Abstract
Purpose
The 2007 World Cancer Research Fund/American Institute for Cancer Research expert report concluded that foods containing vitamin C probably protect against esophageal cancer and fruits probably protect against gastric cancer. Most of the previous evidence was from case–control studies, which may be affected by recall and selection biases. More recently, several cohort studies have examined these associations. We conducted a systematic literature review of prospective studies on citrus fruits intake and risk of esophageal and gastric cancers.
Methods
PubMed was searched for studies published until 1 March 2016. We calculated summary relative risks and 95 % confidence intervals (95 % CI) using random-effects models.
Results
With each 100 g/day increase of citrus fruits intake, a marginally significant decreased risk of esophageal cancer was observed (summary RR 0.86, 95 % CI 0.74–1.00, 1,057 cases, six studies). The associations were similar for squamous cell carcinoma (RR 0.87, 95 % CI 0.69–1.08, three studies) and esophageal adenocarcinoma (RR 0.93, 95 % CI 0.78–1.11, three studies). For gastric cancer, the nonsignificant inverse association was observed for gastric cardia cancer (RR 0.75, 95 % CI 0.55–1.01, three studies), but not for gastric non-cardia cancer (RR 1.02, 95 % CI 0.90–1.16, four studies). Consistent summary inverse associations were observed when comparing the highest with lowest intake, with statistically significant associations for esophageal (RR 0.77, 95 % CI 0.64–0.91, seven studies) and gastric cardia cancers (RR 0.62, 95 % CI 0.39–0.99, three studies).
Conclusions
Citrus fruits may decrease the risk of esophageal and gastric cardia cancers, but further studies are needed.
Electronic supplementary material
The online version of this article (doi:10.1007/s10552-016-0755-0) contains supplementary material, which is available to authorized users.
Keywords: Esophageal cancer, Gastric cancer, Citrus fruits, Meta-analysis, Systematic literature review
Introduction
Esophageal and gastric cancers are the eight and the fifth most common cancers worldwide, respectively [1]. Esophageal cancer accounted for 456,000 new cancer cases in 2012 [1]—it is the sixth most common cause of cancer mortality, with 400,000 deaths in 2012 reflecting its poor prognosis, and has a 5-year survival rate of 15–25 % [2]. Squamous cell carcinoma (SCC) is the predominant histological type of esophageal cancer worldwide but in USA, UK, Australia, and some Western European countries, and the incidence of esophageal adenocarcinomas now exceeds that of SCC [3, 4]. Gastric cancer is more common in low- and middle-income countries, and although incidence rates are declining in most parts of the world, almost one million new cases occurred worldwide in 2012 [1]. The incidence of cancers of the gastric cardia has remained stable or increased at least in Western countries. Gastric cancer is usually diagnosed at advanced stages. This makes the disease the third leading cause of cancer death globally, with an estimated 723,000 deaths in 2012 [1].
Tobacco use is a risk factor for esophageal and gastric cancers. Alcohol and tobacco use are the main risk factors for esophageal SCC [5]. Due to close anatomical proximity and similar etiology, esophageal adenocarcinomas and cancers of the gastric cardia have other risk factors in common, including obesity and gastro-esophageal reflux disease [5, 6]. Helicobacter Pylori infection is the major risk factor for non-cardia gastric cancer. Approximately, 80 % of non-cardia gastric cancers are attributable to Helicobacter Pylori infection. Despite the possibility of preventing non-cardia gastric cancer by treating H. Pylori infection, there are concerns with possible adverse consequences of the antibiotic treatment, such as development of antibiotic resistance and alterations of the intestinal microbiota [7]. There is no effective screening for early detection of these cancers.
Diet may also play a role on the development of esophageal and gastric cancers. In 2007, the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Second Expert Report concluded that there was evidence that high total intake of salt probably increases the risk of gastric cancer, and that vegetables and fruits intake probably protects against esophageal and gastric cancers [8]. With respect to fruit intake, recent meta-analyses of cohort studies reported significant inverse associations with gastric cancer [9] and esophageal SCC [10] but not with adenocarcinomas of the esophagus [11].
Citrus fruits are rich in vitamin C, and foods containing vitamin C were judged probably to protect against esophageal cancer in the WCRF/AICR Second Expert Report [8]. Much of the previous evidence on citrus fruits was based on case–control studies. More recently, a publication from an integrated network of case–control studies [12], conducted in Italy and Switzerland, reported a significantly inverse association between citrus fruits intake and risk of esophageal cancer.
A recent meta-analysis of cohort studies reported nonsignificant inverse association between citrus fruits intake and the risk of gastric cancer for the comparison of the highest versus the lowest intakes [9]. However, there is no recent meta-analysis of cohort studies on citrus fruits intake and risk of esophageal cancer or subtypes of esophageal and gastric cancers. As part of the WCRF/AICR Continuous Update Project (CUP) [13], we conducted a systematic literature review and meta-analysis of cohort studies to investigate the association between citrus fruits intake and the risk of esophageal cancer, adenocarcinomas and squamous cell carcinomas, and total gastric, cardia, and non-cardia gastric cancers.
Methods
Search strategy
All cohort studies identified in the systematic literature review for the WCRF/AICR Second Expert Report [8] were indexed in PubMed. Therefore, we updated the search using the same search strategy in PubMed for studies published until 1st March 2016. Searches for esophageal and gastric cancers were carried out separately following protocols that can be accessed at http://www.wcrf.org/int/research-we-fund/continuous-update-project-cup. In addition, reference lists of relevant reviews identified in the search and of the studies included in the meta-analysis were screened for any further publications.
Study selection
The following inclusion criteria were applied for studies included in this meta-analysis: (a) cohort, nested case–control or case-cohort design; (b) reported estimates of the relative risk (hazard ratio, odds ratio, or risk ratio) with confidence intervals (CI); (c) reported quantifiable measure of citrus fruits intake. If several publications using the same study population were identified, the one with the largest number of cases was selected.
Data extraction
The following data were extracted from each study: the first author’s last name, publication year, country in which the study was conducted, study name, follow-up period, sample size, sex, age, number of cases, dietary assessment method (type, number of food items, validation), exposure, frequency or amount of intake, associated RR and corresponding 95 % CI, and adjustment variables. The search and data extraction for the systematic literature reviews of esophageal cancer and gastric cancer prior to January 2006 was conducted by the WCRF/AICR Second Expert Report teams at the Pennsylvania State University and the University of Leeds, respectively [8]. The search and data extraction from January 2006 to 1 March 2016 was conducted by the CUP team at Imperial College London. All extracted data are stored in the CUP database [13].
Statistical analyses
We conducted dose–response meta-analyses and summarized the associations for the highest compared to the lowest citrus fruits intake reported in the studies using random-effects models [14].
When not provided in the publications, the linear dose–response trends were derived from the natural logs of the RRs and CIs across categories of citrus fruits intake, using the method by Greenland and Longnecker [15]. For this method, the distribution of person-years, cases, RRs, and CIs for at least three categories is required. When not available, person-years per quantile were estimated by dividing total person-years by the number of quantiles. Means or medians of intake were assigned to each category, and when a study reported only the range of intake per category, the midpoint was estimated. For open-ended uppermost or lowermost intake categories, we computed the midpoint by assigning the width to match the nearest category. When intake was reported per unit of energy intake [16, 17], we estimated the absolute intake per quantile using the mean energy intake of the whole study population provided in the paper. When intake was reported in times or servings per day or per week, we used a standard portion size of 80 g to convert frequency to grams (http://www.wcrf.org/sites/default/files/protocol_oesophageal_cancer.pdf). The dose–response was expressed for an increment of 100 g/day of citrus fruits. We used the multivariable adjusted RR from each study. The EPIC study [18, 19] reported calibrated relative risk estimates to account for possible diet measurement error, and we used these calibrated risk estimates for the linear dose–response meta-analysis.
We first estimated summary RR for all esophageal and gastric cancers, respectively. For these analyses, the RRs for men and women were combined using fixed-effect meta-analysis before pooling. When RRs were reported by cancer subtypes only, we estimated the combined RR of gastric cardia and non-cardia or esophageal adenocarcinoma and squamous cell carcinoma using Hamling’s method [20]. The meta-analyses were also conducted by sex and cancer type, for which we combined the RRs of esophageal adenocarcinoma and gastric cardia cancers using fixed-effect models. The extent of heterogeneity in the meta-analyses was assessed using Cochran Q test and I2 statistics, with low and high heterogeneity extent indicated by I2 values below 30 % or substantially higher than 50 % [21].
Subgroup analyses were conducted to assess possible sources of heterogeneity, as well as study quality. The predefined factors to explore were sex, outcome type, geographic location, duration of follow-up, number of cases, publication year, and adjustment for confounders including smoking, alcohol intake and adiposity (as measured by BMI), when the number of studies allowed it.
Publication bias was assessed with Egger’s test [22] and visually by using funnel plot. All analyses were conducted using Stata version 12 software (Stata Corp, College Station, TX).
Results
Flowcharts of the search are provided as an online resource (Fig. 1a, b). Seven potentially relevant cohort studies [16, 18, 23–27] on esophageal and eight studies (seven publications) [17, 19, 24–26, 28, 29] on gastric cancer were identified (Table 1). For the linear dose–response meta-analysis, one publication including two cohort studies [28] investigated non-cardia gastric cancer only and was excluded from the analysis of all gastric cancers; one study on esophageal cancer was also excluded because it did not provide quantifiable measure of exposure [23]. Hence, six studies [16, 18, 24–27] were included in the dose–response for esophageal cancer and six studies [17, 19, 24–26, 29] for gastric cancer (Figs. 1a, 2b).
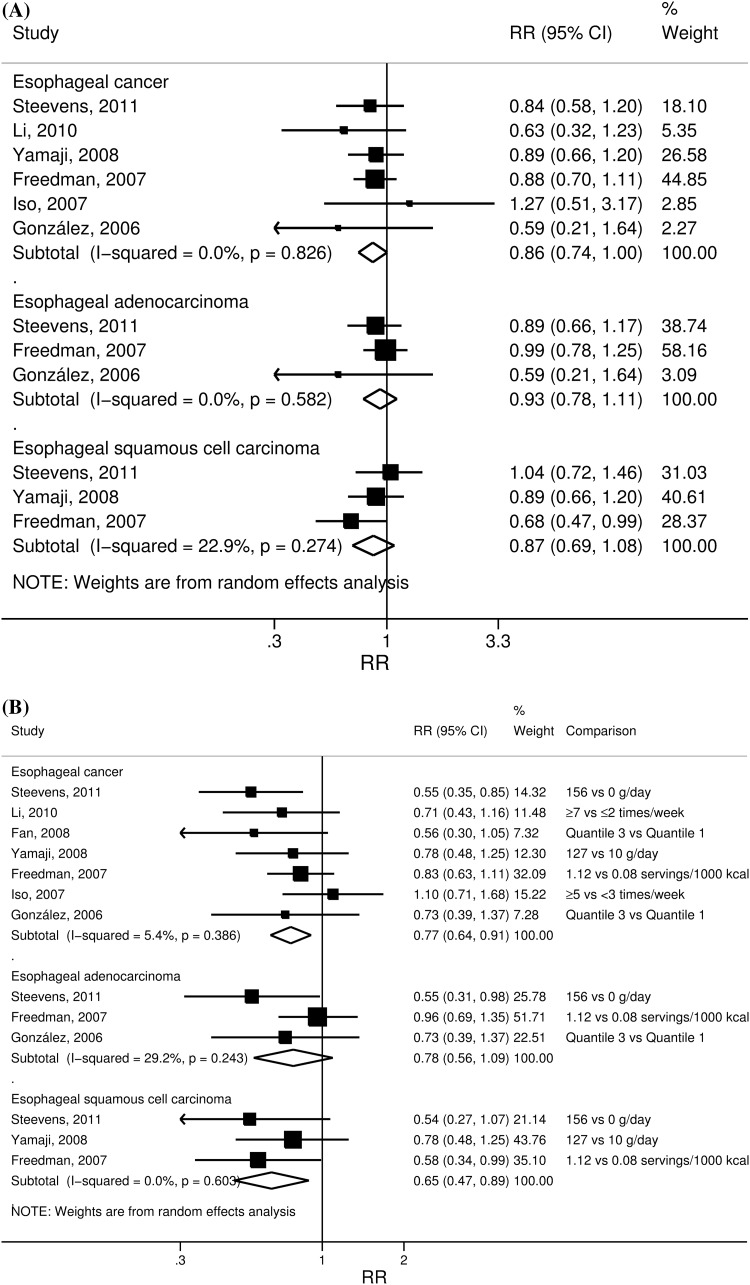
Fig. 1.
Summary RRs of esophageal cancer, esophageal adenocarcinoma, and squamous cell carcinoma per 100 g/day increase in citrus fruits intake (a) and in the highest versus lowest analysis (b)
Table 1.
Prospective cohort studies of citrus fruits intake and esophageal and gastric cancer risk
| Author, year, country (ref) | Study name, characteristics | Follow-up period (years of follow-up) | Study size, sex, number of cases | Dietary assessment | Outcome | Quantity | RR | Adjustment for confounders |
|---|---|---|---|---|---|---|---|---|
| Esophageal cancer | ||||||||
| Steevens 2011, Netherlands [26] | NLCS Case cohort Age: 55–69 years |
1986–2002 (16.3) | 4 035 Men and women, 144 | Validated FFQ, 150 food items, fresh lemon juice, grapefruits, grapefruit juice, mandarins, oranges, fresh orange juice | Incidence Esophageal AC |
156 versus 0 g/day Per 25 g/day |
0.55 (0.31–0.98) Ptrend: 0.37 0.97 (0.90–1.04) |
Age, sex, smoking status, cigarettes/day, smoking duration, alcohol, red meat, fish, vegetable, all other fruits |
| 101 | Esophageal SCC | 156 versus 0 g/day Per 25 g/day |
0.54 (0.27–1.07) Ptrend: 0.38 1.01 (0.92–1.10) |
|||||
| Li, 2010, Japan [25] | NHI Prospective Cohort Age: 40–79 years |
1995–2003 (9) | 42,470 Men and women, 151 | Validated FFQ, 40 food items, citrus fruit | Incidence Esophageal cancer |
≥7 versus ≤2 times/week | 0.71 (0.43–1.16) Ptrend: 0.18 |
Age, sex, BMI, smoking, alcohol, employment, education, walking, exercise or sports, diabetes, gastric ulcer, hypertension, family history of cancer, energy intake, intake of tea, coffee, miso soup, rice, soybean, dairy products, fish, meat, vegetables, and other fruits |
| Yamaji, 2008, Japan [27] | JPHC Prospective Cohort Age: 40–69 years |
1995/1998–2004 (7.7) | 38 790 Men, 116 | Validated FFQ, 138 food and beverage items, mandarin oranges, other oranges, 100 % orange juice | Incidence Esophageal SCC |
127 versus 10 g/day Per 100 g/day |
0.78 (0.48–1.25) 0.89 (0.66–1.20) |
Age, study area, cigarette smoking, alcohol drinking |
| Freedman, 2007, USA [16] | NIH-AARP Prospective Cohort Age: 50 years |
1995/1996–2000 (4.5) | 490,802 Men and women, 213 | Validated FFQ, 124 food items, oranges, tangerines, tangelos, grapefruits | Incidence Esophageal AC |
1.12 versus 0.08 serving/ 1,000 kcal |
0.96 (0.69–1.35) | Age, sex, BMI, alcohol, education, smoking dose, total energy intake, usual activity throughout the day, vigorous physical activity |
| 103 | Esophageal SCC |
0.58 (0.34–0. 99) Ptrend: 0.05 |
||||||
| Iso, 2007, Japan [24] | JACC Prospective cohort Age: 40–79 years |
N/A-2003 (15) | 43,011 Men, 139 | Validated FFQ, 39 food items, citrus fruit | Mortality Esophageal cancer |
≥5 versus < 3 times/week | 1.18 (0.73–1.89) | Age, area of study |
| 59 504 Women, 25 | 0.80 (0.30–2.11) | |||||||
| González, 2006, 10 European countries [18] | EPIC Prospective cohort Age: 35–70 years |
1992/1998–2002 (6.5) | 481 518 Men and women, 67 | Country-specific validated questionnaires, 88–266 items; food record, citrus fruit, juices excluded | Incidence Esophageal AC |
≥43.40 versus ≤10.68 g/day (M) ≥60.71 versus ≤17.43 g/day (W) Per 50 g/day |
0.73 (0.39–1.37) Ptrend: 0.22 (observed) 0.77 (0.46–1.28) (calibrated) |
Age, sex, center, education level, energy intake, height, leisure, physical activity, red meat intake, weight, work, physical activity, alcohol intake, processed meat intake, smoking |
| Author, year, country (ref) | Study name, characteristics | Follow-up period (years of follow-up) | Study size, sex, number of cases | Dietary assessment | Outcome | Quantity | RR | Adjustment for confounders |
| Gastric cancer | ||||||||
| González, 2012, 10 European countries [19] | EPIC Prospective Cohort Age: 35–70 years |
1992/1998–2010 (11.02) 240 206 225 |
477 312 Men and women, 683 | Country-specific validated questionnaires, 88–266 items; food record, citrus fruit, juices excluded | Incidence Gastric AC |
103.6 versus 10.8 g/day (M) 84.2 versus 22.7 g/day (W) Per 50 g/day |
0.87 (0.68–1.12) Ptrend: 0.07 0.91 (0.82–1.01) (calibrated) 0.97 (0.90–1.04) (observed) |
Age, sex, BMI, center, educational level, energy intake, physical activity, total vegetable consumption, alcohol intake, other fruits, red and processed meat, smoking, other fresh fruits |
| Never smokers Former smokers Current smokers |
Highest versus lowest Per 50 g/day Highest versus lowest Per 50 g/day Highest versus lowest Per 50 g/day |
(0.64–1.51) 1.00 (0.90–1.11) 0.90 (0.57–1.40) 1.01 (0.90–1.14) 0.69 (0.44–1.10) 0.86 (0.74–1.01) |
||||||
| 201 | Gastric cardia AC | 103.6 versus 10.8 g/day (M) 84.2 versus 22.7 g/day (W) Per 50 g/day |
0.61 (0.38-1.00) Ptrend: 0.01 0.82 (0.64–1.05) (calibrated) 0.85 (0.71–1.02) (observed) |
|||||
| 323 | Gastric non-cardia AC | 103.6 versus 10.8 g/day (M) 84.2 versus 22.7 g/day (W) Per 50 g/day |
1.25 (0.86–1.80) Ptrend: 0.46 0.99 (0.85–1.15) (calibrated) 1.03 (0.95–1.13) (observed) |
|||||
| Steevens, 2011, Netherlands [26] | NLCS Case cohort Age: 55–69 years |
1986–2002 (16.3) | 4 035 Men and women, 156 | Validated FFQ, 150 food items, fresh lemon juice, grapefruits, grapefruit juice, mandarins, oranges, fresh orange juice | Incidence Gastric cardia AC |
156 versus 0 g/day Per 25 g/day |
0.38 (0.21–0.69) Ptrend: 0.003 0.88 (0.81–0.97) |
Age, sex, smoking status, cigarettes/day, smoking duration, alcohol, red meat, fish, vegetable, all other fruits |
| 460 | Gastric non-cardia AC | 156 versus 0 g/day Per 25 g/day |
0.80 (0.56–1.15) Ptrend: 0.46 0.99 (0.95–1.03) |
|||||
| Li, 2010, Japan [25] | NHI Prospective cohort Age: 40–79 years |
1995–2003 (9) | 42 470 Men and women, 806 | Validated FFQ, 40 food items, citrus fruit | Incidence Gastric cancer |
≥7 versus ≤2 times/week | 0.99 (0.80–1.21) Ptrend: 0.90 |
Age, sex, BMI, smoking, alcohol, employment, education, walking, exercise or sports, diabetes, gastric ulcer, hypertension, family history of cancer, energy intake, intake of tea, coffee, miso soup, rice, soybean, dairy products, fish, meat, vegetables, and other fruits |
| Epplein, 2010, China [28] | SMHS Prospective cohort Age: 40–74 years |
2002/2006–2007 (3.6) | 59 247 Men, 132 | Validated FFQ, 81 food items, tangerines, oranges, grapefruit | Incidence Distal (i.e., non-cardia) gastric cancer |
>18.0 versus ≤1.6 g/day | 0.70 (0.41–1.18) Ptrend: 0.34 |
Age, education level, smoking, total energy intake |
| SWHS Prospective cohort Age: 40–70 years |
1996/2000–2007 (9.2) | 73 064 Women, 206 | Validated FFQ, 77 food items, tangerines, oranges, grapefruit | >31.9 versus ≤ 6.1 g/day | 0.94 (0.62–1.42) Ptrend: 0.86 |
|||
| Freedman, 2008, USA [17] | NIH-AARP Prospective cohort Age: 50–71 years Retired |
1995/1996–2000 (4.5) | 490 802 Men and women, 198 | Validated FFQ, 124 food items, oranges, tangerines, tangelos, grapefruits | Incidence Gastric cardia cancer |
1.12 versus 0.08 serving/1,000 kcal | 0.88 (0.62–1.23) | Age, sex, BMI, ethnicity, alcohol intake, cigarette dose, education, total energy, usual activity throughout the day, vigorous physical activity |
| 196 | Gastric non-cardia cancer | 1.36 (0.96–1.94) | ||||||
| Iso, 2007, Japan [24] | JACC Prospective cohort Age: 40–79 years |
N/A-2003 (15) | 43 011 Men, 715 | Validated FFQ, 39 food items, citrus fruit | Mortality Gastric cancer |
≥5 versus <3 times/week | 1.06 (0.86–1.30) | Age, area of study |
| 59 504 Women, 344 | 1.29 (0.95–1.74) | |||||||
| McCullough, 2001, USA [29] | CPS II Prospective cohort Age: 30 years |
1982–1996 (14) | 436 654 Men, 910 | FFQ, 32 food items, citrus fruit, juices | Mortality Gastric cancer |
>7 versus 0–1.9 times/week | 0.88 (0.75–1.03) | Age, BMI, educational level, family history of stomach cancer, multivitamin supplement, smoking habits, aspirin use, ethnicity/race, vitamin C supplement |
| 533 391 Women, 439 | >7 versus 0–2.9 times/week | 0.97 (0.78–1.21) | ||||||
Main characteristics of studies included in the linear dose–response meta-analysis
AC adenocarcinoma, SCC squamous cell carcinoma, BMI body mass index, FFQ Food Frequency Questionnaire, NLCS the Netherlands Cohort Study on diet and cancer, NHI Ohsaki National Health Insurance Cohort, JPHC Japan Public Health Center-based Prospective Study, JAAC Japan Collaborative Cohort study, NIH-AARP National Institute of Health (NIH)-AARP(formerly the American Association for Retired Persons) Diet and Health Study, EPIC European Prospective Investigation into Cancer and Nutrition, SMHS Shanghai Men’s Health Study, SWHS Shanghai Women’s Health Study
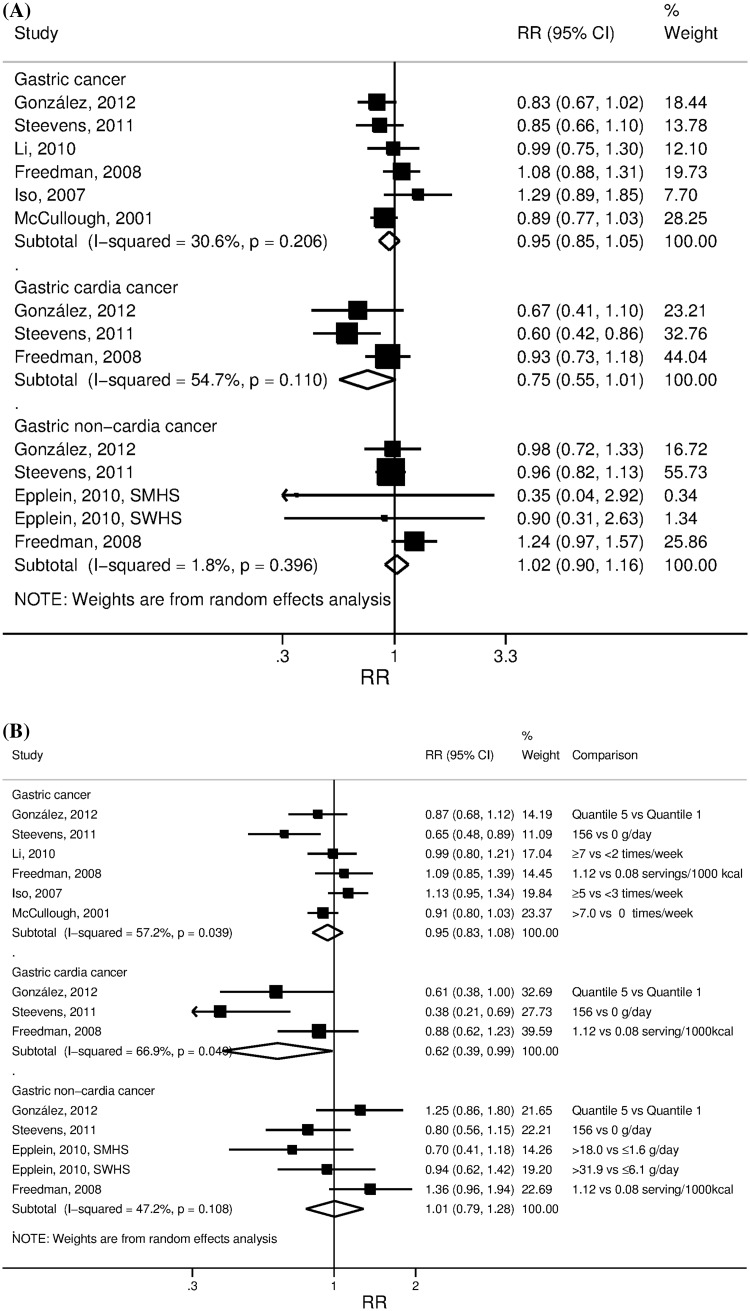
Fig. 2.
Summary RRs of gastric, gastric cardia and non-cardia cancers per 100 g/day increase in citrus fruits intake (a) and in the highest versus lowest analysis (b)
Main study characteristics are shown in Table 1. Citrus fruits intake was assessed using food frequency questionnaires. The definition of citrus fruits exposure varied slightly across the studies; in three studies, it included citrus fruits juice [26, 27, 29] (Table 1).
All measures of association included in the meta-analyses were adjusted for multiple confounding factors, albeit defined differently in the studies, including alcohol [16–19, 25–27], BMI and physical activity [16–19, 25, 29], socioeconomic status [16, 18, 19, 25, 29], smoking status [16–19, 25–27, 29], number of cigarettes [11, 16–19, 26, 27], and duration of smoking [19, 26, 27] with the exception of a Japanese study with cancer mortality as endpoint that adjusted only for age and geographic area [24]. None of the studies adjusted for gastric reflux disease (GERD), Table 3. The lack of information about gastric reflux was indicated in one publication [16]. One study on gastric cancer mortality [29] investigated regular use of antacids but did not include it in the final model due to lack of confounding.
Table 3.
Subgroup meta-analyses of citrus fruits and risk of esophageal and gastric cancers
| Per 100 g/day | Esophageal cancer | Gastric cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| N | RR (95 % CI) | I 2 (%) | P heterogeneity | N | RR (95 % CI) | I 2 (%) | P heterogeneity | |
| All studies | 6 | 0.86 (0.74–1.00) | 0 | 0.83 | 6 | 0.95 (0.85–1.05) | 31 | 0.21 |
| Sex | ||||||||
| Men | 2 | 0.93 (0.70–1.24) | 0 | 0.34 | 2 | 0.91 (0.76–1.09) | 8 | 0.30 |
| Women | 1 | 0.63 (0.08–5.23) | – | – | 2 | 1.20 (0.67–2.15) | 65 | 0.09 |
| Outcome type | ||||||||
| Incidence | 5 | 0.85 (0.73–0.99) | 0 | 0.84 | 4 | 0.93 (0.82–1.07) | 23 | 0.27 |
| Mortality | 1 | 1.27 (0.51–3.17) | – | – | 2 | 1.03 (0.73–1.46) | 70 | 0.07 |
| Geographic location | ||||||||
| Asia | 3 | 0.87 (0.67–1.13) | 0 | 0.45 | 2 | 1.10 (0.85–1.41) | 21 | 0.26 |
| Europe | 2 | 0.80 (0.57–1.13) | 0 | 0.54 | 2 | 0.84 (0.71–0.98) | 0 | 0.86 |
| North America | 1 | 0.88 (0.70–1.11) | – | – | 2 | 0.97 (0.81–1.16) | 55 | 0.13 |
| Europe and North America | 3 | 0.86 (0.71–1.04) | 0 | 0.75 | 4 | 0.91(0.82–1.02) | 23 | 0.28 |
| Duration of follow-up | ||||||||
| <10 years | 4 | 0.85 (0.72–1.02) | 0 | 0.70 | 2 | 1.05 (0.89–1.23) | 0 | 0.62 |
| ≥10 years | 2 | 0.88 (0.63–1.24) | 0 | 0.40 | 4 | 0.90 (0.79–1.03) | 32 | 0.22 |
| Number of cases | ||||||||
| <100 | 1 | 0.59 (0.21–1.65) | – | – | – | |||
| 100–<200 | 3 | 0.87 (0.67–1.13) | 0 | 0.45 | – | |||
| 200–<500 | 2 | 0.87 (0.71–1.05) | 0 | 0.81 | 1 | 1.08 (0.88–1.31) | – | – |
| 500–<1,000 | – | 3 | 0.87 (0.76–1.00) | 0 | 0.60 | |||
| ≥1,000 | – | 2 | 1.03 (0.73–1.46) | 70 | 0.07 | |||
| Publication year | ||||||||
| <2,010 | 4 | 0.89 (0.74–1.06) | 0 | 0.75 | 3 | 1.02 (0.85–1.24) | 56 | 0.10 |
| ≥2,010 | 2 | 0.78 (0.57–1.08) | 0 | 0.47 | 3 | 0.87 (0.76–1.00) | 0 | 0.60 |
| Adjustment for confounders | ||||||||
| Socioeconomic status | ||||||||
| Yes | 3 | 0.84 (0.68–1.04) | 0 | 0.52 | 4 | 0.93 (0.83–1.04) | 21 | 0.29 |
| No | 3 | 0.89 (0.71–1.11) | 0 | 0.70 | 2 | 1.02 (0.69–1.53) | 69 | 0.07 |
| Smoking | ||||||||
| Yes | 5 | 0.85 (0.73–0.99) | 0 | 0.84 | 5 | 0.92 0.84–1.01 | 4 | 0.39 |
| No | 1a | 1.27 (0.51–3.17) | – | – | 1a | 1.29 0.89–1.85 | – | – |
| Alcohol intake | ||||||||
| Yes | 5 | 0.85 (0.73–0.99) | 0 | 0.84 | 4 | 0.93 (0.82–1.07) | 23 | 0.27 |
| No | 1a | 1.27 (0.51–3.17) | – | – | 2b | 1.03 (0.73–1.46) | 70 | 0.07 |
| BMI | ||||||||
| Yes | 3 | 0.84 (0.68–1.04) | 0 | 0.52 | 4 | 0.93 (0.83–1.04) | 21 | 0.29 |
| No | 3 | 0.89 (0.71–1.11) | 0 | 0.70 | 2 | 1.02 (0.69–1.53) | 69 | 0.07 |
| Physical activity | ||||||||
| Yes | 3 | 0.84 (0.68–1.04) | 0 | 0.52 | 3 | 0.96 (0.81–1.13) | 39 | 0.20 |
| No | 3 | 0.89 (0.71–1.11) | 0 | 0.70 | 3 | 0.94 (0.78–1.14) | 46 | 0.16 |
| Total energy intake | ||||||||
| Yes | 3 | 0.84 (0.68–1.04) | 0 | 0.52 | 3 | 0.96 (0.81–1.13) | 39 | 0.20 |
| No | 3 | 0.89 (0.71–1.11) | 0 | 0.70 | 3 | 0.94 (0.78–1.14) | 46 | 0.16 |
| Ethnicity | ||||||||
| Yes | – | 2 | 0.97 (0.81–1.16) | 55 | 0.13 | |||
| No | 6 | 0.86 (0.74–1.00) | 0 | 0.83 | 4 | 0.93 (0.79–1.11) | 37 | 0.19 |
Gastric or esophageal cancers were primary outcomes in all but two studies [24, 25] that reported on multiple cancer sites.
Five studies were conducted in Asia [24, 25, 27, 28], two in Europe [18, 19, 26], and two in North America [16, 17, 29] (Table 1). All studies were included men and women apart from Yamaji et al., 2008 [27] which was only included men (Table 1). A summary of the results of meta-analyses by cancer type is presented in Table 2.
Table 2.
Summary table of meta-analyses of citrus fruits and esophageal and gastric cancers
| Cancer type | Esophageal cancer | Esophageal squamous cell carcinoma | Esophageal adenocarcinoma | Esophageal adenocarcinoma, gastric cardia | Gastric cancer | Gastric cardia cancer | Gastric non-cardia cancer |
|---|---|---|---|---|---|---|---|
| Linear dose–response meta-analysis per 100 g/day | |||||||
| No. of studies | 6 | 3 | 3 | 3 | 6 | 3 | 4 |
| No. of cases | 1,057 | 320 | 422 | 1,348 | 4,907 | 555 | 1,317 |
| Person-years | 7,513,150 | 2,542,187 | 5,354,570 | 7,507,530 | 22,949,089 | 7,507,530 | 8,393,008 |
| RR (95 % CI) | 0.86 (0.74–1.00) | 0.87 (0.69–1.08) | 0.93 (0.78–1.11) | 0.83 (0.67–1.02) | 0.95 (0.85–1.05) | 0.75 (0.55–1.01) | 1.02 (0.90–1.16) |
| I 2, P heterogeneity | 0 %, 0.83 | 23 %, 0.27 | 0 %, 0.58 | 50 %, 0.14 | 31 %, 0.21 | 55 %, 0.11 | 2 %, 0.40 |
| Highest versus lowest analysis | |||||||
| No. of studies | 7 | 3 | 3 | 3 | 6 | 3 | 4 |
| No. of cases | 1 158 | 320 | 422 | 1 348 | 4 907 | 555 | 1 317 |
| RR (95 % CI) | 0.77 (0.64–0.91) | 0.65 (0.47–0.89) | 0.78 (0.56–1.09) | 0.67 (0.44–1.01) | 0.95 (0.83–1.08) | 0.62 (0.39–0.99) | 1.01 (0.79–1.28) |
| I 2, P heterogeneity | 5 %, 0.39 | 0 %, 0.60 | 29 %, 0.24 | 77 %, 0.01 | 57 %, 0.04 | 67 %, 0.05 | 47 %, 0.11 |
Esophageal cancer
Six studies [16, 18, 24–27] with a total of 1,057 cases among 1,160,130 participants were included in the linear dose–response meta-analysis. Citrus fruit was inversely associated with esophageal cancer risk; the association was statistically significant only in the highest versus lowest analysis. The summary RR for an increase of 100 g/day of citrus fruits intake was 0.86 (95 % CI 0.74–1.00), with no evidence of heterogeneity (I2 = 0 %, Pheterogeneity = 0.83) (Fig. 1a). There was no evidence of publication or small study bias (p = 0.55). The summary RR for the highest compared with the lowest intake was 0.77 (95 % CI 0.64–0.91), with low heterogeneity (I2 = 5 %, Pheterogeneity = 0.39) (Fig. 1b).
In analyses by cancer type, three studies could be included in the analyses of adenocarcinoma [16, 18, 26] and SCC [16, 26, 27] of the esophagus, respectively. Similar not statistically significant inverse associations were observed for both cancer types in linear dose–response meta-analyses. The summary RR per 100 g/day increase in citrus fruits intake was 0.93 (95 % CI 0.78–1.11, 422 cases, three studies) for esophageal adenocarcinoma, with no evidence of heterogeneity (I2 = 0 %, Pheterogeneity = 0.58) and 0.87 (95 % CI 0.69–1.08, 320 cases, three studies) for SCC with low heterogeneity (I2 = 23 %, Pheterogeneity = 0.27) (Fig. 1a). The summary RR for the highest compared with the lowest intake was 0.78 (95 % CI 0.56–1.09) for adenocarcinomas and 0.65 (95 % CI 0.47–0.89) for SCC (Fig. 1b).
Only two studies in men [24, 27], one incidence and one on mortality from esophageal cancer and one study on esophageal cancer mortality in women [24] were available. There is not enough data to examine the association of citrus fruits and esophageal cancer risk by sex (Table 3).
In subgroup analysis (all esophageal cancers), no differences emerged across study characteristics, including adjustment factors (Table 3). There is some suggestion that more adjusted studies tend to report stronger associations, but the number of studies is low. A positive not significant association was observed in the only study [24] that did not adjust for tobacco, smoking, and alcohol intake in which the outcome was mortality for esophageal cancer. When this study was omitted from the analysis, the summary RR for an increase of 100 g/day of citrus fruits intake was 0.85 (95 % CI 0.73–0.99) with no heterogeneity.
Gastric cancer
Six studies [17, 19, 24–26, 29] investigated the association between citrus fruits intake and gastric cancer risk with a total of 4,907 cases among 2,087,179 participants. No significant association with gastric cancer was observed. The summary RR per 100 g/day increment was 0.95 (95 % CI 0.85–1.05), with moderate [21] heterogeneity (I2 = 31 %, Pheterogeneity = 0.34) (Fig. 2a). The summary RR for the highest compared to the lowest intake was 0.95 (95 % CI 0.83–1.08) with evidence of heterogeneity (I2 = 57 %, Pheterogeneity = 0.04) (Fig. 2b).
In subgroup analyses by cancer type, inverse association was observed for cancers of the gastric cardia, but not for non-cardia gastric cancers. Three studies [17, 19, 26] investigated the association between citrus fruits intake and gastric cardia cancer risk with a total of 555 cases among 972,149 participants. The summary RR for 100 g/day increment was 0.75 (95 % CI 0.55–1.01), with moderate [21] heterogeneity (I2 = 55 %, Pheterogeneity = 0.11) (Fig. 2a), and it was 0.62 (95 % CI 0.39–0.99) comparing the highest with lowest intake, with high heterogeneity (I2 = 67 %, Pheterogeneity = 0.05) (Fig. 2b).
Five studies [17, 19, 26, 28] investigated the association between citrus fruits intake and non-cardia gastric cancer risk with a total of 1,317 cases among 1,104,460 participants. The summary RR for 100 g/day increment was 1.02 (95 % CI 0.90–1.16), with low heterogeneity (I2 = 2 %, Pheterogeneity = 0.4) (Fig. 2a), and it was 1.01 (95 % CI 0.79–1.28) for the highest compared with the lowest intake (Fig. 2b).
When the analyses were restricted to the three studies [17, 19, 26] that reported on both cardia and non-cardia gastric cancers, the RRs for an increase of 100 g/day were 0.75 (95 % CI 0.55–1.01) and 1.04 (95 % CI 0.89–1.22), respectively.
It was not possible to formally explore the source of heterogeneity in the analyses on cardia gastric cancer. Visual inspection of the forest plot shows that heterogeneity is driven by the American NIH-AARP study [17] that reported no association of citrus fruits with cardia gastric cancer. The reasons for the different results are unclear. The NIH-AARP study [17] categorized intake by servings/1,000 kcal, whereas the two other studies [19, 26] reported in continuous increments in g/day.
Esophageal adenocarcinoma and gastric cardia cancers
We estimated the summary RR of esophageal adenocarcinomas and gastric cardia cancers (three studies, five publications) [16–19, 26]. When combined, these cancers totaled to 1,348 cases among 5,268,049 participants. The summary RR per 100 g/day increment was 0.83 (95 % CI 0.67–1.02), with moderate [21] heterogeneity (I2 = 50 %, Pheterogeneity = 0.14) (Table 2). The summary RR was 0.67 (95 % CI 0.44–1.01) for the highest compared with the lowest intake (Table 2).
Summary risk estimates observed in subgroup analyses for all gastric cancers were mostly similar to that in the overall analysis, with exceptions in some subgroups where a positive association was observed. Estimates of risk were below 1 in studies adjusted for smoking and alcohol and BMI but not in the unadjusted studies (Table 3). Significant associations were observed in subgroup analyses for all gastric cancers among European studies [19, 26] and studies with 500–<1,000 cases [19, 25, 26]. Inverse not significant associations were observed in men but not in women (2 studies) [24, 29]. There was no evidence of small study effects such as publication bias (p = 0.25).
Interaction with smoking
One study reported on the interaction of smoking status and citrus fruits intake in relation to esophageal or gastric cancers. In the EPIC study [19], the inverse association of citrus fruits for gastric cancer was restricted to current smokers and not observed in never or former smokers (p for interaction =0.07). Other studies in the review explored the interaction of smoking and intake of total fruits and vegetables, or fruits. In general, no significant interactions with smoking were observed. In the NIH-AARP study, the risk estimates of adenocarcinoma and SCC for total fruits and vegetable intakes appeared similar in smokers, non-smokers, and current smokers [17]. In the study in Japanese men, esophageal SCC risk was inversely associated with total fruits or vegetables intake in never, current and former smokers [27]. In the Netherlands Cohort Study, slightly greater inverse associations of fruit intake with SCC and adenocarcinomas of esophagus and gastric cardia cancer were reported in smokers that in never smokers, but the interaction was not significant (p for interaction = 0.25; 0.15; and 0.49, respectively) [26]. In a Chinese study in men and women, a significant reduction in risk of distal gastric cancer from increased fruit intake was significant among ever smokers and inverse but nonsignificant in never smokers, but the interaction by smoking status was not statistically significant (p for interaction = 0.27) [28].
Discussion
In these meta-analyses of cohort studies, citrus fruits intake was marginally associated with reduced risks of esophageal and gastric cardia cancers. No association with non-cardia gastric cancers was observed. Similar results were observed for adenocarcinomas and SCC of esophagus.
Citrus fruits are rich in vitamin C that could influence cancer risk by scavenging reactive oxygen species, protecting mucosal tissues from the damaging effects of oxidative stress, and inhibiting nitrosamine formation in the stomach [30]. The results of this meta-analysis are consistent with the inverse association of prediagnostic plasma vitamin C concentration and risk of gastric cardia cancer (215 cases) observed in the EPIC study [31] and in a study in a high-risk Chinese population (467 cases) [32]. In the EPIC study, the associations were more pronounced for gastric cardia than non-cardia cancer, although the associations were not statistically significant when stratified by subtype. Further evidence is provided by the Shandong Intervention Trial of vitamin supplementation (vitamin C, E and selenium), in which supplemented individuals had a lower risk of esophageal and gastric cancers [33] and in a meta-analysis of 20 randomized controlled trials of antioxidant supplementation (vitamins A, C, E, and selenium) inverse but not significant lower risk of gastrointestinal cancers was observed [34]. In the NIH-AARP, use of vitamin C supplements was associated with reduced risk of gastric non-cardia adenocarcinomas, but no association was observed with multivitamin supplements use that usually contains vitamin C [35]. Finally, in recent meta-analyses, total fruit intake was associated with significantly lower risk of gastric cancer [9] and esophageal squamous cell carcinoma [10].
In addition to high vitamin C content, citrus fruits contain a wide range of bioactive compounds such as citrus flavonoids, carotenoids, and limonoids. Experimental studies have demonstrated that these bioactive components may protect DNA, regulate cell growth, and induce apoptosis [36–38].
The main limitation of this meta-analysis is the small number and limited power of published studies on citrus fruits intake, esophageal and gastric cancer risks, and the unexplained heterogeneity of the inverse association of citrus fruits intake and gastric cancer cardia in the three studies identified [17, 19, 26].
The observed inverse associations could be due to residual confounding by smoking. In the EPIC study [19], the inverse association of citrus fruits for gastric cancer was restricted to current smokers and not observed in never or former smokers (p for interaction =0.07). However, other studies included in the meta-analysis [16, 17, 19, 26] reported no evidence of interaction of effect modification by smoking status. In the NIH-AARP, the association between fruit and vegetable intake with ESCC [16] was similar in the limited number of non-drinkers and non-smokers; in a study on gastric cancer [17], there was no evidence of effect modification by cigarette smoking status or alcohol drinking; in the NLCS study, the risk estimates for total fruit intake and risk of all types of gastric and esophageal cancers were further below 1 in current smokers compared to never and former smokers, but the interaction was not significant (p for interaction >0.15) [26]. On the other hand, smokers tend to eat less fruits and vegetables [39, 40], have lower concentration of serum antioxidants [41], and may benefit more from higher citrus fruits intake [27].
Measurement error of diet may have attenuated the risk estimates. Only the EPIC cohort corrected for dietary measurement error [18, 19]. When non-calibrated risk estimates from the EPIC cohort were used in the sensitivity analysis, the association became significant for gastric cardia cancer (RR 0.75; 95 % CI 0.57–0.99), and the risk estimates did not change for esophageal cancer and remained similar for all gastric cancers (RR 0.96; 95 % CI 0.88–1.05) and gastric non-cardia cancer (RR 1.04; 95 % CI 0.94–1.16). Strengths of this meta-analysis include the prospective design of the included studies, which are less prone to bias than other observational studies, detailed dose–response and categorical meta-analyses, and the increased statistical power to detect modest but statistically significant inverse associations.
Conclusions
In conclusion, there is evidence from cohort studies that citrus fruits may decrease the risk of esophageal and cardia gastric cancers, but the data are still limited.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
TN is the principal investigator of the Continuous Update Project at Imperial College London. CS managed the database for the Continuous Update Project. SV did the literature search, study selection, and data extraction. DSMC and SV carried out the statistical analyses. SV wrote the first draft of the original manuscript. All authors revised the manuscript. SV and DSMC take responsibility for the integrity of the data and the accuracy of the data analysis. We thank the systematic literature review teams at the Pennsylvania State University and the University of Leeds for their contributions to the esophageal and gastric cancer databases, respectively.
Funding
This work was supported by the World Cancer Research Fund International as part of the Continuous Update Project (Grant Number: 2007/SP01) (http://www.wcrf-uk.org/). The funder of this study had no role in the decisions about the design and conduct of the study; collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. The views expressed in this review are the opinions of the authors. They may not represent the views of WCRF International/AICR and may differ from those in future updates of the evidence related to food, nutrition, physical activity, and cancer risk.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal Carcinoma. Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Steevens J, Botterweck AA, Dirx MJ, van den Brandt PA, Schouten LJ. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol. 2010;22(6):669–678. doi: 10.1097/MEG.0b013e32832ca091. [DOI] [PubMed] [Google Scholar]
- 4.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23(12):3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 5.Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6(5):112–120. doi: 10.4251/wjgo.v6.i5.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomark Prev. 2014;23(5):700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrero R, Park JY, Forman D. The fight against gastric cancer—the IARC Working Group report. Best Pract Res Clin Gastroenterol. 2014;28(6):1107–1114. doi: 10.1016/j.bpg.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research (2007) Food, nutrition, physical activity, and the prevention of cancer: a global perspective. AIRC, Washington, DC
- 9.Wang Q, Chen Y, Wang X, Gong G, Li G, Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer. 2014;50(8):1498–1509. doi: 10.1016/j.ejca.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer. 2013;133(2):473–485. doi: 10.1002/ijc.28024. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Jiang G, Zhang G, Xue Q, Zhang H, Wang C, et al. Intake of vegetables and fruit and risk of esophageal adenocarcinoma: a meta-analysis of observational studies. Eur J Nutr. 2014;53(7):1511–1521. doi: 10.1007/s00394-014-0656-5. [DOI] [PubMed] [Google Scholar]
- 12.Foschi R, Pelucchi C, Dal ML, Rossi M, Levi F, Talamini R, et al. Citrus fruit and cancer risk in a network of case–control studies. Cancer Causes Control. 2010;21(2):237–242. doi: 10.1007/s10552-009-9454-4. [DOI] [PubMed] [Google Scholar]
- 13.World Cancer Research Fund International (2015) Continuous update project (CUP). 2015. http://www.wcrf.org/int/research-we-fund/continuous-update-project-cup. Accessed on 18th March 2015
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 16.Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, et al. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int J Cancer. 2007;121(12):2753–2760. doi: 10.1002/ijc.22993. [DOI] [PubMed] [Google Scholar]
- 17.Freedman ND, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. Fruit and vegetable intake and gastric cancer risk in a large United States prospective cohort study. Cancer Causes Control. 2008;19(5):459–467. doi: 10.1007/s10552-007-9107-4. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez CA, Pera G, Agudo A, Bueno-de-Mesquita HB, Ceroti M, Boeing H, et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int J Cancer. 2006;118(10):2559–2566. doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Duell EJ, Agudo A, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. Int J Cancer. 2012;131(12):2910–2919. doi: 10.1002/ijc.27565. [DOI] [PubMed] [Google Scholar]
- 20.Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27(7):954–970. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan Y, Yuan JM, Wang R, Gao YT, Yu MC. Alcohol, tobacco, and diet in relation to esophageal cancer: the Shanghai Cohort Study. Nutr Cancer. 2008;60(3):354–363. doi: 10.1080/01635580701883011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iso H, Kubota Y. Nutrition and disease in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Asian Pac J Cancer Prev. 2007;8(Suppl):35–80. [PubMed] [Google Scholar]
- 25.Li WQ, Kuriyama S, Li Q, Nagai M, Hozawa A, Nishino Y, et al. Citrus consumption and cancer incidence: the Ohsaki cohort study. Int J Cancer. 2010;127(8):1913–1922. doi: 10.1002/ijc.25203. [DOI] [PubMed] [Google Scholar]
- 26.Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer. 2011;129(11):2681–2693. doi: 10.1002/ijc.25928. [DOI] [PubMed] [Google Scholar]
- 27.Yamaji T, Inoue M, Sasazuki S, Iwasaki M, Kurahashi N, Shimazu T, et al. Fruit and vegetable consumption and squamous cell carcinoma of the esophagus in Japan: the JPHC study. Int J Cancer. 2008;123(8):1935–1940. doi: 10.1002/ijc.23744. [DOI] [PubMed] [Google Scholar]
- 28.Epplein M, Shu XO, Xiang YB, Chow WH, Yang G, Li HL, et al. Fruit and vegetable consumption and risk of distal gastric cancer in the Shanghai Women’s and Men’s Health studies. Am J Epidemiol. 2010;172(4):397–406. doi: 10.1093/aje/kwq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCullough ML, Robertson AS, Jacobs EJ, Chao A, Calle EE, Thun MJ. A prospective study of diet and stomach cancer mortality in United States men and women. Cancer Epidemiol Biomark Prev. 2001;10(11):1201–1205. [PubMed] [Google Scholar]
- 30.Duell EJ, Lujan-Barroso L, Llivina C, Munoz X, Jenab M, Boutron-Ruault MC, et al. Vitamin C transporter gene (SLC23A1 and SLC23A2) polymorphisms, plasma vitamin C levels, and gastric cancer risk in the EPIC cohort. Genes Nutr. 2013;8(6):549–560. doi: 10.1007/s12263-013-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Carcinogenesis. 2006;27(11):2250–2257. doi: 10.1093/carcin/bgl096. [DOI] [PubMed] [Google Scholar]
- 32.Lam TK, Freedman ND, Fan JH, Qiao YL, Dawsey SM, Taylor PR, et al. Prediagnostic plasma vitamin C and risk of gastric adenocarcinoma and esophageal squamous cell carcinoma in a Chinese population. Am J Clin Nutr. 2013;98(5):1289–1297. doi: 10.3945/ajcn.113.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104(6):488–492. doi: 10.1093/jnci/djs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Systematic review: primary and secondary prevention of gastrointestinal cancers with antioxidant supplements. Aliment Pharmacol Ther. 2008;28(6):689–703. doi: 10.1111/j.1365-2036.2008.03785.x. [DOI] [PubMed] [Google Scholar]
- 35.Dawsey SP, Hollenbeck A, Schatzkin A, Abnet CC. A prospective study of vitamin and mineral supplement use and the risk of upper gastrointestinal cancers. PLoS ONE. 2014;9(2):e88774. doi: 10.1371/journal.pone.0088774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56(15):6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 37.Silalahi J. Anticancer and health protective properties of citrus fruit components. Asia Pac J Clin Nutr. 2002;11(1):79–84. doi: 10.1046/j.1440-6047.2002.00271.x. [DOI] [PubMed] [Google Scholar]
- 38.Tundis R, Loizzo MR, Menichini F. An overview on chemical aspects and potential health benefits of limonoids and their derivatives. Crit Rev Food Sci Nutr. 2014;54(2):225–250. doi: 10.1080/10408398.2011.581400. [DOI] [PubMed] [Google Scholar]
- 39.Dallongeville J, Marecaux N, Fruchart JC, Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr. 1998;128(9):1450–1457. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- 40.Dehghan M, Akhtar-Danesh N, Merchant AT. Factors associated with fruit and vegetable consumption among adults. J Hum Nutr Diet. 2011;24(2):128–134. doi: 10.1111/j.1365-277X.2010.01142.x. [DOI] [PubMed] [Google Scholar]
- 41.Alberg AJ, Chen JC, Zhao H, Hoffman SC, Comstock GW, Helzlsouer KJ. Household exposure to passive cigarette smoking and serum micronutrient concentrations. Am J Clin Nutr. 2000;72(6):1576–1582. doi: 10.1093/ajcn/72.6.1576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.