Abstract
The ability of glycinecin A, a bacteriocin derived from Xanthomonas campestris pv. glycines 8ra, to kill closely related bacteria has been demonstrated previously by our group (S. G. Heu et al., Appl. Environ. Microbiol. 67:4105-4110, 2001). In the present study, we aimed at determining the glycinecin A-induced cause of death. Treatment with glycinecin A caused slow dissipation of membrane potential and rapid depletion of the pH gradient. Glycinecin A treatment also induced leakage of potassium ions from X. campestris pv. vesicatoria YK93-4 cells and killed sensitive bacterial cells in a dose-dependent manner. Sensitive cells were killed within 2 h of incubation, most likely due to the potassium ion efflux caused by glycinecin A. These results suggest that the bactericidal mechanism of action of glycinecin A is correlated with the permeability of membranes to hydroxyl and potassium ions, leading to the lethal activity of the bacteriocin on the target bacteria.
Bacteriocins, extracellular proteinaceous bactericidal substances that are produced by many species of bacteria, trigger the killing of strains or species closely related to their producers. Their narrow specificity of action and their proteinaceous nature distinguish them from other antibiotics (3).
The modes of action of bacteriocins differ (1, 21). Some bacteriocins inhibit the synthesis of macromolecules, for example, colicin E3, which specifically inhibits protein synthesis (26). Others have nuclease activity: colicin E2 and pyocin S3 induce DNA degradation (5, 22), and colicin E5 cleaves a specific group of tRNAs (15). Two-component bacteriocins, such as lacticin 3147, plantaricin EF, plantaricin JK, and lactococcin G, require the complementary actions of two components for activity and target the cell membrane (16, 19, 20). Lactococcin G selectively forms potassium channels in target bacterial membranes (20), and the plantaricins EF and JK form pores in the membranes of target cells, dissipating the transmembrane electrical potential and pH gradient (19). The cytoplasmic membrane is the primary target of colicins A, E1, K, Ia, and Ib (3, 4, 6). These and other related colicins disrupt transport and induce the leakage of ions, such as potassium and magnesium ions, by forming voltage-dependent channels in phospholipid bilayers, destroying the potential of the cell. These actions result in the inhibition of protein or nucleic acid biosynthesis and uncoupling of electron transport from active transport of thiomethyl-β-d-galactoside and potassium. The loss of these ions has been implicated as the primary cause of cell death (3). The bactericidal activities of enterocoliticin and serracin P have been shown to have a phage tail-like action that forms pores in target cell membranes (11, 24).
Xanthomonas campestris pv. glycines 8ra has antibacterial activity against most phytopathogenic Xanthomonas species tested, including Xanthomonas axonopodis, Xanthomonas campestris pv. campestris, Xanthomonas campestris pv. citri, Xanthomonas campestris pv. pruni, and Xanthomonas campestris pv. vesicatoria (25). X. campestris pv. glycines 8ra produces a bacteriocin named glycinecin A, a heterodimeric protein consisting of 39- and 14-kDa subunits encoded by the glyA and glyB genes, respectively. Coexpression of the two subunits in the same host is essential for the activity of this bacteriocin (9). Glycinecin A treatment was as effective in the control of Xanthomonas oryzae pv. oryzae and X. campestris pv. vesicatoria as chemical treatment with copper hydroxide (12). A chimeric glycinecin A, consisting of concatemerized GlyA and GlyB, has been demonstrated to have bactericidal activity comparable to that of wild-type glycinecin A, and the protein was more stable with varying pH and temperature than the wild-type protein (13). Understanding the mode of action of the bacteriocin will be important to allow effective utilization of this protein. The purpose of this study was to determine the mode of action of glycinecin A on X. campestris pv. vesicatoria cells. Purified chimeric glycinecin A was used in this study.
MATERIALS AND METHODS
Chemicals, glycinecin A, bacterial strains, and growth conditions.
Luria-Bertani medium (LB) broth, nutrient agar, K-HEPES, 3,3-dipropylthiocarbocyanine [DiSC3(5)], 2′,7′-bis-(2-carboxyethyl)-5(and -6)-carboxylfluorescein (BCECF), and the K ionophores valinomycin and nigericin (Nig) were purchased from Sigma (St. Louis, Mo.).
Chimeric glycinecin A was expressed and purified from Escherichia coli DH5α transformed with the glyAB genes as described previously (9). X. campestris pv. vesicatoria YK93-4 was used as a sensitive organism to test the bactericidal mode of action. E. coli cultures were grown in LB broth at 37°C, and X. campestris pv. vesicatoria was grown in nutrient broth medium (NB) at 28°C.
Purification of glycinecin A.
Glycinecin A was expressed in E. coli DH5α transformed with pSGEB as described previously (9) with slight modifications. Briefly, E. coli DH5α carrying the glyAB genes (pSGEB) was grown for 48 h at 37°C in LB medium containing ampicillin (50 μg/ml). The bacterial culture was centrifuged, and the supernatant was collected and precipitated with ammonium sulfate (30 to 60%). The precipitate was resuspended in 50 mM Tris-HCl (pH 8.0) and dialyzed overnight against 20 mM Tris-HCl (pH 8.0). The dialyzed solution was applied to a 3.0- by 15-cm Q-Sepharose column (Pharmacia, Uppsala, Sweden), the column was washed with 20 mM Tris-HCl (pH 8.0) until the A280 signal returned to baseline, and the bound proteins were eluted with a linear gradient of 0 to 1.0 M NaCl in the same buffer at a flow rate of 2 ml/min. The fractions were tested for activity against X. campestris pv. vesicatoria by spotting 10 μl of each fraction onto agar plates and overlaying the plates with 7 ml of 0.7% agar containing 100 μl of a solution of the test bacterium at an optical density at 600 nm (OD600) of 0.4. Active fractions were pooled, desalted, and concentrated with a Centricon 10 concentrator (Millipore, Bedford, Mass.). The concentrate was applied to a Mono Q HR 5/5 (Pharmacia) column preequilibrated with 20 mM Tris-HCl (pH 8.0). The column was washed with the same buffer and eluted with a linear gradient of 0 to 1.0 M NaCl at a flow rate of 1 ml/min. Fractions corresponding to A280 peaks were tested for activity against sensitive strains and concentrated by using Centricon concentrators as described above. The final concentrate was applied to a Sephacryl S-200 gel filtration column (Pharmacia) and eluted with 0.15 M NaCl in 20 mM Tris-HCl (pH 8) at a flow rate of 0.5 ml/min. Active fractions were tested and concentrated as described above. This partially purified glycinecin A was used in this study.
Quantification of bacteriocin activity.
The lethal activity of bacteriocin suspensions was determined by spot tests. Serial dilutions (twofold) of the glycinecin A preparation in sterilized 20 mM Tris-HCl (pH 8.0) were made, and 10 μl of each dilution was spotted onto LB agar and allowed to dry for 10 min. The plate was overlaid with 7 ml of soft agar (0.7%, wt/vol) containing 0.1 ml of the indicator strain (OD600, 0.4) and was incubated overnight at 28°C. The bacteriocin titer was defined as the reciprocal of the highest dilution factor that showed inhibition of the indicator strain. The activity was calculated as titer−1 × 100 and was expressed in arbitrary units (AU) per milliliter.
Effect of glycinecin A on the viability of Xanthomonas cells.
X. campestris pv. vesicatoria cells were grown to mid-exponential phase at 28°C in NB, centrifuged (3,000 × g, 10 min), washed twice with 20 mM Tris-HCl (pH 8.0), and diluted to an OD600 of 0.3 (corresponding to 108 to 109 cells/ml) in the same buffer. The cells were then diluted 10-fold in 20 mM Tris-HCl (pH 8.0) containing glycinecin A at a final concentration of 5,120 AU/ml. Samples were taken at the indicated times and diluted 10- to 10,000-fold in 20 mM Tris-HCl (pH 8.0). Aliquots (20 μl) were spotted in duplicate onto NB agar plates for overnight culture at 28°C, and CFU were counted manually.
The relationship between the glycinecin A concentration and its lethal activity on X. campestris pv. vesicatoria cells was tested as follows. X. campestris pv. vesicatoria cells were grown with shaking overnight at 28°C, harvested, washed with 20 mM Tris-HCl (pH 8.0), and resuspended in the same buffer to an OD600 of 1.0. Glycinecin A was then added at varying concentrations to 1 ml of the bacterial culture, and the mixtures were incubated for 120 min. The cultures were centrifuged, and the pellets were resuspended in 10 ml of fresh NB and incubated at 28°C for 10 h in a rotary shaker. The OD600 was monitored every 2 to 3 h by using a Tecan (Salzburg, Austria) ELISA reader.
Effects of glycinecin A on membrane proton motive force of sensitive cells. (i) Measurement of ΔΨ.
The membrane potential (ΔΨ) of X. campestris pv. vesicatoria YK93-4 cells was measured by monitoring the fluorescence intensity of the fluorescent probe DiSC3(5) as described previously (7) with the following modifications. The cells were harvested in the exponential phase of growth (OD600, 0.4) by centrifugation (3,000 × g, 10 min) at 4°C, washed twice in 50 mM K-HEPES (pH 7.0), resuspended in the same buffer to approximately 1/20 of the original volume, and stored on ice until use. The cell suspension was diluted to a final OD600 of 0.3 in 50 mM K-HEPES containing 10 mM glucose and 5 μM DiSC3(5). The fluorescence intensity was measured with an F-4500 spectrophotometer (Hitachi Co. Ltd., Tokyo, Japan) at 30°C in a stirred cuvette (excitation wavelength of 643 nm, slide width of 5 nm; emission wavelength of 666 nm, slide width of 5 nm). The pH gradient (ΔpH) was depleted by addition of the H/K exchanger nigericin to a final concentration of 5 μM. After a steady-state ΔΨ was reached, glycinecin A was added to the reaction mixture at a final concentration of 5,120 AU/ml. The K ionophore valinomycin (final concentration, 5 μM) was used to obtain control samples with no ΔΨ.
(ii) Membrane ΔpH measurement.
The transmembrane ΔpH of target cells was measured by monitoring the fluorescence intensity of the pH-sensitive fluorescent probe BCECF as described previously (18). Measurements were made with an F-4500 spectrophotometer (Hitachi) in a stirred cuvette (excitation wavelength of 502 nm, slide width of 5 nm; emission wavelength of 525 nm, slide width of 10 nm).
Measurement of the extracellular K+ ion concentration.
The effects of glycinecin A on the efflux of K+, Mg2+, and PO43− ions were measured as described previously with slight modifications (24). Briefly, a 100-ml culture of X. campestris pv. vesicatoria cells was grown with shaking at 28°C to an OD600 of 0.6. The cells were harvested, washed three times in 20 mM Tris-HCl (pH 8), and resuspended to their original volume in the same buffer. Glycinecin A was then added to a final concentration of 5,120 AU/ml. Samples were collected at the indicated time after the addition of glycinecin A and immediately centrifuged at 10,000 × g for 5 min, and the supernatants were frozen in liquid nitrogen. Negative-control samples were not treated with glycinecin A, and positive control samples were heated to 100°C for 10 min. The concentrations of K+, Mg2+, and PO43− ions were determined on an atomic absorbance spectrometer (Perkin-Elmer 3100; Perkin-Elmer Optoelectronics, Fremont, Calif.), and each treatment was performed in triplicate.
RESULTS
Effects of glycinecin A on the viability of sensitive Xanthomonas cells.
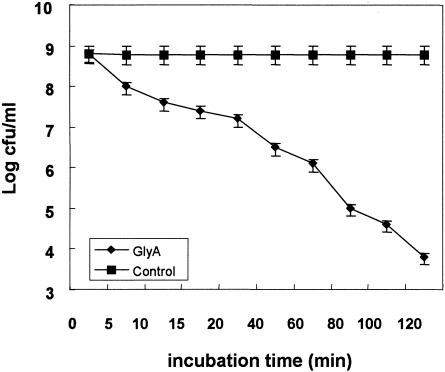
The antibacterial activity of glycinecin A on X. campestris pv. vesicatoria cells was assayed by incubating the cells with glycinecin A at a concentration of 5,120 AU/ml. As shown in Fig. 1, the survival of cells decreased as a function of time, diminishing gradually after 20 min of treatment with glycinecin A. Survival decreased markedly after 60 to 120 min of incubation, with decreases in viability of 1,000- to 100,000-fold.
FIG. 1.
Effects of glycinecin A on the viability of X. campestris pv. vesicatoria cells as a function of time. ⧫, viability of X. campestris pv. vesicatoria cells incubated in 20 mM Tris-HCl (pH 8.0) after exposure to 5,120 AU of glycinecin A/ml; ▪, viability of control cells incubated in 20 mM Tris-HCl (pH 8.0) alone. Error bars, standard deviations for three independent experiments.
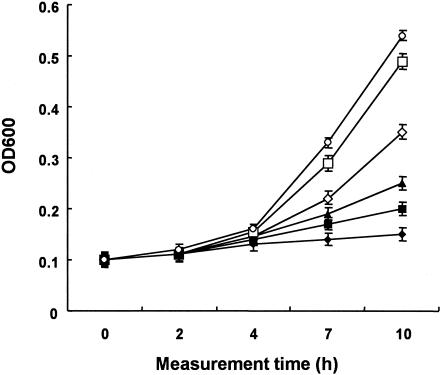
Figure 2 shows the relationship between the killing activity and the concentration of glycinecin A. A decrease in the concentration of glycinecin A led to a decrease in its bactericidal activity during 120 min of treatment. A glycinecin A concentration of 5,120 AU/ml inhibited the growth of X. campestris pv. vesicatoria cells almost completely after 120 min of incubation. However, glycinecin A at a concentration of 2,560 AU/ml did not kill all of the X. campestris pv. vesicatoria cells after 120 min of treatment; and the culture had an OD600 of 0.2 after 10 h of recovery incubation with shaking at 28°C. Further reductions of the glycinecin A concentration showed further increases in the OD600. The lethal activity of the bacteriocin was very low at a final concentration of 320 AU/ml, and a recovery OD600 of 0.5 was measured.
FIG. 2.
Effects of varying doses of glycinecin A on X. campestris pv. vesicatoria cells. Glycinecin A was added to a 1-ml sample of an overnight culture to a final concentration of 5,120 (⧫), 2,560 (▪), 1,280 (▴), 640 (◊), 320 (□), or 0 (•) AU/ml, and the cultures were incubated for 120 min. Next, 10 ml of fresh NB were added, the cultures were shaken at 28°C, and OD600 was monitored every 1 to 2 h. Error bars, standard deviations for three independent experiments.
Effects of glycinecin A on the membrane proton motive force of target cells. (i) Effects of glycinecin A on the ΔpH of X. campestris pv. vesicatoria cells.
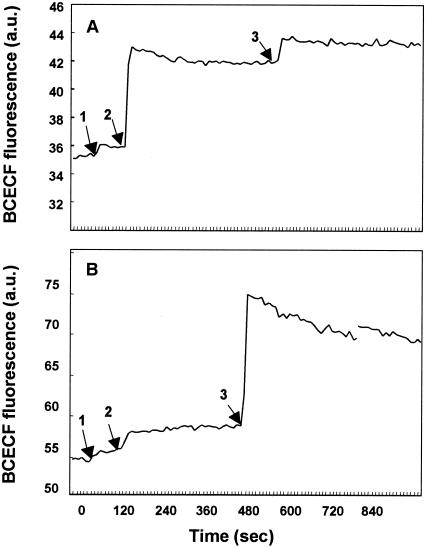
The fluorescent probe BCECF can be used to measure the transmembrane ΔpH. Changes in the fluorescence intensity of this reagent, due to extrusion or loss of intracellular BCECF, indicate depletion of the membrane ΔpH (18). Glycinecin A treatment caused a marked increase in the fluorescence intensity of BCECF-loaded X. campestris pv. vesicatoria cells, indicating depletion of the membrane ΔpH of these cells (Fig. 3A). The ΔpH of sensitive cells was dissipated almost completely by glycinecin A treatment, as indicated by a small increase in the fluorescence intensity due to the H+/K+ exchanger nigericin, which rapidly caused the pH of the cells to revert to that of the medium (7). In contrast, glycinecin A did not dissipate the ΔpH of E. coli cells (Fig. 3B), although treatment with 5 μM nigericin was able to completely dissipate the ΔpH of these cells. These results suggest that glycinecin A selectively induces leakage of ions in X. campestris pv. vesicatoria cells but not in E. coli cells.
FIG. 3.
Effects of glycinecin A and nigericin on the ΔpH of X. campestris pv. vesicatoria (A) and E. coli (B) cells, as indicated by the efflux of the fluorescent dye BCECF. BCECF-loaded and glucose-energized cells were diluted in 50 mM potassium phosphate buffer. Valinomycin was added to a concentration of 5 μM at the time indicated by arrow 1 to dissipate ΔΨ, and glycinecin A (5,120 AU) and nigericin (5 μM) were added at the times indicated by arrows 2 and 3, respectively. Data represent results of three independent experiments.
(ii) Effects of glycinecin A on the ΔΨof X. campestris pv. vesicatoria cells.
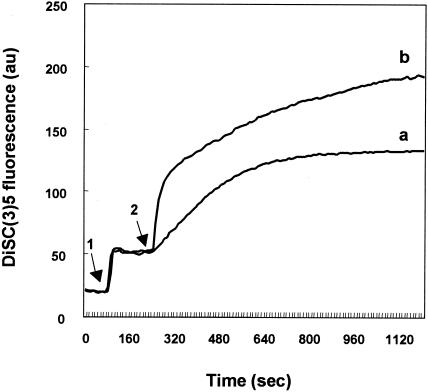
Many bacteriocins dissipate the transmembrane potential related to active transport by first inducing leakage of ions through the formation of voltage-dependent channels in phospholipid bilayer membranes. This leads to the loss of ions, such as potassium and magnesium, which is the primary cause of cell death. The effect of glycinecin A on the ΔΨ of Xanthomonas cells was measured by using the fluorescence intensity of the potentiometric dye DiSC3(5). Changes in the fluorescence of the dye indicate the generation or disruption of ΔΨ (2). In cells energized with glucose, a rapid quenching of fluorescence was observed after addition of the dye (data not shown), indicating the generation of ΔΨ. The ΔΨ of X. campestris pv. vesicatoria cells was dissipated upon addition of glycinecin A to a concentration of 5,120 AU/ml. The increase in the fluorescence intensity of DiSC3(5) reflected the disruption of the ΔΨ. Valinomycin at 5 μM dissipated the ΔΨ of X. campestris pv. vesicatoria cells almost completely after 15 min of treatment. Glycinecin A at a concentration of 5,120 AU/ml disrupted the ΔΨ of similar cells more gradually than did valinomycin at 5 μM (Fig. 4).
FIG. 4.
Effects of glycinecin A (a) and valinomycin (b) on the ΔΨ of X. campestris pv. vesicatoria cells, as indicated by the fluorescence intensity of the dye. Cells were energized with 10 mM glucose and labeled with DiSC3(5). Next, the cell suspension was treated with 5 μM nigericin at the time indicated by arrow 1 to dissipate the transmembrane ΔpH, and valinomycin at 5 μM or glycinecin A at 5,120 AU/ml was added at the time indicated by arrow 2. Data represent results of three independent experiments.
Glycinecin A was observed to dissipate the ΔΨ further after 20 min of measurement (data not shown). This result implies that the bactericidal action of glycinecin A progresses slowly, as the survival of sensitive cells decreased with increasing incubation time. ΔΨ may be a primary indicator of the further efflux of ions, such as potassium ions, in X. campestris pv. vesicatoria cells after glycinecin A treatment.
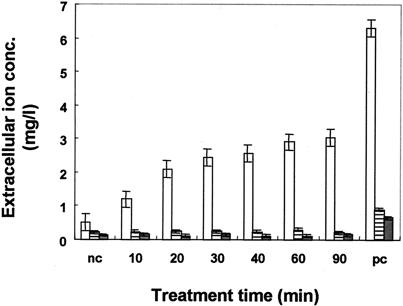
Effects of glycinecin A on the effluxes of K+, Mg2+, and PO43− ions from X. campestris pv. vesicatoria cells.
To confirm the activity of glycinecin A on X. campestris pv. vesicatoria cells, the effluxes of K+, Mg2+, and PO43− ions were measured as described above. Figure 5 shows the effluxes of these ions with time after glycinecin A treatment. The total intracellular K+ ion content of the bacterial suspension (108 to 109 cells/ml) was released by boiling, to a concentration equal to 6.4 mg/liter. Samples treated with glycinecin A for 10 min displayed slightly greater changes in the extracellular potassium ion concentration than untreated controls. This suggests that the K+ voltage channels of sensitive cells were nonfunctional and open after 10 min of incubation with glycinecin A, as indicated by the extracellular K+ ion concentration, which increased markedly after 10 min, up to 2.9 mg/liter after 60 min of incubation, representing nearly half of the total K+ in the cells. These data demonstrate a strong correlation between the release of K+ ions and the percentage of surviving cells in cultures incubated for varying times with glycinecin A. The significant increase in K+ efflux was observed during a period of 10 to 60 min of treatment, corresponding to a marked increase in cell death (Fig. 1).
FIG. 5.
Effects of glycinecin on the effluxes of K+, Mg2+, and PO43− ions. Indicator cells were incubated with glycinecin A at a final concentration of 5,120 AU/ml, and samples were taken at the indicated times to measure the extracellular K+ (open bars), Mg2+(striped bars), and PO43− (shaded bars) ion concentrations. The negative control (nc) was a bacterial suspension incubated in 20 mM Tris-HCl (pH 8) alone for 120 min. The positive control (pc) was a boiled bacterial suspension. Error bars, standard deviations for three independent experiments.
Interestingly, the extracellular concentrations of Mg2+ and PO43− ions showed no changes during the time of the treatment in comparison to untreated controls. This indicates that the increase in the extracellular concentration of K+ is not due to the death of the cells (Fig. 5).
DISCUSSION
In general, pore-forming bacteriocins have lethal activity because they alter the permeability of the cell membrane, disrupting the membrane proton motive force and the efflux of ions, such as potassium, magnesium, sodium, and chloride (3, 7, 8, 20). As with other known two-component bacteriocins, including the plantaricin A system, plantaricin S, lactococcin S, and lactococcin M, which cause the efflux of intracellular potassium ions, the dissipation of proton motive force leads to the hydrolysis of ATP in susceptible bacteria (20). Glycinecin A also induces the dissipation of ΔΨ, depletion of ΔpH, and efflux of potassium ions. Plantaricin JK and EF disrupt the proton motive force (ΔΨ and ΔpH) of sensitive cells. However, ΔpH depletion is more effective at inhibiting cell growth than the dissipation of ΔΨ. The depletion of ΔpH leads to a drop in the intracellular pH and subsequent inhibition of cellular metabolism (19). Our results indicate that glycinecin A causes slow dissipation of ΔΨ but rapid depletion of ΔpH. This suggests that glycinecin A inhibits the growth of sensitive cells by the dissipation of ion channels, leading to efflux of hydroxyl ions followed by leakage of potassium ions. The antimicrobial activities of lactococcins A, B, and G and other class II bacteriocins are thought to require a receptor-like factor (8). The disruption of the ΔpH of X. campestris pv. vesicatoria cells by glycinecin A, which is not observed with E. coli cells, may be due to a specific receptor that is present in the former cells but not in the latter.
The circulation of potassium ions is important in several homeostatic mechanisms, such as the regulation of intracellular pH and osmotic strength. Electrogenic potassium efflux causes the collapse of ΔΨ, followed by inhibition of the uptake of amino acids. Release of potassium ions causes an increase in ATP hydrolysis by F0F1-ATPase in the cytoplasm of sensitive strains (20). The loss of intracellular K+ and the dissipation of proton motive force (ΔΨ and ΔpH) cause ATP depletion but not ATP efflux, leading to disruption of the active transport of organic and inorganic molecules into and out of the cell, which causes the death of susceptible strains (7, 17, 19). On the basis of ion selectivity, two-peptide bacteriocins are divided into two subgroups: the first contains monovalent cation-conducting systems, such as lactococcin and plantaricin EF, while the second contains bacteriocins with a preference for anions, such as plantaricin JK and possibly acidocin J1132 (19). Glycinecin A treatment caused slight leakage of cations, such as K+ and H+, as indicated by depletion of ΔpH and increases in the extracellular potassium ion concentration following its addition. Glycinecin A causes a slow efflux of potassium ions, and its lethal action is strictly dose dependent. Pore-forming peptides may cause the efflux of macromolecules, such as β-galactosidase (10, 23), and the release of A280-absorbing materials (14, 17). Our studies indicate that glycinecin A does not cause the efflux of macromolecules or induce the release of A280-absorbing materials (data not shown) from sensitive cells, which suggests that the release of K+ is not due to cell death.
In conclusion, the bactericidal activity of glycinecin A on X. campestris pv. vesicatoria cells may depend on the formation of voltage channels, which leads to the efflux of potassium ions and the dissipation of ΔΨ and ΔpH. The time-dependent killing activity and the release of potassium ions observed in this study indicate that potassium ion leakage occurs simultaneously with cell death. Meanwhile, the bacteriocin did not cause the release of Mg2+ and PO43− ions. These suggests that potassium leakage is the main cause of death of X. campestris pv. vesicatoria cells triggered by glycinecin A treatment.
Acknowledgments
We are grateful to Youngmee Kim, Hee Kyoung Lim, and Yoon Suk Kang for technical assistance in the purification of glycinecin A.
This work was supported by the Korean Science and Engineering Foundation (KOSEF) through the Subtropical Horticulture Research Center at Cheju National University and by a Korea Institute of Science and Technology Evaluation and Planning (KISTEP) grant (M6-0105-00-0055).
REFERENCES
- 1.Braun, V., H. Pilsl, and P. Gross. 1994. Colicins, structures, modes of action, transfer through membranes, and evolution. Arch. Microbiol. 161:199-206. [DOI] [PubMed] [Google Scholar]
- 2.Breeuwer, P., and T. Abee. 2000. Assessment of viability of microorganisms employing fluorescence techniques. Int. J. Food Microbiol. 55:193-200. [DOI] [PubMed] [Google Scholar]
- 3.Daw, M. A., and F. R. Falkiner. 1996. Bacteriocins: nature, function and structure. Micron 27:467-479. [DOI] [PubMed] [Google Scholar]
- 4.Duche, D. 2002. The pore-forming domain of colicin A fused to a signal peptide: a tool for studying pore-formation and inhibition. Biochimie 84:455-464. [DOI] [PubMed] [Google Scholar]
- 5.Duport, C., C. Baysse, and Y. M. Briand. 1995. Molecular characterization of pyocin S3, a novel S-type pyocin from Pseudomonas aeruginosa. J. Biol. Chem. 270:8920-9827. [DOI] [PubMed] [Google Scholar]
- 6.Hechard, Y., and H. G. Sahl. 2002. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84:545-557. [DOI] [PubMed] [Google Scholar]
- 7.Herranz, C., L. M. Cintas, P. E. Hernandes, G. N. Moll, and A. J. M. Driessen. 2001. Enterocin P causes potassium ion efflux from Enterococcus faecium T36 cells. Antimicrob. Agents Chemother. 45:901-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herranz, C., Y. Chen, H. J. Chung, L. M. Cintas, P. E. Hernandez, T. J. Montville, and M. L. Chikindas. 2001. Enterocin P selectively dissipates the membrane potential of Enterococcus faecium T136. Appl. Environ. Microbiol. 67:1689-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heu, S. G., J. H. Oh, Y. S. Kang, S. R. Ryu, S. K. Cho, Y. S. Cho, and M. J. Cho. 2001. gly gene cloning and expression and purification of glycinecin A, a bacteriocin produced by Xanthomonas campestris pv. glycines 8ra. Appl. Environ. Microbiol. 67:4105-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisham, R. I., Y. Sugimoto, and T. Aoki. 2000. Ovotransferrin antimicrobial peptide (OTAP-92) kills bacteria through a membrane damage mechanism. Biochim. Biophys. Acta 1523:196-205. [DOI] [PubMed] [Google Scholar]
- 11.Jabrane, A., A. Sabri, P. Compere, P. Jacques, I. Vandenberghe, J. Van Beeumen, and P. Thonart. 2002. Characterization of serracin P, a phage-tail-like bacteriocin, and its activity against Erwinia amylovora, the fire blight pathogen. Appl. Environ. Microbiol. 68:5704-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon, Y. H., M. J. Cho, Y. S. Cho, and I. G. Hwang. 2001. Effect of glycinecin A on the control of bacterial leaf spot of pepper and bacterial leaf blight of rice. Korean J. Plant Pathol. 17:249-256. [Google Scholar]
- 13.Kim, Y. M., S. K. Cho, and M. J. Cho. 2001. Improvement in the stability of glycinecin A through protein fusion of the two structural components. Korean J. Microbiol. 39:177-180. [Google Scholar]
- 14.Manca de Nadra, M. C., D. D. Sandino, and A. M. Strasser de Saad. 1998. Pediocin N5p from Pediococcus pentosaceus: adsorption on bacterial strains. Int. J. Food Microbiol. 39:79-85. [DOI] [PubMed] [Google Scholar]
- 15.Masaki, H., T. Ogawa, K. Tomita, T. Ueda, K. Watanabe, and T. Uozumi. 1997. Colicin E5 as a new type of cytotoxin, which cleaves a specific group of tRNAs. Nucleic Acids Symp. Ser. 37:287-288. [PubMed] [Google Scholar]
- 16.McAuliffe, O., M. P. Ryan, R. P. Ross, C. Hill, P. Breeuwer, and T. Abee. 1998. Lacticin 3147, a broad spectrum bacteriocin which selectively dissipates the membrane potential. Appl. Environ. Microbiol. 64:439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minahk, C. J., M. E. Farias, F. Sesma, and R. D. Morero. 2000. Effect of enterocin CRL35 on Listeria monocytogenes cell membrane. FEMS Microbiol. Lett. 192:79-83. [DOI] [PubMed] [Google Scholar]
- 18.Molenaar, D., T. Abee, and W. N. Konings. 1991. Measurement of intracellular pH in bacteria with a fluorescent probe. Biochim. Biophys. Acta 1115:75-83. [DOI] [PubMed] [Google Scholar]
- 19.Moll, G., E. van den Akker, H. H. Hauge, J. N. Meyer, I. F. Nes, W. N. Konings, and A. J. M. Driessen. 1999. Complementary and overlapping selectivity of the two-peptide bacteriocins plantaricin EF and JK. J. Bacteriol. 181:4848-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moll, G., T. Ubbink-Kok, H. Hildeng-Hauge, J. Niseen-Meyer, I. F. Nes, W. N. Konings, and A. J. M. Driessen. 1996. Lactococcin G is a potassium ion-conducting, two-component bacteriocin. J. Bacteriol. 178:600-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montville, T. J., and M. E. C. Bruno. 1995. Evidence that dissipation of proton motive force is a common mechanism of action for bacteriocins and other antimicrobial proteins. Int. J. Food Microbiol. 24:53-74. [DOI] [PubMed] [Google Scholar]
- 22.Ringose, P. 1970. Sedimentation analysis of DNA degradation products resulting from the action of colicin E2 on Escherichia coli. Biochim. Biophys. Acta 213:320-334. [DOI] [PubMed] [Google Scholar]
- 23.Silvestro, L., N. W. Jeffrey, and P. H. Axelsen. 2000. Antibacterial and antimembrane activities of cecropin A in Escherichia coli. Antimicrob. Agents Chemother. 44:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauch, E., H. Kaspar, C. Schaudinn, P. Dersch, K. Madela, C. Gewinner, S. Hertwig, J. Wecke, and B. Appel. 2001. Characterization of enterocoliticin, a phage tail-like bacteriocin, and its effect on pathogenic Yersinia enterocolitica strains. Appl. Environ. Microbiol. 67:5634-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo, J., S. G. Heu, and Y. S. Cho. 1998. Influence of growth conditions on the production of a bacteriocin, production by Xanthomonas campestris pv. glycines 8ra. Korean J. Plant Pathol. 14:376-381. [Google Scholar]
- 26.Zarivach, R., E. Ben-Zeev, N. Wu, T. Auerbach, A. Bashan, K. Jakes, K. Dickman, A. Kosmidis, F. Schluenzen, A. Yonath, M. Eisenstein, and M. Shoham. 2002. On the interaction of colicin E3 with the ribosome. Biochimie 84:447-454. [DOI] [PubMed] [Google Scholar]