Abstract
Background
New chemotherapy combinations are being tested for the treatment of women with advanced, persistent or recurrent cervical cancer. We sought to evaluate the cost effectiveness of some newer combination therapies in cervical cancer.
Patients and methods
A cost effectiveness decision model was used to analyze Gynecologic Oncology Group 240. All regimens were modeled for seven cycles. The regimens studied are as follows: regimen 1, cisplatin/paclitaxel (CP); regimen 2, CP with bevacizumab (CP+B); regimen 3, paclitaxel/topotecan (PT); and regimen 4, PT with bevacizumab (PT+B). Overall survival, cost, and complications were studied. Sensitivity analyses were performed.
Results
Mean chemotherapy costs over mean total costs for seven cycles of each follows: CP $571/$32,966; CP+B $61,671/$96,842; PT $9,211/$71,620; and PT+B $70,312/$109,211. Incremental cost-effectiveness ratio (ICER) for CP+B was $133,559/quality adjusted life year (QALY). ICER for PT+B was $124,576/QALY. To achieve an incremental ICER for CP+B:CP of <$50,000/QALY gained, the mean overall survival has to increase from 1.1 years with CP to 3.5 years with CP+B. An ICER <$50,000/QALY for the other regimens would take a survival of >10 years for PT and 4.1 years for PT+B. Treating 1,000 women with cervical cancer with CP+B would cost almost double the cost of treating >18,000 women with ovarian cancer annually (carboplatin/paclitaxel).
Conclusion
CP is the most cost effective regimen. A 12-month increase in overall survival will not even make the newer combinations cost effective. Currently, the use of bevacizumab is not sustainable at today’s costs.
Keywords: cervical cancer, chemotherapy, bevacizumab, cost-effectiveness
Introduction
Health care providers in the US always want to give the latest/greatest therapies to their individual patients. Is this a sustainable model? Many studies have shown that cancer patients are much more likely to declare bankruptcy than patients with other diseases.1 Although we may add a few months of progression free survival or even actual survival, the price tag may be so exorbitant that we cause other long-term side-effects in the family such as debt, bankruptcy, and even depression.2 The discussion of finances is ignored by most physicians but is as important as the discussion of the risk and benefits of therapy.
Cervical cancer is related to lower socio-economic status and poor access to preventative health care.3,4 A study from Belgium demonstrated that the average cost of treating a patient with early stage disease was >$13,000.5 This amount increases remarkably if patients no longer have early stage cancer.
Gynecologic Oncology Group (GOG) 204 was created to look at the role of cisplatin doublets in the care of these women.6 This study found that the overall survival, progression free survival, and relative risk all favored the use of cisplatin/paclitaxel (CP) for this population. Thus, CP was established to be the standard of care for this population. GOG 240 compared the new standard with or without the addition of bevacizumab (B) to the doublet of paclitaxel/topotecan (PT) with or without B (PT or PT+B).7
We sought to evaluate the cost effectiveness of combination therapies from GOG 240 data that were released at the 2013 American Society of Clinical Oncology meeting.7
Materials and methods
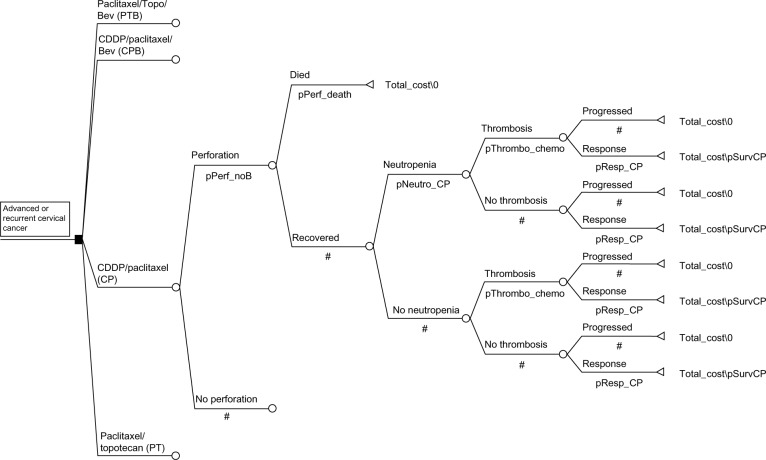
Costs and outcomes of treating women with advanced, recurrent or persistent squamous cell carcinoma of the cervix were modeled. Regimens studied were: CP, CP with B (CP+B), PT, or PT+B (Figure 1). No access to results from GOG 240 beyond what was published in the reference was available. All patients modeled were treated with seven cycles of chemotherapy. The average patient was assumed to be a 65-year old (US Medicare coverage) with a body surface area of 2 m2, height of 165 cm, weight of 100 kg, and a creatinine of 0.8 mg/dL (Cockcroft and Gault actual weight).
Figure 1.
Decision tree outlining the major decision points in the analysis.
Note: Regimens are defined in the image 100–p=#.
Abbreviations: Bev, bevacizumab; CDDP, cisplatin; Perf, perforation; Neutro, neutropenia; Thrombo, thrombosis; chemo, chemotherapy; Resp, Response; Surv, survival; CP, cisplatin/paclitaxel; pPerf_noB, % perforated without bevacizumab; p, % perforated.
The overall survival for the CP regimen varied quite a bit from GOG 169 to GOG 204 in a similar population (9.7 months to 13 months).6,8 Complications, recurrence data, and available survival data were derived from the results of GOG 240 with the topotecan arm supplemented by further data.7,9 We did not adjust complication, recurrence, or survival rates for age, and we assumed that experiencing a grade three or worse complication did not affect recurrence or survival rates. Utility values were estimated from the data in the literature.10,11
Estimated complication costs were based on 2012 Medicare costs (average sale price January 1, 2012 – March 31, 2012) as well as published figures.12,13 For values not readily found in the literature, patient reimbursements from Center for Medicare/Medicaid Services were examined for the specific problems. The values used in the base case, as well as the sensitivity analysis ranges, are shown in Table 1.
Table 1.
Mean survival by chemotherapy
Values were varied widely in sensitivity analysis to determine the effect on results. One-way sensitivity analysis was performed. Incremental cost-effectiveness ratio (ICER) was measured as cost per quality adjusted life year (QALY) survival, calculated by dividing the difference in cost between the strategies by the difference in survival among the strategic. A willingness to pay threshold (WTP) was based on published literature. An amount of $50,000/QALY was used.
Results
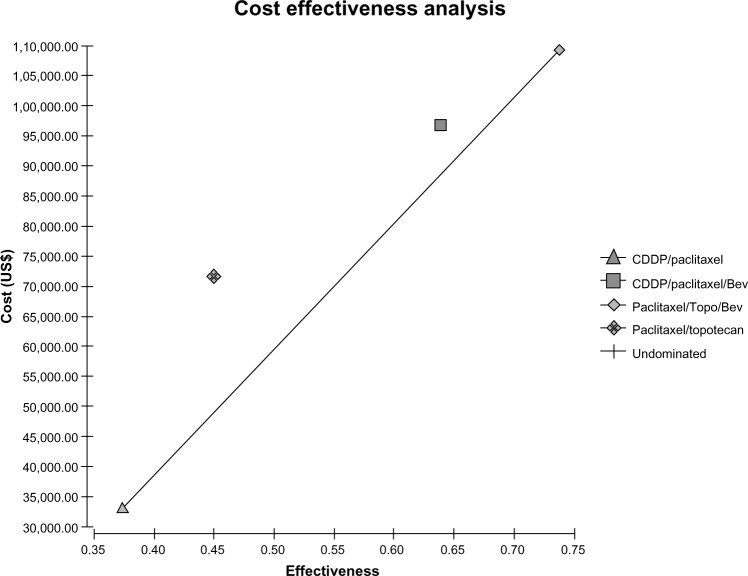
Although survival varied significantly among GOG 169, 179, and 204, the survival from GOG 204 for CP was used.6,8,14 Table 1 lists the mean survivals used in the cost effectiveness analysis. Figure 2 is the cost effectiveness analysis diagram at baseline costs and known survivals. As can be seen, none of the regimens are dominated, so there is potential for each regimen to become a cost-effective alternative. However, the cost frontier from CP to PT+B is steep showing that the ICERs will be high.
Figure 2.
Cost effectiveness diagram with cost on the vertical axis in dollars and relative effectiveness on the horizontal axis.
Note: The shape legends are contained on the figure.
Abbreviations: CDDP, cisplatin; Bev, bevacizumab; Topo, topotecan.
Table 2 has two major parts. Column 2 is the actual chemotherapy cost for each of the five chemo regimes studied. As can be seen, the costs vary widely from $571 for seven cycles of cisplatin to $70,312 for seven cycles of PT+B. Column three includes all direct and indirect costs for seven cycles used in the model (chemotherapy costs, infusion costs, complication rates and costs). Hence, again there is a wide range of costs starting with CP at $32,966 and extending to PT+B at $109,211. As can be seen from the comparison of columns two and three, changes in cost stem from both the medications (direct costs) and the complications (indirect costs).
Table 2.
Incremental cost effectiveness ratio by quality adjusted life year
| Treatment | Cost of medications for seven cycles | Mean total costs for seven cycles (US$) | ICER | Reference |
|---|---|---|---|---|
| CP | $571 | $32,966 | – | 12,17 |
| CP+B | $61,671 | $96,842 | $133,559/QALY | 12,17 |
| PT | $9,211 | $71,620 | $511,947/QALY | 12,17 |
| PT+B | $70,312 | $109,211 | $124,576/QALY | 12,17 |
Abbreviations: CP, cisplatin/paclitaxel; CP+B, cisplatin/paclitaxel/bevacizumab; PT, paclitaxel/topotecan; PT+B, paclitaxel/topotecan/bevacizumab; ICER, incremental cost-effectiveness ratio; QALY, quality adjusted life year.
The ICER for CP+B was $133,559/QALY. The ICER for PT+B was $124,576/QALY and the ICER for PT was $511,947/QALY. All of these are significantly above a WTP of $50,000/QALY.15
The exact number of patients with cervical cancer annually is unknown. An analysis of cost can be estimated by looking at 1,000 women requiring treatment for this disease annually. Figure 3 compares the cost of treating 1,000 women with this disease by each of the GOG 240 regimens. Figure 4, compares the cost of treating 1,000 women with cervical cancer with CP+B versus treating all of the women requiring chemotherapy after surgery for ovarian cancer annually (18,598 women).16 As seen in Figure 4, treating 1,000 women with CP+B for cervical cancer costs ∼67% more than treating over 18,000 women with first-line chemotherapy (carboplatin and paclitaxel) for ovarian cancer.
Figure 3.
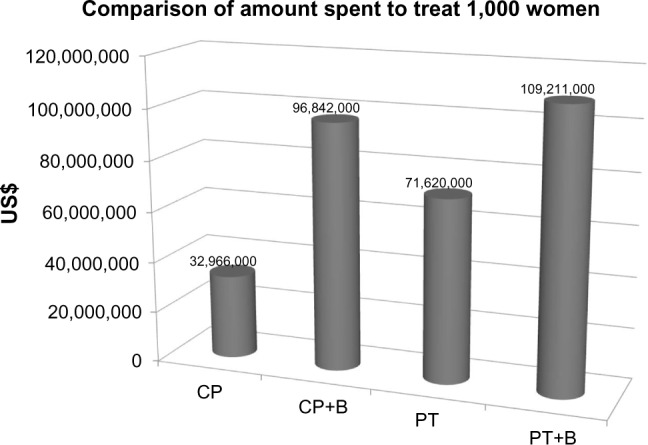
Comparison of GOG 240 regimens when treating 1,000 women.
Note: Costs are on the diagrams vertical axis.
Abbreviations: GOG, Gynecologic Oncology Group; CP, cisplatin/paclitaxel; CP+B, cisplatin/paclitaxel/bevacizumab; PT, paclitaxel/topotecan; PT+B, paclitaxel/topotecan/bevacizumab.
Figure 4.
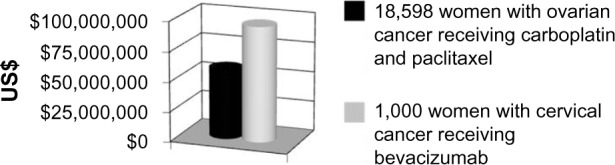
Comparison of treating 1,000 women with advanced or recurrent cervical cancer with cisplatin/paclitaxel/bevacizumab to treating 18,598 women with ovarian cancer and carboplatin/paclitaxel after initial debulking surgery.
Discussion
Over the past decade the standard of care for this unfortunate patient population has changed from cisplatin (6.5–8.8 months average overall survival), to cisplatin/topotecan (9.4 months average overall survival) to CP (12.9 months average overall survival). Therefore, CP chemotherapy is now commonly used for the treatment of advanced cancer of the cervix because of GOG 204.6 Geisler et al previously demonstrated that CP was an acceptable alternative to cisplatin alone for women with this advanced disease.17 The other doublets, although more effective than cisplatin alone, were less effective and more expensive than CP. This fact strengthens the use of the CP regimen as standard of care. This result brought forth the question about the addition of bevacizumab to CP. Thus, GOG 240 was created. With the expense of bevacizumab, does the added survival found in the bevacizumab arms make it cost effective and sustainable? While getting a 4-month increase in overall survival in GOG 240 is statistically significant, it comes at the addition of huge costs. Figure 4 shows that it would cost more to treat 1,000 women with advanced or recurrent cervical cancer than to treat 18,000 women with newly diagnosed ovarian cancer. The goal of cost effectiveness analysis is to determine when a paradigm shift should occur because a new regimen is more efficient and has an acceptable cost. Although economically this concept is easy to understand, medically it is not easily understood or accepted. Doctors want the latest and greatest treatments for their patients even if the small incremental increase in survival cannot justify an exorbitant price tag.
In the United Kingdom, the National Institute for Health and Clinical Excellence (NICE) approved cisplatin/topotecan for use in a very select sub-population.18 This group included women without prior platin-analog exposure. That specific population was not a primary end point in GOG 179. NICE also accessed data from GOG 179 which were not generally available. Despite the acceptance of cisplatin/topotecan (CT) by NICE, the newer data from GOG 204, show that CP, not CT, is the standard regimen.
Looking at Tables 1 and 2, one can see that the survival for the arms containing bevacizumab have an increased survival of approximately 4 months. However, the ICER is over $100,000/QALY. In Shiroiwa et al’s study from 2010, an acceptable WTP in the US was $62,500.15 This estimate is far below the ICER for the addition of bevacizumab in these patients. To get the ICER down to this study’s WTP of $50,000/QALY, the survival increase would have to go from 4 months to 12 months.
Geisler et al have further demonstrated that if targeted therapies (anti-angiogenesis drugs, tyrosine kinase inhibitors, etc) are to become standard of care over CP, either larger improvements in survival or lower prices ($100s instead of $1,000s or $10,000s) will be necessary.19 All physicians want better survival for their patients, but it has to be sustainable. Most countries, including the US do not have the resources to treat patients regardless of the cost. Rationing health care is not ethical, but neither is spending money on treatments that are not worth the expense. We need to rationally approach changes in standards of care so that we can treat the most patients with the best possible treatments. This fact would make one compliment the authors of GOG 240 for the improvement they found by adding bevacizumab, but recommend holding off changing the standard of care away from CP until either a regimen with longer survival or much cheaper cost is found. Only cost effective care can be sustainable in a situation in which money is finite.
Footnotes
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32(6):1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amir Z, Wilson K, Hennings J, Young A. The meaning of cancer: implications for family finances and consequent impact on lifestyle, activities, roles and relationships. Psychooncology. 2012;21(11):1167–1174. doi: 10.1002/pon.2021. [DOI] [PubMed] [Google Scholar]
- 3.Robinson KM, Christensen KB, Ottesen B, Krasnik A. Socio- demographic factors, comorbidity and diagnostic delay among women diagnosed with cervical, endometrial or ovarian cancer. Eur J Cancer Care (Engl) 2011;20(5):653–661. doi: 10.1111/j.1365-2354.2011.01259.x. [DOI] [PubMed] [Google Scholar]
- 4.Drolet M, Boily MC, Greenaway C, et al. Sociodemographic inequalities in sexual activity and cervical cancer screening: implications for the success of human papillomavirus vaccination. Cancer Epidemiol Biomarkers Prev. 2013;22(4):641–652. doi: 10.1158/1055-9965.EPI-12-1173. [DOI] [PubMed] [Google Scholar]
- 5.Annemans L, Remy V, Lamure E, et al. Economic burden associated with the management of cervical cancer, cervical dysplasia and genital warts in Belgium. J Med Econ. 2008;11(1):135–150. doi: 10.3111/13696990801961611. [DOI] [PubMed] [Google Scholar]
- 6.Monk BJ, Sill MW, McMeekin DS, et al. Phase III Trial of Four Cisplatin-Containing Doublet Combinations in Stage IVB, Recurrent, or Persistent Cervical Carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2009;27(28):4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tewari K, Sill M, Long HJ, et al. Incorporation of bevacizumab in the treatment of recurrent and metastatic cervical cancer: A phase III randomized trial of the Gynecologic Oncology Group. ASCO 2013. J Clin Oncol. 2013;(Suppl) abstr 3. [Google Scholar]
- 8.Moore DH, Blessing JA, McQuellon RP, et al. Phase III Study of Cisplatin With or Without Paclitaxel in Stage IVB, Recurrent, or Persistent Squamous Cell Carcinoma of the Cervix: A Gynecologic Oncology Group Study. J Clin Oncol. 2004;22(15):3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 9.Tiersten AD, Selleck MJ, Hershman DL, et al. Phase II study of topotecan and paclitaxel for recurrent, persistent, or metastatic cervical carcinoma. Gynecol Oncol. 2004;92(2):635–638. doi: 10.1016/j.ygyno.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Versteegh MM, Leunis A, Uyl-de Groot CA, Stolk EA. Condition-specific preference-based measures: benefit or burden? Value Health. 2012;15(3):504–513. doi: 10.1016/j.jval.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Jewell EL, Smrtka M, Broadwater G, et al. Utility Scores and Treatment Preferences for Clinical Early-Stage Cervical Cancer. Value Health. 2011;14(4):582–586. doi: 10.1016/j.jval.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services [homepage on the Internet] Medicare Part B Drug Average Sales Price Manufacturer reporting of Average Sales Price (ASP) data. [Accessed February 24, 2015]. Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrug-AvgSalesPrice/
- 13.Cohn DE, Kim KH, Resnick KE, O’Malley DM, Straughn JM., Jr At what cost does a potential survival advantage of bevacizumab make sense for the primary treatment of ovarian cancer? A cost-effectiveness analysis. J Clin Oncol. 2011;29(10):1247–1251. doi: 10.1200/JCO.2010.32.1075. [DOI] [PubMed] [Google Scholar]
- 14.Long HJ, 3rd, Bundy BN, Grendys EC, Jr, et al. Randomized Phase III Trial of Cisplatin with or without Topotecan in Carcinoma of the Uterine Cervix: A Gynecologic Oncology Group Study. J Clin Oncol. 2005;23(21):4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19(4):422–437. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]
- 16.Walter AC, Manahan KJ, Geisler JP. Annual cost of bevacizumab in the adjuvant treatment of ovarian cancer to the U.S. Medicare system. Gynecologic Oncology. 2012;125(Suppl 1):S15. [Google Scholar]
- 17.Geisler JP, Swathirajan J, Wood KL, Manahan KJ. Treatment of Advanced or Recurrent Cervical Cancer with Cisplatin or Cisplatin Containing Regimens: A Cost Effective Analysis. J Cancer. 2012;3:454–458. doi: 10.7150/jca.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paton F, Paulden M, Saramago P, et al. Topotecan for the treatment of recurrent stage IB carcinoma of the cervix. Health Technol Assess. 2010;14(Suppl 1):55–62. doi: 10.3310/hta14Suppl1/08. [DOI] [PubMed] [Google Scholar]
- 19.Geisler JP, Walter AC, Manahan KJ. Eur Soc Gynecol Oncol – Liverpool. UK: 2013. An economic analysis of new regimens in the treatment of women with advanced or recurrent cervical cancer. [PubMed] [Google Scholar]