Abstract
There has been no previous prospective study evaluating dual antiplatelet therapy (DAPT) duration shorter than 6 months after cobalt-chromium everolimus-eluting stent (CoCr-EES) implantation. STOPDAPT trial is a prospective multi-center single-arm study evaluating 3-month DAPT duration after CoCr-EES implantation. The primary endpoint was a composite of cardiovascular death, myocardial infarction (MI), stroke, definite stent thrombosis (ST) and TIMI major/minor bleeding at 1 year. Between September 2012 and October 2013, a total of 1525 patients were enrolled from 58 Japanese centers, with complete 1-year follow-up in 1519 patients (99.6 %). Thienopyridine was discontinued within 4 months in 1444 patients (94.7 %). The event rates beyond 3 months were very low (cardiovascular death: 0.5 %, MI: 0.1 %, ST: 0 %, stroke: 0.7 %, and TIMI major/minor bleeding: 0.8 %). Cumulative 1-year incidence of the primary endpoint was 2.8 % [upper 97.5 % confidence interval (CI) 3.6 %], which was lower than the pre-defined performance goal of 6.6 % (P < 0.0001). Using the CoCr-EES group in the RESET trial as a historical comparison group, where nearly 90 % of patients had continued DAPT at 1 year, cumulative incidence of the primary endpoint tended to be lower in the STOPDAPT than in the RESET (2.8 versus 4.0 %, P = 0.06) and adjusted hazard ratio was 0.64 (95 % CI 0.42–0.95, P = 0.03). The cumulative incidence of definite/probable ST was lower in the STOPDAPT than in the RESET [0 patient (0 %) versus 5 patients (0.3 %), P = 0.03]. In conclusion, stopping DAPT at 3 months in selected patients after CoCr-EES implantation was at least as safe as the prolonged DAPT regimen adopted in the historical control group.
Electronic supplementary material
The online version of this article (doi:10.1007/s12928-015-0366-9) contains supplementary material, which is available to authorized users.
Keywords: Dual antiplatelet therapy, Everolimus-eluting stent, Coronary artery disease, Coronary stent
Introduction
Several previous randomized controlled trials comparing short (3–6 months) dual antiplatelet therapy (DAPT) with pro1onged (12 months or longer) DAPT after coronary stent implantation demonstrated similar ischemic event risk and lower bleeding event risk with shorter course of DAPT [1–5]. Therefore, the current ESC/EACTS guideline recommend 6-month DAPT after new generation coronary drug-eluting stent (DES) implantation in patients with stable coronary artery disease [6]. Two previous trials (RESET and OPTIMIZE) suggested the safety and efficacy of 3-month DAPT after implantation of one of the first generation (G1) DES, Endeavor™ zotarolimus-eluting stent (E-ZES), which was associated with relatively large late lumen loss (neointimal hyperplasia) similar to bare-metal stents (BMS) [3, 4]. Second-generation drug-eluting stent (G2-DES) with small late lumen loss, cobalt-chromium everolimus-eluting stent (CoCr-EES) in particular, has been reported to have lower risk for stent thrombosis (ST) compared with G1-DES or BMS [7]. Therefore, the optimal DAPT duration after G2-DES implantation could be shorter than 6–12 months currently recommended in the guidelines [6, 8]. However, there has been no previous prospective study evaluating DAPT duration shorter than 6 months after G2-DES implantation.
In the current study, we sought to evaluate the safety of 3-month DAPT duration after CoCr-EES implantation in a prospective multicenter single-arm trial.
Methods
Study population
ShortT and OPtimal duration of Dual AntiPlatelet Therapy after everolimus-eluting cobalt-chromium stent (STOPDAPT) trial is a prospective multi-center single-arm trial enrolling patients who agreed to follow the 3-month DAPT protocol (discontinuation of clopidogrel at 2–4 months and aspirin monotherapy thereafter) after successful CoCr-EES implantation. Patients who underwent successful percutaneous coronary intervention (PCI) using CoCr-EES were to be enrolled, if the physicians in charge judged 3-month DAPT duration to be appropriate for the patient. Patients who had previous history of PCI using DES other than CoCr-EES were excluded. The study sponsor (Abbott vascular) was involved in the discussion on the study design, and gave final approval for submission of the manuscript. However, patient enrollment, data collection, statistical analysis, and manuscript preparation were conducted independent of the study sponsor. The relevant review boards or ethics committees in all participating centers approved the research protocol. The trial was registered with ClinicalTrials.gov number, NCT01303640.
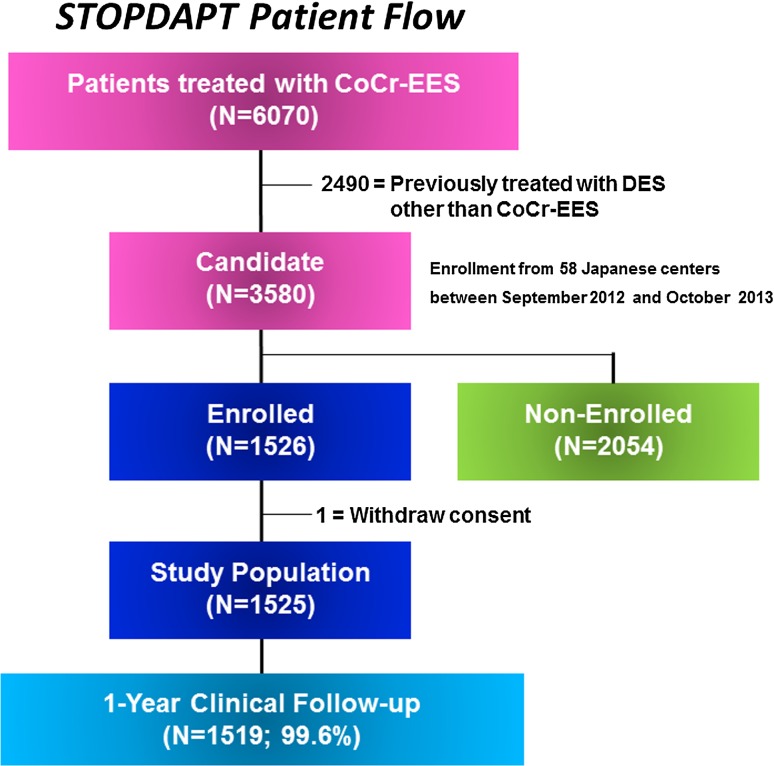
Between September 2012 and October 2013, 6070 patients underwent PCI using CoCr-EES in 58 Japanese centers (Supplemental Appendix A). We excluded 2490 patients who were previously treated with DES other than CoCr-EES. Among 3580 eligible patients, 1526 patients (43 %) were enrolled in this study. Excluding 1 patient who withdrew consent for study participation, 1525 patients constituted the current study population (Fig. 1). Among 2054 patients who were not enrolled in this study, 62 % of patients were judged by the attending physicians not suitable for the study and 14 % of patients refused study participation (Table 1).
Fig. 1.
Study flow chart. CoCr-EES, Cobalt-chromium everolimus-eluting stent; DES, drug-eluting stent
Table 1.
Baseline characteristics: enrolled versus non-enrolled patients
| Enrolled (N = 1525) | Non-enrolled (N = 2054) | P value | |
|---|---|---|---|
| Age (years) | 70.0 ± 10.6 | 70.0 ± 11.0 | 0.97 |
| Age ≥75 years | 570 (37 %) | 776 (38 %) | 0.81 |
| Male gender | 1117 (73 %) | 1553 (76 %) | 0.11 |
| Body mass index | 24.1 ± 3.6 | 23.9 ± 3.6 (2010) | 0.04 |
| Coexisting condition | |||
| Hypertension | 1260 (83 %) | 1574 (77 %) | <0.0001 |
| Diabetes mellitus | 604 (40 %) | 824 (40 %) | 0.76 |
| Insulin-treated diabetes | 119 (7.8 %) | 176 (8.6 %) | 0.41 |
| Treated with oral medication only | 360 (24 %) | 482 (23 %) | 0.92 |
| Treated with diet therapy only | 125 (8.2 %) | 166 (8.1 %) | 0.9 |
| ESRD (eGFR < 30 mL/min/1.73 m2) not on hemodialysis | 35/1521 (2.3 %) | 93/2054 (4.5 %) | 0.0003 |
| Hemodialysis | 56 (3.7 %) | 141 (6.9 %) | <0.0001 |
| Cardiac risk factor | |||
| Current smoker | 315 (21 %) | 430 (21 %) | 0.84 |
| Prior Stroke | 168 (11 %) | 243 (12 %) | 0.45 |
| Heart failure | 101 (6.6 %) | 191 (9.3 %) | 0.004 |
| Peripheral vascular disease | 142 (9.3 %) | 177 (8.6 %) | 0.47 |
| Clinical characteristics | |||
| Clinical presentation | |||
| Stable coronary artery disease | 1040 (68 %) | 1277 (62 %) | 0.0002 |
| Unstable angina | 229 (15 %) | 299 (15 %) | 0.7 |
| Acute myocardial infarction | 256 (17 %) | 478 (23 %) | <0.0001 |
| Target-vessel location | |||
| Left main coronary artery | 17 (1.1 %) | 160 (7.8 %) | <0.0001 |
| Left anterior descending coronary artery | 866 (57 %) | 1108 (54 %) | 0.09 |
| Left circumflex coronary artery | 361 (24 %) | 460 (22 %) | 0.37 |
| Right coronary artery | 405 (27 %) | 614 (30 %) | 0.03 |
| Bypass graft | 4 (0.3 %) | 17 (0.8 %) | 0.02 |
| Complexity of coronary artery disease | |||
| Number of treated lesions per patient | 1.21 ± 0.48 | 1.43 ± 0.74 | <0.0001 |
| Multi-vessel treatment | 130 (8.5 %) | 315 (15 %) | <0.0001 |
| Reasons for non-enrollment | |||
| Physicians’ judgment not to be suitable for the study | NA | 1276 (62 %) | |
| Patients’ refusal for study participation | NA | 292 (14 %) | |
| Others | NA | 486 (24 %) | |
Values are expressed as mean ± SD or number (%)
ESRD end stage renal disease, eGFR estimated glomerular filtration rate
As a historical control group, we selected the CoCr-EES group in the RESET (Randomized Evaluation of Sirolimus-eluting versus Everolimus-eluting stent Trial) trial (a randomized controlled trial comparing CoCr-EES with sirolimus-eluting stent conducted by the same study group in 2010), where nearly 90 % of patients had continued DAPT at 1 year [9]. The eligibility criteria of the RESET were comparable to that of the STOPDAPT except for the inclusion of patients with previous DES implantation. Among 1597 patients in the CoCr-EES group in the RESET, 38 patients with in-hospital primary endpoint events were excluded from the historical control group in this study, because patients in the STOPDAPT were enrolled after completion of successful PCI. A total of 1559 patients were selected as a historical control group.
Procedures
Antiplatelet regimen included aspirin (≥81 mg daily) indefinitely and thienopyridine (75 mg clopidogrel daily) for 3 months after stent implantation. Ticlopidine 200 mg/day was only allowed for those who did not tolerate clopidogrel. Patients were instructed to discontinue thienopyridine at 3-month hospital visit. Acceptable time window for the discontinuation of thienopyridine therapy was within ±1 month. Status of antiplatelet therapy was evaluated throughout the follow-up period as previously described [10]. Persistent discontinuation of thienopyridine was defined as withdrawal lasting for at least 2 months [10].
Endpoints and definitions
The primary endpoint in this trial was a composite of cardiovascular death, myocardial infarction (MI), stroke, definite ST and Thrombolysis in Myocardial Infarction (TIMI) major/minor bleeding at 1 year. Primary endpoint events were adjudicated by the independent clinical event committee (Supplemental Appendix B). Major secondary endpoints were TIMI major/minor bleeding and a composite of cardiovascular death, MI, stroke or definite ST at 1 year. Secondary endpoints included death, MI, stroke, possible/probable/definite ST, TIMI/Global Utilization of Streptokinase and Tissue plasminogen activator for Occluded coronary arteries (GUSTO) bleeding, target-lesion revascularization (TLR), target-vessel revascularization (TVR), coronary artery bypass grafting, and any coronary revascularization.
Death was regarded as cardiac in origin unless obvious non-cardiac causes could be identified. MI and ST were defined according to the Academic Research Consortium definitions [11]. Stroke during follow-up was defined as ischemic or hemorrhagic stroke requiring hospitalization with symptoms lasting >24 h. Bleeding was defined according to the TIMI [12] and GUSTO classifications [13]. TLR was defined as either PCI or coronary artery bypass grafting due to restenosis or thrombosis of the target lesion that included the proximal and distal edge segments as well as the ostium of the side branches.
Data collection and follow-up
Demographic, angiographic, and procedural data were collected from hospital charts or databases in each participating center according to the pre-specified definitions by experienced clinical research coordinators in the participating centers (Supplemental Appendix B) or in the study management center (Supplemental Appendix B). Follow-up data on the clinical events were collected from the hospital charts in the participating centers (74 %), letters to patients (20 %), and telephone call to referring physicians (8.4 %).
Angiographic analysis
For the STOPDAPT, qualitative and quantitative coronary angiography was evaluated at the same angiographic core laboratory as in the RESET (Cardiocore, Tokyo, Japan) with use of CAAS 5.9 (Pie Medical Imaging, Maastricht, The Netherlands). Baseline angiograms in the STOPDAPT were assessed in 350 patients randomly selected at the time of enrollment. The target segment was defined as the entire segment involving the implanted stent and the 5-mm proximal and distal edges adjacent to the stent. A segment to be treated with multiple overlapping stents was regarded as a single target segment. In addition to the standard angiographic parameters, SYNTAX (Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery) score was also evaluated [14].
Statistical analysis
The event rate for the primary endpoint in this single-arm trial was compared against a pre-specified performance goal using an exact test through the binominal distribution. To determine the sample size in this study, we used the data from the 1559 patients in the CoCr-EES group in the RESET trial [9]. Actual event rate of the CoCr-EES group in the RESET trial was 4 % and its upper one-sided 80 % confidence limit was 4.4 %. We assumed the true rate 4.4 % and we set the performance goal to be 6.6 % by adding delta of 2.2 % (50 % of 4.4 %) to 4.4 % of true rate. A total of 1455 patients would yield 95 % power at a level of one-sided type 1 error of 0.025 to achieve 6.6 % of performance goal. We finally rounded up to 1500 patients to take into account for dropouts.
Categorical variables were presented as number and percentage and continuous variables were expressed as mean value ± SD or median with inter-quartile range. We used the exact binomial test to compare the incidence of primary endpoint to the performance goal of 6.6 % using one-sided alpha of 0.025. Then, we compared the STOPDAPT group with the RESET group using the Chi-square test or Fisher’s exact test for categorical variables, and Student’s t test or Wilcoxon rank sum test based on their distributions for continuous variables. Cumulative incidence was estimated by the Kaplan–Meier method and differences were assessed with the log-rank test. To evaluate the events beyond 3 months, we also conducted the landmark analyses at 3 months. Those patients who had the individual endpoint events before 3 months were excluded in the landmark analyses. Due to the presence of differences in baseline characteristics between the 2 studies, we also used Cox proportional hazard models to estimate the risk of the STOPDAPT relative to the RESET for the primary endpoint. In the multivariable analysis, we chose 10 clinically relevant factors indicated in Table 1 as the risk adjusting variables. The continuous variables were dichotomized by clinically meaningful reference values or median values. The study (STOPDAPT or RESET) and the 10 risk adjusting variables were simultaneously included in the Cox proportional hazard model. The effect of the STOPDAPT compared to the RESET was expressed as hazard ratios (HR) and their 95 % confidence intervals (CI). In the pre-specified sub-group analysis, we also conducted the formal interaction test between the study and subgroup factors.
Statistical analyses were conducted by a physician (Natsuaki M) and by a statistician (Morimoto T) with the use of JMP 10.0 and SAS 9.4 (SAS Institute Inc, Cary, NC, USA) software. We used one-sided P values <0.025 as statistically significant level in the evaluation of performance goal, and two-sided P values <0.05 as statistically significant for other comparisons.
Results
Baseline Characteristics: Enrolled versus Non-enrolled Patients in the STOPDAPT
Baseline characteristics were significantly different in several aspects between the enrolled and non-enrolled patients (Table 1). Chronic kidney disease, hemodialysis, heart failure, and acute myocardial infarction (AMI) presentation were more prevalent in the non-enrolled group, while higher body mass index (BMI) and hypertension were more often found in the enrolled group. Patients with treatment of left main coronary artery were less often enrolled in the study. Regarding the complexity of coronary artery disease, the number of treated lesions was greater and multi-vessel treatment was more often performed in the non-enrolled group than in the enrolled group (Table 1).
Baseline characteristics: STOPDAPT versus RESET
Baseline characteristics were also significantly different in several aspects between the STOPDAPT and RESET (Table 2). Patients in the STOPDAPT were significantly older than those in the RESET. Female gender, hypertension, dyslipidemia, atrial fibrillation, anemia, and AMI presentation were more often found in the STOPDAPT than in the RESET, while diabetes, hemodialysis, family history of coronary artery disease, prior MI, heart failure, prior PCI, and multi-vessel disease were more prevalent in the RESET than in the STOPDAPT. Patients with treatment of left main coronary artery and chronic total occlusion were less often enrolled in the STOPDAPT than in the RESET. Total stent length per patient was significantly longer in the STOPDAPT, while multi-vessel treatment was more often performed in the RESET. Regarding the medications at hospital discharge, β-blockers and anticoagulants were more often prescribed in the STOPDAPT than in the RESET (Table 2).
Table 2.
Baseline Characteristics: STOPDAPT versus RESET
| STOPDAPT (N = 1525) | RESET (N = 1559) | P value | |
|---|---|---|---|
| Age (years) | 70.0 ± 10.6 | 68.9 ± 9.7 | 0.002 |
| Age ≥75 yearsa | 570 (37 %) | 480 (31 %) | 0.0001 |
| Male gendera | 1117 (73 %) | 1213 (78 %) | 0.003 |
| Body mass index | 24.1 ± 3.6 | 24.3 ± 3.6 (1542) | 0.25 |
| Coexisting condition | |||
| Hypertensiona | 1261 (83 %) | 1238 (79 %) | 0.02 |
| Diabetes mellitusa | 604 (40 %) | 707 (45 %) | 0.001 |
| Insulin-treated diabetes | 119 (7.8 %) | 171 (11 %) | 0.003 |
| Treated with oral medication only | 360 (24 %) | 343 (22 %) | 0.29 |
| Treated with diet therapy only | 125 (8.2 %) | 193 (12 %) | 0.0001 |
| Dyslipidemia | 1209 (79 %) | 1164 (75 %) | 0.002 |
| ESRD (eGFR < 30 mL/min/1.73 m2) not on hemodialysis | 35/1521 (2.3 %) | 31/1552 (2.0 %) | 0.56 |
| Hemodialysisa | 56 (3.7 %) | 90 (5.8 %) | 0.006 |
| Atrial fibrillation | 172 (11 %) | 104 (6.7 %) | <0.0001 |
| Anemia (hemoglobin <11.0 g/dL)a | 241 (16 %) | 190 (12 %) | 0.004 |
| Cardiac risk factor | |||
| Current smoker | 315 (21 %) | 329 (21 %) | 0.76 |
| Family history of coronary artery disease | 192 (13 %) | 248/1343 (18 %) | <0.0001 |
| Prior myocardial infarction | 267 (18 %) | 459 (29 %) | <0.0001 |
| Prior strokea | 168 (11 %) | 176 (11 %) | 0.81 |
| Heart failure | 101 (6.6 %) | 138 (8.9 %) | 0.02 |
| Peripheral vascular disease | 142 (9.3 %) | 140 (9.0 %) | 0.75 |
| Prior percutaneous coronary intervention | 468 (31 %) | 741 (48 %) | <0.0001 |
| Prior coronary artery bypass grafting | 41 (2.7 %) | 61 (3.9 %) | 0.06 |
| Clinical characteristics | |||
| Clinical presentation | |||
| Stable coronary artery disease | 1040 (68 %) | 1282 (82 %) | <0.0001 |
| Unstable angina | 229 (15 %) | 175 (11 %) | 0.002 |
| Acute myocardial infarctiona | 256 (17 %) | 102 (6.5 %) | <0.0001 |
| Left ventricular ejection fraction <30 % | 17/1315 (1.3 %) | 24/1345 (1.8 %) | 0.3 |
| Multi-vessel disease | 578 (38 %) | 759 (49 %) | <0.0001 |
| Target-vessel location | |||
| Left main coronary arterya | 17 (1.1 %) | 46 (3.0 %) | 0.0002 |
| Left anterior descending coronary artery | 866 (57 %) | 762 (49 %) | <0.0001 |
| Left circumflex coronary artery | 361 (24 %) | 393 (25 %) | 0.32 |
| Right coronary artery | 405 (27 %) | 511 (33 %) | 0.0002 |
| Bypass graft | 4 (0.3 %) | 6 (0.4 %) | 0.55 |
| Complexity of coronary artery disease | |||
| Number of treated lesions per patient | 1.21 ± 0.48 | 1.23 ± 0.51 | 0.16 |
| Medications | |||
| Aspirin | 1524 (99.9 %) | 1553 (99.6 %) | 0.049 |
| Thienopyridines | 1522 (99.8 %) | 1552 (99.6 %) | 0.21 |
| Clopidogrel | 1508 (99.1 %) | 1350 (87 %) | <0.0001 |
| Ticlopidine | 14 (0.9 %) | 200 (13 %) | |
| Statins | 1223 (80 %) | 1207 (77 %) | 0.06 |
| B-blockers | 620 (41 %) | 566 (36 %) | 0.01 |
| ACE-I/ARB | 939 (62 %) | 967 (62 %) | 0.8 |
| Calcium-channel blockers | 675 (44 %) | 670 (43 %) | 0.47 |
| Nitrates | 219 (14 %) | 426 (27 %) | <0.0001 |
| Anticoagulantsa | 168 (11 %) | 125 (8.0 %) | 0.005 |
| Warfarin | 125 (8.2 %) | 125 (8.0 %) | |
| Dabigatran | 34 (2.2 %) | 0 (0 %) | |
| Rivaroxaban | 9 (0.6 %) | 0 (0 %) | |
| Lesion and Procedural characteristics | |||
| Before index procedure | |||
| Chronic total occlusion | 72 (4.7 %) | 109 (7.0 %) | 0.007 |
| Culprit for STEMI | 203 (13 %) | 69 (4.4 %) | <0.0001 |
| Bifurcation | 317 (21 %) | 337 (22 %) | 0.57 |
| After index procedure | |||
| Number of stents used per patient | 1.37 ± 0.65 | 1.5 ± 0.77 (1554) | <0.0001 |
| Total stent length per patient (mm) | 32.9 ± 20.9 | 30.8 ± 18.9 (1554) | 0.004 |
| Multi-vessel treatment | 130 (8.5 %) | 183 (12 %) | <0.0001 |
Values are expressed as mean ± SD or number (%)
ESRD end stage renal disease, eGFR estimated glomerular filtration rate, ACE-I angiotensin converting enzyme inhibitors, ARB angiotensin II receptor blockers, STEMI ST-segment elevation myocardial infarction
aPotential independent variables selected for multivariable analysis
Angiographic characteristics: STOPDAPT versus RESET
In angiographic characteristics, thrombus and bifurcation lesions were more often found in the STOPDAPT, while in-stent restenosis was more prevalent in the RESET. Lesion length was significantly longer and reference vessel diameter was significantly larger in the STOPDAPT than in the RESET. There were small, but significant differences in in-segment minimum lumen diameter, in-segment percent diameter stenosis, and in-segment acute gain between the 2 groups. SYNTAX score was not significantly different between the 2 groups (Table 3).
Table 3.
Baseline angiographic characteristics: STOPDAPT versus RESET
| STOPDAPT (N = 350) | RESET (N = 1744) | P value | |
|---|---|---|---|
| Before index procedure | |||
| Lesion length, mm | 19.7 ± 12.6 (307) | 17.0 ± 11.5 (1643) | 0.0001 |
| Reference vessel diameter, mm | 2.69 ± 0.56 | 2.58 ± 0.63 (1737) | 0.002 |
| Minimum lumen diameter, mm | 0.8 ± 0.44 | 0.82 ± 0.48 | 0.6 |
| Percent diameter stenosis, % | 70.1 ± 15.1 | 69.1 ± 16.4 (1743) | 0.27 |
| Thrombus | 37 (11 %) | 78 (4.5 %) | <0.0001 |
| Chronic total occlusion | 12/349 (3.4 %) | 72/1725 (4.2 %) | 0.52 |
| In-stent restenosis | 13 (3.7 %) | 192 (11 %) | <0.0001 |
| Bifurcation | 176 (50 %) | 681 (39 %) | 0.0001 |
| Moderate or heavy calcification | 74 (21 %) | 346 (20 %) | 0.58 |
| Small vessel (reference vessel diameter ≤2.75 mm) | 189/350 (54 %) | 1114/1737 (64 %) | 0.0004 |
| Long lesion (lesion length >18 mm) | 124/307 (40 %) | 559/1643 (34 %) | 0.03 |
| SYNTAX score | 9 (6–15) (346) | 10 (6–16) (1458) | 0.06 |
| After index procedure | |||
| Number of stents used | |||
| Per lesion | 1.16 ± 0.41 (350) | 1.27 ± 0.57 (1743) | 0.0008 |
| Bifurcation 2-stent approach | 6 (1.7 %) | 18 (1.0 %) | 0.3 |
| Minimum lumen diameter, mm | |||
| In-stent | 2.5 ± 0.46 | 2.46 ± 0.49 (1730) | 0.19 |
| In-segment | 2.15 ± 0.51 | 2.06 ± 0.55 (1730) | 0.006 |
| Percent diameter stenosis, % | |||
| In-stent | 10.2 ± 7.5 | 10.7 ± 8.8 (1729) | 0.26 |
| In-segment | 19.9 ± 10.8 | 22.5 ± 12.0 (1729) | 0.002 |
| Acute gain, mm | |||
| In-stent | 1.7 ± 0.53 | 1.65 ± 0.54 (1730) | 0.1 |
| In-segment | 1.34 ± 0.56 | 1.24 ± 0.58 (1730) | 0.002 |
Values are expressed as mean ± SD, median (interquartile range) or number (%)
SYNTAX score, synergy between percutaneous coronary intervention with taxus and cardiac surgery score
Discontinuation of Thienopyridine
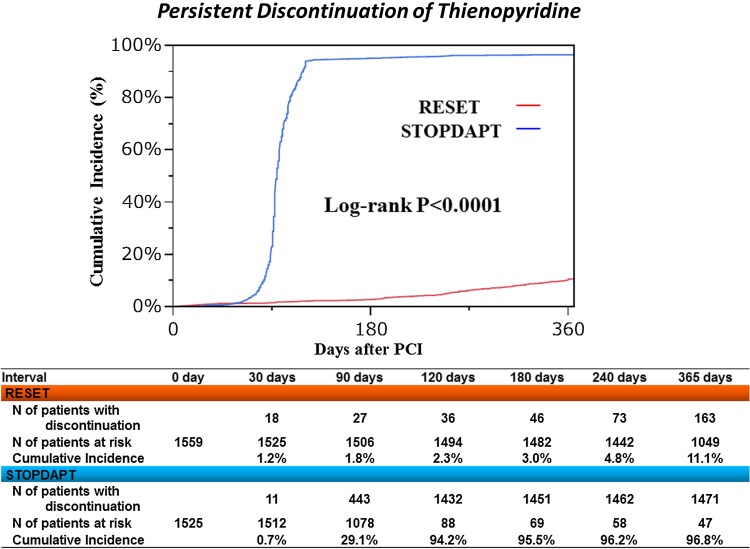
In the STOPDAPT, thienopyridine was discontinued within 4 months in 1444 patients (94.7 %). The reasons for not stopping thienopyridine within 4 months (protocol violation) in 81 patients included the decisions by the attending physician (16 patients), by the patient (8 patients), and by the general practitioner (33 patients), occurrence of events (14 patients; death: 4 patients, stroke: 3 patients, PCI: 6 patients, and peripheral artery disease: 1 patient), aspirin discontinuation (7 patients) and no hospital visit (3 patients). Cumulative 4-month and 1-year incidence of persistent discontinuation of thienopyridine was 94.2 and 96.8 %, respectively, in the STOPDAPT and 2.3 and 11.1 %, respectively, in the RESET (Fig. 2).
Fig. 2.
Cumulative incidence of persistent discontinuation of thienopyridine: STOPDAPT versus RESET
Clinical outcomes through 1 year
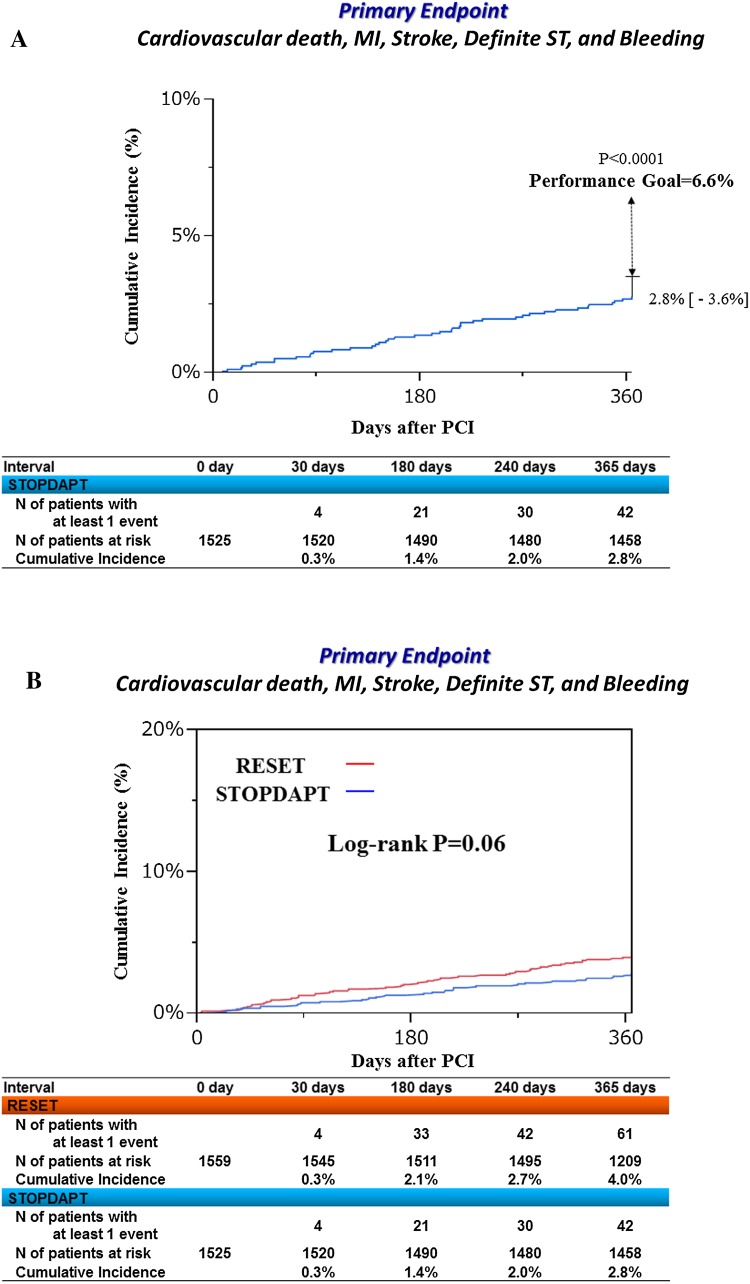
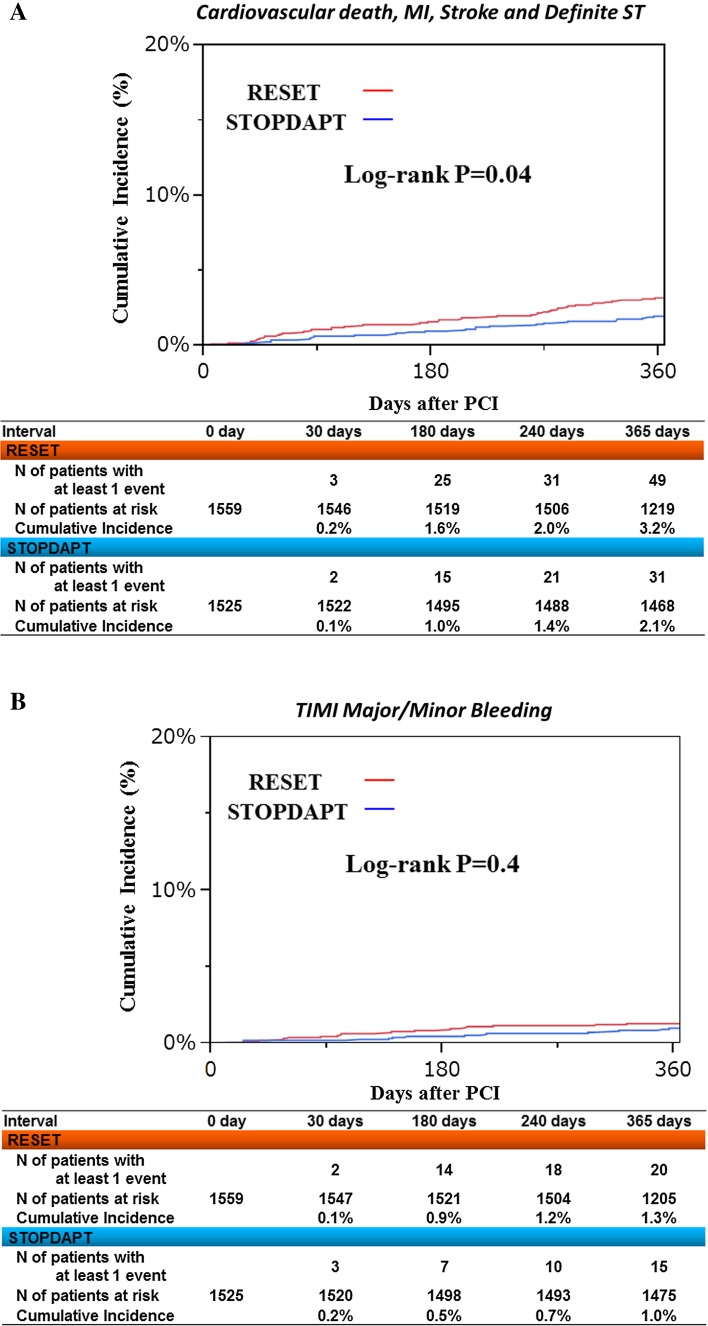
Complete 1-year clinical follow-up was achieved in 1519 patients (99.6 %) (Fig. 1). The cumulative 1-year incidence of the primary endpoint was 2.8 % (upper 97.5 % CI 3.6 %), which was significantly lower than the pre-defined performance goal of 6.6 % (P < 0.0001) (Fig. 3a). Cumulative 1-year incidence of the primary endpoint tended to be lower in the STOPDAPT than in the RESET (2.8 versus 4.0 %, P = 0.06) (Fig. 3b; Table 4). In the multivariable analysis, the risk for the primary endpoint was significantly lower in the STOPDAPT than in the RESET [adjusted HR 0.64 (95 % CI 0.42–0.95), P = 0.03] (S1 Table). The cumulative 1-year incidence of definite/probable ST was lower in the STOPDAPT than in the RESET [0 patient (0 %) versus 5 patients (0.3 %), P = 0.03] (Table 4). Regarding the major secondary endpoint, the cumulative incidence of a composite of cardiovascular death, MI, stroke and definite ST was significantly lower in the STOPDAPT than in the RESET, while the cumulative incidence of TIMI major/minor bleeding was not significantly different between the 2 groups (Fig. 4; Table 4).
Fig. 3.
a Cumulative incidence of the primary endpoint. Primary endpoint, a composite of cardiovascular death, MI, stroke, definite ST and TIMI major/minor bleeding. b Cumulative incidence of the primary endpoint: STOPDAPT versus RESET. Primary endpoint, a composite of cardiovascular death, MI, stroke, definite ST and TIMI major/minor bleeding; MI myocardial infarction, ST stent thrombosis, TIMI thrombolysis in myocardial infarction
Table 4.
Clinical outcomes at 12 months
| No. of patients with at least one event (cumulative incidence) | P value | ||
|---|---|---|---|
| STOPDAPT (N = 1525) | RESET (N = 1559) | ||
| Primary Endpoint | 42 (2.8 %) | 61 (4.0 %) | 0.06 |
| Death | |||
| All-cause | 30 (2.0 %) | 25 (1.6 %) | 0.49 |
| Cardiac death | 9 (0.6 %) | 13 (0.9 %) | 0.4 |
| Cardiovascular death | 10 (0.7 %) | 15 (1.0 %) | 0.33 |
| Non-cardiac death | 21 (1.4 %) | 12 (0.8 %) | 0.11 |
| Myocardial infarction | 4 (0.3 %) | 18 (1.2 %) | 0.003 |
| Stroke | |||
| Any | 17 (1.1 %) | 21 (1.4 %) | 0.51 |
| Ischemic | 14 (0.9 %) | 15 (1.0 %) | 0.86 |
| Hemorrhagic | 4 (0.3 %) | 8 (0.5 %) | 0.24 |
| Bleeding | |||
| TIMI major | 12 (0.8 %) | 12 (0.8 %) | 0.99 |
| TIMI minor/major | 15 (1.0 %) | 20 (1.3 %) | 0.4 |
| TIMI minimal/minor/major | 37 (2.5 %) | 38 (2.5 %) | 0.9 |
| GUSTO severe | 10 (0.7 %) | 16 (1.0 %) | 0.23 |
| GUSTO moderate/severe | 16 (1.1 %) | 19 (1.2 %) | 0.61 |
| Definite stent thrombosis | |||
| All patients | 0 (0 %) | 4 (0.3 %) | 0.046 |
| Acute (0–1 day) | 0 (0 %) | 0 (0 %) | |
| Subacute (2–30 days) | 0 (0 %) | 1 (0.06 %) | |
| Late (31–365 days) | 0 (0 %) | 3 (0.2 %) | |
| Stent thrombosis | |||
| Possible | 6 (0.4 %) | 7 (0.5 %) | 0.78 |
| Probable | 0 (0 %) | 1 (0.07 %) | 0.32 |
| Definite/probable | 0 (0 %) | 5 (0.3 %) | 0.03 |
| Definite/probable/possible | 6 (0.4 %) | 12 (0.8 %) | 0.16 |
| Death or myocardial infarction | 34 (2.2 %) | 40 (2.6 %) | 0.49 |
| Cardiovascular death or myocardial infarction | 14 (0.9 %) | 30 (2.0 %) | 0.02 |
| Cardiovascular death, MI or stroke | 31 (2.1 %) | 49 (3.2 %) | 0.045 |
| Cardiovascular death, MI, stroke and definite ST | 31 (2.1 %) | 49 (3.2 %) | 0.045 |
| Target-lesion revascularization | 30 (2.0 %) | 62 (4.2 %) | 0.0007 |
| Target-vessel revascularization | 55 (3.7 %) | 102 (6.9 %) | <0.0001 |
| Coronary revascularization | |||
| Any | 109 (7.3 %) | 175 (11.8 %) | <0.0001 |
| Coronary artery bypass grafting | 3 (0.2 %) | 7 (0.5 %) | 0.2 |
Values are expressed as number (%)
TIMI thrombolysis in myocardial infarction, GUSTO global utilization of streptokinase and tissue plasminogen activator for Occluded coronary arteries, MI myocardial infarction, ST stent thrombosis
Fig. 4.
a Cumulative incidence of a composite of cardiovascular death, MI, stroke and definite ST: STOPDAPT versus RESET. MI myocardial infarction, ST stent thrombosis. b Cumulative incidence of TIMI major/minor bleeding: STOPDAPT versus RESET. TIMI thrombolysis in myocardial infarction
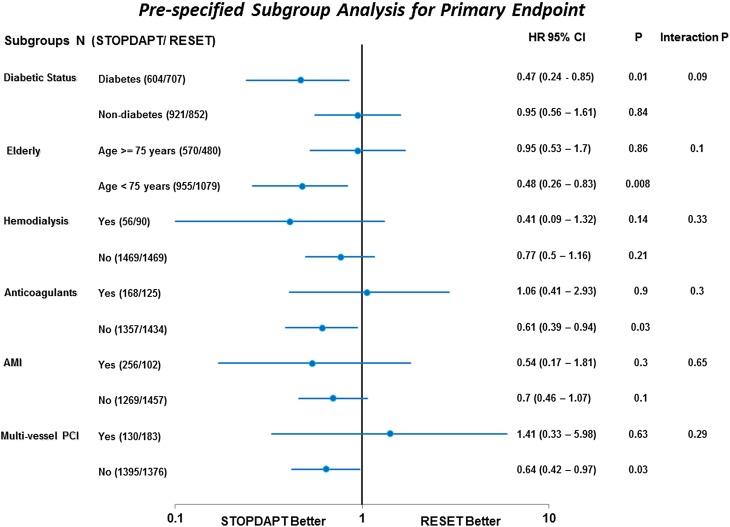
In the subgroup analysis, the STOPDAPT was associated with significantly lower risk for the primary endpoint compared with the RESET in those with diabetes and <75 years of age as well as those without anticoagulants and multivessel PCI. However, the interaction between the study (STOPDAPT or RESET) and the subgroup factor was not significant for any of the pre-specified subgroup factors (Fig. 5).
Fig. 5.
Forrest plot for the hazard ratios of STOPDAPT relative to RESET for the primary endpoint in the pre-specified subgroups. Primary endpoint, a composite of cardiovascular death, MI, stroke, definite ST and TIMI major/minor bleeding; MI myocardial infarction, ST stent thrombosis, TIMI thrombolysis in myocardial infarction, AMI acute myocardial infarction, PCI percutaneous coronary intervention
Clinical outcomes between 3 and 12 months
Between 3 and 12 months, the cumulative incidence of the primary endpoint was not significantly different between the STOPDAPT and the RESET (2.0 versus 2.7 %, P = 0.19). No patients had definite or probable ST in the STOPDAPT, while 4 patients (0.3 %) had definite or probable ST in the RESET between 3 and 12 months (Table 5). The cumulative incidence of TIMI major/minor bleeding between 3 and 12 months was not significantly different between the 2 groups (Table 5).
Table 5.
Clinical outcomes between 3 and 12 months
| No. of patients with at least one event (cumulative incidence) | P value | ||
|---|---|---|---|
| STOPDAPT | RESET | ||
| Primary Endpoint | 30 (2.0 %) | 41 (2.7 %) | 0.19 |
| Death | |||
| All-cause | 25 (1.7 %) | 18 (1.2 %) | 0.28 |
| Cardiac death | 9 (0.6 %) | 13 (0.9 %) | 0.4 |
| Cardiovascular death | 8 (0.5 %) | 11 (0.7 %) | 0.5 |
| Non-cardiac death | 17 (1.1 %) | 8 (0.5 %) | 0.07 |
| Myocardial infarction | 2 (0.1 %) | 13 (0.9 %) | 0.004 |
| Stroke | |||
| Any | 11 (0.7 %) | 11 (0.7 %) | 0.97 |
| Ischemic | 9 (0.6 %) | 8 (0.5 %) | 0.82 |
| Hemorrhagic | 3 (0.2 %) | 4 (0.3 %) | 0.68 |
| Bleeding | |||
| TIMI major | 10 (0.7 %) | 7 (0.5 %) | 0.48 |
| TIMI minor/major | 12 (0.8 %) | 13 (0.9 %) | 0.84 |
| TIMI minimal/minor/major | 26 (1.7 %) | 25 (1.7 %) | 0.92 |
| GUSTO severe | 7 (0.5 %) | 10 (0.7 %) | 0.44 |
| GUSTO moderate/severe | 11 (0.7 %) | 12 (0.8 %) | 0.81 |
| Definite stent thrombosis | 0 (0 %) | 3 (0.2 %) | 0.08 |
| Stent thrombosis | |||
| Possible | 6 (0.4 %) | 4 (0.3 %) | 0.53 |
| Probable | 0 (0 %) | 1 (0.07 %) | 0.32 |
| Definite/probable | 0 (0 %) | 4 (0.3 %) | 0.045 |
| Definite/probable/possible | 6 (0.4 %) | 8 (0.5 %) | 0.59 |
| Death or myocardial infarction | 27 (1.8 %) | 28 (1.9 %) | 0.89 |
| Cardiovascular death or myocardial infarction | 10 (0.7 %) | 21 (1.4 %) | 0.049 |
| Cardiovascular death, MI or stroke | 21 (1.4 %) | 32 (2.1 %) | 0.13 |
| Cardiovascular death, MI, stroke and definite ST | 21 (1.4 %) | 32 (2.1 %) | 0.13 |
| Target-lesion revascularization | 29 (1.9 %) | 57 (3.8 %) | 0.002 |
| Target-vessel revascularization | 52 (3.5 %) | 93 (6.3 %) | 0.0004 |
| Coronary revascularization | |||
| Any | 98 (6.6 %) | 158 (10.8 %) | <0.0001 |
| Coronary artery bypass grafting | 3 (0.2 %) | 6 (0.4 %) | 0.31 |
Values are expressed as number (%)
Abbreviations are as in Table 4
Discussion
The main finding of the current study is that stopping DAPT at 3 months in selected patients after CoCr-EES implantation was at least as safe as the prolonged DAPT regimen adopted in the historical control group.
Several previous randomized controlled trials compared 6-month versus ≥12-month DAPT after implantation of G1- and G2-DES, demonstrating similar ischemic event risk and lower bleeding event risk with 6-month DAPT [1, 2, 15–17]. Regarding the DAPT duration shorter than 6-month, 3-month DAPT with E-ZES (G1-DES) was non-inferior to 12-month DAPT with the other G1- or G2-DES with respect to the primary composite endpoints in the RESET and OPTIMIZE trials [3, 4]. In this first prospective study stopping DAPT at 3 months after CoCr-EES implantation, cumulative incidence of the primary endpoint was significantly lower than the pre-defined performance goal and tended to be lower than that in the historical control of the RESET, where nearly 90 % of patients continued DAPT at 1 year.
It was noteworthy that no definite or probable ST occurred in patients enrolled in the STOPDAPT. CoCr-EES is reported to be less thrombogenic compared with BMS by the bench testings [18]. In clinical trials and registries, the rates of late and very late ST were consistently very low after implantation of G2-DES, CoCr-EES in particular [7, 19, 20]. Given the extremely low incidence of late and very late ST, it might not be clinically appealing to extend DAPT duration to reduce the risk for ST. The cumulative 1-year incidences of cardiovascular death and MI were also very low with 3-month DAPT, which has also been demonstrated in the RESET and OPTIMIZE trials [3, 4]. Therefore, 3-month DAPT might be sufficient to protect patients from ischemic events within 1 year after implantation of G2-DES, if the patients have low ischemic event risk, like those enrolled in the current study.
The cumulative 1-year incidences of TIMI major/minor bleeding and other bleeding endpoints were not significantly different between the STOPDAPT and the RESET. Patients in the STOPDAPT included more patients with high bleeding risks such as advanced age, hypertension and anticoagulants usage than those in the RESET. The different bleeding risk profiles between the STOPDAPT and RESET trials might have led to the similar bleeding incidences between the 2 trials. In addition, the current study as well as the RESET and OPTIMIZE trials did not have enough statistical power to demonstrate the difference in the rates of bleeding events [3, 4]. However, shorter as compared with prolonged DAPT duration was clearly associated with lower risk of bleeding in the meta-analysis [5].
Recently, the DAPT trial demonstrated that 30-month DAPT, as compared with 12-month DAPT, reduced the rates of ST and major adverse cardiovascular and cerebrovascular events [21]. It might be important to distinguish the mandatory DAPT duration to protect patients against ST from long-term antiplatelet therapy as a secondary prevention. Considering the increased bleeding events and a signal suggesting increasing mortality [21], systematic implementation of prolonged DAPT would not be appropriate. The mandatory DAPT duration after coronary stent implantation would remain to be shorter than 1 year. We should continue to ask who would be the appropriate candidates for intensive long-term antiplatelet therapy, and what would be the optimal long-term antiplatelet regimen.
Study limitation
There are several important limitations in the current study. First, and most importantly, this study was not a randomized controlled trial, but a single-arm study comparing with a historical control group. We could not draw any definitive conclusions from a single-arm study. The current study was designed as a pilot study to investigate the safety of 3-month DAPT in patients receiving G2-DES, because the study sponsor had planned a large randomized controlled trial comparing 3 months versus longer DAPT duration after G2-DES implantation. Second, selection bias toward inclusion of patients with lower ischemic risk should be considered when interpreting the result of this study. Multivariable analysis could not fully adjust the measured and unmeasured confounders. Third, detailed information of PCI such as final balloon size, balloon dilatation pressure and intravascular ultrasound use was not collected in this study. Finally, we could not exclude the possibility of underreporting of the clinical events in this investigator-driven study. However, the method of follow-up data collection was exactly the same in the STOPDAPT as in the RESET.
Conclusion
Stopping DAPT at 3 months in selected patients after CoCr-EES implantation was at least as safe as the prolonged DAPT regimen adopted in the historical control group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We appreciate the supports of the co-investigators participating in the STOPDAPT study.
Compliance with ethical standards
Conflict of interest
Abbott Vascular is the funding source of this study. Takeshi Kimura, Keiichi Igarashi, Kazushige Kadota, Kengo Tanabe, Yoshihiro Morino, and Ken Kozuma were advisory board members of Abbott Vascular.
Human rights statement
Written informed consents were obtained from all the study patients.
Footnotes
On behalf of the STOPDAPT trial investigators.
References
- 1.Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the efficacy of Xience/Promus versus Cypher to reduce late loss after stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125:505–513. doi: 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–2026. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- 3.Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation) J Am Coll Cardiol. 2012;60:1340–1348. doi: 10.1016/j.jacc.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 4.Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310:2510–2522. doi: 10.1001/jama.2013.282183. [DOI] [PubMed] [Google Scholar]
- 5.Stefanini GG, Siontis GC, Cao D, Heg D, Juni P, Windecker S. Short versus long duration of DAPT after DES implantation: a meta-analysis. J Am Coll Cardiol. 2014;64:953–954. doi: 10.1016/j.jacc.2014.06.1158. [DOI] [PubMed] [Google Scholar]
- 6.Authors/Task Force members. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;2014(35):2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 7.Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D’Ascenzo F, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 8.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: A report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124:2574–2609. doi: 10.1161/CIR.0b013e31823a5596. [DOI] [PubMed] [Google Scholar]
- 9.Kimura T, Morimoto T, Natsuaki M, Shiomi H, Igarashi K, Kadota K, et al. Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the randomized evaluation of sirolimus-eluting versus everolimus-eluting stent trial (RESET) Circulation. 2012;126:1225–1236. doi: 10.1161/CIRCULATIONAHA.112.104059. [DOI] [PubMed] [Google Scholar]
- 10.Kimura T, Morimoto T, Nakagawa Y, Tamura T, Kadota K, Yasumoto H, et al. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation. 2009;119:987–995. doi: 10.1161/CIRCULATIONAHA.108.808311. [DOI] [PubMed] [Google Scholar]
- 11.Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 12.Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, et al. Thrombolysis in myocardial infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase: Clinical findings through hospital discharge. Circulation. 1987;76:142–154. doi: 10.1161/01.CIR.76.1.142. [DOI] [PubMed] [Google Scholar]
- 13.Simoons ML, et al. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 14.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. Euro Inter. 2005;1:219–227. [PubMed] [Google Scholar]
- 15.Colombo A, Chieffo A, Frasheri A, Garbo R, Masotti-Centol M, Salvatella N, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64:2086–2097. doi: 10.1016/j.jacc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Gilard M, Barragan P, Noryani AA, Noor HA, Majwal T, Hovasse T, et al. Six-month versus 24-month dual antiplatelet therapy after implantation of drug eluting stents in patients non-resistant to aspirin: ITALIC, a randomized multicenter trial. J Am Coll Cardiol. 2015;65:777–786. doi: 10.1016/j.jacc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Schulz-Schupke S, Byrne RA, Ten Berg JM, Neumann FJ, Han Y, Adriaenssens T, et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 versus 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. 2015;36:1252–1263. doi: 10.1093/eurheartj/ehu523. [DOI] [PubMed] [Google Scholar]
- 18.Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–1409. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Inter. 2013;6:1267–1274. doi: 10.1016/j.jcin.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125:1110–1121. doi: 10.1161/CIRCULATIONAHA.111.058560. [DOI] [PubMed] [Google Scholar]
- 21.Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.