Targeting neural stem cells (NSCs) in the adult brain represents a promising approach for developing new regenerative strategies. The NFL-TBS.40-63 peptide targets NSCs in vitro and in vivo when injected into the cerebrospinal fluid. These results indicate that the NFL-TBS.40-63 peptide represents a new molecular tool to target NSCs to develop new strategies for regenerative medicine and the treatment of brain tumors.
Keywords: Neural stem cells, NFL-TBS.40-63 peptide, Targeting, Subventricular zone, Differentiation, Proliferation
Abstract
Targeting neural stem cells (NSCs) in the adult brain represents a promising approach for developing new regenerative strategies, because these cells can proliferate, self-renew, and differentiate into new neurons, astrocytes, and oligodendrocytes. Previous work showed that the NFL-TBS.40-63 peptide, corresponding to the sequence of a tubulin-binding site on neurofilaments, can target glioblastoma cells, where it disrupts their microtubules and inhibits their proliferation. We show that this peptide targets NSCs in vitro and in vivo when injected into the cerebrospinal fluid. Although neurosphere formation was not altered by the peptide, the NSC self-renewal capacity and proliferation were reduced and were associated with increased adhesion and differentiation. These results indicate that the NFL-TBS.40-63 peptide represents a new molecular tool to target NSCs to develop new strategies for regenerative medicine and the treatment of brain tumors.
Significance
In the present study, the NFL-TBS.40-63 peptide targeted neural stem cells in vitro when isolated from the subventricular zone and in vivo when injected into the cerebrospinal fluid present in the lateral ventricle. The in vitro formation of neurospheres was not altered by the peptide; however, at a high concentration of the peptide, the neural stem cell (NSC) self-renewal capacity and proliferation were reduced and associated with increased adhesion and differentiation. These results indicate that the NFL-TBS.40-63 peptide represents a new molecular tool to target NSCs to develop new strategies for regenerative medicine and the treatment of brain tumors.
Introduction
It is a major challenge to treat chronic diseases of the central nervous system, because most active drugs do not pass the blood-brain barrier efficiently to allow sufficient bioavailability amounts in the brain. Another important issue is to deliver the highly bioactive molecules specifically to the desired site of action to avoid secondary or toxic effects. One promising strategy is to inject such molecules in the cerebrospinal fluid to target neural stem cells (NSCs). These cells are located in the neurogenic regions of the adult brain (subventricular zone [SVZ], dentate gyrus of the hippocampus) and along the central canal of the spinal cord [1–8]. The SVZ represents the most important pool of endogenous NSCs in the brain. They are characterized by their capacity to self-renew, form neurospheres in culture, proliferate, and differentiate into neurons, astrocytes, and oligodendrocytes [9–11]. The use of normal or genetically modified NSCs has been described for the treatment of neurodegenerative disorders (Parkinson’s, Huntington’s, and Alzheimer’s diseases, amyotrophic lateral sclerosis), spinal cord lesions, and malignant glioma [12, 13]. Several studies have also showed the recruitment of endogenous NSCs in several brain disorders. For instance, in patients with multiple sclerosis, endogenous mobilization of NSCs from the SVZ to striatal lesions was observed [14]. Endogenous migration of NSCs to the glioma was also demonstrated [15]. A recent review of clinical trials reported the use of NSCs as a possible treatment of neurodegenerative diseases, including amyotrophic lateral sclerosis, stroke, and spinal cord injury [16]. Therefore, the capacity to target and manipulate endogenous NSCs represents a promising avenue for regenerative-based therapies to increase their mobilization, stimulate neurogenesis, and avoid the occurrence of side effects [17].
A potential candidate to target NSCs is the NFL-TBS.40-63 peptide, which corresponds to the tubulin-binding site located on the light subunit of neurofilaments [18]. The peptide alone, as well as nanoparticles functionalized with the peptide, can massively enter glioblastoma cells in vitro and in vivo; however, the uptake is very low in healthy cells (astrocytes and neurons) [19–21]. Moreover, this peptide disrupts the microtubule network of glioblastoma cells and reduces the tumor volume in animals bearing glioma but has no major toxicity on healthy cells. The peptide did not damage the tissue when injected into healthy brain [19–21]. Recent works have also shown that this peptide promotes the differentiation and maturation of oligodendrocytes derived from newborn rats in vitro [22, 23].
In the present study, we investigated the targeting capacity and potential effects of this peptide on NSCs. We show that the peptide is able to target NSCs isolated from newborn and adult animals in vitro and in vivo after its injection into the lateral ventricle of adult rats. Although the peptide showed a cytotoxic effect on the glioblastoma cells, it has no major effect on NSCs at concentrations less than 10 µmol/l. At higher concentrations, the peptide affects their self-renewal and proliferation ability and increases their adhesion and differentiation. Together, these results indicate that the NFL-TBS.40-63 peptide represents a promising tool for targeting the delivery of biologically active proteins into NSCs and/or manipulating these cells.
Materials and Methods
Cell Culture and Materials
Primary cultures of NSCs were derived from the SVZ of newborn (1–5 days) and adult (<4 months) Wistar rats, as previously described [24]. The dissociated cells were grown in minimal essential medium-α (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com) supplemented with 25 mmol/l d-glucose (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), 1 mmol/l sodium pyruvate, 15 mmol/l HEPES, 5% penicillin/streptomycin, 1% B27 (Thermo Fisher Scientific Life Sciences), and 20 ng/ml epidermal growth factor (EGF; Promega, Madison, WI, http://www.promega.com). After 5–7 days, the stem cells formed floating neurospheres.
The NFL-TBS.40-63 peptide (YSSYSAPVSSSLSVRRSYSSSSGS) is biotinylated or coupled to 5-carboxyfluorescein (5-FAM) and was synthesized by Millegen (Toulouse, France), Eurogentec (Seraing, Belgium, http://www.eurogentec.com), or GeneCust (Dudelange, Luxembourg, http://www.genecust.com). The peptides from these three companies showed similar results. A scrambled peptide (NFL-SCR: SLGSPSSSVRASYSSSRSYVYSSS) coupled to 5-FAM was used as a control peptide. The composition of the NFL-SCR peptide is the same as the NFL-TBS.40-63 peptide but the sequence is in disorder (GeneCust). These peptides were dissolved in sterile water at a concentration of 1 mmol/l as stock solutions.
Chlorpromazine hydrochloride (50 µmol/l), phorbol 12-myristate 13-acetate (PMA; 10 g/ml), and 5-(N,N-dimethyl) amiloride hydrochloride (DAM; 1 mmol/l; Sigma-Aldrich) were used to inhibit endocytosis. Wortmannin (100 nmol/l), U0126 (40 µmol/l), sunitinib (1 µmol/l; Sigma-Aldrich), genistein (400 µmol/l; Merck, Kenilworth, NJ, http://www.merck.com), and gefitinib (50 µmol/l; Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com) were used to inhibit the signaling pathway implicated in the endocytic machinery. These reagents do not induce major toxicity in cells at these concentrations.
Analysis of Neurosphere Formation and Self-Renewal
The neurosphere-forming properties were analyzed according to a previously described protocol [25] to quantify the number of primary and secondary neurospheres formed in culture. In brief, 5 × 103 SVZ cells were seeded per well in a 24-well plate in media containing 20 ng/ml EGF. The cells were incubated in the absence or presence of increasing concentrations of biotinylated NFL-TBS.40-63 peptide or with 1 µg/ml colchicine (Sigma-Aldrich). After 5–7 days, the total number of primary neurospheres and the percentage of adherent primary neurospheres were determined in each condition using microscope analysis. For the self-renewal assay, primary neurospheres were collected in each condition, dissociated into single cells, and seeded in media containing 20 ng/ml EGF. After 5 days, the total number of secondary neurospheres was counted in each condition using microscope analysis. The morphology of primary neurospheres was observed with an inverted microscope (Leica DMI6000; Leica Biosystems, Nussloch, Germany, http://www.leicabiosystems.com) equipped with a CoolSNAP-HQ2 camera and analyzed using MetaMorph, version 7.1.7.0, software (Molecular Devices, Sunnyvale, CA, http://www.moleculardevices.com). They were also observed using scanning electron microscopy.
Flow Cytometry (Fluorescence-Activated Cell Sorting)
The uptake of 5-FAM-labeled NFL-TBS.40-63 and NFL-SCR peptides was quantified in NSCs derived from newborn and adult rats. A total of 200–300 neurospheres were seeded in 35-mm dishes and cultured for 30 minutes at 37°C in media containing 5-FAM-labeled peptides. To quench the extracellular signal of the 5-FAM-labeled peptides, 0.4% trypan blue (Sigma-Aldrich) was added before analysis. To investigate the uptake molecular mechanism, neurospheres were preincubated at 4°C for 30 minutes or with 10 mmol/l sodium azide in the presence of 6 mmol/l 2-deoxy-d-glucose to deplete cellular ATP or with different endocytosis and signaling pathway inhibitors for 30 minutes at 37°C, as previously described [21]. Next, 20 µmol/l 5-FAM-labeled NFL-TBS.40-63 peptide was added to the cells for 30 minutes at 37C°. Subsequently, after centrifugation (5 minutes at 42g), the cells were dissociated mechanically and washed twice with PBS and then resuspended in 50 µg/ml propidium iodide (PI; Sigma-Aldrich). The fluorescent-positive viable cells that incorporated the 5-FAM-labeled peptide were analyzed by flow cytometry (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ, http://www.bd.com). The gating schemes for the flow cytometry analysis of each sample were included in supplemental online Figure 1.
The cell cycle of NSCs was analyzed after incubation for 48 hours with the biotinylated NFL-TBS.40-63 peptide or with colchicine, and the DNA content was evaluated by flow cytometry. In brief, 200–300 neurospheres were seeded in 35-mm dishes and then treated with increasing concentrations of biotinylated peptide or with 1 µg/ml colchicine for 48 hours at 37°C. The neurospheres were then collected in a microcentrifuge tube. After centrifugation (5 minutes at 42g), the cells were dissociated mechanically and permeabilized with PBS-Tween 0.5% and fixed with ethanol 70% before adding 1 mg/ml RNase (Thermo Fisher Scientific Life Sciences) for 30 minutes at 37°C. The cell suspension was diluted in 10 µg/ml PI before DNA content analysis by flow cytometry to quantify the cells in the G1, S, and G2/M phases. The gating schemes for the flow cytometry analysis of each sample are shown in supplemental online Figure 2.
The expression of specific markers was evaluated after treating NSCs with the biotinylated NFL-TBS.40-63 peptide for 7 days. Typically, 200–300 neurospheres were seeded in 35-mm dishes and cultured for 7 days at 37°C in media containing biotinylated peptide at increasing concentrations or containing 1% newborn calf serum (NBCS). After dissociation, the cells were incubated with the anti-polysialylated-neural cell adhesion molecule (PSA-NCAM), the anti-A2B5 antibodies conjugated to R-phycoerythrin (Miltenyi Biotech, Bergisch Gladbach, Germany, http://www.miltenyibiotech.com), or the anti-CD90.1-PerCP-Cy5.5 (Molecular Probes; Thermo Fisher Scientific Life Sciences) for 10 minutes at 4°C, washed, and resuspended in 50 µg/ml PI or with calcein violet AM (Thermo Fisher Scientific Life Sciences). The fluorescent-positive viable cells that expressed the marker were analyzed by flow cytometry. The gating schemes for the flow cytometry analysis of each sample are shown in supplemental online Figure 3.
Cell Viability Assay
NSC viability was determined using the trypan blue (Sigma-Aldrich) exclusion technique. In brief, 200–300 neurospheres were seeded in 24-well plate and then treated with increasing concentrations of biotinylated NFL-TBS.40-63 peptide or 1 µg/ml colchicine for 72 hours at 37°C. Next, the cells were harvested and dissociated mechanically. The number of viable cells (i.e., those not labeled with trypan blue) was manually counted and quantified by Trypan blue exclusion in each sample using a Zeiss microscope.
CyQUANT Cell Proliferation Assay
To analyze the peptide effect on the proliferation of NSCs, primary neurospheres were dissociated and 5 × 103 cells per well were plated on BD Cell-Tak (3.5 µg/cm2 and 1.4 mg/ml; BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) on a 96-well microplate. The cells were treated with increasing concentrations of biotinylated NFL-TBS.40-63 peptide or 1 µg/ml colchicine for 72 hours at 37°C. After 3 washes with PBS, the cells were frozen at −80°C. Finally, the DNA concentration was analyzed using the CyQUANT cell proliferation assay kit (Molecular Probes; Thermo Fisher Scientific Life Sciences).
Immunofluorescence and Microscopy
The uptake of the 5-FAM-labeled NFL-TBS.40-63 peptide was visualized in dissociated NSCs incubated for 30 minutes with 20 µmol/l peptide and then plated on BD Cell-Tak-coated coverslips in 24-well plates (5 × 104 cells per well) for 1 hour. After PBS washing, the cells were fixed with 4% paraformaldehyde for 15 minutes and washed three times in PBS, before mounting coverslips with ProLong Gold antifade solution (Thermo Fisher Scientific Life Sciences). NSCs were observed with a confocal microscope (Leica TCS SP8; Leica Biosystems).
To visualize the peptide uptake by NSCs and the possible effect on their microtubule network, 100 neurospheres were plated in a 24-well plate on BD Cell-Tak coverslips in 24-well plates for 24 hours. They were then incubated for 30 minutes with 20 µmol/l 5-FAM-labeled NFL-TBS.40-63 peptide to evaluate the percentage of cells containing the peptide or for 6 hours with 40 µmol/l 5-FAM-labeled NFL-TBS.40-63 peptide to evaluate the effect of the peptide on the microtubule network. After PBS washing, the cells were fixed with 4% paraformaldehyde for 15 minutes and washed three times in PBS. They were then incubated in a 0.5% Triton X-100 permeabilization solution for 30 minutes and washed three times in PBS before incubation in a blocking solution (1% bovine serum albumin in 0.1% Triton X-100) for 1 hour. The neurospheres were then incubated overnight at 4°C with a mouse anti-α-tubulin antibody (Sigma-Aldrich) 1/1,000 or with a rabbit anti-SOX2 antibody (Thermo Fisher Scientific Life Sciences) 1/80. After three washes in PBS, tubulin or SOX2, respectively, were revealed using Alexa Fluor 568-labeled anti-mouse or anti-rabbit antibodies (Thermo Fisher Scientific Life Sciences) at 1/200 dilution, for 2 hours. Neurospheres were washed three times with PBS before adding 3 µmol/l 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 5 minutes. After PBS washing, coverslips were mounted with a ProLong Gold antifade solution. Finally, neurospheres were observed with an inverted fluorescent microscope (Leica DMI6000; Leica Biosystems) equipped with a CoolSNAP-HQ2 camera and analyzed using MetaMorph, version 7.1.7.0, software (Molecular Devices), or with a confocal microscope (Leica TCS SP8; Leica Biosystems).
The morphology of neurospheres was observed by incubating dissociated NSCs on 10 µg/ml of poly-l-lysine (Sigma-Aldrich)-coated coverslips in 24-well plates (5 × 103 cells per well). The NSCs were treated with 0, 20, or 60 µmol/l biotinylated NFL-TBS.40-63 peptide for 7 days to obtain neurospheres. Fluorescent neurospheres were obtained following washes, fixation, permeabilization, and saturation, as previously described, and incubated overnight at 4°C with rabbit anti-nestin antibody (R&D Systems, Minneapolis, MN, http://www.rndsystems.com) 1/100 and Alexa Fluor 568 anti-rabbit antibody (Thermo Fisher Scientific Life Sciences) 1/200 for 2 hours. After PBS washing, the neurospheres were incubated with 3 µmol/l DAPI for 5 minutes, and coverslips were mounted with a ProLong Gold antifade solution. They were finally observed with a fluorescent microscope (Leica DMR; Leica Biosystems) equipped with a Scion Corporation camera (BioVision Technologies, Frederick, MD, http://www.biovision-technologies.com) and analyzed with ImageJ, version 1.43, software (NIH, Bethesda, MD, http://www.imagej.nih.gov). Treated neurospheres were also prepared for scanning electron microscopy observation, after washes with 0.2 mol/l cacodylate buffer (pH 7.4), fixation with the cacodylate buffer supplemented with 2.5% glutaraldehyde for 16 hours at 4°C, and, finally, washes with this buffer.
RNA Isolation and Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
The relative expression level of lineage-specific genes (SSEA-1, NESTIN, S100β, GFAPα, DCX, TUBB3, CNP, and MBP), described in Table 1, was quantified by reverse transcription-polymerase chain reaction (RT-PCR) assay. The sequence of the primers were optimized by Plateforme d’Analyses Cellulaire et Moléculaire, University of Angers. The RT-PCR experimental protocol and the gene expression analysis were performed by this platform. Typically, dissociated NSCs (5 × 105 cells) were treated for 7 days at 37°C with or without biotinylated NFL-TBS.40-63 peptide (20 µmol/l and 60 µmol/l) or with 1% NBCS. After cell scraping, washing with PBS, and cryopreservation, total RNA was extracted and purified using the RNeasy Micro kit (Qiagen, Courtaboeuf, France, http://www.qiagen.com) and then converted into cDNA using the SuperScriptTM II Reverse Transcriptase (Thermo Fisher Scientific Life Sciences). The cDNAs were purified (Qiaquick PCR purification kit; Qiagen), and quantitative RT-PCR analysis was performed using Maxima SYBR Green qPCR Master Mix (Fermentas; Thermo Fisher Scientific Life Sciences) and primer mix. The amount of cDNA was normalized using the rat-specific glyceraldehyde-3-phosphate dehydrogenase mRNA. The gene relative expression levels were compared with the control condition.
Table 1.
Primer sequences used for reverse transcription-polymerase chain reaction
Stereotaxic Injection of the Peptide and In Vivo Investigation
All experimental procedures and animal care were achieved in conformity with the guidelines of the French Government, with the approval of the Local Committee for Ethics on Animal Experiments (Comité d’Ethique en Expérimentation Animale des Pays-de-la-Loire). The peptide localization in the subventricular zone of adult Wistar rats was analyzed following its stereotaxic injection according to a previously described protocol [19].
Adult Wistar female rats were anesthetized by intraperitoneal injection of a mixture of ketamine 10% (80 mg/kg) and xylazine 2% (10 mg/kg). The rats were then placed on a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, http://www.kopfinstruments.com), and a sagittal incision was made through the skin to expose the cranium in which a small hole was made using a dental drill at the appropriate coordinates (−0.8 mm anterior and 1.6 mm lateral or 2 mm lateral to the bregma to inject in the right lateral ventricle or in the subventricular zone, respectively). A 20-µl volume of the 5-FAM peptide 1 mmol/l was injected using a 10-µl Hamilton syringe (Hamilton glass syringe 70 RN) with a 32-gauge needle (VWR International, Radnor, PA, http://www.us.vwr.com) at a depth of 4.3 mm from the outer border of the cranium and connected through a cannula to a 100-µl Hamilton 22-gauge syringe (Hamilton glass syringe 810 RN; VWR International) containing the peptide. A slow infusion was performed by convection-enhanced delivery with an osmotic pump (Harvard Apparatus, Holliston, MA, http://www.harvardapparatus.com) at a flow rate of 0.5 µl/minute. After the injection and slow withdrawal of the needle (0.5 mm/minute), the head skin was sutured. The rats were killed 1, 24, or 48 hours after the injection, and the brains were removed and rapidly frozen using isopentane and liquid nitrogen. They were conserved in a −80°C freezer before sectioning them using a Leica cryostat (Leica Biosystems).
For immunohistochemistry, the brain sections (12 µm thick) were fixed with cold methanol for 10 minutes, washed three times in PBS, and blocked with PBS containing 5% bovine serum albumin at room temperature for 1 hour. The sections were then incubated overnight with mouse anti-glial fibrillary acidic protein (GFAP), mouse anti-vimentin (Sigma-Aldrich), mouse anti-nestin (R&D Systems), mouse anti-βIII tubulin (Sigma-Aldrich), rabbit anti-CD133 (Proteintech, Rosemont, IL, http://www.ptglab.com), or rabbit anti-Olig2 (Abcam, Cambridge, U.K., http://www.abcam.com) antibodies diluted 1/200, 1/200, 1/50, 1/200, 1/100, and 1/100, respectively, in PBS with 5% bovine serum albumin. After washing three times in PBS, primary antibodies were revealed using Alexa Fluor 568-labeled anti-mouse or anti-rabbit antibodies (Thermo Fisher Scientific Life Sciences) at a 1/200 dilution in PBS containing 5% bovine serum albumin. They were incubated for 90 minutes at room temperature and then washed in PBS. The brain sections were then counterstained with 3 µmol/l DAPI for 5 minutes and rinsed twice with PBS. Finally, the slides were mounted with the ProLong Gold antifade reagent and observed with an inverted fluorescent microscope (Leica DMI6000; Leica Biosystems) equipped with a CoolSNAP-HQ2 camera and analyzed with the MetaMorph, version 7.1.7.0, software (Molecular Devices) or with a confocal microscope (Leica TCS SP8; Leica Biosystems).
Statistical Analysis
All experiments were repeated at least three times. For fluorescence-activated cell sorting (FACS) analysis, 20,000 events per sample were analyzed. The results are presented as the mean ± SEM, and the data are presented as bar graphs. Statistical analysis was performed with the Student t test using Prism, version 3.00, software (GraphPad, San Diego, CA, http://www.graphpad.com).
Results
We first investigated the in vitro and in vivo internalization of the NFL-TBS.40-63 peptide in NSCs from newborn and adult rats and then characterized the cellular uptake molecular mechanism. We also analyzed the possible cellular and molecular effects of the peptide, in particular on the microtubule network, cell cycle, and viability of NSCs. Finally, we evaluated the consequences of the peptide uptake on the fundamental properties of NSCs, including the neurosphere formation, self-renewal, proliferation, and differentiation.
In Vitro Peptide Uptake in NSCs Derived From Newborn Rats Occurs Through a Passive Transport
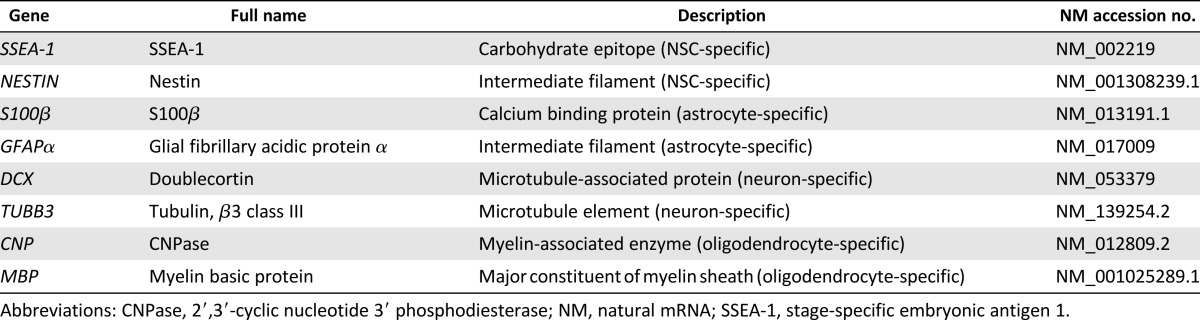
NSCs isolated from the SVZ from newborn rats were incubated with increasing concentrations of 5-FAM-labeled peptide for 30 minutes, and the peptide incorporation was evaluated using the sensitive FACS technique. Figure 1A shows a typical dose-dependent uptake of the NFL-TBS.40-63 peptide in NSCs. At 5 µmol/l 5-FAM-labeled peptide, 38.72% ± 9.08% of NSCs internalized the peptide. At 40 µmol/l, almost all NSCs were labeled with the peptide (91.07% ± 5.13%). When 0.4% trypan blue was added before the FACS reading to quench the surface-bound fluorescence of the 5-FAM-labeled peptide. The results confirmed the intracellular fluorescence of the peptide (Fig. 1A). These results were also confirmed by confocal microscopy examination of dissociated NSCs or neurospheres (Fig. 1B, 1C). The observations clearly indicated that the peptide is mainly internalized in NSCs located in the neurosphere center (Fig. 1C).
Figure 1.
Uptake of the NFL-TBS.40-63 peptide in neural stem cells from newborn rats. (A): The percentage of neural stem cells (NSCs) incorporating 5-FAM-labeled NFL-TBS.40-63 peptide, with (dark curve) and without (dotted curve) 0.4% trypan blue, was analyzed using the fluorescence-activated cell sorting technique. (B): Confocal microscopy of NSCs incubated with or without 20 µmol/l 5-FAM-labeled NFL-TBS.40-63 peptide (green). Scale bars = 10 µm. (C): Confocal microscopy of neurospheres incubated without (control) or with 20 µmol/l 5-FAM-labeled NFL-TBS.40-63 peptide (green) immunostained with anti-α-tubulin (red) to reveal the microtubule network. The nuclei were stained with DAPI (blue). Scale bars = 20 µm (original magnification ×63) and 5 µm (original magnification ×100). (D): Confocal microscopy of neurospheres incubated with 20 µmol/l 5-FAM-labeled NFL-TBS.40-63 peptide (green) immunostained with an anti-SOX2 antibody (red) to reveal stem cells. The nuclei were stained with DAPI (blue). Scale bars = 50 µm. (E): Percentages of NSCs that incorporate 5-FAM-labeled NFL-TBS.40-63 or scrambled peptides (20 µmol/l). Percentages of NSCs that incorporate 5-FAM-labeled NFL-TBS.40-63 peptide (20 µmol/l), after pretreatment in an ATP-depleted buffer or at 4°C (F) or with different endocytosis and signaling pathway inhibitors (G). Data are presented as mean ± SEM. ∗∗, p < .01. Abbreviations: 5-FAM, 5-carboxyfluorescein; DAM, 5-(N,N-dimethyl) amiloride hydrochloride; DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; PMA, phorbol 12-myristate 13-acetate.
The specific uptake of the peptide by NSCs was also confirmed using the stem cell-specific marker (SOX2). Figure 1D clearly shows colocalization of the FAM-fluorescent peptide (green) and the SOX2-labeled NSCs (red), which are mainly localized in the center of the neurosphere (Fig. 1D). As a control, we used a scrambled peptide (NFL-SCR), which contains the same amino acids as the NFL-TBS.40-63 peptide but in a disorganized sequence. Previous studies showed that the NFL-SCR peptide enters much less in glioblastoma cells compared with the massive uptake of the NFL-TBS.40-63 peptide [19]. Using FACS analysis, Figure 1E shows that the NFL-SCR peptide uptake in NSCs is significantly lower than that of the NFL-TBS.40-63 peptide, with 51.23% ± 5.23% uptake for NFL-SCR and 87.57% ± 3.75% for NFL-TBS.40-63 (at 20 µmol/l peptide). These results have confirmed the importance of the sequence and structure of the NFL-TBS.40-63 peptide for its selective entry into NSCs, which was also demonstrated for glioblastoma cells [19, 26].
Next, we investigated the molecular mechanism responsible for the selective uptake of the NFL-TBS.40-63 peptide. In particular, we evaluated endocytosis and direct translocation, two main pathways known for the uptake of cell-penetrating peptides [27]. The cellular uptake was first evaluated by incubating the cells at 4°C or in an ATP-depleted buffer for 30 minutes before adding the peptide. Then, the peptide was added, and the cells were further incubated in the same conditions for 30 minutes. In these conditions, it is known that endocytosis, an active transport (energy- and temperature-dependent), is blocked, but direct translocation, a passive transport (energy- and temperature-independent), is not [28, 29]. The peptide uptake was not significantly affected by incubation at 4°C or in the ATP-depletion buffer, indicating that the internalization of the peptide in NSCs occurs mainly through a passive mechanism (Fig. 1F). To further confirm the passive transport of the peptide into NSCs, we also tested a panel of inhibitors for each endocytic pathway, as well as inhibitors of signaling pathways involved in the endocytic mechanism. Chlorpromazine, PMA, and DAM inhibit clathrin-dependent endocytosis, caveolin-dependent endocytosis, and micropinocytosis, respectively [30–32]. Genistein inhibits receptor tyrosine kinase activation, sunitinib inhibits VEGF receptor/platelet-derived growth factor receptor activation, and gefitinib inhibits EGF receptor activation. The endocytosis-associated PI3K/Akt and mitogen-activated protein kinase signaling pathways are inhibited with Wortmannin and U0126, respectively [33–36]. Figure 1G shows that NFL-TBS.40-63 peptide uptake is poorly affected by the presence of these inhibitors and confirms that endocytosis is not responsible for the internalization of this peptide in NSCs.
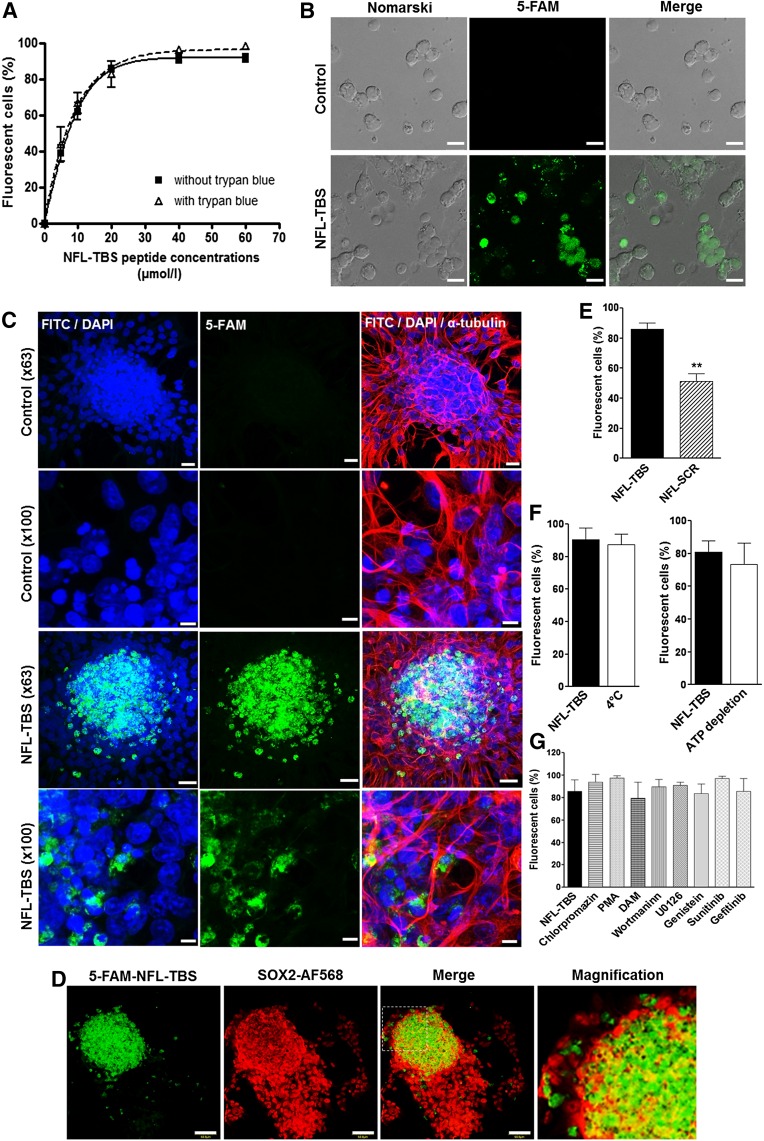
NFL-TBS.40-63 Peptide Has No Major Effect on the Cell Cycle or Microtubule Network of NSCs Derived From Newborn Rats but Decreases Their Viability
To investigate the possible effects of the NFL-TBS-40.63 peptide on NSCs, we analyzed their cell cycle using FACS. Apart from colchicine, used as a positive control to disrupt the microtubule network, the cell cycle of NSCs was not significantly affected by the presence of the NFL-TBS.40-63 peptide (60%–65% of cells in G1 phase, 10% in S phase, and 7% in G2/M phases; Fig. 2A). These results clearly show that in contrast to glioblastoma cells [18], the peptide has no major effect on the cell cycle of NSCs. Moreover, examination with the inverted fluorescent microscope (Leica DMI6000; Leica Biosytems) indicates that the cell shape and microtubule network of NSCs are not affected by the peptide, and colchicine provokes profound alterations (Fig. 2B).
Figure 2.
The NFL-TBS.40-63 peptide has no detectable effect on the microtubule network and reduces the viability of neural stem cells. (A): The percentages of neural stem cells (NSCs) at G1, S, or G2/M phases of the cell cycle after treatment with colchicine or increasing concentrations of the NFL-TBS.40-63 peptide. (B): NSCs were incubated with 20 µmol/l 5-FAM-labeled NFL-TBS.40-63 peptide (green) and immunostained to reveal the microtubule network with an anti-α-tubulin (red). The nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). A magnification of a region of interest is included (dotted framed images). Scale bars = 20 µm. (C): Percentages of viable NSCs after treatment with colchicine or increasing concentrations of the NFL-TBS.40-63 peptide. Data are presented as mean ± SEM. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. Abbreviation: 5-FAM, 5-carboxyfluorescein.
To evaluate the viability of NSCs in the presence of increasing concentrations of the peptide, we used the trypan blue exclusion technique (trypan blue penetrates only in dead cells). Typically, in the presence of low concentrations of the NFL-TBS.40-63 peptide (10 and 20 µmol/l), no major effect on NSC viability is observed after 72 hours at 37°C (Fig. 2C). However, the number of viable NSCs was significantly reduced following their incubation with higher concentrations of the peptide. At 40 µmol/l peptide, only 44.71% ± 7.046% of the cells were viable (compared with 71.36% ± 7.923% of viable cells in the control condition). A strong cytotoxic effect was observed with 100 µmol/l NFL-TBS.40-63 peptide (only 29.53% ± 6.536% of NSCs were viable). As a positive control, we incubated NSCs with 1 µg/ml colchicine, which provokes a strong cytotoxic effect (only 15.70% ± 2.277% of viable cells; Fig. 2C).
Other Effects of the NFL-TBS.40-63 Peptide on NSCs Derived From Newborn Rats
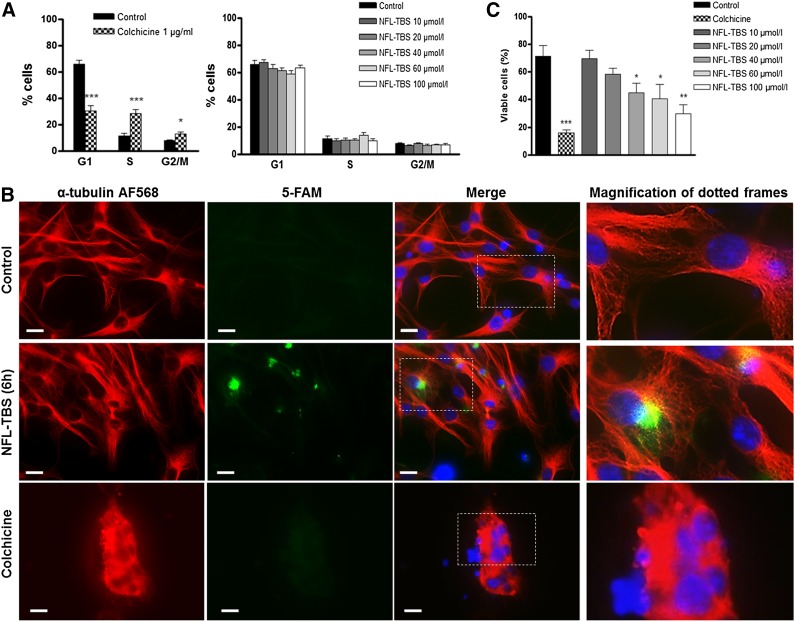
When NSCs are exposed to increasing concentrations of the peptide, the number of primary neurospheres (manually counted using the inverted Leica microscope) was not profoundly affected, except at 100 µmol/l (Fig. 3A). In contrast, when primary neurospheres were first cultured in the presence of the peptide and were then dissociated and incubated only in the normal culture medium, NSCs generated a significantly lower number of secondary neurospheres compared with the control (Fig. 3B). As a positive control, NSCs were incubated with 1 µg/ml colchicine, which disrupts both primary and secondary neurosphere formation (Fig. 3A, 3B). Together, these results show that the peptide has no major effect on the formation of primary neurospheres (except at high concentrations) but affects their self-renewal.
Figure 3.
Effects of the NFL-TBS.40-63 peptide on neural stem cell properties. (A): Number of primary neurospheres formed after incubation of neural stem cells (NSCs) with colchicine or increasing concentrations of the NFL-TBS.40-63 peptide. (B): Self-renewal was evaluated by manually counting the numbers of secondary neurospheres after dissociation of the primary neurospheres that were previously treated with colchicine or increasing concentrations of peptide. (C): Proliferation was evaluated by measuring the DNA concentration of NSCs treated with colchicine or increasing concentrations of the NFL-TBS.40-63 peptide. (D): Morphology of neurospheres after incubation of NSCs without or with 20 or 60 µmol/l NFL-TBS.40-63 peptide. Scale bars = 30 µm. (E): Percentages of adherent primary neurospheres after incubation of NSCs with increasing concentrations of the NFL-TBS.40-63 peptide. (F): Scanning electron microscopy of NSCs treated without or with 20 µmol/l NFL-TBS.40-63 peptide. Scale bars = 2 µm. (G): Primary neurospheres, treated without (control) or with 20 or 60 µmol/l NFL-TBS.40-63 peptide, were immunostained with anti-nestin (green) and DAPI (blue). Scale bars = 40 µm. Percentages of NSCs that expressed PSA-NCAM (H), CD90, or A2B5 (I) after treatment with increasing concentrations of the NFL-TBS.40-63 peptide or 1% new born calf serum (serum). Data are presented as mean ± SEM. (J): The relative expression level of SSEA-1, NESTIN, S100β, GFAPα, DCX, TUBB3, CNP, and MBP was quantified via quantitative reverse transcription-polymerase chain reaction analysis in NSCs treated without (control) or with 20 µmol/l and 60 µmol/l the NFL-TBS.40-63 peptide or with 1% new born calf serum (serum). The gene relative expression levels were compared with the control condition. Data are presented as mean ± SEM. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; PSA-NCAM, polysialylated-neural cell adhesion molecule.
The self-renewal disruption of NSCs was associated with a significant decrease in their proliferation when incubated with the peptide (Fig. 3C). Colchicine also significantly decreased the NSC proliferation (Fig. 3C); however, unlike the peptide, this effect was associated with cell cycle alteration and microtubule disruption (Fig. 2A, 2B).
When NSCs were treated for 7 days with increasing concentrations of peptide, the typical spherical shape of neurospheres was modified into a more flattened shape, surrounded by increased adherent cells and cellular extensions. This phenomenon was first observed at 20 µmol/l and was amplified with higher concentrations of the peptide (Fig. 3D). The number of adherent neurospheres was significantly increased at peptide concentrations of 40 µmol/l and 60 µmol/l and no floating neurospheres could be observed (Fig. 3E). Using scanning electron microscopy, we confirmed this atypical shape of the adherent NSCs, together with multiple cellular extensions, compared with the typical spherical shape of NSCs incubated without the NFL-TBS.40-63 peptide (Fig. 3F; supplemental online Fig. 3). Some of these adherent cells were more elongated with high peptide concentrations, and they still expressed nestin, which is the intermediate filament expressed specifically by NSCs [37] (Fig. 3G).
The increased attachment of NSCs at high peptide concentrations is related to a weak decrease of the PSA-NCAM expression, which was also observed when NSCs were incubated with 1% NBCS (Fig. 3H). Finally, higher amounts of glial and neuronal progenitor markers (A2B5 and CD90, respectively) were detected by FACS when NSCs were incubated with increasing concentrations of the peptide (Fig. 3I).
Interestingly, RT-PCR analysis revealed that the lineage of NSC differentiation was differently affected after incubation with the NFL-TBS.40-63 peptide for 7 days. A significant downward expression of the astrocyte-lineage specific genes (S100β and GFAPα) is observed when primary NSCs were incubated with 20 or 60 µmol/l the NFL-TBS.40-63 peptide, but these genes were overexpressed when NSCs were incubated with serum (Fig. 3J). Moreover, for some genes, differential expression was observed depending on the peptide concentration. For instance, with the NFL-TBS.40-63 peptide at 20 µmol/l, an increased expression of stem cell markers (SSEA-1 and NESTIN), neuronal-lineage markers (DCX and TUBB3), and oligodendrocyte-lineage markers (CNP) was observed. However, at a higher concentration (60 µmol/l), the peptide reduces the expression of DCX and TUBB3, the markers of mature neurons. Finally, at high concentration, the peptide promoted an increased expression of CNP, a marker of premature oligodendrocytes. In contrast, the expression of MBP, a marker of mature oligodendrocytes, was downregulated (Fig. 3J). Together, these results indicate that the NFL-TBS.40-63 peptide modulates the different NSC differentiation pathways in a concentration-dependent fashion.
In Vitro and In Vivo Peptide Uptake in NSCs Derived From Adult Rats
We also analyzed the NFL-TBS.40-63 peptide uptake in adult NSCs in vitro by flow cytometry and in vivo by stereotaxic injection of the peptide in the subventricular zone or in the lateral ventricle of adult rats, followed by immunohistochemistry.
The NSCs were isolated from the SVZ of adult rats and incubated with increasing concentrations of 5-FAM-labeled peptide for 30 minutes. The incorporation evaluated by the sensitive FACS technique showed a dose-dependent uptake of the peptide in these cells, with typically 92.74% ± 2.51% of cells containing the peptide at 40 µmol/l, results similar to the uptake observed for NSCs isolated from newborn rats (Figs. 1A, 4A).
Figure 4.
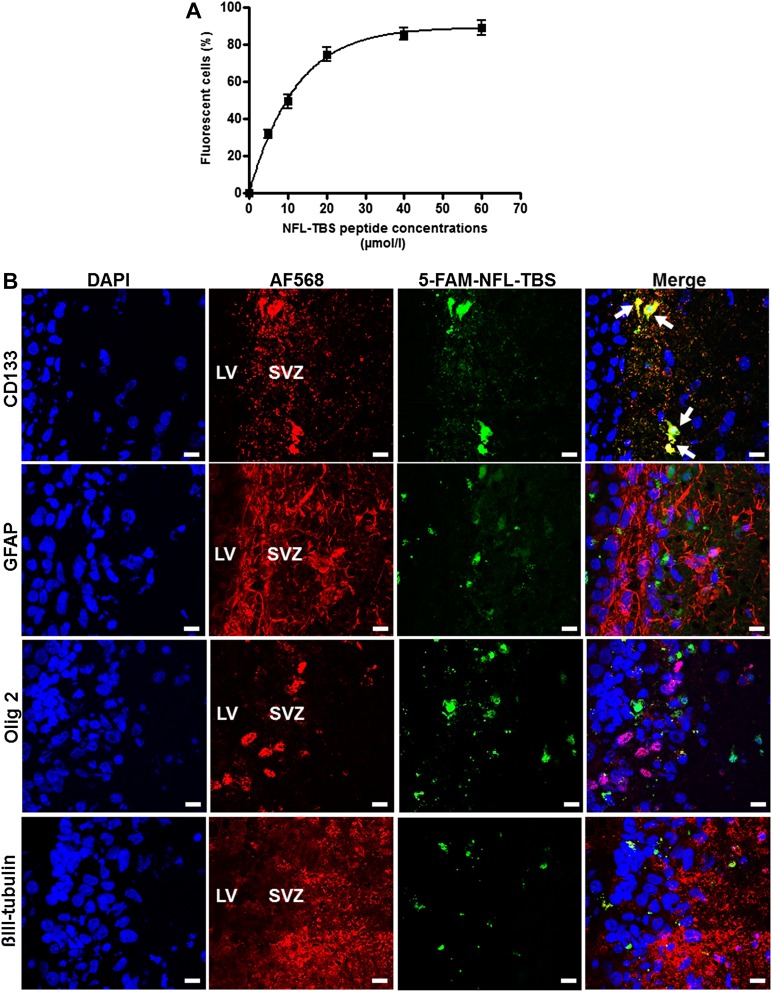
In vitro and in vivo uptake of the NFL-TBS.40-63 peptide in neural stem cells (NSCs) from adult rats. (A): Percentages of adult NSCs that incorporated the 5-FAM-labeled NFL-TBS.40-63 peptide. Data are presented as mean ± SEM. (B): Confocal microscope localization of the 5-FAM-labeled NFL-TBS.40-63 peptide (green) after its injection in the SVZ of adult rats. At 48 hours after peptide injection, immunohistochemistry was performed to reveal neural stem cells (CD133; red), astrocytes (GFAP; red), oligodendrocytes (Olig 2; red), neurons (βIII-tubulin; red), and nuclei (DAPI; blue). White arrows indicate colocalization of the peptide with NSCs. Scale bars = 10 µm. Abbreviations: 5-FAM, 5-carboxyfluorescein; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; LV, lateral ventricle; SVZ, subventricular zone.
The possibility to target in vivo the uptake of the peptide by adult NSCs was then tested. Typically, when the NFL-TBS.40-63 peptide (20 µl, at 1 mmol/l) is directly injected in the subventricular zone, 48 hours later, the peptide was colocalized mainly with the neural stem cell marker (CD133) but poorly or not at all with differentiated cells (astrocytes [GFAP], oligodendrocytes [Olig 2], and neurons [βIII-tubulin; Fig. 4B).
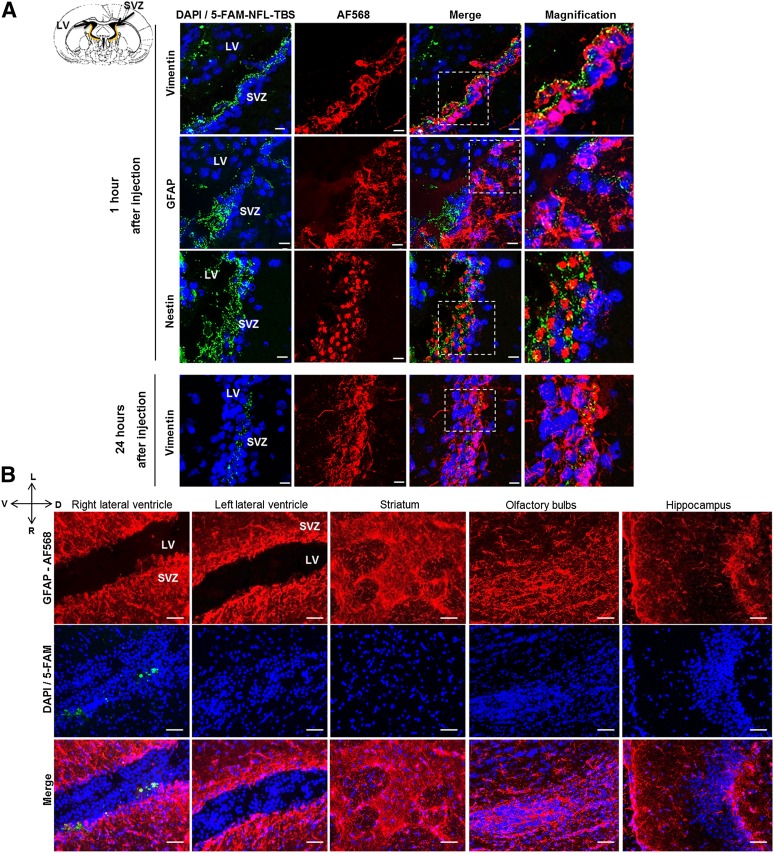
The NFL-TBS.40-63 peptide (20 µl, at 1 mmol/l) was also injected in the right lateral ventricle of adult rats, and its localization was examined 1 and 24 hours after its injection (Fig. 5A). The SVZ was separated from the lateral ventricles by a layer of ciliated ependymal cells that express vimentin, with GFAP-expressing cells in the subependymal zone [7]. NSCs can be recognized with the anti-nestin antibody. The brain sections revealed that 1 hour after its injection, the peptide was localized in the right lateral ventricle, in the ependymal cells of the SVZ, and in the subependymal zone (Fig. 5A). At high magnification, confocal microscope analysis indicated that the peptide is present in ependymal cells (vimentin-positive cells), and some amount of peptide can cross the ependymal barrier to penetrate the subependymal zone (GFAP-positive cells), where NSCs and progenitors are found (nestin-positive cells), without causing detectable cytotoxicity, even 24 hours later. When other areas of the brain were examined at different time points (typically 24 hours later; Fig. 5B) and at the studied concentration (20 µl, at 1 mmol/l), the peptide was found only in the subventricular zone close to the right lateral ventricle, but no peptide was observed in the other brain areas (left lateral ventricle, striatum, olfactory bulbs, and hippocampus).
Figure 5.
In vivo localization of the NFL-TBS.40-63 peptide after injection in the right lateral ventricle of adult rats. (A): Confocal microscope localization of the 5-FAM-labeled NFL-TBS.40-63 peptide (green) in the subventricular zone of adult rats 1 and 24 hours after its injection in the right lateral ventricle. Immunofluorescence analysis of vimentin (red; ependymal cells), GFAP (red; subependymal cells), nestin (red; neural stem cells), and DAPI (blue). Scale bars = 10 µm. (B): Confocal microscope localization of the 5-FAM-labeled NFL-TBS.40-63 peptide (green) in different brain areas after injection in the right lateral ventricle. Immunohistochemistry was performed to reveal astrocytes (GFAP; red) and DAPI (nuclei; blue). Scale bars = 50 µm. Abbreviations: 5-FAM, 5-carboxyfluorescein; D, dorsal; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; L, left; LV, lateral ventricle; R, right; SVZ, subventricular zone; V, ventral.
Discussion
In the present study, we showed that the NFL-TBS.40-63 peptide is taken up by a high percentage of NSCs derived from newborn and adult rats through a dose-dependent uptake, as already described for glioblastoma cells [19]. Recent studies also showed in vitro peptide uptake by astrocytes and neurons but at a much lower amount compared with glioblastoma cells [18, 19]. However, when injected in the striatum of normal rats, the peptide is not found in healthy tissue [19]. These observations were confirmed after the injection of the peptide directly in the subventricular zone, where the peptide only colocalized with NSCs but not with differentiated neural cells. Recent studies also indicated that the peptide can penetrate in vitro in oligodendrocytes, preferentially differentiated oligodendrocytes than progenitors and can promote their growth and survival [23]. Taken together, these data indicate that the NFL-TBS.40-63 peptide massively targets glioblastoma and NSCs both in vitro and in vivo, but it can also penetrate in low amounts in other type of neural cells. In vivo, when injected in the lateral ventricle, the NFL-TBS.40-63 peptide is found in vimentin-positive ependymal cells and can cross the ependymal wall to target NSCs that are nestin-positive (Fig. 5). Finally, when the peptide is directly injected into the subventricular zone, it preferentially targets CD133-positive NSCs compared with other neural cells (Fig. 4).
As previously suggested, the high proliferative activity of glioblastoma cells could account for the massive internalization of the peptide; however, interestingly, the uptake molecular mechanism in NSCs was fundamentally different from that of glioblastoma cells. In glioblastoma cells, the peptide is internalized mainly by endocytosis [21], and in NSCs, it can translocate directly through the membrane by a passive transport, which is temperature- and energy-independent. This was also confirmed using a complete panel of inhibitors of endocytosis, which did not affect the peptide uptake. It indicates that the peptide uses a unique entry pathway that is different from other cell-penetrating peptides, such as R8 and Tat, which transduce in induced pluripotent stem cells through macropinocytosis, an endocytic pathway [38]. One possibility could be that a particular composition of these cells for lipids or phospho-lipids that might modulate the translocation of the peptide [39, 40]. Moreover, when injected in the lateral ventricle of adult rats, the NFL-TBS.40-63 peptide is located in the ependymal layer and a part of it in the subependymal area, showing that the peptide can also enter in vivo in the SVZ to target NSCs. It is important to note that the ependymal cells of the SVZ express CD133, a stem cell marker, and can also represent a population of NSCs in the SVZ [41]. To our knowledge, the present study is the first time that a peptide has been shown to enter so specifically and massively in vitro and in vivo in NSCs.
As previously described, the NFL-TBS.40-63 peptide has several specific effects on glioblastoma cells, in particular the disruption of their microtubule network associated with a decrease of proliferation, migration, and viability at low peptide concentrations (from 5 to 10 µmol/l) [18, 19]. Such a disruption of the microtubule network and, consequently, the alteration of the cell cycle were not observed in NSCs. Tubulin immunostaining in NSCs revealed a normally organized microtubule network after peptide incubation. Longer treatment with the peptide did not affect the cell cycle of NSCs. This suggests that the peptide interacts with a tubulin strongly expressed in glioblastoma cells that is absent or at low concentration in healthy cells. High levels of βIII-tubulin were found in glioblastoma, and βII-tubulin was expressed mainly in NSCs [42, 43]. However, the viability of NSCs is affected at high concentrations of the peptide, as observed in glioblastoma cells, but no major toxicity was observed in other healthy neural cells at high peptide concentrations (100 µmol/l and greater) [19]. Recent work has shown a beneficial effect of the peptide on the maturation and differentiation of oligodendrocytes, as well as their protection against lysophosphatidylcholine treatment [22, 23].
Finally, we investigated the effects of the NFL-TBS.40-63 peptide on neurosphere formation, self-renewal proliferation, and differentiation. When incubated in serum-free medium and EGF, NSCs proliferated and formed typical neurospheres. After dissociation, secondary spheres were formed from NSCs. Our results show that the peptide has no major effect on primary neurosphere formation, except at very high concentrations (100 µmol/l). Moreover, at high concentrations (greater than 40 µmol/l), the peptide disturbs the self-renewal capacity of NSCs, decreasing the number of secondary neurospheres compared with untreated cells. This effect is associated with fewer viable cells and decreased expression of PSA-NCAM, together with increased cell attachment. The PSA-NCAM protein characterizes the NSC growth with limited cell-cell interactions and lower interactions of cells with the extracellular matrix [44]. Increased NSC adhesion was also reported when the cells were cultured on phospholipid bilayers functionalized with different RGD-containing peptides, probably because of increased peptide interactions with receptors on the NSC surface [45]. Unlike these RGD peptides, the NFL-TBS.40-63 peptide was internalized in NSCs, promoting adhesion. Moreover, the peptide can modulate NSC differentiation in a concentration-dependent manner. The differentiation of neurons and oligodendrocytes can be induced with a low peptide concentration. A similar effect (at 10 µmol/l NFL-TBS.40-63 peptide) has already been described for the maturation and differentiation of oligodendrocytes from glial cell cultures derived from newborn rats [22, 23]. However, at higher concentrations, the peptide can only promote the differentiation of cells from the oligodendrocyte lineage but reduces their maturation. Further investigations are necessary to determine the molecular mechanism involved in these effects. As demonstrated in a previous study, the NFL-TBS.40-63 peptide can modulate the expression of a particular microRNA involved in glioblastoma pathogenesis [46]. Thus, one possibility could be that this peptide could induce post-translational regulation of NSCs, especially on microRNAs, which represent key regulators of NSC properties [47].
Conclusion
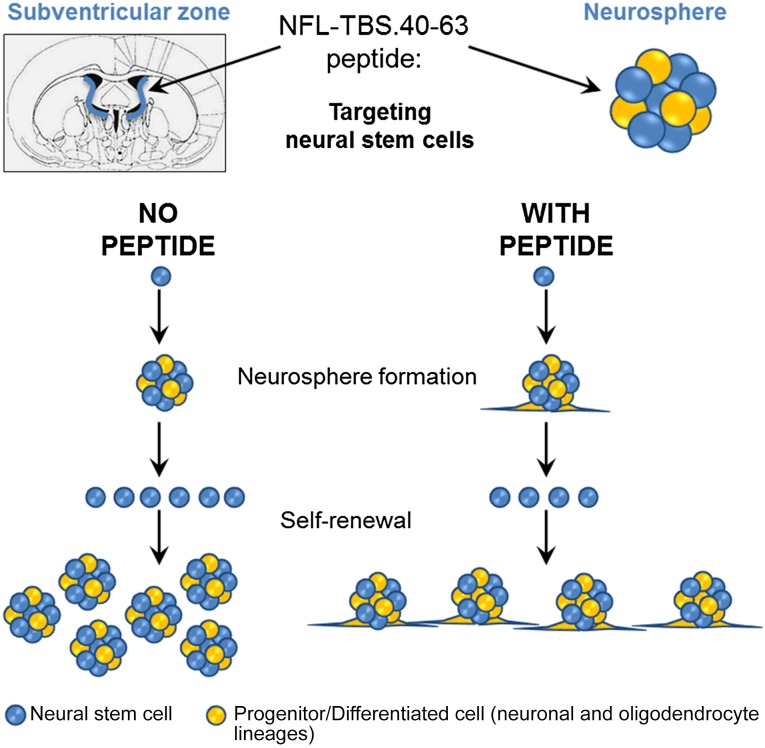
Taken together, our data show that the NFL-TBS.40-63 peptide can target in vitro and in vivo NSC and modulate their properties (Fig. 6), as previously shown with the Tat and RGD peptides for mesenchymal stem cells [48–50]. However, to our knowledge, the Tat peptide did not show the same in vitro and in vivo NSC targeting capacity as that with the NFL-TBS.40-63 peptide. One possibility could be to use the NFL-TBS.40-63 peptide to functionalize lipid nanoparticles to target their entry into NSCs, as previously shown for glioblastoma cells [20]. Complexes of plasmid DNA and nanoparticles functionalized with the cell-penetrating Tat peptide were proposed to genetically modify glioblastoma and NSC cell lines [51]. Therefore, the NFL-TBS.40-63 peptide represents a promising new selective delivery system for NSCs and a new potential application for stem cell-based therapeutic strategies in several pathological conditions such as brain tumors and neurodegenerative disorders.
Figure 6.
Schematic representation of the NFL-TBS.40-63 peptide effects on neural stem cells (NSCs). The NFL-TBS.40-63 peptide targets NSCs in vitro and in vivo. In culture, the neurosphere formation was not affected by the peptide, and the self-renewal capacity was reduced, with increased stem cell attachment and differentiation.
Supplementary Material
Acknowledgments
We acknowledge the assistance of Rodolphe Perrot and Guillaume Mabilleau from Service Commun d’Imageries et d’Analyses Microscopiques, Catherine Guillet and Jérôme Cayon from Plateforme d’Analyses Cellulaire et Moléculaire, and the staff from Service Commun d’Animalerie Hospitalo-Universitaire. This work was supported by Association Française contre les Myopathies, Association pour la Recherche sur le Cancer, Ciblage Moléculaire et Thérapeutique, Maturation & Accelerating Translation With Industry, L’Université Nantes, Angers, Le Mans, and “Région des Pays de la Loire” to J.E. C.L.-C. was supported by the “Ministère de l’Enseignement Supérieur et de la Recherche,” by Association pour la Recherche sur le Cancer, and by the “Sociétés d’Accélération du Transfert de Technologie Ouest Valorisation.” K.B. is supported by a grant from the University of Angers, in the framework of NanoFar “European Doctorate in Nanomedicine” Erasmus Mundus Joint Doctorates program funded by the Education, Audiovisual and Culture Executive Agency.
Author Contributions
C.L.-C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; K.B.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.E.: conception and design, financial support, provision of study material, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 4.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton LK, Truong MK, Bednarczyk MR, et al. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience. 2009;164:1044–1056. doi: 10.1016/j.neuroscience.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Miller FD, Gauthier-Fisher A. Home at last: Neural stem cell niches defined. Cell Stem Cell. 2009;4:507–510. doi: 10.1016/j.stem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Hall PA, Watt FM. Stem cells: The generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 11.Azari H, Rahman M, Sharififar S, et al. Isolation and expansion of the adult mouse neural stem cells using the neurosphere assay. J Vis Exp. 2010:2393. doi: 10.3791/2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SU, Lee HJ, Kim YB. Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology. 2013;33:491–504. doi: 10.1111/neup.12020. [DOI] [PubMed] [Google Scholar]
- 13.Bexell D, Svensson A, Bengzon J. Stem cell-based therapy for malignant glioma. Cancer Treat Rev. 2013;39:358–365. doi: 10.1016/j.ctrv.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Nait-Oumesmar B, Picard-Riera N, Kerninon C, et al. Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc Natl Acad Sci USA. 2007;104:4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller FJ, Snyder EY, Loring JF. Gene therapy: Can neural stem cells deliver? Nat Rev Neurosci. 2006;7:75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- 16.Nam H, Lee KH, Nam DH, et al. Adult human neural stem cell therapeutics: Current developmental status and prospect. World J Stem Cells. 2015;7:126–136. doi: 10.4252/wjsc.v7.i1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. 2014;32:795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bocquet A, Berges R, Frank R, et al. Neurofilaments bind tubulin and modulate its polymerization. J Neurosci. 2009;29:11043–11054. doi: 10.1523/JNEUROSCI.1924-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berges R, Balzeau J, Peterson AC, et al. A tubulin binding peptide targets glioma cells disrupting their microtubules, blocking migration, and inducing apoptosis. Mol Ther. 2012;20:1367–1377. doi: 10.1038/mt.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balzeau J, Pinier M, Berges R, et al. The effect of functionalizing lipid nanocapsules with NFL-TBS.40-63 peptide on their uptake by glioblastoma cells. Biomaterials. 2013;34:3381–3389. doi: 10.1016/j.biomaterials.2013.01.068. [DOI] [PubMed] [Google Scholar]
- 21.Lépinoux-Chambaud C, Eyer J. The NFL-TBS.40-63 anti-glioblastoma peptide enters selectively in glioma cells by endocytosis. Int J Pharm. 2013;454:738–747. doi: 10.1016/j.ijpharm.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Fressinaud C, Eyer J. Neurofilament-tubulin binding site peptide NFL-TBS.40-63 increases the differentiation of oligodendrocytes in vitro and partially prevents them from lysophosphatidyl choline toxicity. J Neurosci Res. 2014;92:243–253. doi: 10.1002/jnr.23308. [DOI] [PubMed] [Google Scholar]
- 23.Fressinaud C, Eyer J. Neurofilaments and NFL-TBS.40-63 peptide penetrate oligodendrocytes through clathrin-dependent endocytosis to promote their growth and survival in vitro. Neuroscience. 2015;298:42–51. doi: 10.1016/j.neuroscience.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Coronas V, Bantubungi K, Fombonne J, et al. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J Neurochem. 2004;91:1292–1301. doi: 10.1111/j.1471-4159.2004.02823.x. [DOI] [PubMed] [Google Scholar]
- 25.Nicoleau C, Benzakour O, Agasse F, et al. Endogenous hepatocyte growth factor is a niche signal for subventricular zone neural stem cell amplification and self-renewal. Stem Cells. 2009;27:408–419. doi: 10.1634/stemcells.2008-0226. [DOI] [PubMed] [Google Scholar]
- 26.Berges R, Balzeau J, Takahashi M, et al. Structure-function analysis of the glioma targeting NFL-TBS.40-63 peptide corresponding to the tubulin-binding site on the light neurofilament subunit. PLoS One. 2012;7:e49436. doi: 10.1371/journal.pone.0049436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart KM, Horton KL, Kelley SO. Cell-penetrating peptides as delivery vehicles for biology and medicine. Org Biomol Chem. 2008;6:2242–2255. doi: 10.1039/b719950c. [DOI] [PubMed] [Google Scholar]
- 28.Mano M, Teodósio C, Paiva A, et al. On the mechanisms of the internalization of S4(13)-PV cell-penetrating peptide. Biochem J. 2005;390:603–612. doi: 10.1042/BJ20050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derossi D, Calvet S, Trembleau A, et al. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271:18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 30.Duchardt F, Fotin-Mleczek M, Schwarz H, et al. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 31.Anderson HA, Chen Y, Norkin LC. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koivusalo M, Welch C, Hayashi H, et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol. 2010;188:547–563. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eierhoff T, Hrincius ER, Rescher U, et al. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010;6:e1001099. doi: 10.1371/journal.ppat.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimitropoulos K, Giannopoulou E, Argyriou AA, et al. The effects of anti-VEGFR and anti-EGFR agents on glioma cell migration through implication of growth factors with integrins. Anticancer Res. 2010;30:4987–4992. [PubMed] [Google Scholar]
- 35.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 36.Colin D, Limagne E, Jeanningros S, et al. Endocytosis of resveratrol via lipid rafts and activation of downstream signaling pathways in cancer cells. Cancer Prev Res (Phila) 2011;4:1095–1106. doi: 10.1158/1940-6207.CAPR-10-0274. [DOI] [PubMed] [Google Scholar]
- 37.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 38.Yukawa H, Noguchi H, Nakase I, et al. Transduction of cell-penetrating peptides into induced pluripotent stem cells. Cell Transplant. 2010;19:901–909. doi: 10.3727/096368910X509031. [DOI] [PubMed] [Google Scholar]
- 39.Terrone D, Sang SL, Roudaia L, et al. Penetratin and related cell-penetrating cationic peptides can translocate across lipid bilayers in the presence of a transbilayer potential. Biochemistry. 2003;42:13787–13799. doi: 10.1021/bi035293y. [DOI] [PubMed] [Google Scholar]
- 40.Henriques ST, Castanho MA. Translocation or membrane disintegration? Implication of peptide-membrane interactions in pep-1 activity. J Pept Sci. 2008;14:482–487. doi: 10.1002/psc.1003. [DOI] [PubMed] [Google Scholar]
- 41.Coskun V, Wu H, Blanchi B, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura Y, Yamamoto M, Oda E, et al. Expression of tubulin beta II in neural stem/progenitor cells and radial fibers during human fetal brain development. Lab Invest. 2003;83:479–489. doi: 10.1097/01.lab.0000063930.75913.b3. [DOI] [PubMed] [Google Scholar]
- 43.Katsetos CD, Dráberová E, Legido A, et al. Tubulin targets in the pathobiology and therapy of glioblastoma multiforme. I. Class III beta-tubulin. J Cell Physiol. 2009;221:505–513. doi: 10.1002/jcp.21870. [DOI] [PubMed] [Google Scholar]
- 44.Johnson CP, Fujimoto I, Rutishauser U, et al. Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J Biol Chem. 2005;280:137–145. doi: 10.1074/jbc.M410216200. [DOI] [PubMed] [Google Scholar]
- 45.Ananthanarayanan B, Little L, Schaffer DV, et al. Neural stem cell adhesion and proliferation on phospholipid bilayers functionalized with RGD peptides. Biomaterials. 2010;31:8706–8715. doi: 10.1016/j.biomaterials.2010.07.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivalin R, Lépinoux-Chambaud C, Eyer J, et al. The NFL-TBS.40-63 anti-glioblastoma peptide disrupts microtubule and mitochondrial networks in the T98G glioma cell line. PLoS One. 2014;9:e98473. doi: 10.1371/journal.pone.0098473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji F, Lv X, Jiao J. The role of microRNAs in neural stem cells and neurogenesis. J Genet Genomics. 2013;40:61–66. doi: 10.1016/j.jgg.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Shah BS, Clark PA, Moioli EK, et al. Labeling of mesenchymal stem cells by bioconjugated quantum dots. Nano Lett. 2007;7:3071–3079. doi: 10.1021/nl071547f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei Y, Tang H, Yao L, et al. Applications of mesenchymal stem cells labeled with Tat peptide conjugated quantum dots to cell tracking in mouse body. Bioconjug Chem. 2008;19:421–427. doi: 10.1021/bc0700685. [DOI] [PubMed] [Google Scholar]
- 50.Jo J, Song H, Park SG, et al. Regulation of differentiation potential of human mesenchymal stem cells by intracytoplasmic delivery of coactivator-associated arginine methyltransferase 1 protein using cell-penetrating peptide. Stem Cells. 2012;30:1703–1713. doi: 10.1002/stem.1146. [DOI] [PubMed] [Google Scholar]
- 51.Song HP, Yang JY, Lo SL, et al. Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials. 2010;31:769–778. doi: 10.1016/j.biomaterials.2009.09.085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.