Abstract
V-ATPases are ATP-driven proton pumps that function within both intracellular compartments and the plasma membrane in a wide array of normal physiological and pathophysiological processes. V-ATPases are composed of a peripheral V1 domain that hydrolyzes ATP and an integral V0 domain that transports protons. Regulated assembly of the V-ATPase represents an important mechanism of regulating V-ATPase activity in response to a number of environmental cues. Our laboratory has demonstrated that glucose-dependent assembly of the V-ATPase complex in yeast is controlled by the Ras/cAMP/PKA pathway. By contrast, increased assembly of the V-ATPase during dendritic cell maturation involves the PI-3 kinase and mTORC1 pathways. Recently, we have shown that amino acids regulate V-ATPase assembly in mammalian cells, possibly as a means to maintain adequate levels of amino acids upon nutrient starvation. V-ATPases have also been implicated in cancer cell survival and invasion. V-ATPases are targeted to different cellular membranes by isoforms of subunit a, with a3 targeting V-ATPases to the plasma membrane of osteoclasts. We have shown that highly invasive human breast cancer cell lines express higher levels of the a3 isoform than poorly invasive lines and that knockdown of a3 reduces both expression of V-ATPases at the plasma membrane and in vitro invasion of breast tumor cells. Moreover, overexpression of a3 in a non-invasive breast epithelial line increases both plasma membrane V-ATPases and in vitro invasion. Finally, specific ablation of plasma membrane V-ATPases in highly invasive human breast cancer cells using either an antibody or small molecule approach inhibits both in vitro invasion and migration. These results suggest that plasma membrane and a3-containing V-ATPases represent a novel and important target in the development of therapeutics to limit breast cancer metastasis.
Introduction
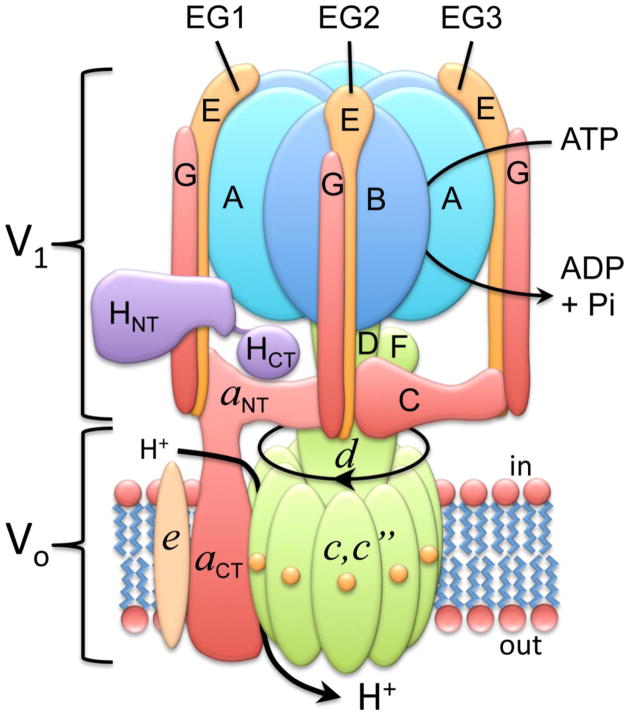
The vacuolar ATPases (V-ATPases) are ATP-driven proton pumps that play important roles in both normal and disease processes [1–5]. V-ATPases within cells function in such processes as intracellular membrane traffic, protein processing and degradation, coupled transport of small molecules and the entry of various viruses and toxins, including influenza virus and diphtheria toxin [1–3,6]. V-ATPases present in the plasma membrane of specialized cells are important for acid secretion in the kidney, sperm maturation in the epididymus and degradation of bone by osteoclasts [1,2,4,5]. The V-ATPases are large, multi-subunit complexes composed of a peripheral V1 domain that hydrolyzes ATP and an integral V0 domain that translocates protons [1]. The V1 domain is composed of eight subunits (A-H) in a stoichiometry of A3B3C1D1E3F1G3H1 while the V0 domain (in mammals) contains five subunits in a stoichiometry of a1c9c″1d1e1 (see Fig. 1, adapted from reference [7]). The V-ATPases operate by a rotary mechanism in which ATP hydrolysis at catalytic sites located at the interface of the A and B subunits drives rotation of a central rotor composed on subunits D and F of V1 connected to the ring of proteolipid subunits (c,c″) in V0 [1,2]. Each proteolipid subunit contains a single buried glutamate residue which undergoes reversible protonation during proton transport and is the site of modification by the inhibitor dicyclohexylcarbodiimide (DCCD) [8]. Protons reach these buried residues by way of a proton-conducting hemi-channel located in the C-terminal hydrophobic domain of subunit a [9]. Following ATP-driven rotation of the proteolipid ring, the buried glutamate residues are deprotonated through interaction with a single buried arginine residue located in subunit a [10] and then exit via a second hemi-channel in subunit a to the luminal side. Subunit a is held fixed relative to the A3B3 catalytic head through interactions between the N-terminal domain of subunit a and subunits C, H and the EG heterodimers [11,12].
Figure 1. Structure and mechanism of the V-ATPase.
The V-ATPase is composed of a peripheral V1 domain responsible for ATP hydrolysis and an integral V0 domain that carries out proton transport. V1 contains eight subunits (A–H) while V0 (in mammals) contains five subunits (a,c,c″,d,e). The V-ATPases operate by a rotary mechanism in which ATP hydrolysis drives rotation of a central complex composed of subunits D,F,d, c and c″ relative to the remainder of the complex. Rotation of buried glutamate residues on the proteolipid subunits (c,c″) relative to proton-conducting hemi-channels in subunit a drive proton transport (see text for details). Adapted from reference [7].
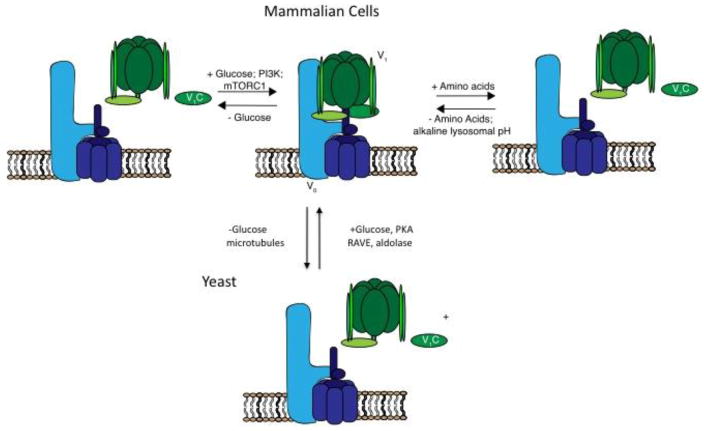
An important mechanism of regulating V-ATPase activity in vivo involves reversible dissociation and reassembly of the V1 and V0 domains (Fig. 2). The first part of this article will focus on recent advances from our laboratory on understanding the regulation of V-ATPase assembly in yeast in response to changes in glucose concentration [13], during maturation of dendritic cells [14] and in mammalian cells in response to changes in amino acid levels [15]. Targeting of V-ATPases to different cellular membranes is controlled by isoforms of subunit a [1,4,5]. In mammals, subunit a exists as four isoforms (a1–a4), with a3 and a4 responsible for targeting of V-ATPases to the plasma membrane of osteolcasts and renal intercalated cells, respectively [4,16]. Results from a variety of laboratories have suggested a role of V-ATPases in cancer cell survival and invasion [17–20; For reviews see 1,21,22]. The second part of this article will focus on recent advances from our laboratory on the role of plasma membrane and a3-containing V-ATPases in breast cancer cell migration and invasion [17–19].
Figure 2. Regulation of V-ATPase assembly in yeast and mammalian cells.
V-ATPase assembly in yeast is regulated by glucose as a way to conserve ATP [23]. This process is controlled by the Ras/cAMP/PKA pathway [13], requires catalytic activity [29], intact microtubules [28], interaction with aldolase [26] and the RAVE complex [24]. In dendritic cells, V-ATPase assembly increases during maturation to promote antigen processing [34]. Assembly in dendritic cells is regulated by PI-3 kinase and mTORC1 [14]. Glucose also regulates V-ATPase assembly in mammalian cells in a PI-3 kinase dependent process [40]. Amino acid depletion increases V-ATPase assembly in mammalian cells in a PI-3 kinase and mTORC1-independent manner [15], likely as a means to increase amino acid availability from lysosomal protein degradation.
Regulation of V-ATPase assembly in yeast
Regulated assembly of the V-ATPase represents an important and widely employed mechanism of controlling V-ATPase activity in vivo (Fig. 2). In response to a variety of stimuli, the V-ATPase undergoes reversible changes in the degree of assembly of the V1 and V0 domains, which, in the dissociated state, remain inactive with respect to ATP hydrolysis and proton translocation [1]. In yeast, dissociation of the V-ATPase complex occurs in response to glucose depletion, is rapid and reversible, and does not require new protein synthesis [23]. Reassembly requires interaction with the RAVE complex, which interacts with the V-ATPase in a glucose-dependent manner and whose interactions have now been carefully mapped [24]. Interestingly RAVE promotes the assembly of only V-ATPase complexes containing the yeast isoform of subunit a which targets the complex to the vacuole (Vph1p), and not those containing Stv1p, which are targeted to the Golgi [25]. Similarly, glucose-dependent assembly also requires interaction of the V-ATPase with the glycolytic enzyme aldolase [26]. Interactions with PI(3,5)P2 appear to stabilize assembly of the V-ATPase on the vacuolar membrane through interaction with the N-terminal domain of subunit a [27].
It was previously demonstrated that dissociation of the V-ATPase in response to glucose depletion in yeast requires an intact microtubular network [28] and a catalytically active enzyme [29]. Interestingly, mutations in the non-homologous region of the catalytic subunit A are able to block dissociation of the V-ATPase without altering catalytic activity [30]. In addition, dissociation of the V-ATPase is sensitive to the membrane environment in which the V-ATPase resides, with V-ATPase complexes present in the yeast vacuole but not the Golgi undergoing dissociation in response to glucose starvation [31]. Moreover, dissociation of the complex in the vacuole requires a sufficiently acidic vacuolar pH [30], which may ensure a sufficiently acidic vacuolar lumen, even under limiting nutrient conditions.
Using a novel genetic screen to identify yeast mutants that are defective in dissociation of the V-ATPase in response to glucose depletion, we identified Ira2p as a protein whose absence results in constitutive assembly of the V-ATPase, even in the absence of glucose [13]. Ira2p is a Ras GAP that activates GTP hydrolysis by Ras, thus converting active, GTP-bound Ras to the inactive, GDP-bound form. The involvement of Ira2p in glucose-regulated assembly of the V-ATPase suggested the involvement of Ras, an important glucose-responsive signaling molecule in yeast. To test the involvement of Ras in this process, we next expressed a constitutively active form of Ras (Val19) in yeast and demonstrated that, like disruption of Ira2p, expression of constitutively active Ras maintains the V-ATPase in an assembled state, even in the absence of glucose [13]. An important downstream target of Ras in yeast is adenylate cylclase, which, when activated, increases the cellular levels of cAMP. Increased cAMP in turn increases protein kinase A (PKA) activity. PKA is a heterotetramer composed of two catalytic and two regulatory subunits, the latter encoded by the BCY1 gene. In yeast mutants in which BCY1 is deleted, the catalytic subunits of PKA are constitutively active. Our laboratory demonstrated that in bcy1Δ mutant yeast, which express high levels of PKA activity, V-ATPase assembly was again high, independent of the presence or absence of glucose [13]. Thus, activation of PKA by either disruption of Ira2p, expression of constitutively active Ras or constitutive activation of PKA by disruption of the BCY1 gene results in loss of regulated assembly of the V-ATPase in response to glucose availability [13]. Whether activation of PKA blocks dissociation or enhances assembly is unknown. It is also not known whether PKA maintains the pump in an assembled state through direct phosphorylation of a V-ATPase subunit or associated protein or whether there are additional signaling molecules between glucose-dependent activation of PKA and V-ATPase assembly. Interestingly, increased assembly has also been reported to increase PKA activity [32], suggesting the possibility that there is a positive feedback loop between V-ATPase assembly and PKA.
Regulation of V-ATPase assembly in dendritic cells
Dendritic cells function in the immune system by taking up foreign antigens, degrading them within lysosomes and presenting the peptide fragments on their surface in complex with MHC class II molecules [33]. Recognition of antigen-bound MHC complexes by T cells results in their stimulation to mount an immune response to the foreign pathogen. Because antigen processing occurs within lysosomes, it is dependent upon the V-ATPase to maintain the low lysosomal pH required for the activity of acid-dependent proteases involved in lysosomal protein breakdown. Exposure of dendritic cells to bacterial products, such as LPS, activates toll like receptors (TLRs) which leads to maturation of dendritic cells to become more active in antigen presentation. It was found that during LPS-stimulated maturation, increased assembly of the V-ATPase occurs, resulting in decreased lysosomal pH and increased protein degradation [34]. Dendritic cells can also be induced to achieve a semi-mature state by disruption of cell-cell contacts that normally occurs in the periphery [35]. In this semi-mature state, dendritic cells take up and process self-antigens, which suppresses the immune response and is thus important in immune tolerance [36]. We recently demonstrated that increased V-ATPase assembly and lysosomal acidification is also a characteristic of this semi-mature state of dendritic cells [14]. Moreover, increased assembly and activity of the V-ATPase is dependent upon both PI-3 kinase and mTORC1 (Fig. 2) [14]. The mechanism by which these important signaling molecules control V-ATPase assembly remains to be determined, although factors identified as interacting with the V-ATPase may be involved [37].
Regulation of V-ATPase assembly in response to changes in amino acid levels
A central regulator of cell growth and metabolism is mTORC1 [38]. mTORC1 integrates signals from nutrient availability and growth factor receptors to control such processes as protein and lipid synthesis and autophagy. Activation of mTORC1 by growth factors requires adequate levels of amino acids, which are sensed at the lysosome. The V-ATPase, in complex with the Ragulator, senses amino acid levels and signals to the Rag GTPases to recruit mTORC1 to the lysosomal membrane, where it is activated by Rheb [39]. Thus, inhibition of the V-ATPase with concanamycin results in inhibition of mTORC1 activity. Recently, our laboratory demonstrated that V-ATPase assembly on lysosomes is controlled by amino acid levels [15]. Thus, amino acid starvation increases both V-ATPase assembly and V-ATPase-dependent lysosomal acidification in a reversible manner [15]. Amino acid-dependent changes in assembly require V-ATPase activity and are blocked by neutralization of the lysosome. Unlike changes in assembly in response to glucose [40], during dendritic cell maturation [14] or during influenza infection [41,42], changes in assembly in response to amino acids are not dependent upon either PI-3 kinase or mTORC1 activity [15]. Thus, a novel signaling pathway controls amino acid-dependent changes in V-ATPase assembly (Fig. 2). The fact that amino acid activation of mTORC1 requires V-ATPase activity whereas elevated levels of amino acids actually decrease V-ATPase activity suggests that amino acid-dependent changes in V-ATPase assembly are not involved in changes in mTORC1 activity [15]. In support of this, there is little correlation in the response of V-ATPase assembly and mTORC1 activity to the withdrawal of individual amino acids or to neutralization of lysosomes with chloroquine [15]. We have suggested that the increased V-ATPase-dependent lysosomal acidification that we observe upon amino acid starvation increases lysosomal protein breakdown, thus helping to restore amino acids to normal levels. Interestingly, the V-ATPase-Ragulator complex is also involved in lysosomal recruitment and activation of AMP kinase in response to glucose depletion [43], although the involvement of changes in V-ATPase assembly in this process are unknown. The V-ATPase is thus an integral part of the nutrient sensing machinery of cells.
Function of V-ATPases in breast cancer cell invasion
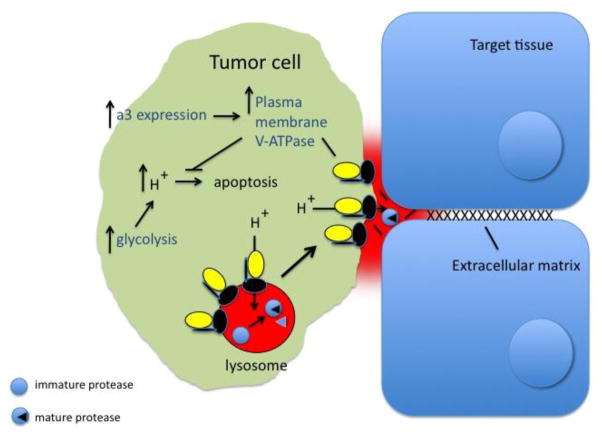
A variety of studies have suggested that V-ATPases play a role in the survival and invasiveness of tumor cells (Fig. 3) (for reviews see [1,21]). For human breast cancer cells, comparison of the highly invasive MB231 line with the poorly invasive MCF7 line revealed that the highly invasive line expressed much higher levels of V-ATPase at the plasma membrane than the poorly invasive line [44]. Moreover, the in vitro invasiveness of the highly invasive cells but not the poorly invasive cells was inhibited by specific V-ATPase inhibitors (bafilomycin and concanamycin). Targeting of V-ATPases to different cellular membranes is controlled by isoforms of subunit a (in mammals, a1–a4), with a3 and a4 directing V-ATPase complexes to the plasma membrane of osteoclasts and renal intercalated cells, respectively [1,4,5,16]. Our laboratory has shown that MB231 cells express 70-fold higher levels of a3 mRNA and 20-fold higher levels of a4 mRNA relative to MCF7 cells, whereas mRNA levels for a1 and a2 were comparable [18]. Moreover, knockdown of both a3 and a4 using isoform-specific siRNAs inhibited in vitro invasion by MB231 cells [18], suggesting that expression of a subunit isoforms capable of targeting V-ATPase to the plasma membrane was important in breast cancer cell invasion.
Figure 3. Function of V-ATPases in tumor cells.
Tumor cells employ V-ATPases at the plasma membrane both as a means to promote invasion and migration [19] and to aid in cell survival [20]. Plasma membrane V-ATPases may promote tumor cell invasion by activation of extracellular proteases (including secreted cathepsins) that facilitate invasion through degradation of extracellular matrix. Intracellular V-ATPases may also aid in activation of cathepsins, which normally reside within lysosomes. V-ATPases are important in tumor cell survival by facilitating the secretion the large amount of metabolic acid generated by the dependence of tumor cells on glycolytic metabolism. An important mechanism by which tumor cells increase expression of V-ATPases at the plasma membrane is by over-expression of the a3 isoform [17,18], which is able to target V-ATPases to the cell surface.
In order to compare more closely related human breast cancer cell lines, MCF10a and MCF10CA1a cells were next employed [17]. MCF10a cells are a non-tumorigenic, non-invasive human breast epithelial cell line whereas MCF10CA1a cells were derived by transfection of MCF10a cells with Ras and repeated selection for cells able to metastasize in mice [45,46]. Our laboratory showed that the in vitro invasion of the highly metastatic MCF10CA1a cells but not the parental MCF10a cells was inhibited by concanamycin [17]. In addition, MCF10CA1a cells expressed much higher levels of both a3 mRNA and plasma membrane V-ATPases than MCF10a cells. Moreover, siRNA-mediated knockdown of a3 but not other a subunit isoforms significantly inhibited both plasma membrane V-ATPase expression and in vitro invasion of MCF10CA1a cells [17]. Importantly, over-expression of a3 (but not the other a subunit isoforms) in the parental MCF10a cells dramatically increased both invasiveness and the expression of V-ATPases at the plasma membrane [17]. These results suggest that highly invasive tumor cells express high levels of a3 which target V-ATPases to the plasma membrane, where they aid in invasion. How a3 expression is upregulated in tumor cells is not known but is a question of great interest. Transcription factors such as TFEB [47] have been shown to upregulate expression of V-ATPase subunits, and this is linked to the dependence of many tumors on autophagy as a source of amino acids [48], but it is not known whether up-regulation occurs in an isoform specific manner. It is also possible that a3 expression is under the control of factors which regulate the expression of genes involved in invasion or migration, but the nature of such factors remains to be elucidated.
Because the V-ATPase inhibitors that have been employed in these studies (including bafilomycin and concanamycin), are membrane permeant, they inhibit all the V-ATPases in the cell. This is important since it is possible that intracellular V-ATPases, in addition to those present at the plasma membrane, might be involved in promoting tumor cell invasion. Similarly, knockdown of a3 using siRNA might reduce plasma membrane V-ATPase expression in tumor cells either because a3 is itself present in plasma membrane V-ATPase complexes or a3-containing V-ATPases within intracellular compartments (such as secretory vesicles), are involved in the trafficking of V-ATPases to the cell surface. To directly test whether V-ATPases present at the plasma membrane of breast tumor cells are involved in invasion, two approaches were taken to specifically ablate plasma membrane V-ATPase activity [19]. First, MB231 cells were stably transfected with a V5-epitope tagged version of subunit c, part of the integral V0 domain [19]. The V5 epitope tag was located at the C-terminus of subunit c, ensuring that it would be exposed on the outside of the cell for V-ATPases present at the plasma membrane [19]. Subunit c was chosen because of its presence in 9 copies per complex [11], thus making it likely that nearly all V-ATPase complexes in the cell would contain at least one copy of the V5-tagged c subunit. In fact, V5-tagged complexes could be detected at the plasma membrane of non-permeabilized cells, indicating that the tagged subunit was assembled and trafficked normally [19]. Next, it was shown that addition of an antibody against the V5-tag inhibited V-ATPase dependent proton flux across the plasma membrane of cells expressing the V5-tagged c subunit but not control, untransfected cells [19]. Importantly, addition of the V5 antibody inhibited both invasion and migration of transfected but not untransfected cells [19]. As a second approach to address the role of plasma membrane V-ATPases in breast tumor cell invasion, a membrane impermeant inhibitor (streptavidin-conjugated biotin-bafilomycin) was employed. Consistent with the above results, this inhibitor significantly reduced both invasion and migration of MB231 cells [19]. These results suggest that it is plasma membrane rather than intracellular V-ATPases that play the most important role in breast tumor cell invasion and migration.
Our results have led us to hypothesize that breast tumor cells (by an unknown mechanism) up-regulate the a3 isoform, which results in increased trafficking of V-ATPase complexes to the plasma membrane, where they increase tumor cell invasion (Fig. 3) [17–19]. One possible mechanism by which they might achieve this is by increasing the activity of extracellular proteases that participate in invasion. Cathepsins are an important class of proteases that have been shown to be secreted by tumor cells and have been implicated in tumor metastasis [49–52]. Secreted cathepsins facilitate tumor cell invasion both by directly degrading extracellular matrix and by activating other matrix-degrading proteases (such as MMPs) which do so. Moreover, because cathepsins normally reside in lysosomes, they require an acidic pH both for their activity and for their proteolytic activation [50,53,54]. Thus, plasma membrane V-ATPases may in part facilitate tumor cell invasion by activating secreted cathepsins involved in this process. How plasma membrane V-ATPases participate in tumor cell migration is less clear, but could involve interactions with cytoskeletal proteins at the leading edge of migrating cells. Plasma membrane V-ATPases also aid in tumor cell survival [20] by facilitating the secretion of excess metabolic acid generated through the reliance of tumor cells on glycolytic metabolism. It is interesting to speculate that tumor cells may also increase assembly of V-ATPases at the plasma membrane as a means to increase cell surface V-ATPase activity, and this represents an important but unanswered question.
Our results suggest that plasma membrane and a3-containing V-ATPases represent an important and novel target in the development of drugs to limit breast cancer metastasis. That knock-down of a3 has been shown to reduce metastasis of melanoma cells in an in vivo mouse model suggests that this role may not be restricted to breast cancer [55].
Highlights.
V-ATPases are ATP-driven proton pumps important in many normal and disease processes.
Regulated assembly of V-ATPases is an important mechanism of controlling activity.
V-ATPase assembly in mammalian cells increases in response to amino acid starvation.
Plasma membrane V-ATPases are important for tumor cell invasion and migration.
Plasma membrane and a3-containing V-ATPases are novel targets to block metastasis.
Acknowledgments
This work was supported by NIH grant GM34478 (to MF), Tufts University Sackler Families Collaborative Cancer Biology Awards (to KC and LS), Predoctoral Ruth L. Kirschstein National Research Service Awards CA192500 (to KC) and CA189321 (to LS), and the Tufts University Sackler Dean’s Award (to CM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cotter K, Stransky L, McGuire C, Forgac M. Recent Insights into the Structure, Regulation, and Function of the V-ATPases. Trends Biochem Sci. 2015;40:611–622. doi: 10.1016/j.tibs.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 3.Kane PM. The long physiological reach of the yeast vacuolar H+-ATPase. J Bioenerg Biomembr. 2007;39:415–421. doi: 10.1007/s10863-007-9112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breton S, Brown D. Regulation of luminal acidification by the V-ATPase. Physiol. 2013;28:318–329. doi: 10.1152/physiol.00007.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun-Wada G-H, Wada Y. Vacuolar-type proton pump ATPases: acidification and pathological relationships. Histol Histopathol. 2013;28:805–815. doi: 10.14670/HH-28.805. [DOI] [PubMed] [Google Scholar]
- 6.Gruenberg J, van der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol. 2006;7:495–504. doi: 10.1038/nrm1959. [DOI] [PubMed] [Google Scholar]
- 7.Couoh-Cardel S, Milgrom E, Wilkens S. Affinity Purification and Structural Features of the Yeast Vacuolar ATPase Vo Membrane Sector. J Biol Chem. 2015;290:27959–27971. doi: 10.1074/jbc.M115.662494. jbc.M115.662494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai H, Berne M, Forgac M. Inhibition of the coated vesicle proton pump and labeling of a 17,000-dalton polypeptide by N,N′-dicyclohexylcarbodiimide. J Biol Chem. 1987;262:11006–11011. [PubMed] [Google Scholar]
- 9.Toei M, Toei S, Forgac M. Definition of membrane topology and identification of residues important for transport in subunit a of the vacuolar ATPase. J Biol Chem. 2011;286:35176–35186. doi: 10.1074/jbc.M111.273409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawasaki-Nishi S, Nishi T, Forgac M. Arg-735 of the 100-kDa subunit a of the yeast V-ATPase is essential for proton translocation. Proc Natl Acad Sci. 2001;98:12397–12402. doi: 10.1073/pnas.221291798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Benlekbir S, Rubinstein JL. Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase. Nature. 2015;521:241–245. doi: 10.1038/nature14365. [DOI] [PubMed] [Google Scholar]
- 12.Oot RA, Wilkens S. Subunit interactions at the V1-Vo interface in yeast vacuolar ATPase. J Biol Chem. 2012;287:13396–13406. doi: 10.1074/jbc.M112.343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bond S, Forgac M. The Ras/cAMP/Protein Kinase A Pathway Regulates Glucose-dependent Assembly of the Vacuolar (H+)-ATPase in Yeast. J Biol Chem. 2008;283:36513–36521. doi: 10.1074/jbc.M805232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberman R, Bond S, Shainheit MG, Stadecker MJ, Forgac M. Regulated assembly of vacuolar ATPase is increased during cluster disruption-induced maturation of dendritic cells through a phosphatidylinositol 3-kinase/mTOR-dependent pathway. J Biol Chem. 2014;289:1355–1363. doi: 10.1074/jbc.M113.524561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stransky LA, Forgac M. Amino Acid Availability Modulates Vacuolar H+-ATPase Assembly. J Biol Chem. 2015;290:27360–27369. doi: 10.1074/jbc.M115.659128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, et al. From lysosomes to the plasma membrane: localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem. 2003;278:22023–22030. doi: 10.1074/jbc.M302436200. [DOI] [PubMed] [Google Scholar]
- 17.Capecci J, Forgac M. The Function of Vacuolar ATPase (V-ATPase) a Subunit Isoforms in Invasiveness of MCF10a and MCF10CA1a Human Breast Cancer Cells. J Biol Chem. 2013;288:32731–32741. doi: 10.1074/jbc.M113.503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinton A, Sennoune SR, Bond S, Fang M, Reuveni M, Sahagian GG, et al. Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem. 2009;284:16400–16408. doi: 10.1074/jbc.M901201200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotter K, Capecci J, Sennoune S, Huss M, Maier M, Martinez-Zaguilan R, et al. Activity of Plasma Membrane V-ATPases is Critical for the Invasion of MDA-MB231 Breast Cancer Cells. J Biol Chem. 2015;290:3680–3692. doi: 10.1074/jbc.M114.611210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Schwarzenberg K, Wiedmann RM, Oak P, Schulz S, Zischka H, Wanner G, et al. Mode of Cell Death Induction by Pharmacological Vacuolar H+-ATPase (V-ATPase) Inhibition. J Biol Chem. 2013;288:1385–1396. doi: 10.1074/jbc.M112.412007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sennoune SR, Luo D, Martínez-Zaguilán R. Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell Biochem Biophys. 2004;40:185–206. doi: 10.1385/CBB:40:2:185. [DOI] [PubMed] [Google Scholar]
- 22.Hinton A, Bond S, Forgac M. V-ATPase functions in normal and disease processes. Pflüg Arch - Eur J Physiol. 2009;457:589–598. doi: 10.1007/s00424-007-0382-4. [DOI] [PubMed] [Google Scholar]
- 23.Kane PM. Disassembly and reassembly of the yeast vacuolar H(+)-ATPase in vivo. J Biol Chem. 1995;270:17025–17032. [PubMed] [Google Scholar]
- 24.Smardon AM, Nasab ND, Tarsio M, Diakov TT, Kane PM. Molecular Interactions and Cellular Itinerary of the Yeast RAVE (Regulator of the H+-ATPase of Vacuolar and Endosomal Membranes) Complex. J Biol Chem. 2015;290:27511–27523. doi: 10.1074/jbc.M115.667634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smardon AM, Diab HI, Tarsio M, Diakov TT, Nasab ND, West RW, et al. The RAVE complex is an isoform-specific V-ATPase assembly factor in yeast. Mol Biol Cell. 2014;25:356–367. doi: 10.1091/mbc.E13-05-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu M, Ammar D, Ives H, Albrecht F, Gluck SL. Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump. J Biol Chem. 2007;282:24495–24503. doi: 10.1074/jbc.M702598200. [DOI] [PubMed] [Google Scholar]
- 27.Li SC, Diakov TT, Xu T, Tarsio M, Zhu W, Couoh-Cardel S, et al. The signaling lipid PI(3,5)P2 stabilizes V1-Vo sector interactions and activates the V-ATPase. Mol Biol Cell. 2014;25:1251–1262. doi: 10.1091/mbc.E13-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu T, Forgac M. Microtubules are involved in glucose-dependent dissociation of the yeast vacuolar [H+]-ATPase in vivo. J Biol Chem. 2001;276:24855–24861. doi: 10.1074/jbc.M100637200. [DOI] [PubMed] [Google Scholar]
- 29.Parra KJ, Kane PM. Reversible association between the V1 and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol Cell Biol. 1998;18:7064–7074. doi: 10.1128/mcb.18.12.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao E, Forgac M. Involvement of the nonhomologous region of subunit A of the yeast V-ATPase in coupling and in vivo dissociation. J Biol Chem. 2004;279:48663–48670. doi: 10.1074/jbc.M408278200. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki-Nishi S, Nishi T, Forgac M. Yeast V-ATPase complexes containing different isoforms of the 100-kDa a-subunit differ in coupling efficiency and in vivo dissociation. J Biol Chem. 2001;276:17941–17948. doi: 10.1074/jbc.M010790200. [DOI] [PubMed] [Google Scholar]
- 32.Dechant R, Binda M, Lee SS, Pelet S, Winderickx J, Peter M. Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J. 2010;29:2515–2526. doi: 10.1038/emboj.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 34.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 35.Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vander Lugt B, Beck ZT, Fuhlbrigge RC, Hacohen N, Campbell JJ, Boes M. TGF-β Suppresses β-Catenin-Dependent Tolerogenic Activation Program in Dendritic Cells. PLoS ONE. 2011;6:e20099. doi: 10.1371/journal.pone.0020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merkulova M, Păunescu TG, Azroyan A, Marshansky V, Breton S, Brown D. Mapping the H+ (V)-ATPase interactome: identification of proteins involved in trafficking, folding, assembly and phosphorylation. Sci Rep. 2015;5:14827. doi: 10.1038/srep14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sautin YY, Lu M, Gaugler A, Zhang L, Gluck SL. Phosphatidylinositol 3-Kinase-Mediated Effects of Glucose on Vacuolar H+-ATPase Assembly, Translocation, and Acidification of Intracellular Compartments in Renal Epithelial Cells. Mol Cell Biol. 2005;25:575–589. doi: 10.1128/MCB.25.2.575-589.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohio HP, Adamson AL. Glycolytic control of vacuolar-type ATPase activity: a mechanism to regulate influenza viral infection. Virology. 2013;444:301–309. doi: 10.1016/j.virol.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Marjuki H, Gornitzky A, Marathe BM, Ilyushina NA, Aldridge JR, Desai G, et al. Influenza A virus-induced early activation of ERK and PI3K mediates V-ATPase-dependent intracellular pH change required for fusion: ERK and PI3K regulate V-ATPase activity. Cell Microbiol. 2011;13:587–601. doi: 10.1111/j.1462-5822.2010.01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C-S, Jiang B, Li M, Zhu M, Peng Y, Zhang Y-L, et al. The Lysosomal v-ATPase-Ragulator Complex Is a Common Activator for AMPK and mTORC1, Acting as a Switch between Catabolism and Anabolism. Cell Metab. 2014;20:526–540. doi: 10.1016/j.cmet.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 44.Sennoune SR, Bakunts K, Martínez GM, Chua-Tuan JL, Kebir Y, Attaya MN, et al. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol. 2004;286:C1443–1452. doi: 10.1152/ajpcell.00407.2003. [DOI] [PubMed] [Google Scholar]
- 45.Soule HD, Maloney TM, Wolman SR, Peterson WD, Brenz R, McGrath CM, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 46.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, et al. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat. 2001;65:101–110. doi: 10.1023/a:1006461422273. [DOI] [PubMed] [Google Scholar]
- 47.Tognon E, Kobia F, Busi I, Fumagalli A, De Masi F, Vaccari T. Control of lysosomal biogenesis and Notch-dependent tissue patterning by components of the TFEB-V-ATPase axis in Drosophila melanogaster. Autophagy. 2016 doi: 10.1080/15548627.2015.1134080. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524:361–365. doi: 10.1038/nature14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. 2011;21:228–237. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 51.Rothberg JM, Bailey KM, Wojtkowiak JW, Ben-Nun Y, Bogyo M, Weber E, et al. Acid-mediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia. 2013;15:1125–1137. doi: 10.1593/neo.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Victor BC, Anbalagan A, Mohamed MM, Sloane BF, Cavallo-Medved D. Inhibition of cathepsin B activity attenuates extracellular matrix degradation and inflammatory breast cancer invasion. Breast Cancer Res. 2011;13:R115. doi: 10.1186/bcr3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turk V, Turk B, Guncar G, Turk D, Kos J. Lysosomal cathepsins: structure, role in antigen processing and presentation, and cancer. Adv Enzyme Regul. 2002;42:285–303. doi: 10.1016/s0065-2571(01)00034-6. [DOI] [PubMed] [Google Scholar]
- 54.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 55.Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y, et al. The a3 isoform vacuolar type H+-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res. 2011;9:845–855. doi: 10.1158/1541-7786.MCR-10-0449. [DOI] [PubMed] [Google Scholar]