To clarify the underlying mechanisms of how mesenchymal stem cells (MSCs) promote skin expansion and help ameliorate complications, the authors identified the differentially expressed genes between mechanically stretched human MSCs and controls. Microarray analysis suggested upregulation of genes related to hypoxia, vascularization, and cell proliferation, enhancing transplanted MSC homing to the expanded skin and transdifferentiation into epidermal basal cells and endothelial cells.
Keywords: Skin regeneration, Stem cell therapy, Mesenchymal stem cell, Mechanical stretching, Tissue expansion
Abstract
Skin tissue expansion is a clinical procedure for skin regeneration to reconstruct cutaneous defects that can be accompanied by severe complications. The transplantation of mesenchymal stem cells (MSCs) has been proven effective in promoting skin expansion and helping to ameliorate complications; however, systematic understanding of its mechanism remains unclear. MSCs from luciferase-Tg Lewis rats were intravenously transplanted into a rat tissue expansion model to identify homing and transdifferentiation. To clarify underlying mechanisms, a systematic approach was used to identify the differentially expressed genes between mechanically stretched human MSCs and controls. The biological significance of these changes was analyzed through bioinformatic methods. We further investigated genes and pathways of interest to disclose their potential role in mechanical stretching-induced skin regeneration. Cross sections of skin samples from the expanded group showed significantly more luciferase+ and stromal cell-derived factor 1α (SDF-1α)+, luciferase+keratin 14+, and luciferase+CD31+ cells than the control group, indicating MSC transdifferentiation into epidermal basal cells and endothelial cells after SDF-1α-mediated homing. Microarray analysis suggested upregulation of genes related to hypoxia, vascularization, and cell proliferation in the stretched human MSCs. Further investigation showed that the homing of MSCs was blocked by short interfering RNA targeted against matrix metalloproteinase 2, and that mechanical stretching-induced vascular endothelial growth factor A upregulation was related to the Janus kinase/signal transducer and activator of transcription (Jak-STAT) and Wnt signaling pathways. This study determines that mechanical stretching might promote skin regeneration by upregulating MSC expression of genes related to hypoxia, vascularization, and cell proliferation; enhancing transplanted MSC homing to the expanded skin; and transdifferentiation into epidermal basal cells and endothelial cells.
Significance
Skin tissue expansion is a clinical procedure for skin regeneration to cover cutaneous defects that can be accompanied by severe complications. The transplantation of mesenchymal stem cells (MSCs) has been proven effective in promoting skin expansion and ameliorating complications. This study, which sought to provide a systematic understanding of the mechanism, determined that mechanical stretching could upregulate MSC expression of genes related to hypoxia, vascularization, and cell proliferation; enhance transplanted MSC homing to the expanded skin tissue; and promote their transdifferentiation into epidermal basal cells and endothelial cells.
Introduction
The technique of skin expansion with a tissue expander for reconstructing large skin defects was developed by Neumann [1] in the 1950s and has since been widely used in plastic and reconstructive procedures. Skin regenerated through tissue expansion is used to replace the lost tissue and can be an ideal match in terms of color, texture, and structure [2]. In some cases, because this procedure is strictly limited by the regenerative capacity of skin, the expansion of the donor-site skin is unsatisfactory owing to complications such as skin attenuation, infection, ulceration, and even necrosis of the expanded tissue [3]. These complications can lead to suspension of the expansion process, failure of skin-defect coverage, and increased financial and emotional burden on patients [4].
To manage these problems, adjunctive agents such as prostaglandin E2, papaverine, cytochalasin D, and dimethyl sulfoxide (DMSO) have been used to facilitate the process of skin expansion, but none of them show sufficient clinical efficacy [5–7]. Recently, as a promising therapeutic approach of cell-based therapy, mesenchymal stem cells (MSCs) in preclinical and clinical studies have been shown to be highly efficient in treating various diseases [8]. We previously showed that bone marrow-derived MSC transplantation could efficiently enhance skin tissue expansion [9, 10] by transdifferentiating into epidermal and vascular components in the expanded skin [11], but the underlying mechanism remains to be clarified. Our recent study proved that mechanical stretching upregulates stromal cell-derived factor (SDF)-1α in the skin and recruits circulating MSCs through the SDF-1α/C-X-C chemokine receptor 4 (CXCR4) pathway [11]. However, alterations in the gene expression patterns in MSCs caused by mechanical stretching and the pathways involved in this process have not yet been systematically analyzed.
Thus, in this study, we transplanted MSCs into an animal model of skin tissue expansion and tracked MSC migration in vivo to confirm the contribution of migrating MSCs to skin regeneration in the presence of mechanical stretching. To clarify the mechanism underlying this process, a systematic approach was used to identify the differentially expressed genes between human MSCs cultured in the presence and absence of mechanical strain, and the biological significance of these changes were analyzed through bioinformatic methods. We further investigated genes and pathways of interest to disclose their potential role in mechanical stretching-induced skin regeneration.
Materials and Methods
Ethics Statement
The Guide for the Care and Use of Laboratory Animals was followed in all animal procedures. The protocol was approved by the Committee on the Ethics of Animal Experiments of Shanghai Jiao Tong University School of Medicine.
Isolation and Culture of Luciferase-MSCs
Bone marrow cells from 3-week-old luciferase (Luc)-Tg Lewis rats (ROSA/Luciferase-LEW; Sino-British SIPPR/BK Laboratory Animal, Shanghai, China, http://www.bku.com) [12] were harvested by flushing the femurs of the rats with phosphate-buffered saline (PBS) and cultured in 100-mm culture dishes in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific Life Sciences, Waltham, MA, www.thermofisher.com) supplemented with 10% fetal bovine serum (GE Healthcare Life Sciences, Pittsburgh, PA, http://www.gelifesciences.com) and 1% penicillin/streptomycin (Thermo Fisher Scientific Life Sciences, Waltham, MA, www.thermofisher.com). The medium was changed every 2 days, and the cultured MSCs were further propagated for 3 passages.
Tissue Expansion Model and Intravenous Transplantation of MSCs
Twelve wild-type female Lewis rats (6 weeks old; Sino-British SIPPR/BK Laboratory Animal) were randomized into two groups: an expanded group and a control group. All rats were anesthetized, and 10-ml silicone expanders (Shanghai Xinsheng Bio-medical Co., Shanghai, China, http://www.xinsheng-sh.net) were subcutaneously implanted in the middle of the dorsal part of all rats. Intravenous MSC transplantation was performed 7 days postimplantation. Each rat was intravenously injected with 1 × 107 luc-MSCs (suspended in PBS) through the retrobulbar venous plexus. For the expanded group, inflation began immediately after MSC transplantation and was conducted every other day with 10 ml saline each time. Rats in the control group underwent no inflation.
Immunofluorescence and Immunohistochemical Staining
The whole area of skin tissue (expanded or nonexpanded) was harvested after 21 days in both groups. After fixing with paraformaldehyde, 10-μm sections were prepared and stained with antibodies against firefly luciferase (Novus Biologicals, Littleton, CO, http://www.novusbio.com), keratin14 (KRT14; Santa Cruz Biotechnology, Dallas, TX, http://www.scbt.com), CD31 (Abcam, Cambridge, MA, www.abcam.com), SDF-1α (Abcam), and hypoxia-inducible factor (HIF)-1α (Abcam). Fluorescence staining was detected using a confocal laser scanning fluorescence microscope (Carl Zeiss, Jena, Germany, http://www.zeiss.com). Immunohistochemistry quantification was determined through image analysis by a Nikon Eclipse E800 microscope (Nikon, Melville, NY, http://www.nikonusa.com) and measured as percentage of the scanned area using Image-pro Analyzer 6.3 (Media Cybernetics, Silver Spring, MD, http://www.mediacy.com). The average of 10 random high-power fields was used for each specimen.
Dataset Acquisition and Microarray Experiment
The microarray dataset was downloaded from ArrayExpress (https://www.ebi.ac.uk/arrayexpress; accession number E-MEXP-3124). Data from samples DMSO 1_1, DMSO 1_2, DMSO 1_3, Mec-DMSO 1_2, and Mec-DMSO 1_3 was used for this investigation.
Based on the protocol description in the dataset, hMSCs were maintained in DMEM containing 10% fetal bovine serum, 50 U/ml penicillin, 50 μg/ml streptomycin, and 2 mM l-glutamine on a circular (diameter, 21 mm) membrane coated with fibronectin (Sigma-Aldrich, St Louis, MO, http://www.sigmaaldrich.com) in a humidifier incubator at 37°C and 5% CO2. Upon 90% confluence, cells were subjected to 5% strain for 6 hours in a humidified incubator with 5% CO2 at 37°C. Experiments without strain were treated identically but not exposed to mechanical stress. Sample preparation, hybridization, washing, and scanning of Illumina Human Sentrix-6 BeadChip arrays (Illumina, San Diego, CA, http://www.illumina.com) were carried out according to Illumina’s recommended protocols.
Differentially Expressed Gene Filtration
Gene expression data were normalized using the quantile normalization algorithm in Chipster version 1.3.0, and differentially expressed genes (DEGs) in the two groups were defined using the Significance Analysis of Microarray (SAM) package in R (version 3.1.3). Genes identified using the SAM method were filtered with a 2.0-fold change cutoff at a false discovery rate of <0.01. Filtration of DEGs between MSCs with or without mechanical stretching was realized using the two-tailed Student’s t test.
Functional Annotation and Pathway Analysis
Upregulated and downregulated genes were separately analyzed using functional annotation and pathway analysis. Gene Ontology (GO) is an international standardized functional gene-classification system that describes the properties of genes and gene products in any organism. The GO terms of DEGs were enriched using the Database for Annotation, Visualization and Integrated Discovery (https://david.ncifcrf.gov) [13, 14], which was applied for pathway analysis by the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/pathway.html).
hMSC Culture Under Mechanical Stretching and Drug Treatment
hMSCs (PromoCell, Heidelberg, Germany, http://www.promocell.com) were cultured according to the protocol described above. After cells were 90% confluent at the second passage, they were subjected to 5% elongation using an FX-4000T Flexcell Tension Plus unit (Flexcell, Hillsborough, NC, http://www.flexcellint.com) for 6 hours in a humidified incubator with 5% CO2 at 37°C. The unstretched hMSCs were treated identically but without exposure to mechanical strain. To evaluate the role of the Janus kinase/signal transducer and activator of transcription (Jak-STAT) and Wnt signaling pathways in inducing vascular endothelial growth factor A (VEGFA) expression in response to mechanical stretching, the Jak inhibitor AG490 (50 μM; Selleck Chemicals, Houston, TX, http://www.selleckchem.com) and Wnt-pathway inhibitor ICG-001 (10 μM; Selleck Chemicals) were added to the medium 6 hours before strain. DMSO was added as control. hMSCs were then collected for real-time reverse-transcription polymerase chain reaction (RT-PCR) after exposure to mechanical strain.
Short Interfering RNA and Transfection
Short interfering RNA (siRNA) oligonucleotides were designed using BLOCK-iT RNAi Designer (http://rnaidesigner.thermofisher.com/rnaiexpress) and provided by GenePharma (Shanghai, China, http://www.genepharma.com). The sequences were as follows: rat matrix metalloproteinase 2 (MMP2), 5′-GGAAACCAAGAUGUGGCAATT-3′ (sense) and 5′-UUGCCACAUCUUGGUUUCCTT-3′ (antisense); rat MMP2 Scramble, 5′-GGAAACCGUAGGGUAACAA-3′ (sense) and 5′-UUGUUACCCUACGGUUUCC-3′ (antisense); rat HIF-1α, 5′-CCGUUGUACAAUGAUGUAA-3′ (sense) and 5′-UUACAUCAUUGUACAACGG-3′ (antisense); and rat HIF-1α Scramble, 5′-CCGCAUGGUAAGUAUUUAA-3′ (sense) and 5′-UUAAAUACUUACCAUGCGG-3′ (antisense). Lipofectamine 2000 (Thermo Fisher Scientific Life Sciences) was used for transient transfections according to the instructions provided by the manufacturer. Rat MSCs were collected 6 hours posttransfection and transplanted into the expansion model described above. In vivo siRNA transfection was conducted just before expansion and continued every 3 days during the expansion process. The expanded skin area was collected after 21 days.
Real-Time RT-PCR
Transcript levels of genes of interest were confirmed by real-time RT-PCR. Total RNA was extracted with Trizol (Thermo Fisher Scientific Life Sciences), and reverse transcription into cDNA was performed with an RT-PCR kit (TaKaRa, Shiga, Japan, http://www.takara.com) on an ABI HT7900 instrument (Thermo Fisher Scientific Life Sciences). A NanoDrop Spectrophotometer (ND-1000; Thermo Fisher Scientific Life Sciences) was used to determine RNA concentration. The expression levels of different genes relative to glyceraldehyde-3-phosphate dehydrogenase was determined using SYBR green dye (04673484001; Roche, Indianapolis, IN, http://www.roche.com) and an ABI StepOne plus real-time PCR machine (Thermo Fisher Scientific Life Sciences). Primers are listed in supplemental online Table 1.
Western Blot Analysis
The total protein of skin samples and cells was extracted using Protein Extraction Reagent (Thermo Fisher Scientific Life Sciences). An equal amount of protein (40 μg) from each sample was separated on a 10% sodium dodecyl sulfate polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, and blocked using 5% bovine serum albumin in Tris-buffered saline. The membranes were then incubated overnight at 4°C with primary antibodies against MMP2 (Abnova Corp., Taipei City, Taiwan, http://www.abnova.com), HIF-1α (Cell Signaling Technology, Danvers, MA, http://www.cellsignal.com), SDF-1α (Cell Signaling Technology), VEGFA (Novus Biologicals), p-GSKS3β (Abcam), and p-STAT3 (Abcam) and incubated with secondary antibodies for 2 hours at 37°C. Protein expression was detected with an enhanced chemiluminescence detection system (Tanon, Shanghai, China, http://www.biotanon.com). The β-actin signal was used as a loading control.
Statistical Analysis
All statistical analyses were performed using PASW Statistics 18.0 (SPSS, Chicago, IL, http://www.ibm.com). Statistical differences were determined by a two-tailed Student’s t test. p values less than .05 were considered statistically significant.
Results
Enhanced Homing and Transdifferentiation of MSCs Under Mechanical Stretching
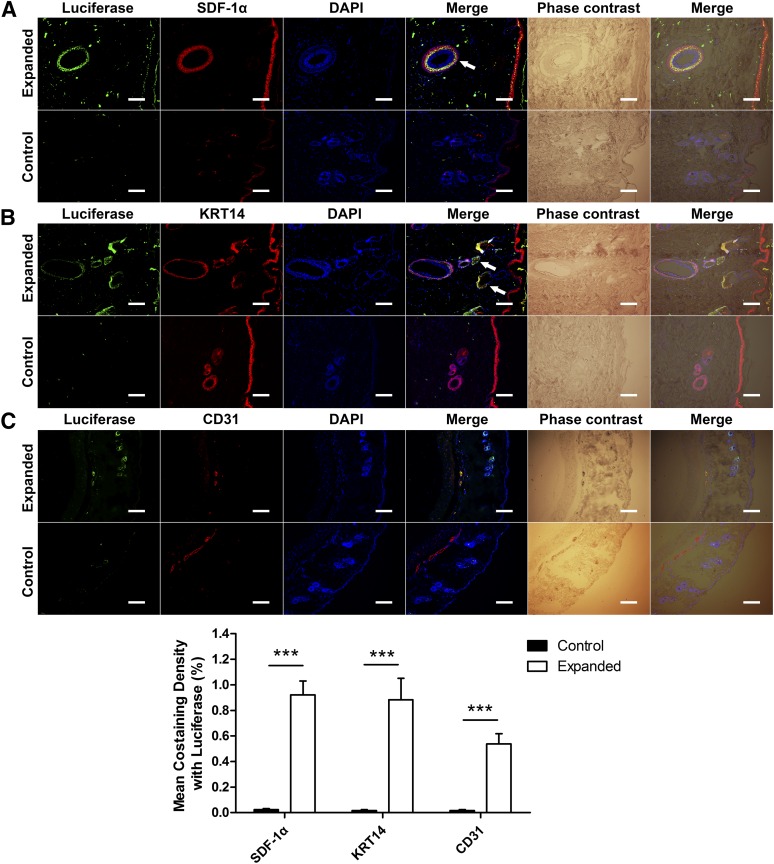
Transplanted MSCs in the skin of both groups were located by fluorescence staining with antiluciferase antibody. We evaluated the effect of mechanical stretching on SDF-1α expression in the expanded skin and its chemotactic effect during circulating MSC homing. Skin from the expanded group showed significantly more SDF-1α and luciferase colocalization than that of the control group, especially in the outer root sheath of the hair follicles (Fig. 1A), showing that mechanical stretching could significantly increase the expression of SDF-1α in the expanded skin and that MSCs were recruited to sites where SDF-1α was most highly expressed.
Figure 1.
Transplanted mesenchymal stem cells (MSCs) transdifferentiated into KRT14+ cells and CD31+ cells after SDF-1α-induced homing in the expanded skin. Luciferase was stained to locate the transplanted MSCs. (A): Skin from the expanded group showed significantly more SDF-1α and luciferase colocalization than the control group, especially the outer root sheath of the hair follicles (arrow), verifying the essential role of SDF-1α in MSC homing under mechanical stretching. (B): More KRT14+/luciferase+ cells were observed in the skin from the expanded group than the control group, especially in the outer root sheath of the hair follicles (arrow). (C): More CD31+/luciferase+ cells were observed in blood vessels (arrow) of the skin from the expanded group than the control group. ∗∗∗, p < .001; scale bars = 100 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; KRT, keratin; SDF, stromal cell-derived factor.
Furthermore, CD31 and KRT14 staining were performed to examine MSC transdifferentiation in the expanded skin. In the expanded group, luciferase+/KRT14+ cells were detected and mainly located in the outer root sheath of hair follicles, whereas there were few costaining cells in the control group (Fig. 1B), indicating that mechanical stretching might play an essential role in the transdifferentiation of homed MSCs into epidermal basal cells. Luciferase+/CD31+ cells were also observed in blood vessels in the skin from the expanded group and not in the control group (Fig. 1C). This suggested that, under mechanical stretching, the transplanted MSCs might transdifferentiate into endothelial cells in the expanded skin tissue.
Upregulation of SDF-1α Might Be Induced by HIF-1α in the Expanded Skin
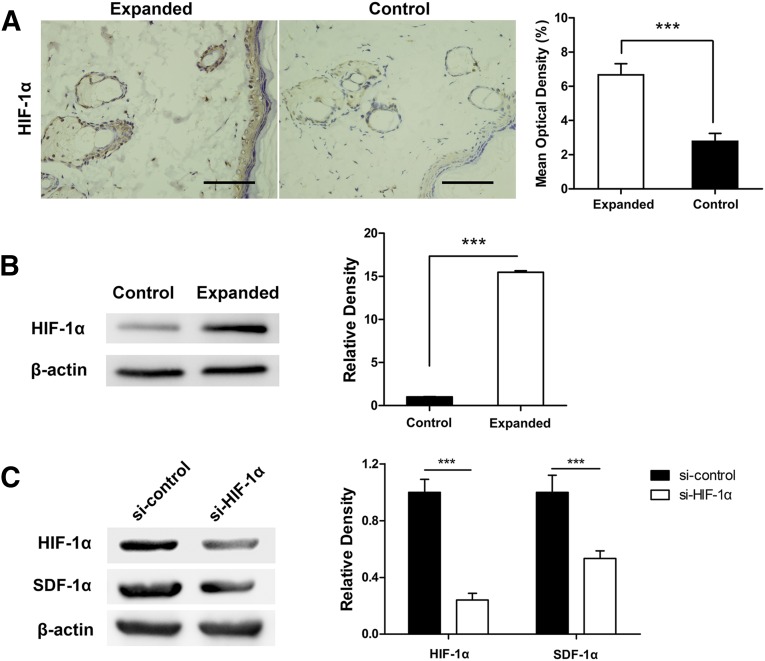
Considering that the implanted tissue expander induces temporary hypoxia and ischemia in the overlying skin and that SDF-1α is a hypoxia-related chemokine [15–19], HIF-1α was stained to verify the hypoxic environment in the expanded skin. Immunohistochemical staining showed that the expanded skin had significantly more HIF-1α-positive cells than control (fold change, 2.376; p < .001) (Fig. 2A). We also measured HIF-1α expression by Western blot analysis and observed higher levels of HIF-1α in the expanded skin than nonexpanded skin (Fig. 2B). To evaluate the correlation between SDF-1α and HIF-1α, we used siRNA targeted against HIF-1α (si-HIF-1α) to knock down its expression in the expanded skin. Scrambled siRNA was used as control (si-control). After 21-day expansion, the expanded skin samples were subjected to Western blot to analyze the expression of SDF-1α in both groups. The results showed that SDF-1α expression was significantly lower when HIF-1α was knocked down in the expanded skin, suggesting that the upregulation of SDF-1α might be induced by HIF-1α under mechanical stretching (Fig. 2C).
Figure 2.
Upregulation of SDF-1α might be induced by HIF-1α in the expanded skin. (A): Immunohistochemistry staining showed enhanced HIF-1α expression after skin tissue expansion. (B): Western blot results also showed higher HIF-1α in the expanded skin. (C): We used small interfering RNA to knock down HIF-1α expression in the expanded skin. After 21-day expansion, the expanded skin samples were subjected to Western blot. The results showed that SDF-1α expression was significantly lower when HIF-1α was knocked down in the expanded skin. ∗∗∗, p < .001; scale bars = 100 μm. Abbreviations: HIF, hypoxia-inducible factor; SDF, stromal cell-derived factor.
Functional Annotation of DEGs Between Stretched and Control hMSCs
To investigate the mechanisms underlying the MSC response to mechanical stretching, we used an in vitro stretching model on human MSCs to mimic the stretching environment in the expanded skin. Then we performed microarray analysis on mechanically stretched human MSCs and control MSCs to uncover potentially relevant genes and pathways, which could be further investigated in the animal expansion model. We detected DEGs comprising 178 upregulated genes and 410 downregulated genes between the stretched hMSCs and control hMSCs. Compared with the control hMSCs, there were 17 highly expressed genes with least threefold upregulation in the stretched hMSCs: DHRS9, CCND2, ISLR, EGR1, CCL2, IL8, MMP10, GPR68, SFRP4, TSPAN8, GAL, HPGDS, GPNMB, BHLHE40, RARRES2, NMB, and LRP1.
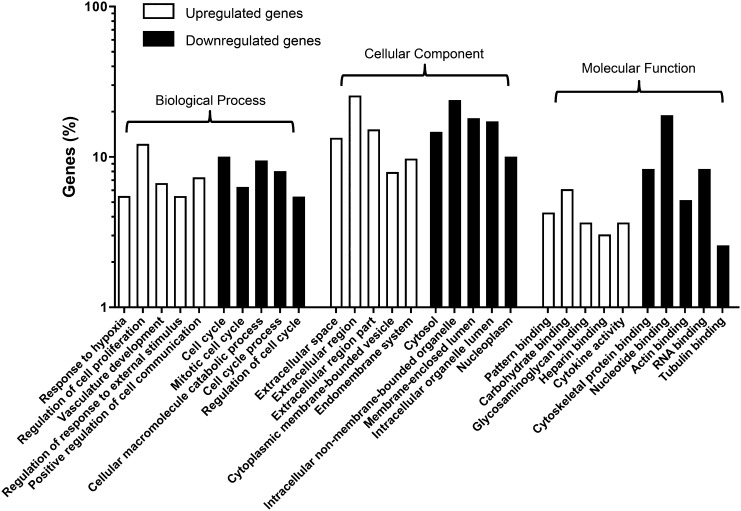
To investigate the functional changes in the mechanically stretched hMSCs, the 588 overlapping DEGs were mapped to the GO database. The significant GO of the 178 upregulated DEGs in the stretched hMSCs included various terms, and those such as “response to hypoxia,” “regulation of cell proliferation,” “vasculature development,” and “cytokine activity” were of interest. For the 410 downregulated DEGs in the stretched hMSCs, the significant GO included terms such as “regulation of cell cycle,” “cellular macromolecule catabolic process,” “cytoskeletal protein binding,” and “intracellular non-membrane-bounded organelle.”
DEGs in hMSCs with or without mechanical stretching were classified into categories based on biological process, molecular function, and cellular components. The top five significantly (based on p value) upregulated and downregulated GO terms in biological process, molecular function, and cellular components are shown in Figure 3.
Figure 3.
Functional categorization of significantly differentially expressed genes in mechanically stretched hMSCs. Differentially expressed genes from hMSCs cultured in the presence and absence of mechanical stretching were classified into the following categories: biological process, molecular function, and cellular components. Abbreviation: hMSC, human mesenchymal stem cell.
Genes with upregulated expression in terms of biological process were mainly involved in “response to hypoxia,” “regulation of cell proliferation,” and “vasculature development.” This indicated that mechanical stretching might induce hypoxia in hMSCs, which could stimulate the expression of genes related to vascularization and cell proliferation. The genes involved in the three GO terms are shown in supplemental online Tables 2–4.
Activation of the Jak-STAT and Wnt Signaling Pathways in Mechanically Strained hMSCs
DEGs were classified according to KEGG functional annotations to identify the pathways involved in the mechanical stretching process. The results from KEGG pathway analysis showed that the upregulated genes were annotated for six pathways, among which the Jak-STAT and Wnt signaling pathways were the most significant, with seven and six genes involved, respectively, including CCND2, LIF, and WNT5B (Table 1). Other KEGG pathway annotations of the upregulated and downregulated genes are presented in supplemental online Tables 5 and 6.
Table 1.
DEGs involved in the Jak-STAT and Wnt signaling pathways

Data Confirmation by Real-Time RT-PCR
To confirm the effectiveness of microarray identification, real-time RT-PCR was performed to analyze genes of interest from the upregulated categories. The results were consistent with that of microarray detection (supplemental online Fig. 1).
MMP2 Is a Crucial Factor in MSC Homing
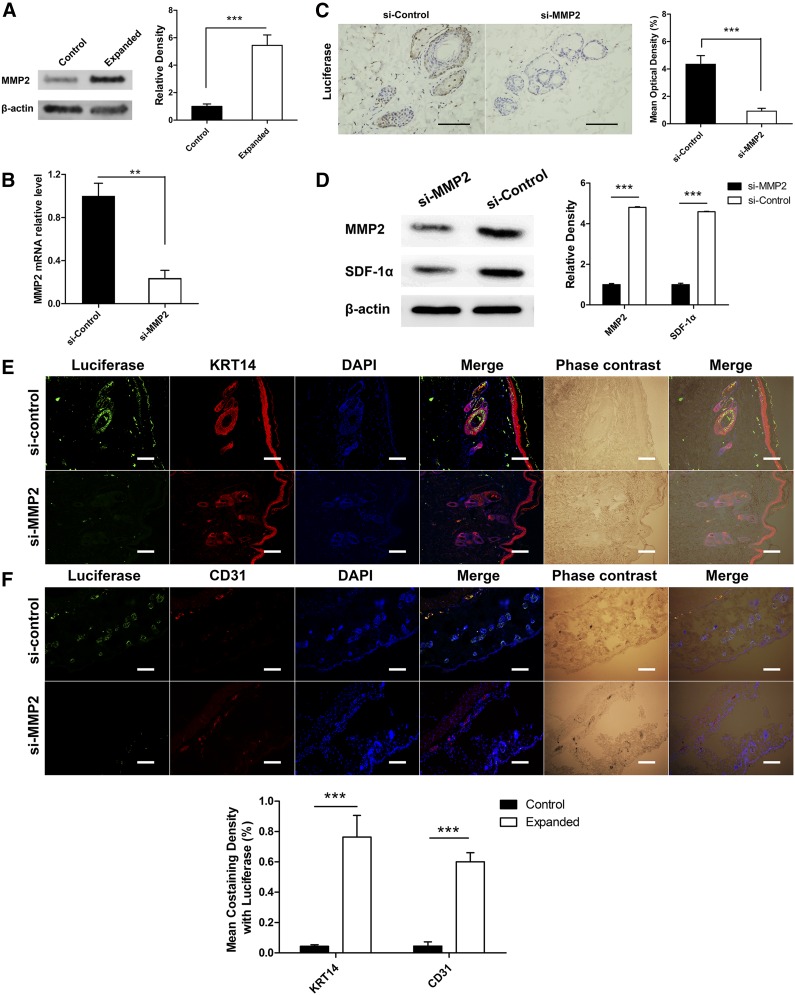
As shown by the results of the microarray analysis, the upregulated DEG MMP2 was enriched in two GO terms (“response to hypoxia” and “vasculature development”). Previous study [20] had revealed a close relationship between the SDF-1α/CXCR4 axis and MMP2. Therefore, we next investigated whether MMP2 is involved in MSC homing to the expanded skin in the rat skin expansion model. The total tissue lysate of the expanded skin and normal skin transplanted with MSCs were analyzed by Western blot using anti-MMP2 antibody, and the result showed enhanced MMP2 expression in the expanded skin (Fig. 4A). Furthermore, 21-bp siRNA sequences targeted against MMP2 (si-MMP2) were used to knock down MMP2 expression in the transplanted MSCs to see whether their homing was impaired compared with si-control. We found that the expanded skin transplanted with si-MMP2 MSCs had significantly fewer luciferase-positive cells than control (fold change, 0.219; p < .001) (Fig. 4C), suggesting that MMP2 is a crucial factor in MSC homing. We then performed fluorescence staining to determine the fate of siRNA-treated MSCs after transplantation into the rat expansion model. The results showed that skin transplanted with si-MMP2 MSCs had significantly fewer luciferase+/KRT14+ or luciferase+/CD31+ cells than that transplanted with si-control MSCs (p < .001) (Figs. 4E, 4F). Western blot analysis of the skin samples suggested that SDF-1α expression in the expanded skin was also decreased when transplanted with si-MMP2-treated MSCs (Fig. 4D), while HIF-1α expression was not affected (supplemental online Fig. 2). This implies a correlation between MMP2 and SDF-1α in MSC homing.
Figure 4.
MMP2 is a crucial factor for mesenchymal stem cells (MSCs) homing. (A): Western blot analysis showed higher MMP2 expression in the expanded skin after MSC transplantation. (B): MSCs were transfected with siRNAs targeting MMP2, and the expression levels of the target mRNAs were analyzed by reverse transcription-polymerase chain reaction. (C): si-MMP2-transfected MSCs and si-control-transfected MSCs were transplanted into the rat skin expansion model. The expanded skin area was collected after 21 days. Immunohistochemistry staining showed that expanded skin transplanted with si-MMP2-transfected MSCs contained significantly fewer luciferase-positive cells than control. (D): Western blot analysis showed that expanded skin transplanted with si-MMP2-transfected MSCs had significantly lower MMP2 and SDF-1α expression levels than control. (E,F): Costaining of luciferase/KRT14 and luciferase/CD31 were performed to determine the fate of siRNA-treated MSCs after transplantation into the rat expansion model. The results showed that skin transplanted with si-MMP2 MSCs had significantly fewer luciferase+/KRT14+ and luciferase+/CD31+ cells than that with si-control MSCs. ∗∗, p < .01; ∗∗∗, p < .001; scale bars = 100 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; KRT, keratin; MMP, matrix metalloproteinase; mRNA, messenger RNA; SDF, stromal cell-derived factor; si, small interfering.
The Jak-STAT and Wnt Signaling Pathways Are Involved in Mechanical Stretching-Induced VEGFA Upregulation
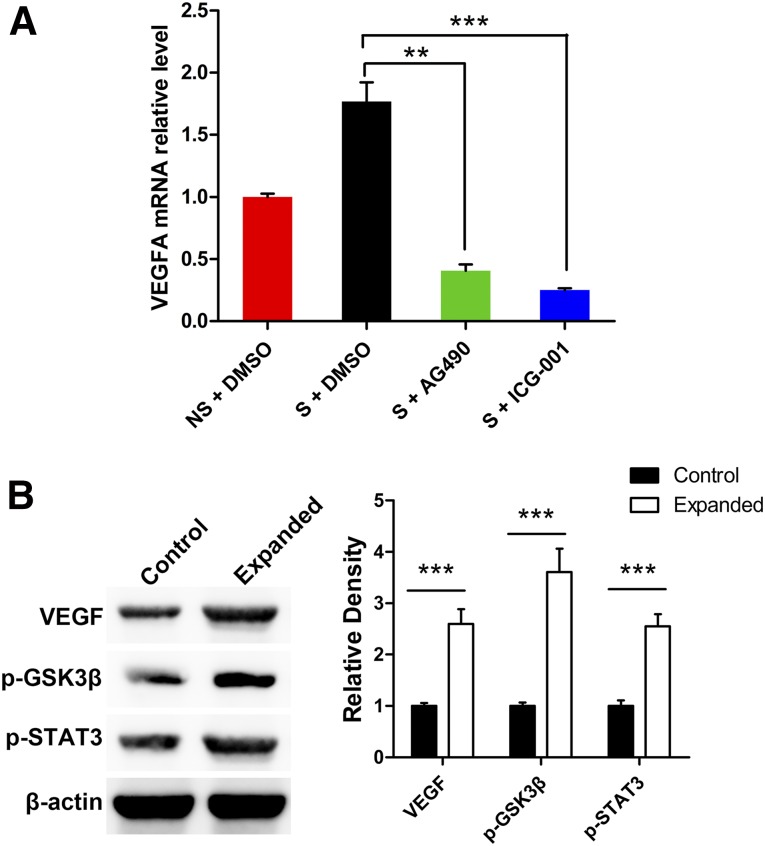
According to the microarray analysis, the Jak-STAT and Wnt signaling pathways might be activated in stretched MSCs. To verify the role of the Jak-STAT and Wnt signaling pathways in inducing VEGFA expression in response to mechanical stretching, mechanically strained hMSCs were treated with the Jak inhibitor AG490 and the Wnt-pathway inhibitor ICG-001. RT-PCR results showed that hMSCs under mechanical stretching treated with AG490 or ICG-001 expressed significantly lower (p < .01) VEGFA messenger RNA levels than those with DMSO (Fig. 5A), which validated our hypothesis that the Jak-STAT and Wnt signaling pathways are involved in mechanical stretching-induced VEGFA upregulation in hMSCs.
Figure 5.
The Jak-STAT and Wnt signaling pathways are involved in mechanical stretching-induced VEGFA upregulation. (A): Human mesenchymal stem cells (hMSCs) under mechanical stretching were treated with the Jak inhibitor AG490 and the Wnt-pathway inhibitor ICG-001. Reverse transcription-polymerase chain reaction results showed that hMSCs under mechanical stretching treated with AG490 or ICG-001 expressed significantly lower VEGFA mRNA levels than those treated with DMSO. (B): Rat skin samples from the expanded and control groups were analyzed by Western blot to evaluate the expression of VEGFA, p-STAT3 (marker of the Jak-STAT signaling pathway), and p-GSK3β (marker of the Wnt signaling pathway); the results showed that expression of VEGFA and markers of the Jak-STAT and Wnt signaling pathways were upregulated in the expanded skin compared with unexpanded skin after MSC transplantation. ∗∗, p < .01; ∗∗∗, p < .001. Abbreviations: DMSO, dimethyl sulfoxide; Jak-STAT, Janus kinase/signal transducer and activator of transcription; mRNA, messenger RNA; NS, no strain; S, strain; VEGFA, vascular endothelial growth factor A.
Furthermore, to test whether similar changes were involved in the rat expansion model, Western blot was performed on the rat skin samples (expanded and control groups) to analyze the expression level of VEGFA, p-STAT3 (marker of the Jak-STAT signaling pathway), and p-GSK3β (marker of the Wnt signaling pathway). Consistent with microarray and real-time RT-PCR results, the expression of VEGFA and markers of the Jak-STAT and Wnt signaling pathways were significantly higher in the expanded skin compared with unexpanded skin (p < .001), indicating an upregulation of VEGFA and activation of the Jak-STAT and Wnt signaling pathways in the expanded skin after MSC transplantation.
Discussion
Optimal coverage of skin defects can be achieved by skin regenerated through tissue expansion. However, the capacity for skin tissue regeneration using this procedure is limited and can result in severe complications during the expansion process. Previously, we found that MSC transplantation was effective in promoting skin tissue expansion [9, 10]. In this study, systematic investigation was carried out to illustrate the molecular mechanism of intravenously transplanted MSC contribution to skin regeneration during tissue expansion.
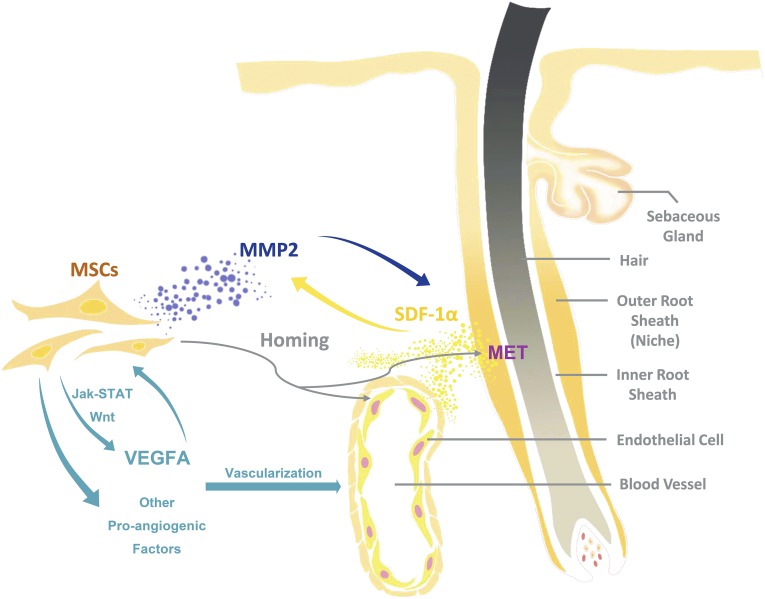
The homing of infused MSCs to the target site is crucial in regenerative medicine [21]. In the present study, SDF-1α was expressed in the basal layer of the epidermis and the outer root sheath of hair follicles after mechanical stretching. Considering that the implanted tissue expander induces temporary hypoxia and ischemia in the overlying skin, which was verified by HIF-1α staining, and that SDF-1α is a hypoxia-related chemokine that contributes to MSC migration in multiple tissues [15–19], our results suggest that mechanical stretching-induced hypoxia elevates SDF-1α secretion in the expanded skin and enhances the homing of intravenously transplanted MSCs. Furthermore, microarray analysis revealed another upregulated gene closely related to MSC homing: MMP2, which is a member of MMPs that degrade extracellular matrix components and promote cell migration [22]. Previous study has also revealed that MMP2 could upregulate the expression of SDF-1α and its receptor CXCR4 in endothelial cells [20]. Because the impaired homing of si-MMP2-transfected MSCs was observed in the skin expansion model in this study, we determine that MMP2 could be a promoting factor for MSC homing during the skin expansion process. Decreased SDF-1α expression in the expanded skin was also observed when transplanted with si-MMP2-treated MSCs, which implies a correlation between MMP2 and SDF-1α in MSC homing. Thus, we infer that mechanical stretching-induced hypoxia might trigger a feedback mechanism of MMP2 and SDF-1α between the homed MSCs and the expanded skin tissue (i.e., MMP2 secreted by homed MSCs induces the expression of SDF-1α in the expanded skin), which enhances the homing of circulating MSCs (Fig. 6).
Figure 6.
Schematic diagram of the homing and transdifferentiation of transplanted MSCs. Mechanical stretching might enhance MSC homing to the outer root sheath of hair follicles and blood vessels through feedback between MMP2 and the SDF-1α/C-X-C chemokine receptor 4 axis. MSCs transdifferentiate into KRT14+ cells in the microenvironment of the hair follicle stem cell niche, and into CD31+ cells in blood vessels. MSCs promote vascularization in the expanded skin through upregulation of VEGFA (via the Jak-STAT and Wnt signaling pathways) and other proangiogenic factors. Abbreviations: Jak-STAT, Janus kinase/signal transducer and activator of transcription; KRT, keratin; MET, mesenchymal-epithelial transition; MMP, matrix metalloproteinase; MSC, mesenchymal stem cell; SDF, stromal cell-derived factor; VEGFA, vascular endothelial growth factor A.
After their recruitment to the outer root sheath of hair follicles in the expanded skin, the transplanted MSCs expressed KRT14, as shown by the results of immunofluorescence staining. In skin tissue, KRT14 is a marker of basal cells, including epidermal stem cells, transient amplifying cells, and others [23, 24], and it is expressed in the basal layer of the epidermis and the outer root sheath of hair follicles [25, 26]. Hence, our results indicate that intravenously transplanted MSCs might directly transdifferentiate into epidermal basal cells after homing and contribute to skin regeneration (Fig. 6). Once homed to the outer root sheath of hair follicles, the mesenchymal-derived MSCs express the epithelial marker KRT14, undergoing mesenchymal-epithelial transition (MET). As no such results were observed in the control group, we hypothesize that this MET of MSCs might be closely related to the mechanical stretching. Previous studies had reported that the microenvironment around the homed MSCs, namely the stem cell niche, plays an important role in MSC transdifferentiation into organ-specific cells [27, 28]. Therefore, identifying alterations in the microenvironment of hair follicles in the expanded skin is crucial for clarifying the MET of MSCs under mechanical stretching.
In addition to direct improvement of skin regeneration through MET, intravenously transplanted MSCs also accelerate the process of skin expansion by promoting vascularization, which alleviates ischemia in the expanded skin tissue. It has been reported that MSCs can directly transdifferentiate into vascular cells and act as an important regulator of neovascularization by secreting proangiogenic factors [29, 30]. In this study, more luciferase+/CD31+ cells were observed in the blood vessels in the expanded group, evidence that mechanical stretching might enhance the transdifferentiation of MSCs into endothelial cells after homing to the expanded skin tissue. Furthermore, in the in vitro stretched hMSCs, gene functional annotation revealed that 11 upregulated genes were enriched in the GO term “vasculature development” (supplemental online Table 4), which implies a paracrine effect of MSCs on vascularization induced by mechanical stretching. Moreover, genes enriched in the Jak-STAT and Wnt signaling pathways, which are closely related to vascularization [31, 32], also showed elevated expression. These results validate mechanical stretching as an important contributing factor to the angiogenic capacity of MSCs. Among the genes of interest, VEGFA is recognized as one of the most potent regulators of angiogenesis [33, 34]. It can be upregulated via either the Jak-STAT or Wnt signaling pathway [32, 35–38], and autocrine VEGFA leads to MSC transdifferentiation into endothelial cells [39]. In our observation, the mechanical strain-induced upregulation of VEGFA in hMSCs was abrogated by Jak inhibitor AG490 or Wnt-pathway inhibitor ICG-001, and the expression of VEGFA and markers of the Jak-STAT and Wnt signaling pathways were upregulated in the expanded skin compared with unexpanded skin after MSC transplantation. Therefore, our results indicate that mechanical stretching might enhance MSC-mediated vascularization in expanded skin tissue by inducing MSC direct transdifferentiation into endothelial cells, upregulating their expression of the proangiogenic factor VEGFA through the Jak-STAT and Wnt signaling pathways (Fig. 6).
Although isolating the transplanted MSCs from the expanded skin tissue is an ideal way to analyze their systemic change in gene expression, it is difficult to dissociate skin tissues into single cells and sort them. Nevertheless, in vitro mechanical strain was designed to mimic the environment under mechanical stretching in the expanded skin, and the results were valuable for providing an avenue for future clinical interventions.
Conclusion
This study determined that mechanical stretching upregulates MSC expression of genes related to hypoxia, vascularization, and cell proliferation; enhances transplanted MSC homing to the expanded skin tissue; and promotes transdifferentiation into epidermal basal cells and endothelial cells.
Supplementary Material
Acknowledgments
We thank Oxford Science Editing for language support. This work was supported by the National Natural Science Foundation of China (Grant 2012BAI11B03) and Doctoral Innovation Fund Projects from Shanghai Jiao Tong University School of Medicine (Grant bxj201431).
Author Contributions
X.L. and X.H.: conception and design, manuscript writing, data analysis and interpretation, final approval of manuscript; Y.Z. and R.J.: conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; Q.L.: conception and design, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Neumann CG. The expansion of an area of skin by progressive distention of a subcutaneous balloon; use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg (1946) 1957;19:124–130. doi: 10.1097/00006534-195702000-00004. [DOI] [PubMed] [Google Scholar]
- 2.De Filippo RE, Atala A. Stretch and growth: The molecular and physiologic influences of tissue expansion. Plast Reconstr Surg. 2002;109:2450–2462. doi: 10.1097/00006534-200206000-00043. [DOI] [PubMed] [Google Scholar]
- 3.Huang X, Qu X, Li Q. Risk factors for complications of tissue expansion: A 20-year systematic review and meta-analysis. Plast Reconstr Surg. 2011;128:787–797. doi: 10.1097/PRS.0b013e3182221372. [DOI] [PubMed] [Google Scholar]
- 4.Hallock GG. Safety of clinical overinflation of tissue expanders. Plast Reconstr Surg. 1995;96:153–157. doi: 10.1097/00006534-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Wickman M. Comparison between rapid and slow tissue expansion in breast reconstruction. Plast Reconstr Surg. 1993;91:663–670. [PubMed] [Google Scholar]
- 6.Netscher DT, Spira M, Peterson R. Adjunctive agents to facilitate rapid tissue expansion. Ann Plast Surg. 1989;23:412–416. doi: 10.1097/00000637-198911000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Lee P, Squier CA, Bardach J. Enhancement of tissue expansion by anticontractile agents. Plast Reconstr Surg. 1985;76:604–610. doi: 10.1097/00006534-198510000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Ren G, Chen X, Dong F, et al. Concise review: Mesenchymal stem cells and translational medicine: Emerging issues. Stem Cells Translational Medicine. 2012;1:51–58. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M, Li Q, Sheng L, et al. Bone marrow-derived mesenchymal stem cells transplantation accelerates tissue expansion by promoting skin regeneration during expansion. Ann Surg. 2011;253:202–209. doi: 10.1097/SLA.0b013e3181f9ba1ah. [DOI] [PubMed] [Google Scholar]
- 10.Zhou S, Chiang C, Liu K, et al. Intravenous transplantation of bone marrow mesenchymal stem cell could effectively promote vascularization and skin regeneration in mechanical stretched skin. Br J Dermatol. 2015;172:1278–1285. doi: 10.1111/bjd.13251. [DOI] [PubMed] [Google Scholar]
- 11.Zhou SB, Wang J, Chiang CA, et al. Mechanical stretch upregulates SDF-1α in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin. Stem Cells. 2013;31:2703–2713. doi: 10.1002/stem.1479. [DOI] [PubMed] [Google Scholar]
- 12.Hakamata Y, Murakami T, Kobayashi E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation. 2006;81:1179–1184. doi: 10.1097/01.tp.0000203137.06587.4a. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 14.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji JF, He BP, Dheen ST, et al. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Li Y, Chen J, et al. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002;30:831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Li Y, Chen X, et al. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 18.Abbott JD, Huang Y, Liu D, et al. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 19.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 20.Maddirela DR, Kesanakurti D, Gujrati M, et al. MMP-2 suppression abrogates irradiation-induced microtubule formation in endothelial cells by inhibiting αvβ3-mediated SDF-1/CXCR4 signaling. Int J Oncol. 2013;42:1279–1288. doi: 10.3892/ijo.2013.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tebebi PA, Burks SR, Kim SJ, et al. Cyclooxygenase-2 or tumor necrosis factor-alpha inhibitors attenuate the mechanotransductive effects of pulsed focused ultrasound to suppress mesenchymal stromal cell homing to healthy and dystrophic muscle. Stem Cells. 2015;33:1173–1186. doi: 10.1002/stem.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badiga AV, Chetty C, Kesanakurti D, et al. MMP-2 siRNA inhibits radiation-enhanced invasiveness in glioma cells. PLoS One. 2011;6:e20614. doi: 10.1371/journal.pone.0020614. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Fuchs E. Epidermal differentiation: The bare essentials. J Cell Biol. 1990;111:2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunnwald M, Tomanek-Chalkley A, Alexandrunas D, et al. Isolating a pure population of epidermal stem cells for use in tissue engineering. Exp Dermatol. 2001;10:45–54. doi: 10.1034/j.1600-0625.2001.100106.x. [DOI] [PubMed] [Google Scholar]
- 25.Coulombe PA, Kopan R, Fuchs E. Expression of keratin K14 in the epidermis and hair follicle: Insights into complex programs of differentiation. J Cell Biol. 1989;109:2295–2312. doi: 10.1083/jcb.109.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam H, Sehgal L, Kundu ST, et al. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell. 2011;22:4068–4078. doi: 10.1091/mbc.E10-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 28.Backly RM, Cancedda R. Bone marrow stem cells in clinical application: Harnessing paracrine roles and niche mechanisms. Adv Biochem Eng Biotechnol. 2010;123:265–292. doi: 10.1007/10_2010_78. [DOI] [PubMed] [Google Scholar]
- 29.Lin CS, Lue TF. Defining vascular stem cells. Stem Cells Dev. 2013;22:1018–1026. doi: 10.1089/scd.2012.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Au P, Tam J, Fukumura D, et al. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jee SH, Chu CY, Chiu HC, et al. Interleukin-6 induced basic fibroblast growth factor-dependent angiogenesis in basal cell carcinoma cell line via JAK/STAT3 and PI3-kinase/Akt pathways. J Invest Dermatol. 2004;123:1169–1175. doi: 10.1111/j.0022-202X.2004.23497.x. [DOI] [PubMed] [Google Scholar]
- 32.Skurk C, Maatz H, Rocnik E, et al. Glycogen-synthase kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res. 2005;96:308–318. doi: 10.1161/01.RES.0000156273.30274.f7. [DOI] [PubMed] [Google Scholar]
- 33.Duh EJ, Yang HS, Haller JA, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: Implications for ocular angiogenesis. Am J Ophthalmol. 2004;137:668–674. doi: 10.1016/j.ajo.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 35.Huang SP, Wu MS, Shun CT, et al. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11:517–527. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- 36.Wei LH, Kuo ML, Chen CA, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 37.Easwaran V, Lee SH, Inge L, et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–3153. [PubMed] [Google Scholar]
- 38.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–6054. [PubMed] [Google Scholar]
- 39.Oswald J, Boxberger S, Jørgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.