Freshly isolated, autologous, adipose-derived stromal cells were combined with a fat-graft procedure to treat lymphedema in a woman who had undergone lymphadenectomy and radiation therapy 4 years previously. The patient experienced no adverse events from the treatment, and arm volume and need for compression therapy were reduced after 4 months. A phase II study is under way to further test the feasibility and safety of this treatment.
Keywords: Lymphedema, Stromal vascular fraction, Adipose-derived stromal cells, Adipose-derived stem cells, Adipose-derived regenerative cells, Mesenchymal stem cell transplantation, Regenerative medicine, Cell-based therapy, Tissue-based therapy
Abstract
Lymphedema is one of the most frequent side effects following cancer treatment, and treatment opportunities for it are currently lacking. Stem cell therapy has been proposed as a possible novel treatment modality. This study was the first case in which freshly isolated adipose-derived stromal cells were used to treat lymphedema. Treatment was given as a cell-assisted lipotransfer in which 4.07 × 107 cells were injected with 10 ml of lipoaspirate in the axillary region. Four months after treatment, the patient reported a great improvement in daily symptoms, reduction in need for compression therapy, and volume reduction of her affected arm. There were no adverse events. The outcome for this patient provides support for the potential use of cellular therapy for lymphedema treatment. We have begun a larger study to further test the feasibility and safety of this procedure (ClinicalTrials.gov Identifier NCT02592213).
Significance
Lymphedema is a very debilitating side effect of cancer treatment and has very few treatment options. Stem cell therapy has the potential to change the treatment paradigm from a conservative to a more curative approach. Freshly isolated, autologous, adipose-derived stromal cells were combined with a fat-graft procedure to treat lymphedema. The treated patient had great improvement in daily symptoms, a reduced need for compression therapy, and a reduction in arm volume after 4 months. There were no adverse events. The use of cellular therapy for lymphedema treatment is supported by this patient’s outcome. A phase II study has begun to further test its feasibility and safety.
Introduction
In the developed world, cancer treatment is the leading cause of lymphedema. It is a debilitating condition associated with recurrent infections [1] and reduced quality of life [2, 3]. The present standard of care is compression therapy, which depends on continuous adherence to be effective [4]. Accordingly, new therapeutic approaches are needed to treat this condition. One such approach is stem cell therapy; in a recent review, we summarized all preclinical and clinical studies that used stem cells for therapeutic lymphangiogenesis [5]. All reported clinical studies used bone marrow-derived mesenchymal stem cells (MSCs). Adipose tissue is the most abundant and easiest accessible source of MSCs, but it has not been used previously for lymphedema treatment. To our knowledge, we are the first to report the use of cell-assisted lipotransfer using freshly isolated adipose-derived stromal cells (the stromal vascular fraction [SVF]) to treat lymphedema.
Materials and Methods
The patient was a 48-year-old woman with breast cancer-related lymphedema of her arm due to lymphadenectomy and radiation therapy 4 years ago. Besides conservative management, there were no treatment opportunities available; therefore, she was offered experimental stem cell treatment, which she accepted. Hospital exemption was acquired from the Danish Health and Medicines Authority to allow for the autologous cell treatment. No further approvals were necessary.
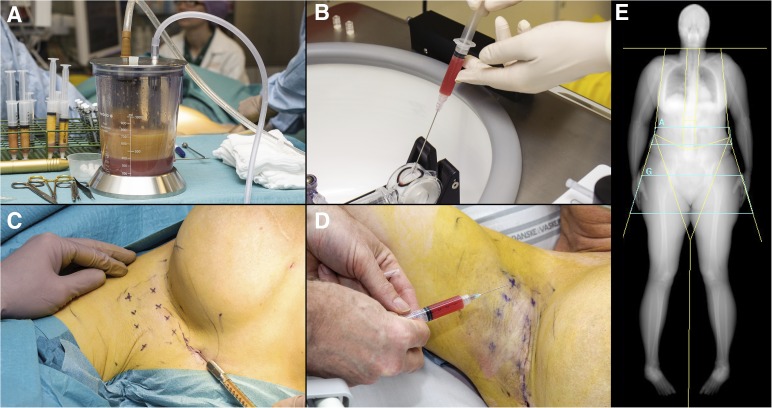
The procedure was performed under general anesthesia and involved liposuction (body-jet; Human med AG, Schwerin, Germany, http://www.humanmed.com) of 320 ml of lipoaspirate for SVF isolation using the automated Celution 800/CRS System (Cytori Therapeutics, San Diego, CA, http://www.cytori.com) according to the manufacturer’s instructions. The SVF was isolated within 2 hours and resuspended in 5 ml of lactated Ringer’s solution with a syringe, and 1 ml was saved for characterization. An additional 10 ml of pure centrifuged lipoaspirate was used for fat grafting to the axillary region using a technique incorporating fan-shaped injections to release axillary scar formation. The remaining 4 ml of SVF was injected into the same region in the subcutaneous plane immediately after isolation at 8 premarked points. Figure 1 presents intraoperative photographs. The patient was discharged the same afternoon.
Figure 1.
Photographs and dual-energy x-ray absorptiometry (DXA) scan of the injection of the stromal vascular fraction of freshly isolated adipose-derived stromal cells in the procedure described in this study. (A): Lipoaspirate (320 ml) was obtained using water-assisted liposuction. This was transferred to 60-ml syringes and then injected into the Celution System (Cytori Therapeutics). (B): The adipose-derived stromal cells were isolated within 2 hours and were suspended in 5 ml of lactated Ringer’s solution. (C): During the surgical procedure, 10 ml of lipoaspirate was used for fat grafting to the axillary region. (D): The adipose-derived stromal cells were injected into the same region at eight prespecified points immediately after isolation; the patient was awake at this time. (E): Whole-body DXA scans were used to calculate arm volume pre- and postoperatively. The lines isolating the arms, including the shoulders, represent the areas from which volume was calculated.
As previously described by Haahr et al. [6], SVF was characterized by nucleated cell count and viability, the percentage of fibroblastoid colony-forming units (CFU-F), and surface marker analysis using the following markers from BD Biosciences (Albertslund, Denmark, http://www.bd.com): anti-CD34 (PE-CF594, clone 581), anti-CD45 (fluorescein isothiocyanate, clone HI30), CD31 (Alexa Fluor 647, clone WM59), CD73 (allophycocyanin [APC], clone AD2), CD90 (APC, clone 5E10), and appropriate isotypes according to the manufacturer’s recommendations. Sample acquisition was performed on a BD LSRII flow cytometer and analyzed using the FACSDiva software version 8.0.1 (BD Biosciences) and FlowJo 7.6.5 (Tree Star, Ashland, OR, http://www.flowjo.com). Cell doublets were excluded from all analyses by sequential gating through forward- and side-scatter height/width plots. A nucleated cell count including viability analysis was performed using three technical replicates with a NucleocounterNC-200 (ChemoMetec, Allerod, Denmark, http://chemometec.com). The percentage of CFU-F was determined by seeding cells at low density (4 concentrations in triplicate ranging from 28 to 280 live nucleated cells per cm2) in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) with 1 g/l glucose, 10% fetal bovine serum (Sigma-Aldrich), 1% penicillin-streptomycin (PS) (Sigma-Aldrich), culturing for 14 days, and counting hematoxylin-stained colonies comprising more than 50 cells.
The outcome was evaluated by pre- and postoperative circumference measurements, dual-energy x-ray absorptiometry (DXA) scans, and evaluation of adverse events. DXA scans were performed as whole-body scans and each arm was measured separately; based on known densities of bone, fat, and nonfat tissue, a volume was calculated as described in the literature [7]. Adverse events were evaluated immediately following the SVF injection, before discharge, and after 1 and 4 months with a combination of clinical examination of the injected area, open-ended questioning (experience of any discomfort) of the patient, and more specific questioning regarding infection, pain, and swelling.
Results
A total of 4.07 × 107 viable cells were administered to the patient. The CFU-F was 0.7%. Flow cytometry on stored, fixed cells showed that cells were 14.3% CD45+, 60.4% CD34+, 9.6% CD31+, 44.5% CD73+, and 69.5% CD90+.
The patient was seen after 1 and 4 months, and she reported a great reduction in her symptoms of arm heaviness and tension. The patient no longer needed to use intermittent pneumatic compression therapy, which she had used on a daily basis before treatment. She still wore her compression garment.
Circumference measurements after 4 months showed a reduction of 1 cm at the wrist and lower arm and 2 cm at the upper arm. Pre- and postoperative DXA scans showed a reduction of 292 ml in the lymphedema-affected arm, whereas that of the healthy arm was increased by 50 ml. There were no postoperative complications or adverse events related to the procedure.
Discussion
Treatment of lymphedema remains one of the greatest challenges in reconstructive surgery for which efficient treatment possibilities are lacking. The outcome of this patient supports the results of previous studies using bone marrow-derived cells and provides a foundation for the potential use of cellular therapy for lymphedema treatment [8, 9].
The DXA scan was performed as a whole-body scan. Software limitations meant that the arm-volume measurements included the shoulder, thereby increasing the risk for variability in the volume measurements. This could explain the increase of 50 ml in the healthy arm volume at follow-up. To minimize variability, we now scan each arm separately and isolate the arm with subregional analysis, a technique that was not available to us when this patient was scanned. Potential changes in lymph drainage were not assessed in this patient, and this is a limitation when interpreting the results, because we cannot conclude that the cell therapy resulted in improved lymph drainage. We recommend that all future studies seeking to treat lymphedema include standardized, subjective evaluations with validated questionnaires and scales, objective measurements of arm volume through circumference measurements and imaging studies, and functional measurements to evaluate any change in drainage.
Based on the promising preclinical and clinical results, we have initiated a phase II study in our department to further test the feasibility and safety of this procedure (ClinicalTrials.gov Identifier: NCT02592213).
Author Contributions
N.M.T.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; C.H.J.: provision of study material or patients, collection and/or assembly of data, manuscript revision for intellectual content, final approval of manuscript; S.P.S.: financial support, administrative support, provision of study material or patients, manuscript revision for intellectual content, final approval of manuscript; J.A.S.: conception and design, financial support, administrative support, provision of study material or patients, collection and/or assembly of data, manuscript revision for intellectual content, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg (cellulitis): Case-control study. BMJ. 1999;318:1591–1594. doi: 10.1136/bmj.318.7198.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McWayne J, Heiney SP. Psychologic and social sequelae of secondary lymphedema: A review. Cancer. 2005;104:457–466. doi: 10.1002/cncr.21195. [DOI] [PubMed] [Google Scholar]
- 3.Tobin MB, Lacey HJ, Meyer L, et al. The psychological morbidity of breast cancer-related arm swelling. Psychological morbidity of lymphoedema. Cancer. 1993;72:3248–3252. doi: 10.1002/1097-0142(19931201)72:11<3248::aid-cncr2820721119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Lasinski BB, McKillip Thrift K. et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R. 2012;4:580–601. doi: 10.1016/j.pmrj.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Toyserkani NM, Christensen ML, Sheikh SP, et al. Stem cells show promising results for lymphoedema treatment—a literature review. J Plast Surg Hand Surg. 2015;49:65–71. doi: 10.3109/2000656X.2014.964726. [DOI] [PubMed] [Google Scholar]
- 6.Haahr MK, Jensen CH, Toyserkani NM, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: An open-label phase I clinical trial. EBioMedicine. 2016;5:204–210. doi: 10.1016/j.ebiom.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gjorup C, Zerahn B, Hendel HW. Assessment of volume measurement of breast cancer-related lymphedema by three methods: Circumference measurement, water displacement, and dual energy X-ray absorptiometry. Lymphat Res Biol. 2010;8:111–119. doi: 10.1089/lrb.2009.0016. [DOI] [PubMed] [Google Scholar]
- 8.Hou C, Wu X, Jin X. Autologous bone marrow stromal cells transplantation for the treatment of secondary arm lymphedema: A prospective controlled study in patients with breast cancer related lymphedema. Jpn J Clin Oncol. 2008;38:670–674. doi: 10.1093/jjco/hyn090. [DOI] [PubMed] [Google Scholar]
- 9.Maldonado GEM, Pérez CAA, Covarrubias EEA, et al. Autologous stem cells for the treatment of post-mastectomy lymphedema: A pilot study. Cytotherapy. 2011;13:1249–1255. doi: 10.3109/14653249.2011.594791. [DOI] [PubMed] [Google Scholar]