Abstract
Immunochromatography test (ICT) (Paracheck Pf) for diagnosis of Plasmodium falciparum (Pf) infection was compared with the conventional smear examination method. A total of 350 specimens of blood from cases of fever were investigated (falciparum malaria 220, vivax malaria 100, controls 30). Paracheck Pf ICT was found to have enormous advantages over smear examination due to its high degree of sensitivity, specificity, speed and ease of performance. Paracheck Pf ICT test kits are stable at room temperature. Regimental medical officers (RMOs) and nursing assistants with minimal training can safely practise Paracheck Pf ICT method. Introduction of this test method in the Armed Forces can facilitate early diagnosis and specific treatment of falciparum malaria even at far flung places. This will have enormous beneficial effect in reducing morbidity due to malaria and saving precious lives. In short as well as long term, it is a viable cost effective option.
Key Words: Falciparum malaria, Pf HRP-2 antigen, Paracheck Pf ICT method, Rapid diagnosis of malaria
Introduction
Malaria is one of the most important causes of morbidity and mortality amongst troops. Infection with P falciparum has its own hazards. Timely diagnosis and prompt treatment with specific drugs can save lives. However, in far-flung areas, early diagnosis by blood smear examination is not possible. The main restrain is non-availability of laboratory support, microscope and trained manpower. Treatment in such situations is empirical, bordering on both sides, over and under treatment. Availability of rapid, reliable, simple and cost effective diagnostic test for malaria is the need of the hour. ICT for detection of malaria antigen has been devised but is still not used extensively. This study was undertaken to compare utility of ICT method with conventional smear examination for diagnosis of Pf infection.
Material and Methods
350 specimens of blood from cases of fever formed the study material. Out of these, 320 were from cases suspected to be suffering from malaria. 30 specimens from cases of viral fever, urinary tract infection, respiratory tract infection, enteric fever, postoperative infection etc, formed the control. Specimen of blood from all cases was obtained for detection of malaria parasite by thin, stained blood smear and for Pf HRP-2 antigen by ICT method. In a number of cases of Pf infection, the tests were repeated periodically up to 30 days to compare the level and stage of parasitemia with ICT positivity.
The blood smears were stained in the conventional manner using Leishman's stain [1]. These were examined under binocular light microscope by trained laboratory assistants and by pathologists. The findings (type, stage and parasite density) were noted. Average time taken for detection of parasite by this method was determined. Problems and difficulties encountered in diagnosis by smear examination were recorded.
Principle and mechanism of immunochromatography test : ICT for diagnosis of Pf infection is based on the principle of detection of histidine rich protein - 2 (HRP-2) in the blood. This protein molecule is specific for Pf species and is released in large amount during rupture of schizont. Therefore, it is considered to be pathognomonic of trophozoite and merozoite stages of Pf infection and causing malaria. In the test procedure, specimen of blood is reacted with the antibodies of HRP-2 antigen. Chromogenic substance tagged to the antibodies makes this reaction visible. Non-development of specific colour band denotes absence of HRP-2 antigen and is considered to exclude Pf infection. Paracheck Pf (manufactured by Orchid Biomedical Systems) was used for ICT. Schematic representations of the Paracheck Pf test strips are shown in Fig. 1, Fig. 2. Test procedure recommended by the manufacturer for Paracheck Pf (Table 1) was followed.
Fig. 1.
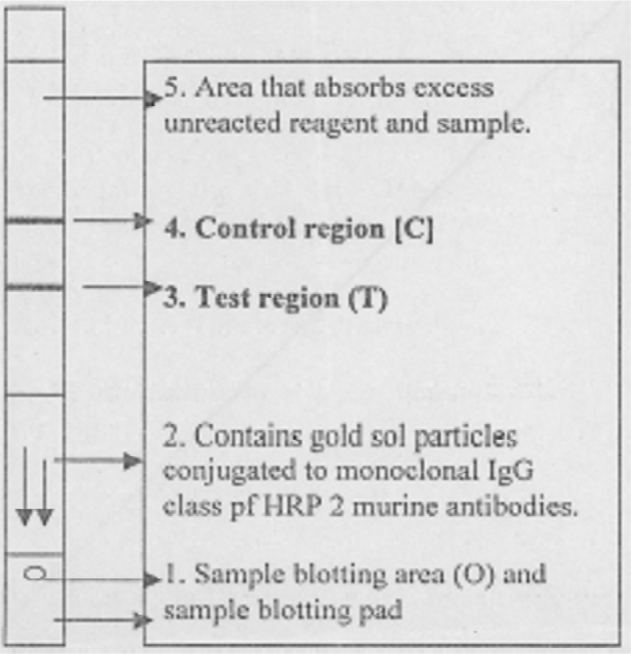
Schematic representation of the Paracheck Pf dipstick
Fig. 2.
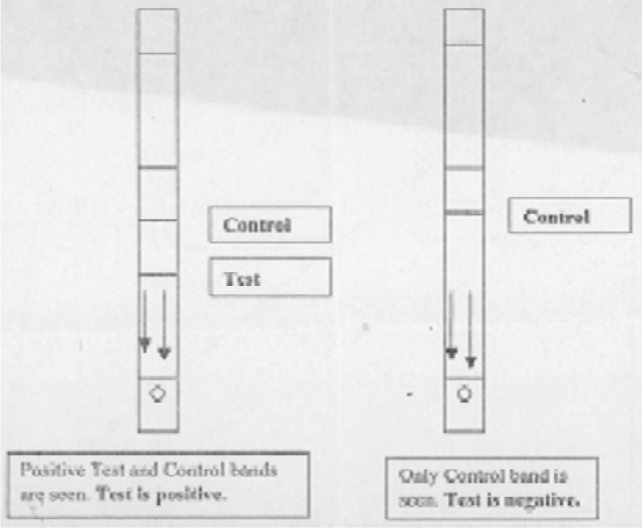
Schematic representation of Paracheck Pf positive and negative test result
Table 1.
Procedure of Paracheck Pf test
| Step No. | Procedure |
|---|---|
| 1. | Bring the Paracheck Pf kit component to room temperature. If the pouch has been stored at 2°-8°C, it may take up to 30 minutes depending on ambient temperature. |
| 2. | Check the colour of the desiccant. It should be blue. If it has turned colourless or faint blue, discard the device (dipstick) and use another device |
| 3. | Open the pouch and remove the device (dipstick) |
| 4. | After gently evenly mixing the anticoagulated blood sample, touch the applicator pipette to the surface of the blood. Blot this small quantity of blood on the sample pad. It delivers approximately 5 μl of whole blood. OR |
| In case of finger prick method, touch the sample applicator pipette to the blood on the finger prick and immediately blot the specimen on the sample pad. Avoid clotting of blood. | |
| 5. | Dispense six drops (300 μl) of clearing buffer into the clean glass tube |
| 6. | Place the dipstick arrows pointing down in the test tube to immerse the sample pad in the clearing buffer. Wait for 15 miunutes |
| 7. | Read the control line and the test line and interpret the result by comparing with Fig. 2 |
The laboratory assistant initially performed the Paracheck Pf tests. Subsequently nursing assistants were trained to use the test kit. The training of nursing assistants comprised demonstration and hands-on practice sessions lasting 30 to 45 minutes. Whenever possible, the test was performed at the bedside while obtaining blood for the smear. In some cases the specimens of blood were collected in anticoagulant mixture and stored at 4°C pending test when delay was anticipated. Some specimens of blood in anticoagulant were preserved at 4°C up to 4 weeks. In a small number of cases, Paracheck Pf was performed on serum samples also, to determine suitability of the specimen. Some Paracheck Pf kits, instead of 4°C, were stored at room temperature varying from 8°C in winter to 40°C in summer. This was to determine the viability of the kit on storage at fluctuating room temperature. Feedback was obtained from all workers to determine their perception about the case of performance and interpretation of the test.
Results
A total of 350 blood samples from cases of fever were studied. Out of this, 30 were negative controls while 320 were from suspected cases of malaria (P falciparum 220 and P vivax 100). P vivax (Pv) was detected in 75 smears while 105 smears had Pf parasites. Five smears had double infection (Pv and Pf). The smear positivity and Paracheck Pf positivity/negativity is compared in Table 2. The observations in performing smear examination and Paracheck Pf test are as per Table 3.
Table 2.
Comparison of smear and Paracheck Pf
| Clinical diagnosis & No | Smear Report | Paracheck Pf | ||
|---|---|---|---|---|
| Pf (%) | Pv (%) | Pos (%) | Neg (%) | |
| Control 30 | nil | nil | nil | 30 (100) |
| P falciparum 220 | 100 (45.45) | 05* (2.5) | 200 (90.90) | 20** (9.01) |
| P vivax 100 | 05* (5) | 70 (70) | 05* (5) | 95 (95) |
| Total 350 | 105 | 75 | 205 | 145 |
Mixed infection of P falciparum and P vivax
Smears with only gametocytes
Table 3.
Comparison between microscopy and Paracheck Pf for diagnosis of P falciparum infection
| Remark | Microscopy | Paracheck Pf |
|---|---|---|
| Slide and spreader | Required | Not required |
| Stain | Required | Not required |
| Microscope | Required | Not required |
| Test tube | Not required | Required |
| Trained manpower | Required | Not required |
| Interpretation | Subjective | Objective |
| Time | 60 min | 15-20 min |
| Optimum time for specimen collection | While febrile | Any time |
| Quality of smear | Good | Not required |
| Staining of artifacts | Interfere | Nil |
| No of fields examined | Minimum100 | Single reading |
| Observer error | Possible | Not likely |
| Positive control | Nil | Available for comparison |
| Ambiguous result | Frequent | Nil |
| Sensitivity for trophozoite and merozoite stages | Low | Near 100% |
| Sensitivity for trophozoite and merozoite stages | Good | Near 100% |
| Parasite type and stage detected | All types, sexual and asexual | Only asexual phase of P falciparum |
Paracheck Pf tests gave equally good results with specimen of whole blood directly applied on the strip as well as with citrate-oxalate anticoagulant mixture. Anticoagulated blood stored at 4°C up to 4 weeks also continued to give satisfactory results. However, haemolysed blood and the serum samples were not found to be suitable for Paracheck Pf tests. Storage of Paracheck Pf test kits at room temperature (8°C to 40°C) up to a period of six months did not adversely affect their performance.
Discussion
Mosquito bite and subsequent acquisition of plasmodium infection is an accepted occupational hazard for soldiers. Malaria thus plays an important role in the health status of troops. Pf infection is most feared due to its severe complications, high morbidity and mortality. Certain parts of India are endemic and hyperendemic zones of Pf infection [2]. The remoteness of these areas and lack of diagnostic facilities precludes prompt treatment. More often than not, the clinician, usually a young RMO or even a nursing assistant has to depend on his limited clinical skill to diagnose and treat these cases. Empirical diagnosis is always fraught with the danger of under/over treatment [3]. This sometimes leads to loss of precious life, while it mostly increases the cost of medical cover.
The mainstay of diagnosis of malaria has been demonstration of the parasite in blood/bone marrow smears [1]. Subjectivity, need for considerable expertise for interpretation and delays associated with it are unavoidable. This historically important method, though not very sensitive and reproducible, has been accorded the status of “gold standard” [4]. Various attempts have been made to improve the sensitivity and specificity of smear examination. All these and some other developments in diagnosis of malaria require sophisticated, high cost gadgets, modern infrastructure and specially trained manpower [5, 6, 7, 8, 9, 10, 11, 12, 13]. Due to this rider, these developments have mostly remained confined to research laboratories or to a few diagnostic centres. The majority of malaria cases occur at places where these technological developments have not reached. Reliable diagnosis of malaria at remote locations still remains a dream. Introduction of rapid and relatively faster diagnostic methods like ICT is perhaps the light at the end of this tunnel. Unfortunately, their potential is still underexploited.
Detection of malaria parasite in a blood smear depends on three important variables; (a) parasite density, (b) quality of smear and its staining and (c) proficiency of the smear examiner. The degree of parasitemia varies with phases in the life cycle of the parasite as well as the vector. Many a times the parasites remain sequestered and are not present in peripheral blood [4]. Best results are achieved when the time of specimen collection is in tandem with peripheral parasitemia. If not, the diagnosis eludes despite repeated smear examinations.
The time and effort required to detect malaria parasite in a smear is inversely proportional to the quality of smears, its staining and the level of parasitemia. The quality of smear is highly variable depending on the availability of good quality slides, spreaders, quantity of blood taken on each slide and of course the expertise of the paramedical staff. Artifacts may add to the tribulations of the examiner. Preparation of suitable smear thus, is of paramount importance. In our study, many a smear was of extremely poor quality and we were forced to obtain fresh specimen. Variations in the quality of smears are too wide and despite our best efforts at training, could not be avoided. Technical expertise in detection of malaria parasite is acquired gradually with experience and diligence. Besides technical difficulties, examining a bad smear leads to frustration and lowered efficiency of the laboratory technician. Diagnosis of malaria by smear examination is considered to be cost effective [4]. However, if the cost of trained manpower, establishment of infrastructure, delayed diagnosis/missed diagnosis leading to avoidable morbidity and mortality is taken into account then this method does not remain cost effective or cheap at all.
In our study, two trained and proficient microscopists examined all the smears independently on different occasions to avoid bias. Detection of gametocytes of Pf was easy but ring stages required longer time and more diligent search. In few cases, differentiating Pf from Pv rings was more difficult. In cases with mixed infection (Pv with Pf), detection of Pv trophozoites leads to complacency and missing Pf ring stages. The time at which these smears were examined too affected the detection. The chance of missing parasites in the smears seen at the end of a tiring day or at the middle of night by emergency duty laboratory assistant was much higher than when examined during early working hours. This highlights the tremendous variations attributable to subjective observer factors in detection of malaria parasite.
Selection of ICT kit : ICT for diagnosis of Pf infection is based on the principle of detection of HRP-2 in the blood [14, 15]. This protein molecule is specific for Pf species and is released in largest amount during rupture of schizont. Therefore, it is considered to be pathognomonic of trophozoite and merozoite stages of Pf infection and clinical malaria. A number of ICT kits are available in the market for detection of HRP-2 antigen. ICT Malaria (Shweta Chemical), ParaSight (Diagnostics India), Paracheck Pf (Orchid Biomedical Systems), Rapid-MP (Biolab Diagnostics), OptiMAL (Morepen) and Quorum (Quorum Diagnostics) are some prominent ones. We undertook limited trial of some of these kits before launching this study. Paracheck Pf (Orchid Biomedical Systems) was found to have advantage of simplicity and was thus selected for this study.
A total of 350 blood specimens from cases of fever were studied. This included Pv and Pf infection as well as control cases of fever due to causes other than malaria (Table 2). Antigen detection test for Pf by Paracheck Pf dipstick method was found to be highly sensitive and specific. It was able to detect parasitemia of very low level (100 parasites/microliter of blood) also very easily. This aspect has been proved by other studies [16, 17] and our findings corroborated it. In cases with high clinical suspicion of Pf infection, this test was positive earlier than the detection of parasites in the peripheral smear. The test remained positive as long as the patient had detectable trophozoite and merozoite stages of parasitemia and for some time there after. This aspect is very important as it demonstrates higher sensitivity of Paracheck Pf. Other workers too have encountered similar situations [17]. ICT is likely to be false positive in patients with rheumatoid factor [18]. Our study did not encounter any case of rheumatoid arthritis.
The Paracheck Pf test was strongly positive and correlated well with the trophozoite and merozoite stages and degree of parasitemia. In blood specimen from the patients showing only gametocytes in smear examination, the test was either weakly positive or negative. The weak positivity perhaps was due to persistent low antigenemia. Paracheck Pf cannot be used for diagnosis of gametocytic stage of parasitemia. This limitation of the test must be appreciated as the patient is in infective stage. Detection of gametocytes by smear examination is relatively easy due to their larger size and peculiar morphology. There was no false positivity with patients suffering from Pv infection or from other causes of fever. The Paracheck Pf test could detect antigen from the oxalated blood stored for over 4 weeks at 4°C also. The test could not detect antigen properly from haemolysed blood or from serum samples. The only precaution while using this kit is that the test strip must be kept vertical during the test time of 15 minutes.
Feed back from laboratory technicians and nursing assistants on performance of Paracheck Pf was analyzed. Paracheck Pf test was found to be easy to master and perform even by the nursing assistant. Simplicity and rapidity of the test were highly appreciated. The Paracheck Pf kit is self sufficient except for one small test tube. The additional item is required to hold four drops of clearing buffer solution. Any clean glass test tube or even an empty clean vial can be used. This is in contrast with diagnosis by smear examination that requires slides and spreader, special stain, specialized equipment like microscope and trained manpower. The time required for Paracheck Pf was 15 minutes. However, the actual time spent by the worker is only 2 minutes while remaining waiting time is required for the reaction and development of colour. The smear examination on the other hand required upto 60 minutes of technician's time (30 minutes for staining and 15-30 minutes for microscopy). Thus Paracheck Pf test is economical in terms of time as well.
Interpretation of Paracheck Pf is easy as it is visual macroscopic comparison of test band with positive control band (Fig 2). This does not require any expertise. Smear examination can on the other hand require expertise and is subjective to a great extent, leading to misdiagnosis at times. Storage of Paracheck Pf kit at room temperature (8°C to 40°C) upto 6 months did not adversely affect its performance. Thus the procurement, storage and transportation of this kit to the farthest outpost poses no special problem.
Rapid diagnosis of Pf infection at the peripheral medical setup is the need of the hour. Conventional slide examination is not possible at these places. Our study has confirmed that Paracheck Pf is superior to conventional microscopy for diagnosis of Pf infection. Based on this study, following conclusions are drawn :
-
(a)
The RMO or even a nursing assistant in a peripheral Medical Inspection room / Regimental Aid Post can perform Paracheck Pf test easily.
-
(b)
The test kit is self sufficient except for a small test tube, which is available at all MI rooms.
-
(c)
The Paracheck Pf kit is stable at temperature 8°C to 40°C upto 6 months, hence its storage and transportation does not need cold chain.
-
(d)
Specimen of blood obtained by prick method or mixed with oxalate-citrate anticoagulant can be used for Paracheck Pf test.
-
(e)
The test is highly sensitive and specific for diagnosis of Pf infection.
-
(f)
Paracheck Pf is a better alternative to conventional microscopy for early diagnosis of Pf infection.
It is recommended that Paracheck Pf be introduced and made available at all health care set-ups where early diagnosis of Pf infection is desired. Its importance at places bereft of laboratory facilities is re-emphasized. This will facilitate prompt and scientific treatment of Pf infection. The expense on provision of this facility will be offset by the reduction in morbidity and mortality due to Pf infection.
Acknowledgement
This study was undertaken as AFMRC Project No 2289/99. I thank the Armed Forces Medical Research Committee for the financial support. I am grateful to the patients, the doctors, nursing and laboratory staff, Commandants and Commanding Officers of the units that participated in this study. I sincerely hope that the findings of this study will pave the way for reducing the morbidity and mortality due to malaria.
References
- 1.Warhurst DC, Williams JE. Laboratory diagnosis of malaria. J Clin Pathol. 1996;49:533–538. doi: 10.1136/jcp.49.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park K. Park’s Text Book of Preventive and Social Medicine. 15th ed. Banarasidas Bhanot; Jabalpur (India): 1997. pp. 188–202. [Google Scholar]
- 3.WHO A rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. Bull of WHO. 1996;74:47–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Chander Y, Nagendra A, Subramanya H. Malaria — Diagnostics Today (Editorial) MJAFI. 2001;57:185–187. doi: 10.1016/S0377-1237(01)80038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nlabandin RM, Sammons DW, Manley M. A molecular based magnet test for malaria. Am J Clin Pathol. 1995;103:57–64. doi: 10.1093/ajcp/103.1.57. [DOI] [PubMed] [Google Scholar]
- 6.Benito A, Roche J, Molina R, Amela C, Alvar J. Application and evaluation of QBC malaria diagnosis in a holoendemic area. Applied Parasitol. 1994;35:266–272. [PubMed] [Google Scholar]
- 7.Gay F, Traore B, Zanoni J, Danis M, Fribourg-Blanc A. Direct acridine orange fluorescence examination of blood slides compared to current techniques of malaria diagnosis. Trans R Soc Trop Med Hyg. 1996;90:516–518. doi: 10.1016/s0035-9203(96)90300-4. [DOI] [PubMed] [Google Scholar]
- 8.Kawamoto F. Rapid diagnosis of malaria by fluorescence microscopy with light microscopy and interference filters. Lancet. 1991;i:202–212. doi: 10.1016/0140-6736(91)92159-y. [DOI] [PubMed] [Google Scholar]
- 9.Lowe BS, Jeffia NF, New L, Pedersen C, Engback K, Marsh K. Acridine orange fluorescence technique as alternative to traditional Giemsa staining for the diagnosis of malaria in developing countries. Trans R Soc Trop Med Hyg. 1996;90:34–36. doi: 10.1016/s0035-9203(96)90470-8. [DOI] [PubMed] [Google Scholar]
- 10.Makler MT, Ries LK, Horton RJ, Hinrichs DJ. Detection of Plasmodium falciparum infection with the fluorescent dye benzthiocarboxypurine. Am J Trop Med Hyg. 1991;44:11–16. doi: 10.4269/ajtmh.1991.44.11. [DOI] [PubMed] [Google Scholar]
- 11.Cooke AH, Moody AH, Lemon K, Chiodini PN, Horton J. Use of fluorochrome benzthiocarboxypurine in malaria diagnosis. Trans R Soc Trop Med Hyg. 1992;86:378. doi: 10.1016/0035-9203(92)90228-5. [DOI] [PubMed] [Google Scholar]
- 12.Barker RH., Jr DNA probe diagnosis of parasitic infections. Exp Parasitol. 1990;70:494–499. doi: 10.1016/0014-4894(90)90136-z. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto F, Miyake K, Kaneko O. Sequence variation in the 18S rRNA gene, a target for PCR based malaria diagnosis, in Plasmodium ovale from Southern Vietnam. J Clin Microbiol. 1996;34:2287–2289. doi: 10.1128/jcm.34.9.2287-2289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowling MAC, Shute GT. A comparative study of thick and thin blood films in the diagnosis of scanty malaria parasitemia. Bulletin of World Health Organisation. 1966;34:249–267. [PMC free article] [PubMed] [Google Scholar]
- 15.Howard RJ, Uni S, Aikawa M. Secretion of malarial histidine rich protein (Pf HRP-2) from Plasmodium falciparum infected erythrocytes. J Cell Biol. 1986;103:1269–1277. doi: 10.1083/jcb.103.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rock EP, Marsh K, Saul SJ. Comparative analysis of the Plasmodium falciparum histidine rich proteins HRP-1, HRP-@, HRP # in malaria diagnosis of diverse origin. Parasitol. 1987;87:283–289. doi: 10.1017/s0031182000057681. [DOI] [PubMed] [Google Scholar]
- 17.Parra ME, Evans CB, Taylor DW. Identification of Plasmodium falciparum histidine rich protein 2 in the plasma of humans with malaria. J Clin Microbiol. 1991;29:1629–1634. doi: 10.1128/jcm.29.8.1629-1634.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra B, Samantaray JC, Nirdha BR. Evaluation of rapid antigen capture assay for the diagnosis of falciparum malaria. Ind J Med Res. 1999;109:16–19. [PubMed] [Google Scholar]
Uncited References
- 19.Gupta MK, Misra RN, Chawla N. Immunochromatographic test: A new dimension in diagnosis of Plasmodium falciparum malaria. MJAFI. 2001;57:188–190. doi: 10.1016/S0377-1237(01)80039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto MJW, Pereira NF, Rodrigues S, Kharangate NV, Verenkar MP. Rapid diagnosis of falciparum malaria by detection of Plasmodium falciparum HRP 2 antigen. JAPI. 1999;47:1076–1078. [PubMed] [Google Scholar]
- 21.Grobusch MP, Alperman U, Schwenke S, Jelinek T, Warhurst DC. False positive rapid tests for malaria in patients with rheumatoid factor. Lancet. 1999;353:2997–2998. doi: 10.1016/s0140-6736(05)74930-8. [DOI] [PubMed] [Google Scholar]


