This phase I clinical trial evaluated the safety and clinical efficacy of adipose-derived stromal cells (ASCs) in osteoarthritis. Eighteen patients with severe knee osteoarthritis were treated with a single intra-articular injection of autologous ASCs at low (2 × 106 cells), medium (10 × 106), or high (50 × 106) doses (n = 6 each). After 6 months, no serious adverse events were reported, and patients treated with low-dose ASCs significantly improved in pain and function.
Keywords: Osteoarthritis, Adipose mesenchymal stromal cells, Intra-articular injection, Therapeutic potential, Regenerative medicine, Phase I clinical trial
Abstract
Osteoarthritis (OA) is the most widespread musculoskeletal disorder in adults. It leads to cartilage damage associated with subchondral bone changes and synovial inflammation, causing pain and disability. The present study aimed at evaluating the safety of a dose-escalation protocol of intra-articular injected adipose-derived stromal cells (ASCs) in patients with knee OA, as well as clinical efficacy as secondary endpoint. A bicentric, uncontrolled, open phase I clinical trial was conducted in France and Germany with regulatory agency approval for ASC expansion procedure in both countries. From April 2012 to December 2013, 18 consecutive patients with symptomatic and severe knee OA were treated with a single intra-articular injection of autologous ASCs. The study design consisted of three consecutive cohorts (six patients each) with dose escalation: low dose (2 × 106 cells), medium dose (10 × 106), and high dose (50 × 106). The primary outcome parameter was safety evaluated by recording adverse events throughout the trial, and secondary parameters were pain and function subscales of the Western Ontario and McMaster Universities Arthritis Index. After 6 months of follow-up, the procedure was found to be safe, and no serious adverse events were reported. Four patients experienced transient knee joint pain and swelling after local injection. Interestingly, patients treated with low-dose ASCs experienced significant improvements in pain levels and function compared with baseline. Our data suggest that the intra-articular injection of ASCs is a safe therapeutic alternative to treat severe knee OA patients. A placebo-controlled double-blind phase IIb study is being initiated to assess clinical and structural efficacy.
Significance
Although this phase I study included a limited number of patients without a placebo arm, it showed that local injection of autologous adipose-derived stem cells was safe and well tolerated in patients with knee osteoarthritis. This study also provides encouraging preliminary evidence of efficacy. Larger and controlled long-term studies are now mandatory to confirm whether this new strategy of cell therapy can improve pain and induce structural benefit in osteoarthritis.
Introduction
Osteoarthritis (OA) is a multifactorial, slowly progressive degenerative disorder of the joints leading to irreversible damage of the cartilage, sclerosis of subchondral bone, and synovial inflammation [1]. As a consequence of increasing longevity and obesity, the cost of OA to the health care system rapidly grows. Current treatment strategies have no impact on the progressive degeneration of joint tissues. In this context, the use of mesenchymal stromal stem cells (MSCs) is an attractive therapeutic option thanks to their chondrogenic and anti-inflammatory properties [2]. Adipose tissue-derived MSCs (ASCs) share similar properties with bone marrow-derived MSCs but are easier to collect for clinical application, with higher isolation yields. Indeed, intra-articular (IA) injection of ASCs prevented OA onset in a collagenase-induced murine knee OA model and reduced synovitis, osteophyte formation, and cartilage degeneration [3]. Furthermore, intra-articular injection of 2 or 6 million autologous ASCs improved the cartilage degradation score and significantly reduced knee synovitis in a biomechanical induced OA rabbit model [4].
Using an established Good Manufacturing Practice (GMP) procedure based on ASCs expanded for 2 weeks in the presence of platelet lysate [5], we conducted a proof-of-concept phase I clinical trial to assess the safety and efficacy of intra-articular injection of autologous ASCs in patients with active and severe knee OA.
Patients and Methods
Study Design
A phase I, prospective, bicentric, single-arm, open-label, dose-escalating clinical trial of a single injection of autologous ASCs in patients with severe primary knee OA was conducted from March 2012 to April 2014 in two hospitals: CHRU Montpellier (France) and the Department of Orthopedic Surgery at the University of Würzburg (Germany). No placebo group was scheduled because of ethical issues (including late-stage knee OA patients associated with liposuction procedure without active therapy benefit). The study protocol was approved by the local ethics committees of both institutions (Comité de Protection des Personnes of Montpellier [UF8606-120203] and Ethik-Kommission bei der Medizinischen of Würzburg) and by the national competent authorities (TC301; EudraCT no. 2011-000183-10).
Patient Selection and Enrollment
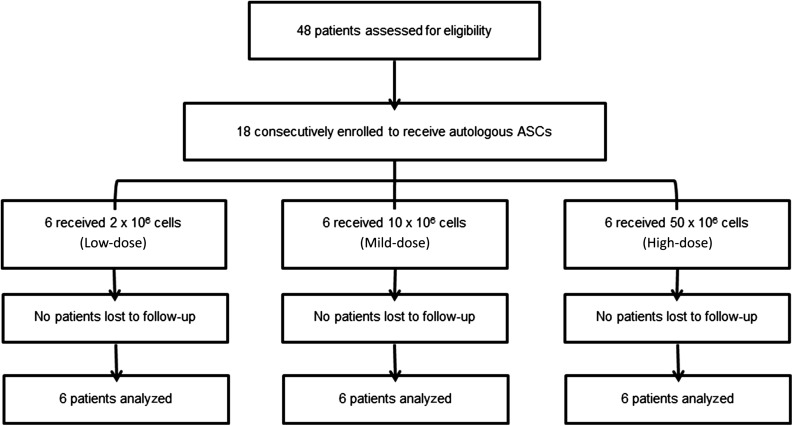
A total of 48 outpatients with knee OA were screened (Fig. 1). Eighteen consecutive patients with primary femorotibial knee OA diagnosed according to the clinical and radiological criteria of the American College of Rheumatology were enrolled in this study after written informed consent was obtained [6].
Figure 1.
Flow chart of the clinical trial. Abbreviation: ASC, adipose-derived stromal cell.
Inclusion Criteria
Patients 50–75 years of age with symptomatic primary knee OA and radiographic changes of grade 3 to 4 according to the Kellgren-Lawrence scale in the targeted knee were included [7]. To obtain histologic analysis for safety issues, the medical board required end-stage knee OA patients with an indication of knee prosthesis in the year after inclusion. Symptomatic primary knee OA was defined by daily knee pain for at least 12 months before study inclusion.
Exclusion Criteria
Patients were excluded if they had secondary arthritis (related to rheumatoid arthritis, spondyloarthritis, previous articular fractures, postinfectious arthritis, and crystal arthropathies), autoimmune disorders, or previous malignancies in the past 5 years. Previous administration of oral/intra-articular corticosteroids and injection of hyaluronic acid derivatives within 6 months before screening examination were also exclusion criteria.
Treatment Allocation
Eligible patients were consecutively allocated to the treatment groups, three arms with different doses (2 × 106, 10 × 106, and 50 × 106 cells) (Fig. 1). The starting dose of 2 × 106 cells has been defined based on the No Observed Adverse Effect Level obtained after IA administration determined in preclinical studies performed in goat and rabbit models of OA, adjusted by allometric factors (weight and size of the knee joint compared with human) [4] (data not shown).
First, the patients underwent outpatient liposuction under local anesthesia, and autologous ASCs were produced and prepared at a single GMP facility (Etablissement Français du Sang Midi-Pyrénnées, France), as summarized in the supplemental online data. Fourteen days after isolation, ASCs were recovered and underwent defined quality control before shipping (supplemental online data). A single IA dose of ASCs was injected into the knee joint (volume, 5 ml) under ultrasound control.
Cell Preparation and Expansion of ASCs
The procedure has been described [5]. The stromal vascular fraction (SVF) was obtained by means of collagenase digestion. Aliquots of 10 g of adipose tissue were mixed with 34 ml of collagenase solution (NB6; Coger, Paris, France, http://www.cogerbio.com) and incubated at 37°C for 45 minutes. Enzymatic digestion was stopped by the addition of complete culture medium (CCM) containing minimum essential medium (MacoPharma, Tourcoing, France, http://www.macopharma.com), human platelet growth factor-enriched plasma, 10 mg/ml ciprofloxacin, and 1 U/ml heparin. After homogenization, the digested suspension was passed through sterile 100-µm filters. The cells were centrifuged at room temperature for 10 minutes at 600g. The supernatant was discarded, and the SVF was resuspended in 20 ml of CCM. An aliquot of the SVF was removed for quality control: cell count, viability, phenotyping (CD34, CD45, and CD14), and sterility.
Cells from the SVF were then seeded in a 1,270-cm2 CellStack culture chamber (MacoPharma) at a density of 4 × 103 cells per cm2 in CCM, by use of a seeding kit (MacoPharma), at 37°C in an atmosphere saturated with moisture and 5% CO2. After an initial 24-hour incubation, the nonadherent cells were removed. The adherent cells were washed once with Dulbecco’s phosphate-buffered saline (PBS), and CCM medium was added for 7 days. The medium was completely replaced at days 4 and 6 of culture with the use of medium exchange kits (MacoPharma). At day 8 (primary culture, P0), the cells were harvested with the use of a detachment kit (MacoPharma) according to the following protocol. After aspiration of the medium and washing with Dulbecco’s PBS, 50 ml of irradiated trypsin solution was added for 5 minutes at room temperature. After inhibition of trypsin activity by the addition of CCM, the cells were collected in a transfer bag (MacoPharma). An aliquot of the cell suspension was aseptically removed for cell count, viability, phenotyping (CD34, CD45, and CD14), measures of hTERT messenger RNA contents by quantitative reverse-transcription polymerase chain reaction, and assessment of microbial testing.
The cells were seeded in 1,270-cm2 CellStack culture chambers at a density of 2 × 103 cells per cm2 and incubated for 6 days. The CCM was completely replaced at days 11 and 13. At day 11, an aliquot of culture medium was aseptically removed for mycoplasma and endotoxin testing. At day 14, the cells were harvested according to the procedure described above. The cell suspension was placed in a transfer bag (MacoPharma) and washed with Dulbecco’s PBS. The ASCs were then resuspended in a solution containing 3.6% human albumin (provided by Laboratoire Français du Fractionnement et des Biotechnologies, Courtaboeuf, France) and a polyionic solution containing glucose. An aliquot of the ASC suspension was aseptically removed for cell count, and its quality was evaluated as described above.
Flow cytometry analyses were performed as follows. Briefly, ASCs (2 × 105 cells) were stained with saturating amounts of monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) and their respective isotype controls for 30 minutes in the dark at 4°C in PBS/0.5% human albumin and 0.1% sodium azide. After washing, the labeled cells were analyzed by flow cytometry (EPICS XL-MCL flow cytometer; Beckman-Coulter, Nyon, Switzerland, http://www.beckmancoulter.com). FITC anti-CD14, FITC anti-CD45, PE anti-CD34, PE anti-CD73, PE anti-CD90, PE anti-CD105, and immunoglobulin G1 PE and FITC were from BD Pharmingen (Le Pont de Claix, France, http://www.bdbiosciences.com).
Release Criteria of ASCs
Release criteria were defined as negative for microbial testing on SVF, intermediate product (P0), and final product (P1); negative for mycoplasma testing on adipose tissue and culture medium at day 11; endotoxin testing negative on culture medium at day 11; and absence of hTERT detection by quantitative reverse-transcription polymerase chain reaction on intermediate product (P0). Finally, on active substance, cellular viability had to be >90%. The percentage of positive cells for hematopoietic markers (CD45 and CD14) had to be lower than 2%, and for mesenchymal markers, higher than 90% for CD90 and CD73 and higher than 80% for CD105. The percentage of positive cells for CD34 had to be less than 10%. Karyotype analyses were performed, on final product, for 15 productions. Because of the time required for performing the karyotype analysis, results were obtained after release. Karyotype analyses revealed no clonal abnormalities. Results for release criteria obtained for the three cohorts are presented in the supplemental online Appendix.
Outcome Measures
Primary Endpoint
Incidence, relatedness, and severity of treatment-emergent suspected unexpected serious adverse reactions, serious adverse events, and adverse events (AEs) were documented at each visit throughout the study. Laboratory tests (hematology, blood chemistry, and urinalysis), vital signs, and physical examinations of the patients were assessed systematically. A 12-week safety period was implemented between subject 1 and subject 2 of the first cohort receiving the low dose, and the safety medical board authorized continuation with patients 2 to 6. A further 4-week safety period was scheduled between the other two cohorts.
Secondary Endpoints
Secondary efficacy endpoints were assessed by measuring the Western Ontario and McMaster Universities Arthritis Index (WOMAC), pain visual analog scale (VAS), the Patient Global Assessment (PGA), the Short Arthritis Assessment Scale (SAS), and the Knee Injury and Osteoarthritis Outcome Score (KOOS index) [8]. A 0- to 100-mm VAS was used to assess WOMAC pain (5 questions), physical function (17 questions), and stiffness (2 questions) subscales. Osteoarthritis Research Society International (OARSI)/Outcome Measures in Rheumatology response was defined as 20% improvement compared with baseline VAS and WOMAC [9]. Quality of life was measured by the short-form 36 (SF-36) questionnaire [10].
Secondary imaging endpoints included delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) and T1rho MRI for selected German patients at 3–4 months after ASC injection [11]. MRIs were evaluated by a radiologist blinded to the administered dose. dGEMRIC and T1rho maps were motion corrected and zerofilled and then derived using anatomical landmarks and an automated fit algorithm [12].
Histology
Upon request of the ethics committee, a total knee arthroplasty (TKA) was originally scheduled 3 months after ASC injection for all patients to obtain histologic analysis. However, if a patient refused TKA, knee arthroscopy with biopsy could be performed. No standardized protocol was planned for biopsies. Cartilage and synovial samples were fixed for 24 hours in 10% neutral formol and embedded in paraffin. Sections of 5-µm thickness were stained with hematoxylin and eosin, Alcian blue, or Toluidine blue. Immunohistochemistry was performed on a Benchmark Ultra Ventana automat with the following antibodies: protein S100 (1:3,200, polyclonal; Dako, Carpinteria, CA, http://www.dako.com), CD34 (1:100, QBEND/10; Dako), and Ki67 (1:100, monoclonal mouse, clone Mib-1; Dako). The OARSI cartilage OA histopathology grading system was performed by an experienced anatomopathologist who was blinded to the treatments [13].
Statistical Analysis
All values are expressed as mean ± SD. The significance of differences was assessed by Wilcoxon test or one-way analysis of variance and corresponding nonparametric tests. A value of p < .05 was considered statistically significant. All analyses were performed using GraphPad Prism software version 6.0 (GraphPad Software, La Jolla, CA, http://www.graphpad.com).
Results
Characteristics of Patients
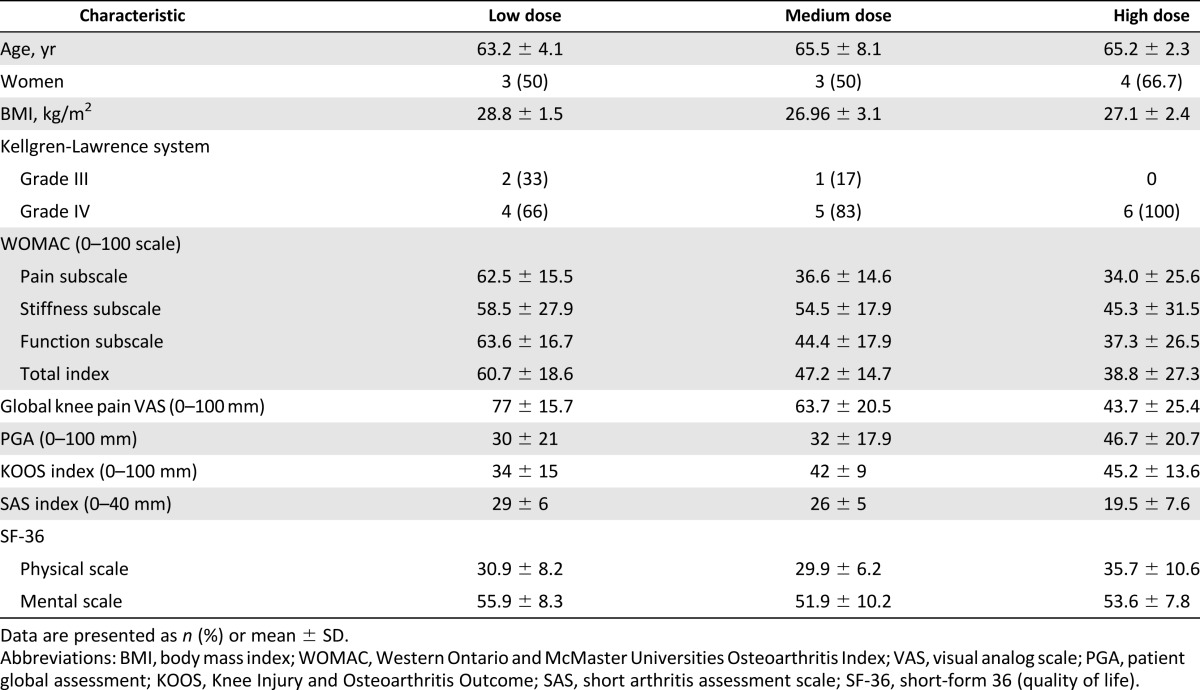
All three cohorts had similar baseline characteristics for age, sex, and body mass index, and 83% of patients were grade IV on the Kellgren-Lawrence scale (Table 1). Eleven patients were included in France and seven in Germany. Baseline levels for pain and function (WOMAC, KOOS, SAS scores) were different between the cohorts (Table 1). Disease activity at baseline was higher in the group of patients injected with the low dose of ASCs, with higher VAS and WOMAC values. All patients completed the 6-month follow-up. Only one patient with persistent joint swelling and knee pain underwent TKA surgery at 6 months.
Table 1.
Patient demographic and baseline characteristics of each group (low, medium, and high dose, n = 6 each)
Safety and Tolerance Profile of IA Injection of Autologous ASCs
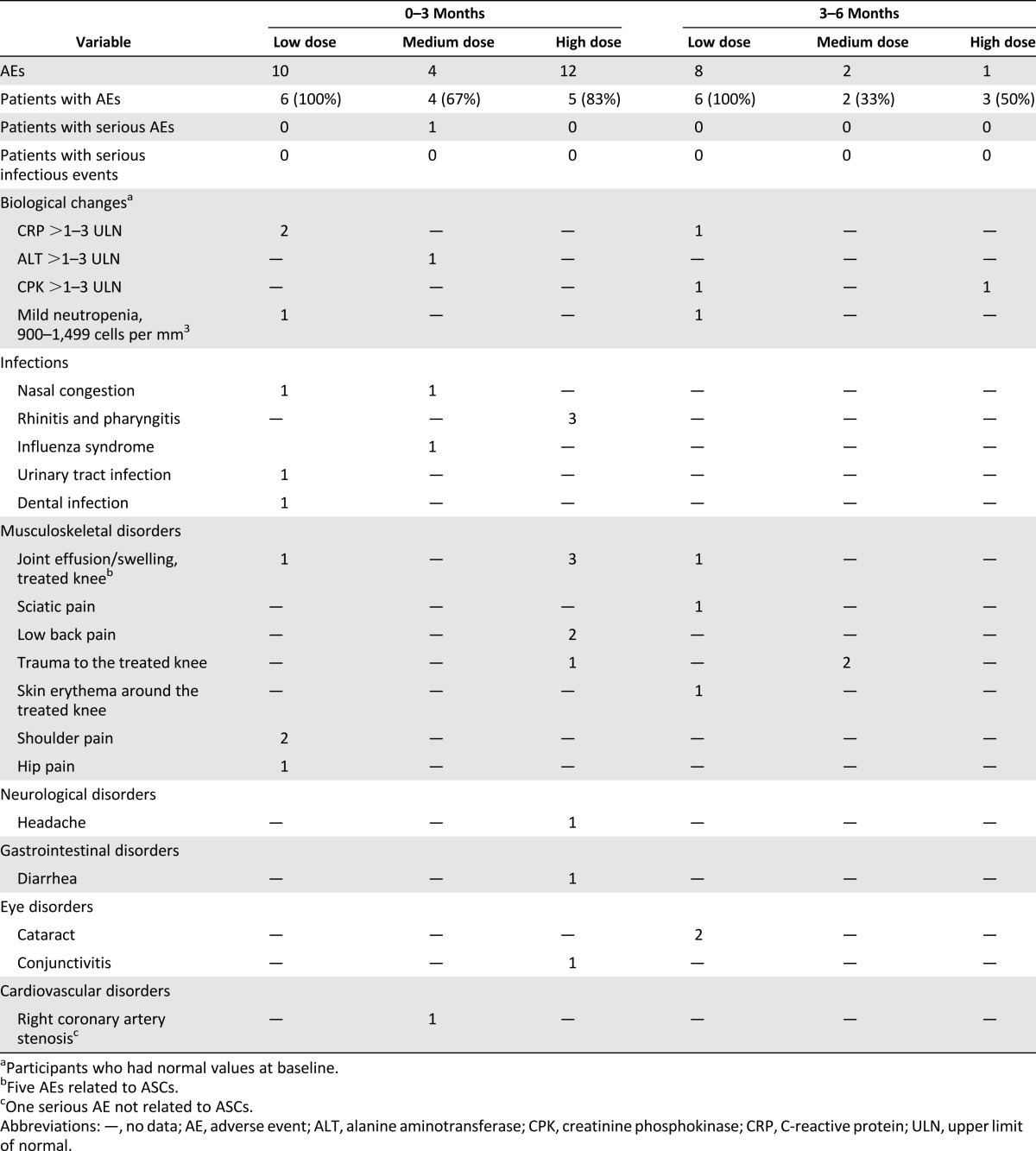
No AE associated with liposuction and IA injection was observed in this study (Table 2). No serious infectious AEs related to ASC injection occurred during follow-up (Table 2). Laboratory tests, vital signs, and electrocardiograms indicated no local or systemic safety concerns.
Table 2.
Summary of adverse events during the clinical trial
One severe adverse event, unstable angina pectoris without increased cardiac markers, was reported in 1 patient 3 months after ASC injection. The patient’s risk factors included hypertension and hyperlipidemia. Five minor AEs reported by four patients were potentially related to the procedure: slight knee pain/joint effusion occurred during the first week after ASC injection that resolved with nonsteroidal anti-inflammatory drugs in three patients and spontaneously (without medication) in one patient (Table 2).
Otherwise, a small increase in creatinine phosphokinase was observed in two patients and in alanine aminotransferase in one patient. There was also a mild decrease of neutrophil count in one patient who presented with a low baseline count (1,500/mm3) and high variability of neutrophil count, independent of IA injection, during follow-up.
Efficacy Profile of Autologous ASC Injection on OA Clinical Outcomes
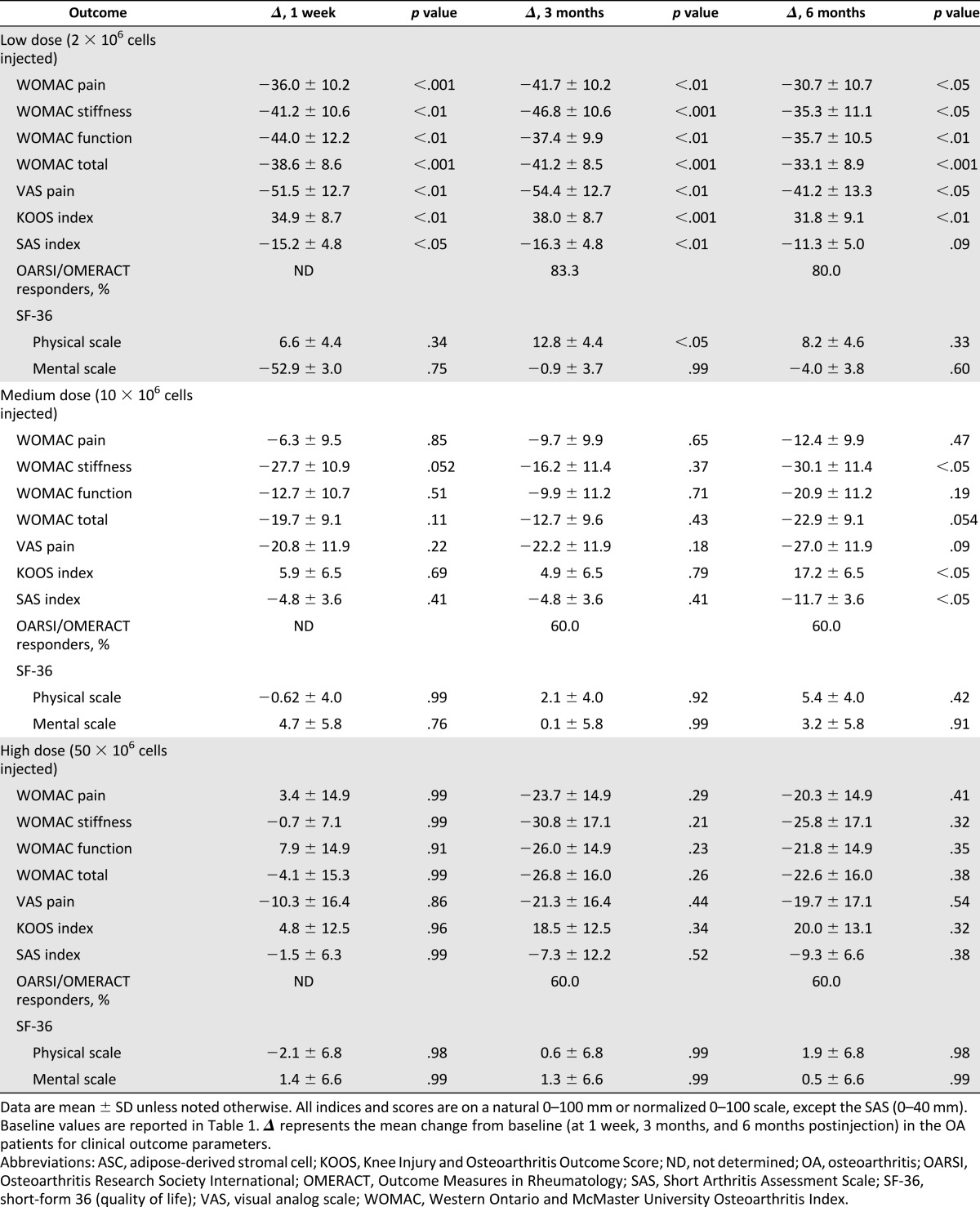
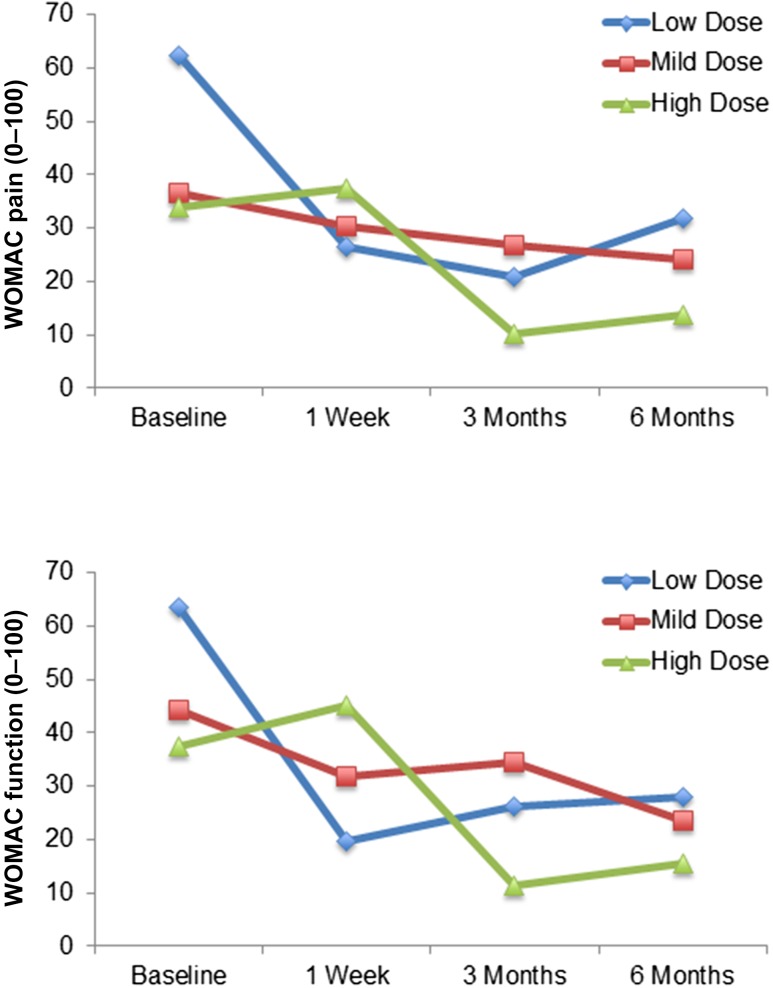
Mean changes from baseline to 1 week, 3 months, and 6 months in clinical outcomes are summarized in Table 3. Improvement for all clinical outcome parameters (pain, function, and mobility) regardless of the injected dose was observed (Fig. 2). However, statistical significance was detected only for patients treated with the low dose. Finally, all patients except one refused to have the previously scheduled TKA.
Table 3.
Effect of autologous ASC injection on OA clinical outcomes.
Figure 2.
WOMAC pain and function improvement during the study. Abbreviation: WOMAC, Western Ontario and McMaster Universities Arthritis Index.
MRI Evaluation
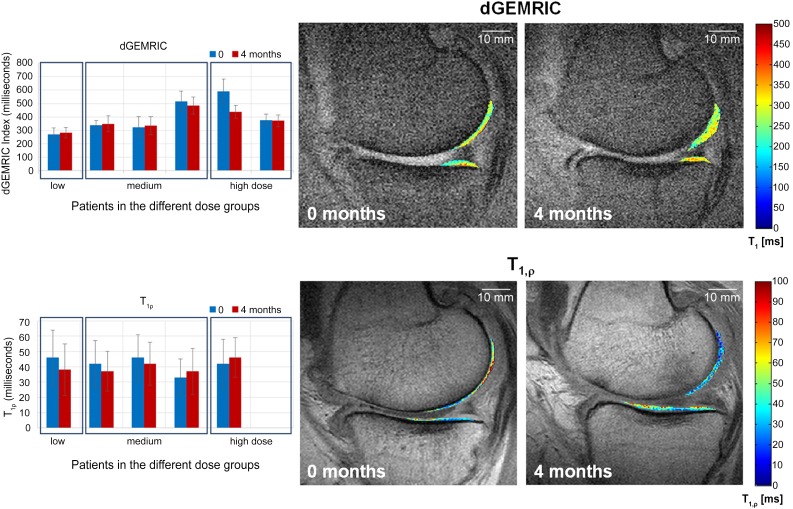
Among the 7 patients included in Germany, quantitative dGEMRIC (6 patients) and T1rho (5 patients) maps were acquired and analyzed before and 4 months after therapy (Fig. 3). In these parameter maps, the dGEMRIC index increased in three selected patients with time, whereas the T1rho values decreased at the same time. For the other three patients, the opposite effect was observed. Thus, the positive changes were only limited and suggested a possible cartilage improvement in three of six patients. In conclusion, within this small number of patients, we did not observe any correlation between MRI and clinical changes.
Figure 3.
dGEMRIC and T1rho magnetic resonance imaging (MRI) of selected patients. The graphs on the left show the dGEMRIC (n = 6) and T1rho (n = 5) values before and 4 months after cell therapy. Increasing dGEmRIC and decreasing T1rho values are each known to correspond to increasing glycosaminoglycan/proteoglycan content and thus improved cartilage condition. On the right, the corresponding dGEMRIC and T1rho maps are shown as a color-coded overlay on an anatomical MRI for a patient receiving a low cell dose. The observed values in the cartilage change in the time course can be easily seen and correspond to an increase in cartilage condition. Abbreviation: dGEMRIC, delayed gadolinium-enhanced magnetic resonance imaging of cartilage.
Histologic Analysis
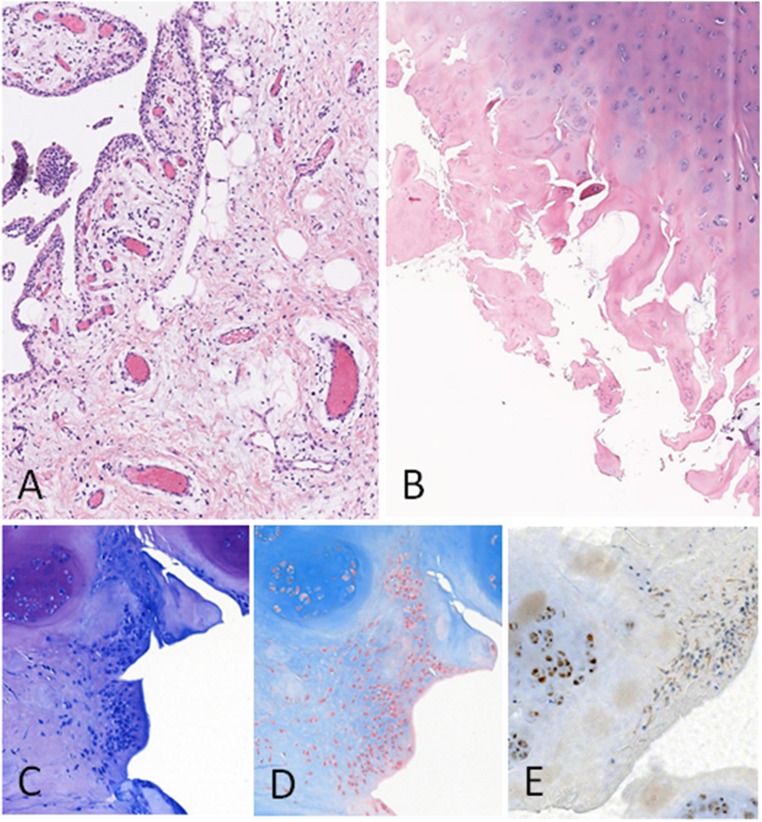
Histologic analysis of cartilage and synovium at 3 months was available for 11 of 18 patients after arthroscopy. All samples showed signs of severe OA (OARSI histologic grading >3). Osteoarthritic chondrocytes stained positive for PS100 and negative for CD34 or Ki67 (Fig. 4). Significant synovial inflammation was absent in two cases, whereas weak or moderate inflammation and synovial hyperplasia with diffuse interstitial lymphocytic infiltrate were observed in five and four cases, respectively. In one patient (case 2) who received a low dose of ASCs, we observed a sheet of cells that could be interpreted as a stem cell graft on cartilage surface (Fig. 4). These cells showed rare Ki67 nuclear staining and weak PS100 staining and were CD34 negative. Finally, none of the synovial or cartilage samples showed any tumor proliferation.
Figure 4.
Histologic findings. (A): Vascular congestion and weak lymphocytic infiltrate of the synovial (case 8) (magnification, ×50). (B): Osteoarthritic cartilage OARSI grade >3 (case 4) (×25). (C): Toluidine blue staining (case 2) (magnification, ×100). (D): Stem cell stroma shows an Alcian blue depleted matrix compared with the strong staining of osteoarthritic cartilage (case 2) (magnification, ×100). (E): Weak PS100 staining of possible stem cells on the cartilage surface and strong PS100 staining of chondrocytes (case 2) (magnification, ×100). Abbreviations: OARSI, Osteoarthritis Research Society International.
Discussion
This pilot trial reached its predetermined primary outcome parameters, i.e., safety of IA injection of ASCs in patients with knee OA. Our results are similar to those reported from other studies, in critical limb ischemia or fistulae in inflammatory bowel disease, where ASCs have been injected locally without reported side effects [5, 14]. Additionally, we report clinical improvement with a reduction in pain levels and WOMAC score in all three groups, even though statistically significant results were obtained only in the low-dose group. Actually, the large variability in the range of the initial clinical parameters as well as the limited sample size may explain why statistical significance was not reached at 6 months. However, when compared with historical control studies, our approach seems very encouraging. For example, in a thoroughly double-blind study on hyaluronic acid treatment into the knee, the WOMAC pain score decreased by 22.9 ± 1.4 mm between baseline and 6 months [15]. In the present study, WOMAC pain score decreased by 30.7 ± 10.7 mm in the group receiving low-dose ASCs. Furthermore, the average difference from baseline to 6 months on the WOMAC subscale scores (pain, function, and stiffness) is higher than the recommended minimal perceptible clinical improvement of 10 mm [16]. Additionally, a study comparing hyaluronic acid with saline solution reported 54.6% OARSI responders in the saline group after 13 weeks [17]. This score is lower than the OARSI response obtained with the three different ASC doses at the same time point in the present study, with 83.3% in low-dose, 60% in medium-dose, and 60% in high-dose groups. In a recent controlled study with steroid as comparator, the magnitude of the placebo effect led to a decrease in WOMAC pain score of 20 mm at 6 months versus baseline [18]. They recorded 52.1% OARSI responders at 6 months, which is lower than obtained in our groups. These studies suggest that ASC therapy might be more efficient than a possible placebo effect.
Our results are also consistent with those obtained in a recent study in patients with a larger heterogeneity in age and less severe forms of OA [11]. In a recent similar study from Jo et al., the highest efficiency was found at the highest dose (100 × 106 cells) in patients who presented the highest levels of pain at baseline (VAS and WOMAC) [19]. In our study, the group of patients injected with 2 × 106 cells exhibited the best response to ASC treatment, whereas they had higher baseline pain and WOMAC scores compared with those receiving higher doses. One possible reason for this inverse dose effect of ASC therapy might be the higher level of inflammation in the lowest dose group, as reflected by the highest level of pain at baseline. The inflammatory milieu might have primed the injected ASCs to exert their immunomodulatory functions more efficiently than in the groups where the inflammation was lower. We therefore cannot rule out that the treatment response was partly dependent on the initial disease activity. Orozco et al. published another interesting study on the treatment of knee OA with autologous MSCs derived from bone marrow [20]. They injected 40 × 106 cells into the knee joint. Improvement of cartilage morphology and quality was observed in almost all patients using MRI T2 mapping, suggesting a possible structural benefit of stem cell therapy.
The potential mode of action of ASCs for the treatment of OA includes at least three different biological effects. The first is direct differentiation of ASCs into chondrocytes, whereas the others are related to a possible paracrine effect of secreted bioactive molecules, including anti-inflammatory and chondroprotective mediators. However, the capacity of MSCs to differentiate into chondrocytes is probably not critical in the observed therapeutic effect. Preliminary studies in rabbits and goats have shown that cartilage regeneration did not occur at the expense of chondrogenic differentiation of the injected cells but may be strongly related to a secondary stimulation of endogenous progenitor cells through paracrine effects [21]. MSCs contributed to the repair of damaged articular cartilage through homing, engraftment, production of cartilage matrix, and reduction of local inflammation [22–25]. Stromal cells have been shown to possess immunomodulatory and antifibrotic properties, to protect cells from oxidative stress and apoptosis, and to stimulate proliferation and chondrogenic differentiation in coculture through secretion of growth factors [23]. In preclinical models of OA or experimental models of inflammatory diseases such as arthritis and experimental encephalitis, the benefit of ASC injection was related to secretion of anti-inflammatory factors including hepatocyte growth factor, human leukocyte antigen G5, or interleukin-1 receptor antagonist [26]. The immunomodulatory properties of adipose-derived MSCs are even stronger than those from other tissue sources [27]. Whether the in vitro capabilities of MSCs from different tissue sources reflect the in vivo situation has still to be elucidated. Nevertheless, there is an obvious variation among donors that could be related to differences in isolation, expansion, and freezing/thawing procedures. Altogether, these data suggest that MSCs can reduce synovitis and favor an appropriate environment for tissue regeneration through expression of active growth factors or recruitment of endogenous progenitors.
Conclusion
Although this phase I study included a limited number of patients without a placebo arm, we were able to show that this innovative treatment was safe and well tolerated in patients with knee OA. We also provided encouraging preliminary evidence of efficacy. Larger and controlled long-term studies are now mandatory to confirm whether this new strategy of cell therapy can improve pain and induce structural benefit. Moreover, it is likely that similar therapeutic procedures based on autologous ASCs can be extended in the future to other joints, such as the hip joint, or indications such as intervertebral disc degeneration.
Supplementary Material
Acknowledgments
We thank all the patients for their participation in the study and Dr. Mazen Hamoui and Prof. Andrea Facchini for their cooperation and support during the study. M.R. and U.N. are currently affiliated with the Department of Orthopaedic and Trauma Surgery, Evangelisches Waldkrankenhaus Spandau, Berlin, Germany. Trial registration number: NCT01585857. The research leading to these results has received funding from the European Union Seventh Framework Programme FP7/2007-2013 under grant agreement 241719. Work in the laboratory INSERM U844 was also supported by the Inserm Institute, the University of Montpellier, and the Agence Nationale pour la Recherche for support of the national infrastructure: “ECELLFRANCE: Development of a national adult mesenchymal stem cell based therapy platform.”
Author Contributions
Y.-M.P.: provision of study patients, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; L.R.: provision of study patients, collection and assembly of data, manuscript writing, final approval of manuscript; R.F.: administrative support, provision of study patients, collection and assembly of data, data analysis and interpretation, final approval of manuscript; O.P.: administrative support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; C.D., F.C., and C.C.: provision of study material, final approval of manuscript; F.B.: conception and design, administrative support, manuscript writing, final approval of manuscript; L.S., S.F., and P.B.: conception and design, administrative support, provision of study material, final approval of manuscript; L.C. and G.L.: conception and design, administrative support, final approval of manuscript; D.N.: conception and design, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript; J.S. and D.H.: provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.D.: collection of data, manuscript writing, final approval of manuscript; U.N.: conception and design, administrative support, provision of study patients, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; C.J.: conception and design, financial support, provision of study patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
S.F. has uncompensated research funding from European Union Seventh Framework Programme FP7/2007-2013, grant agreement 241719. D.H. is employed by MRB Research Center. The other authors indicated no potential conflicts of interest.
References
- 1.Findlay DM. If good things come from above, do bad things come from below? Arthritis Res Ther. 2010;12:119. doi: 10.1186/ar3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen C, Djouad F, Bouffi C, et al. Multipotent mesenchymal stromal cells in articular diseases. Best Pract Res Clin Rheumatol. 2008;22:269–284. doi: 10.1016/j.berh.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 3.ter Huurne M, Schelbergen R, Blattes R, et al. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64:3604–3613. doi: 10.1002/art.34626. [DOI] [PubMed] [Google Scholar]
- 4.Desando G, Cavallo C, Sartoni F, et al. Intra-articular delivery of adipose derived stromal cells attenuates osteoarthritis progression in an experimental rabbit model. Arthritis Res Ther. 2013;15:R22. doi: 10.1186/ar4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bura A, Planat-Benard V, Bourin P, et al. Phase I trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 7.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 9.Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12:389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 11.Koh YG, Choi YJ, Kwon OR, et al. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med. 2014;42:1628–1637. doi: 10.1177/0363546514529641. [DOI] [PubMed] [Google Scholar]
- 12.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: Degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 13.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Lee WY, Park KJ, Cho YB, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells. 2013;31:2575–2581. doi: 10.1002/stem.1357. [DOI] [PubMed] [Google Scholar]
- 15.Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis. 2012;71:1454–1460. doi: 10.1136/annrheumdis-2011-200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635–2641. [PubMed] [Google Scholar]
- 17.Strand V, Baraf HS, Lavin PT, et al. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20:350–356. doi: 10.1016/j.joca.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Leighton R, Akermark C, Therrien R, et al. DUROLANE Study Group NASHA hyaluronic acid vs. methylprednisolone for knee osteoarthritis: A prospective, multi-centre, randomized, non-inferiority trial. Osteoarthritis Cartilage. 2014;22:17–25. doi: 10.1016/j.joca.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 20.Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: Two-year follow-up results. Transplantation. 2014;97:e66–e68. doi: 10.1097/TP.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 21.Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 22.Hoogduijn MJ, Crop MJ, Peeters AM, et al. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007;16:597–604. doi: 10.1089/scd.2006.0110. [DOI] [PubMed] [Google Scholar]
- 23.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 24.Wolbank S, Peterbauer A, Fahrner M, et al. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173–1183. doi: 10.1089/ten.2006.0313. [DOI] [PubMed] [Google Scholar]
- 25.Yañez R, Lamana ML, García-Castro J, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 26.Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: Role of secretome and exosomes. Biochimie. 2013;95:2229–2234. doi: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Mattar P, Bieback K. Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol. 2015;6:560. doi: 10.3389/fimmu.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.