Abstract
Although there have been tremendous advances in the understanding of human dysfunctions in the brain circuitry for self-reflection, emotion, and cognitive control, a brain-based taxonomy for mental disease is still lacking. As a result, these advances have not been translated into actionable clinical tools, and the language of brain circuits has not been incorporated into training programmes. To address this gap, I present this synthesis of published work, with a focus on functional imaging of circuit dysfunctions across the spectrum of mood and anxiety disorders. This synthesis provides the foundation for a taxonomy of putative types of dysfunction, which cuts across traditional diagnostic boundaries for depression and anxiety and includes instead distinct types of neural circuit dysfunction that together reflect the heterogeneity of depression and anxiety. This taxonomy is suited to specifying symptoms in terms of underlying neural dysfunction at the individual level and is intended as the foundation for building mechanistic research and ultimately guiding clinical practice.
Introduction
Technical and conceptual advances in brain imaging have provided new insights into the brain circuits that underlie complex cognitive, emotional, and self-reflective functions,1–4 the very functions that define human experience. Dysfunctions within and between these circuits give rise to the symptoms that characterise mental disease. Understanding these dysfunctions offers the opportunity to specify symptoms at the level of the organ that generates them—the brain. By doing so, the notion of mental disease could be revolutionised and treatments could be tailored according to individual experiences and underlying neural disconnections.
Why therefore is there such a gap between these advances and their application in practice? In psychiatry, language about brain circuits has not been incorporated into clinically meaningful taxonomies, training programmes do not provide doctors with training in neural circuit terminology, and practitioners do not have neuroscience-based tools to inform their decision making. This gap remains despite substantial efforts on many fronts. Federally, the US National Institute of Mental Health is pioneering the Research Domain Criteria project, the aim of which is to generate a neurobiologically valid classification for mental disorders.5 The aim of the White House’s BRAIN Initiative is to develop neurotechnologies for de mystifying brain disorders, including depression.6 The National Institute of Mental Health and American Psychiatric Association worked hard to develop the new, fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), and to incorporate neuroscience not covered in previous editions.7
In this Personal View, I propose that the development of a neural circuit taxonomy suited to clinical actions is one way to address the gap between brain imaging advances and practice. There is a compelling need for change, in view of the escalating burden of mental disease. Globally, 405 million people experience depression, and 274 million have anxiety disorders.8 These disorders are the main causes of disability and lost productivity, with a staggering economic cost in the USA of US$42–53 billion per year.8 Other medical specialties, such as cardiovascular medicine, face similar challenges in translating complex biological signals into clinical care models,9 but are further ahead in linking a taxonomy based on the organ of interest to subjective symptoms and treatment indications. For example, electro cardiography can be used to quantify types of arrhythmia due to electrical circuit dysfunctions (too fast, too slow, irregular) and to indicate specific treatments (pacemaker), and angiography can be used to quantify types of blockage due to coronary heart disease (clots, stroke, heart attack) and to indicate treatments (lifestyle changes, medications, surgery).
A neural circuit approach to mental disorder
As a broad hypothesis, it is proposed that specific dysfunctions in the functional and structural connectivity of large-scale circuits that govern emotional, cognitive, and self-reflective functions define unique biotypes of depression and anxiety. These biotypes will probably differ from traditional diagnostic categories and are likely to overlap, interact, or co-occur in individual patients. A single mechanism is unlikely to underlie a broad descriptive diagnosis such as depression. Two people in whom major depressive disorder is diagnosed might share only one symptom. The current symptom-based categories arguably conflate several types of individuals in whom diverse brain dysfunctions drive symptoms. If a taxonomy based on brain dysfunctions is articulated, then it can be mapped onto the profiles of symptoms to which these dysfunctions give rise.
Mental disorders have not typically been thought of as brain disorders. Instead, the term brain disorder normally refers to a neurological condition associated with a discrete lesion or degenerative process. This usage might reflect limited understanding of the real-time coordination of the brain. With the advent of brain-imaging techniques with sufficient spatial and temporal resolution to quantify neural connections in vivo, now is the right time to reformulate understanding of mental disorders as neural circuit dysfunctions.
Which large-scale circuits?
Researchers have identified an intrinsic neural circuit architecture that might underlie domain-general processes of self-reflection, salience perception, and attention, as well as sensorimotor, visual, and auditory function.1,3,4,10 The universality of this intrinsic architecture has been demonstrated with large-scale functional connectivity analysis of hundreds of brain regions encompassing every major brain system at rest and across 64 task-evoked states.1 These brain regions have been identified with parcellation and meta-analysis.1 During rest, the default mode circuit tends to be upregulated, and other circuits downregulated.1,2 This intrinsic architecture might also have a major role in shaping task processes— such as controlling cognition—or reaction to threatening or rewarding stimuli, with a smaller amount of variance contributed by extrinsic task-general and task-specific evoked changes.1,11–15 This extrinsic architecture has been shown by conjunction analysis of multiple datasets with multiple tasks.15 Task-general profiles include a down-regulation of within-circuit functional connectivity during task performance and an anticorrelation between default mode and circuits involved in attention and cognitive control,1,15 although there could be positive correlations with demanding shifts in task sets.16 Task-specific activations and connectivity correlate with overt behavioural performance.17,18
In this Personal View, I focus on six circuits that have been implicated in dysfunctions expressed in depression and anxiety: default mode, salience, negative affect, positive affect (reward), attention, and cognitive control (figure 1). I summarise available evidence as a basis for proposing putative biotypes of dysfunction that might account for the natural heterogeneity of these disorders.
Figure 1. Large-scale intrinsic and task-evoked circuits identified in published work.
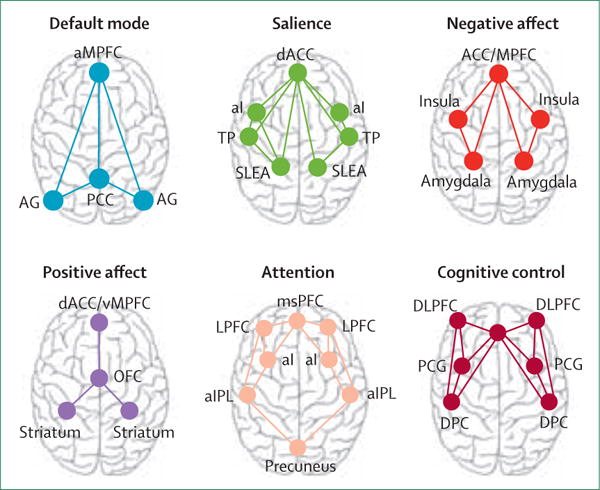
aMPFC=anterior medial prefrontal cortex. AG=angular gyrus. PCC=posterior cingulate cortex (includes precuneus). dACC=dorsal anterior cingulate cortex. aI=anterior insula. TP=temporal pole. SLEA=sublenticular extended amygdala. ACC/MPFC=dorsal medial prefrontal cortex (includes dorsal ACC and vMPFC, including ventral—subgenual and pregenual—and rostral ACC). msPFC=medial superior prefrontal cortex. LPFC=lateral prefrontal cortex. aIPL=anterior inferior parietal lobule. MPFC=medial prefrontal cortex. vMPFC=ventromedial prefrontal cortex. OFC=orbitofrontal cortex. ACC=anterior cingulate cortex. DLPFC=dorsolateral prefrontal cortex (includes anterior prefrontal cortex and inferior frontal cortex). PCG=precentral gyrus. DPC=dorsal parietal cortex.
Types of neural circuit dysfunction underlying depression and anxiety
The summary of existing evidence is based on published meta-analyses and reviews and on circuits and circuit dysfunctions that have been identified in at least two well powered studies. So far, the focus of brain imaging research has mainly and appropriately been on case-control comparisons, in which mood and anxiety disorder are defined by traditional criteria. Activation within specific brain regions of interest was also a focus. In the past 5 years, there has been an escalation in the study of dysfunctions in connectivity within and between circuits in depression and anxiety, partly as a result of advances in precision imaging and analysis techniques, including those developed within the Human Connectome Project.19,20
Existing findings tend to be inconsistent, and reveal profiles of neural hyporeactivity and hyper-reactivity, and both hypoconnectivity and hyperconnectivity, within the broad diagnostic categories of depression and anxiety. These variations might show the contribution of multiple biotypes of underlying neural circuit dysfunction that cut across existing diagnostic categories. Group-average results might reflect different combinations of these dysfunctions depending on the nature of the sample being investigated. Because future clinical translational applications will rely on a system for classifying individual patients, neural biotypes are conceptualised here as extremes of hypoactivation or hyperactivation and connectivity within and between underlying neural circuit dimensions (figure 2). Structural anatomical abnormalities can also ground or contribute to neural circuit biotypes defined by functional activation or connectivity, or both, as noted in early important work on the subgenual cingulate.21
Figure 2. Proposed taxonomy of putative biotypes of neural circuit dysfunction for depression and anxiety based on published work.
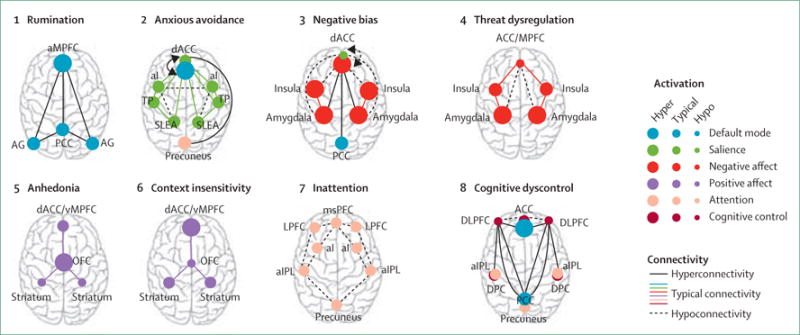
The proposed phenotype associated with each proposed neural dysfunction is mentioned above each type of dysfunction. aMPFC=anterior medial prefrontal cortex. AG=angular gyrus. PCC=posterior cingulate cortex (includes precuneus). dACC=dorsal anterior cingulate cortex. aI=anterior insula. TP=temporal pole. SLEA=sublenticular extended amygdala. ACC/MPFC=dorsal medial prefrontal cortex (includes dorsal ACC and vMPFC, including ventral—subgenual and pregenual— and rostral ACC). msPFC=medial superior prefrontal cortex. LPFC=lateral prefrontal cortex. aIPL=anterior inferior parietal lobule. MPFC=medial prefrontal cortex. vMPFC=ventromedial prefrontal cortex. OFC=orbitofrontal cortex. ACC=anterior cingulate cortex. DLPF=dorsoateral prefrontal cortex (includes anterior prefrontal cortex and inferior frontal cortex). PCG=precentral gyrus. DPC=dorsal parietal cortex.
Default mode circuit
The default mode circuit (typically known as the default mode network) is defined by the anterior medial prefrontal cortex, posterior cingulate cortex, and angular gyrus.22 Connectivity between these regions has been observed under task-free conditions when participants are asked to rest and reflect on their thoughts.22,23 Independent-components analysis suggests that the anterior and posterior regions define sub-networks of the default mode circuit.23 This circuit also has a basis in structural matter connections between the same regions.24
Putative biotype 1: rumination
Resting-state analyses of depression have shown a consistent profile of functional overactivation and hyperconnectivity of the default mode circuit in depression.25,26 Hyperfunctioning of the default mode circuit in major depressive disorder has been associated with higher levels of maladaptive rumination about depressive thoughts (figure 2).26 Anatomical abnormalities might contribute to default mode circuit hyper-function. Structurally, major depressive disorder has been associated with disruptions to both grey matter27 and white matter28 within the default mode circuit, particularly within the posterior sub-network.
Salience circuit
The so-called salience circuit is defined by core nodes in the anterior cingulate cortex (ACC), anterior insula, and sublenticular extended amygdala4 (figure 1). This circuit detects salient changes in the environment, both interoceptive and external. When these changes are detected, the salience circuit might signal the need for additional processing and initiation of appropriate cognitive control.4,29
In addition to the salience circuit, a distinct cingulo-opercular circuit has also been defined. The circuit is defined by nodes in the anterior medial prefrontal cortex, dorsal ACC, anterior insula, frontal operculum, and anterior thalamus, and is implicated in the detection of potential mismatches and conflict.4 These regions and functions show overlap with the default mode and salience circuits even though the cingulo-opercular circuit is articulated as a distinct circuit.4
Putative biotype 2: anxious avoidance
Insula hypoconnectivity within the salience circuit has been noted in depression, social anxiety disorder, and panic disorder (figure 2).23,30 It has been inversely associated with symptom severity.23 Insula–amygdala hypoconnectivity, which is correlated with anxious avoidance, has also been observed (figure 2).23 Hyper-connectivity between the insula and anterior nodes of the default mode circuit (figure 2) has been reported in both depression23 and social anxiety disorder.30 Dorsal nodes of the salience circuit show both hyper connectivity and hypoconnectivity with the posterior precuneus node of the attention circuit (figure 2).31 The direction of altered connectivity between salience and attention circuits can fluctuate with the nature of interoceptive or external events, consistent with the view that the salience-circuit guides the switching of attention according to stimulus importance. These salience circuit dysfunctions might contribute to so-called anxious avoidance biotypes characterised by difficulty distinguishing relevant salient cues and an avoidance of situations that could generate interoceptive or environmental stimulus overload.
Negative affect circuit
The circuit engaged by negatively valenced stimuli comprises subcortical nodes in the amygdala, brainstem regions, hippocampus, insula, and both dorsal and ventral prefrontal nodes—ie, dorsal medial prefrontal cortex, dorsal ACC, ventral medial prefrontal cortex, and ventral (subgenual and pregenual)-rostral ACC connections (figure 1).14,32 Dorsal and rostral nodes have been preferentially implicated in appraisal and expression of emotion and can be thought of as an aversive amplification sub-network,32 whereas the ventral nodes are implicated in automatic regulation of negative emotion.14,33 These sub-networks can be engaged even in the absence of conscious sensory awareness.13,14 In view of their commonly observed coactivation,14 the negative affect circuit might subserve the perception of negative emotion cues and the salience circuit—the arousal aspects of feeling these emotions.
Putative biotype 3: negative bias
Several studies of negative-affect-circuit dysfunction suggest biases that are congruent with altered subjective mood. These biases are elicited during the processing of stimuli such as negative facial expressions. Heightened insula activation has been observed in major depressive disorder in response to mood-congruent facial emotion stimuli such as sadness and disgust (figure 2).34 Individuals with generalised social anxiety disorder also show exaggerated insula activation and insula–ACC hypoconnectivity when attending to emotional faces.35 Hyper-responsivity of the amygdala has also been reported in response to sad faces, consistent with a mood-congruent negative bias (figure 2).36–38
At rest, hyperconnectivity between the anterior (subgenual ACC and dorsal medial prefrontal cortex) nodes of the negative affect circuit and the default mode has been observed in depression.25,26 This state of intrinsic hyperconnectivity is thought to drive rumination and the negative attributions that underlie negative biases.
Putative biotype 4: threat dysregulation
Several diagnoses have been associated with amygdala hyperactivation and cortical hypoactivation elicited by threat-relevant negative emotion stimuli. Amygdala hyperactivation during non-conscious threat processing has been reported in current depressive disorder,39 generalised anxiety disorder,40 generalised social phobia– anxiety disorder,40–42 specific phobia,43 and panic disorder.40,43 ACC hypoactivation during threat processing has been observed in generalised anxiety disorder33,44 and generalised social anxiety,44 and task context-dependent ACC hyperactivation in anxiety disorder.33
Correspondingly, reduced connectivity between the amygdala and ventral prefrontal nodes (subgenual–ventral ACC) has been noted during implicitly processed threat in unmedicated major depressive disorder,45 generalised social anxiety disorder,46 and generalised anxiety disorder.32 Amygdala–ACC connectivity correlated with anxious symptoms for threat-related faces in a task-specific manner.32
This profile of altered activation and connectivity suggests a core dysfunction of threat dysregulation (figure 2). Anatomical abnormalities might contribute to threat dysregulation. A reduction in the uncinate fasciculus white matter connections that support functional communication between the amygdala and ACC has been observed in major depressive disorder.47 An ongoing state of poor emotion regulation might also contribute to the often-noted loss of hippocampal grey matter in depression and anxiety.48
Positive affect circuit
Reward-processing components of the affective circuits are defined by the striatal nucleus accumbens and ventral tegmental areas (collectively referred to as the striatum) and their projections to the orbitofrontal cortex and medial prefrontal cortex12 (figure 1). These regional components can be preferentially engaged by different types of reward processing, including sensitivity to the presence of salient reward stimuli and the anticipation of these stimuli. Evidence so far suggests distinct profiles of dysfunctional positive-affect processing in depression and anxiety.
Putative biotype 5: anhedonia
Consistent findings of striatal hypoactivation in some people with depression49 suggest a distinct loss of sensitivity to reward stimuli that characterises an anhedonia biotype (figure 2). Striatal hypoactivation in these patients is apparent in response to socially rewarding stimuli (such as happy faces) and during reward-motivated decision making.49 Anatomically, a loss of striatal grey matter might contribute to a functional anhedonia biotype.50 Anhedonia has also been associated with greater activation of the orbitofrontal cortex (ventral medial prefrontal cortex) during the processing of happy faces51 and reward outcomes,52 which might reflect compensation for striatal hypoactivation. Anhedonia might encompass negative-affect-circuit dysfunction as suggested by reports of amygdala hypoactivation to happy faces in unmedicated major depressive disorder,53 generalised anxiety disorder,54 and panic disorder.55
Putative biotype 6: context insensitivity
In remitted depression, overactivation of the anterior cingulate and midfrontal region has been observed during the anticipation of primary rewards.56 These findings suggest a context-insensitivity biotype of neural dysfunction (figure 2), in which there is a heightened anticipation of reward without sensitivity to the surrounding context or the capacity to differentiate emotional context. This dysfunction can persist even during remission.56
Attention circuit
The frontoparietal attention circuit is defined by nodes in the medial superior frontal cortices, anterior insula, anterior inferior parietal lobule, and precuneus (figure 1).57 It is implicated in alertness, sustained attention, and the support of recollection.57 A close interplay between the attention and default mode circuits could be important to configuration of the switching between resting and task-context processing.57
Putative biotype 7: inattention
Hypoconnectivity within the frontoparietal attention circuit has been noted in major depressive disorder and in social anxiety,58 and suggests the presence of an inattention biotype (figure 2). In anxiety disorder, frontoparietal circuit hypoconnectivity has been correlated with a specific behavioural profile of false alarm errors59 consistent with disruption to sustained attention.
Cognitive control circuit
The cognitive control circuit comprises the dorsolateral prefrontal cortex, ACC, dorsal parietal cortex, and precentral gyrus (figure 1). Together these regions and their interconnectivity are implicated in the support of higher cognitive functions, such as working memory and selective attention.11,60 Under task-specific demands the cognitive control circuit is implicated in cognitive flexibility.17
Putative biotype 8: cognitive dyscontrol
Dysfunction of the cognitive control circuit during effortful selective processing of relevant stimuli while inhibiting irrelevant stimuli suggests a cognitive dyscontrol biotype (figure 2). Hypoactivation of the dorsolateral prefrontal cortex and dorsal anterior cingulate cortex has been observed in depression.61,62 Such hypoactivation persists with later-life depression,63 suggesting that a cognitive dyscontrol biotype might have a trait-like status.
ACC and dorsolateral prefrontal cortex hypoactivation during an inhibition task has been associated with slowing of the time to inhibit responses for people with higher anxiety.18 Problems with selective inhibition in depression could also be suggested by problems suppressing default mode rumination, and a profile of positive correlation (rather than anticorrelation) between dorsal prefrontal cognitive control regions and posterior cingulate default mode regions.25,64
Exploratory biotypes of neural circuit dysfunction
Emerging lines of evidence suggest additional neural circuit biotypes that for now might be deemed more exploratory. Although most studies suggest default mode hyperconnectivity in depression and anxiety, default mode hypoconnectivity has been noted in some.25,65 Default mode hypoconnectivity has been correlated with overgeneral autobiographical memory65 and social anxiety,58 and might reflect impairments in self-representation. Future studies might explore dysfunctions on the basis of interactions between negative and positive affective circuits given, for example, that the striatum of the reward circuit has also been implicated in aversive processing.13
With regard to attentional dysfunction, hyper connectivity (rather than hypoconnectivity) of the frontoparietal attention circuit has been observed in social anxiety.66 The attention circuit shows hypoconnectivity with the positive affect circuit, and together these dysfunctions might reflect an exploratory hypervigilance biotype. Within the cognitive control circuit, task-evoked dorsolateral prefrontal cortex hyperactivation (rather than hypoactivation) has been observed in the absence of behavioural performance deficits in unmedicated depression.67 This dysfunction might show a compensatory form of cognitive dyscontrol associated with cognitive overdrive or a distinct dysfunction dependent on task demands.
Use of neural circuit dysfunction to guide treatment
To advance a neural circuit model, it is essential to understand how neural-circuit-based biotypes relate to interventions. In the future, on the basis of increasingly precise evidence about the mechanistic actions of each circuit and which dysfunctions predict response to treatment, I envision that these neural circuit biotypes could be used to help guide choice of intervention (figure 3). I present three examples focused on pharmacotherapy, because drugs are the most common type of intervention for both depression and anxiety. Choice of pharmacotherapy is based on clinical interview at present, and typically only around 30% of patients recover with the first drug they are prescribed.68
Figure 3. Speculative connection between neural circuit biotypes for depression and anxiety and potentially suitable interventions.
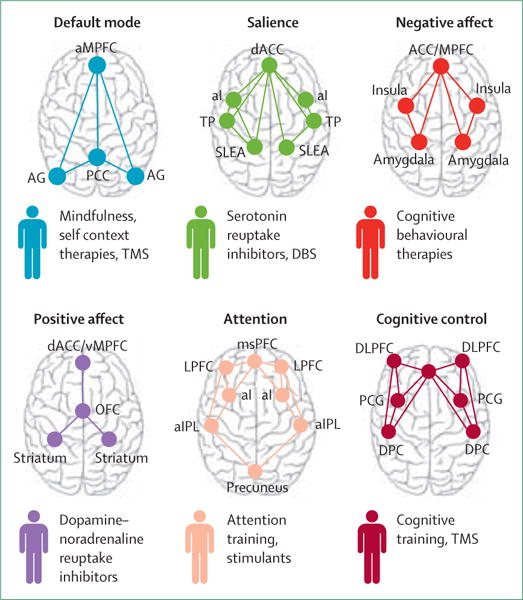
aMPFC=anterior medial prefrontal cortex. AG=angular gyrus. PCC=posterior cingulate cortex (includes precuneus). dACC=dorsal anterior cingulate cortex. aI=anterior insula. TP=temporal pole. SLEA=sublenticular extended amygdala. ACC/mPFC=dorsal medial prefrontal cortex (includes dorsal ACC and vMPFC, including ventral—subgenual and pregenual—and rostral ACC). msPFC=medial superior prefrontal cortex. LPFC=lateral prefrontal cortex. aIPL=anterior inferior parietal lobule. mPFC=medial prefrontal cortex. vMPFC=ventromedial prefrontal cortex. OFC=orbitofrontal cortex. ACC=anterior cingulate cortex. DLPF=dorsolateral prefrontal cortex (includes anterior prefrontal cortex and inferior frontal cortex). PCG=precentral gyrus. DPC=dorsal parietal cortex. TMS=transcranial magnetic stimulation. DBS=deep-brain stimulation.
In seminal studies, anterior insula hyperactivation during resting metabolism (quantified by PET and relevant to the negative bias biotype) has been identified as a differential biomarker of remission on citalopram (vs cognitive behavioural therapy).69 These findings show that neural circuit biomarkers can be assessed for their clinical utility with metrics such as number needed to treat (3·6 in this case69). Another large biomarker prediction study suggests that amygdala hyperactivation consistent with a negative bias biotype might also help to identify individuals who are unlikely to respond to alternative types of antidepressants such as a dual-avction serotonin–noradrenaline reuptake inhibitor.53
Relevant to the threat dysregulation biotype, response to fluoxetine has been associated with improved amygdala–ACC connectivity during an implicit emotion task.70 In a biomarker prediction study, pre-treatment hyporeactivity of the amygdala during implicit emotion threat processing was predictive of subsequent response to escitalopram and sertraline, and normalised after treatment.53
Patients with a striatally mediated anhedonia biotype of dysfunction might benefit from antidepressants that facilitate plasticity in striatal dopamine pathways. For example, pramipexole has antidepressant efficacy for depression and, in animal models, PET shows that it binds to extrastriatal dopamine receptors and modulates striatal function when probed by a reward task.71 Bupropion is also thought to act on dopamine and modulate striatal function.71 A relation between positive-affect-circuit dysfunction, dopamine-related plasticity, and a phenotype of anhedonia might explain why antidepressants that act on serotonin do not seem to improve anhedonia.71
Future directions
Future studies might escalate progress towards a clinically applicable neural circuit model of mental disorder by pursuing several issues. First, quantitative metrics should be used to define cohesive groups of people on the basis of their brain dysfunctions and then to identify the specific symptoms generated by these dysfunctions irrespective of traditional diagnostic categories. Second, large, multisite investigations should be done using standardised protocols, integrative analytic models, and shared databases. These approaches have been implemented in several imaging studies.61,72 Standardised imaging protocols will be essential for the future viability of routine scans for mental health assessment.73
Third, advancing an understanding of biotypes suited to the clinic is essential, and can be achieved by establishing metrics (such as Z scores) for the normative distribution of neural circuit variables in healthy people and across dimensions of familial risk. Methods to establish the reproducibility of imaging data across people, sites, and time are also needed.72 Fourth, the relations between activation, connectivity and structure, and the more nuanced interactions between circuits within the same patient samples, need to be elucidated. Information about brain-behaviour and symptom correlations should be incorporated, as should the modulation of neural circuit dysfunctions by more distal factors, such as variation in genetics, other omics analyses, and life events. This approach will necessitate imaging of the same people under various resting and task conditions, along with the acquisition of several other data modalities, thereby generating big datasets.
Fifth, modern computational techniques, such as data-driven machine-learning approaches, should be used to test, validate, and refine taxonomies for mental disease. Finally, further applications of neural circuit taxonomy for a wider array of interventions should be considered. Because antidepressant drugs are not the only treatments with antidepressant effects, expansion of understanding of both predictive and mechanistic neural-circuit–intervention relations is essential. For example, default mode hyperconnectivity, together with hypoconnectivity of cognitive control circuits, is predictive of response to transcranial magnetic stimulation (figure 3).74 Deep-brain stimulation of the subcallosal cingulate has been associated with a persistent normalisation of hyper-responsivity to negative emotion stimuli that might characterise negative bias biotypes (figure 3).75 Investigation of new lines of evidence implicating additional neural circuits in treatments for depression and anxiety is also important. For example, connectivity of visual circuits could distinguish treatment-resistant patients from treatment-sensitive patients.74
Conclusion
With the escalation of insights into large-scale neural circuits that govern the flexibility of human self-reflective, emotional, and cognitive functions, a foundation has been laid upon which a neural circuit taxonomy for mental diseases such as depression and anxiety can be built. A neural circuit taxonomy can be used to close the gap between insights about the mechanisms of mental disease and delivery of these insights into the hands of clinicians as an actionable brain-based system for improving treatment outcomes. By using such an approach, we can also undertake novel prospective investigations of neural-circuited guided treatment delivery in real-world settings.
Search strategy and selection criteria.
I first did a scoping search to determine the extent of the primary research literature and seminal articles on brain-imaging-defined neural networks or circuits in human beings. This scoping search provided key words for neural networks or circuits. I then searched PubMed for articles published before Jan 5, 2016, with the terms default mode AND (activation OR connectivity) AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); default mode AND (activation OR connectivity) AND treatment OR drug OR brain stimulation AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); salience OR cingulo-opercular AND (activation OR connectivity) AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); salience OR cingulo-opercular AND (activation OR connectivity) AND treatment OR drug OR brain stimulation AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); negative affect OR negative emotion OR threat OR limbic AND (activation OR connectivity) AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); negative affect OR negative emotion OR threat OR limbic AND (activation OR connectivity) AND treatment OR drug OR brain stimulation AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); positive affect OR positive emotion OR reward AND (activation OR connectivity) AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); positive affect OR positive emotion OR reward AND (activation OR connectivity) AND treatment OR drug OR brain stimulation AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); attention OR frontoparietal AND (activation OR connectivity) AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); attention OR frontoparietal AND (activation OR connectivity) AND treatment OR drug OR brain stimulation AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); cognitive control OR central executive AND (activation OR connectivity) AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); cognitive control OR central executive AND (activation OR connectivity) AND treatment OR drug OR brain stimulation AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); intrinsic OR resting AND (activation OR connectivity) AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); intrinsic OR resting AND (activation OR connectivity) AND treatment OR drug OR brain stimulation AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); extrinsic OR task AND (activation OR connectivity) AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); extrinsic OR task AND (activation OR connectivity) AND treatment OR drug OR brain stimulation AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); prefrontal cortex OR DLPFC or MPFC OR anterior cingulate OR ACC OR insula OR amygdala OR striatum OR nucleus accumbens OR posterior cingulate OR PCC OR precentral OR precuneus AND (activation OR connectivity) AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia); prefrontal cortex OR DLPFC or MPFC OR anterior cingulate OR ACC OR insula OR amygdala OR striatum OR nucleus accumbens OR posterior cingulate OR PCC OR precentral OR precuneus AND (activationOR connectivity) AND treatment OR drug OR brain stimulation AND (depression OR depressive disorder OR mood disorder OR anxiety OR anxiety disorder OR generalized anxiety OR social anxiety OR phobia). In the resulting references I prioritised meta-analyses and reviews and empirical findings reproduced in at least two well powered studies.
Acknowledgments
I thank Andrea Goldstein-Piekarski (Stanford, CA, USA) for comments on an early draft, and Nowreen Chowdhry (Stanford, CA, USA) and Katherine Grisanzio (Stanford, CA, USA) for their contributions to referencing and to figures. I was supported by a National Institute of Mental Health grant (R01MH101496).
Footnotes
Contributors
LMW conceived and wrote this Personal View.
Declaration of interests
I have received fees from Brain Resource and Humana for projects not related to this work.
References
- 1.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–51. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–78. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 6.Markoff J. Obama seeking to boost study of human brain. http://www.nytimes.com/2013/02/18/science/project-seeks-to-build-map-of-human-brain.html (accessed Feb 18, 2013)
- 7.Kupfer DJ, Regier DA. Neuroscience, clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168:672–74. doi: 10.1176/appi.ajp.2011.11020219. [DOI] [PubMed] [Google Scholar]
- 8.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 9.Gladding PA, Cave A, Zareian M, et al. Open access integrated therapeutic and diagnostic platforms for personalized cardiovascular medicine. J Pers Med. 2013;3:203–37. doi: 10.3390/jpm3030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–37. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 11.Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–60. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 12.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26:9264–71. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical–subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hugdahl K, Raichle ME, Mitra A, Specht K. On the existence of a generalized non-specific task-dependent network. Front Hum Neurosci. 2015;9:430. doi: 10.3389/fnhum.2015.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crittenden BM, Mitchell DJ, Duncan J. Recruitment of the default mode network during a demanding act of executive control. eLife. 2015;4:e06481. doi: 10.7554/eLife.06481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roalf DR, Ruparel K, Gur RE, et al. Neuroimaging predictors of cognitive performance across a standardized neurocognitive battery. Neuropsychology. 2014;28:161–76. doi: 10.1037/neu0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forster S, Nunez Elizalde AO, Castle E, Bishop SJ. Unraveling the anxious mind: anxiety, worry, and frontal engagement in sustained attention versus off-task processing. Cereb Cortex. 2015;25:609–18. doi: 10.1093/cercor/bht248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barch DM, Burgess GC, Harms MP, et al. the WU-Minn HCP Consortium Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–89. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Essen DC, Smith SM, Barch DM, et al. the WU-Minn HCP Consortium The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–27. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 22.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–58. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: a review. Neurosci Biobehav Rev. 2015;56:330–44. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Horn A, Ostwald D, Reisert M, Blankenburg F. The structural-functional connectome and the default mode network of the human brain. Neuroimage. 2014;102:142–51. doi: 10.1016/j.neuroimage.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 25.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107:11020–25. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78:224–30. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh MK, Kesler SR, Hadi Hosseini SM, et al. Anomalous gray matter structural networks in major depressive disorder. Biol Psychiatry. 2013;74:777–85. doi: 10.1016/j.biopsych.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014;76:567–74. doi: 10.1016/j.biopsych.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson A, Thome J, Frewen P, Lanius RA. Resting-state neuroimaging studies: a new way of identifying differences and similarities among the anxiety disorders? Can J Psychiatry. 2014;59:294–300. doi: 10.1177/070674371405900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson OJ, Krimsky M, Lieberman L, Allen P, Vytal K, Grillon C. The dorsal medial prefrontal (anterior cingulate) cortex–amygdala aversive amplification circuit in unmedicated generalised and social anxiety disorders: an observational study. Lancet Psychiatry. 2014;1:294–302. doi: 10.1016/S2215-0366(14)70305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1:10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biol Mood Anxiety Disord. 2013;3:7. doi: 10.1186/2045-5380-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–38. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu CH, Williams SC, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 38.Arnone D, McKie S, Elliott R, et al. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am J Psychiatry. 2012;169:841–50. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- 39.Jaworska N, Yang XR, Knott V, Macqueen G. A review of fMRI studies during visual emotive processing in major depressive disorder. World J Biol Psychiatry. 2015;16:448–71. doi: 10.3109/15622975.2014.885659. [DOI] [PubMed] [Google Scholar]
- 40.Fonzo GA, Ramsawh HJ, Flagan TM, et al. Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. Br J Psychiatry. 2015;206:206–15. doi: 10.1192/bjp.bp.114.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 42.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–29. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Killgore WD, Britton JC, Schwab ZJ, et al. Cortico-limbic responses to masked affective faces across PTSD, panic disorder, and specific phobia. Depress Anxiety. 2014;31:150–59. doi: 10.1002/da.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blair KS, Geraci M, Smith BW, et al. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry. 2012;72:476–82. doi: 10.1016/j.biopsych.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 46.Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013;30:234–41. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steffens DC, Taylor WD, Denny KL, Bergman SR, Wang L. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PLoS One. 2011;6:e22697. doi: 10.1371/journal.pone.0022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao YJ, Du MY, Huang XQ, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med. 2014;44:2927–37. doi: 10.1017/S0033291714000518. [DOI] [PubMed] [Google Scholar]
- 49.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MJ, Hamilton JP, Gotlib IH. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Res. 2008;164:114–22. doi: 10.1016/j.pscychresns.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 52.Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012;136:1126–34. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams LM, Korgaonkar MS, Song YC, et al. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology. 2015;40:2398–408. doi: 10.1038/npp.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blair K, Shaywitz J, Smith BW, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165:1193–202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ottaviani C, Cevolani D, Nucifora V, et al. Amygdala responses to masked and low spatial frequency fearful faces: a preliminary fMRI study in panic disorder. Psychiatry Res. 2012;203:159–65. doi: 10.1016/j.pscychresns.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 56.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–39. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 57.Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci USA. 2012;109:12788–93. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu C, Liao W, Ding J, et al. Regional homogeneity changes in social anxiety disorder: a resting-state fMRI study. Psychiatry Res. 2011;194:47–53. doi: 10.1016/j.pscychresns.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Sylvester CM, Corbetta M, Raichle ME, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–35. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–68. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korgaonkar MS, Grieve SM, Etkin A, Koslow SH, Williams LM. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38:863–71. doi: 10.1038/npp.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 63.Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bartova L, Meyer BM, Diers K, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18. doi: 10.1016/j.jpsychires.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–17. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 66.Arnold Anteraper S, Triantafyllou C, Sawyer AT, Hofmann SG, Gabrieli JD, Whitfield-Gabrieli S. Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connect. 2014;4:81–90. doi: 10.1089/brain.2013.0180. [DOI] [PubMed] [Google Scholar]
- 67.Matsuo K, Glahn DC, Peluso MA, et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158–66. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- 68.Saveanu R, Etkin A, Duchemin AM, et al. The international Study to Predict Optimized Treatment in Depression (iSPOT-D): outcomes from the acute phase of antidepressant treatment. J Psychiatr Res. 2015;61:1–12. doi: 10.1016/j.jpsychires.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 69.Dunlop BW, Mayberg HS. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin Neurosci. 2014;16:479–90. doi: 10.31887/DCNS.2014.16.4/bdunlop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen CH, Suckling J, Ooi C, et al. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33:1909–18. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- 71.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–37. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 72.Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–39. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siegle GJ. Beyond depression commentary: wherefore art thou, depression clinic of tomorrow? Clin Psychol (New York) 2011;18:305–10. doi: 10.1111/j.1468-2850.2011.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2014;172C:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hilimire MR, Mayberg HS, Holtzheimer PE, et al. Effects of subcallosal cingulate deep brain stimulation on negative self-bias in patients with treatment-resistant depression. Brain Stimulat. 2015;8:185–91. doi: 10.1016/j.brs.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


