Abstract
Background: To explore whether endometrial receptivity is determined by osteopontin (OPN) and integrin αvβ3 expression in women with elevated serum progesterone (P) and/or oestradiol (E2) who are undergoing in vitro fertilisation (IVF). Methods: According to serum hormone levels on the day of HCG administration, 33 infertile women were divided into 3 groups: the high E2, high P, and high E2 and P groups. The control group included 11 fertile, healthy women. Endometrial biopsy was performed on ovulation day + 7 to + 8 for all study participants, and the mRNA and protein expression levels of OPN and integrin αvβ3 were analyzed. Result: No statistically significant differences regarding OPN and integrin αvβ3 expression were found between infertile patients in the high P, high E2, high E2 and P and control groups. There was no significant correlation between OPN and integrin αvβ3 staining intensity during the implantation window biopsy in any of the groups studied. Conclusion: Endometrial OPN and integrant αvβ3 expression/co-expression is not impaired during the window of implantation in patients with high P, high E2, or high E2 and P levels. The clinical value of assessing endometrial receptivity with OPN and integrin αvβ3 seems to be uncertain.
Key words: endometrial receptivity, IVF, elevated hormone levels, osteopontin, integrin αvβ3
Abstract
Zusammenfassung
Hintergrund: Ziel der Studie war es zu untersuchen, ob die Rezeptivität des Endometriums von der Osteopontin- (OPN-) und Integrin αvβ3-Expression bestimmt wird bei Frauen mit erhöhten Progesteron-(P-) und/oder Östradiol-(E2-)Konzentrationen, die sich einer In-vitro-Fertilisation (IVF) unterziehen. Methoden: Basierend auf der Messung des Hormonspiegels am Tage der hCG-Gabe wurden 33 unfruchtbare Frauen in 3 Gruppen wie folgt eingeteilt: hoher E2-, hoher P- und hoher E2- und P-Spiegel. Die Kontrollgruppe bestand aus 11 gesunden fruchtbaren Frauen. Eine Biopsie des Endometriums wurde am 7. oder 8. Tag nach der Ovulation bei allen Studienteilnehmende durchgeführt, und die mRNA und Protein-Expressionsspiegeln von OPN und Integrin αvβ3 wurden ausgewertet. Ergebnisse: Bezüglich der Exprimierung von OPN und Integrin αvβ3 fanden sich keine statistisch signifikanten Unterschiede zwischen den unfruchtbaren Patientinnen in den Gruppen mit hohen P- oder E2-Spiegeln, der Gruppe mit hohen E2- und P-Spiegeln und der Kontrollgruppe. In den entnommenen Biopsaten konnte bei keiner der untersuchten Gruppen eine signifikante Korrelation zwischen der Färbungsintensität für OPN und Integrin αvβ3 und dem Implantationsfenster festgestellt werden. Schlussfolgerungen: Die Expression und Koexpression von OPN und Integrin αvβ3 im Endometrium sind während des Implantationsfensters nicht beeinträchtigt bei Patientinnen mit hohen P- oder E2-Spiegeln bzw. hohen E2- und P-Spiegeln. Der klinische Nutzen einer auf die Messung der Expression von OPN- und Integrin αvβ3 basierenden Bewertung der Rezeptivität des Endometriums scheint fraglich zu sein.
Schlüsselwörter: Rezeptivität des Endometriums, IVF, erhöhte Hormonspiegel, Osteopontin, Integrin αvβ3
Introduction
Successful embryo implantation requires a receptive endometrium, while the ovaries provide the hormonal stimulus for establishment of a successful pregnancy 1. During in vitro fertilization (IVF) cycle, endometrium and embryo are exposed to supra-physiological concentrations of oestradiol (E2) and progesterone (P) under ovarian stimulation, which could influence pregnancy outcome. Detection of elevated serum P on the day of human chorionic gonadotropin (HCG) administration has been reported to occur in 20–40 % of IVF and embryo transfer (ET) cycles 2. Moreover, many studies have described an adverse relationship between elevated P concentration on the day of HCG administration and IVF pregnancy outcome 3. In addition, a negative association between the probability of pregnancy and E2 concentration on the day of HCG administration has also been reported 4. However, the potential effect of these subtle hormone increases in implantation remains controversial. A more recent publication by the current study group 5 demonstrated that elevated P was detrimental to pregnancy rate, while high serum E2 concentration at the time of HCG administration had no effect on IVF pregnancy outcome. A high E2 concentration, combined with elevated P, had the potential negative effect and higher ectopic pregnancy rate. In these prior studies, the theory of reduced endometrial receptivity with high P concentration was supported in the fresh embryo-transfer cycle.
Endometrial receptivity has been extensively studied, and a number of architectural, cellular, biochemical, and molecular events occurred in the endometrium within the implantation window (i.e. 6–8 days after ovulation) 6. In particular, integrin αvβ3 and its extracellular matrix ligand, osteopontin (OPN), are two of the best characterised endometrial receptivity biomarkers. OPN is the main ligand for integrin αvβ3, and several studies have identified OPN as a putative biomarker for uterine receptivity in human endometrium 7. Moreover, OPN and integrin αvβ3 have been found to be simultaneously expressed in the human endometrium throughout the menstrual cycle in normally cycling fertile women, with both glycoproteins being maximally expressed during the implantation window 8. The maximal expression of these two molecules during the implantation window in human endometrial epithelial cells and the secretion of OPN into the uterine cavity suggest that these factors play a role in the regulation of endometrial function and embryo implantation 8, 9. Detection of both integrin αvβ3 and OPN has been proposed as means of distinguishing receptive from non-receptive endometria in clinical practice and as a new method to investigate impaired endometrial receptivity in a certain group of infertile patients 10, 11, 12.
Different studies have reported that integrin αvβ3 is not expressed in the endometria of women with certain conditions, such as endometriosis, hydrosalpinges, polycystic ovary syndrome (PCOS), and unexplained infertility 8, 13, 14, 15, 16. Recently, Casals et al. 17 studied the expression of OPN and integrin αvβ3 in seven groups of women who received either CC, ovarian stimulation for IVF, oral contraception, dehydrogesterone for endometrial luteal phase defect, two different regimens of hormone replacement therapy, or no treatment. There were no significant differences between spontaneous and treatment cycles in the seven experimental groups with respect to the expression of OPN and integrin αvβ3. However, there have been limited studies on IVF cycle, and more prospective clinical controlled studies are needed to investigate these receptivity markers. To our knowledge, there have been no previous controlled investigations on the expression of these two endometrial receptivity markers in IVF patients with elevated P and/or E2 level in the same cycle. Therefore, it is currently unknown whether elevated levels of P, E2, or both P and E2 detected on the day of HCG administration influence the expression of OPN and integrin αvβ3 during the implantation window. Accordingly, the mechanisms underlying impaired implantation in IVF patients with high levels of P and/or E2 require further exploration. Thus, the aim of the present study was to investigate the endometrial expression and co-expression of OPN and integrin αvβ3 in infertile women with high sex hormone levels during IVF cycle.
Materials and Methods
Patients and study design
All participants underwent a timed endometrial biopsy at the Reproductive Medical Center of the Affiliated Hospital of Kunming University of Science and Technology. The inclusion criteria were as following: women < 38 years of age, no previous diagnosis of adnexal masses, basal serum follicle-stimulation hormone (FSH) on day 2 < 10 IU/L, regular menstruation, and no hormone treatment during the previous 3 months; all enrolled patients were unable to conceive due to tubal obstruction or male infertility. In this prospective study, patients with endocrinopathies, organic diseases, and other factors affecting endometrial receptivity, such as PCOS, ovarian tumor, polyps, fibroids, endometriosis, and hydrosalpinges, were excluded. Enrolled patients underwent IVF-ET with a gonadotropin-releasing hormone agonist and recombinant FSH; moreover, in hopes of improving the chance for success during frozen ET cycle, fresh ET for all patients was cancelled to avoid ovarian hyperstimulation syndrome (OHSS) or extraordinary high P level (serum P levels ≥ 2.1 ng/ml, twice the threshold of an elevated P concentration {i.e., 1.05 ng/ml on the day of HCG administration, as defined by our previous clinical research 5}).
According to our previous study 5, a serum P concentration ≥ 1.05 ng/ml on the day of HCG administration was defined as an elevated P concentration, and E2 elevation was defined as a serum E2 concentration exceeding 5210.9 ng/ml. Patients were divided into 3 groups according to their serum P and E2 concentrations on the day of HCG administration: the high E2 group (E2 ≥ 5210.9 pg/ml and P < 1.05 ng/ml), the high P group (E2 < 5210.9 pg/ml and P ≥ 1.05 ng/ml), and the high E2 and P group (E2 ≥ 5210.9 pg/ml and P ≥ 1.05 ng/ml). Each group included 11 infertile women; the sample size was decided arbitrarily, but in keeping with previous studies on the subject 17, 18. For the specific purpose of this study, the luteal phase was supported with micronized vaginal progesterone soft capsules (TROGESTAN, 100 mg per capsule; Besins Manufacturing Belgium, Capsugel, France), given 400 mg/day for 12 days and commenced on the day of oocyte retrieval.
We also included a control group of 11 fertile healthy women (mean parity 1.3; range 1–2), who were undergoing tubal sterilization and had no history of miscarriage. All of the women had regular menstrual cycle (every 27–32 days) without steroid treatment or other medication for at least 3 months prior to endometrium collection. Endometrial biopsy was performed on ovulation day + 7 to + 8 for all study participants. Transvaginal ultrasonography (PHILIPS HD5; Philips Co., Holland) was performed to monitor follicular growth beginning from day 8–10 of the natural cycle until follicles disappear. For all patients, the largest diameter was measured in both the longitudinal and transverse dimensions in all follicles. The day of ovulation was designated as the day of maximum follicular enlargement, which was followed by sudden disappearance of this follicle and its loss of clear wall demarcation and intrafollicular echoes on the next day 19, 20. The chronological day of each patient was determined by counting forward from the ovulation day, as detected by ultrasonographic scan.
For all women, luteal serum concentrations of E2 and P and endometrial biopsies were obtained to assess luteal function, according to a previously reported 21 method of evaluation. Serum hormones were quantified on the day of HCG administration and the same day as endometrial sampling. All samples were obtained between 8:00 a. m. and 10:00 a. m., and the clinical data for the four groups were analysed.
All patients signed an informed consent before receiving IVF treatment and biopsy, and this study was approved by the Ethics Committee of the Hospital.
Endometrial samples
Timed endometrial biopsies were taken from the uterine fundus using the Pipelle biopsy device (Cooper Surgical, USA). Endometrial samples were divided into two parts: the first one was fixed in 10 % formalin and embedded in paraffin for immunohistochemical analysis of OPN and integrin αvβ3 expression and histological dating, according to Noyes criteria 22. The second portion was immediately snap-frozen in liquid nitrogen and used for real time quantitative polymerase chain reaction (RT-QPCR).
Endometrial dating
For endometrial dating, 5-µm sections were stained with haematoxylin and eosin and evaluated according to the histopathological criteria of Noyes et al. 22. All endometrial samples were evaluated by the experienced pathologist (H. Y.) who was blinded with regard to the ovulatory day and study group. An out-of-phase biopsy was defined as a lag of ≥ 3 days between the chronological and histological day 23, 24.
Immunohistochemical analysis
Immunohistochemistry for both OPN and integrin αvβ3 were performed on 5-µm sections of formalin-fixed, paraffin-embedded endometrial biopsies, using the EnVision™ FLEX + system (Dako Co., Denmark). Mouse monoclonal anti-OPN (Akm2A1, dilution 1 : 100; Santa Cruz, CA, USA) and anti-integrin αvβ3 (23C6, dilution 1 : 40; Santa Cruz, CA, USA) antibodies were applied. Paraffin sections were deparaffinised and rehydrated in xylene and graded alcohols, followed by heat-induced epitope retrieval (High PH). Endogenous peroxidase activity was blocked by incubating slides in 3 % hydrogen peroxide for 10 minutes at room temperature. The slides were then incubated with the primary antibodies for 60 minutes, followed by incubation with the secondary antibody from the EnVision™ FLEX + kit (Dako NenmarkA/S, Glostrup, Denmark) for 30 minutes. The reaction product was visualised with the prepared liquid diaminobenzidine substrate chromogen solution for 5 minutes; slides were then washed in distilled water, counterstained with haematoxylin, washed, dehydrated, and mounted. As previously reported 9, in every case a negative control was performed by omission of incubation with the specific primary antibody.
Immunostaining results were scored semi-quantitatively in each section. The staining intensity and positive percentage were evaluated, and the mean values were obtained. A staining score was calculated over 5 high-power fields using the following equation: HSCORE = ΣPi (i + 1), where i was the intensity of staining with a value of 1, 2, or 3 (weak, moderate, or strong, respectively), and Pi was the percentage of stained luminal and glandular epithelial cells for each intensity, ranging from 0–100 % 25, 26, 27. Previous reports have demonstrated low intra-observer (r = 0.983; p < 0.0001) and inter-observer (r = 0.994; p < 0.0001) differences for HSCORE in uterine tissues using this technique 25. Endometrial samples were considered to express OPN and/or integrin αvβ3 when these glycoproteins were detected with any intensity in both the endometrial glands and luminal surface epithelium 17, 24, 28.
Real-time quantitative polymerase chain reaction
Total RNA was extracted from endometrial tissues with TRIzol reagent (Sigma, USA) according to the manufacturerʼs instructions. The concentration and quality of RNA were determined using ultraviolet spectrophotometry at 260 nm and 280 nm. Equal amounts of total RNA (2 µg) were reverse transcribed using the Superscript First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA), and the resulting first-strand cDNA was diluted and used as a template in the RT-QPCR analysis. All measurements were performed in triplicate. The mRNA levels of OPN, β3 integrin, and GAPDH (internal control to normalise for variances in input cDNA) were measured. The following gene-specific primers and probes were designed with the Oligo Primer Analysis 4.0 software, and the specificity of each primer was determined using the NCBI BLAST module (http://www.ncbi.nlm.nih.gov/BLAST/). The primer sequences were as following: OPN sense (5′-CTAAGAAGTTTCGCAGACC-3′), OPN antisense (5′-CATTCAA CTCCTCGCTTT-3′); β3 integrin sense (5′-TGCCGTGACGAGATTGAG-3′), β3 integrin antisense (5′-GAGCAGGACCACCAGGAT-3′); and GAPDH sense (5′-ATCATCAGCAATGCCTCC-3′), GAPDH antisense (5′-CATCACGCCACAGTTTCC-3′). Detection of expression was performed with SYBR Green (FS Universal SYBR Green Master; Roche, Switzerland) and an ABI PRISM 7300 Real Time PCR instrument (Applied Biosystems, USA) using the relative standard curve method. For sample analysis, the threshold was set based on the exponential phase of products, and the 2-ΔΔCT method was performed to analyse the data as previously described 29. The expression level of each gene was normalised to GAPDH mRNA and expressed as the n-fold difference relative to the control.
Hormone assay
Hormones were measured using commercially available kits. P and E2 levels in serum were measured using a competitive chemiluminescent assay (Access Immunoassay System, UniCel DXI 800; Beckman Coulter, USA). The sensitivity was 20 pg/ml for E2 and 0.10 ng/ml for P, and the inter-assay coefficients of variation for E2 and P were 12 % and 6.1 %, respectively.
Statistical analysis
Values were expressed as mean ± standard error of the mean. The χ2 test and Kruskal-Wallis test were used to analyze categroriacal data and continuous variables, respectively. The correlation between OPN and integrin αvβ3 expression was evaluated using the Spearman rank correlation coefficient test. Statistical analyses were performed with the Statistical Package for Social Sciences version 11.5 (SPSS, Chicago, IL, USA). A p-value < 0.05 was considered to be statistically significant.
Results
Characteristics of the enrolled volunteers
The mean age of the women in the high E2, high P, high E2 and P, and control groups were 30.27 ± 1.25, 28.91 ± 1.11, 28.55 ± 1.28, and 29.45 ± 1.18 years (mean ± standard error of the mean), respectively. All cycles included in the present study were ovulatory, according to oocyte retrieval or ultrasonographic criteria and mid-luteal serum P concentration > 10 mg/ml. Accordingly, the endometrial specimens were noted to be clearly progestational fundal sample in all instances.
The serum levels of E2 and P
Serum hormone concentrations are presented in Table 1. As expected, E2 and P serum concentrations were significantly higher in cycles treated with ovarian stimulation than in control during the implantation window. The E2 concentration in the control group was much lower than that of the other three groups (p < 0.05), but there was no difference in E2 concentration among the high E2, high P, and high E2 and P groups. However, the P level did not reach statistical significance among the four groups during the implantation window (Table 1).
Table 1 Serum hormone concentrations on the HCG day and the biopsy day in four groups.
| Group | HCG day | Biopsy day | ||
|---|---|---|---|---|
| Estradiol (pg/ml) | Progesterone (ng/ml) | Estradiol (pg/ml) | Progesterone (ng/ml) | |
| Values are expressed as mean ± SEM. a–h Figures with common superscripts are significantly different (p < 0.05). | ||||
| Group control | 190.28 ± 18.11f, g, h | 18.13 ± 1.51 | ||
| Group high E2 | 6 210.81 ± 383.24a, b | 0.87 ± 0.07d | 1 563.55 ± 334.57f | 32.69 ± 8.46 |
| Group high P | 3 972.19 ± 313.36a, c | 2.8 ± 0.37d, e | 1 569.45 ± 385.65g | 31.34 ± 6.14 |
| Group both high E2 + P | 7 432.27 ± 252.07b, c | 1.63 ± 0.12e | 1 830.27 ± 489.45h | 30.41 ± 4.62 |
The expression level of β3 integrin and the immunohistochemical analysis of integrin αvβ3
Compared to the control group, the expression level of β3 integrin mRNA in endometrial tissues was up-regulated during the implantation window in patients with high E2, high P, and both high E2 and P (Fig. 1). Immunohistochemical analysis revealed that integrin αvβ3 was mainly expressed on the membrane of glandular epithelial cells, and the staining was scattered and weakly positive (Fig. 3). There were no significant differences with regard to mRNA β3 integrin expression or β3 integrin protein expression and intensity among the four groups (Table 2).
Fig. 1.

Expression of β3 integrin in the endometrial tissue during implantation window. The RT-QPCR analysis of β3 integrin mRNA expression in endometrium of the four groups reveals an increase in transcript level during the implantation window in patients with high E2, high P, and both high E2 and high P.
Fig. 3.
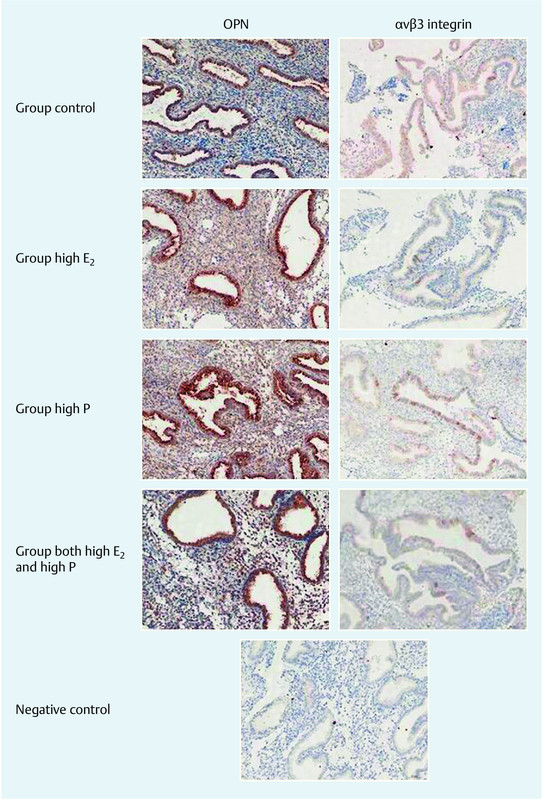
Immunohistochemical staining of endometrial OPN and αvβ3 integrin during the implantation window in four groups. Magnification: × 200. The immunohistochemical staining intensity was measured using the HSCORE scoring system as described in Materials and Methods section. Data are pooled from three independent experiments with a total of eleven in each group, p > 0.05, compared with the control.
Table 2 Endometrial biopsy and epithelial OPN and αvβ3 integrin expression and their co-expression in the four groups studied during the implantation window.
| Parameter | Group control (n = 11) | Group high E2 (n = 11) | Group high P (n = 11) | Group both high E2 + P (n = 11) | p value |
|---|---|---|---|---|---|
| Values are expressed as mean ± SEM or n (%). | |||||
| Endometrial biopsy | |||||
| Chronological dating | 7.09 ± 0.09 | 7.27 ± 0.14 | 7.18 ± 0.12 | 7.27 ± 0.14 | NS |
| In-phase endometrial | 10 (90.9) | 8 (72.7) | 10 (90.9) | 9 (81.8) | NS |
| OPN expression | |||||
| Positive samples | 10 (90.9) | 11 (100) | 11 (100) | 11 (100) | NS |
| HSCORE | 1.18 ± 0.26 | 1.59 ± 0.26 | 1.41 ± 0.32 | 1.10 ± 0.14 | NS |
| αvβ3 integrin expression | |||||
| Positive samples | 4 (36.36) | 3 (27.27) | 6 (54.54) | 2 (18.18) | NS |
| HSCORE | 0.54 ± 0.23 | 0.27 ± 0.18 | 0.45 ± 0.18 | 0.29 ± 0.19 | NS |
| OPN/αvβ3 co-expression | |||||
| OPN (−)/αvβ3 (−) | 1 (9.09) | 0 | 0 | 0 | NS |
| OPN (+)/αvβ3 (−) | 6 (54.54) | 8 (72.72) | 5 (45.45) | 9 (81.81) | NS |
| OPN (−)/αvβ3 (+) | 0 | 0 | 0 | 0 | NS |
| OPN (+)/αvβ3 (+) | 4 (36.36) | 3 (27.27) | 6 (54.54) | 2 (18.18) | NS |
The mRNA level of OPN
The RT-QPCR analysis of OPN mRNA was performed using the same samples as those used for the β3 integrin analysis (Fig. 2). We indeed demonstrated a marked increase in OPN expression during the implantation window in the high E2, high P, and high E2 and P groups, especially in the high E2 group (p < 0.05); however, there was no significant difference in OPN level among the high E2, high P and high E2 and P groups.
Fig. 2.

Expression of OPN in the endometrial tissue during implantation window. The RT-QPCR analysis of OPN mRNA expression in endometrium of the four groups reveals a marked increase in transcript level during the implantation window in patients with high P, and both high E2 and high P, especially in group high E2.
Up-regulation of OPN expression was confirmed by immunohistochemistry (Fig. 3). OPN staining was mostly moderate to strong in all patients, concentrated in the cytoplasm, and confined to the glandular and luminal epithelial compartments. Although OPN expression was higher in the high E2 group, no significant difference was found in OPN level among the control, high E2, high P, and high E2 and P groups (all p > 0.05, Table 2).
Correlation between integrin αvβ3 and OPN during the implantation window biopsies
Table 2 summarises the data related to endometrial histology and OPN and integrin αvβ3 expression or co-expression during the implantation window biopsies carried out in the control, high E2, high P, and high E2 and P groups. No statistically significant differences were found among the four groups with respect to histology or, expression of endometrial markers during the implantation window. The simultaneous presence of both markers was observed in only 34.1 % (15/44) of mid-luteal biopsies, with no differences among the four groups. There was no significant correlation between integrin αvβ3 and OPN staining intensity during the implantation window biopsies in any of the study groups (Fig. 4).
Fig. 4.

Correlation between staining intensity for OPN and αvβ3 integrin expression during the implantation window in endometrial biopsies in patients of high E2, high P, both high E2 and high P, and control group.
Discussion
Successful implantation of embryos is an important physiologic event in the establishment of pregnancy 30. During IVF cycle, high serum P concentrations on the day of HCG administration could have an adverse effect on endometrial condition, as could the combination of high P, E2 level, a pattern observed through our clinical research 5. However, the question remains as to which factors affect endometrial receptivity of patients with high P and/or E2. In recent years, many studies have investigated potential causes of reduction in endometrial receptivity 31. Several adhesion molecules considered essential for uterine competence during implantation have been proposed as markers of endometrial receptivity 32, 33, 34. In particular, integrin αvβ3 and its ligand OPN have been intensively studied in reproductive medicine 18, 35, 36; assessment of these two glycoproteins has been proposed as a novel approach to determine uterine receptivity for various causes of infertility 21. However, there are very few published studies investigating the cellular mechanism of action of high serum P and E2 in IVF cycle. In the present study, we employed OPN and integrin αvβ3 as markers of emdometrial receptivity. To our knowledge, there have been no prior studies on the effect of elevated serum P and/or E2 levels on the expression of OPN and integrin αvβ3 during the implantation window.
It is surprising that our findings demonstrated that there was no significant difference among the four study groups in the expression of OPN and integrin αvβ3 during the implantation window. Furthermore, there was no significant correlation between integrin αvβ3 and OPN staining intensity during the implantation window in any of the groups studied.
Nevertheless, we confirmed the temporal and spatial expression of these two major markers of endometrial receptivity. Both OPN and integrin αvβ3 demonstrate an abrupt onset of staining in the luminal and glandular epithelium from the early to mid-secretory phase onward 18. Maximal expressions of both OPN and integrin αvβ3 during the mid to late secretory phase in human endometrial epithelial cells and secretion of OPN into the uterine cavity suggest both factors play a role in the regulation of endometrial function and embryo implantation 8, 9. Previous studies 8, 37 reported that OPN and integrin αvβ3 were differentially regulated by ovarian steroids along with epidermal growth factor (EGF) and heparin-binding EGF (HB-EGF). They demonstrated that E2 and E2 in combination with P reduced the expression of β3 integrin. Inhibition of E2 by the antagonist ICI 182780 increased the relative expression of β3, whereas the antiprogestin Ru-486 had little effect. In addition, EGF and HB-EGF dramatically increased β3 subunit expression. On the other hand, expression of OPN was modulated by P and dramatically increased with both E2 and P, whereas E2 alone also appeared to antagonise its expression. Ru-486 reduced the stimulatory effect of P on OPN, whereas HB-EGF had little if any effect on its expression 8.
We found no significant difference in levels of β3 mRNA or integrin αvβ3 expression during the receptive phase when comparing the high E2, high P, high E2 and P, and control groups. Research has shown that there was no significant difference in αvβ3, α4β1, and α1β1 integrin expression between patients undergoing IVF or intracytoplasmic sperm injection (ICSI) and the control group 38. These data add to the increasing uncertainty about the clinical value of assessing the endometrium with only integrins. Thomas et al. 39 studied the endometrial expression of these three integrins in 66 women undergoing ICSI treatment (at LH + 7–9 days) and the subsequent success rate. The results demonstrated that there was significantly increased expression of integrin αvβ3 in the luminal epithelium of patients for whom treatment was successful compared to patients for whom treatment was unsuccessful. However, treatment was successful in some patients with negative expression. They concluded that the clinical value of assessing the endometrium before treatment has certain drawbacks, and the expression of α1, α4, and integrin αvβ3 s appears to have no prognostic value with regard to subsequent IVF treatment.
Using the same samples, we confirmed a significant up-regulation of OPN mRNA during the implantation window in the high E2, high P, and high E2 and P groups. OPN has been found to be consistently up-regulated in the endometrium during the window of implantation in different studies of the transcriptome 18, 40, 41, 42. In a study by Li et al. 41, a microRNA array and microarray were performed to evaluate the endometrial receptivity in 12 patients with high serum P level and 7 fertile women with normal P on the day of HCG administration. Spp1 (osteopontin precursor) and ang (angiogenin precursor) were up-regulated genes, and RT-PCR verified the array results. Immunohistochemical analysis indicated that elevated OPN and decreased vascular endothelial growth factor in patients with high P levels on the day of HCG administration had poor pregnancy rate. However, in the present study, no significant difference in endometrial OPN immunoreactivity was observed among patients with high P, high E2, or high E2 and P, compared with the control group.
Our immunohistochemical analysis of OPN and its receptor integrin αvβ3 did not show different expression of these two markers, either alone or in combination, among the specimens of the high P, high E2, high E2 and P, and control groups. Discrepancies between different studies may be explained by the following facts:
First, discordant results were often obtained in infertile patients because of the heterogeneity of patients included in the same study group. To avoid this circumstance, the inclusion of patients in the current study was restricted to patients unable to conceive because of tubal obstruction or male infertility. Cases with endometriosis, hydrosalpinges, PCOS and unexplained infertility were excluded.
Second, the clinical value of assessing the endometrium before treatment has certain drawbacks 39. Endometrial samples obtained during the implantation window in spontaneous cycle have been included in some investigations 18, 38, 39; however, we and others demonstrated changes in the endometrial expression of OPN and integrin αvβ3 during the implantation window in the same IVF cycle 17, 41.
Third, the analysis of mRNA or protein expression could produce discrepant results because not all mRNA molecules could be translated into protein 43. Finally, the simultaneous presence of both OPN and integrin αvβ3 was observed in only 34.1 % (15/44) of cases during the implantation window in our study. In studies by Cacals et al. 21, 44, two biopsies were performed during a single menstrual cycle in each subject. The simultaneous presence of both OPN and integrin αvβ3 was only observed in 33.33–41.18 % of cases in the mid-luteal phase, but 98–100 % of cases in the late-luteal phase. Nevertheless, previous research confirmed that there was dynamic expression of several integrins in the human endometrium 28. It was found that integrin αvβ3 expression was closely correlated with histological maturation of the endometrium, with expression occurring primarily at postovulatory days 6–7, whereas OPN expression occurred primarily at post-ovulatory days 4–5. Both markers were expressed by all endometria dated post-ovulatory day ≥ 8. The intensity of their expression also increased from mid-luteal to late post-ovulatory days. These changes in OPN and integrin αvβ3 expression occurred irrespective of endometria being in-phase or out-of-phase 21. In our study, only one biopsy was performed at 7–8 days after oocyte retrieval or ovulation. The staining of integrin αvβ3 was scattered and weakly positive, but the staining of OPN was mostly moderate to strong, with positive expression in all patients during the implantation window, consistent with previous studies.
While we observed that serum P level at the time of biopsy was significantly increased in stimulated patients, there was no difference among the four groups. Ovarian stimulation is known to advance endometrial maturation, and P may hasten the closure of the implantation window 45. A recent study by Papanikolaou et al. 46 showed that increased P level on the day of HCG administration impaired pregnancy outcome in day-3 single ETs, whilst it had no effect on day-5 single-blastocyst transfer. It was proposed that high follicular P concentration greatly advances the endometrium; therefore, the placement of a day-3 embryo in an asynchronous endometrium (earlier than a natural pregnancy) resulted in failure to establish an embryo endometrium cross-dialogue and a failed implantation. The negative impact of premature luteinization on pregnancy rate with blastocyst transfer suggested that the endometrium has already significantly recovered from the violation induced from the supra-physiological steroid concentration on the fifth luteal day. Thus, a proposed strategy for cases with P elevation on the day of HCG administration is the selection for ET on day 5. Our present study showed that endometrial OPN and integrin αvβ3 expression or co-expression during the window of implantation were not impaired in patients with elevated P and/or high E2 levels. However, whether these results indicate that normal endometrial receptivity has also recovered from the violation induced from the supra-physiological steroid concentration on the fifth luteal day, which needs further exploration.
According to our results, if OPN and integrin αvβ3 are proven to be accurate markers of uterine receptivity, it may be concluded that there is no endometrial impairment in patients with high P and/or E2. However, some investigations have reported uncertainty about the value of OPN and integrins in assessing endometrial receptivity in the clinical setting 17, 21, 28, 38, 44. Furthermore, a limitation of this study was the relatively small number of study participants, and these results should be confirmed in a large population. The HSCORE values may become more significant if a larger study population is used; nevertheless, the finding of no difference in staining among patients with high P and/or E2 indicated that elevated hormone levels on the day of HCG administration has no impact on uterine receptivity during the implantation window.
In conclusion, the results of the present study show that OPN and integrin αvβ3 expression or co-expression during the window of implantation are not impaired in patients with elevated P and/or E2 on the day of HCG administration.
Conflict of Interest The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
Co-author: Zhenlin He and Yanping Ma are joint first authors of this article.
References
- 1.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–746. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
- 2.Ubaldi F, Camus M, Smitz J. et al. Premature luteinization in in vitro fertilization cycles using gonadotropin-releasing hormone agonist (GnRH-a) and recombinant follicle-stimulating hormone (FSH) and GnRH-a and urinary FSH. Fertil Steril. 1996;66:275–280. doi: 10.1016/s0015-0282(16)58453-2. [DOI] [PubMed] [Google Scholar]
- 3.Bosch E, Valencia I, Escudero E. et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. 2003;80:1444–1449. doi: 10.1016/j.fertnstert.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Arslan M, Bocca S, Arslan E. et al. Cumulative exposure to high estradiol levels during the follicular phase of IVF cycles negatively affects implantation. J Assist Reprod Genet. 2007;24:111–117. doi: 10.1007/s10815-006-9101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, Li R, Ma Y P. et al. Effect of HCG-day serum progesterone and oestradiol concentrations on pregnancy outcomes in GnRH agonist cycles. Reprod Biomed Online. 2012;24:511–520. doi: 10.1016/j.rbmo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Castelbaum A J, Wheeler J, Coutifaris C. et al. Timing of the endometrial biopsy may be critical for the accurate diagnosis of luteal phase deficiency. Fertil Steril. 1994;61:443–447. [PubMed] [Google Scholar]
- 7.Borthwick J M, Charnock-Jones D S, Tom B D. et al. Determination of the transcript profile of human endometrium. Mol Hum Reprod. 2003;9:19–33. doi: 10.1093/molehr/gag004. [DOI] [PubMed] [Google Scholar]
- 8.Apparao K B, Murray M J, Fritz M A. et al. Osteopontin and its receptor alphavbeta(3) integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. J Clin Endocrinol Metab. 2001;86:4991–5000. doi: 10.1210/jcem.86.10.7906. [DOI] [PubMed] [Google Scholar]
- 9.von Wolff M, Strowitzki T, Becker V. et al. Endometrial osteopontin, a ligand of beta3-integrin, is maximally expressed around the time of the “implantation window”. Fertil Steril. 2001;76:775–781. doi: 10.1016/s0015-0282(01)02015-5. [DOI] [PubMed] [Google Scholar]
- 10.Lessey B A. Implantation defects in infertile women with endometriosis. Ann N Y Acad Sci. 2002;955:265–280. doi: 10.1111/j.1749-6632.2002.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 11.Makker A, Singh M M. Endometrial receptivity: clinical assessment in relation to fertility, infertility, and antifertility. Med Res Rev. 2006;26:699–746. doi: 10.1002/med.20061. [DOI] [PubMed] [Google Scholar]
- 12.Strowitzki T, Germeyer A, Popovici R. et al. The human endometrium as a fertility-determining factor. Hum Reprod Update. 2006;12:617–630. doi: 10.1093/humupd/dml033. [DOI] [PubMed] [Google Scholar]
- 13.Boroujerdnia M G, Nikbakht R. Beta3 integrin expression within uterine endometrium and its relationship with unexplained infertility. Pak J Biol Sci. 2008;11:2495–2499. doi: 10.3923/pjbs.2008.2495.2499. [DOI] [PubMed] [Google Scholar]
- 14.Ceydeli N, Kaleli S, Calay Z. et al. Difference in alpha(v)beta3 integrin expression in endometrial stromal cell in subgroups of women with unexplained infertility. Eur J Obstet Gynecol Reprod Biol. 2006;126:206–211. doi: 10.1016/j.ejogrb.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 15.Lessey B A, Castelbaum A J, Sawin S W. et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–649. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 16.Savaris R F, Pedrini J L, Flores R. et al. Expression of alpha 1 and beta 3 integrins subunits in the endometrium of patients with tubal phimosis or hydrosalpinx. Fertil Steril. 2006;85:188–192. doi: 10.1016/j.fertnstert.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 17.Casals G, Ordi J, Creus M. et al. Osteopontin and alphavbeta3 integrin as markers of endometrial receptivity: the effect of different hormone therapies. Reprod Biomed Online. 2010;21:349–359. doi: 10.1016/j.rbmo.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 18.DuQuesnay R, Wright C, Aziz A A. et al. Infertile women with isolated polycystic ovaries are deficient in endometrial expression of osteopontin but not alphavbeta3 integrin during the implantation window. Fertil Steril. 2009;91:489–499. doi: 10.1016/j.fertnstert.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 19.Peters A J, Lloyd R P, Coulam C B. Prevalence of out-of-phase endometrial biopsy specimens. Am J Obstet Gynecol. 1992;166:1738–1745. doi: 10.1016/0002-9378(92)91564-q. [DOI] [PubMed] [Google Scholar]
- 20.Shoupe D, Mishell D RJ, Lacarra M. et al. Correlation of endometrial maturation with four methods of estimating day of ovulation. Obstet Gynecol. 1989;73:88–92. [PubMed] [Google Scholar]
- 21.Casals G, Ordi J, Creus M. et al. Osteopontin and alphavbeta3 integrin expression in the endometrium of infertile and fertile women. Reprod Biomed Online. 2008;16:808–816. doi: 10.1016/s1472-6483(10)60146-0. [DOI] [PubMed] [Google Scholar]
- 22.Noyes R W, Hertig A T, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 23.Creus M, Ordi J, Fábregues F. et al. alphavbeta3 integrin expression and pinopod formation in normal and out-of-phase endometria of fertile and infertile women. Hum Reprod. 2002;17:2279–2286. doi: 10.1093/humrep/17.9.2279. [DOI] [PubMed] [Google Scholar]
- 24.Ordi J, Creus M, Ferrer B. et al. Midluteal endometrial biopsy and alphavbeta3 integrin expression in the evaluation of the endometrium in infertility: implications for fecundity. Int J Gynecol Pathol. 2002;21:231–238. doi: 10.1097/00004347-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Budwit-Novotny D A, McCarty K S, Cox E B. et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–5425. [PubMed] [Google Scholar]
- 26.Lessey B A, Albelda S, Buck C A. et al. Distribution of integrin cell adhesion molecules in endometrial cancer. Am J Pathol. 1995;146:717–726. [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan H C, Zhu X M, Luo Q. et al. Ovarian stimulation with GnRH agonist, but not GnRH antagonist, partially restores the expression of endometrial integrin beta3 and leukaemia-inhibitory factor and improves uterine receptivity in mice. Hum Reprod. 2006;21:2521–2529. doi: 10.1093/humrep/del215. [DOI] [PubMed] [Google Scholar]
- 28.Creus M, Balasch J, Ordi J. et al. Integrin expression in normal and out-of-phase endometria. Hum Reprod. 1998;13:3460–3468. doi: 10.1093/humrep/13.12.3460. [DOI] [PubMed] [Google Scholar]
- 29.Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Salamonsen L A. Role of proteases in implantation. Rev Reprod. 1999;4:11–22. doi: 10.1530/ror.0.0040011. [DOI] [PubMed] [Google Scholar]
- 31.Nikas G, Develioglu O H, Toner J P. et al. Endometrial pinopodes indicate a shift in the window of receptivity in IVF cycles. Hum Reprod. 1999;14:787–792. doi: 10.1093/humrep/14.3.787. [DOI] [PubMed] [Google Scholar]
- 32.Giudice L C. Potential biochemical markers of uterine receptivity. Hum Reprod. 1999;14 02:3–16. doi: 10.1093/humrep/14.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 33.Lessey B A. Adhesion molecules and implantation. J Reprod Immunol. 2002;55:101–112. doi: 10.1016/s0165-0378(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 34.Lessey B A. Two pathways of progesterone action in the human endometrium: implications for implantation and contraception. Steroids. 2003;68:809–815. doi: 10.1016/j.steroids.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Franchi A, Zaret J, Zhang X. et al. Expression of immunomodulatory genes, their protein products and specific ligands/receptors during the window of implantation in the human endometrium. Mol Hum Reprod. 2008;14:413–421. doi: 10.1093/molehr/gan029. [DOI] [PubMed] [Google Scholar]
- 36.Quenby S, Anim-Somuah M, Kalumbi C. et al. Different types of recurrent miscarriage are associated with varying patterns of adhesion molecule expression in endometrium. Reprod Biomed Online. 2007;14:224–234. doi: 10.1016/s1472-6483(10)60791-2. [DOI] [PubMed] [Google Scholar]
- 37.Somkuti S G, Yuan L, Fritz M. et al. Epidermal growth factor and sex steroids dynamically regulate a marker of endometrial receptivity in Ishikawa cells. J Clin Endocrinol Metab. 1997;82:2192–2197. doi: 10.1210/jcem.82.7.4102. [DOI] [PubMed] [Google Scholar]
- 38.Thomas K, Thomson A J, Wood S J. et al. Endometrial integrin expression in women undergoing IVF and ICSI: a comparison of the two groups and fertile controls. Hum Reprod. 2003;18:364–369. doi: 10.1093/humrep/deg104. [DOI] [PubMed] [Google Scholar]
- 39.Thomas K, Thomson A, Wood S. et al. Endometrial integrin expression in women undergoing in vitro fertilization and the association with subsequent treatment outcome. Fertil Steril. 2003;80:502–507. doi: 10.1016/s0015-0282(03)00792-1. [DOI] [PubMed] [Google Scholar]
- 40.Kao L C, Tulac S, Lobo S. et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 41.Li R, Qiao J, Wang L. et al. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol. 2011;9:29. doi: 10.1186/1477-7827-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riesewijk A, Martín J, van Os R. et al. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003;9:253–264. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- 43.Cho S, Ahn Y S, Choi Y S. et al. Endometrial osteopontin mRNA expression and plasma osteopontin levels are increased in patients with endometriosis. Am J Reprod Immunol. 2009;61:286–293. doi: 10.1111/j.1600-0897.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 44.Casals G, Ordi J, Creus M. et al. Expression pattern of osteopontin and alphavbeta3 integrin during the implantation window in infertile patients with early stages of endometriosis. Hum Reprod. 2012;27:805–813. doi: 10.1093/humrep/der432. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann G E, Bergh P A, Guzman I. et al. Premature luteinization is not eliminated by pituitary desensitization with leuprolide acetate in women undergoing gonadotrophin stimulation who demonstrated premature luteinization in a prior gonadotrophin-only cycle. Hum Reprod. 1993;8:695–698. doi: 10.1093/oxfordjournals.humrep.a138122. [DOI] [PubMed] [Google Scholar]
- 46.Papanikolaou E G, Kolibianakis E M, Pozzobon C. et al. Progesterone rise on the day of human chorionic gonadotropin administration impairs pregnancy outcome in day 3 single-embryo transfer, while has no effect on day 5 single blastocyst transfer. Fertil Steril. 2009;91:949–952. doi: 10.1016/j.fertnstert.2006.12.064. [DOI] [PubMed] [Google Scholar]


