out where evidence is missing or when there are gaps in our research knowledge.77 This systematic review provided a comprehensive overview of our current knowledge of the prevalence and severity of pain in HD patients, with actionable guidance for future studies on this topic. Based on the available evidence, prevalence and severity of pain varied widely between studies. It is thus necessary to explore factors associated with pain in HD patients to gain insight into the reasons behind such heterogeneity in pain prevalence and severity.
Conclusion
The findings of this systematic review indicated a high prevalence of pain in HD patients, and thus pain in this population should be recognized as a considerable health concern. This review should encourage the nephrology community to promote pain management in HD patients as a clinical and research priority for improving quality of life and pain-related disability. However, there are considerable gaps in the literature that future studies should address when devising a study protocol.
Acknowledgments
We are very grateful to Ms Ana Utrobicic for helping us to retrieve full texts of the manuscripts. Thanks to Ms Dalibora Behmen for professional English-language editing.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hoerger TJ, Simpson SA, Yarnoff BO, et al. The future burden of CKD in the United States: a simulation model for the CDC CKD Initiative. Am J Kidney Dis. 2015;65(3):403–411. doi: 10.1053/j.ajkd.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Mehrotra R, Kalantar–Zadeh K. Battleground: chronic kidney disorders mineral and bone disease – calcium obsession, vitamin D, and binder confusion. Clin J Am Soc Nephrol. 2008;3(1):168–173. doi: 10.2215/CJN.03850907. [DOI] [PubMed] [Google Scholar]
- 3.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BE. Epidemiology of chronic kidney disease and anemia. J Am Med Dir Assoc. 2006;7(9 Suppl):S3–S6. doi: 10.1016/j.jamda.2006.09.004. quiz S17–S21. [DOI] [PubMed] [Google Scholar]
- 5.Hsu HJ, Yen CH, Hsu KH, et al. Factors associated with chronic musculoskeletal pain in patients with chronic kidney disease. BMC Nephrol. 2014;15:6. doi: 10.1186/1471-2369-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong A, Wong G, McTaggart S, et al. Quality of life of young adults and adolescents with chronic kidney disease. J Pediatr. 2013;163(4):1179–1185.e5. doi: 10.1016/j.jpeds.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 7.Kul M, Çengel-Kültür SE, Şenses-Dinç G, Bilginer Y, Uluç S, Baykan H. Quality of life in children and adolescents with chronic kidney disease: a comparative study between different disease stages and treatment modalities. Turk J Pediatr. 2013;55(5):493–499. [PubMed] [Google Scholar]
- 8.Feng L, Yap KB, Ng TP. Depressive symptoms in older adults with chronic kidney disease: mortality, quality of life outcomes, and correlates. Am J Geriatr Psychiatry. 2013;21(6):570–579. doi: 10.1016/j.jagp.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Lapane KL, Quilliam BJ, Benson C, Chow W, Kim MS. Impact of noncancer pain on health-related quality of life. Pain Pract. 2015;15(4):333–342. doi: 10.1111/papr.12184. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CY, Vittinghoff E, Lin F, Shlipak MG. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med. 2004;141(2):95–101. doi: 10.7326/0003-4819-141-2-200407200-00007. [DOI] [PubMed] [Google Scholar]
- 11.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17(8):2275–2284. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 12.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69(2):375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 14.Davison SN. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42(6):1239–1247. doi: 10.1053/j.ajkd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198–3203. doi: 10.1681/ASN.2006050477. [DOI] [PubMed] [Google Scholar]
- 16.Santoro D, Satta E, Messina S, Costantino G, Savica V, Bellinghieri G. Pain in end-stage renal disease: a frequent and neglected clinical problem. Clin Nephrol. 2013;79(Suppl 1):S2–S11. [PubMed] [Google Scholar]
- 17.Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Health Care. York, UK: CRD; 2009. [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.André MB, Rembold SM, Pereira CM, Lugon JR. Prospective evaluation of an in-center daily hemodialysis program: results of two years of treatment. Am J Nephrol. 2002;22(5–6):473–479. doi: 10.1159/000065280. [DOI] [PubMed] [Google Scholar]
- 22.Borgel F, Benhamou PY, Zmirou D, Balducci F, Halimi S, Cordonnier D. Assessment of handicap in chronic dialysis diabetic patients (Uremidiab section study) Scand J Rehabil Med. 1992;24(4):203–208. [PubMed] [Google Scholar]
- 23.Devins GM, Armstrong SJ, Mandin H, et al. Recurrent pain, illness intrusiveness, and quality of life in end-stage renal disease. Pain. 1990;42(3):279–285. doi: 10.1016/0304-3959(90)91140-E. [DOI] [PubMed] [Google Scholar]
- 24.Celik G, Ozbek O, Yilmaz M, Duman I, Ozbek S, Apiliogullari S. Vapocoolant spray vs lidocaine/prilocaine cream for reducing the pain of venipuncture in hemodialysis patients: a randomized, placebo-controlled, crossover study. Int J Med Sci. 2011;8(7):623–627. doi: 10.7150/ijms.8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Figueiredo AE, Viegas A, Monteiro M, Poli-de-Figueiredo CE. Research into pain perception with arteriovenous fistula (AVF) cannulation. J Ren Care. 2008;34(4):169–172. doi: 10.1111/j.1755-6686.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- 26.Andersen C, Danielson K, Ladefoged J. EMLA cream for pain prevention in hemodialysis patients. Dial Transplant. 1989;18(12):684–685. [Google Scholar]
- 27.Asgari MR, Motlagh NH, Soleimani M, Ghorbani R. Effect of lidocaine spray on the pain intensity during insertion of vascular needles in hemodialysis patients. Faslnamahi Kumish. 2012;14(3):271–279. [Google Scholar]
- 28.Crespo R, Rivero MF, Contreras MD, Guisado C. Influence of bevel position of the needle on puncture pain in haemodialysis. EDTNA ERCA J. 1994;20(4):21–23. [Google Scholar]
- 29.Ludwig K, Ruder H, Wendt MA, Richter R. Pain reduction in children and adolescents on hemodialysis with local anesthetic cream. Monatsschr Kinderheilkd. 1997;145(1):60–62. [Google Scholar]
- 30.McPhail S. Hemodialysis needles can be pain free: use of a topical anaesthetic cream. J CANNT. 1992;2(4):19–20. [PubMed] [Google Scholar]
- 31.Peiffer-Labauge A, Marcelli C, Mourad G, et al. [Painful leg syndrome in hemodialyzed patients for chronic renal insufficiency with recent kidney transplantation] Syndrome douloureux des membres inférieurs chez l’insuffisant rénal chronique hémodialyse récemment transplanté. Rev Rhum Mal Osteoartic. 1991;58(3):187–191. French. [PubMed] [Google Scholar]
- 32.Mercadante S, Ferrantelli A, Tortorici C, et al. Incidence of chronic pain in patients with end-stage renal disease on dialysis. J Pain Symptom Manage. 2005;30(4):302–304. doi: 10.1016/j.jpainsymman.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Al-Hilali N, Al-Humoud HM, Ninan VT, Nampoory MR, Ali JH, Johny KV. Profiled hemodialysis reduces intradialytic symptoms. Transplant Proc. 2004;36(6):1827–1828. doi: 10.1016/j.transproceed.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Caplin B, Alston H, Davenport A. Does online haemodiafiltration reduce intra-dialytic patient symptoms? Nephron Clin Pract. 2014;124(3–4):184–190. doi: 10.1159/000357050. [DOI] [PubMed] [Google Scholar]
- 35.Vergne H, Darnis MC, Ostertag A, Poux JM. Douleur à la ponction de la fistule artério-veineuse en hémodialyse. 2002. [Accessed April 27, 2016]. Available from: http://cnrd.fr/IMG/pdf/VERGNE.pdf.
- 36.Bouattar T, Skalli Z, Rhou H, et al. [The evaluation and analysis of chronic pain in chronic hemodialysis patients] Évaluation et analyse de la douleur chez les hémodialysés chroniques. Nephrol Ther. 2009;5(7):637–641. doi: 10.1016/j.nephro.2009.06.004. French. [DOI] [PubMed] [Google Scholar]
- 37.El Harraqui R, Abda N, Bentata Y, Haddiya I. [Evaluation and analysis of pain in chronic hemodialysis] Évaluation et analyse de la douleur en hémodialysé chronique. Nephrol Ther. 2014;10(7):500–506. doi: 10.1016/j.nephro.2014.06.005. French. [DOI] [PubMed] [Google Scholar]
- 38.Fortina F, Agliata S, Ragazzoni E, et al. [Chronic pain during dialysis: pharmacological therapy and its costs] Il dolore chronico in dialisi. Minerva Urol Nefrol. 1999;51(2):85–87. Italian. [PubMed] [Google Scholar]
- 39.Antoniazzi AL, Bigal ME, Bordini CA, Speciali JG. [Headache and hemodialysis: evaluation of the possible triggering factors and of the treatment] Cefaléia relacionada à hemodiálise: análise dos possíveis fatores desencadeantes e do tratamento empregado. Arq Neuropsiquiatr. 2002;60(3A):614–618. Portuguese. [PubMed] [Google Scholar]
- 40.Braz AD, Duarte AL. Musculoskeletal manifestations in hemodialysis patients. Rev Bras Reumatol. 2003;43(4):223–231. [Google Scholar]
- 41.Djurić M, Zidverc-Trajković J, Sternić N, et al. [Hemodialysis-related headaches] Hemodijalizne glavobolje. Vojnosanit Pregl. 2007;64(5):319–323. doi: 10.2298/vsp0705319d. Serbian. [DOI] [PubMed] [Google Scholar]
- 42.Nikić PM, Zidverc-Trajković J, Andrić BR, Djurić M, Stojimirović BB. [Headache associated with haemodialysis] Glavobolja kod bolesnika na hemodijalizi. Srp Arh Celok Lek. 2008;136(7–8):343–349. doi: 10.2298/sarh0808343n. Serbian. [DOI] [PubMed] [Google Scholar]
- 43.Calls J, Rodríguez Calero M, Hernández Sánchez D, et al. [An evaluation of pain in haemodialysis patients using different validated measurement scales] Evaluación del dolor en hemodiálisis mediante diversas escalas de medición validadas. Nefrologia. 2009;29(3):236–243. doi: 10.3265/Nefrologia.2009.29.3.5120.en.full. Spanish. [DOI] [PubMed] [Google Scholar]
- 44.Armendáriz MP, Terceño AM, García ME, Blázquez SF. Evaluation of pain in patients undergoing hemodialysis. Rev Soc Esp Enferm Nefrol. 2010;13(4):264–266. [Google Scholar]
- 45.Rodriguez Calero MA, Sánchez DH, Navarro JG, Amer FJ, Ginesta JC. [Evaluation of chronic pain in a population of patients on haemodialysis] Evaluación del dolor crónico en una población de pacientes hemodializado. Rev Soc Esp Enferm Nefrol. 2007;10(2):65–71. Spanish. [Google Scholar]
- 46.Rodriguez Calero MA, Sánchez DH, Navarro JG, Amer FJ, Ginesta JC, Llull JS. [Assessment and management of intra-dialysis pain] Evaluación y manejo del dolor intradiálisis. Rev Soc Esp Enferm Nefrol. 2006;9(2):65–70. Spanish. [Google Scholar]
- 47.Rodriguez Calero MA, Sánchez DH, Navarro JG, Ginesta JC. Dolor intrasesión y dolor crónico en pacientes que reciben hemodiálisis. Metas Enferm. 2009;12(2):12–18. Spanish. [Google Scholar]
- 48.Antoniazzi AL, Bigal ME, Bordini CA, Speciali JG. Headache associated with dialysis: the International Headache Society criteria revisited. Cephalalgia. 2003;23(2):146–149. doi: 10.1046/j.1468-2982.2003.00510.x. [DOI] [PubMed] [Google Scholar]
- 49.Antoniazzi AL, Bigal ME, Bordini CA, Tepper SJ, Speciali JG. Headache and hemodialysis: a prospective study. Headache. 2003;43(2):99–102. doi: 10.1046/j.1526-4610.2003.03025.x. [DOI] [PubMed] [Google Scholar]
- 50.Cristofolini T, Draibe S, Sesso R. Evaluation of factors associated with chronic low back pain in hemodialysis patients. Nephron Clin Pract. 2008;108(4):c249–c255. doi: 10.1159/000124328. [DOI] [PubMed] [Google Scholar]
- 51.Jesus A, Oliveira HA, Paixão MO, Fraga TP, Barreto FJ, Valença MM. Clinical description of hemodialysis headache in end-stage renal disease patients. Arq Neuropsiquiatr. 2009;67(4):978–981. doi: 10.1590/s0004-282x2009000600003. [DOI] [PubMed] [Google Scholar]
- 52.Golan E, Haggiag I, Os P, Bernheim J. Calcium, parathyroid hormone, and vitamin D: major determinants of chronic pain in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(8):1374–1380. doi: 10.2215/CJN.00680109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konishiike T, Hashizume H, Nishida K, Inoue H, Nagoshi M. Shoulder pain in long-term haemodialysis patients: a clinical study of 166 patients. J Bone Joint Surg Br. 1996;78(4):601–605. [PubMed] [Google Scholar]
- 54.Malaki M, Mortazavi FS, Moazemi S, Shoaran M. Insomnia and limb pain in hemodialysis patients: what is the share of restless leg syndrome? Saudi J Kidney Dis Transpl. 2012;23(1):15–20. [PubMed] [Google Scholar]
- 55.Aitken E, McLellan A, Glen J, Serpell M, Mactier R, Clancy M. Pain resulting from arteriovenous fistulae: prevalence and impact. Clin Nephrol. 2013;80(5):328–333. doi: 10.5414/CN107917. [DOI] [PubMed] [Google Scholar]
- 56.Verhallen AM, Kooistra MP, van Jaarsveld BC. Cannulating in haemodialysis: rope-ladder or buttonhole technique? Nephrol Dial Transplant. 2007;22(9):2601–2604. doi: 10.1093/ndt/gfm043. [DOI] [PubMed] [Google Scholar]
- 57.Bourbonnais FF, Tousignant KF. The pain experience of patients on maintenance hemodialysis. Nephrol Nurs J. 2012;39(1):13–19. quiz 20. [PubMed] [Google Scholar]
- 58.Milinković M, Zidverc-Trajković J, Sternić N, et al. Hemodialysis headache. Clin Nephrol. 2009;71(2):158–163. doi: 10.5414/cnp71158. [DOI] [PubMed] [Google Scholar]
- 59.Goksel BK, Torun D, Karaca S, et al. Is low blood magnesium level associated with hemodialysis headache? Headache. 2006;46(1):40–45. doi: 10.1111/j.1526-4610.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 60.Caplin B, Kumar S, Davenport A. Patients’ perspective of haemodialysis-associated symptoms. Nephrol Dial Transplant. 2011;26(8):2656–2663. doi: 10.1093/ndt/gfq763. [DOI] [PubMed] [Google Scholar]
- 61.Binik YM, Baker AG, Kalogeropoulos D, et al. Pain, control over treatment, and compliance in dialysis and transplant patients. Kidney Int. 1982;21(6):840–848. doi: 10.1038/ki.1982.108. [DOI] [PubMed] [Google Scholar]
- 62.Claxton RN, Blackhall L, Weisbord SD, Holley JL. Undertreatment of symptoms in patients on maintenance hemodialysis. J Pain Symptom Manage. 2010;39(2):211–218. doi: 10.1016/j.jpainsymman.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Krishnan AV, Phoon RK, Pussell BA, Charlesworth JA, Bostock H, Kiernan MC. Altered motor nerve excitability in end-stage kidney disease. Brain. 2005;128(Pt 9):2164–2174. doi: 10.1093/brain/awh558. [DOI] [PubMed] [Google Scholar]
- 64.Laaksonen S, Metsärinne K, Voipio-Pulkki LM, Falck B. Neurophysiologic parameters and symptoms in chronic renal failure. Muscle Nerve. 2002;25(6):884–890. doi: 10.1002/mus.10159. [DOI] [PubMed] [Google Scholar]
- 65.Baumgaertel MW, Kraemer M, Berlit P. Neurologic complications of acute and chronic renal disease. Handb Clin Neurol. 2014;119:383–393. doi: 10.1016/B978-0-7020-4086-3.00024-2. [DOI] [PubMed] [Google Scholar]
- 66.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Bay WH, Van Cleef S, Owens M. The hemodialysis access: preferences and concerns of patients, dialysis nurses and technicians, and physicians. Am J Nephrol. 1998;18(5):379–383. doi: 10.1159/000013380. [DOI] [PubMed] [Google Scholar]
- 68.Ferrans CE, Powers MJ. Quality of life of hemodialysis patients. ANNA J. 1993;20(5):575–581. discussion 582. [PubMed] [Google Scholar]
- 69.Grudzinski A, Mendelssohn D, Pierratos A, Nesrallah G. A systematic review of buttonhole cannulation practices and outcomes. Semin Dial. 2013;26(4):465–475. doi: 10.1111/sdi.12116. [DOI] [PubMed] [Google Scholar]
- 70.Wong B, Muneer M, Wiebe N, et al. Buttonhole versus rope-ladder cannulation of arteriovenous fistulas for hemodialysis: a systematic review. Am J Kidney Dis. 2014;64(6):918–936. doi: 10.1053/j.ajkd.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 71.Atkar RK, MacRae JM. The buttonhole technique for fistula cannulation: pros and cons. Curr Opin Nephrol Hypertens. 2013;22(6):629–636. doi: 10.1097/MNH.0b013e328365ae9e. [DOI] [PubMed] [Google Scholar]
- 72.Bana DS, Yap AU, Graham JR. Headache during hemodialysis. Headache. 1972;12(1):1–14. doi: 10.1111/j.1526-4610.1972.hed1201001.x. [DOI] [PubMed] [Google Scholar]
- 73.Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia. 1988;8(Suppl 7):1–96. No authors listed. [PubMed] [Google Scholar]
- 74.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 75.Kobrin SM, Berns JS. Quinine – a tonic too bitter for hemodialysis-associated muscle cramps? Semin Dial. 2007;20(5):396–401. doi: 10.1111/j.1525-139X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 76.Canzanello VJ, Burkart JM. Hemodialysis-associated muscle cramps. Semin Dial. 1992;5(4):299–304. [Google Scholar]
- 77.Palmer SC, Craig JC, Jones A, Higgins G, Willis N, Strippoli GF. Celebrating 20 years of evidence from the Cochrane Collaboration: what has been the impact of systematic reviews on nephrology? Nephrol Dial Transplant. 2015;30(6):871–877. doi: 10.1093/ndt/gfu232. [DOI] [PubMed] [Google Scholar]
- 78.Alessandri M, Massanti L, Geppetti P, Bellucci G, Cipriani M, Fanciullacci M. Plasma changes of calcitonin gene-related peptide and substance P in patients with dialysis headache. Cephalalgia. 2006;26(11):1287–1293. doi: 10.1111/j.1468-2982.2006.01217.x. [DOI] [PubMed] [Google Scholar]
- 79.Barrett BJ, Vavasour HM, Major A, Parfrey PS. Clinical and psychological correlates of somatic symptoms in patients on dialysis. Nephron. 1990;55(1):10–15. doi: 10.1159/000185911. [DOI] [PubMed] [Google Scholar]
- 80.Carreon M, Fried LF, Palevsky PM, Kimmel PL, Arnold RM, Weisbord SD. Clinical correlates and treatment of bone/joint pain and difficulty with sexual arousal in patients on maintenance hemodialysis. Hemodial. 2008;12(2):268–274. doi: 10.1111/j.1542-4758.2008.00264.x. [DOI] [PubMed] [Google Scholar]
- 81.Chattopadhyay C, Ackrill P, Clague RB. The shoulder pain syndrome and soft-tissue abnormalities in patients on long-term haemodialysis. Br J Rheumatol. 1987;26(3):181–187. doi: 10.1093/rheumatology/26.3.181. [DOI] [PubMed] [Google Scholar]
- 82.Davison SN, Jhangri GS. The impact of chronic pain on depression, sleep, and the desire to withdraw from dialysis in hemodialysis patients. Journal of Pain & Symptom Management. 2005;30(5):465–473. doi: 10.1016/j.jpainsymman.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 83.Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. Journal of Pain & Symptom Management. 2010;39(3):477–485. doi: 10.1016/j.jpainsymman.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Elsurer R, Afsar B, Mercanoglu E. Bone pain assessment and relationship with parathyroid hormone and health-related quality of life in hemodialysis. Ren Fail. 2013;35(5):667–672. doi: 10.3109/0886022X.2013.780617. [DOI] [PubMed] [Google Scholar]
- 85.Er MS, Eroǧlu M, Altinel EC, Altinel L. Hemodialysis and pain. The Turkish Nephrology, Dialysis and Transplantation Journal. 2013;22(2):167–170. [Google Scholar]
- 86.Fidan F, Alkan BM, Tosun A, Altunoǧlu A, Ardiçoǧlu O. Quality of life and correlation with musculoskeletal problems, hand disability and depression in patients with hemodialysis. Int J Rheum Dis. 2016;19(2):159–166. doi: 10.1111/1756-185X.12171. [DOI] [PubMed] [Google Scholar]
- 87.Gamondi C, Galli N, Schonholzer C, et al. Frequency and severity of pain and symptom distress among patients with chronic kidney disease receiving dialysis. Swiss Med Wkly. 2013;143:w13750. doi: 10.4414/smw.2013.13750. [DOI] [PubMed] [Google Scholar]
- 88.Goksan B, Karaali-Savrun F, Ertan S, Savrun M. Haemodialysis-related headache. Cephalalgia. 2004;24(4):284–287. doi: 10.1111/j.1468-2982.2004.00668.x. [DOI] [PubMed] [Google Scholar]
- 89.Harris TJ, Nazir R, Khetpal P, et al. Pain, sleep disturbance and survival in hemodialysis patients. Nephrol Dial Transplant. 2012;27(2):758–765. doi: 10.1093/ndt/gfr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iacono SA. Chronic Pain in the Hemodialysis Patient Population. Dial Transplant. 2004;33(2):92–101. [Google Scholar]
- 91.Khan MA. Frequency of symptomatology in patients on hemodialysis: A single center experience. Rawal Med J. 2012;37(1):24–26. [Google Scholar]
- 92.Kimmel PL, Emont SL, Newmann JM, Danko H, Moss AH. ESRD patient quality of life: symptoms, spiritual beliefs, psychosocial factors, and ethnicity. American Journal of Kidney Diseases. 2003;42(4):713–721. doi: 10.1016/s0272-6386(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 93.Parfrey PS, Vavasour HM, Henry S, Bullock M, Gault MH. Clinical features and severity of nonspecific symptoms in dialysis patients. Nephron. 1988;50(2):121–128. doi: 10.1159/000185141. [DOI] [PubMed] [Google Scholar]
- 94.Shayamsunder AK, Patel SS, Jain V, Peterson RA, Kimmel PL. Sleepiness, sleeplessness, and pain in end-stage renal disease: distressing symptoms for patients. Sem Dialysis. 2005;18(2):109–118. doi: 10.1111/j.1525-139X.2005.18218.x. [DOI] [PubMed] [Google Scholar]
- 95.Weisbord SD, Fried LF, Arnold RM, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16(8):2487–2494. doi: 10.1681/ASN.2005020157. [DOI] [PubMed] [Google Scholar]