Abstract
Background
Foot ulcers and their complications are an important cause of morbidity and mortality in diabetes. The present study examines the amputation risk criterion and the long term outcome in terms of amputations and mortality in patients with diabetic foot.
Methods
27 patients with diabetic foot lesions were studied. There were 15 patients with early lesions and 10 with advanced lesions. 15 patients were managed conservatively including local amputations and 12 with lower extremity amputations. 80% patients were males in 45-59 years of age group and all patients had more than 6 years of poorly controlled diabetes.
Results
Precipitating factors included walking barefoot, history of minor trauma, infection, callosities or burns in 86% of patients. Major lower limb amputations were common in irregularly treated, poorly controlled diabetics due to infection in a limb devitalized by angiopathy and desensitised by neuropathy.
Conclusion
Diabetic foot ulcers are associated with high morbidity and mortality. Mortality was higher in ischaemic ulcers than neuropathic ulcers.
Key Words: Amputations, Diabetic foot, Aggressiveness
Introduction
Amputations of lower limbs in diabetic patients are more common than in non-diabetics and five out of six amputations occur in diabetes [1]. Statistics reveal that 25% of the hospital admissions among diabetics are for the foot lesions and of those presenting with diabetic foot, 40% require amputations [2]. 50-70% of all non traumatic amputations occur in diabetics [3].
The management includes control of diabetes, infections and assessment of the vascular status. This can be done by clinical assessment of the venous filling time and measurement of ankle brachial index as calculated by the hand held Doppler. A Doppler spectrum analysis will reveal spectral broadening, increase in the peak systolic velocity and a monophasic wave pattern [4]. The extent of infection in the tissue planes and the underlying tissues was assessed by plain X-ray of the part but CT scan, MRI or radionucleide scan was rarely used [5]. All patients were classified according to Wagner's grade and management protocol was modified as per grade of involvement [6]. The purpose of this study is to analyse and study the risk factors of lower limb amputations in diabetic patients.
Material and Methods
27 patients of diabetic foot managed between Sep 96 to May 2003 at different hospitals were studied. Foot lesions studied included gangrene of foot, chronic indolent ulcers and auto amputation of toes. Age, sex, duration and severity of diabetes and regularity of the anti diabetic treatment was recorded. Clinical examination included presence of peripheral pulses, trophic changes and features of neuropathy like loss of ankle jerk, reduced perception and sensory loss.
A hand held Doppler was used to calculate the ankle brachial index and venous filling time. ABI<0.9 and VFT>20 seconds were considered ischaemic. Radiograph of the feet were taken to see the joint degeneration, soft tissue infection and vessel calcification.
Angiography could not be carried out. Fundoscopy was done in all cases to detect retinopathy. All patients were managed as per grade of involvement by dressing with or without split skin grafting (SSG), wound debridement/drainage followed by wound care with or without SSG, local amputations and lower extremity amputations below or above knee. Two patients were sent to vascular surgery center for vascular intervention.
Results
There were 24 males and 3 females with a sex ratio of 8:1. The average age was 61.9 years with a range of 28 to 82 years. 60% of the patients were in the age group of 40-59 years. The average duration of the disease was 8 years and majority of the patients had diabetes for more than 5 years. 5 patients presented with foot lesion as their first symptom. The duration of the foot lesion ranged from 20 to 54 days with a mean of 38 days at time of presentation. Distribution of patients according to age, sex duration and severity of diabetes is summarized in Table 1.
Table 1.
Age, sex, duration and severity of diabetes in the patients
| Age group | Sex | Duration | Severity | |||||
|---|---|---|---|---|---|---|---|---|
| M | F | 1-5 | 5-10 | > 10 | Mild | Moderate | Severe | |
| 30-39 | 3 | − | − | 1 | 2 | − | − | 3 |
| 40-49 | 5 | 1 | 3 | 2 | 1 | − | 2 | 4 |
| 50-59 | 8 | 1 | 2 | 3 | 4 | 2 | 3 | 4 |
| 60-69 | 6 | 1 | 2 | 4 | 1 | 2 | 4 | 1 |
| 70 & above | 2 | − | − | 1 | 1 | − | 2 | − |
The precipitating factors included minor trauma 65%, burns 10%, infection 15% and callosities 10%. The mean fasting and 2 hours postprandial sugar was 251.2 ± 64.5 and 320.5 ± 68.75 mg/dl. 2 patients presented with ketosis. The incidence of associated complications other than foot lesions are shown in Table 2. The culture of aerobic organisms from the foot lesions was carried out in all patients. The nature of organisms isolated are shown in Table 3. The type of treatment offered is summarized in Table 4. Most of the patients with raised venous filling time, low ankle brachial index, proteinuria, raised serum cholesterol and an abnormal lipid profile underwent a lower extremity amputation. The distribution is shown in Table 5. 6 patients succumbed to infections or other diabetic complications either in preoperative or postoperative period. 5 patients with early foot lesions required only wound debridement and dressings.
Table 2.
Prevalence of diabetic complications in patients
| Complications | No. of patients | Percentage |
|---|---|---|
| Neuropathy | 12 | 40% |
| Retinopathy | 18 | 67% |
| Peripheral vascular disease | 3 | 10% |
| Nephropathy | 6 | 20% |
| Hypertension | 7 | 22% |
| Ischaemic heart disease | 14 | 52% |
| Other infections | 5 | 18% |
Table 3.
Nature of invading organisms
| Organisms | No. of patients | Percentage |
|---|---|---|
| Staphylococcus | 9 | 34% |
| Proteus | 6 | 23% |
| Pseudomonas | 4 | 15% |
| Streptococci | 7 | 25% |
| E Coli | 1 | 3% |
Table 4.
Type of treatment
| Wound debridement/drainage/SSG | 5 |
| Local amputations | |
| Ray | 5 |
| Toe | 2 |
| Forefoot | 3 |
| Lower extremity amputations | |
| below knee | 10 |
| above knee | 2 |
Table 5.
Qualitative variables in patients requiring lower extremity amputation
| Variable | Value | No. of patients |
|---|---|---|
| Venous filling time | > 20 secs | 10 |
| < 20 secs | 2 | |
| Ankle branchial index | > 0.9 | 3 |
| < 0.9 | 9 | |
| Proteinuria | + | 11 |
| − | 01 | |
| Lipid profile | Abnormal | 8 |
| Normal | 4 |
Discussion
Foot lesions are amongst the commonest indication for hospitalisation amongst diabetic patients [7]. 60-80% of the non-traumatic amputations are performed in diabetics and there is 15 fold risk of major amputations. 45-85% of lower extremity amputations are preventable if aggressive, prompt and correct line of treatment is followed [8]. Management includes control of diabetes, treatment of infections as per culture report from deep tissue curetting and assessment of vascular status. The Doppler spectral analysis revealed spectral broadening, increase in peak systolic velocity and a monophasic wave pattern. Arterial blocks are localized with recording of segmental pressure and pulse volume traces [9]. Diabetic neuropathy usually develops after many years of hyperglycemia. This was present in 60% of patients in this study. Although macrovascular disease was the major cause of morbidity and mortality, microvascular complications were also present at the time of diagnosis [10]. More sophisticated evaluation is done by transcutaneous oxygen measurement [11], Xenon 131 measured skin blood flow and skin temperature measurements [12]. This helps in designing thermographically controlled amputation flaps. Abnormal pressure points should be tackled with optical phlebiography [13], an optical method of assessing pressures and loads under the feet confirming that pathologically higher pressures occur under the metatarsal heads of the diabetic neuropathic foot during standing and walking [14]. The incidence of lower extremity amputation in a diabetic patient can be predicted by assessing various risk factors [15]. Risk factors significant in this study were rural origin, duration of diabetes, insulin therapy, poor compliance, irregular foot wear habit/walking barefoot, absent pedal pulses, retinopathy, proteinuria and abnormal lipid profile.
Male sex and age were not found to be significant risk factors in our study while smoking, obesity and hypertension were. History of trauma and family history of diabetes were significant risk factors. Below knee amputation is indicated for extensive infection, tissue destruction and intractable rest pain and was done in 10 patients. Toe amputation was done in patients with persistent toe ulceration, gangrene limited to toe and osteomyelitis of the distal or middle phalanx or a septic interphalangeal joint. Ray amputation was done in 5 patients where the necrotic process involved the base of the toe. Transmetatarsal amputation has practically no advantage over multiple ray amputations but was performed for foot salvage when the infection involved multiple toes or the metatarsophalangeal joint or the skin cover was inadequate [16].
Major amputations were required in patients with overwhelming sepsis or deep compartment abscesses with extensive forefoot gangrene or impending toe loss as shown in Fig 1. Aim of amputation is to preserve the extremity length, as longer the stump the better are the rehabilitation results [17].
Fig. 1.
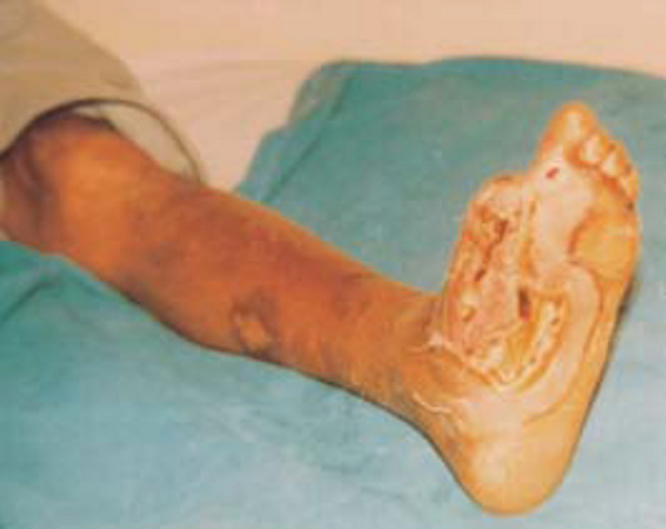
Involvement of sole in diabetic foot after great toe amputation
Broad spectrum parentral antibiotics were started empirically and later specific antibiotics were added as per culture report. Aerobic and anaerobic culture was done in patients with foot lesions. This reduces the rate of contralateral limb loss in patients with diabetes mellitus as the rate of contralateral limb loss ranges from 6-15% per year and up to 56% over five years after major lower extremity amputation (Fig 2). There is a greater need to devote more time in the examination of the contralateral limb as well as patient education in prophylactic skin and foot care [18]. These education programmes reduce the number of major amputations in patients with diabetes mellitus up to 50% as patients developing foot lesions have significantly less knowledge of diabetes including foot care.
Fig. 2.
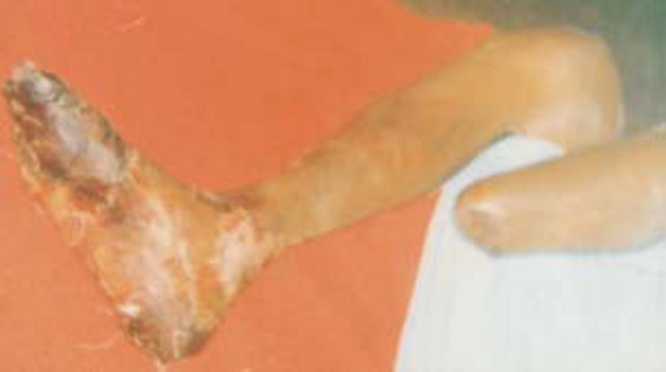
Extensive gangrene of forefoot and heel with left BK amputation
“Care your feet as your face or you will bury your feet before your face”
References
- 1.Campbell WB, Ponette D, Sugiono M. Long-term results following operation for diabetic foot problems: arterial disease confers a poor prognosis. Eur J Vase Endovasc Surg. 2000 Feb;19(2):174–177. doi: 10.1053/ejvs.1999.1006. [DOI] [PubMed] [Google Scholar]
- 2.Cavallini M, Murante G, Caterino S, Volpini M, Montesi M, Fallucca F. The diabetic foot seen by the surgeon. Personal experience. Minerva Chir. 2003, Mar;55(3):147–152. [PubMed] [Google Scholar]
- 3.Moulik PK, Mtonga R, Gill GV. Amputation and Mortality in. New Onset. Diabetic Foot Ulcers Stratified by Etiology. Diabetes Care. 2003;26:491–494. doi: 10.2337/diacare.26.2.491. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer DD, Pels S, Payne WG, Mannari RJ. Decreasing amputation rates in patients with diabetes mellitus. An outcome study. J Am Podiatr Med Assoc. 2002 Sep;92(8):425–428. doi: 10.7547/87507315-92-8-425. [DOI] [PubMed] [Google Scholar]
- 5.Van Damme H, Rorive M, Martens De Noorthout BM. Amputations in diabetic patients: a plea for footsparing surgery. Acta Chir Belg. 2001 May–June;101(3):123–129. [PubMed] [Google Scholar]
- 6.Vayussairat M, Le Devehat C. Critical analysis of vascular explorations in diabetic complications. J Mal Vasc. 2001 Apr;26(2):122–125. [PubMed] [Google Scholar]
- 7.Peng CW, Tan SG. Perioperative and rehabilitative outcomes after amputation for ischaemic leg gangrene. Ann Acad Med Singapore. 2000 Mar;29(2):168–172. [PubMed] [Google Scholar]
- 8.Todd WF, Armstrong DG, Liswood PJ. Evaluation and treatment of the infected foot in a community teaching hospital. J Am Podiatr Med Assoc. 1996;86(9):421–426. doi: 10.7547/87507315-86-9-421. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong DG, Lavery LA, Harkless LB. Who is at risk for diabetic foot ulceration? Clin Podiatr Med Surg. 1998;15(1):11–19. [PubMed] [Google Scholar]
- 10.Sykes MT, Godsey JB. Vascular evaluation of the problem diabetic foot. Clin Podiatr Med Surg. 1998;15(1):49–83. [PubMed] [Google Scholar]
- 11.Day MR, Harkless LB. Factors associated with pedal ulceration in patients with diabetes mellitus. J Am Podiatr Med Assoc. 1997;87(8):365–369. doi: 10.7547/87507315-87-8-365. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Clinical practice recommendations 2001, report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2001;24(suppl 1):S12. [PubMed] [Google Scholar]
- 13.Lavery LA, Vela SA, Lavery DC. Reducing dynamic foot pressures in high-risk diabetic subjects with foot ulcerations. Diabetes Care. 1996;19(8):818–821. doi: 10.2337/diacare.19.8.818. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Foot care in patients with diabetes mellitus. Diabetes care. 1998;21(Suppl 1):54–55S. [Google Scholar]
- 15.Frykberg RG. Diabetic foot ulcers: Current concepts. J Foot Ankle Surg. 1998;37(5):440–446. doi: 10.1016/s1067-2516(98)80055-0. [DOI] [PubMed] [Google Scholar]
- 16.Wieman TJ, Smiell JM, Su Y. Efficacy and safely of a topical gel formulation of recombination human platelet-derived growth factor-BB(becaplermin) in patients with chronic neuropathic diabetic ulcers: a phase III randomised placebo-controlled double-blind study. Diabetes care. 1998;21(15):822–829. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 17.Frykberg RG. Team approach toward lower extremity amputation prevention in diabetes. J Am Podiatr Med Assoc. 1997;87(7):305–312. doi: 10.7547/87507315-87-7-305. [DOI] [PubMed] [Google Scholar]
- 18.Patout CA, Jr, Birke JA, Horswell R. Effectiveness of a comprehensive diabetes lower-extremity amputation prevention (LEAP) program in a predominantly low income African American population. Diabetes Care. 2000;23:13339–13342. doi: 10.2337/diacare.23.9.1339. [DOI] [PubMed] [Google Scholar]


