Abstract
Molecular nanotechnology is destined to become the core technology in 21st century medicine. Nanotechnology mean, controlling biologically relevant structures with molecular precision. Nanomedicine is exploring how to use carbon buckyballs, dendrimers and other cleverly engineered nanoparticles in novel drugs to combat viruses, bacteria, cancer and delivery of drugs. Medical nanorobots will be of the size of a microbe, capable of self-replication, containing onboard sensors, computers, manipulators, pumps, pressure tanks and power supplies. Building such sophisticated molecular machine systems will require molecular manufacturing to using massively parallel assembly lines in nanofactories.
Key Words: Nanotechnology, Nanodevices, nanomedicine
Introduction
Nanomedicine – the science of diagnosing, treating and preventing disease with the use of molecular biology combined with nanotechnology is an emerging sub-discipline.
The prefix nano- (Greek word nanos= “dwarf”) means one-billionth (10-9). In 1989, Don Eigler, an engineer at IBM, unfurled his company's initials out of xenon atoms, creating the smallest-ever logo [1].
Nanoscale-structured materials and devices hold great promise for advanced diagnostics targeted drug delivery, smart drugs, and immuno-isolation therapies [2, 3]. Biomaterials available are given in Table-1. Nano-biotechnology offers the benefits of molecular medicine through genomics, proteomics, and artificially engineered microbes [4, 5]. Medical (biological robots) allow micromanipulation, transportation of molecules and communication with physicians by molecular-scale computers. In long term this will allow instant pathogen diagnosis and extermination, chromosome replacement and individual cell surgery [6, 7]. Nanorobots are computer-driven while biobots are driven by Microtubule-Associated-Proteins (MAP) and include kinesins and dynein. The volume of either is < 100 cubic nanometers. In this dimension, a 10,000 element logic system with a small processor occupies <100 nanometers/side3. This compares with the volume of a typical cell (thousands of cubic microns) and is even substantially smaller than subcellular organelles. Such a computer uses less than 10-9 watts/hour while operating continuously at gigahertz frequencies. In comparison, the human body uses about 100 watts/hour at rest and more during exercise [4, 8].
Table 1.
Some accepted biomaterials
| Metals | Ceramics | Polymers |
|---|---|---|
| 316L stainless steel | Alumina | Ultra high molecular weight |
| Co-Cr Alloys | Zirconia | polyethylene |
| Titanium | Carbon | Polyurethane |
| Ti6Al4V | Hydroxyapatite |
The concepts of general-purpose, self-replicating manufacturing systems have been modelled on the work by von Neumann (1961) whose simplified version is the convergent assembly consisting of universal computer containing a program that directs the behaviour of the universal constructor. Once construction is finished, the original program contained in the universal computer is copied to the new universal computer and program execution started [9]. Von Neumann later added a kinematical constructor with a robotic arm (Fig 1). This arm is capable of moving and grasping specific components in three-dimension which could be assembled into another kinematic constructor and control computer. The state of the cell at the tip could also be changed, thereby allowing the assembly of a broad range of structures with flexible programme control [10].
Fig. 1.
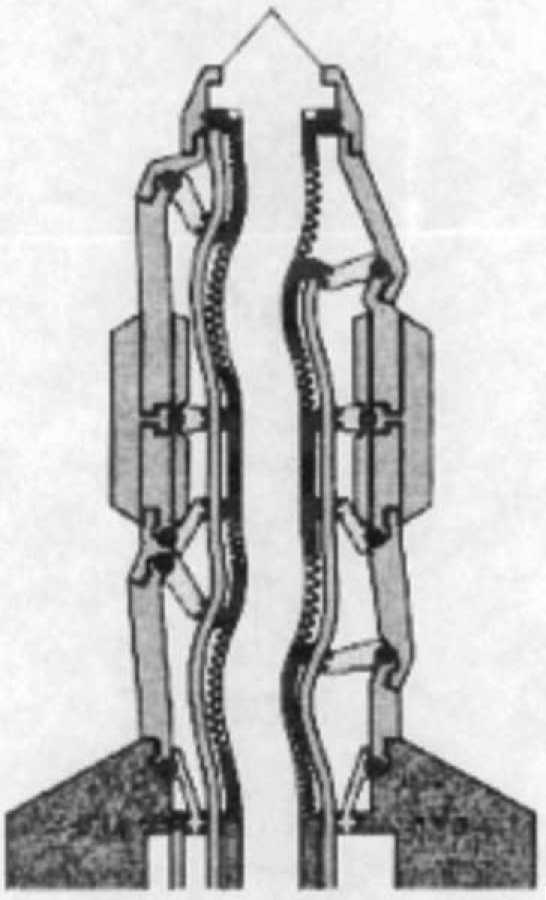
Robotic arm
Drexler followed the von Neumann kinematical architecture by an assembler that is very small, ∼ 0.1 microns (100 nanometers) in size. The constructor has additional details in order to manipulate molecular structures with atomic precision (Fig. 2, Fig. 3) [11]. Nanorobots and biobots are fashioned with this as a prototype [12].
Fig. 2.

Basic design for nanorobots
Fig. 3.

Respirocyte
Applications of Nanotechnology in medicine
Respirocyte
A simple method of providing metabolic support and preventing tissue damage due to hypoxia despite reduced blood flow is by the use of an “artificial red blood cell.” Respirocytes are blood-borne 1-micron-diameter diamondoid storage tanks designed by Robert A. Freitas Jr. for transporting respiratory gases throughout the human body. These spherical nanodevices can be reversibly pressurised up to 1000 atm [13]. The respirocyte available today consists of 18 billion precisely arranged structural atoms plus 9 billion temporarily-resident molecules when fully loaded. It can be remotely reprogrammed via externally applied signals to the onboard nanocomputer and through chemical and pressure sensors [Fig 3].
Twelve pumping stations powered by endogenous serum glucose, are spaced evenly along an equatorial circle. Each station has its own independent glucose-metabolising powerplant, glucose tank, environmental glucose sensors and an array of 3-stage molecular sorting rotor assemblies [Fig 4] for reversibly pumping O2, CO2, and H2O between the ambient medium and an interior chamber [14]. The equatorial pumping station network occupies ∼50% of respirocyte surface. On the remaining surface, a universal “bar code” consisting of concentric circular patterns of shallow rounded ridges is embossed around the poles for easy product-identification by an electron microscope.
Fig. 4.

3-stage molecular sorting rotor assembly
A litre of blood normally contains ∼0.2 litres of oxygen, while one litre of these spheres contains ∼530 litres of oxygen. Thus, they are >2,000 times efficient/unit volume than blood. Taking into account that blood is only about half occupied by red blood cells, these spheres are over 10,000 times more efficient than red blood cells.
Artificial mitochondria
Increased oxygen levels in the presence of ischaemically injured mitochondria, will be ineffective in restoring the tissue to its healthy state. More direct metabolic support in the form of ATP, coupled with selective release or absorption of critical metabolites would be effective. Artificial mitochondria enclosed in hollow polymer nano-capsules with gold-coated glass beads respond to externally applied light. The gold heats up and deforms, thereby releasing the payload by swelling or contrating [15]. Gilead Sciences ™is selling another drug delivery mechanism using lipid spheres (nanosomes) of 100 nanometers in diameter that encase even antibiotics and anticancer drugs [16]. The first quasi-viral component molecular “delivery bags” are under study at Rice University [17].
Artificial Phagocytes
The microbivore is an experimental nanorobotic mechanical phagocyte [18]. It is an oblate spheroidal nanomedical device consisting of 610 billion precisely arranged structural atoms plus another ∼150 billion molecules (mostly gas or water) when fully loaded. Microbivore measures 3.4 microns by 2.0 microns, thus ensuring passage through the narrowest of human capillaries, which are ∼4 microns in diameter. It includes two normally empty processing chambers namely morcellation chamber and digestion chamber totalling 4 micron3 in displaced volume. The nanodevice consumes 100-200 pW of continuous power while in operation and can completely digest trapped microbes at a maximum throughput of 2 micron3 / 30-second cycle, large enough to internalise a single microbe from virtually any major bacteraemic species. The microbivore has a dry mass of 12.2 picograms (Table 2).
Table 2.
Microbivore Baseline Design: External Surface Area, Internal Volume, and Maximum Power Allocations
| Microbivore Subsystem | Nanorobot Hull Area Allocation (micron2) | Internal Volume Allocation (micron3) | Maximum Power Draw (pW) |
|---|---|---|---|
| Reversible Microbobial Binding Sites | |||
| 20,000 Receptor Blocks | 8.82 | 0.0882 | 0.02 |
| Telescoping Grapples | |||
| 300 Grapple Arms in Silos | 0.589 | 0.177 | 180 |
| Power Supply and Buffer Storage | |||
| 10 Powerplants | −- | 2.0 | −- |
| Glucose Buffer Storage Tank | −- | 1.0 | −- |
| Oxygen Buffer Storage Tank | −- | 1.3 | −- |
| Sensors | |||
| External sensors | 0.45 | 0.1 | 1 |
| Acoustic sensors | 0.44 | 0.01 | −- |
| Internal sensors | −- | 0.04 | 0.4 |
| Computers | |||
| Computer and Memory Storage | −- | 0.11 | < 70 |
| Structural Support | |||
| External Microbivore Hull | −- | 1.2443 | −- |
| Unspecified Other Structure | 5.825 | 0.3799 | −- |
| Total | 26.885 | 12.1056 | < 382.66 |
During each cycle of operation, a target bacterium is bound to the surface of the microbivore via a species specific reversible binding site. Telescoping robotic grapples emerge from silos on the device surface, establish secure anchorage to the microbe's plasma membrane transport the pathogen to the ingestion port at the front of the device where the pathogen cell is internalized into a 2 micron3 morcellation chamber. After sufficient mechanical mincing, the morcellated remains of the cell are pistoned into a 2 micron3 digestion chamber where a preprogrammed sequence of 40 engineered enzymes are successively injected and extracted six times, progressively reducing the morcellate ultimately to mono-residue amino acids, mononucleotides, glycerol, free fatty acids and simple sugars. These simple molecules are then harmlessly discharged back into the bloodstream through an exhaust port at the rear of the device, completing the 30-second digestion cycle. This “digest and discharge” protocol is conceptually similar to the internalization and digestion process practiced by natural phagocytes, except that the artificial process is faster and cleaner.
Artificial Platelets
The baseline clottocyte or artificial mechanical platelets [19] is a serum oxy-glucose-powered spherical nanorobot producing “instant” haemostasis. It is ∼2 microns in diameter (∼4 micron3 volume) containing a compact fibre mesh that is folded onboard. Surface sensors detect decreased serum levels of fibrinogen, plasminogen, alpha2antiplasmin, antithrombin III, factor VII, protein C, protein S. The control computer then commands the device to promptly unfurl its mesh packet in the immediate vicinity of an injured blood vessel. Soluble thin films, dissolve upon contact with plasma, revealing sticky sections complimentary to blood group antigens. Blood cells are immediately trapped in the overlapping artificial nettings released by multiple neighbouring activated clottocytes and bleeding halts at once. Special control protocols are present in which carefully specified constellations of sensor readings must be observed before device activation is permitted so that clottocytes do not release their mesh packets at the wrong place or at an inappropriate time.
There is a small risk of triggering disseminated intravascular coagulation (DIC). One solution is to equip clottocytes with sensors to detect elevated levels of thrombin and various fibrin/fibrinogen-derived degradation products. If DIC conditions arise, nanorobots might respond by absorbing and metabolising the excess thrombin or by releasing thrombin inhibitors such as antithrombin III, hirudin or anticoagulants that reduce thrombin generation such as danaparoid to interrupt the cascade.
Diamond is chemically inert and non-inflammatory in relation to the complement system [20]. Clottocytes enveloped with diamonds and externally coated with autologous platelet membrane are as biocompatible as native platelets and also assists in the recruitment of intrinsic coagulation mechanisms. Also, the bio-resorbable netting is capable of being broken up into non-immunogenic phagocytosable pieces, either by natural enzymatic pathways or by artificial fibrinolytic enzymes (analogous to fibrinolytic plasmin) released from the clottocyte at the appropriate time. Total bleeding time ranges from 2-5 minutes. Clottocytes may allow complete haemostasis in as little as ∼1 second even in moderately large wounds which is 100-1000 times faster than the natural system.
Drug Delivery Methods:
Norwood has three device-based drug delivery technologies which can be applied to drug delivery problems such as poor absorption and acid degradation [21]. In contrast to traditional drug administration, controlled drug delivery systems designed for long-term administration maintain the blood drug levels constant between the desired maximum and minimum for an extended period of time.
-
•
Laser Assisted Drug Delivery (LAD) – is designed to painlessly and temporarily alter the stratum corneum, so that drugs are delivered trans-dermally, avoiding the gastric side effects associated with oral administration. A wide range of drugs can be delivered [22]. The LAD device has been cleared for marketing by the Australian TGA and the USA FDA.
-
•
Needle-free Drug Delivery – Norwood and its research partner at Massachusetts Institute of Technology (MIT) have developed a needle-free injector system for both human and veterinary application [23]. A small hand-held device with a novel, patented, extremely fast and powerful contractile fibre-activated pump fires the drug at the skin with sufficient velocity to penetrate without the use of needles. Key features of the Norwood device include safety, low cost, silent operation and control of dosage. Nanotechnology is working on the development of skin impedance measurement, the control of delivery force and depth of delivery.
-
•
Micro-needle Drug Delivery – provides precise delivery of pharmaceuticals over time via micron-scale holes in the skin [24]. Drugs that are either too large or too hydrophobic to diffuse through intact skin may be delivered effectively, because micro-needles penetrate only into the upper layers of skin (where the primary transport barrier resides) and contact with nerves and capillaries is avoided, making the delivery painless.
Smart Drugs
Yoshihisa Suzuki at Kyoto University [25] has bound gentamicin to a hydrogel using a newly developed peptide linker which can be cleaved by a proteinase enzyme manufactured by Pseudomonas aeruginosa, a gram-negative bacillus. When this hydrogel is applied to a wound where P. aeruginosa are present, then the microbial proteolytic enzyme cleaves the linker and releases gentamicin. The antibiotic is not released if P. aeruginosa bacteria are absent. This specific action is highly desirable to prevent the emergence of drug-resistant bacteria secondary to indiscriminate use of antibiotics.
Tissue scanning
“Quantum dots” are tiny semiconductor nanocrystals which can be excited to fluorescence with white light liberating a wide range of sharply defined wavelengths of light that depends on their size. Biological events can be tracked by simultaneously tagging different biological components (e.g., different proteins or DNA sequences) with nanodots of a specific color. In mid 2000, Genentech began evaluating the dots for commercial utility in a variety of cellular and molecular assays [26]. Today, a ‘Quantun Dot Corporation’ already exists.
A related technology called PEBBLES (Probes Encapsulated by Biologically Localised Embedding), pioneered by Raoul Kopelman at the University of Michigan, allows dye-tagged nanoparticles to be inserted into living cells to monitor metabolism or disease conditions [27]. Many metabolic diseases like Cushing's disease, Grave's disease, Paget's disease, Addison's disease, Conn's syndrome, Prader-Labhart-Willi syndrome are due to the mis-regulation of the signaling molecules. Synthesis of these regulatory proteins can be affected by incorporating the relevant gene into the genome. The vehicle proposed to transport the gene proteins intracellular are Starburst dendrimers. These are tree-shaped synthetic molecules with a regular branching structure emanating outward from a core. Dendrimers form, nanometer by nanometer, so the exact number of synthetic steps or “generations” dictates the size of the particles in a batch and can be constructed upto 30 “generations”, incorporating more than 100,000 atoms. The peripheral layer and the outermost branches of the dendrimer particle form a dense field of molecular groups that serve as hooks for attaching other useful molecules. Beyond a certain size, dendrimers trigger endocytosis and the vesicle formed is then admitted into the cell's interior. Once inside, the DNA is released and migrates to the nucleus where it becomes part of the cell's genome. The technique has been tested on a variety of mammalian cell types, and James R. Baker Jr. hopes to begin clinical human trials of dendrimer gene therapy [28].
Nanomaterials
Dental and Bone Replacement
“Biomimicry” or the growth of new bio-structures in a mould or directly in/on the injured body part (teeth or bone), can develop from either nano-physical or nano-chemical structures. A nano-physical structure develops from a single nano-crystal, whereas a nano-chemical structure develops from an array of large reactive molecules attached to a surface (Table 1). These nanostructures are used as seed molecules, or seed crystals, to drive materials to grow by themselves. Biomimicry is already the basis of new tough and light materials for bullet-proof vests and other defence applications.
Researchers hope to use nano-patterned polymers to grow adult stem cells that will turn into bone. Once the process of growing tissue on patterned scaffolding is perfected, nano-structured devices can be attached to further improve bone growth rates and reduce healing time. This could eliminate the long recovery times, scarring, and infection associated with bone grafts. A low intensity electric current can be provided to stimulate bone growth. Tiny channels are present along which controlled doses of growth-enhancing proteins can be pumped [29].
Nanoparticles in Bone Cement
All commercial polymethylmethacrylate bone cement contains <10% of 0.5-3nm size, barium sulfate or zirconium oxide particles to increase the toughness of bone cements and reduce the incidence of cement fractures. Research is currently being conducted on the use of 100nm size barium sulphate nanoparticles as radiopacifiers which could be uniformly dispersed in the cement and that have the potential to improve its fracture toughness and have a substantially longer fatigue life.
In addition, manipulation of calcium and phosphate in hydroxyapatite at the molecular level led to the production of a new nanoparticle that is identical in structure and composition to natural bone. This novel synthetic bone can be used in areas where natural bone is damaged or removed, such as in the treatment of fractures and soft tissue injuries [29].
Summary
Initial work with medical nanoscale-structured materials and devices, nano-biotechnology and medical nanorobots shows performance improvement of 100 to 1000 fold, over natural biological systems. When doctors gain access to medical robots, they will be able to quickly cure most known diseases that affect people today, to rapidly repair most physical injuries our body suffers, and to extend the human health span.
Conflicts of Interest
None identified
References
- 1.Klafter RD, Chmielewski TA, Negin M. Proceedings of Supercomputing Conference. Prentice Hall ACM Press; 1989. Robotic engineering: an integrated approach. [Google Scholar]
- 2.Schweizer EK. Positioning single atoms with a scanning tunnelling microscope. Nature. 1990;344:524–526. [Google Scholar]
- 3.Black CT, Bezencenet O. Nanometer-Scale Pattern Registration and Alignment by Directed Diblock Copolymer Self-Assembly. Nanotechnology. 2004;3:412–415. [Google Scholar]
- 4.Allender CJ, Richardson C, Woodhouse B. Pharmaceutical applications for molecularly imprinted polymers. Int J Pharm. 2000;195:39–43. doi: 10.1016/s0378-5173(99)00355-5. [DOI] [PubMed] [Google Scholar]
- 5.Silva GA. Introduction to nanotechnology and its applications to medicine. Surg Neurol. 2004;61:216–220. doi: 10.1016/j.surneu.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Crandall BC. Nanotechnology: Molecular Speculations on Global Abundance. 1st ed. MIT Press; Cambridge, Massachusetts: 1996. [Google Scholar]
- 7.Freitas Robert A., Jr The future of nanofabrication and molecular scale devices in nanomedicine. Studies in Health Technology and Informatics. 2002;80:45–59. [PubMed] [Google Scholar]
- 8.Merkle RC. Self replicating systems and molecular manufacturing. Journal of the British Interplanetary Society. 1992;45:407–413. [Google Scholar]
- 9.Von Neumann J, Burks AW. Theory of self reproducing automata. 1st ed. University of Illinois Press; 1966. [Google Scholar]
- 10.Merkle RC. Reversible electronic logic using switches. Nanotechnology. 1993;4:21–40. [Google Scholar]
- 11.Drexler Erik K. Machine-Phase Nanotechnology. Scientific American. 2001;74:156–168. doi: 10.1038/scientificamerican0901-74. [DOI] [PubMed] [Google Scholar]
- 12.Montemagno CD, Bachand GD. Constructing nanomechanical devices powered by biomolecular motors. Nanotechnology. 1999;10:225–231. [Google Scholar]
- 13.Freitas Robert A., Jr Exploratory design in medical nanotechnology: A mechanical artificial red cell. Biotechnol Bioeng. 1998;26:411–430. doi: 10.3109/10731199809117682. [DOI] [PubMed] [Google Scholar]
- 14.Kelly TR, De Silva H, Silva RA. Unidirectional rotary motion in a molecular system. Nature. 1999;401:150–152. doi: 10.1038/43639. [DOI] [PubMed] [Google Scholar]
- 15.Koumura N, Zijlstra RW, van Delden RA. Light-driven monodirectional molecular rotor. Nature. 1999;401:152–155. doi: 10.1038/43646. [DOI] [PubMed] [Google Scholar]
- 16.Desai TA, Chu WH, Tu JK. Microfabricated immunoisolating biocapsules. Biotechnol Bioeng. 1998;57:118–120. doi: 10.1002/(sici)1097-0290(19980105)57:1<118::aid-bit14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Deamer DW, Akeson M. Nanopores and nucleic acids: prospects for ultrarapid sequencing. Trends Biotechnol. 2000;8:147–151. doi: 10.1016/s0167-7799(00)01426-8. [DOI] [PubMed] [Google Scholar]
- 18.Freitas Robert A., Jr Microbivores. Artificial mechanical phagocytes using digest and discharge protocol. Zyvex. 2001:634–645. preprint. [Google Scholar]
- 19.Freitas Robert A., Jr Clottocytes Artificial mechanical platelets. Foresight Update. 2000;41:9–11. [Google Scholar]
- 20.Allender CJ, Richardson CB, Woodhouse CM. Pharmaceutical applications for molecularly imprinted polymers. Int J Pharm. 2000;95:39–43. doi: 10.1016/s0378-5173(99)00355-5. [DOI] [PubMed] [Google Scholar]
- 21.Leon J. Beyond Needles and Pills. Time. 2001:74–86. [Google Scholar]
- 22.Nishizawa M, Menon VP, Martin CR. Metal nanotubule membranes with electrochemically switchable ion-transport selectivity. Science. 1995;268:700–702. doi: 10.1126/science.268.5211.700. [DOI] [PubMed] [Google Scholar]
- 23.Bachand GD, Montemagno CD. Constructing organic/inorganic NEMS devices powered by biomolecular motors. Biomedical Microdevices. 2000;2:179–184. [Google Scholar]
- 24.Meller A, Nivon L, Brandin EJ. Rapid nanopore discrimination between single polynucleotide molecules. Proc Natl Acad Sci. 2000;97:1079–1084. doi: 10.1073/pnas.97.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanihara M, Nishimura Y, Suzuki K. A new drug delivery system with controlled release of antibiotic only in the presence of infection. J Biomed Mater Res. 1998;42:112–116. doi: 10.1002/(sici)1097-4636(199810)42:1<112::aid-jbm14>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Shi H, Ratner BD. Template recognition of protein-imprinted polymer surfaces. J Biomed Mater Res. 2000;49:1–11. doi: 10.1002/(sici)1097-4636(200001)49:1<1::aid-jbm1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Clark HA, Kopelman R, Tjalkens R. Optical nanosensors for chemical analysis inside single living cells. 2. Sensors for pH and calcium and the intracellular application of PEBBLE sensors. Anal Chem. 1997;19:4837–4843. doi: 10.1021/ac990630n. [DOI] [PubMed] [Google Scholar]
- 28.Baker JR, Jr, Kukowska-Latallo JF, Bielinska AU. Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl Acad Sci. (USA) 1999;93:4897–4902. doi: 10.1073/pnas.93.10.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freitas Robert A., Jr The future of nanofabrication and molecular scale devices in nanomedicine. Studies in Health Technology and Informatics. 2002;80:45–59. [PubMed] [Google Scholar]


